University of California, Davis
I sense from the classical debate between Piaget and Chomsky (Piattelli-Palmarini, 1980) that at least some of us are all too prone to think of learning and instinct as being virtually antithetical. According to this common view, behavior is one or the other, but it is rarely, if ever, both. Lower animals display instincts, but our own species, apart from a few very basic drives, displays instincts rarely. Instead, we are supposed to be the manifestation of what can be achieved by the emancipation from instinctive control (Gould & Marler, 1987).
It is self-evident that this antithesis is false. Just as instincts are products of interactions between genome and environment, even the most extreme case of purely arbitrary, culturally transmitted behavior must, in some sense, be the result of an instinct at work. Functions of instincts may be generalized or highly specialized, but without them learning could not occur. Thus, the question I pose is not “Do instincts to learn exist?” but rather “What is their nature, and by what behavioral and physiological mechanism do they operate?” How do they impinge on the pervasive plasticity that behavior displays at so many points in the course of its development? I suggest that concepts from the classical ethology of Konrad Lorenz (1950) and Niko Tinbergen (1951) are instructive in a search for answers to these questions.
Of the several concepts with which Lorenz and Tinbergen sought to capture the essence of instinctive behavior in animals (listed in Table 2.1), I concentrate especially on three. First is the notion of sensitive periods as phases of development with unusual potential for lability. Second and third are the complementary ideas of releasers (or sign stimuli) and innate release mechanisms, invoked by ethologists to explain the remarkable fact that many organisms, especially in infancy, are responsive to certain key stimuli during interactions with their social companions and with their physical environments, when they first encounter them. This responsiveness implies the possession of brain mechanisms that attune them innately to certain kinds of stimulation.
TABLE 2.1
Concepts From Classical Ethology Relevant to the Instinct to Learn
Sensitive Periods
Imprinting
Fixed Action Patterns
Releasers
Innate Release Mechanisms
[Instincts to Learn]
In recent years, I have come to believe that many such mechanisms have richer and more interesting functions than simply to serve as design features for animal as automata. They also provide the physiological machinery to facilitate and guide learning processes, as one set of components in what I think can be appropriately viewed as instincts to learn.
I use birdsong to make the case for instincts to learn as an approach that is productive and logical, even with behavior that is clearly and obviously learned. As a research strategy, it prepares us directly for posing the right kinds of questions in neurophysiological investigations of the underlying mechanisms. It is a position that follows naturally, once the crucial point is appreciated that instincts are not immutable and completely lacking in ontogenetic plasticity, as has so often been assumed in the past, but are themselves, by definition, susceptible to the influence of experience. I present evidence that even the most creative aspects of song development are imbued with instinctive influences, by which I refer to the aspects of the phenotype of the learning organism that are attributable to its genetic constitution (Johnston, 1988). These influences pervade all aspects of ontogeny. We cannot begin to understand how a young bird learning to sing interacts with its social and physical environments, and assimilates information from these interactions, without taking full account of innate contributions to the assimilation process. Each species accommodates most readily to those aspects of experience that are compatible with its nature.
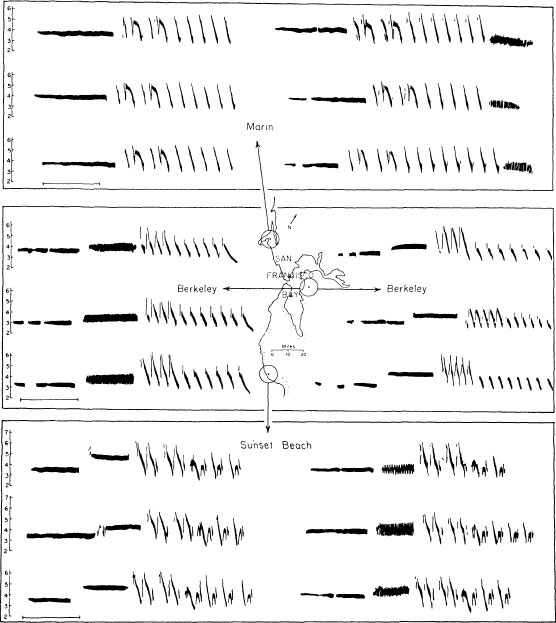
FIG. 2.1. An illustration of song dialects in the white-crowned sparrow in the San Francisco Bay area. Songs of 18 males are illustrated, 6 from Marin County, 6 in the Berkeley area, and 6 from Sunset Beach, to the south. Each male has a single song type, forthe most part. Local dialects are most evident in the second, trilled portion of the song (from Marler, 1970). These dialects have been studied in much greater detail by Baptista (1975).
One of the best illustrations of local dialects in birdsong is the white-crowned sparrow (Fig. 2.1). This is a very simple case. With rare exceptions, each male has a single song type, which has about a 2-second duration. Some song features conform very closely to the local dialect, and others are unique to each individual male. The dialects are so marked that someone with a cultivated ear would be able to tell where he or she was in California, blindfolded, simply by listening to their songs (Baker & Cunningham, 1985; Baptista, 1975, 1977). The fact that the dialects are learned becomes obvious when a male bird is reared without hearing the song of its own kind. A much simpler song develops, lacking all traces of the local accent (Marler, 1970; Petrinovich, 1985). What is the nature of this learning process, and what, if any, are the contributions of instinctive processes? We can detect such contributions in many aspects of the process of learning to sing.
If we present a young bird with an array of different songs or tutors to learn from, are they equipotential as stimuli, or are some preferred over others? If there are preferences, do species differ in the songs they favor, or is a song that is a strong learning stimulus for one species, strong for others as well?
As a key feature of the research on which this report is based, a comparative approach has been taken. The underlying principle is simple. Young males of two species, the swamp and the song sparrow, were brought into the laboratory and reared under identical conditions. This gave us the opportunity to observe whether they interacted similarly or differently with the experimental situations in which they were reared. Despite their close genetic relatedness, their songs are very different (Fig. 2.2). One is simple; the other is complex. They differ in the overall “syntax” of their songs and in the “phonology” of the individual notes. They differ in repertoire size, a male song sparrow having about three times as many song types as a male swamp sparrow (3 in one case, 10 to 12 in the other).
How do males of these two species react if we bring them into the laboratory as nestlings, raise them by hand so that their opportunity to hear song in nature is limited, and expose them to tape recordings with equal numbers of swamp sparrow songs and song sparrow songs? When we analyze the songs that they produce, it becomes clear that each displays a preference for songs of its own species (Fig. 2.3).
In most of the experiments I report on, birds were raised by hand, after being taken as nestlings from the field at an age of 3–5 days. We do this because it is more difficult to raise them from the egg. Might they have learned something in the egg, or the first few days of life before being brought into the laboratory that has an influence on development of singing behavior, perhaps leading them to favor songs of the species heard during that period?
FIG. 2.2. Sound spectrograms of normal song and swamp sparrow songs. They differ in both syntax and phonology, and also in the size of individual song repertoires, which average about 3 song types in swamp sparrows and 10–12 in song sparrows.
FIG. 2.3. Learning preferences of male song and swamp sparrows either raised in the laboratory from the egg or exposed to song in nature during the nestling phase and only then brought into the laboratory. Birds were given a choice of tape recordings to learn, some of their own species’ song and some of the other species. The results show that both have an innate preference for learning songs of their own species, but the preference is stronger in swamp sparrows than in song sparrows. Song experience during the nestling phase evidently has no effect on learning preferences.
To check on the possibility of pre- or peri-natal experience of species-specific song on learning preferences, eggs from wild nests of the same two species were taken early in incubation, hatched in the laboratory, and raised with absolutely no opportunity to hear adult song of their species. They displayed similar learning preferences (Fig. 2.3). The preference for conspecific song is thus innate (Marler & Peters, 1989). Interestingly, the song sparrow preference is less extreme in birds raised under both conditions. Dooling and Searcy (1980) uncovered a similar trend by looking at heart-rate changes in 3-week-old song and swamp sparrows in response to song (Fig. 2.4). It may be that, as found in some other birds (Baptista & Petrinovich, 1984, 1986; Clayton, 1988; Pepperberg, 1988), social interaction with live tutors is more important in song sparrows than in swamp sparrows, because song sparrows are not known to imitate swamp sparrows in nature, even though they live in close proximity. In swamp sparrows, learning from tape recordings and live tutors has been shown to take place in a very similar fashion (Marler & Peters, 1988b). Social influences notwithstanding, in both species the preference can be sustained solely on the basis of acoustic features of song. What are the acoustic features on which these preferences are based? The answer is different in the two species.
By using computer-synthesized songs in which different acoustic features were independently varied, we found that the learning preference of male swamp sparrows is based not on syntactical features of the song but on the phonology of the syllables. As illustrated in Fig. 2.5, male swamp sparrows presented with simplified songs consisting either of swamp sparrow syllables or song sparrow syllables unerringly favor those with conspecific syllables, irrespective of the temporal pattern in which they are presented. They then recast them in the normal syntactical pattern, whether or not this pattern has been available to them in the songs they have heard. In choosing models for learning, the song syllable is clearly the primary focus of interest for a swamp sparrow.
FIG. 2.4. Cardiac responses of young swamp and song sparrows to recorded songs of their own and of the other species. The responses are calibrated in relation to the neutral stimulus of a canary song. Each responds most strongly to songs of its own species. The swamp sparrows discriminated more strongly than the song sparrows, in which the preference was not statistically significant. The trend matches that in song learning preferences (Fig. 2.3). These data were gathered at an age of 3–4 weeks, prior to any song production. (After Doding and Searcy 1980.)
FIG. 2.5. A diagram of song learning preference in male swamp sparrows. Three pairs of computer-synthesized songs are illustrated with the same syntax but composed of syllables either from song sparrow or from swamp sparrow songs. In each case, male swamp sparrows preferred syllables of their own species, irrespective of the syntactical arrangement in which they were presented. In each case, the syllable chosen was produced with typical swamp sparrow syntax, regardless of the syntactical structure of the learned model.
In contrast, song sparrows, with their more complex songs, base their learning preference not only on syllabic structure but also on a number of syntactical features, including the number of segments, their internal phrase structure—whether syllables are trilled or unrepeated, and such attributes as the tempo in which they are delivered. There is no evidence that young male swamp sparrows refer to any of these syntactical features when they choose models for song learning (Marler & Peters, 1980, 1988a, 1989).
The evidence of differences in innate responsiveness to song features from species to species is thus clear and unequivocal, implying the existence of something like Lorenzian “innate-release mechanisms.” This innate responsiveness is employed not to develop fixed behaviors, as we might once have thought, but as the basis for a learning process. Having focused attention on the particular set of exemplars that satisfy the innate criteria, sparrows then learn them, in specific detail, including the local dialect (if this is a species that possesses dialects). In the swamp sparrow, the dialects are defined by the patterning of notes within a syllable (Marler & Pickert, 1984), as displayed in Fig. 2.6. Balaban (1988) has shown that both males and females acquire responsiveness to these dialect variations. Thus, the birds go far beyond the dictates of the initial ethological lock-and-key mechanism.
A further point, the importance of which cannot be overstressed, is that birds are not completely bound by these innate preferences. If conspecific songs are withheld, sparrows can be persuaded to learn nonpreferred songs (Fig. 2.7), especially if these are accompanied by further, strong stimulation, as with a live interactive tutor of another species (Baptista & Petrinovich, 1984, 1986). Thus, the process of choosing models for song learning is probabilistically controlled, not absolutely determined. Given the normal ecology of the species, however, conspecific song tutoring will usually be available for innate preferences to be exercised, thus establishing a certain predictable trajectory to the learning process
How might one model the mechanisms underlying such learning preferences? There is ample experimental evidence that birds can hear the songs of other species perfectly well and can discriminate between them with precision, even at the level of individual differences (Dooling, 1989). Yet they either fail to learn them in retrievable form in the normal course of song acquisition, or, if they do learn them, they forget them again. One caveat here is that we still lack a direct test of what has been memorized, and we have to rely instead on what is produced as a memorization index. Even in the earliest productions of imitations, in plastic song, copies of songs of other species are not usually in evidence. By this criterion, these sparrows behave as though any song presented as a stimulus is subjected to normal sensory processing but is then quickly lost from memory in the usual course of events, unless the exposure is massive, continuing day after day, and associated with strong arousal. There is an urgent need to develop memorization assays that are independent of song production.
FIG. 2.6. Swamp sparrow songs are constructed from 6 basic note types (I—VI), present in sim in different populations (histograms at the bottom). Two typical songs from New York and illustrated, with different rules for ordering note types within syllables. In New York three-note I notes are typically in first position and type VI notes in final position, with one of the other note In Minnesota three-note songs an opposite rule tends to prevail, as illustrated (Marler & Picke males and females are both responsive to these differences in syllable construction (Balaba, 1998).
FIG. 2.7. If song and swamp sparrows are raised in the laboratory and presented only with tape-recorded songs of the other species, on rare occasions they will imitate them. Examples are illustrated of a swamp sparrow copy (B) of part of a song sparrow model (A) and a song sparrow copy (D) of a swamp sparrow model (C). Male swamp sparrows rarely imitate song sparrow song. Song sparrows imitate swamp sparrow song more often (cf. Fig. 2.3), but when they do so they usually recast the swamp sparrow syllables into song sparrow-like syntax (cf. Fig. 2.20).
When conspecific stimuli are presented, it is as though the bird suddenly becomes attentive, and a brief time window is opened during which the stimulus cluster in view becomes more salient, more likely to be memorized, and probably destined to be used later for guiding song development. One tends to think in terms of parallel processing, with certain circuits responsible for general auditory processing and others committed to the identification of stimuli as worthy of the special attention of the general processing machinery, if and when they are encountered. This interaction might be thought of as a teaching process, with special mechanisms serving—especially in infancy—to instruct general mechanisms about what to pay special attention to during learning and about how the learning process can most efficiently be structured. In adulthood, once their function of establishing certain developmental trajectories has been accomplished, special mechanisms may cease to function or even cease to exist.
One may think of the sign stimuli present in conspecific songs operating not only as behavioral triggers but also as cues for learning, serving as what might be thought of as “enabling signals,” their presence increasing the probability of learning other associated stimuli that might otherwise be neglected (Rauschecker & Marler, 1987). I believe that this function is served by many ethological “releasers,” and it may even be the primary function for many of them.
Vocal Learning Templates
FIG. 2.8. Sound spectrograms illustrating typical songs of swamp and song produced under four conditions. First row: normal learning in the wild [(A) an one syllable of a second dialect also shown in (A)]. Row 2: acoustic isolation, but hearing [(B) and (F)]. Row 3: isolated, but trained with tape recordings of norm their species [(C) and (G)]. Row 4: isolated and deafened [(D) and (H)]. Frequen indicate 1 kHz intervals and the time marker 0.5 sec.
Sparrows are able to generate some aspects of normal, species-specific song syntax irrespective of the syntax of the models to which they have been exposed in the past. This potential is most clearly displayed in the songs of birds raised in isolation, completely deprived of access to adult song of their own or any other species. Figure 2.8 shows examples of natural song and examples of the simpler form of song that develops in males reared in isolation. There are many abnormalities in the songs of males raised in isolation, and quantitative study reveals that the variation is great. Nevertheless, by using a comparative approach, it can be clearly shown that each species is capable of generating some basic features of normal song syntax irrespective of whether these have been experienced in the form of song stimulation by others. The syntax of a swamp sparrow is rather resistant to change by experience, in comparison with the song sparrow, although stimulation by multipartite songs does result in the production of a certain proportion of bipartite song patterns (Marler & Peters, 1980). Male swamp sparrows copy syllables more readily than whole songs. This is less true of song sparrows. When they are allowed to hear conspecific song, they will sometimes imitate the entire syntax of the particular model experienced (Fig. 2.8), even though they are innately responsive to conspecific syntax. Once more, the invocation of innate influences in no way implies a commitment to immutability.
Again, we may pose the question, “What kind of physiological mechanism underlies this ability?”. Some insight is gained by studying the singing behavior of birds that are deaf. We know that the sense of hearing is important not only to permit a bird to hear the songs of others but also to enable it to hear its own voice (Konishi, 1965; Nottebohm, 1968). Male sparrows deafened early in life, prior to any singing, develop songs that are highly abnormal, exceedingly variable, almost amorphous in structure (Fig. 2.8), although certain basic species differences are sometimes still detectable (Marler & Sherman, 1983).
This highly degraded form of song results both if a male is deafened before song stimulation and also after song stimulation but before the development of singing (Konishi, 1965). Thus, there seems to be no internal brain circuitry that makes memorized songs directly available to guide motor development. To transform a memorized song into a produced song, the bird must be able to hear its own voice.
FIG. 2.9. The developmental sequence is different in bird species with learned and unlearned songs. Subsong is radically different from mature song in structure, and undergoes a metamorphosis in the progression through plastic song.
This contrast between the songs of hearing and deaf birds inspired the concept of vocal learning templates, existing in two forms: one innate and the other acquired. Acquired templates, resulting from enrichment, modification, substitution, or interaction with other mechanisms as a consequence of experience, were originally conceived of as transforms of the same basic mechanisms as innate templates (Konishi, 1965; Marler, 1976; Marler & Sherman, 1983). It now seems possible that they are functionally and neuroanatomically separate, although interconnected and interreactive, as indicated earlier. Innate auditory song templates have a potential direct influence on early learning preferences, in some circumstances, and on the later production of songs. They also serve as a vehicle for bringing innate influences to bear on the effects of intervening experience. Auditory templates for vocal learning provide one model of the kind of brain mechanisms underlying this particular instinct to learn. Many of the attributes of this model are applicable to other systems of behavioral development. Ontogeny is guided by sensory feedback from motor activity, with referral of this feedback to templates with specifications that can be supplemented, modified, or overridden by experience. The specifications incorporate innate contributions that may be unique to one species, as is the case with those stressed in this paper, or they may be more generally distributed across species, such as specifications for the tonality that characterizes many birdsongs (Nowicki & Marler, 1988).
Plans for Motor Development
Songs of many birds, such as sub-oscine flycatchers, develop completely normally in isolation. When such a song begins to be performed, the first efforts are clearly identifiable as immature versions of what will ultimately be the normal crystallized song. These early attempts may be noisy and fragmented, but the maturational progression is clear and predictable (Kroodsma, 1984). In birds that learn their songs, the developmental progression is quite different. There is a more complex ontogenetic sequence, from subsong, through plastic song, to crystallized song (Fig. 2.9). The general pattern of song development in 16 male swamp sparrows in the laboratory is diagrammed in Fig. 2.10. There is considerable individual variation, but a modal pattern can nevertheless be discerned that comprises three stages: subsong, plastic song, and crystallized song. This program unfolds similarly in males raised in isolation, suggesting that it is hormonally controlled (but see Marler, Peters, Ball, Dufty, & Wingfield, 1988).
We still know less about subsong than any other aspect of birdsong development. Figure 2.11 shows examples of early subsong from male swamp and song sparrows. It illustrates the fact that the structure of subsong is quite different from that of mature song. It is typical of bird species with learned songs that a kind of metamorphosis intervenes between subsong and later stages of song development. The amorphous structure and noisy spectral organization of sparrow subsong is typical.
FIG. 2.10. Patterns of song development in 16 male swamp sparrows, each raised in individual isolation. They are displayed with the latest developers at the top and the earliest developers at the bottom. Despite considerable individual variation, a species-typical pattern can be discerned.
Despite its lack of structure, careful analysis reveals subtle species differences. Auditory templates appear to be operating even at this early stage. A difference in note duration present in normal song and in those of isolates (Marler & Sherman, 1985) also occurs in the subsong of hearing song and swamp sparrows (Fig. 2.11) but is lacking in the early subsong of deaf birds (Fig. 2.12). Subsong is believed to be critical for several aspects of the development of the general motor skills of singing and also for honing the ability to guide the voice by the ear, which is a prerequisite for vocal imitation (Nottebohm, 1972; Marler & Peters, 1982b); however, direct evidence has been hard to obtain.
Only in the second stage, plastic song, do the more obvious signs of mature song structure appear. Figure 2.13 presents samples of developing song in a single male swamp sparrow, starting with subsong and proceeding through plastic song to the stable form of crystallized song. As plastic song progresses, rehearsal of previously memorized song patterns begins. These continue to stabilize gradually until crystallization occurs. Note that normal species-specific syntax—a single trill—emerges late in swamp sparrows, irrespective of whether such patterns have been heard from others or not, suggesting that an innately specified central motor program is accessed at this stage.
FIG. 2.11. Sound spectrograms of early subsong from swamp and song sparrows with hearing intact, as compared with subsong produced after early deafening. In the birds with hearing intact, note duration averages longer in the song sparrows. This difference is absent in subsong of deaf birds produced at the same age.
Larger repertoires of songs occur during plastic song than in crystallized song (Marler & Peters, 1982a). Male swamp sparrows greatly overproduce song material at intermediate stages of development, as can be seen more clearly by summing data on numbers of songs present in an individual repertoire during the transition from plastic to crystallized song (Fig. 2.14). A typical crystallized repertoire consists of two or three song types, but in early plastic song the repertoire may be four or five times greater. Thus, more is memorized than is manifest in the final products of motor development.
The process of discarding songs during crystallization is not a random one. For one thing, birds that have been persuaded to learn songs of other species by “hybridizing” them with conspecific song elements are more likely to reject these “hybrid” songs during the attrition process (Marler & Peters, 1982a). In addition, there are also opportunities for experience to interact with development to influence the final outcome. There is often a premium in songbirds on countersinging against rivals with similar themes if they are available. The transition from plastic song to full song takes place at a stage of life when a young male is striving to establish his first territory, and, by a “pseudolearning” process, stimulation by the songs of rivals at this time may favor the retention of song themes that most closely match those of rivals in the attrition process. There is also a fascinating suggestion from the work of King and West (1988) on the brown-headed cowbird that females can influence the choice of crystallized song by giving courtship responses to song types that they favor during the plastic song phase.
FIG. 2.12. Histograms of mean note durations in subsong of song and swamp sparrows with hearing intact and after deafening. It is evident that auditory song templates are already operating even at this early age, to generate species differences in subsong structure.
FIG. 2.13. Samples from the process of song development in a single male swamp sparrow, ranging from subsong to crystallized song. The age of the bird is indicated on the right ranging from 252 to 316 days of age. This bird was trained with tape-recorded songs, syllables of some of which are indicated in the boxed insert (1–5). As indicated by the labels, early efforts to reproduce imitations of these months later are imperfect in early plastic song, but they improve as progress towards crystallized song is made. The overproduction of song types during plastic song can also be seen. The two song types in the crystallized repertoire of this male consisted of syllable types 2 and 3.
FIG. 2.14. A plot of mean syllable repertoires of 16 male swamp sparrows at different stages of song development, arranged around day 0 as the time of crystallization. There is extensive overproduction of song types during plastic song, and the repertoire is drastically reduced as development proceeds towards crystallization of the mature repertoire, averaging three song types per bird (from Marler & Peters, 1982).
Steps in Learning to Sing
FIG. 2.15. Steps in the process of learning and reproducing a song.
FIG. 2.16. The period of storage of learned songs without rehearsal in 16 male swamp sparrows trained with tape-recorded song prior to 60 days of age. Songs were recorded and analyzed every 2 weeks, and the age was noted at which the first identifiable imitations were reproduced, some 8 months after last exposure to the models.
The diverse strategies that different birds use in learning to sing are accompanied by certain underlying consistencies. For example, there are always several phases in the process of learning to sing. Sensory and perceptual processing tends to precede production (Fig. 2.15). Songs pass into storage during the acquisition phase, when a bird subjects songs to auditory processing, and commits some of them to memory. It seems logical that the knowledge necessary to develop patterns of action should be acquired before development of these actions commences (Gelman, chapter 10, this volume). After acquisition, internalized representations of songs, or parts of them, may be stored for an appreciable time before the male embarks on the process of retrieving them and generating imitations. In Fig. 2.16, time intervals are plotted between the last exposure to tape-recorded songs of 16 male swamp sparrows, each separately housed, ending at about 60 days of age, and production of the very first hints of identifiable imitations. This storage interval was surprisingly long, on the order of 8 months, an impressive achievement.
The period of storage before retrieval of stored representations from memory begins varies greatly from species to species. It is not known whether this is a phase of passive storage or whether consolidation or active reorganization of memorized material is taking place. Subsong may occur during storage, and even during acquisition, but the onset of rehearsal is the sign that plastic song has begun. Themes are rehearsed and stabilized, and eventually song crystallization occurs.
Sensitive Periods for Acquisition
Another aspect of instincts to learn is the timing of the acquisition phase. Is it brief, or extended? Does it occur only once, or repeatedly during life? There are striking differences between species in the timing of song acquisition (Fig. 2.17). In some birds, acquisition is age-dependent and is restricted to a short period early in life. In other species, song remains changeable from year to year, apparently with a continuing ability to acquire new songs throughout life. Even close relatives, such as sparrows and canaries, may differ strikingly in the timing of sensitive periods, providing ideal opportunities for comparative investigation of variations in the neural and hormonal physiology that correlate with song acquisition. Such species differences can have a direct and profound impact on the potential for behavioral plasticity.
FIG. 2.17. Examples of bird species with age-dependent and age-independent song learning.
Much of the behavioral information on sensitive periods is inadequate to serve well as a springboard for comparative physiological investigation. In an effort to develop a more systematic, experimental approach to this problem, we played tape-recorded songs to male sparrows in the laboratory throughout their first year of life, changing song types every week or two (Marler & Peters, 1987, 1988b; Marler, 1987). By recording and analyzing the songs produced, we were able to extrapolate back to the time when acquisition occurred. Figure 2.18 shows the results for a group of male swamp sparrows, with a clear sensitive period for song acquisition beginning at about 20 days of age and then closing out about 3–4 months later, before the onset of plastic song. A similar picture of song acquisition was obtained with a changing roster of live tutors, brought into song by testosterone therapy (Fig. 2.18). Differences between species in the timing of sensitive periods are sometimes gross but may also be subtle, as can be seen by comparing the timing of song acquisition from tape recordings in male song sparrows (Fig. 2.18). Here, the sensitive period is even more compressed into early adolescence. These birds provide ideal opportunities for pursuing questions about the neural and hormonal changes that are correlated with these sensitive periods and perhaps bear a causal relationship with them (Marler et al., 1987, 1988; Nordeen, Marler, & Nordeen, 1988).
Although sensitive periods for song acquisition are clearly significant components of instincts to learn, it is important to be aware once again that these are not fixed traits (Marler, 1987). There are degrees of lability, depending on such factors as the strength of stimulation—whether a tape recording or a live tutor is used (Baptista & Petrinovich, 1984, 1986). Physiological factors that correlate with the season are also relevant. In some species, young may be hatched so late that singing, which is a seasonal activity in most species, has ceased for the year. In such cases, it has been shown that closure of the sensitive period may be delayed until the following spring, apparently in response to the changing photoperiod (Kroodsma & Pickert, 1984). Deprivation of access to conspecific models can also delay closure of the sensitive period (Clayton, 1988). Once more, the invocation of innate influences does not mean sacrifice of the potential for behavioral flexibility; rather, instincts to learn set a species-specific context within which experience operates.
FIG. 2.18. The sensitive period for song acquisition peaks in male song sparrows between 20 and 50 days of age (top). The peak is attenuated somewhat in male swamp sparrows and extends to a later age, both when they are tutored with tape recordings (middle) and when they are given live tutors (bottom). These results were obtained by training birds with a constantly changing program of either tape recordings or live tutors and then inferring the age at which acquisition occurred from analvses of sonas produced later (from Marler & Peters, 1987, 1988b).
Thus far in this account of song learning, the emphasis has been placed on the production of more-or-less precise imitations of songs heard from other birds. In fact, an element of inventiveness often intrudes. This may take several forms. One revelation from the sensitive period experiments described in the previous section is that sparrows are able to recombine components both of the same song and of songs acquired at different times. Recasting or re-editing of components of learned models into new sequences is commonly exploited as one means for generating novelty and also for producing the very large individual repertoires that some birds possess (Krebs & Kroodsma, 1980). Often, models are broken down into phrases or syllables and then reordered into several different sequences that become stable themes (Marler, 1984). Song sparrows are especially prone to indulge in such recombinations with songs acquired in later phases of the sensitive period (Marler & Peters, 1988b). This correlates with a decline in the completeness with which entire learned songs are accurately reproduced (Fig. 2.19). This tendency to recombine segments of learned models has the effect of creating new songs from old, by reuse of the same basic raw materials.
Species differ greatly in the faithfulness with which they adhere to learned models, although imitations are rarely identical with their models, even in the best mimics. Some species imitate learned models closely, and local dialects are common in birds, but a degree of personal individuality is also virtually universal. In every case examined, this individuality has proved to provide a basis in nature for personal identification of companions and for distinguishing neighbors from strangers (reviewed in Falls, 1982).
FIG. 2.19. When song sparrows reproduce songs acquired early in the sensitive period, they are more likely to reproduce them with the original syntax of the model than with songs acquired later in the sensitive period. For each age block, two sets of data are illustrated, from tape recorded songs heard for a 6-week period (left) and for a 1-week period (right). Songs acquired later are more likely to be broken up into separate phrases that are then recombined in different ways to produce new songs (from Marler & Peters, 1987).
Some degree of inventiveness is, in fact, universal, but species differ greatly in the extent to which they indulge in creative activity in song development. Figure 2.20 illustrates just one example of a song sparrow exposed in the laboratory to a variety of simple synthetic songs. This bird generated an approximation of typical song sparrow syntax in highly creative fashion by drawing two components from one model and one from another model. Some species provide abundant illustrations of this kind of innovative process, both in the laboratory and in the field.
FIG. 2.20. Song sparrows often create new themes by breaking learned songs down into their component syllables and recombining them in various ways. Illustrated here is the song of a laboratory-reared song sparrow exposed to an array of synthetic songs. It learned two of these 1(A) and (C)] and recombined parts of them, as illustrated, to create a crude approximation of normal song sparrow song syntax.
The rules for parsing acquired songs down into components and recombining them are species-specific, however. There is also species variation in the faithfulness with which a bird adheres to the structure of a given imitation. Some, like sparrows, are conservative. They recast syllables often, but they adhere to the basic syllabic structure, which makes them good subjects for studies of learning. Other species, such as the red-winged blackbird, are compulsive improvisers (Marler, Mundinger, Waser, & Lutjen, 1972), subjecting themes to continuous experimentation and embroidery during development, until the originals are barely recognizable.
Even more intriguing is the suggestion that improvisation and invention may be most consistently applied to certain segments of songs, with other segments left as pure, unadulterated imitations. A species like the white-crowned sparrow, in which birds in a given locality adhere closely to a given dialect, nevertheless has song segments or features that are more free for individual improvisation. Thus cues for personal identification may be encoded in one segment or feature, cues for the local dialect in another, and cues for species recognition in yet another set, the arrangement varying from species to species (Marler, 1960).
CONCLUSIONS
It is less illogical than it first appears to speak of instincts for inventiveness. Song development is a creative process, but the inventiveness that birds often display is governed by sets of rules. Each species has its own distinctive set of physiological mechanisms for constraining or facilitating improvisation, guiding learning preferences, directing motor development, and establishing the timing of sensitive periods. Songs are learned, and yet instinctive influences on the learning process intrude at every turn.
Instincts to learn offer priceless opportunities to pinpoint the ways in which physiological or neuroanatomical changes can affect the process of learning a new behavior. Given the striking contrasts in song development in birds that are very close genetic relatives and are otherwise very similar in structure and physiology, presumably quite limited changes in neural organization or the timing of a hormonal event can have profound effects on the course of learning. Already the proverbial bird brain has yielded many secrets about the neural biology of vocal plasticity (Konishi, 1985; Nottebohm, 1987). Yet there is a sense in which we have hardly begun to exploit the potential of comparative studies as a source of new insights into the role of innate species differences in structure and physiology in the operation of instincts to learn.
There is a need in studies of behavioral development to overcome behavioristic prejudice against the invocation of innate contributions. It is as a consequence of such prejudice against the term “innate” that most students of animal behavior have eschewed its use altogether. The result is that ethological investigations of processes of behavioral epigenetics have, for the most part, been rendered impotent. The initiative has been left to geneticists and developmental biologists, who take it for granted that the genome plays a major role in all aspects of behavioral development (Marler & Sherman, 1985).
There is nothing illogical in applying the term “innate” to differences between organisms. As Hinde (1970) asserted, “Evidence that a difference in behavior is to be ascribed to genetic differences must come ultimately from the rearing of animals, known to differ genetically, in similar environments” (p. 431). It is both valid and productive for students of development to address Dobzhansky’s (1962) question, “To what extent are the differences observed between persons due to genotypic or to environmental causes?” (p. 44).
ACKNOWLEDGMENTS
Research was conducted in collaboration with Susan Peters and supported in part by grant No. BRSG SO7 RR07065, awarded by the Biomedical Research Support Grant Program, Division of Research Resources, National Institutes of Health, and by grant number MH 14651. Esther Arruza prepared the figures and typed the manuscript. I thank Judith and Cathy Marler and Eileen McCue for rearing the birds. I am indebted to Susan Peters, Stephen Nowicki, Susan Carey, and Rochel Gelman for discussion and valuable criticism of the manuscript and to the New York Botanical Garden Institute of Ecosystem Studies at the Mary Flagler Cary Arboretum for access to study areas.
References
Baker, M. C., & Cunningham, M. A. (1985). The biology of birdsong dialects. Behavioral and Brain Sciences, 8, 85–133.
Balaban, E. (1988). Cultural and genetic variation in swamp sparrows (Melospiza georgiana). II. Behavioral salience of geographic song variants. Behaviour, 105, 292–322.
Baptista, L. F. (1975). Song dialects and demes in sedentary populations of the white-crowned sparrow (Zonotrichia leucophrys nuttalli). University of California Publications in Zoology, 105, 1–52.
Baptista, L. F. (1977). Geographic variation in song and dialects of the Puget Sound white-crowned sparrow. Condor, 79, 356–370.
Baptista, L. F., & Petrinovich, L. (1984). Social interaction, sensitive phases and the song template hypothesis in the white-crowned sparrow. Animal Behaviour, 32, 172–181.
Baptista, L. F., & Petrinovich, L. (1986). Song development in the white-crowned sparrow: social factors and sex differences. Animal Behaviour, 34, 1359–1371.
Clayton, N. S. (1988). Song tutor choice in zebra finches and Bengalese finches: the relative importance of visual and vocal cues. Behaviour, 104, 281–299.
Dobzhansky, T. (1962). Mankind evolving. New Haven, CT: Yale University Press.
Dooling, R. J. (1989). Perception of complex, species-specific vocalizations by birds and humans. In R. J. Dooling & S. Hulse (Eds.), The comparative psychology of audition (pp. 423–444). Hillsdale, NJ: Lawrence Erlbaum Associates.
Dooling, R. J., & Searcy, M. H. (1980). Early perceptual selectivity in the swamp sparrow. Developmental Psychobiology, 13, 499–506.
Falls, J. B. (1982). Individual recognition by sounds in birds. In D. E. Kroodsma, & E. H. Miller (Eds.), Acoustic communication in birds (Vol. 2, pp. 237–278). New York: Academic Press.
Gould, J. L., & Marler, P. (1987). Learning by instinct. Scientific American, 256, 74–85.
Hinde, R. A. (1970). Animal behaviour: A synthesis of ethology and comparative psychology (2nd ed.). New York: McGraw-Hill.
Johnston, T. D. (1988). Developmental explanation and the ontogeny of birdsong: Nature/nurture redux. Behavioural and Brain Sciences, 11, 631–675.
King, A. P., & West, J. J. (1988). Searching for the functional origins of song in eastern brown-headed cowbirds, Molothrus ater ater. Animal Behaviour, 36, 1575–1588.
Konishi, M. (1965). The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift für Tierpsychologie, 22, 770–783.
Konishi, M. (1985). Birdsong: From behavior to neuron. Annual Review of Neuroscience, 8, 125–170.
Krebs, J. R., & Kroodsma, D. E. (1980). Repertoires and geographical variation in bird song. In J. S. Rosenblatt, R. A. Hinde, C. Beer, & M.-C. Busnel (Eds.), Advances in the study of behavior (pp. 143–177). New York: Academic Press.
Kroodsma, D. E. (1984). Songs of the alder flycatcher (Empidonax alnorum) and willow flycatcher (Empidonax traillii) are innate. Auk, 101, 13–24.
Kroodsma, D. E., & Pickert, R. (1984). Sensitive phases for song learning: Effects of social interaction and individual variation. Animal Behaviour, 32, 389–394.
Lorenz, K. Z. (1950). The comparative method in studying innate behavior patterns. Symposium Society Experimental Biology, 4, 221–268.
Marler, P. (1960). Bird songs and mate selection. In W. N. Tavolga (Ed.), Animal sounds and communication (pp. 348–367). American Institute of Biological Sciences Symposium Proceedings.
Marler, P. (1970). A comparative approach to vocal learning: song development in white-crowned sparrows. Journal of Comparative and Physiological Psychology, 71, 1–25.
Marler, P. (1976). Sensory templates in species-specific behavior. In J. Fentress (Ed.), Simpler networks and behavior (pp. 314–329). Sunderland, MA: Sinauer Associates.
Marler, P. (1984). Song learning: Innate species differences in the learning process. In P. Marler, & H. S. Terrace (Eds.), The biology of learning (pp. 289–309). Berlin: Springer-Verlag.
Marler, P. (1987). Sensitive periods and the role of specific and general sensory stimulation in birdsong learning. In J. P. Rauschecker & P. Marler (Eds.), Imprinting and cortical plasticity (pp. 99–135). New York: John Wiley & Sons.
Marler, P., Mundinger, P., Waser, M. S., & Lutjen, A. (1972). Effects of acoustical stimulation and deprivation on song development in red-winged blackbirds (Agelaius phoeniceus). Animal Behaviour, 20, 586–606.
Marler, P., & Peters, S. (1980). Birdsong and speech: evidence for special processing: In P. Eimas, & J. Miller (Eds.), Perspectives on the study of speech (pp. 75–112). Hillsdale, NJ: Lawrence Erlbaum Associates.
Marler, P., & Peters, S. (1982a). Developmental overproduction and selective attrition: new processes in the epigenesis of birdsong. Developmental Psychobiology, 15, 369–378.
Marler, P., & Peters, S. (1982b). Subsong and plastic song: their role in the vocal learning process. In D. E. Kroodsma & E. H. Miller (Eds.), Acoustic communication in birds: Vol. 2 (pp. 25–50). New York: Academic Press.
Marler, P., & Peters, S. (1987). A sensitive period for song acquisition in the song sparrow, Melospiza melodia: a case of age-limited learning. Ethology, 76, 89–100.
Marler, P., & Peters, S. (1988a). The role of song phonology and syntax in vocal learning preferences in the song sparrow, Melospiza melodia. Ethology, 77, 125–149.
Marler, P., & Peters, S. (1988b). Sensitive periods for song acquisition from tape recordings and live tutors in the swamp sparrow, Melospiza georgiana. Ethology, 77, 76–84.
Marler, P., & Peters, S. (1989). Species differences in auditory responsiveness in early vocal learning. In S. Hulse & R. Dooling (Eds.), The comparative psychology of audition (pp. 243–273). Hillsdale, NJ: Lawrence Erlbaum Associates.
Marler, P., Peters, S., Ball, G. F., Dufty, A. M., Jr., & Wingfield, J. C. (1988). The role of sex steroids in the acquisition of birdsong. Nature, 336, 770–772.
Marler, P., Peters, S., & Wingfield, J. (1987). Correlations between song acquisition, song production, and plasma levels of testosterone and estradiol in sparrows. Journal of Neurobiology, 18, 531–548.
Marler, P., & Pickert, R. (1984). Species-universal microstructure in the learned song of the swamp sparrow (Melospiza georgiana). Animal Behaviour, 32, 673–689.
Marler, P., & Sherman, V. (1983). Song structure without auditory feedback: Emendations of the auditory template hypothesis. Journal of Neuroscience, 3, 517–531.
Marler, P., & Sherman, V. (1985). Innate differences in singing behaviour of sparrows reared in isolation from adult conspecific song. Animal Behaviour, 33, 57–71.
Nordeen, K. W., Marler, P., & Nordeen, E. J. (1988). Changes in neuron number during sensory learning in swamp sparrows. Society of Neuroscience Abstracts, 14, 89.
Nottebohm, F. (1968). Auditory experience and song development in the chaffinch (Fringilla coelebs). Ibis, 110, 549–568.
Nottebohm, F. (1972). Neural lateralization of vocal control in a passerine bird. II. Subsong, calls and a theory of vocal learning. Journal of Experimental Zoology, 1979, 35–49.
Nottebohm, F. (1987). Plasticity in adult avian central nervous system: possible relation between hormones, learning, and brain repair. In F. Plum (Ed.), Higherfunctions of the nervous system (pp. 85–108). Washington: American Physiological Society.
Nowicki, S., & Marler, P. (1988). How do birds sing? Music Perception, 5, 391–26.
Pepperberg, I. M. (1988). The importance of social interaction and observation in the acquisition of communicative competence: Possible parallels between avian and human learning. In T. R. Zentall & B. G. Galef, Jr. (Eds.), Social learning: A comparative approach (pp. 279–299). Hillsdale, NJ: Lawrence Erlbaum Associates.
Petrinovich, L. (1985). Factors influencing song development in the white-crowned sparrow (Zonotrichia leucophrys). Journal of Comparative Psychology, 99, 15–29.
Piattelli-Palmarini, M. (Ed.). (1980). Language and learning. Cambridge, MA: Harvard University Press.
Rauschecker, J. P., & Marler, P. (1987). Cortical plasticity and imprinting: Behavioral and physiological contrasts and parallels. In J. P. Rauschecker & P. Marler (Eds.), Imprinting and cortical plasticity (pp. 349–366). New York: John Wiley & Sons.
Tinbergen, N. (1951). The study of instinct. Oxford: Clarendon Press.