ABSTRACT
In this study, it deals with 'Leptin' hormone, one of the most important endocrine hormones in the human body. Its working principle is on the physiology and the factors that it is effective on. It aims to show the physiology of leptin in an understandable and easily transferable way. It is one of the biggest reasons for me to choose this topic to show how great the role of this hormone is in our lives.
Internationally accepted articles, reviews and studies were based on and analyzed in this regard. Recent theories, concluded experiments and accepted transferred information are included. It was asked to convey this information to the reader in the cleanest way possible.
The importance of the working principle of leptin hormone for our vital continuities and its results are shown. In addition, information about its principles and exceptional circumstances was included.
Key Words:
Leptin, Hormone, Physiology
TABLE OF CONTENTS
SYMBOLS, LIST OF ABBREVIATIONS
CNS Central Nervous System
PNS Peripheral Nervous System
∝
It is the alpha symbol.
∝
MSH Alpha Melanocyte Stimulating Hormone
CCK Cholecystokinin
NPY Neuropeptide
AgRP Agouti Related Peptide
Ob / ob Obese gene
Ra Rat
MAPK Mitogenic Interacting Protein Kinase
NTS Solitary Path Core
IL Interleukin
A CoA Acetyl Coenzyme A
Β Beta Symbol
DM Diabetes Mellitus
GH Growth Hormone
GIS Gastroinstestinal System
TRH Thyroid Relasing Hormone
IBF Insulin Growth Factor
PTP1B Protien Tyrosine Phosphatase 1 B
POMC Proopiomelanocortin
LepR Leptin Reseptör
Ng Nanogram
mL Mililitre
PEM Protein Energy Malnutrition
1.
INTRODUCTION
Leptin, also called "satiety hormone", is a peptide hormone that regulates the appetite and fat stocks in the organism by creating a feeling of satiety. Leptin is secreted from fat (adipose) tissue, which is the body's largest endocrine organ. It acts on receptors in the hypothalamus. Adjusts the appetite to get energy homeostasis. Leptin, The importance of leptin was discovered in 1994 by Jeffrey M. Friedman and his colleagues at Rockefeller University in experiments on mice in a laboratory (Fève and Bastard, 2012).
Scientists started this discovery by realizing that mice with more leptin in their bodies were thinner and did not need to eat much. Leptin is mainly secreted by white adipose tissue and levels are positively correlated with the amount of body fat. Like many other hormones, leptin is secreted hazely and has a significant diurnal
variation with higher levels in the evening and early morning. Circulating leptin levels reflect primarily the amount of energy stored in fat and secondarily acute changes in calorie intake. Leptin serves a critical evolutionary function by maintaining the relative constancy of adipose tissue mass, thus protecting individuals from the risks associated with being too underweight or overweight. Its concentration in the blood is generally proportional to body fat mass. It is more associated with testerone in men than in women. Hunger and chronic malnutrition cause a lower level of leptin in the blood. Leptin deficiency results in hunger, allowing the individual to eat to survive and also inhibit reproduction until their food and fat stores are sufficient to provide energy for pregnancy and breastfeeding. Therefore, leptin is important for the survival of the individual and for the survival of the species (Brennan & Mantzoros, 2006).
2. EFFECTS ON SYSTEMS
In this section, the effect of leptin on body systems, its importance and the situations in which it plays a role are given.
2.1 EFFECT ON THE CENTRAL NERVOUS SYSTEM
The nervous system in vertebrates consists of two main parts: the central nervous system (CNS) and the peripheral nervous system (PNS). The primary action of leptin is on the hypothalamus, which is part of the central nervous system. Leptin receptors are expressed not only in the hypothalamus but also in other brain regions, particularly the hippocampus. Thus, some leptin receptors in the brain are classified as central (hypothalamic) and some as peripheral (non-hypothalamic) (Halaas and Gajiwala, 1995).
As has been scientifically known so far, the general effects of leptin on the central nervous system are:
Leptin deficiency has been shown to alter brain proteins and neuronal functions of obese mice that can be restored with leptin injection. In humans, low circulating plasma leptin has been associated with cognitive changes associated with anorexia, depression and Alzheimer's disease. Studies in transgenic mouse models of Alzheimer's disease chronic administration of leptin
improves brain pathology and cognitive has shown that it can improve performance (Leib et al., 2009).
In general, leptin is currently thought to enter the brain in the choroid plexus, where the intense expression of a leptin receptor molecule can act as a transport mechanism. Increased melatonin levels cause leptin regulation, however, melatonin is known to increase leptin levels in the presence of insulin and therefore cause a decrease in appetite during sleep. Partial sleep deprivation has also been associated with decreased leptin levels. Mice with type 1 diabetes treated with leptin or leptin plus insulin had better metabolic profiles compared to insulin alone: blood sugar was not fluctuated much; Cholesterol levels have been found to decrease (Pelleymounter and Cullen, 1995).
Leptin acts on receptors in the lateral hypothalamus to inhibit hunger and medial hypothalamus to stimulate satiety.
• In the lateral hypothalamus, leptin inhibits hunger.
• It has the ability to counteract the effects of neuropeptide Y, a strong hunger promoter secreted by cells in the gut and hypothalamus.
• Resists the effects of anandamide, another potent hunger promoter that binds to the same receptors as THC.
• Stimulates leptin satiety in the medial hypothalamus
• Promotes hunger suppressing α-MSH synthesis (Thomas, 2007).
Thus, a lesion in the lateral hypothalamus causes anorexia (due to the absence of hunger signals) and a lesion in the medial hypothalamus causes extreme hunger (due to the absence of satiety signals). This appetite inhibition is long-term, in contrast to the rapid inhibition of hunger by cholecystokinin (CCK) and slower suppression of hunger between meals mediated by PYY3-36. The absence of leptin (or its receptor) leads to uncontrolled hunger and ultimately obesity. Fasting or eating a very low-calorie diet lowers leptin levels. Leptin levels change more when food intake decreases than they increase (Augustyniak, Anderson, & Thomas, 2008).
Due to an acute change in energy balance, leptin dynamics may be associated with appetite and ultimately food intake rather than fat stores. Leptin binds to neuropeptide Y (NPY) neurons in the curved nucleus, reducing the activity of these neurons. Leptin signals the
hypothalamus, which gives a feeling of fullness. What's more, leptin signals can make it easier for people to resist the lure of high-calorie foods. Leptin receptor activation inhibits neuropeptide Y and agouti-related peptide (AgRP) and activates α-melanocyte stimulating hormone (α-MSH). Npy neurons are an important element in the regulation of hunger; Selective destruction of npy neurons in mice makes them anorexic while small doses of npy injected into the brains of experimental animals stimulate feeding. Conversely, α-MSH is an important mediator of satiety, and differences in the gene for the α-MSH receptor have been linked to obesity in humans. Leptin interacts with six types of receptors Ob-Ra – Ob-Rf or LepRa-LepRf encoded by a single gene, LEPR. Ob-Rb is the only receptor isoform capable of intracellular signaling via the jack-Stat and MAPK signal transduction pathways and is found in the hypothalamic nuclei. After leptin binds to the Ob-Rb receptor, it is phosphorylated and activates stat3 to the nucleus to affect changes in gene expression, one of the main effects is down-regulation of the expression of endocannabinoids responsible for increasing hunger. In response to leptin, receptor neurons have been shown to reshape themselves by changing the number and types of synapses firing at them (Doherty, Beccano-Kelly, Yan, Gunn-Moore, & Harvey, 2013).
2.2 IMPACTS ON THE CIRCULATION SYSTEM
The role of leptin / leptin receptors in modulation of T cell activity and the innate immune system in experiments with mice shown. It modulates the immune response against atherosclerosis, where obesity is a predisposing and mitigating factor.
Exogenous leptin can promote angiogenesis by increasing vascular endothelial growth factor levels.
Hyperleptinemia, produced by infusion or adenoviral gene transfer, has been observed to reduce blood pressure in rats. Leptin microinjections into the nucleus of the solitary pathway (NTS) have been shown to elicit sympathoexcitatory responses and potentiate cardiovascular responses to chemoreflex activation (De Felice and Vieira 2019).
2.3 EFFECT ON THE REPRODUCTIVE SYSTEM
The effects of leptin on the reproductive system extend from the ovulation cycle to puberty. The effects of leptin, which has a wide range of effects, on this system have been stated (Hehir and Glavey SV, 2003).
2.3.1 The ovulation cycle
In mice and humans (to a lesser extent in humans), the hormone leptin is required for male and female fertility. Ovulation cycles in women are much more dependent on energy balance (positive or negative depending on whether a woman loses weight or gains weight) and energy flux (how much energy is consumed and spent) than energy status (fat levels). When the energy balance is too negative (meaning the woman's starvation point) or the energy flux is too high (meaning the woman is exercising excessively but is still consuming enough calories), the ovarian cycle stops and cannot live
her period as it should. If a woman has an extremely low body fat percentage, her energy status affects menstruation. Leptin levels outside of an ideal range can have a negative impact on egg quality and outcome during in vitro fertilization. Leptin participates in reproduction by stimulating the hormone that releases gonadotropin from the hypothalamus (Dhillo, Comninos, & Jayasena, 2014).
2.3.2 PREGNANCY
Placenta produces leptin. Leptin levels rise during pregnancy and fall after birth. Uterine contractions are inhibited by leptin. Leptin plays a role in hyperemesis gravidarum (severe morning sickness of pregnancy), polycystic ovary syndrome and hypothalamic leptin plays a role in bone growth in mice (Zhao, Townsend, Schulz, & Kunz, 2004)
2.3.3 BREASTFEEDING
Immunoreactive leptin has been seen in the breast milk of humans, animals, and the blood of newborns.
2.3.4 ADOLESCENCE
Leptin, along with kisspeptin, controls the onset of puberty. High leptin levels, often seen in obese women, can trigger the neuroendocrine cascade resulting in early menarche. This can lead to shorter stature when estrogen secretion begins during menarche and causes premature closure of the epiphyses (Zhao, Townsend, Schulz, & Kunz, 2004).
2.4 EFFECT ON THE SKELETON SYSTEM
Leptin's role in regulating bone mass was discovered in 2000. Leptin can affect bone metabolism through direct signaling from the brain. Leptin decreases the cancellous bone and increases the cortical bone. This "cortical-spongy bifurcation" triggers a mechanism to increase bone size and therefore bone resistance to cope with increased body weight (Hamrick and Ferrari, 2008).
Bone metabolism can be regulated by the central sympathetic
outflow because sympathetic pathways innervate bone tissue. A number of brain signaling molecules (neuropeptides and neurotransmitters) have been found in bone, including adrenaline, noradrenaline, serotonin, calcitonin gene-linked peptide, vasoactive gut peptide, and neuropeptide y. Leptin binds to its receptors in the hypothalamus, acting through the sympathetic nervous system to regulate bone metabolism. Leptin can also act directly on bone metabolism through a balance between energy intake and the IGF-I pathway. It has potential for the treatment of bone formation diseases such as fracture healing with leptin (Hamrick & Ferrari, 2008).
2.5 EFFECTS ON THE IMMUNE SYSTEM
Factors that acutely affect leptin levels are also factors that affect other inflammatory markers, such as testosterone, sleep, emotional stress, calorie restriction and body fat levels. Although it has been well discovered that leptin is involved in the regulation of the inflammatory response, it has been further theorized that leptin's role as an inflammatory marker is specifically responsive to fat-derived inflammatory cypolines (Heiman et al., 1997).
In both structure and function, leptin resembles IL-6 and is a member of the cytokine superfamily. Circulating leptin appears to affect the HPA axi and suggests a role for leptin in the stress response. High leptin concentrations are associated with high white blood cell counts in both men and women (Perrier S, Caldefie-Chézet F & Vasson, 2009).
Similar to those observed in chronic inflammation, chronically elevated leptin levels are associated with obesity, overeating, and inflammation-related diseases, including hypertension, metabolic syndrome and cardiovascular disease. However, it is interesting that while leptin is associated with body fat mass, the size of individual fat cells and the act of overeating is not affected by exercise (for comparison, IL-6 is released in response to muscle contractions). For this reason, leptin is claimed to respond specifically to oil-derived inflammation. Leptin is a Pro-angiogenic, pro-inflammatory, and mitogenic factor, and its actions are enhanced by mixing with IL-1 Family cytokines in cancer. (Otsuka et al., 2005).
3. EFFECTS ON OTHER HORMONES
3.1 LEPTINE and INSULIN
Insulin is the most important regulator of energy balance. It enables the use of glucose, free fatty acids and amino acids by tissues. It shows its main effect on muscle, adipose tissue and liver. Insulin, which is a divisional hormone, enables glucose to enter into the cell from the cell membrane, especially in muscles and adipose tissue, and increases its use (Copinschi & Leproult, 2019).
Excess carbohydrates turn into triglycerides in the liver and adipose tissue with the effect of insulin. It also increases amino acid uptake and protein synthesis in cells. As a result, insulin decreases the lipid level in the circulation and enables the storage of excess energy substrates. When blood glucose decreases, insulin secretion decreases to basal level. Thus, glycogenolysis, gluconeogenesis takes place and triglycerides are converted into free fatty acids, and they are converted into glucose and keto acids via acetyl CoA. This information makes the existence of a relationship between leptin and insulin inevitable in the regulation of energy balance and shows that the effect of insulin on leptin production is important. For this reason, insulin is the most studied of the hormones with which leptin is related. Insulin stimulates the synthesis of leptin directly, showing this with a direct effect at the mRNA level. Prolonged hyperinsulinemia increases the leptin level by approximately 40%. This increase is due to the trophic effect of insulin on adipocytes (Knutson and Spiegel, 2009).
The relationship of leptin with insulin has been studied separately in acute and chronic hyperinsulinemia. Although acute hyperinsulinemia has a positive effect on leptin in rats in vivo, it is not the case in humans. Many studies have shown that neither postprandial physiological hyperinsulinemia nor short-term hyperinsulinemia increase plasma leptin secretion. While plasma leptin is associated with fasting insulin level, it is stated that there is no such relationship in the state of satiety. It increases the plasma leptin level as well as the plasma leptin level. It has been observed that insulin injection increases both plasma leptin and adipose tissue leptin mRNA levels. Insulin given to lean mice acutely increased leptin levels, while no such increase in leptin levels was seen in obese mice. Genetically obese mice have been shown to have a
mutation in the leptin gene, so leptin resistance results in an increase in ob gene transcription and thus serum leptin levels. It has been reported that injected insulin does not cause an increase in leptin because it has a maximum insulin stimulus in cases with hyperinsulinemia (Meek & Morton, 2012).
In patients with type II DM, it was discovered that insulin had no effect up to 4 hours after insulin injection, and that serum leptin level increased approximately 1.5 times after 6 to 8.5 hours. For this reason, it is thought that this situation, in which insulin does not acutely stimulate leptin secretion for as long as 24,72,96 hours, is due to its trophic effect in adipose tissue caused by hyperinsulinemia (Meek, Morton, 2012; Coppari, Bjørbæk, 2012)
Studies have also been made and discovered that leptin also has effects on insulin secretion. It is known that leptin administered for a long time increases the synthesis of insulin and glycogen. Studies have shown that leptin suppresses insulin secretion by activating ATP sensitive K + channels in ß cells. Thus, ß cells become hyperpolarized before they can be depolarized to release insulin (Al Sheik, 2017). It has been shown in many studies and studies that leptin has been shown to decrease basal and glucose-stimulated insulin secretion. This indicates that leptin creates negative feedback on insulin, but it has been noted in the studies that this situation may be related to the dose and there may be situations where it may differ.
Chronic hyperinsulinemia may contribute to the pathogenesis of diabetes in excessively obese individuals whose adipoinulin cycle is impaired as a result of a defect in leptin receptors. Thus, while high insulin secretion can stimulate the production of leptin, high leptin constriction does not stimulate insulin secretion (Kelesidis, Kelesidis, Chou, & Mantzoros, 2010).
3.2 LEPTINE and GHRELINE
Ghrelin is a peptide that was first isolated from the stomachs of mice in 1999 and found to be the natural ligand of the growth hormone (GH) releasing receptor. Ghrelin increases the release of GH as well as appetite increases and causes obesity. In animals given ghrelin, it has been shown to cause weight gain and fat due to excessive food intake. On the contrary, in animals given ghrelin antibody, appetite was found to be reduced. The production and release of ghrelin in
the stomach is regulated by calorie intake, ghrelin release increases in hunger and decreases in satiety. In recent years, it has been stated that leptin plays a role in the regulation of energy metabolism by affecting special neurons in the central nervous system together with ghrelin hormone produced by GIS. Unlike leptin, ghrelin is known to increase appetite and fat content (Strohacker, McCaffery, MacLean, & Wing, 2014; Korek et al., 2013).
3.3 LEPTINE and ADINOPECTINE
CDNA encoding adinopectin was first described in 1995. Adinopectin is a protein with a weight of 30kDa mainly released from white adipocytes. As is known, white adipose tissue is the largest energy store and one of the most important parts of energy homeostasis. Adinopectin is a protein produced specifically from white adipose tissue and has an insulin sensitivity-enhancing effect
(Considine, 2020).
There is a serious link between the white adipose tissue that stores energy in the form of trgilisides in case of overnutrition and the health problems associated with obesity. White adipose tissue is considered to be an endocrine organ in recent years. The reason for this can be attributed to its regulation by numerous hormonal signals, nuclear hormone receptors and the central nervous system (Stern, Rutkowski, Scherer, 2016). Adipose tissue secretes a large number of biologically active adipokines. Some of these adipokines also directly or indirectly affect insulin signaling, glucose and lipid metabolism. There is an inverse correlation between adiponectin levels and circulating leptin concentration and insulin sensitivity. Generally, with the increase in leptin levels, a decrease in adiponectin is observed.
3.4 LEPTIN AND THYROID HORMONES
Thyroid hormones increase metabolic rate in most animals. The increase in thyroid hormones is associated with an increase in oxygen combustion, body temperature, heart rate, systolic blood pressure, lipolysis, and weight loss. It causes changes in energy metabolism and body weight with disruptions in thyroid balance. Increased thermogenesis occurs with hyperthyroidism, while a decrease in body temperature and basal metabolic rate is observed with hypothyroidism. The similar effects of leptin and thyroid hormones on thermogenesis and energy metabolism have emerged with the possibility of both showing these effects in similar
physiological pathways such as sympathetic nervous system activation regulation, mainly adrenergic upper regulation. Thyroid hormones play an important role in the regulation of basal metabolism in the transition from hunger to nutrition. In rodents, conservation of energy and slowing down metabolism significantly reduce T3 and T4 levels during starvation (Flier, Harris, Hollenberg, 2000; Sesmilo, Casamitjana, Halperin, Gomis, & Vilardell, 1998).
Whether thyroid hormones modulate leptin expression and secretion is another research topic. Theoretically, it can be accepted that thyroid hormones have an inhibitory effect on leptin secretion, furthermore, cathelocholamines
It has a permissive role on their effects and leptin expression is suppressed by stimulation of these receptors. This situation is compatible with the inverse relationship between leptin and thyroid hormones, which appears under normal conditions. This theory has been confirmed by animal experiments that thyroid hormones have a negative effect on serum leptin levels (Sesmilo, Casamitjana, Halperin, Gomis, & Vilardell, 1998).
While leptin increases in thyroidectomized rats compared to the control group, the leptin level decreases in these rats after T4 or T3 infusion. Administration of T3 in hypothyroid rats resulted in decreases in leptin mRNA expression and circulating leptin levels in adipose tissue (Hsieh et al., 2002). Thyroid hormone applications increase the lipolytic effect of adrenaline.
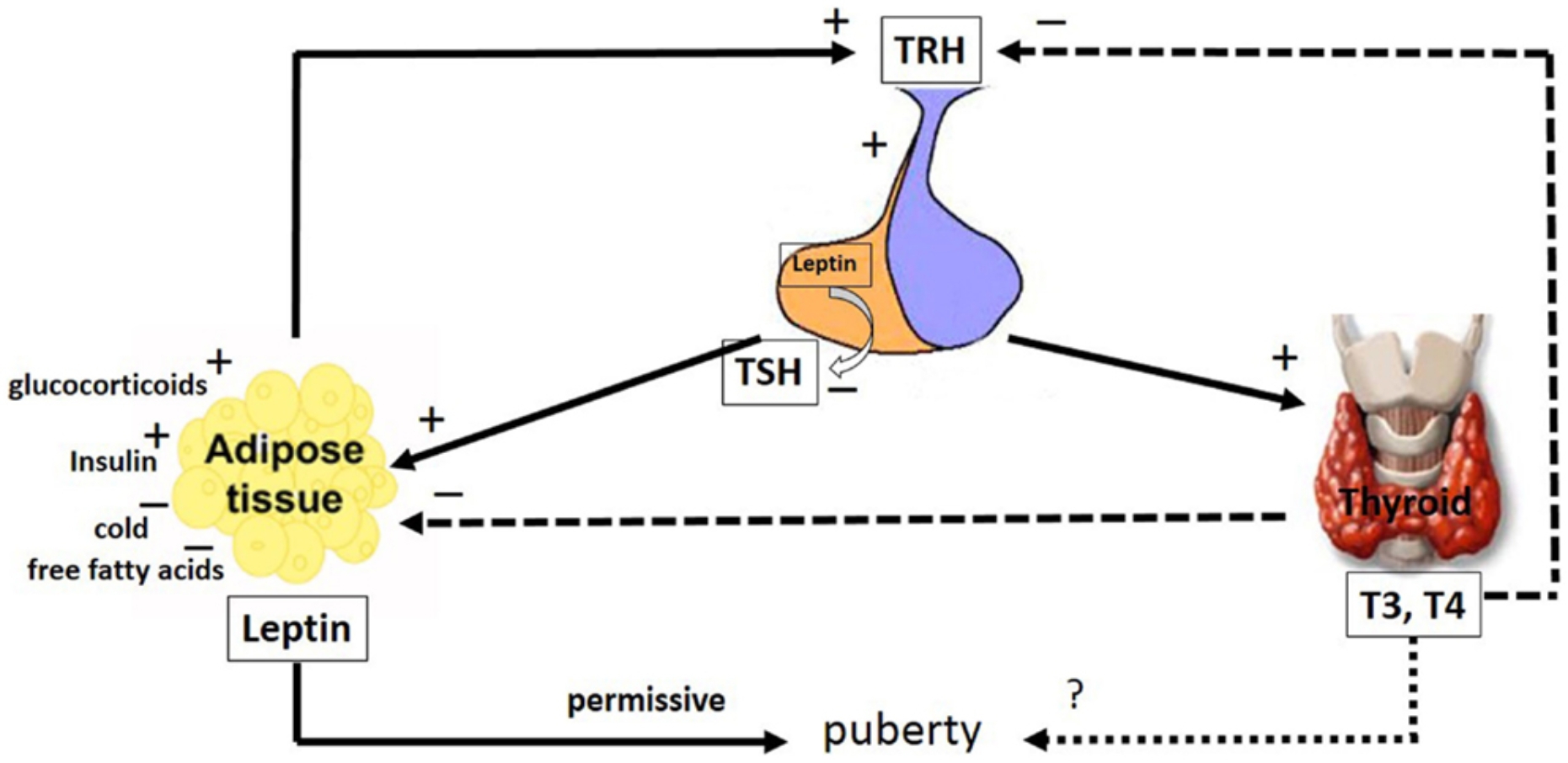
Blocking adrenergic nerves eliminates the calorific effect of thyroid
hormones. Malnutrition decreases both leptin and thyroxine (T4) levels in cattle. The decrease in leptin levels can cause a signal in the brain to reduce the T4 production level by suppressing the release of thyropine releasing hormone (TRH) directly or indirectly. Triiodothyroacetic acid (Triac) and T3 applications inhibit the synthesis and release of leptin from the white and brown adipose tissue of rats. It has been reported that administration of T3 in different doses to rats decreases serum leptin levels and white adipose tissue mRNA levels depending on the dose (Hsieh et al., 2002; Braclık, Marcisz, Giebel, Orzel, 2008). It is known that thyroid hormones play a regulatory role in energy metabolism by increasing thermogenesis. The most important factors in thermogenesis are "uncoupling" proteins (UCP). UCPs are located in the inner membrane of the mitochondria and allow the release of heat instead of ATP synthesis by preventing the protons to match. Thyroid hormones strongly stimulate the expression of UCP2 and UCP3, thus enabling more heat to be generated and consequently more energy expenditure (Guseynova and Mirzazadze, 2010).
Leptin increases the level of thyroid hormones and activation of the sympathetic nervous system, allowing more UCP to be formed and thus increases thermogenesis. Thus, in the prevention of obesity, energy expenditure is increased by taking another very important step in addition to reducing appetite. There was no relationship between serum leptin and thyroid hormones in thyroid gland dysfunctions (Drobniak, Radowicki, & Kanecki, 2014). Although there are studies showing that serum leptin levels are decreased in rats with hyperthyroidism and leptin levels are increased in rats with hypothyroidism, it is possible to find studies showing that leptin levels do not change in the same conditions.
3.5 LEPTIN AND OTHER HORMONES
Growth hormone (GH) has not only effects on growth and development, but also on body composition and fat distribution. This effect is through its lipolytic and nitrogen protective properties. Obesity is associated with distribution within the GH-insulin-like growth factor-1 (IBF-1) axis. In obese subjects, serum values of GH and IBF-binding proteins are generally low and as a result, they have high serum free IBF-1 serum values. In addition, GH secretion
increases during hunger in humans. With the administration of GH, energy use is stimulated and the amount of body fat is reduced. Moreover, leptin is similar in structure to GH (Nogueiras, Tschöp, & Zigman, 2008).
Leptin and GH are both members of the helical cytokine family. Growth hormone administration to developing sheep and cattle increased mRNA in adipose tissue, and also caused an increase in plasma insulin and adipose tissue IGF-I mRNA in cattle. It is thought that the effect of growth hormone on leptin production may be a part of the feedback mechanism. Because in vitro leptin has been observed to reduce the response of cells that secrete growth hormone in sheep pituitary gland to growth hormone-releasing hormone stimulation. However, in vivo injection of leptin increased plasma growth hormone levels in malnourished sheep (Nogueiras, Tschöp, & Zigman, 2008; Esen, Sarandol, Taneli, Yurtsever, & Ozlen, 2008). It has been suggested that the differences between in vivo and in vitro findings may result from numerous interactions such as the effects of GH or cortisol in vivo (Palik, Birkás, Faludi, Karádi, & Cseh, 2005).
Hormone injections such as GH or NPY can increase leptin synthesis. This is thought to indicate a feedback mechanism that will affect leptin's own receptors in the brain to reduce the secretion of hormones such as GH and NPY into the peripheral circulation. Growth hormone release is stimulated by the growth hormone-releasing hormone while inhibited by somatostatin. The role of both factors in the regulation of growth hormone release is controlled by leptin (Popovich et al., 2007; Murashita et al., 2007).
Leptin inhibits somatostatin release and increases growth hormone release from growth hormone releasing hormone in fasted rats. Glucocorticoids stimulated adipose tissue leptin gene synthesis and positive effects were observed for insulin and glucocorticoids in adipose tissue of rats. The complex relationships between insulin and glucocorticoids play an important role in regulating leptin production in humans. Malnutrition decreases plasma leptin level while increasing cortisol level. This increase in cortisol contributes to metabolic adaptations such as protein mobilization and gluconeogenesis in fasting and stimulates refeeding behavior (Murashita et al., 2007).
Nutrition then increases insulin secretion and leptin synthesis. Increasing blood leptin levels reduces insulin and cortisol levels in the blood to normal levels, thus restoring homeostatic balance. Melatonin administration in rats has been shown to reduce intra-abdominal adipose tissue with plasma leptin and insulin. However, the effect of melatonin administration in sheep on plasma leptin levels was not observed.
As a result, recent studies show that adipose tissue-derived leptin hormone has an important role in energy metabolism and is effective. Leptin interacts with many metabolites and it is of great importance to clarify the mechanisms of these interactions. There are still many researches and studies on the functions of leptin. However, the effect of melatonin administration in sheep on plasma leptin levels was observed.
4.THE PLACE OF LEPTIN IN THE ENERGY HOMEOZTASIS
In response to fasting, leptin levels drop disproportionately rapidly before any change in fat mass triggers the neuroendocrine response to acute energy deprivation. In mice and humans, this response includes reducing reproductive hormone levels (a process that requires energy) that prevents pregnancy, decreasing thyroid hormone levels that slow the metabolic rate, increasing the level of growth hormone that can activate energy stores, and reducing insulin-like growth factor-1 (IGF-1) ) is a process that can slow down the processes related to growth. Interactions between leptin, growth hormone and adrenal axes, in humans.
It is less important than animal models because patients with congenital leptin deficiency have normal linear growth and adrenal function in contrast to mice.
Initially, we observed neuroendocrine abnormalities in which fasting-induced decreases in leptin levels reached an average of 0.27 ng / ml, and administration of leptin at physiological replacement doses restored changes in luteinizing hormone pulsatility, decreased testosterone levels, and decreased thyroid stimulating hormone pulsatility (Rosenbaum and Leibel, 2014).
We then determined whether there is a minimum leptin threshold to allow breeding and to maintain other neuroendocrine processes. When inducing leptin deficiency in women of normal weight, higher baseline leptin levels dropped to an average of 2.8 ng / ml. Only modest changes in LH pulsatility were observed. The findings are that the energy stores in the adipose tissue to the brain end pregnancy
It shows that a leptin threshold of 3 ng / ml is required to convey the message that it is sufficient (Rosenbaum, & Leibel, 2014). Reaching a leptin level above this threshold allows the onset of puberty as a child grows up and maintains reproductive and other neuroendocrine processes in older people.
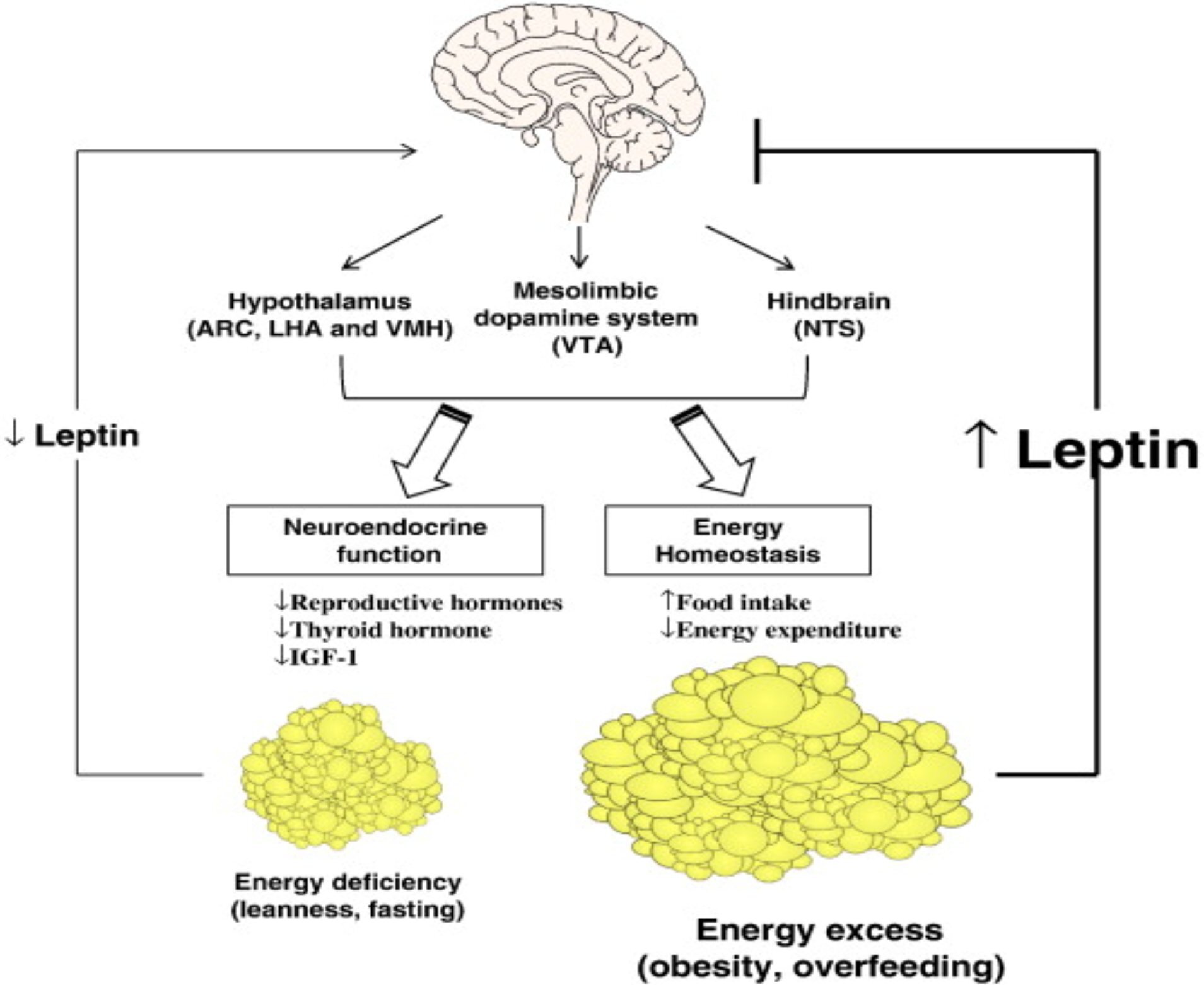
Given that women with anorexia nervosa and exercise-induced amenorrhea were chronically energy-deprived, it was first hypothesized that these conditions were associated with hypoleptinemia. This has been confirmed in observational studies. It was later hypothesized that long-standing hypoleptinemia leads to neuroendocrine dysfunction with subsequent anovulation and osteoporosis (Schwartz, 2003). A proof-of-concept trial of leptin therapy at replacement doses in women with hypothalamic amenorrhea has been conducted and found that it improves or completely normalizes gonadal, thyroid, and to a lesser extent growth hormone axes and bone markers.
5.LEPTIN RESISTANCE
Leptin and its receptors have been identified as key regulators of body weight and energy homeostasis. A decrease in tissue sensitivity to leptin leads to the development of obesity and metabolic disorders such as insulin resistance and dyslipidemia. The
mechanisms underlying the development of leptin resistance include mutations in genes encoding leptin and its receptors, as well as proteins involved in the self-regulation of leptin synthesis and blood-brain barrier permeability. Leptin resistance encompasses a complex pathophysiological phenomenon with a number of potential research lines. Available data on methods used to diagnose leptin resistance were analyzed. Leptin is an adipocyte secreted hormone that regulates appetite and is an important factor in the development of obesity, which is a serious medical, social and economic problem in modern society (Barbarash, Borodkina, Dyleva, & Uchasova, 2019).
More than 20 years ago, leptin and its receptors were identified as key regulators of body weight and energy homeostasis. A small increase in leptin concentration reduces appetite and leads to weight loss; However, in obesity, despite increased leptin, the anorexic effect of leptin is diminished, leptin resistance develops a malfunction of the leptin receptor or intracellular signaling associated with a decrease in the blood transport through the brain barrier (Myers et al., 2012). Clear criteria for defining leptin resistance and its diagnostic use have not been established.
For in vivo studies, it is difficult to elucidate the molecular mechanisms underlying the development of leptin resistance. Most studies examining the effects of leptin resistance on various metabolic processes are performed using mice with a defect in the Ob-leptin receptor gene. This mutation is rare in the human population, making it difficult to identify leptin resistance and possible diagnostic markers. Also, when evaluating leptin resistance, a number of questions need answers (Myers et al., 2012). The threshold for a weak response, including whether the presence of high leptin is sufficient for the diagnosis of leptin resistance, should be considered whether the dynamic changes in concentration over time are defined by either an increase in leptin concentration or leptin resistance (Zieba, Biernat and Barć, 2020).
5.1 LEPTIN HISTORY AND PERSPECTIVES
Leptin identification took place along with experiments on parabiotic animals. In 1950, Ingalls described them as a mutant mice characterized by severe obesity, a reduction in basic metabolism and thermogenesis rates, and reduced physical activity.
Furthermore, in 1973, Coleman et al. Showed that crossing obese mice with wild-type mice resulted in normalization of body weight and basal metabolism in their offspring, and that certain genetic factors underlie the development of obesity with the presence of a circulating "satiety" factor. Twenty years later, he identified the ob gene responsible for the development of obesity in obese mice. The product of the ob gene was later named "leptin" from a Greek word for "thin" (Li, 2011). Administration of recombinant leptin protein to obese and wild type mice resulted in a reduction in adipose tissue volume while preserving lean mass.
Since mice harboring a defective ob gene are associated with the development of severe obesity, subsequent research sought to determine whether mutations in the human form of the gene are associated with obesity in humans. An analysis of the leptin protein structure isolated from human subcutaneous fat cells was performed in 1995 and there was no difference in structure between lean and overweight individuals; however, it has been reported that leptin mRNA levels increase in obese individuals (Wolf & Colditz, 1998; Finkelstein, Fiebelkorn, & Wang, 2004).
Mutation in the OB gene in humans is extremely rare and causes the development of hyperphagia, morbid obesity and hypothalamic hypogonadism soon after birth. One study showed that experimental leptin-deficient rodents were protected from diseases associated with hyperleptinemia, while administration of leptin provided susceptibility and induced obesity. In contrast, hyperinsulinemia, hyperglycemia, and hypothermia were not detected in individuals with leptin deficiency, suggesting that obesity in humans is not associated with leptin-specific defects, but with the insensitivity of leptin target cells to such defects. Similarly, administration of leptin to mice with a defective leptin receptor has been reported not to affect their appetite or weight (Szczesna M and Zieba DA., 2015).
5 .2 LEPTINE RECEPTORS and SIGNALING
Leptin receptors belong to the cytokine Class 1 family and are encoded by the db gene in mice. Multiple splice variants of Ob-R mRNA encode at least six isoforms of leptin receptors (LepRa, LepRb, LepRc, LepRd, LepRe, and LepRf) that have a common leptin binding domain but differ in their intracellular domains. These
leptin-receptor isoforms
Its function is not well understood, but studies have shown that truncated forms are involved in leptin transport (J Mol Endocrinol, 2010).
LepRe does not have a transmembrane domain and is soluble LepR isoform that allows lepr to bind circulating leptin and inhibit leptin transfer. Only lep-rb (long isoform) contains the intracellular motif required for leptin-mediated activation of the jack – STAT signaling pathway in the hypothalamus, making this isoform the primary cause of leptin specific effects (Gorska et al., 2010).
LepRb is a typical class I cytokine receptor without an internal kinase activity. Instead, leptin binding to Leprb allows the phosphorylation of three tyrosine residues in leprb (Y985, Y1077 and Y1138) as well as recruitment and activation of JAK2, which undergoes autophosphorylation. leptin interacts with Sh2-domain-containing signal molecules that bind to the LepRb – JAK2 complex to promote the transmission of specific signals to second-order neurons in the nucleus of the hypothalamus. Each of these phosphorylation domains induces a leptin specific signaling pathway with different physiological functions. Phospho-Y985 activates SHP-2 and MAPK-related signaling and mediates leptin-specific negative feedback signals with shp2 mutations that cause early development of obesity in mice. In addition, phospho-Y1077 activates STAT5 and mediates the reproductive effects of leptin (Kloek, Haq, Dunn, Lavery, Banks, Myers, 2002). While the absence of STAT5 in the central nervous system leads to hyperphagia and obesity, STAT5 activation in hypothalamic neurons suppresses hunger in mice, suggesting that the JAK2 – STAT5 pathway is involved in preventing the development of obesity. Phospho-Y1138 activates the STAT3 signal and mediates the main effects of leptin on energy homeostasis and neuroendocrine functions, but this has little effect on reproduction. In addition, leptin-induced phosphorylation of STAT3 has been used to identify relative changes in leptin sensitivity. STAT3 is then phosphorylated by the LepRb-JAK2 complex, resulting in dimerization and nuclear translocation, where stat3 dimers act as transcription factors to regulate the expression of target genes, including SOCS3.26, which promotes leptin-mediated signaling via the jack2-STAT3 pathway to prevent obesity (including
SOCS3.26). Dunn et al., 2005; Sahu, 2003).
The LepRb signal is associated with two adapter molecules that serve as negative regulators of the leptin signal: SOCS3 and PTP1B. SOCS3 expression is enhanced by leptin-derived phospho-STAT3, and the SOCS3 protein binds to LepRb Y985 and JAK2 to inhibit leptin transfer in the classical inhibitory pathway of the feedback signal.27 Similarly, PTP1B mRNA levels rise after STAT3 nuclear translocation, while low PTP1B levels are associated with leptin. causes increased activity; However, the precise mechanisms involved in the interaction of LepRb with PTP1B are unknown. 28 other genes indirectly regulated by LepRb activity, several hypothalamic neuropeptides such as POMC, CART, AgRP and NPY
includes encoders. Leptin activates sympathetic preganglionic neurons in the thoracic spinal cord and neurons in the retrochiasmatic region, including dorsomedial, ventromedial, paraventricular, and lateral hypertension, and the lateral curved nucleus (Martin, Sanchez, 2001; Sweeney, 2002). The leptin targets in the curved, dorsomedial, ventromedial and ventral pre-aminuclear nuclei are located near the medial heights where leptin can reach neurons by diffusion in the adjacent ventrobasal hypothalamus. In addition, the hormone can be transported to the brain via the cerebrospinal fluid.
Highly expressed in the choroid plexus, ObRa promotes the transport of leptin from blood to cerebrospinal fluid, where the concentration of leptin is 100% lower than plasma, suggesting that cerebrospinal fluid is not the main source of leptin for the brain. The leptin receptors located on the neurons of the curved nuclei are well studied. There are at least two types of neurons in the curved region of the hypothalamus, the first type is responsible for POMC synthesis and the second type is responsible for AgRP and NPY synthesis. Also, these neurons house LepRb receptors. POMC synthesis is stimulated in neurons producing POMC, which then stimulate the synthesis of anorexigenic (appetite suppressing) neuropeptides and α-melanocytostimulatory hormone, and then binding and activating the MC3R and MC41 receptors. A previous study reported that structural changes of the MC4R receptor and an insufficient number of MC3R receptors in mice caused leptin resistance and obesity, and leptin-specific modulation of the melanocortin system caused a
decrease in body weight. Synthesis of AgRP and NPY in other neuron types induces appetite stimulation and damages these neurons responsible for suppressing hunger in mice. Leptin inhibits the synthesis of NPY and AgRP; Therefore, leptin-specific activity in the curved region of the hypothalamus through LepRb receptors modulates the synthesis of neuropeptides that prevent the development of obesity (Bartkowiak J, Walczewska A., 2007; Clement et al., 1998).
5.3 THE MECHANISM OF LEPTIN RESISTANCE

To date, several mechanisms have been identified as potentially underlying leptin resistance. These include a number of molecular and functional changes characterized by structural changes in the molecule, transport to blood across the brain barrier, and impairment and signaling of leptin-receptor function (Guizar et al.,2005 )
This figure schematizes the mechanism of leptin resistance. In short, a gene mutation that causes structural changes in the leptin molecule has been identified as potentially underlying leptin resistance, including leptin transport across the blood brain barrier, hypothalamic inflammation, and impairment of leptin-receptor function accompanied by endoplasmic reticulum stress. It is rarely
possible to establish the hereditary nature of leptin resistance (Palhares et al., 2020).
5.4 GENETIC MUTATION
Mutations in OB and DBU genes in humans are extremely rare and cause hyperphagia in homozygotes, obesity immediately after birth, and hypothalamic hypogonadism. These mutations were described in only three sisters, and resulted in replacing guanine with an adenine in the splice donor region of the exon and producing a truncated leptin receptor devoid of transmembrane and intracellular domains. In high concentrations, mutant receptors circulate and bind leptin. In addition to these results, dysfunctional secretion of thyrotropin and growth hormones may also occur. These findings suggest that mutations in the leptin gene and its receptor are not the main factors inducing the development of leptin resistance in the general population (Ross et al., 2004).
5.6 CARRYING LEPTIN ALONG THE BLOOD BRAIN BARRIER
Leptin resistance can also be developed in the blood-brain barrier, allowing leptin to be transported irregularly from the blood to the brain. Cerebral blood vessels refer to short forms of OBR that bind leptin and transport it from the blood to the interstitial tissue and cerebrospinal fluid of the brain. At serum leptin levels above the 25-30 ng / mL range, the leptin concentration in brain tissues and cerebrospinal fluid does not increase (Di Spiezio et al., 2017). This phenomenon likely plays a role in the development of leptin resistance and obesity, where excessive levels of leptin in the blood cause reduced blood brain barrier permeability. In the spinal fluid of obese individuals, a decrease in leptin concentrations has been observed under these conditions.
5.7 REGULATION OF LEPTIN EXPRESSION
Leptin concentrations and effects related to leptin are directly dependent on the transcription of the OB gene; Therefore, factors affecting this can significantly affect the state of adipose tissue and the development of leptin resistance. A decrease in leptin levels in adipocytes can cause an increase in adipose tissue volume until the required leptin level is reached. In such cases, serum leptin remains at physiological levels even if the individual is clearly obese. This
phenomenon has been observed in Pima Indians who show a predisposition to obesity associated with relatively low serum leptin levels (Flier, 1997).
OB expression levels correlate with lipid content and adipocyte size in cells; however, the mechanism underlying the effects of cellular fat levels on leptin production remains unclear. In addition, cultured adipocytes can store significantly lower amounts of fat compared to their in vivo capacity, and this property prevents a more complete understanding of the associated intracellular signaling pathways (Wran & Rosen, 2012). In addition, external stimuli, including hunger, binge eating, and circadian rhythm, modulate leptin expression and nocturnal leptin levels increase by an average of 30%.
Moreover, leptin itself plays an important role in the development of resistance, and this phenomenon is termed "leptin-dependent leptin resistance". Developing resistance to leptin increases the susceptibility of patients to diet-induced obesity, which contributes to a further increase in leptin levels and an exacerbation of existing leptin resistance in the vicious cycle. In addition, hypothalamic inflammation, endoplasmic reticulum stress, and autophagy disorders play a role in the development of obesity-related leptin resistance (Eferl et al., 2008).
5.8 THE ROLE OF INFLAMMATION IN THE DEVELOPMENT OF LEPTIN RESISTANCE
Considering the functional and anatomical relationship between adipocytes and lymphoid cells, leptin is likely to affect the neuroendocrine and immune systems. Morphologically, lymphoid tissue accumulation, including lymph nodes, omentum, thymus and bone marrow, is associated with adipose tissue. Fat deposits not only exhibit structural, metabolic and thermal insulation functions, but also provide a favorable microenvironment to support immune responses. In particular, lymphoid and adipose tissue common mediators known as adipokines, molecules derived from adipocytes that bind metabolism, and immune homeostasis (among these molecules leptin, adiponectin, chemokines and other proinflammatory cytokines) interact (La Cava, 2017).
Mattace Raso et al. Demonstrated that a high-fat diet causes low-
grade inflammation in peripheral tissues (especially adipose tissue and liver) and leads to an increase in inflammatory cytokines such as IL-6 and tumor necrosis factor (TNF). Leptin is also a proinflammatory cytokine belonging to the long chain helical cytokine family and structurally similar to IL-6, IL-12, IL-15, granulocyte colony stimulating factor, oncostatin M, prolactin, and human growth hormone. Due to its dual nature as a hormone and cytokine, leptin binds the neuroendocrine system to the immune system. A number of studies have shown that leptin, similar to C-reactive protein, IL-1 and IL-6 in their role, is an acute phase inflammatory protein produced at high concentrations during sepsis and fever, and its production is by other inflammatory mediators such as TNF-a and IL-1. can be induced. However, other studies did not report any increase in leptin concentration in people with acute experimental endotoxemia, neonatal sepsis, or HIV infections (Bjorbaek, Elmquist, Frantz, Shoelson, & Flier, 1998). Leptin deficiency (ob / ob) mice and people with congenital leptin deficiency have both metabolic and immune abnormalities, including abnormal cytokine secretion and thymus hypotrophy. In addition, leptin deficiency in ob / mice is associated with immunosuppression and thymus atrophy; this represents a similar pattern observed with acute hunger.
A sharp reduction in calorie intake causes a rapid decrease in serum leptin concentration with a delayed type hypersensitivity reaction and a decrease in thymus atrophy, which is reversible with the introduction of leptin. Also, Farooqi et al. high T cell levels; however, these data were disproved in subsequent studies. Moreover, while people with congenital leptin deficiency have a much higher mortality rate than childhood infection, administration of recombinant leptin in children with congenital leptin deficiency normalizes the absolute number of pure CD4 + / CD45RA + T cells and nearly restores the response to cytokine proliferation and release. Furthermore, the effects of leptin on the adaptive immune response have been extensively studied in human CD4 + T cells, with naive (CD45RA +), memory (CD45RO +), and CD4 + T cells showing different leptin-specific effects on production and proliferation of cytokines (all ObRb '). expresses). In particular, leptin increases IL-2 proliferation and secretion by naive T cells, but memory cells minimally affects its proliferation (Lam & Lu, 2007; Bjorbaek,
Uotani, da Silva, & Flier, 1997).
Although direct leptin-related effects on immune responses have been observed in vitro, its effect on immune responses in vivo is unknown. T cells are sensitive to nutrient input such as glucose because they cannot process glycogen and therefore depend on extracellular sources of glucose to meet their metabolic needs. Leptin contributes to the impairment of starvation-induced T cell function through pathways associated with ERK1 / ERK2 and PI3K-dependent signaling. In congenital immunity, leptin contributes to the activation and phagocytosis of monocytes / macrophages and the release of leukotriene B4, cyclooxygenase 2 (COX2), nitric oxide (NO), and pro-inflammatory cytokines (Vuolteenaho et al., 2009). Products in the form of inducible COX2, prostaglandin, eicosanoids and NO are involved in the regulation of inflammation, chemotaxis and cytokines, and therefore have a significant effect on the immune response. In addition, leptin can induce neutrophil chemotaxis and the release of oxygen radicals such as superoxide anion and hydrogen peroxide, which can be particularly harmful to cells through their ability to denature proteins and damage membrane lipids through the oxidation of unsaturated fatty acids. In human neutrophils, leptin effects are mediated through an indirect mechanism involved in the release of TNF-α from monocytes. Moreover, leptin also affects the development and activation of natural killer cells in vitro and in vivo (Vuolteenaho et al., 2009; Beltowski, 2006).
5.9 SETTING THE CRITERIA FOR DIAGNOSING LEPTIN RESISTANCE
There is currently no method to effectively assess leptin sensitivity in the clinical setting. It is reported that this sensitivity is directly related to obesity in general and adipose tissue volume in particular. A typical obese patient can be characterized by increased leptin levels and overexpression of OB in adipose tissue. Therefore, many studies view hyperleptinemia as a key marker of leptin resistance, and previous relationships between abdominal obesity and leptin concentration have been identified and explained by leptin resistance. However, no clear criteria have been identified for the diagnosis of leptin resistance (Gruzdava et al., 2018).
Each person is likely to have an individual response to binge eating or obesity, with signs of sexual dimorphism associated with circulating leptin concentrations and a decrease in OB expression levels with age. In addition, the focus cannot be solely on serum concentrations of leptin, as these depend not only on OB expression levels, but also on the clearance of the leptin protein and the presence and activity of its receptors (Guerra et al., 2014; Gruzdava et al., 2018).
Based on these findings, many studies suggest that key markers of leptin resistance may include leptin-receptor mRNA and protein levels, as leptin's key metabolic effects depend mainly on the number of receptors available. Obesity leads to a decrease in the expression of short and long isoforms of leptin receptors (OBRa and OBRb, respectively) in the hypothalamus, hepatocytes, adipose tissue, muscles, and in vitro studies, indicating that leptin-receptor levels are associated with leptin concentration (Murugesan, Arunachalam, Ramamurthy, and Subramanian , 2010). Subsequent introduction of exogenous leptin into cell cultures resulted in a decrease in leptin-receptor levels. In addition, a decrease in circulating leptin concentration was accompanied by an increase in OBR expression after prolonged fasting. Therefore, defining the free leptin index as the product of the relationship between leptin levels and soluble OBR by 100 gains diagnostic value. However, although this index has been used since 2003, the reference range limiting its wider application has not been clearly determined (Rizk & Sharif, 2015).
Jacquier et al. Developed a mathematical approach to diagnosing leptin resistance based on the assumption that leptin regulates the activity of its receptors. While the model is based on previous models predicting the dynamics of body weight in rodents, the new model includes the dynamics of leptin, leptin receptors and the regulation of food intake and body weight with two stable equilibrium positions: body weight with normal leptin sensitivity and obesity with leptin resistance (Rizk and Sharif, 2015 ). Using this model to analyze the physiological level of the fat model showed that fixed leptin infusions potentiate a decrease in leptin sensitivity resulting in a decrease in receptor density and an increase in food
intake. The authors suggested further improvement in the method, including the rate of leptin transit across the blood brain barrier (Zhang & Scarpace, 2009).
Leptin receptors are found in many tissues; however, it has not been disclosed which of these are involved in the development of leptin resistance. No relationship has been reported between basal leptin levels in obesity and the expression of leptin receptors in skeletal muscles (especially upper extremity muscles). In contrast, a negative association was found between the concentration of plasma leptin and OBRa levels and OBRb expression in the hypothalamus and liver of rodents. In addition, the contribution of significantly localized adipose tissue to leptin and leptin-receptor levels needs to be studied. In epicardial adipocytes, leptin secretion levels were found to be higher than those of adipocytes in subcutaneous adipose tissue. Thus, not only the number of leptin receptors but also their localization may contribute to the development of leptin resistance (de Queiroz et al., 2014).
6.LEPTIN FUNCTIONS IN INFECTIOUS DISEASES
Leptin is a pleotropic protein, and its importance in energy homeostasis, energy metabolism, neuroendocrine function, and other physiological functions has been shown in studies for a long time, thanks to its effects on peripheral tissues and the central nervous system. To summarize, leptin is secreted by adipose tissue and encoded by the ob gene. It acts as a central tool that regulates immunity and nutrition. One of the most important points is that leptin can modulate both natural and adaptive immune responses. In many studies, it has been stated that leptin deficiency / resistance is related to the decrease and deterioration of cytokine production in the body, increased sensitivity to infectious diseases, autoimmune disorders, malnutrition and inflammatory responses (Martin-Romero, Santos, Governa, & Sanchez, 2000).
It has been determined that energy malnutrition and inadequate unbalanced nutrition increase the tendency to death from infectious diseases and these diseases. Leptin is placed at the midpoint of many interrelated functions in various pathogenic conditions, such as infections caused by bacterial, viral and parasites. The effect of leptin on infectious diseases in parasitic diseases such as
leishmaniasis, trypanosomiasis, amoebiasis and malaria has been emphasized.
Circulating plasma leptin concentration is mostly affected by BMI, metabolic hormones and sex. Women have higher leptin concentrations than men. In short, the function of leptin is metabolic homeostasis, achieved by the information about total body fat mass going to the hypothalamus. In this process, it regulates glococorticoids, insulin hormone, food intake and energy balance. Also, leptin has a very important role. It functions as pro-inflammatory cytokine-like interleukin-1, IL-6, IL-8, IL-18 and tumor necrosis factor-α (TNF-α) as a critical regulator of immunity, and its deficiency increases susceptibility to infectiousness (Maurya, Bhattacharya and Dey Nakhasi, 2020).
In mice, leptin deficiency has been identified as the gene defect responsible for obesity. Most of the biological functions of leptin are implemented through the signal transducer and transcription activator. Generally, leptin antigen presenting cells support the enhancement of immunity by mediating the secretion of very important pro-inflammatory cytokines such as IL-2, IL-6 and TNF-α by activating the function and proliferation of Th1 cells. Leptin deficiency, leptin resistance, and additionally a marked reduction in atrophy were found in mice with defective and immune responses. However, in such cases, it has been stated that leptin administration can reverse this situation in a positive sense (Madej, Boguski, Bryant, 1999; Woodward, 2005).
The relationship between leptin and infectious diseases has become a serious research topic in the last decade. It has been explained that the negative relationship of leptin to malnutrition and malnutrition affects human innate and acquired immunity. This
Therefore, the incidence of mortality has also increased. Limited calorie intake and immune dysfunction in malnutrition have been shown to reduce memory T cells and total CD4 + and CD8 + T cells compared to well-fed and well-fed infected controls. Leptin has an important role in phagocytosis and metabolizing T cell count in both obesity and malnutrition. This is one of the things that makes leptin special. Systemic circulating leptin deficiency in malnutrition is associated with viral, bacterial and parasitic infections such as tuberculosis, pneumonia, sepsis, colitis, viral infection,
leishmaniasis. It has been stated that understanding how leptin changes in malnutrition and infection states has an important place in determining the clinical results of the nutritional status and medical treatment method. As time progresses, the role leptin plays in the pathogenesis of infectious diseases continues to increase both in terms of research and resources (Woodward, 2005; Coleman, 1978).
6.1 LEPTIN AND IMMUNITY
Almost all immune system cells such as neutrophils, monocytes, lymphocytes express the leptin receptor. Leptin regulates hematopoiesis, angiogenesis and innate immunity. It increases the production of IFN- γ, IL-2, TNF -α by inducing the Th1 response. It then leads to the activation of monocytes and macrophages, preventing apoptosis of various immune cells. In innate immunity, leptin increases the activity and function of neutrophils, the release of oxygen free radicals, increased CD11b expression and the level of intercellular adhesion molecule. These effects lead to the migration of immune cells in the areas where inflammation occurs. Leptin also promotes the survival of DCs by triggering nuclear factor-kappa B activation and regulates the B-cell lymphoma 2 and B-cell lymphoma-extra-large gene. In adaptive immunity, it reduces apoptosis by inhibition of the apoptosis pathway. Therefore, it induces the maturation and survival of thymic T cells. In humans and rats, leptin deficiency causes severe immune defects characterized by a decrease in total lymphocytes, an increase in the number of CD4 + helper T cells, and a curvature towards the Th2 phenotype, resulting in an increased susceptibility to intracellular infections. Leptin accompanies the polarization of T cells by inducing the cell-mediated immune response by secretion of IL-2, IL-12 and IFN-and inhibits the production of IL-10 and IL-4. As a result, leptin acts as a Th1 cytokine and affects natural and adaptive immune responses towards the Th1 response by regulating all immune cells with leptin receptors. The role of leptin in the innate immune response is illustrated above (Pulido-Mendez, De Sanctis and Rodríguez-Acosta, 2002).
6.2 MALNUTRITION and LEPTINE
In nutritional deficiency, phagocyte functions, cell immunity, antibody response and complement system are impaired, and in this case, mortality against infectious disease increases. Concomitantly, PEM is one of the most common causes of immunodeficiency. In the case of PEM, there is a serious decrease in fat mass in the body and reduces the concentration of leptin in the circulation. This disrupts the production of proinlamatory mediators. This increases the incidence of infectious diseases. Malnutrition is the primary factor in many infectious diseases. Recent studies show that malnutrition causes worse outcomes of tuberculosis disease. Active tuberculosis is associated with weight loss, cachexia and low serum leptin concentration, which suppresses lymphocyte stimulation and Th1 cytokines such as IL-2, IFN-γ and TNF-α secretion. Lymphocyte stimulation and a drastic reduction in cytokines such as IL-2, IFN-γ and TNF-α were observed in a tuberculosis infected guinea pig. In addition, by limiting the translocation of stress hormones, namely NF-kB, to the nucleus to the decrease in leptin concentration caused by PEM, it also increases glucocorticoids that cause impairment of macrophage functions. Macrophages from experimental PEM mice are less sensitive to lipopolysaccharides due to reduced NF-kB translocation, resulting in impaired production of active phagocytosis, cytokine response, and reactive oxygen intermediates (Schaible and Kaufmann, 2007).
In chronic cirrhosis due to Candida albicans and viral hepatitis and candidiasis, serum leptin concentration decreases as malnutrition becomes more pronounced. This is one of the biomarkers of nutritional status. In addition, the decrease in leptin level due to the decrease in adipose tissue in an individual in a fasted state is disproportionate. Without a specific token, this number drops. According to a study in wild-type mice, while the decrease in leptin level during fasting became fatal due to LPS and TNF-α, it was explained that leptin deficiency did not cause impairment in immune functions during fasting in another wild-type mouse that was in a fasting state despite continuing leptin treatment (Chandra, 1996). In exogenous leptin administration, it modulates T cell responses in mice and prevents hunger-induced immunosuppressions on the delay-type hypersensitivity response and protects against lymphoid atrophies against this condition.
It has been thought that leptin can act in the brain to directly regulate the metabolismic response, thus contributing positively to treatment methods in various infectious diseases. Recent studies show that serum leptin level, bacteria,
reported that it can be used as a promising diagnostic or prognostic marker for critical disease sepsis triggered by an infective agent such as a virus, fungus or parasite (Lord, Matarese, Howard, Bloom, Lechler and Leukoc Biol. 2002).
6.3 LEPTIN AND BACTERIAL INFECTIONS
Mortality was observed to be increased during pulmonary bacterial clearance, pulmonary tuberculosis, bacterial pneumonia, and sepsis in leptin-deficient mice. Decreased serum leptin levels and increased adinopectin in patients suffering from tuberculosis serve as a reliable biomarker to predict the development of pulmonary tuberculosis pathogenesis. In ob / ob mice, mycobacterial infection is inhibited to produce an organized granulomatous response and has been found to be defective in CD4 + and CD8 + T cell function, IFN-and DTY responses. Mechanisms underlying defective leukocyte effector function from leptin-deficient mice have been
associated with a decrease in leukotriene synthesis in the alveolar macrophage and decreased complement receptor synthesis in infected neutrophils. Binding to leptin receptors means that multiple intracellular signaling pathways are activated, including STAT3, STAT5, and ERK1. During long-term stimulation of the leptin receptor long isoform, it activates the transcription of the cytokine signal-3 repressor, a protein that inhibits JAK2 and STAT3 signaling (Neeraj, Sharma, Sudan, & Karunanand, 2017; Al-Fadhli, Saraya, & Qasem, 2014).
It has been described to restore anti-listeria resistance and production of monocyte chemoattractant protein-1 and monocyte chemoattractant protein-2 in leptin-deficient mice. Clostridium difficile colitis is one of the primary causative agents of nosocomial infection in humans and murine. The defective STAT3 signaling pathway leads to susceptibility to infectious colitis and bacterial peritonitis, and leptin therapy must restore the protective mucosal immune response in C. difficile colitis through the STAT3 inflammatory pathway. Infected with Helicobacter pylori-induced pro-inflammatory cytokine response and enhanced leptin secretion from gastric mucosa, which may play a role in weight gain after elimination of H pylori infection, suggesting that leptin has a more local than systemic effect. These findings demonstrate the presence of relevant neuroendocrine control of leptin in systemic immune defense in various bacterial diseases and thus highlight the similar therapeutic potential of leptin to control infectious diseases (Shirsev and Orlova, 2005; Mancuso et al., 2012).
6.4 LEPTIN AND VIRUTIC DISEASES
In mice with impaired leptin receptors or decreased serum leptin levels, viral clearance was impaired, IFN-levels decreased and survival rates decreased in the case of influenza-A infections. Mice lacking functional leptin receptors in T cells limited pH1N1 influenza mortality and infection severity in obese mice, suggesting that leptin signaling in T cells may be a critical mediator of pH1N1 intensity in obese mice. In addition, decreased leptin level in Leptin-resistant obese mice or obese individuals has been observed to increase susceptibility to influenza virus infection by suppressing memory T cell function IFN-α, IFN-β, and IFN-γ mRNA. It has been stated that high levels of leptin receptors in the blood mononuclear cells of
HIV-infected patients, while low levels of leptin in their serum lead to a deficiency in the immune level (Milner, Meyers, Sheridan, Mancuso, & Beck, 2014). One of the most important treatment modalities for HIV-induced HALS patients has been leptin loading and a positive effect has been reported. Leptin has also been discovered to reduce the oxidative status of monocytes, showing that leptin can alter the redox state of monocytes, leading to immunological changes in HIV infection. Collecting this information suggests that leptin can control the viral immune defense and also reveal possible cure potential for leptin analogues in infectious disease (Milner, Meyers, Sheridan, Mancuso, & Beck, 2014; Rebeles et al., 2015).
6.5 LEPTINE AND PARASITIC INFECTIONS
According to the disclosed and recorded reports, most of the deaths from infections caused by parasitic diseases were encountered in poor developing countries. Inadequate, unbalanced and malnutrition causes serum leptin levels to decrease and is a common feature of many infectious parasitic infections. Since leptin has been reported to induce pro-inflammatory cytokines and chemokines, neutrophil chemotaxis, NK cell cytotoxicity, and T cell functions, its deficiency is not susceptible to infectious diseases.
It has been stated that it leads to an increase. Moreover, leptin exerts central effects on hypothalamic-pituitary function and impairment of these effects causes severe parasitic diseases in the host due to immune dysfunction. Also, little is known about the role of leptin in the pathogenesis of parasitic infections (Ortega, Eberhard, & Kris, 2008). There is a need to examine the role of leptin in the control of parasitic infections, as the pressure of such infections is associated with malnutrition, which goes hand in hand in developing countries.
7. RELATION OF LEPTIN AND DIABETES
Diabetes According to the information obtained from the reports made in recent years, it is reported that more than 382 million people worldwide have an increase in the world. People suffering from diabetes are vulnerable to short- and long-term complications such as cardiovascular, kidney disease, blindness, and amputation, making it a serious health problem that needs to be controlled. Given the prevalence and incidence of diabetes, it has been
explained that the pathogenesis still needs to be investigated in order to facilitate the search for strategies and new methods for its treatment. Since the discovery of this disease, external insulin has been the mainstay in the treatment of insulin-deficient diabetes mellitus. Due to the severe beta cell deficiency, administration of exogenous insulin is necessary for survival. However, despite the progress in understanding the causes of diabetes, its consequences and improvements in the development of insulin and its analogs, maintaining tight glycemic control without adverse consequences such as hypoglycemia and weight gain remains a challenge. This also highlights the need for alternative approaches to insulin or excipients (Bandaru & Shankar, 2011).
7.1 LEPTINE GLUCOSE METABOLISM
Studies show that leptin also plays an important role in glucose metabolism in the regulation of energy homeostasis. Studies in rodent models of leptin deficiency have been pioneers in uncovering this information. Leptin therapy lowers blood sugar and insulin levels regardless of changes in food intake and body weight. In both rodents and humans, administration of leptin improves severe insulin resistance and characteristics of other non-obesity-related leptin deficiency models, such as lipodystrophy, which is characterized by fat loss. Taken together, these data show that leptin regulates glycemic control in addition to energy balance in both rodent models and clinical settings (Mastronard, Paz-Filho, Wong, & Licinio, 2012).
It has been proven in recent studies that leptin has a glucose-lowering effect. Since insulin is required for the synthesis and storage of triacylglycerol in adipose tissue, there is a depletion of body fat stores in uDM without insulin therapy. These rapid, progressive losses in body fat stores are accompanied by a marked decrease in plasma leptin levels. Therefore, uDM is characterized by hyperglycemia and hyperphagia, and these responses are deficient in both insulin and leptin. It has been proven that leptin at doses that have no effect when administered peripherally to the brain normalizes blood glucose levels in rodent models. This glucose-lowering effect of leptin occurs by a mechanism independent of reduced food intake, increased urinary glucose loss, or recovery of pancreatic beta cells (Schmidt et al., 2006). Moreover, the glucose-
lowering effect of leptin is mediated by an insulin-independent mechanism characterized by normalization of hepatic glucose production and increased glucose uptake in peripheral tissues including brown adipose tissue, muscle and heart. Collectively, these data suggest that leptin signaling in the brain in uDM is capable of normalizing diabetic hyperglycemia.
7.2 MECHANISMS THAT MEDIATE LEPTIN'S GLUCOSE-REDUCING EFFECTS
The working principle of leptin, which affects the glucose-lowering effects, has not yet been fully clarified and is an active research area. Hyperphagia, hyperglycagonemia, and hypercorticosteronemia are associated with DM, and they are thought to increase glucose production and increase hyperglycemia. The hypothalamic pituitary thyroid axis is inhibited in leptin-deficient rats and mice. This situation can be corrected with leptin treatment. Hyperglycagonemia is one of the hallmarks of diabetes in both humans and rodents. Somatostatin suppression of glucagon has been found to lower blood sugar in a pharmacologically induced canine diabetes model or in people with type 1 diabetes. In addition, recent studies suggest that the glucose-lowering effects of leptin accompany the normalization of plasma glucagon levels (Denroche, Huynh, & Keiffer 2012; Montague et al., 1997).
7.3 NERVOUS EFFECTS CONTROL THE GLUCOSE-REDUCING EFFECTS OF LEPTIN
Leptin receptors are expressed throughout the brain, including various hypothalamic and extrahypotalamic areas involved in the control of energy balance and autonomic function. Neurons in the hypothalamic ventromedial nucleus express leptin receptors and are affected by leptin.
is activated. Injection of leptin in these areas of the brain has been shown to increase glucose uptake in peripheral tissues. Accompanied by these findings, it has been proven that leptin administration to the hypothalamic ventromedial nucleus is sufficient to normalize metabolic disorders associated with DM
(Covey et al., 2006). In additional studies, it was found that the effects of normalizing blood glucose levels in DM require melanocortin signal and are realized with this signal.
8. RESEARCHES ON LEPTIN
This section covers research on leptin. Studies on leptin control, obese adolescents and the skeletal system have been reviewed.
8.2 RELATIONSHIP OF LEPTIN LEVELS WITH SUBCLINIC DYSFUNCTION IN OBESE ADOLESCENTS
Purpose of the Study: The purpose of this study is to verify the effect of that gland on cardiac functions and to evaluate its relationship with leptin and uric acid levels in obese adolescents.
Methods: In this study of 80 participants, the age group of 16-19 was classified as obese or non-obese. Leptin, GLS and uric acid levels were evaluated and compared. It was also analyzed for correlation using regression analysis.
Results: It was observed that the obese group had lower GLS than non-obese. In the obese group, subclinical cardiac dysfunction was worse in the hyperleptinemic group than in the normoleptinemic group. Multivariate regression analysis showed that leptin and triglyceride levels were negatively correlated with absolute GLS.
Conclusion: While subclinical left ventricular systolic dysfunction was found in obese adolescents, GLS was worse in hyperleptinemic cases. Levels of leptin, but not uric acid, have been proven to be associated with worsening of GLS.
8.3 ROLE OF LEPTIN IN HUMAN OBESITY AND ILLNESS: REVIEW OF CURRENT EVIDENCE
Purpose: To review the recent developments in the pathophysiology of leptin, a hormone derived from adipose tissue, and potential clinical applications.
Data Sources: This research is with a MEDLINE search of the literature and related bibliographies on leptin.
Data Synthesis: Leptin is a 16 kilodalton adipocyte hormone that circulates freely and bound in the serum. Serum leptin levels reflect the amount of energy stored in adipose tissue. Serum levels of several cytokines and hormones as well as short-term energy imbalance affect circulating levels of leptin. Leptin acts by binding to specific receptors in the hypothalamus to alter the expression of several neuropeptides that regulate neuroendocrine function and energy intake and expenditure. Therefore, it is thought that leptin plays an important role in the pathogenesis of obesity and eating disorders and mediates the neuroendocrine response to food deprivation. It is expected that the availability of more soluble leptin analogues for clinical studies in humans could significantly advance understanding of the lower layers of energy homeostasis in humans.
Conclusions: Leptin has been shown to significantly expand the understanding of the mechanisms underlying neuroendocrine function, body weight and energy homeostasis. The elucidation of these mechanisms is expected to result in the development of new therapeutic approaches for obesity and eating disorders.
8.4 THE ROLE OF LEPTIN AND BONE METABOLISM IN CHILDREN BETWEEN 8-11 YEARS DEFINING THE BODY FAT OF OTHER HORMONES RELATED TO THE APPEAL REGULATION
It is known that the regulation of body composition in childhood is complex. Numerous hormones potentially play a role. Leptin has been suggested to limit weight gain, but results have been found to be inconsistent.
Objective: It was investigated whether ghrelin, adinopectin, leptin, IGF-I, osteocalcin and parathyroid hormone were related to body composition cross-sectionally and longitudinally at the age of 8-11 years.
Conclusions: Cross-sectional findings support that leptin is produced in proportion to body fat mass, but longitudinal observations indicate that leptin inhibits gains in FMI and FFMI in girls, which may reflect preserved leptin sensitivity predominantly in the
normal weight population.
CONCLUSION AND RECOMMENDATION
The role and physiology of leptin hormone in our body has gained popularity in recent years. Since the year it was discovered, its importance has been understood thanks to the studies and researches. Thanks to leptin, many derivatives in our body have been illuminated. With the physiology of leptin, it is reported that there are very serious issues that are waiting to be discovered and studied, and that there are studies that can take us forward in a positive sense. Leptin related issues are known to have relationships ranging from nutrition to sleep patterns, from living standards to genetic factors. For this reason, it has been explained that it is a very big part of our life and it should be known how hard it is for us.
It is known that leptin secretion level, serum levels and leptin use are interrupted in case of malnutrition and the working principle is affected. These effects have also been explained very precisely that when the malnutrition is eliminated, improvements occur and leptin comes to its own line. As a result, regular, healthy and adequate nutrition is an important step, and the individual should take care and comply with this situation. One of the situations where leptin is regulating and controlling is obesity. It causes obesity to remain in a cycle due to the inadequate use of the secreted leptin in the cells and the undermining of its regulatory effect. It is no coincidence that serum leptin levels and leptin resistance are at the forefront of many health problems.
It is one of the situations that should be examined in creating a diet for individuals, to use the power of leptin, to monitor it, to control it if necessary, to bring the serum level to the point where it should be, to be included in the studies taking into account the effects of the diet plan in addition to the studies, to inform people more about the importance of leptin by the state. It is recommended to encourage hormone tests.
SOURCES
-
-
Brennan, A., & Mantzoros, C. (2020). Drug Insight: the role of leptin in human physiology and pathophysiology—emerging clinical applications. Retrieved24April2020,from https://www.ncbi.nlm.nih.gov/pubmed/16932309
-
-
Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, Roubenoff R, Auerbach S, DeCarli C, Wolf PA, Seshadri S (December 2009).
"Association of plasma leptin levels with incident Alzheimer disease and MRI measures of brain aging"
.
JAMA
.
302
(23): 2565–72.
doi
:
10.1001/jama.2009.1836
.
PMC
2838501
.
PMID
20009056
.
-
Doherty GH, Beccano-Kelly D, Yan SD, Gunn-Moore FJ, Harvey J (January 2013). "Leptin prevents hippocampal synaptic disruption and neuronal cell death induced by amyloid β".
Neurobiol. Aging
.
34
(1): 226–37.
doi
:
10.1016/j.neurobiolaging.2012.08.003
.
PMID
22921154
.
-
-
Ciriello J, Moreau JM (November 2012). "Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in
the rat". Journal of Neuroscience. 229: 88–99.
doi
:
10.1016/j.neuroscience.2012.10.065
.
PMID
23159310
.
-
Torday JS, Rehan VK (October 2006). "Up-regulation of fetal rat lung parathyroid hormone-related protein gene regulatory network down-regulates the Sonic Hedgehog/Wnt/beta-catenin gene regulatory network". Pediatr. Res. 60 (4): 382–88.
doi
:
10.1203/01.pdr.0000238326.42590.03
.
PMID
16940239
.
-
Gruzdeva, O., Borodkina, D., Uchasova, E., Dyleva, Y., & Barbarash, O. (2019). Leptin resistance: underlying mechanisms and diagnosis. Diabetes, metabolic syndrome and obesity : targets and therapy, 12, 191–198. https://doi.org/10.2147/DMSO.S182406
-
-
-
-
Mabuchi T, Yatsuya H, Tamakoshi K, Otsuka R, Nagasawa N, Zhang H, Murata C, Wada K, Ishikawa M, Hori Y, Kondo T, Hashimoto S, Toyoshima H (2005). "Association between serum leptin concentration and white blood cell count in middle-aged Japanese men and women". Diabetes Metab. Res. Rev. 21 (5): 441–47.
doi
:
10.1002/dmrr.540
.
PMID
15724240
.
-
Considine, R. (2020). Leptin and Other Endocrine Systems. Retrieved 20 April 2020, from Department of Medicine, Indiana University, Indianapolis, IN
-
de Salles BF, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E (July 2010). "Effects of resistance training on cytokines". Int J Sports Med. 31 (7): 441–50.
doi
:
10.1055/s-0030-1251994
.
PMID
20432196
.
-
Hickey MS, Considine RV, Israel RG, Mahar TL, McCammon MR, Tyndall GL, Houmard JA, Caro JF (November 1996). "Leptin is related to body fat content in male distance runners". Am. J. Physiol. 271 (5 Pt 1): E938–40.
doi
:
10.1152/ajpendo.1996.271.5.E938
.
PMID
8944684
.
-
Faggioni R, Jones-Carson J, Reed DA, Dinarello CA, Feingold KR, Grunfeld C, et al. . Leptin-deficient (ob/ob) mice are protected from T cell-mediated hepatotoxicity: role of tumor necrosis factor α and IL-18. Proc Natl Acad Sci USA. (2000) 97:2367–72. 10.1073/pnas.040561297 [
PMC free article
] [
PubMed
] [
CrossRef
] [
Google Scholar
]
-
Procaccini C, Jirillo E, Matarese G. Leptin as an immunomodulator. Mol Aspects Med. (2012) 33:35–45. 10.1016/j.mam.2011.10.012
-
Fernandez-Riejos P, Najib S, Santos-Alvarez J, Martín-Romero C, Pérez-Pérez A, González-Yanes C, et al. . Role of leptin in the activation of immune cells. Media Inflamm. (2010) 2010:568343. 10.1155/2010/568343
-
Sharma A, Satyam A, Sharma JB. Leptin, IL-10 and inflammatory markers (TNF-alpha, IL-6 and IL-8) in pre-eclamptic, normotensive pregnant and healthy non-pregnant women. Am J Reprod Immunol. (2007) 58:21–30. 10.1111/j.1600-0897.2007.00486.x
-
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L,
Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature (1994) 372:425–32. 10.1038/372425a0 [
PubMed
] [
CrossRef
] [
Google Scholar
]
-
Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia (1978) 14:141–8. 10.1007/BF00429772 [
PubMed
] [
CrossRef
] [
Google Scholar
]
-
International Diabetes Federation (2013) IDF diabetes atlas, 6th edn. International Diabetes Federation, Brussels
-
Chen L, Magliano DJ, Zimmet PZ (2011) The worldwide epidemiology of type 2 diabetes mellitus—present and future perspectives. Nat Rev Endocrinol 8:228–236
-
The Diabetes Control and Complications Trial Research Group (1993) The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 329:977–986
-
Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL (1999) Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature 401:73–76
-
Imerbtham, T., Thitiwuthikiat, P., Jongjitwimol, J., Nuamchit, T., Yingchoncharoen, T., & Siriwittayawan, D. (2020). <p>Leptin Levels are Associated with Subclinical Cardiac Dysfunction in Obese Adolescents</p>. Retrieved 13 April 2020, from
https://www.ncbi.nlm.nih.gov/pubmed/32273744
-
Dalskov, S., Ritz, C., Larnkjær, A., Damsgaard, C., Petersen, R., & Sørensen, L. et al. (2020). The Role of Leptin and Other Hormones Related to Bone Metabolism and Appetite
Regulation as Determinants of Gain in Body Fat and Fat-Free Mass in 8–11-Year-Old Children. Retrieved 13 April 2020, from
https://www.ncbi.nlm.nih.gov/pubmed/25532044
-
Zhang W, Telemaque S, Augustyniak RA, Anderson P, Thomas GD, An J, Wang Z, Newgard CB, Victor RG (2010). "Adenovirus-mediated leptin expression normalises hypertension associated with diet-induced obesity". J Neuroendocrinol. 22 (3): 175–80.
doi
:
10.1111/j.1365-2826.2010.01953.x
.
PMID
20059648
.
-
-
Ciriello J, Moreau JM (November 2012). "Systemic administration of leptin potentiates the response of neurons in the nucleus of the solitary tract to chemoreceptor activation in the rat". Journal of Neuroscience. 229: 88–99.
-
-
Hamrick, M., & Ferrari, S. (2007). Leptin and the sympathetic connection of fat to bone. Osteoporosis International
, 19
(7), 905-912. doi: 10.1007/s00198-007-0487-9
-
Zhao J, Townsend KL, Schulz LC, Kunz TH, Li C, Widmaier EP (2004). "Leptin receptor expression increases in placenta, but not hypothalamus, during gestation in Mus musculus and Myotis lucifugus".
Placenta
.
25
(8–9): 712–22.
doi
:
10.1016/j.placenta.2004.01.017
.
PMID
15450389
.
-
Morroni M, De Matteis R, Palumbo C, Ferretti M, Villa I, Rubinacci A, Cinti S, Marotti G (2004).
"In vivo leptin expression in cartilage and bone cells of growing rats and adult humans"
.
Journal of Anatomy
.
205
(4): 291–96.
doi
:
10.1111/j.0021-8782.2004.00333.x
.
PMC
1571344
.
PMID
15447688
.
-
-
-
-
Palhares, H., Silva, A., Resende, D., Pereira, G., Rodrigues-Júnior, V., & Borges, M. (2020). Evaluation of clinical and laboratory markers of cardiometabolic risk in overweight and obese children and adolescents. Retrieved 19 April 2020, from
-
-
-
Heiman ML, Ahima RS, Craft LS, Schoner B, Stephens TW, Flier JS (September 1997). "Leptin inhibition of the hypothalamic-pituitary-adrenal axis in response to stress".
Endocrinology
.
138
(9): 3859–63.
doi
:
10.1210/en.138.9.3859
.
PMID
9275075
.
-
Coppari, R., & Bjørbæk, C. (2012). Leptin revisited: its mechanism of action and potential for treating diabetes. Nature reviews. Drug discovery
, 11
(9), 692–708. https://doi.org/10.1038/nrd3757
-
Meek, T. H., & Morton, G. J. (2012). Leptin, diabetes, and the brain. Indian journal of endocrinology and metabolism
, 16
(Suppl 3), S534–S542. https://doi.org/10.4103/2230-8210.105568
-
Perrier S, Caldefie-Chézet F, Vasson MP (January 2009). "IL-1 family in breast cancer: potential interplay with leptin and other adipocytokines". FEBS Lett. 583 (2): 259–65.
doi
:
10.1016/j.febslet.2008.12.030
.
PMID
19111549
.
-
Mabuchi T, Yatsuya H, Tamakoshi K, Otsuka R, Nagasawa N, Zhang H, Murata C, Wada K, Ishikawa M, Hori Y, Kondo T, Hashimoto S, Toyoshima H (2005). "Association between serum leptin concentration and white blood cell count in middle-aged Japanese men and women". Diabetes Metab. Res. Rev. 21 (5): 441–47.
doi
:
10.1002/dmrr.540
.
PMID
15724240
.
-
International Expert Committee International Expert Committee report on the role of the A1C assay in the
diagnosis of diabetes. Diabetes Care 2009; 32: 1327– 1334 [
PMC free article
] [
PubMed
]
-
Al Sheikh M. H. (2017). The Determinants of Leptin Levels in Diabetic and Nondiabetic Saudi Males.
International journal of endocrinology
,
2017
, 3506871.
https://doi.org/10.1155/2017/3506871
-
Strohacker, K., McCaffery, J. M., MacLean, P. S., & Wing, R. R. (2014). Adaptations of leptin, ghrelin or insulin during weight loss as predictors of weight regain: a review of current literature.
International journal of obesity (2005)
,
38
(3), 388–396.
https://doi.org/10.1038/ijo.2013.118
-
Korek, E., Krauss, H., Gibas-Dorna, M., Kupsz, J., Piątek, M., & Piątek, J. (2013). Fasting and postprandial levels of ghrelin, leptin and insulin in lean, obese and anorexic subjects. Przeglad gastroenterologiczny
, 8
(6), 383–389. https://doi.org/10.5114/pg.2013.39922
-
Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997; 20: 1183– 1197
-
Nogueiras, R., Tschöp, M. H., & Zigman, J. M. (2008). Central nervous system regulation of energy metabolism: ghrelin versus leptin. Annals of the New York Academy of Sciences, 1126, 14–19.
https://doi.org/10.1196/annals.1433.054
-
Rodríguez A. (2014). Novel molecular aspects of ghrelin and leptin in the control of adipobiology and the cardiovascular system. Obesity facts, 7(2), 82–95.
https://doi.org/10.1159/000360837
-
Rosenbaum, M., & Leibel, R. L. (2014). 20 years of
leptin: role of leptin in energy homeostasis in humans. The Journal of endocrinology, 223(1), T83–T96.
https://doi.org/10.1530/JOE-14-0358
-
Rosenbaum, M. (2005). Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. Journal Of Clinical Investigation, 115(12), 3579-3586. doi: 10.1172/jci25977
-
Zieba, D., Biernat, W., & Barć, J. (2020). Roles of leptin and resistin in metabolism, reproduction, and leptin resistance. Domestic Animal Endocrinology, 106472. doi: 10.1016/j.domaniend.2020.106472
-
Zieba, D., Biernat, W., & Barć, J. (2020). Roles of leptin and resistin in metabolism, reproduction, and leptin resistance. Domestic Animal Endocrinology, 106472. doi: 10.1016/j.domaniend.2020.106472
-
-
Paz-Filho, G., Mastronardi, C., Wong, M. L., & Licinio, J. (2012). Leptin therapy, insulin sensitivity, and glucose homeostasis. Indian journal of endocrinology and metabolism, 16(Suppl 3), S549–S555.
https://doi.org/10.4103/2230-8210.105571
-
-
Stern, J. H., Rutkowski, J. M., & Scherer, P. E. (2016). Adiponectin, Leptin, and Fatty Acids in the Maintenance of Metabolic Homeostasis through Adipose Tissue Crosstalk.
Cell metabolism
,
23
(5), 770–784.
https://doi.org/10.1016/j.cmet.2016.04.011
-
Flier, J. S., Harris, M., & Hollenberg, A. N. (2000). Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring.
The Journal of clinical investigation
,
105
(7), 859–861.
https://doi.org/10.1172/JCI9725
-
Sesmilo, G., Casamitjana, R., Halperin, I., Gomis, R., & Vilardell, E. (1998). Role of thyroid hormones on serum leptin levels. European Journal Of Endocrinology
, 139
(4), 428-430. doi: 10.1530/eje.0.1390428
-
Hsieh, C., Wang, P., Wang, S., Liu, R., Tung, S., & Chien, W. et al. (2002). Serum leptin concentrations of patients with sequential thyroid function changes. Clinical Endocrinology
, 57
(1), 29-34. doi: 10.1046/j.1365-2265.2002.01543.x
-
Brąclik, M., Marcisz, C., Giebel, S., & Orzeł, A. (2008). Serum Leptin and Ghrelin Levels in Premenopausal Women with Stable Body Mass Index during Treatment of Thyroid Dysfunction. Thyroid
, 18
(5), 545-550. doi: 10.1089/thy.2007.0300
-
Guseynova, R., & Mirzazadze, V. (2010). Effect of duration of diabetes mellitus on the prevalence of diabetic retinopathy in patients with type 2 diabetes and metabolic syndrome. Diabetes Mellitus
, 13
(2), 94-96. doi: 10.14341/2072-0351-5682
-
Palik, E., Birkás, K., Faludi, G., Karádi, I., & Cseh, K. (2005). Correlation of serum ghrelin levels with body mass index and carbohydrate metabolism in patients treated with atypical antipsychotics. Diabetes Research And Clinical Practice
, 68
, S60-S64. doi: 10.1016/j.diabres.2005.03.008
-
Myers, M. G., Jr, Heymsfield, S. B., Haft, C., Kahn, B.
B., Laughlin, M., Leibel, R. L., Tschöp, M. H., & Yanovski, J. A. (2012). Challenges and opportunities of defining clinical leptin resistance. Cell metabolism
, 15
(2), 150–156. https://doi.org/10.1016/j.cmet.2012.01.002
-
Popovic, V., Doknic, M., Maric, N., Pekic, S., Damjanovic, A., & Miljic, D. et al. (2007). Changes in Neuroendocrine and Metabolic Hormones Induced by Atypical Antipsychotics in Normal-Weight Patients with Schizophrenia. Neuroendocrinology
, 85
(4), 249-256. doi: 10.1159/000103868
-
MURASHITA, M., INOUE, T., KUSUMI, I., NAKAGAWA, S., ITOH, K., & TANAKA, T. et al. (2007). Glucose and lipid metabolism of long-term risperidone monotherapy in patients with schizophrenia. Psychiatry And Clinical Neurosciences
, 61
(1), 54-58. doi: 10.1111/j.1440-1819.2007.01610.x
-
Li M. D. (2011). Leptin and beyond: an odyssey to the central control of body weight. The Yale journal of biology and medicine
, 84
(1), 1–7.
-
Wolf AM, Colditz GA. Current estimates of the economic cost of obesity in the United States. Obes Res. 1998;6(2):97–106.
-
Finkelstein EA, Fiebelkorn IC, Wang G. State-level estimates of annual medical expenditures attributable to obesity. Obes Res. 2004;12(1):18–24
-
Gorska, E., Popko, K., Stelmaszczyk-Emmel, A., Ciepiela, O., Kucharska, A., & Wasik, M. (2010). Leptin receptors.
European journal of medical research
,
15 Suppl 2
(Suppl 2), 50–54.
https://doi.org/10.1186/2047-783x-15-s2-50
-
Kloek C, Haq AK, Dunn SL, Lavery HJ, Banks AS,
Myers MG. Regulation of Jak kinases by intracellular leptin receptor sequences. J Biol Chem. 2002;277:41547–55. doi: 10.1074/jbc.M205148200.
-
Dunn S, Björnholm M, Bates SH, Chen Z, Seifert M, Myers MG Jr. Feedback inhibition of leptin receptor/Jak2 signaling via Tyr1138 of the leptin Eeceptor and suppressor of cytokine signaling 3. Mol endocrinol. 2005;19:925–38. doi: 10.1210/me.2004-0353.
-
Sahu A. Leptin signaling in the hypothalamus: emphasis on energy homeostasis and leptin resistance. Front Neuroendocrinol. 2003;24:225–53. doi: 10.1016/j.yfrne.2003.10.001.
-
Martin-Romero C, Sanchez-Margarel V. Human leptin activates PI3K and MAPK pathways in human peripheral blood mononuclear cells: possible role of Sam 68. Cell immunol. 2001;212:83–91. doi: 10.1006/cimm.2001.1851.
-
Sweeney G. Leptin signalling. Cell Signal. 2002;14:655–63. doi: 10.1016/S0898-6568(02)00006-2.
-
Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte J-M, Basdevant A, Bougneres P, Lebouc Y, Froguel P, Guy-Grand B. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911.
-
Ross JA, Kevin C, Oeffinger KC, Davies SM, Mertens AC, Langer EK, Kiffmeyer WR, Sklar CA, Stovall M, Yasui Y, Robison LL. Genetic variation in the leptin receptor gene and obesity in survivors of childhood acute lymphoblastic
leukemia: A Report from the childhood cancer survivor study. J Clin Oncol. 2004;22:3558–62. doi: 10.1200/JCO.2004.11.152.
-
Guizar-Mendoza JM, Amador-Licona N, Flores-Martinez SE, Lopez-Gardona MG, Ahuatzin-Tremary R, Sanchez-Corona J. Association analysis of the Gln223Arg polymorphism in the human leptin receptor gene, and traits related to obesity in Mexican adolescents. J Hum Hypertens. 2005;19:341–6. doi: 10.1038/sj.jhh.1001824.
-
Di Spiezio, A., Sandin, E. S., Dore, R., Müller-Fielitz, H., Storck, S. E., Bernau, M., Mier, W., Oster, H., Jöhren, O., Pietrzik, C. U., Lehnert, H., & Schwaninger, M. (2018). The LepR-mediated leptin transport across brain barriers controls food reward.
Molecular metabolism
,
8
, 13–22.
https://doi.org/10.1016/j.molmet.2017.12.001
-
Flier J. S. (1997). Leptin expression and action: new experimental paradigms.
Proceedings of the National Academy of Sciences of the United States of America
,
94
(9), 4242–4245.
https://doi.org/10.1073/pnas.94.9.4242
-
-
-
Eferl R, Hasselblatt P, Rath M, Popper H, Zenz R, Komnenovic V, et al. Development of pulmonary fibrosis through a pathway involving the transcription factor Fra-2/AP-1. Proc Natl Acad Sci U S A. 2008;105:10525–30. doi: 10.1073/pnas.0801414105.
-
Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier
JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625.
-
Bjorbaek C, Uotani S, da Silva B, Flier JS. Divergent signaling capacities of the long and short isoforms of the leptin receptor. J Biol Chem. 1997;272:32686–32695.
-
Lam QL, Lu L. Role of leptin in immunity. Cell Mol Immunol. 2007;4:1–13.
-
Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Päivärinta U, Moilanen T, Moilanen E. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage-mediator role of NO in leptin-induced PGE 2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838.
-
Beltowski J. Leptin and atherosclerosis. Atherosclerosis. 2006;189:47–60.
-
Gruzdeva O, Uchasova E, Dyleva Y, et al. Relationships between epicardial adipose tissue thickness and adipofibrokine indicator profiles post-myocardial infarction. Cardiovasc Diabetol. 2018;17(1):40.
-
Guerra B, Ponce-González JG, Morales-Alamo D, et al. Leptin signaling in skeletal muscle after bed rest in healthy humans. Eur J Appl Physiol. 2014;114(2):345–357.
-
Murugesan D, Arunachalam T, Ramamurthy V, Subramanian S. Association of polymorphisms in leptin receptor gene with obesity and type 2 diabetes in the local population of Coimbatore. Indian J Hum Genet. 2010;16:72–77.
-
Rizk NM, Sharif E. Leptin as well as free leptin receptor is associated with polycystic ovary syndrome in young women. Int J Endocrinol. 2015;2015(1):1–10.
-
Zhang J, Scarpace PJ. The soluble leptin receptor
neutralizes leptin-mediated STAT3 signalling and anorexic responses in vivo
. Br J Pharmacol. 2009;158(2):475–482.
-
de Queiroz KB, Guimarães JB, Coimbra CC, et al. Endurance training increases leptin expression in the retroperitoneal adipose tissue of rats fed with a high-sugar diet. Lipids. 2014;49(1):85–96.
-
Alti, D., Sambamurthy, C., & Kalangi, S. K. (2018). Emergence of Leptin in Infection and Immunity: Scope and Challenges in Vaccines Formulation.
Frontiers in cellular and infection microbiology
,
8
, 147.
https://doi.org/10.3389/fcimb.2018.00147
-
Martin-Romero C, Santos-Alvarez J, Governa R, Sanchez-Margalet V. Human leptin enhances activation and proliferation of human circulating T lymphocytes. Cell Immunol. (2000) 199:15–24. 10.1006/cimm.1999.1594
-
Oral EA, Javor ED, Ding L, Uzel G, Cochran EK, Young JR, et al. . Leptin replacement therapy modulates circulating lymphocyte subsets and cytokine responsiveness in severe lipodystrophy. J Clin Endocrinol Metab. (2006) 91:621–8. 10.1210/jc.2005-1220
-
Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia (1978) 14:141–8. 10.1007/BF00429772
-
Madej T, Boguski MS, Bryant SH. Threading analysis suggests that the obese gene product may be a helical cytokine. FEBS Lett. (1995) 373:13–8. 10.1016/0014-5793(95)00977-H
-
Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. (2007) 4:e115. 10.1371/journal.pmed.0040115
-
Chandra RK. Nutrition, immunity and infection: from
basic knowledge of dietary manipulation of immune responses to practical application of ameliorating suffering and improving survival. Proc Natl Acad Sci USA. (1996) 93:14304–7. 10.1073/pnas.93.25.14304
-
Neeraj Indora N, Sharma K, Karunanand B, Sudan DPS, Shah AK. Assessment of metabolic (Adiponectin, Leptin) and inflammatory markers (TNF-α, hs-CRP & IFN-γ) in pulmonary tuberculosis: prospective Study. Int J Clin Biochem Res. (2017) 4:216–9. 10.18231/2394-6377.2017.0051
-
Al-Fadhli MA, Saraya MA, Qasem JA. Evaluation of leptin, interleukin-1 beta and tumor necrosis factor alpha in the serum of malaria patients as prognostic markers of treatment outcome. Asian Pac J Trop Biomed. (2014) 4:441–5. 10.12980/APJTB.4.201414B11
-
Shirshev SV, Orlova EG. Molecular mechanisms of regulation of the functional activity of mononuclear phagocytes by leptin. Biochemistry (2005) 70:841–7. 10.1007/s10541-005-0193-1
-
Mancuso P, Myers MG, Goel D, Serezani CH, O'Brien E, Goldberg J, et al. . Ablation of leptin receptor-mediated ERK activation impairs host defense against gram-negative pneumonia. J Immunol. (2012) 189:867–75. 10.4049/jimmunol.1200465
-
Milner J, Meyers M, Sheridan P, Mancuso P, Beck M. The role of T cell leptin signaling in 2009 pandemic H1N1
influenza infection mortality in obese mice (VIR2P.1013). J Immunol. (2014) 192(Suppl. 1):75.2.
-
Milner JJ, Rebeles J, Dhungana S, Stewart DA, Sumner SC, Meyers MH, et al. . Obesity increases mortality and
modulates the lung metabolome during pandemic H1N1
influenza virus infection in mice. J Immunol. (2015) 194:4846–59. 10.4049/jimmunol.1402295
-
Hur SJ, Kim DH, Chun SC, Lee SK. Effect of adenovirus and influenza virus infection on obesity. Life Sci. (2013) 93:531–5. 10.1016/j.lfs.2013.08.016
-
Qin L, Tan YR, Hu CP, Liu XA, He RX. Leptin is over-secreted by respiratory syncytial virus-infected bronchial epithelial cells and regulates th2 and th17 cell differentiation. Int Arch Allergy Immunol. (2015) 167:65–71. 10.1159/000436966
-
Ortega YR, Eberhard ML, Kris H. Protozoan diseases: cryptosporidiosis, giardiasis and other intestinal protozoan diseases. International Encyclopedia of Public Health. Oxford: Academic Press; (2008) p. 354–66.
-
Bandaru, P., & Shankar, A. (2011). Association between plasma leptin levels and diabetes mellitus.
Metabolic syndrome and related disorders
,
9
(1), 19–23.
https://doi.org/10.1089/met.2010.0037
-
Schmidt MI. Duncan BB. Vigo A. Pnakow JS. Couper D. Ballantyne CM. Hoogeveen RC. Heiss G for the ARIC Investigators. Leptin and incident type 2 diabetes: risk or protection? Diabetologia. 2006;49:2086–2096.
-
-
Montague CT, Farooqi IS, Whitehead JP, et al.
Congenital leptin deficiency is associated with severe
early‐onset obesity in humans. Nature 1997; 387: 903–908
-
Covey SD, Wideman RD, McDonald C, et al.
The pancreatic beta cell is a key site for mediating the effects of leptin on glucose homeostasis. Cell Metab 2006; 4: 291–302


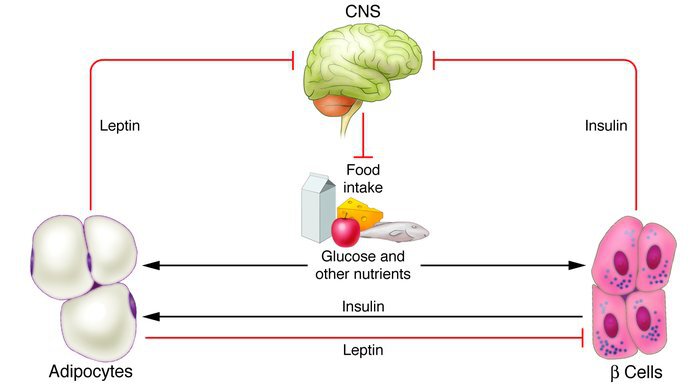

 Blocking adrenergic nerves eliminates the calorific effect of thyroid
hormones. Malnutrition decreases both leptin and thyroxine (T4) levels in cattle. The decrease in leptin levels can cause a signal in the brain to reduce the T4 production level by suppressing the release of thyropine releasing hormone (TRH) directly or indirectly. Triiodothyroacetic acid (Triac) and T3 applications inhibit the synthesis and release of leptin from the white and brown adipose tissue of rats. It has been reported that administration of T3 in different doses to rats decreases serum leptin levels and white adipose tissue mRNA levels depending on the dose (Hsieh et al., 2002; Braclık, Marcisz, Giebel, Orzel, 2008). It is known that thyroid hormones play a regulatory role in energy metabolism by increasing thermogenesis. The most important factors in thermogenesis are "uncoupling" proteins (UCP). UCPs are located in the inner membrane of the mitochondria and allow the release of heat instead of ATP synthesis by preventing the protons to match. Thyroid hormones strongly stimulate the expression of UCP2 and UCP3, thus enabling more heat to be generated and consequently more energy expenditure (Guseynova and Mirzazadze, 2010).
Blocking adrenergic nerves eliminates the calorific effect of thyroid
hormones. Malnutrition decreases both leptin and thyroxine (T4) levels in cattle. The decrease in leptin levels can cause a signal in the brain to reduce the T4 production level by suppressing the release of thyropine releasing hormone (TRH) directly or indirectly. Triiodothyroacetic acid (Triac) and T3 applications inhibit the synthesis and release of leptin from the white and brown adipose tissue of rats. It has been reported that administration of T3 in different doses to rats decreases serum leptin levels and white adipose tissue mRNA levels depending on the dose (Hsieh et al., 2002; Braclık, Marcisz, Giebel, Orzel, 2008). It is known that thyroid hormones play a regulatory role in energy metabolism by increasing thermogenesis. The most important factors in thermogenesis are "uncoupling" proteins (UCP). UCPs are located in the inner membrane of the mitochondria and allow the release of heat instead of ATP synthesis by preventing the protons to match. Thyroid hormones strongly stimulate the expression of UCP2 and UCP3, thus enabling more heat to be generated and consequently more energy expenditure (Guseynova and Mirzazadze, 2010).
 Given that women with anorexia nervosa and exercise-induced amenorrhea were chronically energy-deprived, it was first hypothesized that these conditions were associated with hypoleptinemia. This has been confirmed in observational studies. It was later hypothesized that long-standing hypoleptinemia leads to neuroendocrine dysfunction with subsequent anovulation and osteoporosis (Schwartz, 2003). A proof-of-concept trial of leptin therapy at replacement doses in women with hypothalamic amenorrhea has been conducted and found that it improves or completely normalizes gonadal, thyroid, and to a lesser extent growth hormone axes and bone markers.
Given that women with anorexia nervosa and exercise-induced amenorrhea were chronically energy-deprived, it was first hypothesized that these conditions were associated with hypoleptinemia. This has been confirmed in observational studies. It was later hypothesized that long-standing hypoleptinemia leads to neuroendocrine dysfunction with subsequent anovulation and osteoporosis (Schwartz, 2003). A proof-of-concept trial of leptin therapy at replacement doses in women with hypothalamic amenorrhea has been conducted and found that it improves or completely normalizes gonadal, thyroid, and to a lesser extent growth hormone axes and bone markers. To date, several mechanisms have been identified as potentially underlying leptin resistance. These include a number of molecular and functional changes characterized by structural changes in the molecule, transport to blood across the brain barrier, and impairment and signaling of leptin-receptor function (Guizar et al.,2005 )
To date, several mechanisms have been identified as potentially underlying leptin resistance. These include a number of molecular and functional changes characterized by structural changes in the molecule, transport to blood across the brain barrier, and impairment and signaling of leptin-receptor function (Guizar et al.,2005 )