Intestinal Immunity
The immune response of the intestine and most barrier organs was largely ignored in the early days of immunology. In part, this was because the field was justifiably preoccupied with gaining a basic understanding of immune cells and organs that were experimentally accessible: blood, lymph nodes, spleen, and bone marrow. In part it is due to the difficulty of identifying and isolating immune cells from mucosal tissues, once a daunting task only a few stalwart investigators tackled before the 1980s. However, it was also due to our collective obliviousness to what is now obvious—that those areas that are most exposed to microbes must have a well-developed immune system. The Society for Mucosal Immunology was finally formed in 1985, out of a recognition that the subfield deserved unique attention and its members needed a community to share and advance ideas.
As fetuses, we are sterile organisms. However, from the moment we are born, we begin establishing our microbiome. Maternal microbes acquired during birth and from breast-feeding are important pioneers, but other microbes from contact with skin, milk, and ultimately solid food rapidly join in the colonization effort. For instance, although Peyer’s patches begin to form at the end of gestation, they don’t fully develop until animals switch from milk to a mixed food diet, indicating that exposure to antigens—from food and from microbes—helps drive the maturation of the mucosal immune system.
Although some of these microbes cause disease, many provide benefits we cannot get elsewhere. Benign colonizers provide us with competitive protection from virulent microbes. Certain bacterial species provide us with key nutrients, like vitamins B12 and K. Others digest food that our own cells cannot break down. Some generate metabolites that directly enhance the health of our epithelium. And most importantly to this chapter, our microbial communities tune our own immune system, helping it to mature and establish an all-important balance between tolerance and responsiveness. And perhaps even more intriguingly, the relationship cultivated between our microbiome and our barrier immune systems influences tissues well beyond our barrier organs, and may help regulate autoimmune and inflammatory diseases, as well as neurological health and disease (Clinical Focus Box 13-2).
The Gut Is Organized into Different Anatomical Sections and Tissue Layers
Our gastrointestinal (GI) tract, or “gut,” is essentially a tube that runs continuously from the mouth to the anus; the inside of this long tube is referred to as the lumen. Well known for its role in digestion and absorption of food and water, the GI tract is now also known for its role in maintaining the well-being of our commensal microbiome and regulating both local and systemic immune responses (Figure 13-5).
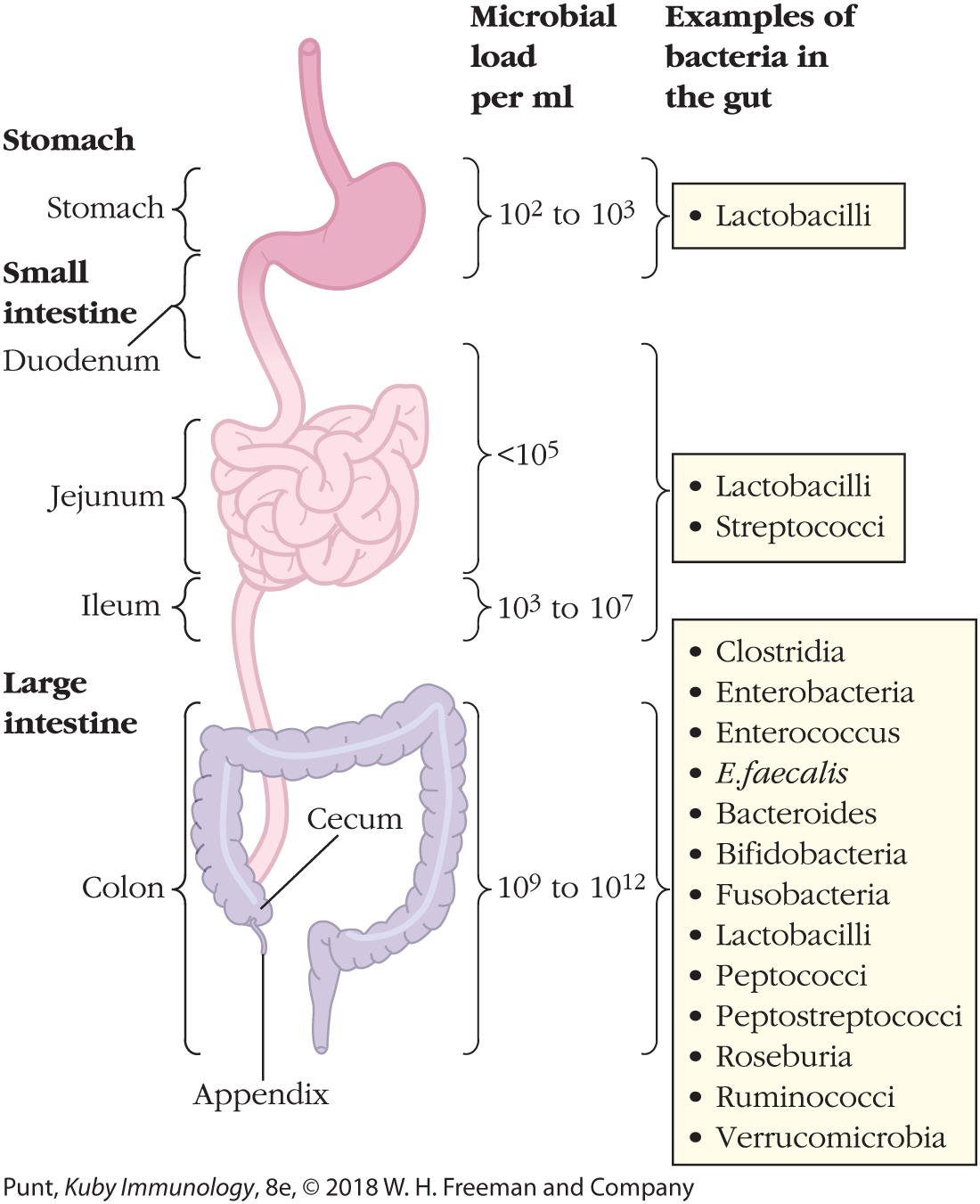
FIGURE 13-5 Gross anatomy of the gastrointestinal (GI) tract. Shown are the major parts of our GI tract, which is a single tube starting at the esophagus (not shown), extending through the stomach, the small intestine, and the large intestine. The small intestine is divided into three main sections: the short duodenum, which receives digestive enzymes from the pancreas, and the longer jejunum and ileum. The ileum connects to the large intestine, also known as the colon. Also shown are examples of commensal bacteria that inhabit different parts of the GI tract, as well as their quantities, which vary between sections and are most abundant in the large intestine.
Gastrointestinal tract is divided into three parts – stomach, small intestine, and large intestine. Microbial load per milliliter in stomach is 10 to the power 2 to 10 to the power 3 and example of bacteria in the gut is lactobacilli. Small intestine consists of duodenum, jejunum, and ileum. Microbial load per milliliter in duodenum and jejunum is less than 10 to the power 5 and that of ileum is 10 to the power 3 to 10 to the power 7. Examples of bacteria in the gut are lactobacilli and streptococci. Large intestine consists of colon with cecum and appendix. Microbial load per milliliter in large intestine is 10 to the power 9 to 10 to the power 12 and examples of bacteria are Clostridia, Enterobacteria, Enterococcus, E.faecalis, Bacteroides, Bifidobacteria, Fusobacteria, Lactobacilli, Peptococci, Peptostreptococci, Roseburia, Ruminococci, and Verrucomicrobia.
The GI tract is teeming with billions of organisms, including viruses, bacteria, and in most parts of the world, parasitic worms. The composition of the total intestinal microbiome is determined by a complex interplay of developmental, environmental, genetic, and immunological influences. An understanding of its gross and cellular anatomy will help our discussion of its immune system considerably.
The small intestine, which receives partially digested food from the stomach, consists of three segments—the duodenum, the jejunum, and the ileum. Digestive enzymes from the pancreas and bile from the gallbladder are excreted into the lumen of the short, C-shaped duodenum. The jejunum and ileum are long and looping—and the major sites of digestion and absorption of food. What we cannot digest in the small intestine enters the large intestine, or colon, which absorbs water as well as some vitamins, and delivers waste to the rectum and anus (see Figure 13-5).
The surfaces of the jejunum and, to a lesser extent, the ileum, are deeply folded and covered with projections known as villi (see Figure 13-3). The folds and villi greatly increase the surface area available for absorption and digestion, as well as the opportunities for our own cells to interact with the microbiome. The large intestine is shorter in length, has fewer folds, much shorter villi, and produces more mucus, which provides an additional protective layer from possible pathogens and lubricates the exit of waste (Figure 13-6).
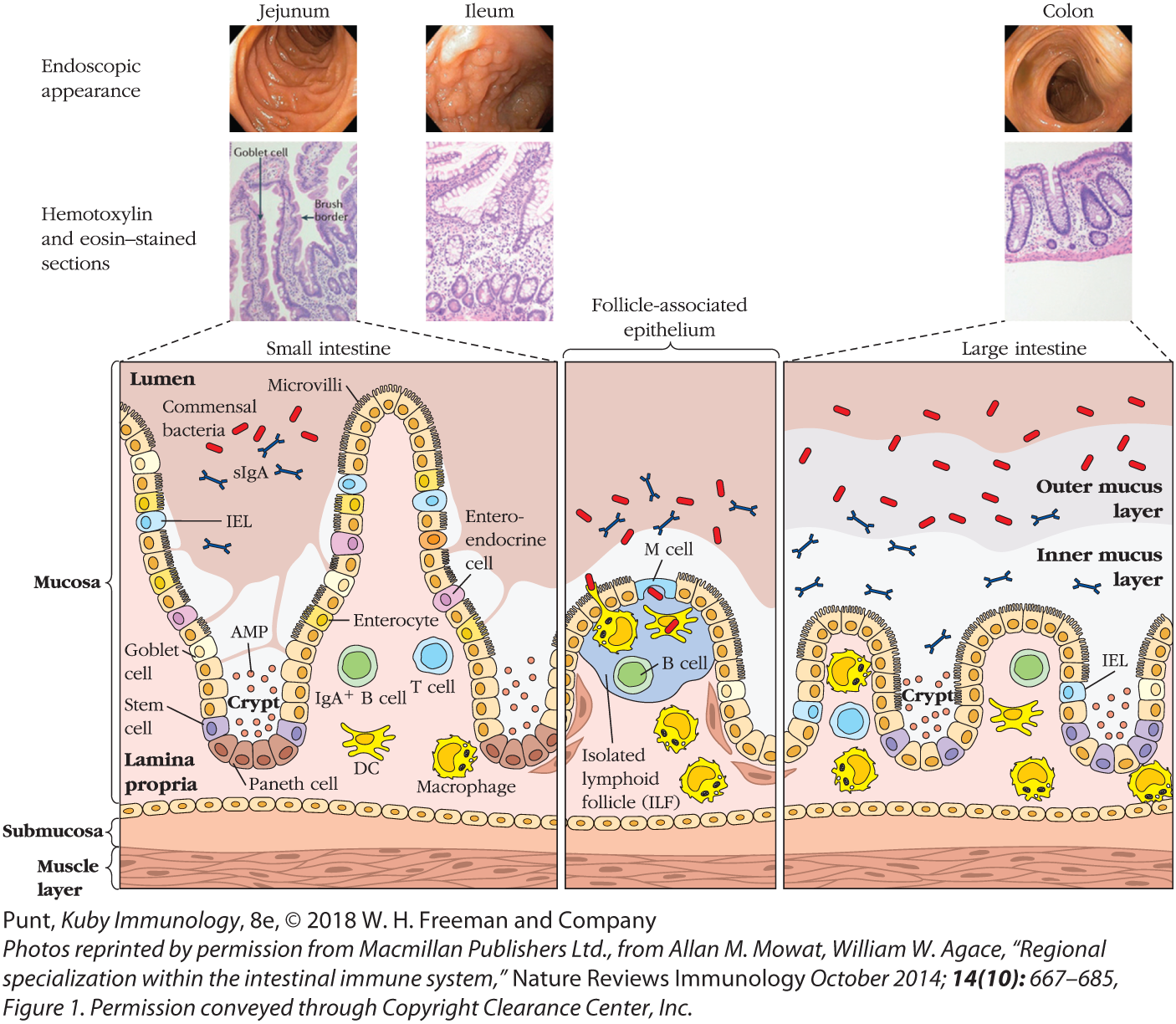
FIGURE 13-6 Cellular anatomy of the small and large intestines. Illustrated here are some of the differences between the small and large intestines, as well as the cells and molecules that populate the immune system of these barrier tissues. See text for details. Briefly, the small intestine has longer villi and deeper crypts. The large intestinal epithelial cells produce more mucus. Epithelial layers in both tissues include a variety of cell types and associate with a similar variety of innate and adaptive immune cells, most of which inhabit the lamina propria and some of which extend processes between the epithelial cells to sample the microorganisms at the surface (see text). Both sections include lymphoid follicles, which are often lined with M cells that deliver antigen to APCs via transcytosis. Peyer’s patches are present only in the small intestine.
Endoscopic appearance
Endoscopic appearance shows the inner layer with raised regions and shallow regions. The inner layer of ileum shows tiny projected regions on the inner wall. The inner layer of colon has a relatively smooth appearance.
Hemotoxylin and eosin–stained sections
The stained section of jejunum shows epithelial cells with brush border and goblet cells. The stained section of ileum shows finger like-projections with columnar cells in the outermost layer and ovoid-shaped cells in the central region. Stained section of colon shows the outer layer folding to form groves in epithelial layer. The center of the layer shows oval-shaped structures with hair like projections on the inner side.
Sectional View
The sectional view of all the three parts shows mucosa, submucosa, and muscle layer, from outside to inside. A layer of cell arranged in a wavy pattern separates the lumen from lamina propina. Furrow-like regions formed by the wavy-layer is labelled as crypt. This layer includes cells such as Goblet cell, Stem cell, Paneth cell, entero endocrine cell, Enterocyte, and I E L cells. Most cells in the epithelial layer have microvilli. AMP particles are shown in the crypt region. Another layer of cells separates the mucosa region from the submucosa region.
The Lumen portion in the mucosa region of the small intestine has commensal bacteria and SIgA. The mucosa region of the small intestine has DC, Macrophage, IGA plus, B cell, and T cell. The crypts are deeper and villi are longer in small intestine when compared to large intestine.
The section representing Follicle-associated epithelium layer shows a large M Cell with isolated lymphoid follicle (IFL) below it. This region has B cell, DC, and macrophage in the lamina propina region and commensal bacteria and s I g A in the lumen region. The section representing large intestine shows the wavy layer with short crests and troughs. IEL is labelled in the epithelial layer. Two layers- inner mucus layer and outer mucus layer are shown above the epithelial layer. In the outer mucus and the inner mucus layer of large intestine, commensal bacteria and SIgA are found.
The walls of the intestinal tract consist of two major layers: the mucosa, the layer in contact with the inside of the intestinal tube, which is known as the lumen, and the submucosa, the layer between the mucosa and the outer, muscular wall of the intestine (see Figures 13-3 and 13-6). The mucosa itself includes two layers: a single layer of diverse epithelial cells, and the lamina propria, a layer of connective tissue populated by resident and migrating immune cells as well as a network of capillaries and lymph vessels. Most immune cells of interest in the intestine are found in the lamina propria and epithelial layers, although some immune tissue also extends into the submucosa. A large number of plasma cells, most of which produce IgA against intestinal antigens, are present in the lamina propria. Most of these IgA-producing B cells are generated in isolated lymphoid follicles (ILFs) (Figure 13-7a).

FIGURE 13-7 IgA shuttled from the lamina propria to the lumen of the intestine. (a) Sections of the small intestine stained with green fluorescent anti-IgA antibodies (left) and hematoxylin and eosin (H&E; right). The ILF that helps generate IgA B cells is indicated in both panels. (b) IgA antibody made by plasma cells binds to polymeric Ig receptors (polyIgRs) and is transcytosed by epithelial cells as a dimer from the lamina propria to the lumen of the intestine.
Two stained sections of the small intestine are shown in the first part of the figure. The first section shows small intestine section stained with green fluorescent anti-IgA antibodies. The clearly visible villi (upper portion) and Lamina propria (lower portion) are labeled. A red-colored oval shaped structure in the lower portion of small intestine is labeled ILFs. The section on the right shows hematoxylin and eosin stained micrograph. In the micrograph, a small oval shaped structure in the lower portion of small intestine is labeled ILFs.
The sectional view shows two interconnected epithelial cells. Three pairs of three interconnected oval structures are shown at the junction of the two epithelial cells and a callout points toward them and reads tight junction. The upper portion of the epithelial cell is labeled apical surface and the lower portion of the epithelial cell is labeled basolateral surface. Each epithelial cell consists of an oval shaped structure labeled nucleus. The portion above the epithelial cell is labeled Lumen and the portion below the epithelial cell is labeled Lamina propria. In the lumen, bacteria, secretory IgA, antigen, and secretory component are present. The tubular structures in the upper end of the epithelial cells are labeled microvilli. Two small oval shaped structures labeled polyIgR are shown on the upper outline of epithelial cell between 2 microvilli. A plasma cell is shown in Lamina propria. An arrow from the plasma cell is connected to Dimeric IgA which has a J-chain at its front end. An arrow from Dimeric IgA points toward polymeric Ig receptor, which is connected to the bottom portion of the epithelial cells. An arrow from polymeric Ig receptor points toward another polymeric Ig receptor inside the epithelial cell and an arrow from polymeric Ig receptor points toward the lumen.
The folds that form the intestinal villi also form the intestinal crypts, the valleys between the villi “mountains.” These crypts contain distinct epithelial cell subsets, including the epithelial stem cells that continually replace the short-lived epithelial cell population (see Figure 13-6).
Key Concepts:
- The gastrointestinal tract contains trillions of microorganisms that influence our health in many different ways.
- The small intestine is the site of most digestion and absorption and has three sections (duodenum, jejunum, and ileum). The large intestine, or colon, is responsible for absorbing water and expelling waste and includes the highest number and diversity of bacteria.
- The small intestine is lined by microscopic folds called villi and crypts.
- The lamina propria is the tissue layer just under the gut epithelial layer and is the site of most immune cell activity.
Gut Epithelial Cells Vary in Phenotype and Function
The epithelial cells in the intestinal mucosa are more diverse in function and form than originally appreciated. Not only are they part of the digestive system, but they are now recognized as a key part of our innate immune system. The various gut epithelial cell types described here are shown in Figure 13-6.
Most epithelial cells are polarized—in other words, they have distinct tops and bottoms. A cell’s apical surface is in contact with the lumen of the gut and is invaginated with hundreds of microvilli. Its basolateral surface is in contact with the lamina propria. Apical and basolateral membranes include very distinct surface proteins, segregated from each other by the tight junctions that connect the epithelial cells to each other. These distinctions help the epithelial cell to convey information and molecules in precise directions. For instance, polymeric immunoglobulin receptors (polyIgRs) on the basolateral surface of epithelial cells capture and internalize IgA antibodies made in the lamina propria (Figure 13-7b). These antibodies are then conveyed via vesicles to the apical surface and released into the intestinal lumen, where they are largely resistant to breakdown by digestive enzymes and help keep bacteria at a healthy distance from the epithelial membranes. This process of shuttling molecules through epithelial cells is known as transcytosis. As we will see, transcytosis of antibodies and antigens across the intestinal epithelium is mediated by several epithelial cell types, and is critical to healthy immune function.
The most common cell in the epithelium of the small intestine, and the one traditionally associated with its digestive function, is the enterocyte. These cells are responsible for transporting nutrients from digested food from the apical layer to the capillaries and lymph vessels present in each villus. We now know that they also have the capacity to respond to microbes via pattern recognition receptors (PRRs) and send signals via cytokines to immune cells in the lamina propria. For example, although enterocytes do not seem to express a lot of surface Toll-like receptor 4 (TLR4), a receptor that binds to products of gram-negative bacteria (see Chapter 4), they do express TLR4 internally. This allows them to ignore some of the commensal bacteria in the lumen, but to alert the immune system if the bacteria have invaded and penetrated their membranes.
Goblet cells are distributed throughout the intestine, but found at highest concentration in the large intestine. Classically characterized by their ability to produce mucus, they also have other important immune functions. They secrete antimicrobial peptides (AMPs) that inhibit the activity of luminal microbes that come too close to their membranes. Indeed, the combination of mucus and AMPs provides a potent barrier between microbes and epithelial surfaces. Goblet cells also sense and transport antigen and live microbes from the lumen to antigen-presenting cells in the lamina propria. Finally, they have the ability to secrete regulatory cytokines.
Microfold (M) cells are highly specialized for the transcytosis of antigen across the epithelium. Their unique structure allows them more intimate contact than most epithelial cells with both the gut lumen and the immune cells in the lamina propria. Their apical surfaces are smoother than those of most epithelial cells because they have blunter microvilli and are covered by only a thin layer of mucus. Their basolateral surfaces are distinct and include a large pocket, or invagination, which is often occupied by immune cells. Most M cells form part of the epithelial layer directly above Peyer’s patches and isolated lymphoid follicles, and transfer antigens from the lumen of the intestine directly to dendritic cells within the follicle.
Paneth cells are secretory cells that inhabit the intestinal crypts. They play at least two critical roles. First, they act as supportive companions of stem cells and secrete factors that sustain them. Second, they secrete a variety of AMPs that protect the gut epithelium. Defects in the ability of Paneth cells to produce AMPs have been associated with inflammatory bowel disease (IBD).
Tuft cells are also highly specialized and bear a resemblance to the sensory taste cells found in our tongues. Typically very rare in the epithelial layer, tuft cells expand in number in response to worm infection. Recent work, discussed later, shows that tuft cells are a critical source of a cytokine that initiates the successful immune response to worms.
Finally, stem cells are found at the bottom of the crypts and give rise to all epithelial cell types, which turn over rapidly in the harsh environment of the gut. With the assistance of Paneth cells, stem cells proliferate and differentiate. Their progeny move smoothly up the walls of the crypt to the top of the villi, continually replacing damaged and dying cells that slough into the intestinal lumen.
Multiple hematopoietic cells are also intimately associated with gut epithelial cells (see Advances Box 13-1). Some antigen-presenting cells (dendritic cells and macrophages) extend processes between epithelial cells and directly sample antigen in the intestinal lumen. Intraepithelial lymphocytes(IELs) are also present in large numbers, particularly in the upper small intestine (the jejunum). Many of these express CD8, and some have been identified as tissue-resident memory cells that can respond rapidly, in an antigen-specific manner.
Key Concepts:
- Both small and large intestines are separated from the gut lumen by a single layer of epithelial cells that are diverse in phenotype and function.
- Many epithelial cells respond directly to microorganisms via PRRs. They cooperate with other innate and adaptive immune cells to sense, sample, and respond flexibly to the microbiome.
- Some epithelial cells secrete mucus and antimicrobial peptides to maintain an additional boundary between the microbiome and cell surfaces.
- Some hematopoietic cells, such as intraepithelial lymphocytes and antigen-presenting cells, are also intimately associated with the epithelial layers and join epithelial cells in communicating with immune cells in the lamina propria.