Immunoprecipitation- and Agglutination-Based Techniques
The multivalency of antibodies has allowed the development of techniques in which antibody-bound molecules can be precipitated from solution, or otherwise separated from nonbound molecules for further analysis. Some of these techniques are quite venerable; other applications are brand new. Nonetheless, all rely on the ability of antibodies to bind to more than one antigenic determinant on a single antigen, thus forming a large complex that will fall out of solution.
Immunoprecipitation Can Be Performed in Solution
When antibodies and soluble antigen are mixed in solution, the bi- or multivalent nature of immunoglobulins allows for a single antibody molecule to bind to more than one antigen (Figure 20-2). If the antigen is polyvalent (has more than one antibody binding site per antigen molecule, represented in Figure 20-2 by the red sites on the brown antigens), it may in its turn bind multiple different antibodies. Eventually, the resulting cross-linked complex becomes so large that it falls out of solution as a precipitate. This precipitate can be spun out of the solution and the antigen separated from the precipitating antibodies by biochemical means.
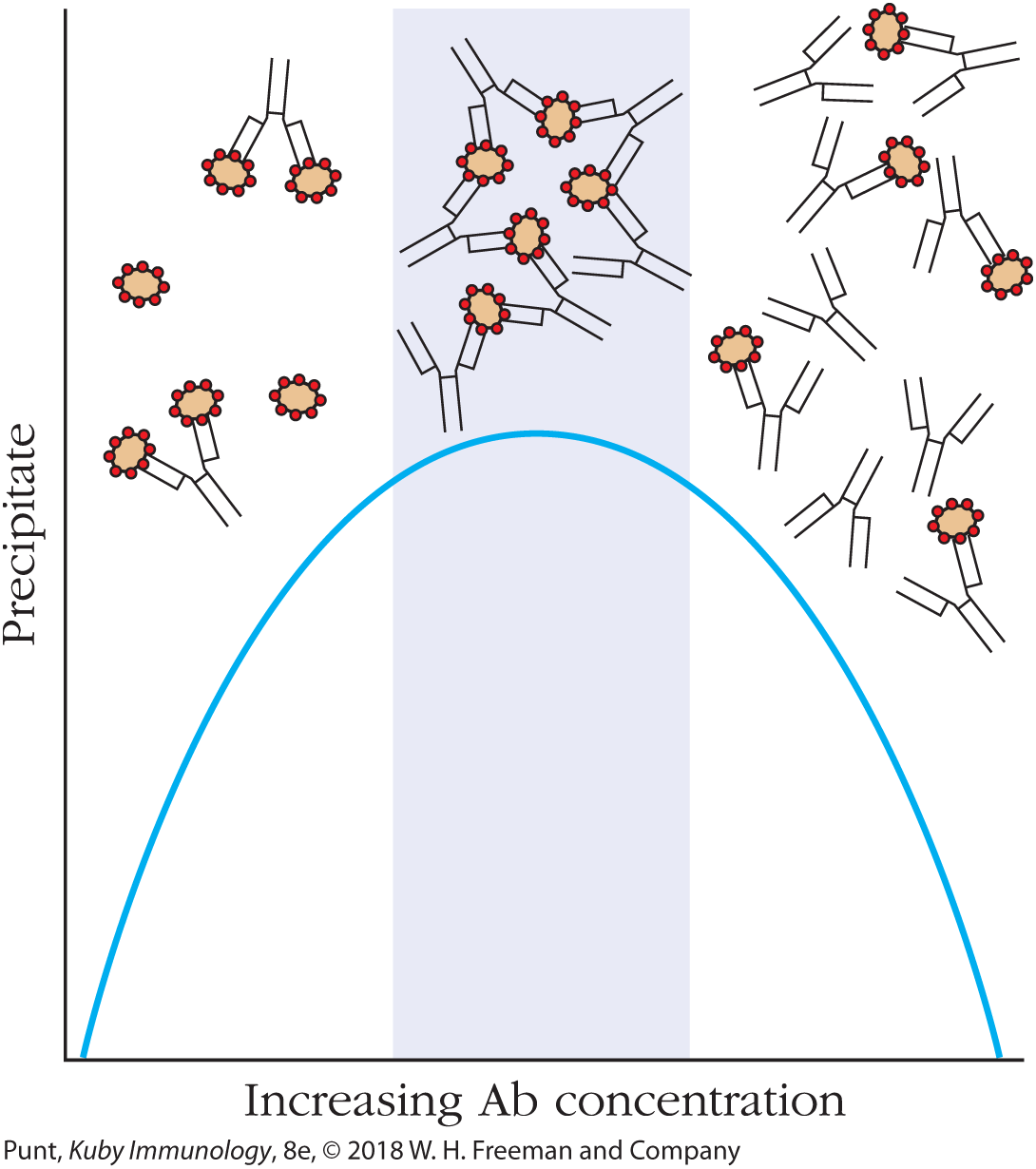
FIGURE 20-2 Immunoprecipitation in solution. When bi- or multivalent antibodies are mixed in solution with antigen, the antibodies can form cross-linkages with two or more antigen molecules, leading to the formation of a cross-linked precipitate (middle portion of graph). Precipitate formation requires that neither antigen (left-hand portion of graph) nor antibody (right-hand portion of graph) molecules are in excess. In either of these two cases, primarily monovalent binding takes place, as shown.
Solution immunoprecipitation can be used to purify antigenic molecules from a heterogeneous mixture of soluble molecules, or to remove particular antigens from a solution. The most efficient immunoprecipitation occurs only when the antibody and antigen concentrations are essentially equivalent (see Figure 20-2, middle). When either the antigen (Figure 20-2, left) or antibody (Figure 20-2, right) is present in excess, monovalent binding is favored that does not result in the formation of a precipitate. Recall from Chapter 3 that Kabat used immunoprecipitation with the ovalbumin antigen to remove anti-ovalbumin antibodies from solution, followed by electrophoresis; this experiment characterized antibodies as belonging to the γ-globulin class of serum proteins.
Immunoprecipitation of Soluble Antigens Can Be Performed in Gel Matrices
Immune precipitates can form not only in solution but also in an agar matrix. When antigen and antibody diffuse toward one another in a gel matrix, a visible line of precipitation will form. As in a precipitation reaction in solution, visible precipitation occurs when the concentrations of antibody and antigen are equivalent to one another.
Immunodiffusion in gels is rapid, easy to perform, and surprisingly accurate. In the Ouchterlony method, the most frequently employed variation of gel immunoprecipitation, both antigen and antibody diffuse radially from wells toward each other, thereby establishing a concentration gradient. At the relative antibody-antigen concentrations at which lattice formation is maximized, termed “equivalence,” a visible line of precipitation, or “precipitin line,” forms in the gel. More sophisticated analyses of Ouchterlony gels can offer information regarding the extent of cross-reactivity of antibody preparations with related antigens (see Figure 20-3).

FIGURE 20-3 Immunodiffusion in agar gels can be used to assay for the presence of antibodies and determine cross-reactivity patterns between complex antigens and antibody samples. A polyclonal antiviral antiserum has been placed in the lower well and viral antigens in the upper two wells. On the left the two antigens are the same, as shown by the smooth precipitin curve. In the middle, the two antigens are partially identical, but the sample on the top left contains antigens not shared by the well on the top right. On the right, two different viral antigens are presented in the two top wells, that are both recognized by the polyclonal antiserum sample. [Obtained from Amrita School of Biotechnology, Amrita Vishwa Vidyapeetham. www.amrita.edu.]
Although various modifications of precipitation reactions were, at one time, the major types of assay used in immunology, other, more sensitive methods are now available for antigen and antibody measurement and are described below. However, Ouchterlony assays are still used in both the research laboratory and the clinic, because of their technical ease and reproducibility. Table 20-1 presents a comparison of the sensitivity, or minimum amount of antibody detectable, of a number of immunoassays.
| Assay | Sensitivity* (μg antibody/ml) |
| Precipitation reaction in fluids | 20–200 |
Precipitation reaction in gels Ouchterlony double immunodiffusion |
20–200 |
Agglutination reactions Direct Agglutination inhibition |
0.006–0.06 0.3 |
| Radioimmunoassay (RIA) | 0.0006–0.006 |
| Enzyme-linked immunosorbent assay (ELISA) | ~0.0001–0.01 |
| ELISA using chemiluminescence | ~0.00001–0.01† |
| Immunofluorescence | 1.0 |
| Flow cytometry | 0.006–0.06 |
Immunoprecipitation Enables Isolation of Specific Molecules from Cell and Tissue Extracts
Immunoprecipitation is frequently used to isolate protein antigens from cell and tissue samples. A detergent extract of cells or tissues is mixed with antibodies to the protein of interest to form an antigen-antibody complex. The detergent is carefully selected to minimize disruption of the antigen-antibody bond. To facilitate efficient retrieval of the antigen-antibody complexes, a secondary antibody or other protein that binds to the primary antibody (such as the bacterial protein A or G) may be added to the mixture. These secondary reagents all bind specifically to the Fc region of the first antibody and are usually pre-attached to a solid-phase support, such as a synthetic bead. In some assays, the primary antibody may be attached directly to the solid-phase support. Since the beads can easily be spun down, the antibody-antigen-bead complex can be collected by centrifugation. Following centrifugation, the protein of interest can be separated from the precipitating antibodies by SDS gel electrophoresis. In a variant of this technique, the beads attached to the secondary reagents may be magnetic, in which case the protein of interest is purified by passage over a magnetic column (see below).
Western blotting (see below) can then be used to ascertain the efficacy of the immunoprecipitation, to estimate the relative abundance of the bound protein in the cell or tissue sample, and to determine which other proteins co-immunoprecipitated and therefore are probably associated with the target protein in its cellular location. Such co-immunoprecipitation studies were the first clue to the multimolecular natures of the TCR and BCR coreceptor complexes.
Hemagglutination Reactions Can Be Used to Detect Any Antigen Conjugated to the Surface of Red Blood Cells
The cross-linking that occurs between di- or multivalent antibodies and multivalent, cellular antigens can result in visible clumping of the complexes formed between the antigens and their cognate (binding) antibodies. This clumping reaction is called agglutination. Agglutination reactions are identical in principle to precipitation reactions; the only difference is that the antigen being bound is associated with a cell surface and the cross-linked product is therefore visible to the naked eye because of the larger size of the particle that contains the antigen.
When antibodies bind antigens on the surface of red blood cells (RBCs), the resultant clumping reaction is referred to as hemagglutination. In the example shown in Figure 20-4, control buffer was added to well 10 of the microtiter tray. Antibodies to sheep red blood cells (SRBCs) were added to well 1 of this tray, and then this antiserum was serially diluted into wells 2 through 9, such that the concentration of antibodies in well 2 was half that in well 1, and so on. The same number of SRBCs was then added to each well.

FIGURE 20-4 Demonstration of hemagglutination, using antibodies against sheep red blood cells (SRBCs). The control well (well 10) contains only SRBCs, which settle into a solid “button.” Experimental wells 1 to 9 contain a constant number of SRBCs plus serial twofold dilutions of anti-SRBC serum. The spread pattern in the experimental series indicates positive hemagglutination through well 3.
In well 10, in the absence of any agglutinating antibody, the SRBCs settle into a tight “button” at the bottom of the well. This tight button represents a negative result in a hemagglutination assay. In well 1, the high concentration of anti-SRBC antibodies induced cross-linking of the SRBCs, so that they form a cross-linked clump of cells that is too misshapen to fall down to the bottom of the well. The diffuse shading of RBCs seen in well 1 represents a positive interaction between the antibodies and the SRBC surface antigen. The concentration of anti-SRBC antibodies in wells 2 and 3 remains high enough to allow hemagglutination, but once the antibodies have been diluted eightfold (well 4), there are too few antibodies to generate cross-links and the SRBCs can again settle into the bottom of the well. The responses in wells 1, 2, and 3 therefore represent a positive hemagglutination reaction.
Hemagglutination reactions are routinely performed to type RBCs. With tens of millions of blood-typing determinations run each year, this is one of the world’s most frequently used immunoassays. In typing for human ABO antigens, human RBCs are mixed with antisera to the A or B blood-group antigens. If the antigen is present on the cells, they agglutinate, forming a visible clump on the slide.
The ease and sensitivity of hemagglutination reactions and the fact that they do not require sensitive instrumentation for data analysis mean that hemagglutination assays can be adapted to measure antibodies directed against any antigen that can be attached to the RBC surface.
Hemagglutination Inhibition Reactions Are Used to Detect the Presence of Viruses and of Antiviral Antibodies
Hemagglutination inhibition reactions are also useful tools in the clinic and in the laboratory for the detection of viruses and of antiviral antibodies. Some viruses (most notably influenza) bear multivalent proteins or glycoproteins on their surfaces that interact with macromolecules on the RBC surface, and induce agglutination. Specifically, the influenza virus envelope bears a trimeric glycoprotein, hemagglutinin (HA). This HA molecule is subjected to mutation and selection, such that different strains of influenza bear different HA types, which in turn are bound by different antibodies. However, all HA molecules bind in a multivalent manner to the sialic acid residues on RBCs and agglutinate them.
To determine whether a patient has antibodies to a particular strain of influenza virus, a technician would perform a serial dilution of the patient’s antiserum in a microtiter plate. The technician would then add the relevant virus and RBCs to each well, at concentrations known to allow hemagglutination. If the patient’s antiserum has anti-HA antibodies that bind the particular influenza strain being tested, the antibodies will attach to the HA molecules on the surface of the virus and prevent those molecules from inducing hemagglutination. The more antibodies in the patient’s serum, the more the serum can be diluted without loss of hemagglutination inhibition.
Other viruses that can cause hemagglutination, and that therefore can be tested by the hemagglutination inhibition assay, include adenoviruses, parvoviruses, togaviruses, some coronaviruses, picornaviruses, other orthomyxoviruses, and paramyxoviruses.
Bacterial Agglutination Can Be Used to Detect Antibodies to Bacteria
A bacterial infection often elicits the production of anti-bacterial antibodies, and such antibodies can be detected by bacterial agglutination reactions. The principle of bacterial agglutination is identical to that for hemagglutination, but in this case the visible pellet is made up of bacteria, cross-linked by antibacterial antibodies directed against antigens on the surfaces of the bacterial cells.
Agglutination reactions can also provide quantitative information about the concentration of antibacterial antibodies in a patient’s serum. The patient’s serum is serially diluted (titrated), as described above. The last well in which agglutination is visible tells us the agglutinin titer of the patient, defined as the reciprocal of the greatest serum dilution that elicits a positive agglutination reaction. The agglutinin titer of an antiserum can be used to diagnose a bacterial infection. Patients with typhoid fever, for example, show a significant rise in the agglutination titer to Salmonella typhi. Agglutination reactions also provide a way to type bacteria. For instance, different species of the bacterium Salmonella can be distinguished by agglutination reactions with a panel of typing antisera.