Chapter Eight
Veno-Venous ECMO
There are times when a patient's lung disease is so severe that adequate gas exchange is either impossible, or can only be accomplished with prohibitively high airway pressures and tidal volumes. When this is the case, veno-venous extracorporeal membrane oxygenation (VV ECMO) should be considered as a rescue therapy. This chapter is simply an overview of the use of extracorporeal support and is designed to familiarize the reader with the rationale for its use. Those desiring to treat patients with ECMO are strongly encouraged to attend a training program sponsored by the Extracorporeal Life Support Organization (ELSO).
VV vs. VA
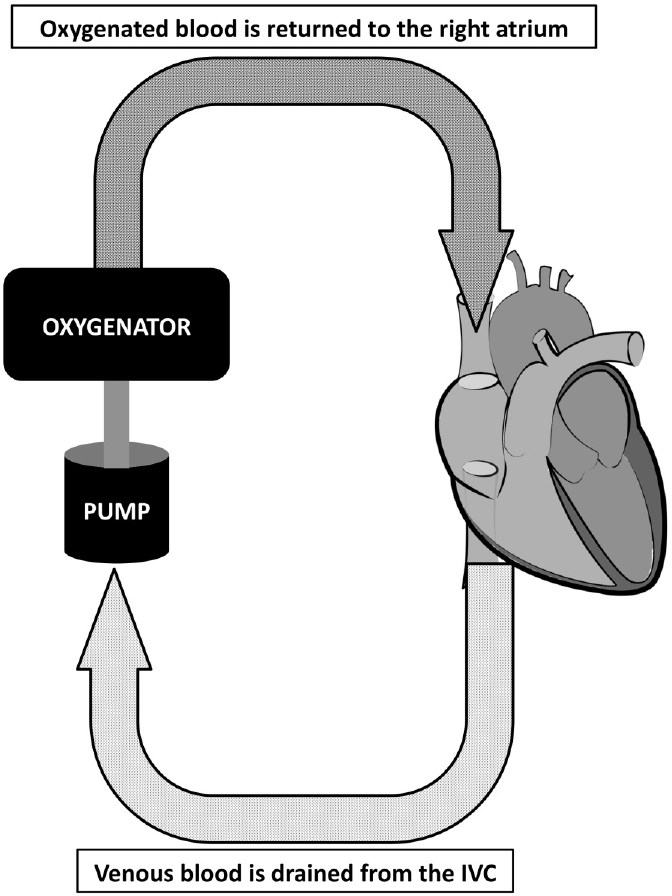
Veno-venous ECMO is considerably different from veno-arterial ECMO (VA ECMO). VA ECMO is similar to heart-lung bypass. Blood is drained from the venous side using a cannula placed in the femoral vein. The blood is pumped through an oxygenator, and the fully oxygenated blood is returned to the patient via a cannula placed in the femoral or subclavian artery (Figure 1). In neonates, the carotid artery is often used; in adults, however, the carotid artery is avoided due to the risk of stroke. With VA ECMO, the extracorporeal circuit can support both the pulmonary and the cardiac systems. The flow through
the pump can make up for even the most severe heart failure. In fact, the primary indication for VA ECMO in adults is refractory cardiogenic shock.
The VA ECMO circuit provides both respiratory and cardiac support by pumping oxygenated blood directly into the aorta. The pump flow is sufficient to replace the entire cardiac output, if necessary
.
VV ECMO, on the other hand, provides no cardiac support. Blood is drained from the inferior vena cava through a cannula placed in the femoral vein. After being pumped through an oxygenator, the blood is returned to the right atrium through a cannula placed in the internal jugular vein.
Dual-lumen cannulas are also available, and function similarly to dual-lumen hemodialysis catheters (albeit much
larger, to accommodate a flow of 4-7 L/min). The dual-lumen cannula is placed via the right internal jugular vein. It passes through the superior vena cava and into the inferior vena cava. The siphon, or drainage, ports are at the tip of the cannula in the IVC. Using transesophageal echocardiography, the cannula is manipulated so that the return port is directed over the tricuspid valve. This helps reduce the risk of recirculation.
How VV ECMO Provides Respiratory Support
The best way to visualize VV ECMO is to consider the entire circuit as an extension of the right atrium. Normally, venous blood returning to the right atrium has an SvO
2
of 70-80%. In a markedly hypoxemic patient, the SvO
2
is much lower—50-60% is the norm. The venous blood goes through the pulmonary vascular system, where oxygen is picked up and CO
2
is unloaded (and ventilated off). Obviously, if a patient has severe ARDS, then the degree to which this gas exchange can occur is quite limited. Blood with an SvO
2
of 50% may only rise to an SaO
2
of 80%, even while the patient is breathing 100% oxygen and receiving high levels of PEEP.
When VV ECMO is initiated, some (but not all) of the venous blood is siphoned into the circuit. A pump drives a blood flow of 4-7 L/min through a membrane oxygenator. As the blood passes through the oxygenator, the hemoglobin becomes fully saturated and the PaO
2
may rise as high as 400-500 mm Hg. When this blood, with an SaO
2
of 100%, is returned to the right atrium, it mixes with the remaining venous blood and then proceeds through the pulmonary circulation. If half of the venous blood has an SvO
2
of 60% and the other half has an SvO
2
of 100% (thanks to the ECMO circuit), the total venous return going through the pulmonary circulation has an SvO
2
of roughly 80%. With higher pump flows, a greater percentage of the venous return is oxygenated using the ECMO circuit, leading to a higher overall SvO
2
. In most patients, the ECMO flow can be increased to the point where the total SvO
2
is 85-90%
.
Here's where VV ECMO becomes really cool. It's also where you have to remember the principles of oxygen delivery [see the earlier chapter in this book]. With a high enough cardiac output and hemoglobin, oxygen delivery to the tissues can be maintained even with mild-to-moderate hypoxemia. In other words, an SaO
2
of 80-85% is perfectly fine so long as cardiac output is sufficient and there is enough hemoglobin to carry the bound oxygen.
If an SaO
2
of 80-85% is enough to get the job done (with adequate cardiac output and hemoglobin), and if the SvO
2
can be kept at 80-85% with VV ECMO, then
there is no need for pulmonary gas exchange whatsoever
. This is a very important point, and is the cornerstone to understanding why VV ECMO can be an effective rescue therapy for severe ARDS. If the venous blood has an SvO
2
of 85% and it flows through lungs that contribute absolutely nothing, then the blood reaching the left atrium will have an SaO
2
of 85%. Since we've already established that an SaO
2
of 85% is sufficient if there's adequate cardiac output and hemoglobin, then there is no need to "beat up the lungs" with high PEEP, high ventilator rates, or any of the other usual things that are done for severe respiratory failure. Instead, the ventilator can be put on what are generally considered "rest settings."
Ventilator Rest Settings on VV ECMO
*
•
Pressure Control Ventilation
•
Rate 10 breaths/minute
•
I-time 1.0-2.0 seconds, adjusted for comfort
•
P
INSP
25 cm H
2
O
•
PEEP 10 cm H
2
O
•
FiO
2
30%
Gas exchange in the ECMO circuit is a function of blood flow through the oxygenator and the gas flow of oxygen across the oxygenator's membrane. The oxygen that is flowing across the membrane is known as the sweep gas because it "sweeps off" CO
2
from the blood in the membrane. CO
2
has a much higher solubility than oxygen, and so it can be rapidly eliminated by increasing the sweep gas flow. Oxygenation can be increased by raising the rate of blood flow through the membrane oxygenator. Put simply, circuit flow controls oxygenation, while the flow of sweep gas controls ventilation. Sweep gas is usually 2-6 L/min. The FiO
2
of the sweep gas is initially set at 1.0 in order to attain the best oxygenation of the blood in the circuit.
Initial VV ECMO Settings
•
Circuit blood flow of 50-60 mL/kg
•
Adjust circuit flow to keep patient SaO
2
80-85%
•
FiO
2
on sweep gas of 100%
•
Set the sweep gas flow about the same as the circuit flow
•
Adjust sweep gas flow to keep PaCO
2
35-45
Most of the time, some ventilator support is necessary. This isn't to provide additional gas exchange support, but instead to improve patient comfort and to prevent complications. If a patient is put on VV ECMO and then extubated, the lack of any positive pressure on the lungs will lead to near-complete alveolar collapse and consolidation. This can cause significant tachypnea and respiratory distress. Alveolar consolidation also prevents the normal clearance of secretions from the pulmonary tree, which can lead to pneumonia or lung abscess.
That said, recent experience with VV ECMO has shown that as patients recover, they can spend more and more time off the ventilator. This is important because it means that physical therapy and mobilization can begin early on, even while on VV ECMO. This is much easier with the dual-lumen cannula placed through the internal jugular vein. Early tracheostomy should be done as soon as it's feasible in order to lessen sedation and begin mobilization. There is nothing like seeing a patient with severe ARDS walk down the hallway, with the ECMO circuit being pushed behind him
.
Weaning VV ECMO
As the patient begins to recover, the FiO
2
of the sweep gas can be lowered. Circuit flow, once established, should not be lowered—lower blood flow increases the risk of thrombosis in the circuit. Keep in mind that VV ECMO is just like a really big right atrium. As the FiO
2
on the sweep gas is reduced, the blood flowing through the oxygenator will pick up less oxygen. That means that the proportion of gas exchange that has to occur through the patient's lungs is increasing. Once the FiO
2
on the sweep gas is 0.21, the ECMO circuit is contributing nothing at all to the patient's oxygenation—it's all being done with low-level ventilator support. Blood is simply flowing through the big right atrium but there's no assistance with oxygenation. If the patient's condition is acceptable, it's time to come off ECMO.
Patient Selection
This is often the most difficult part of using VV ECMO for severe acute respiratory failure. For many years, ECMO was used predominantly for neonates with infant respiratory distress syndrome, meconium aspiration, and congenital diaphragmatic hernia. In recent years, however, ECMO has become more popular for older children and adults. The H1N1 influenza pandemic of 2009 accelerated the interest in ECMO, particularly VV ECMO, as a rescue therapy. The CESAR trial, published in
The Lancet
in 2009, demonstrated a survival benefit for patients with severe influenza-related ARDS transferred to ECMO centers.
36
Only 75% of those randomized to the ECMO arm actually received ECMO, which is an interesting finding. It may be that the true benefit was treating patients in high-volume centers with the appropriate expertise and ability to provide rescue therapies, including ECMO, rather than the provision of ECMO itself
.
Indications for VV-ECMO
•
Hypoxic respiratory failure with a predicted mortality risk ≥ 50%
a.
PaO
2
/FiO
2
< 150 on FiO
2
> 90% despite optimal care for 6 hours or more
b.
Murray Score
*
≥ 3 despite optimal care for 6 hours or more
•
Hypercapnic respiratory failure refractory to treatment with pH < 7.15
•
Acute onset of a potentially reversible cause of respiratory failure
•
Age ≤ 65
•
Immediate respiratory collapse that is unresponsive to optimal care (obstructed airway, etc.)
Contraindications to VV-ECMO
+
•
Mechanical ventilation at high settings (e.g. FiO
2
≥ 90%, P
PLAT
> 30, PEEP ≥ 15) for 7 days or longer
•
Contraindication to anticoagulation
•
Absolute neutrophil count < 500/mm3
•
Major CNS damage or other nonreversible comorbidity
•
Age > 65
•
Progression of chronic respiratory disease to the point of respiratory failur
e
Prior to initiation of VV-ECMO, the following steps should be taken to improve the patient's condition. These are listed in order of preference, although not all are required prior to cannulation for ECMO.
1.
Lung protective ventilation using a tidal volume of 4-6 mL/kg predicted body weight, with PEEP according to the ARDSNet study protocol
2.
Airway pressure release ventilation, with a P
HIGH
up to 35 cm H
2
O
3.
Prone positioning for 16 hours, followed by supine positioning for 8 hours
4.
Diuresis or CRRT to within 105% of dry weight, if hemodynamics permit
5.
Bronchoscopy with therapeutic aspiration of the tracheobronchial tree
If the patient has not improved with the aforementioned therapy, VV ECMO team should be considered. Additional rescue maneuvers that can be tried include:
6.
Inhaled nitric oxide
7.
High frequency oscillatory ventilation
The Extracorporeal Life Support Organization (ELSO) provides extensive expert guidelines for patient selection and referral at its website:
www.elso.org
.
Ventilator Management on Veno-Arterial ECMO
The bulk of this chapter is concerned with VV ECMO as a rescue therapy for severe respiratory failure. The ventilator management in VV ECMO is quite easy—let the circuit do the heavy work, and use the ventilator to simply keep the lungs open without injuring them. It is quite common for the tidal volume
to be less than 100 mL while on the rest settings described earlier, and that's fine—after all, the whole point of VV ECMO is to let the patient's lungs rest and recover.
With VA ECMO, on the other hand, it's important to keep in mind that while the ECMO circuit provides oxygenated blood into the aorta, the blood in the aortic root is more dependent on the patient's native cardiopulmonary function. That means that the blood flowing from the pulmonary circulation into the left atrium and ventricle is delivered preferentially into the coronary ostia and the aortic arch. In other words, oxygen delivery to the coronary arteries still depends on mechanical ventilatory support. The rest of the body, particularly the lower thorax and abdomen, gets its oxygen delivery from the ECMO circuit.
The exact degree to which different areas of the body are perfused depends on how strong the heart is. Consider a patient with zero native cardiac function. All of his arterial flow, including the coronary ostia, depends on the ECMO circuit flow. As he begins to recover cardiac function, the native heart will begin pumping blood into the aortic arch. As the heart grows stronger, it will perfuse more and more blood to the coronary arteries and the vessels coming off the aortic arch. This can lead to a situation where the lower half of the body is well-oxygenated by the ECMO circuit, while the upper body (including the brain) is relatively hypoxic. The management of the "blue nose syndrome" is beyond the scope of this chapter.
Therefore, simply putting a patient receiving VA ECMO on rest settings like you would do with VV ECMO risks myocardial hypoxia. A higher FiO
2
and PEEP may be necessary. Unlike with VV ECMO, ventilator settings should be adjusted to attain adequate gas exchange. The most accurate site for arterial blood gas monitoring depends on where the arterial ECMO cannula is located. In most cases, the cannula is placed in a femoral artery and into the descending aorta. After the coronary ostia, the next branch off the aortic arch is the right brachio cephalic artery. A blood gas specimen from a right radial
arterial line will give the most accurate measure of the native heart's oxygen delivery. In the cases of central subclavian artery cannulation, there is much less discrepancy in regional oxygen delivery. The arterial line is generally placed in the radial artery on the opposite side from the subclavian artery cannula.
*
Always remember that the whole point of VV ECMO for respiratory failure is to let the lungs rest and recover. If they look whited-out on the CXR and the tidal volume on these settings is < 100 mL, so be it! Resist the temptation to use the vent for gas exchange. Let the ECMO circuit do the work.
+
Contraindications are relative, not absolute; however, the presence of these conditions is associated with a higher risk of treatment failure.