Other Barrier Immune Systems
For many good reasons, the intestinal immune system has been the most intensively studied mucosal immune tissue. In sheer size and cell density, it is the largest immune organ in our bodies. It is also relatively easy to access for experimental work. Although we still have much more to learn about the complex networks of cells and cytokines that regulate intestinal immunity, what we do know provides an excellent framework for exploring other barrier immune systems.
In this section, we introduce some of the fundamental features of two other barrier immune systems—the respiratory system and the skin. Many of the cellular and molecular players are the same as those found in the intestine and should be very familiar at this point. Therefore, we will focus most on features and questions that are unique to each.
The Respiratory Immune System Shares Many Features with the Intestinal Immune System
Like the GI tract, the respiratory tract is exposed directly to the external environment. Its total surface area is second in size only to the intestine (Figure 13-16a). The upper respiratory tract includes the nose and mouth. The lower respiratory tract starts with the trachea, which is separated from the oral cavity by a flap called the glottis. The trachea branches into bronchi, and then into smaller and smaller bronchioles, which ultimately dead end in clusters of microscopic sacs called alveoli. These are in intimate contact with capillary beds and are the site of gas exchange.

FIGURE 13-16 Gross and cellular anatomy of the respiratory tract. (a) The main anatomical structures of the respiratory tract, which starts in the nose and mouth, extends through the trachea, and branches into bronchi that serve both lungs. The bronchi divide into smaller areas called bronchioles and end in grapelike bunches of sacs called alveoli, where gas exchange with blood capillaries occurs. Many lymph nodes serve the respiratory tract, including the tonsils and bronchial lymph nodes. The airways are lined by a single epithelial layer (b), which gradually reduces in height and thickness as it reaches the alveoli, where it provides only a very thin layer of protection. The epithelial cells (c) are diverse in phenotype and function (see text) and in the alveoli are joined by a key immune participant, the alveolar macrophage or dust cell (a, c, and d). The dust cell interacts with epithelial cells via a variety of receptors (d) and helps to maintain respiratory homeostasis in part by secreting IL-10.
As in the intestinal tract, the walls of the lower respiratory tract are organized into multiple layers, including a single epithelial cell layer and the underlying lamina propria, which together constitute the respiratory mucosa. As one progresses down the respiratory tract, the walls thin. The height of the epithelial cells, the density of epithelial cell cilia, and the thickness of the lamina propria all diminish (Figure 13-16b). The alveolar walls are lined by very thin epithelium, allowing free passage of gases into surrounding capillaries.
The epithelial layer of the respiratory tract shares many of the same inhabitants as the intestinal tract (Figure 13-16c). These include goblet cells, M cells, and transepithelial processes of antigen-presenting cells. Like the intestinal epithelium, the respiratory epithelium also includes multipotent stem cells that replace damaged and dead epithelial cells. Respiratory epithelium also has some unique features. The apical surfaces of most respiratory epithelial cells have cilia. Together with the mucus produced by goblet cells, these form a mucociliary boundary that actively helps sweep away and expel microbes and particulates that have descended into the airways. The respiratory epithelial layer also includes a unique secretory cell called the club cell, formerly known as a Clara cell,2 which has a variety of protective functions. It is most prevalent in the lower airways of humans and throughout the airways of mice. Club cells also appear to have the ability to act as stem cells.
The alveoli are also the home of specialized alveolar macrophages (dust cells) that monitor the lower airways and alveoli for infection and work with respiratory epithelial cells to regulate the balance between tolerogenic and inflammatory responses in this very delicate site (Figure 13-16d).
The integrity of the respiratory epithelial barrier function is maintained by various antimicrobial proteins and peptides secreted by respiratory mucosal cells and by secretory IgA. Antigen-specific IgA is generated as described for the intestine, and its production depends, as expected, on the previous activation of both innate and T lymphoid cells.
The upper respiratory tract is home to commensal communities of microbes that contribute to immune protection and inspire tolerogenic activities, such as the development of regulatory T cells. The lamina propria of this portion of the respiratory system is home to many of the same subsets of innate and adaptive immune cells found in the intestine. In contrast, a healthy lower respiratory tract (from the trachea down) does not support a community of commensal bacteria and, instead, focuses on ridding itself of microbe visitors.
Secondary lymphoid tissue and isolated follicles can be found in the walls of the entire respiratory tract and is most developed in the nasal tissue (see Figure 13-16a). The nasal-associated lymphoid tissue, or NALT, is part of the ring of tissue that we often refer to as tonsils and adenoids. Comparable in organization to the Peyer’s patches of the intestine, it supports the activation of T cells and B cells triggered by innate cells in the respiratory mucosa. In some animals, such as rabbits and cats, well-organized lymphoid tissue is present in the deeper part of the lungs and is referred to as bronchus-associated lymphoid tissue, or BALT. In other animals, such as humans and mice, this tissue is very loosely organized and requires antigenic stimulation to develop fully. It is therefore referred to as inducible BALT (iBALT).
The lamina propria of the respiratory mucosa is home to populations of innate and lymphoid cells that are also found in the intestine, including CD103+ dendritic cells, CX3CR1+ macrophages, ILCs, regulatory T cells, IgA-secreting plasma cells, and more (Figure 13-17). Natural killer (NK) cells, also known as cytotoxic ILC1 cells, may even be more abundant in the lung than in the intestinal lamina propria. These cells are very effective at identifying and killing virally infected cells. As in the gut, ILC2s also play an important role in maintaining the integrity of the respiratory epithelium. They express the growth factor amphiregulin, which interacts with epidermal growth factor (EGF) receptors on the epithelium, enhancing their health and growth.

FIGURE 13-17 Type 1 and type 2 immune responses in the respiratory tract. Events are very similar to those described for the intestine. Briefly, viruses, and some bacteria and fungi, interact with PRRs (TLRs and NLRs) on antigen-presenting cells to trigger a type 1 response that activates ILC1 and TH1 cells, ILC3 and TH17 cells. These produce type 1 cytokines that activate effector cells, like cytotoxic cells and macrophages, which kill infected cells and engulf pathogens. Type 2 responses are triggered by worms and some allergens. As in the intestine, they induce the production of alarmins by epithelial cells, which activate ILC2, TH2, and TH9 cells, which generate type 2 cytokines and amphiregulin (AREG). See text for details.
As in the intestine, interactions of upper airway epithelial and immune cells with commensal microbes stimulate tolerogenic responses. They enhance the production and activity of regulatory T cells and class switching of B cells to the anti-inflammatory IgA phenotype. Under healthy conditions, alveolar macrophages in the lower airways receive anti-inflammatory signals from the epithelium that expresses ligands for TGF-β (e.g. integrins αvβ6) that interact with TGF-β receptors on the macrophage (see Figure 13-16d).
Interactions with invading microbes trigger a sequence of events roughly similar to those that occur in the intestine (see Figure 13-17). Bacterial and viral microbes engage pattern recognition receptors, including NLRs expressed by epithelium and antigen-presenting cells. This initiates a cascade of proinflammatory signals and cytokines, including IL-17, IL-23, and IL-1β, that program the antigen-presenting cells to induce type 1 responses and activate ILC1, TH1, and TH17 cells, as well as other subsets. As in the intestine, worms and allergens stimulate epithelial cells to produce alarmins, including IL-25, IL-33, and TSLP. These trigger a type 2 response, activating ILC2 and TH2 cells to produce IL-4, IL-5, and IL-15 and recruit effector eosinophils, basophils, and mast cells.
As in the intestine (see Figure 13-9), antigen-specific lymphocytes generated in lymph nodes draining the lung are induced to express homing receptors that send them back to respiratory tissues. CCR4 is one chemokine receptor that directs immune cells back to the lung, which expresses the CCR4 ligands CCL17 and CCL22. Interestingly, other barrier tissues, including the skin, can also attract CCR4+ cells. This behavior offers one mechanistic explanation for how antigen exposure in the lung (or intestine or skin) can have both local and systemic effects. It is also the basis for our hope that mucosal vaccination may help enhance general human health.
Example: Respiratory Immune Response to a Virus
Influenza virus is among the most common invaders of the respiratory epithelium. It gains access when its surface hemagglutinin protein binds to the terminal sugar, sialic acid, of surface carbohydrate side chains on epithelial and other innate immune cells in the epithelium. This interaction induces endocytosis of the virus. The virus fuses its membranes with those of the endocytic vesicle, releasing its single-stranded RNA genome into the cytoplasm of the infected cell.
As expected, cells are alerted to the virus’s presence via pattern recognition receptors (see Chapter 4). For instance, TLR7 and RIG-I bind flu’s RNA genome and NLRs bind to flu-specific surface proteins. These interactions set off signaling cascades that result in the production of inflammatory cytokines, including IL-1β, type I IFNs, and the alarmin IL-33. NK cells (cytotoxic ILC1 cells) specifically recognize and kill infected cells that display flu’s hemagglutinin proteins on their surface, an event that happens just before the budding of new viral particles. This, together with early type I IFN production, limits the initial spread of infection.
In addition, antigen-presenting cells that have been activated by exposure to the flu migrate to the NALT and iBALT, where they activate T and B cells that induce type 1 cytotoxic and humoral responses, locally and systemically. Memory T and B cells are also generated and are fundamental to the success of flu vaccines, which are designed to activate APCs either locally (in the case of nasal delivery) or systemically (in the case of intramuscular injection).
Allergy and Asthma in the Respiratory Tract
Asthma is an inappropriate respiratory immune response to nonpathogenic antigens and conditions. It is one example of a respiratory allergic response. Our understanding of its causes is still incomplete, even as its incidence continues to increase in large parts of the world.
Asthma can be initiated by microbes, pollens, pollution, obesity, and even cold temperatures. Antigen-inspired asthma triggers a type 2 immune response that, interestingly, involves many of the same players responsible for the immune response to worms in the intestine. TH2 cells, ILC2s, eosinophils, basophils, and IgE-producing B cells all contribute to the response, which is mediated by the classic type 2 cytokines, including IL-4, IL-5, and IL-13 (see Figure 13-17). Activation of tissue mast cells by IgE bound to allergens or by other signals triggers degranulation, with release of mediators such as histamine that were prepackaged in granules. The effects of these mediators on bronchial smooth muscle cells, mucus secretion, and blood vessels cause the classic symptoms of allergic responses (see Chapter 15). Chronic exposure to these stimuli induces more permanent changes in the bronchial tissue, resulting in asthma.
Intriguingly, exposure to intestinal worms may ameliorate allergic responses in the airways, including asthma. If you recall from our previous discussion, parasitic worm infection also has the paradoxical benefit of inducing a tolerogenic response in the gut. This response seems to have systemic effects that quell immune responses in distal sites. Attempts to replicate this influence in humans with worm antigens or worms themselves have been less successful than they have been in mice and remain a topic of active research.
Intranasal Vaccines
Mucosal vaccines represent a promising effort to translate basic knowledge of mucosal immunity to the clinics. Intranasal vaccination may be particularly useful because of the relative ease of delivery, as well as its ability to induce both local and systemic immune responses. As shown in Figure 13-18, exposure of respiratory tract APCs to antigen (e.g., either live attenuated virus or lipid-associated protein antigen particles) can result in both local and systemic adaptive immune memory. The APCs travel to draining lymph nodes, stimulating the generation of mucosal tissue–homing T cells and B cells.

FIGURE 13-18 Nasal vaccination: exploiting basic knowledge for therapy. Introducing pathogen antigens nasally can trigger immune responses that theoretically can lead to both systemic and local (mucosal) immune protection and memory. Nasal vaccines for viral infections (flu) and bacterial infections (canine Bordetella) have had mixed success. See text for details.
An intranasal, live attenuated vaccination for flu virus, known as “FluMist,” has been marketed for several years. Although considered effective for earlier flu outbreaks, particularly in young children, it was not recommended for the 2016–2017 flu season. Some speculate that its effectiveness varies by flu strain and that H1N1 flu preparations, in particular, do not stimulate protective immunity.
Intranasal vaccines are common in veterinary medicine. Those who have dogs might be familiar with the intranasal vaccine for kennel cough, which is caused by the gram-negative Bordetella bacterium. This intranasal vaccine often includes nonvirulent versions of three different organisms—Bordetella and two viruses (a parainfluenza and adenovirus)—and protects dogs from kennel cough and other respiratory infections. Immunity is not necessarily long-lasting, and efforts to improve immune memory are ongoing. Intranasal sprays are also used successfully to vaccinate chicken flocks against avian flu and other poultry diseases.
The Skin Is a Unique Barrier Immune System
The skin consists of three major layers—the upper epithelial layers or epidermis, the area directly underneath the epithelium or dermis, and the hypodermis, the deepest layer of our skin (Figure 13-19a). Unlike the epithelium of the mucosal organs, the epithelium of the skin is multilayered and does not produce mucus. The cells and lymphoid tissue that make up the skin’s immune system are therefore not considered part of the mucosal-associated lymphoid tissue (MALT). Not surprisingly, however, the skin’s immune system shares many features with that of the intestinal and respiratory tracts.

FIGURE 13-19 Immune responses in the skin. (a) The skin is covered by multiple layers of specialized epithelial cells known as keratinocytes, which compose the epidermis. A variety of innate and adaptive immune cells, including Langerhans cells and CD8+ TRM cells, reside in the epidermis, but most of the immune cells are in the dermis, the layer just beneath the epithelium. Like the lamina propria of the intestine and respiratory tracts, the dermis includes a now familiar host of innate and adaptive immune cells that coordinate homeostatic and inflammatory responses. Unlike mucosal immune systems, the dermis does not include organized secondary lymphoid tissue (although some follicles can form in inflammatory states). (b and c) Two examples of the relationship between microorganisms on the skin surface and the immune system below: (b) Staphylococcus epidermidis interacts directly and indirectly with dermal myeloid cells to stimulate a type 1 T-cell response that protects us from Leishmania infection. (c) Staphylococcus aureus, on the other hand, produces a toxin that stimulates a type 2 response that enhances allergic dermatitis. See text.
Skin epithelial cells, or keratinocytes, are densely packed in stratified layers in the epidermis. New keratinocytes are generated at the border between epidermis and dermis. These new cells displace the older keratinocyte layers, which shift upward. The surface of our skin, in fact, is a 10- to 15-µm layer of dead keratinocytes and lipids, known as the stratum corneum, which forms a very protective, waterproof, first barrier to infection. Immune cells are present in the epidermis, as well as in the dermis, which also includes hair follicles, sebaceous glands, capillaries, and nerve endings. The hypodermis is a fatty layer occupied by arteries, veins, and sweat glands.
Like epithelial cells of the intestinal and respiratory systems, keratinocytes do not simply provide a physical barrier. Rather, they are active members of the innate immune system; they constitutively produce antimicrobial substances (such as psoriasin) and also recognize and respond to pathogens via pattern recognition receptors (see Chapter 4). Langerhans cells, a type of dendritic cell, are specialized antigen-presenting cells that are found in epidermis. They and other antigen-presenting cells extend processes between keratinocytes and act as sentinels that carry information about microbes from the epidermis to immune cells in the dermis. In addition, they carry skin antigens to the draining lymph nodes, where they activate naïve T and B cells to generate immune responses. Investigators have marveled at the ability of Langerhans cells to migrate surprisingly great distances. Indeed, Langerhans cells activated by skin antigens have shown up in the mesenteric lymph nodes and consequently have the potential to exert an influence on immune responses at other mucosal tissues.
Lymphocytes also reside in the epidermis and human epidermal tissues, including a large population of resident memory CD8+ T cells (CD8+ TRM cells). Although γδ T cells are abundant in the mouse epidermis and extend processes that resemble those of dendritic cells, γδ T cells are only thinly scattered throughout the human epidermis. Interestingly, there are no organized lymphoid follicles in the skin as there are in the intestine.
Most other immune cells are found in the dermal layer (see Figure 13-19a), which includes the full complement of innate and adaptive immune cells present in the lamina propria of mucosal immune tissue. Whereas CD8+ memory cells are present in the epidermis and appear to be long-term residents, CD4+ memory cells are found in the dermis and appear to circulate. Dermal γδ T cells respond to IL-23 produced by dermal APCs and appear to be a major source of IL-17 in the skin, contributing to both protective immunity as well as inflammatory skin diseases, like psoriasis. They play a similar role in skin immune homeostasis as ILC3 and TH17 cells in the gut and respiratory immune systems.
Regulatory T cells abound in mouse and human dermis. They tend to be long-term residents and accumulate over the life span of an individual. As in other barrier sites, they regulate tolerance to commensal bacteria and ameliorate inflammatory skin reactions. However, recent studies suggest that their origin and the timing of their development may differ considerably from those of intestinal and respiratory TREGs.
Rather than develop continually in response to constant sampling of luminal antigens by tolerogenic antigen-presenting cells, as happens in the gut, skin TREG cells may arise in one wave early in development and then take up long-term residence in the skin (Figure 13-20). Recent studies show that tolerance to commensal bacteria arises in a narrow window of time during neonatal development. In fact, mice were not able to generate tolerizing TREGs if they were colonized with commensal bacteria when mature. This observation underscores the possibility that exposure to microbes in infants has a profound influence on our immune health as adults.
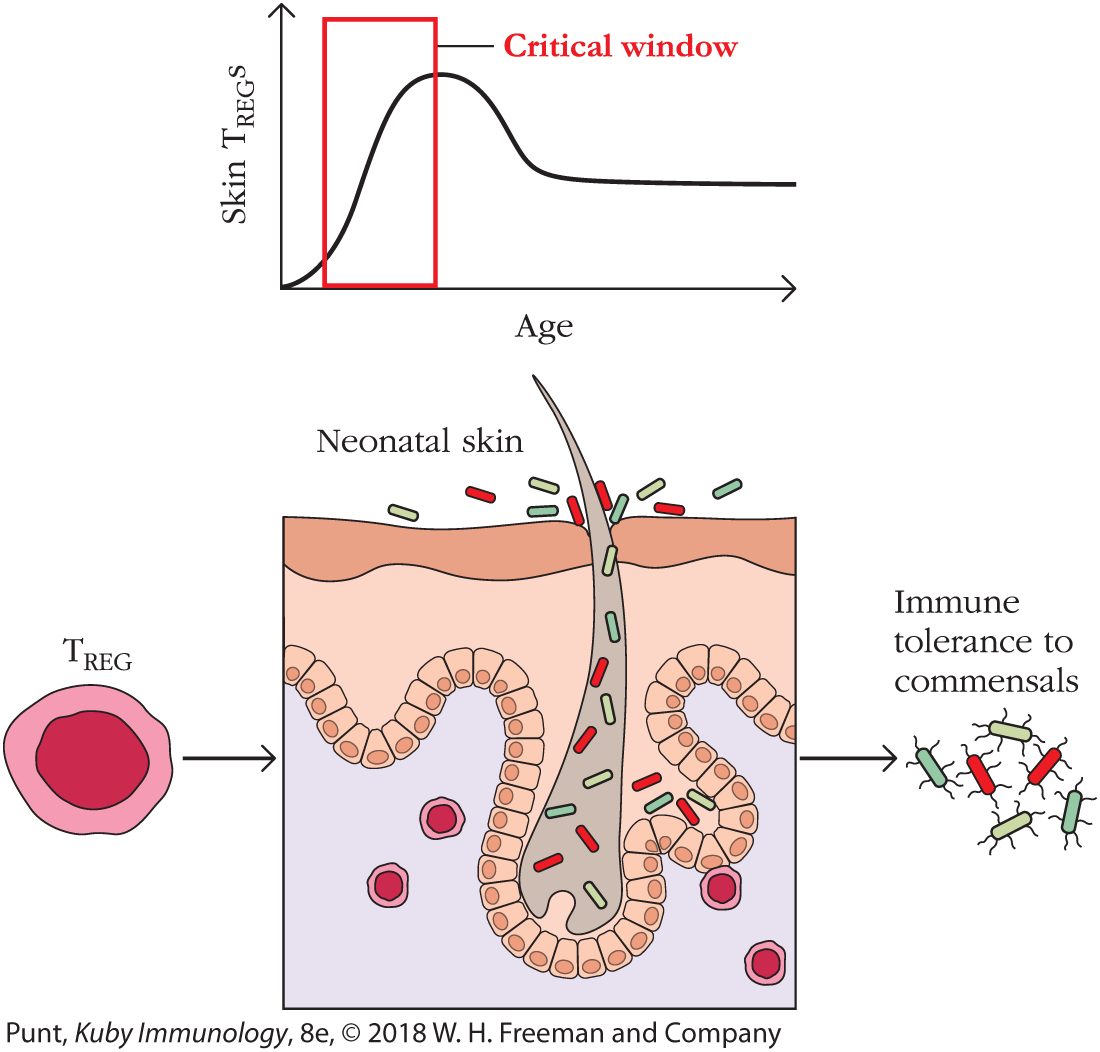
FIGURE 13-20 Developmental regulation of TREG cells in the skin. The generation of skin-specific TREG cells is age dependent and occurs when newborns (neonates) are first colonized by microorganisms (see text).
Although this time-dependent effect of TREG development seems most dramatic in the skin, it is likely that time of exposure to commensal microbes and antigens has an effect on tolerance in all barrier immune systems. For instance, exposure to antigens in the lung during the neonatal period may help individuals avoid IgE hypersensitivities, an observation that provides scientific support for the hygiene hypothesis (see Chapter 1, Clinical Focus Box 1-3).
Interestingly, recent studies also show that skin TREG cells concentrate in hair follicles, which are also host to commensal microbes. As we have learned from the intestinal immune system, interactions between commensal microbes, epithelial cells, and immune cells often favor TREG development. It is tempting to speculate that similar mechanisms are at work in the skin. Perhaps some TREG development occurs continually, too, supplementing the more long-term TREG population that arose during neonatal periods. There is still much to learn.
The surface of human skin is replete with communities of microorganisms including bacteria, which interact with the immune system in many ways (Figure 13-19b and c). Recent work shows that colonization of skin with specific bacterial species can protect animals from infection with Leishmania—a protozoal parasite that is spread by the bite of a sandfly.
Staphylococcus epidermidis is one commensal bacterial species that provides protection and appears to do so by interacting with pattern recognition receptors that induce IL-1 production by dermal (not epidermal!) dendritic cells (see Figure 13-19b). IL-1, in turn, induces IL-17 production by dermal CD8+ T cells, which contribute to a successful immune response against Leishmania parasites. S. epidermidis also protects against other bacterial infections by inducing antimicrobial peptide production by keratinocytes. It even produces its own antimicrobial peptides that can fend off more pathogenic bacteria at the skin surface.
Staphylococcus aureus is associated with multiple skin and systemic disorders, including allergic dermatitis. It can generate inflammation by producing a molecule, δ-toxin, that penetrates the epidermal layer. δ-Toxin interacts with dermal mast cells, causing them to release cytokines that stimulate a type 2 inflammatory reaction (see Figure 13-19c).
Finally, Edward Jenner, an eighteenth century physician who is now considered an early founder of immunological thinking, was among the first to exploit the ability of the skin’s immune system to generate not just a local but a systemic immune response. He successfully generated protective immunity to smallpox, a strictly human disease and scourge, by scratching live cowpox virus into the skin of his patients (and his son). This approach, later dubbed vaccination by Louis Pasteur, was responsible for the worldwide eradication of this fatal and deforming disease. How a local skin response induces systemic effects is still under investigation. However, the ability of the Langerhans cell to travel large distances, carrying antigen to draining lymph nodes, offers one clue.