

methane and mars
While the evidence for biologically produced methane in the atmosphere of Mars is no less controversial than the evidence that Martian nanobacteria produced magnetite grains and deposited them in a Martian rock that later became a meteorite from Mars, the Martian methane debate has had great staying power. For half a century, astronomers have been searching for evidence of methane in the atmosphere of Mars. Why? Methane is a simple molecule that packs a very powerful astrobiological punch: in the oxygen-rich, hydrogen-poor Martian environment, almost no methane should exist without being actively created by living things. Therefore, the discovery of more methane in the atmosphere of Mars than could exist there without life, as is the case in the atmosphere of Earth, could be unequivocal proof that life exists or existed on Mars.
Methane is a colorless, odorless gas that is the simplest possible molecule composed of both and only hydrogen and carbon atoms. (Because methane is odorless, energy companies add an odorant, mercaptan, which smells like rotten eggs, to the methane used for cooking and heating in homes; the smell of the mercaptan helps in detecting gas leaks.) In the language of chemistry, methane is a hydrocarbon. One methane molecule contains one carbon atom bonded to four hydrogen atoms (CH4). Because hydrogen is the most abundant element in the universe and because, after helium, carbon is the third most abundant element in the universe, methane also exists everywhere in the universe where it can survive; it cannot, however, survive everywhere.
Methane is a common and familiar gas on Earth, as it is the most abundant type of molecule found in natural gas extracted from oil and gas wells, shale deposits, and coal seams. It is the product of the decomposition of organic matter, mostly from ancient marine microorganisms deposited in terrestrial sediments over the last half billion years, that was then subjected to immense heat and pressure for millions of years under Earth’s crust. The natural gas used in home heating and cooking is almost pure methane (the gas used in most common barbecue grills is propane, C3H8, which is also extracted from the same underground gas reservoirs). On Earth, virtually all the methane, whether buried in underground deposits or free in the atmosphere or trapped in methane clathrates in permafrost, is biological in origin. Methane on Earth is a clear chemical signature that life exists (or existed in the ancient past) on Earth.
Methane is a fragile molecule. Because it cannot survive if the temperature is too hot (above about 1500 K), methane is not present in the atmospheres of stars, though it is present in the atmospheres of many brown dwarfs (brown dwarfs are more massive than planets but less massive than stars). Methane also requires a hydrogen-rich (reducing) environment in order to survive and cannot endure in the presence of free oxygen atoms or in an environment rich in oxygen-bearing molecules, such as oxygen (O2) or carbon dioxide (CO2). In such an (oxidizing) environment, the carbon-hydrogen bond in the methane molecule is not strong enough to resist the chemically aggressive nature of oxygen. As a result, oxygen atoms rip apart the carbon-hydrogen bonds and then attach themselves separately to both the carbon and the hydrogen atoms. These chemical reactions favor the formation of carbon dioxide (CO2) and water (H2O) molecules at the expense of methane. Because the Martian atmosphere is made of 96 percent carbon dioxide, it is a very strongly oxidizing environment. Methane in the Martian atmosphere simply cannot survive for long. Any ancient methane in the atmosphere of Mars would long ago have been converted to carbon dioxide and water; therefore, any current reservoir of Martian atmospheric methane must have been actively produced or released from an underground reservoir in the very recent past.
Methane is at risk in the atmosphere of Mars for another reason. The methane molecule cannot hold itself together when exposed to ultraviolet light. The bonds that attach the atoms to each other in the methane molecule absorb ultraviolet light energy and as a result are destroyed. Interstellar space, for example, which is bathed by ultraviolet photons produced by hot stars, is devoid of methane, except in the densest cores of giant molecular clouds where the outer layers of these clouds can shield small, internal pockets of methane from the ravages of ultraviolet light. The atmospheres of some planets and some of their moons in the outer solar system also are capable of protecting their methane reservoirs and thus can sustain methane-rich environments. The atmospheres of the planets Uranus (2.3 percent methane) and Neptune (1.5 percent methane) have significant amounts of methane because those planets are rich in hydrogen, while Saturn’s moon Titan has methane lakes and icebergs, methane-rich mud, and a methane-rich atmosphere. The surfaces of Pluto and Neptune’s moon Triton are richly tiled with methane ice, while both Pluto and Triton also have methane in their atmospheres (Pluto even shows evidence for methane frost and snow at high elevations1).
The atmosphere of Earth has only a tiny amount of methane (1800 parts per billion, or 0.00018 percent of Earth’s atmosphere), and our ozone layer only partially protects what little we have. The small amount of ultraviolet sunlight that penetrates Earth’s ozone layer slowly destroys methane atoms in Earth’s atmosphere, such that a typical methane atom can survive for only about twelve years. Once sunlight dissociates the carbon atom from the hydrogen atoms, in the oxygen-rich atmosphere of Earth the free carbon atom bonds with one oxygen molecule (O2) to form carbon dioxide, and the two hydrogen molecules (2H2) react with one oxygen molecule to form two molecules of water.
As for the origin of the methane in Earth’s atmosphere, a tiny amount is produced through natural geological processes, as it is belched out of volcanoes or through a process called serpentinization, in which water, heated by the magma at mid-ocean ridges, reacts with iron-rich and magnesium-rich rocks to form the mineral serpentine. The hydrogen atoms liberated from the water molecules in this process react with carbon dioxide dissolved in ocean water to form methane.
Most of Earth’s atmospheric methane, however, has a biological origin, from among the following sources:
•the effluence that results from the digestive processes of termites (possibly as much as 15 percent of the total methane content of the atmosphere2)
•ruminant livestock, such as cattle, buffalo, sheep, goats, and camels (estimates range as high as 20 percent)
•decomposition of organic waste in landfills, wetlands, wastewater treatment facilities, and manure management systems (possibly as much as or greater than 30 percent)
•microbes that feed off organic material in rice paddies (probably more than 6 percent and perhaps as high as 12 percent)
•production, combustion, and distribution of fossil fuels, including coal mining (most likely more than 15 percent and perhaps as high as 30 percent)3
As a result of past and present biological activity, Earth’s atmospheric methane level, minute as it is, is about one million times greater than the level that volcanic and other geological sources alone can generate. Thus, if life did not exist and had never existed on Earth, the methane level in Earth’s atmosphere would be much less than one part per billion.
Unlike Uranus, Neptune, and Titan, Mars is not one of the methane-rich locales in our solar system. Other than a small fraction of a percent of the meteoritic dust that rains down onto the Martian surface, Mars lacks a rich source of methane—unless Mars has life. Martian volcanic activity appears to be extinct; Mars, unlike Earth, lacks a plate tectonic system; also, unlike Earth, Mars has no large, domesticated herds of livestock roaming the surface.
If Mars has a modern, active reservoir of methane, that source remains obscured. And if Mars has a source of methane, we can reasonably ask what will happen to that methane when it passes from rocks or plants or animals into the Martian atmosphere. Though much thinner than Earth’s atmosphere, the Martian atmosphere is thick and mobile enough to take any injected methane from any source and spread it globally in only a few weeks’ time. Mars also lacks an ozone layer that could shield methane molecules from destructive solar ultraviolet photons, though the abundant CO2 in the lower Martian atmosphere does act as a weak shield from these destructive photons. Once methane molecules bubble above the bulk of the carbon dioxide in the lower atmosphere, which they will do because they are lighter in weight than carbon dioxide molecules, sunlight will destroy them. The destruction of methane also depends on the presence of oxygen-containing species, especially the OH radical, with which it can react. Because the abundance of OH is so low in the Martian atmosphere, methane can survive for much longer there than it could in Earth’s atmosphere. The lifetime for methane in the Martian atmosphere, as a result, is estimated to be 300–600 years, according to the calculations of all Martian atmospheric models.4
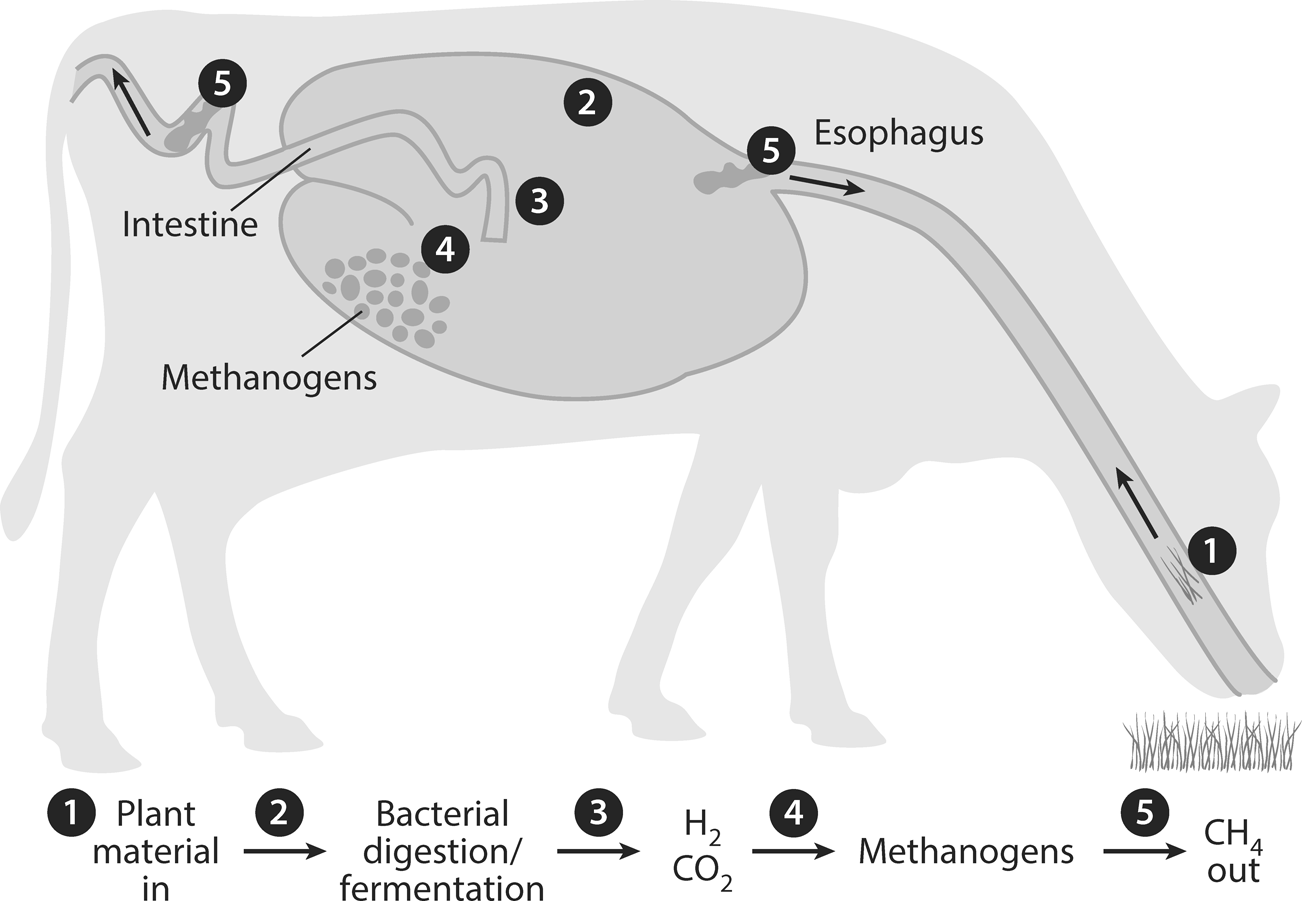
Figure 12.1. Domesticated livestock, especially cows, sheep, goats, and camels, produce methane gas when the bacteria in their digestive tracks helps them digest food.
The Martian atmosphere therefore should contain no methane unless the planet has an active source that is able to continually replenish the atmospheric supply of methane. Furthermore, even if Mars has one or more localized sources of methane, because of the rapid, global circulation of Martian atmospheric gases the entire planet should show about the same amount of methane unless the methane was injected into the atmosphere extremely recently (i.e., within the last few weeks).

Figure 12.2. Pie chart illustrating the sources of methane in the terrestrial environment, all of which ultimately are attributable to past or present life.
Mars does have enormous—but extinct—volcanoes, the largest volcanoes in the entire solar system, in fact. They could inject significant volumes of methane into the atmosphere were they to erupt. But volcanic eruptions do not simply erupt methane. Volcanoes also belch other gases, including sulfur dioxide (SO2) at a level 100 to 1,000 times greater than that of methane. If Mars were volcanically active, the atmosphere would contain easily detectable levels of sulfur dioxide in addition to methane, but it does not.5 The absence of sulfur dioxide in the Martian atmosphere provides very strong evidence that the Martian volcanic system is not active at the present time and that any methane, if it exists at all, did not enter the atmosphere through volcanic eruptions.
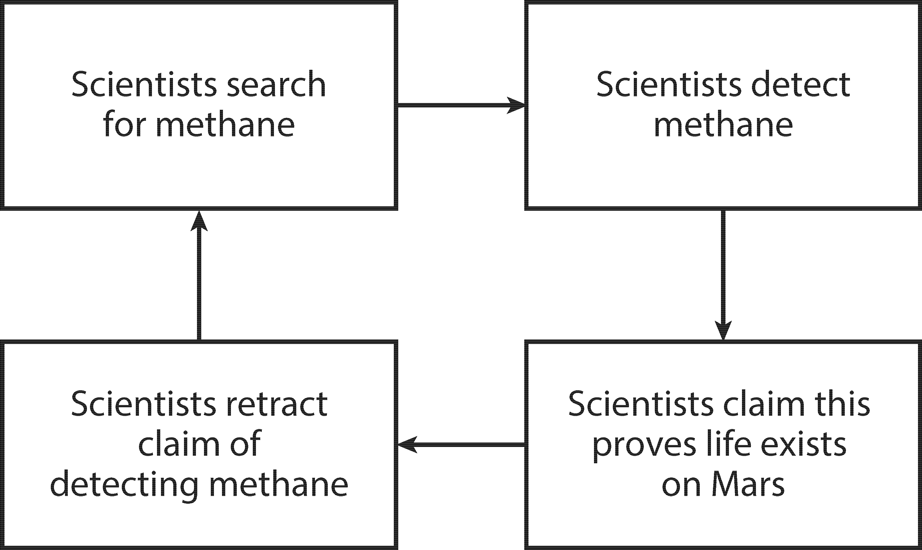
Figure 12.3. The Mars methane science cycle.
The story of what we know about Martian methane is simple.
Chapter One: Scientists detect methane.
Chapter Two: Because Mars has no obvious nonbiological source of methane, they or others then claim that the detected methane is evidence for the existence of methane-generating life-forms on Mars. Naturally, all the major newspapers and media outlets report this historic discovery, because it is, in fact, a discovery of unprecedented importance: life discovered on Mars!
Chapter Three: A few months later, they or others retract the claim for the detection of methane. No methane on Mars and no evidence for life on Mars.
Some years later, the Martian methane story begins anew: other scientists detect methane and claim that the methane proves life exists on Mars. More big headlines. Soon, they too are forced to walk back their claims for having detected methane and for having proven life exists on Mars.
More time passes; another possibly specious methane detection hits the front pages. More unsubstantiated claims are made for life on Mars as a new generation of explorers chases a discovery that they hope will reward them with fame (and perhaps fortune). A new generation of reporters, ignorant of the past and eager to report on the Next Big Thing, happily reports on the Next Big Thing.
Maybe one of these reported discoveries was or will be correct. That would be incredible news. The lesson of history, however, is that we may be fooling ourselves.
martian methane discovered?
On October 17, 1966, Dr. Lewis Kaplan, of Caltech’s Jet Propulsion Laboratory, stepped to the podium in the Jack Tar Hotel in San Francisco and announced news of a stunning scientific discovery. There, before his colleagues at a meeting of the American Chemical Society, he reported that he and his two French colleagues, Pierre and Janine Connes, two astronomers working at the National Center of Scientific Research and the Meudon Observatory, had detected methane gas in the atmosphere of Mars.
The Kaplan/Connes research team had used an instrument designed by Pierre Connes known as a Michelson interferometer that was capable of seeing details in the light from Mars ten times more clearly than astronomers had previously been capable of seeing. Effectively, they had a better detector and a better telescope but were employing the same technique as that used by Huggins a century before in his search for Martian water. From September 1964 through June 1965, using their new instrument at the Observatoire de Haute-Provence, France, they had measured the contents of the atmosphere of Mars. In doing so they claimed to have observed the spectral signatures of previously undetected gases and to have surpassed the work of previous observers of Mars because of the superior quality of their instrumentation.
What they had detected, exactly and indisputably, were absorption bands in the near-infrared spectrum of Mars. Kaplan and the Connes believed these absorption features were best explained by the presence in the Martian atmosphere of “gaseous compounds containing hydrogen.” Such compounds were likely “methane derivatives and perhaps methane itself.”6
According to an article published in the Los Angeles Times the next day, the Kaplan/Connes team’s measurement “suggests the presence of previously unreported methane and methane-like material in the Martian atmosphere.”7 LA Times science writer Irving Bengelsdorf wrote, at the time, “These observations, if correct, suggest that there may be biological activity on Mars producing methane.” Though these astronomers lacked the evidence to prove their case, in 1966 the consensus among astronomers was that the only possible sources of methane on Mars were biological. As a result, Dr. Kaplan most likely did not try to dissuade Bengelsdorf from connecting the dots from methane to life on Mars. The methane supposedly discovered by Kaplan, Connes, and Connes therefore did not merely imply the possible presence of life to the scientists involved in trying to detect methane on Mars. This detection of methane was, in the minds of these discoverers, tantamount to the discovery of life on Mars. Among other outcomes of this premature discovery announcement by the Kaplan team is that the media took control of the story, and the story they told was simply this: methane on Mars equals life on Mars.
Without any doubt, these scientists were confident that the names of the discoverers of life on Mars would someday be written boldly in our history books, and that those names would be Kaplan, Connes, and Connes. Schiaparelli had found canals (well, not really) and was famous, but he had not discovered life. Lowell had found more canals (but no, not really) and claimed he had evidence for life on Mars, and Lowell was now infamous, but he also had not found life. Kuiper’s lichens were merely dust. Sinton’s algae turned out to be terrestrial heavy water and windblown Martian sand. But these methane results had been obtained with a state-of-the-art Michelson interferometer. The work of Kaplan, Connes, and Connes was modern and surely would stand the test of time. Soon, they reported, they would observe Mars again with an instrument yet one hundred times more sensitive than the one they had used for their observations in 1965.
At a meeting held just four months later, in February 1967, at the Institute for Space Studies of the National Aeronautics and Space Administration in New York City, Kaplan again reported “that the air of Mars contains methane, or methane-based gases, whose presence is difficult to explain unless they are generated by living organisms.” At this meeting, he reported that new observations “with Mars much closer had strengthened his belief that the earlier findings are reliable.”8 Kaplan explained that the chemistry of the Martian air “seems to forbid any extended lifetime for methane or its sister gases.” Consequently, “something must be continuously providing new methane. On Earth this is done by bacteria that live in the absence of oxygen—as in decaying material in swamps.”
Pierre and Janine Connes and Lewis Kaplan had announced their initial discovery in an unrefereed letter, known as an announcement, published in Science magazine in August 1966.9 Unfortunately for them, their conclusion that they had detected methane on Mars was wrong. After their public announcements at the American Chemical Society meeting in October 1966 and the ISS/NASA meeting in February 1967, they never again mentioned their claim for discovering methane, though they also never retracted their claim in print. Probably they hoped that their ill-advised and incorrect announcement would be forgotten. They continued to work together for a few years, even publishing several detailed studies of carbon dioxide in the Martian atmosphere,10 but they were very careful to never again mention or even cite their own earlier work on methane. Quietly, undetected, almost like odorless methane itself, this first hint for the presence of methane in the atmosphere of Mars dissipated into thin air. Interest in Martian methane, however, continued.
mariner 7
Mariner 7 was launched from Cape Canaveral in Florida on March 27, 1969. Destination: Mars. In 1969, NASA did not yet have the ability to put a spacecraft into orbit around Mars; instead, Mariner 7 flew past Mars on August 5, just five days after the flyby of its twin, Mariner 6. Notably for the study of Martian methane or any other component of the atmosphere of Mars, whether sent to Mars as a flyby or an orbiter, a spacecraft surveying Mars from just above the Martian atmosphere does not have to also peer through Earth’s atmosphere. Observers using this kind of data to look for evidence of Martian methane therefore do not have to find a way to remove from the data the overwhelmingly strong signature of terrestrial methane, as did Kaplan and his colleagues. As a result, Mariner 6 and 7 and all future missions to Mars would be capable of making much more definitive measurements of the contents of the atmosphere of Mars than would be possible via almost any observations that might be made from ground-based telescopes.

Figure 12.4. Launch of Mariner 7 spacecraft atop an Atlas-Centaur rocket from the Kennedy Space Center on March 7, 1969. Image courtesy of NASA.
Mariner 6 returned seventy-five images of Mars and determined that the composition of the atmosphere was about 98 percent carbon dioxide. Mariner 7 obtained 126 spectacular, but grainy, images of Mars, and some infrared spectroscopic data that generated a great deal of excitement.
Just two weeks after astronauts Neil Armstrong and Michael Collins put the first human footprints on the surface of the Moon, NASA was at the peak of its game. First, men on the Moon; now, close-up pictures and measurements of Mars. The world was eagerly awaiting the latest news from NASA and the Mariner 7 Mars science team, and NASA didn’t waste any time before providing Mariner science updates. Taxpayers, via congressional appropriations, had funded this mission and NASA, quite reasonably, wanted to update the public as soon as possible on the Mariner 7 discoveries. Without question, front-page news about the Mariner 7 mission would be good for the future of NASA and planetary science. The scientific team members were also eager for their just rewards and the publicity that would ensue after the announcement of their discoveries, having worked for years to build and test and calibrate the instruments that now were generating the data that were at last streaming back across the tens of millions of miles of space that separate Earth and Mars. The press was at the door, pens and television cameras at the ready, prepared to turn a handful of geeky engineers and shy scientists into international personalities and possibly even into award-winning heroes.
Data collected on August 5 were quickly (but insufficiently) analyzed and interpreted. The media hordes descended on the Jet Propulsion Laboratory for a press conference that was held on August 7. The Mariner 7 science team did not disappoint them. Walter Sullivan, of the New York Times, convinced his editors to put his article on Mariner 7 science discoveries on the front page of the August 8 issue, with the headline, “2 Gases Associated with Life Found on Mars Near Polar Cap.” Big news indeed! And an even bigger mistake.
Dr. George Pimentel, Professor of Chemistry at the University of California at Berkeley, announced that his team, using the Mariner 7 Infrared Spectrometer (IRS), had detected methane and ammonia in the atmosphere of Mars in a localized region above its south pole. The spectroscopic signature of methane had been detected by the IRS in a “strong” absorption band at a near-infrared wavelength close to 3.3 microns. In a strong band, two things are certain. First, the gas is very efficient in absorbing light at that particular wavelength; second, a great deal of the gas is present and is absorbing large amounts of light. In comparison, the ammonia band the IRS team observed toward Mars at 3.0 microns was also present but weaker than the methane line. As for location on Mars, Pimentel stated, “We are confident that we have detected gaseous methane and gaseous ammonia between approximately 61 degrees and 76 degrees south latitudes on Mars.”11
Having made that measurement, Pimentel and his colleague, Dr. Kenneth C. Herr, also a chemist at the University of California at Berkeley, continued, remarking that the detection “gives no direct clue whatsoever concerning its origin.” That, however, did not stop them from speculating: “I have no clue as to the origin of these gases, but if the readings are true—and I believe they are—we have to face the possibility they could be of biological origin.”12 Impressively, not only were they able to speculate about origins, about which they knew nothing, they could extrapolate to deduce the locality of the Martian life-forms: “The geographic localization of the absorptions suggests that their origin is in this hospitable region” [the edge of the polar cap, between 61 and 76 degrees southern latitude, where they claimed there was a reservoir of water].
The euphoria lasted barely a month. On September 11, Pimentel announced at another news conference that the spectral features previously attributed to methane and ammonia were, in fact, caused by frozen carbon dioxide (dry ice). His team’s improved analysis of the IRS spectral data thereby “remove[d] one of the final hopes scientists held of finding life on the planet.” In his words, the fact that carbon dioxide can mimic the behavior of methane and ammonia was “just a cruel coincidence.”13
Drawing lessons from hasty conclusions is almost too easy. First, science should not be done by press conference. Second, this kind of science is very hard to do right, and takes extreme care, patience, dedication, and perseverance. To their credit, Pimentel and his team eventually demonstrated they had all of these qualities.a They got the science right. In 1972, after several years of effort, they reported an upper limit for methane in the Martian atmosphere of 3.7 parts per million (for easy comparison with later measurements, 3,700 parts per billion).14
In the language of science and statistics, an upper limit is not a detection. Instead, it is a way of reporting the degree of accuracy of measurements made in an experiment. Usually a scientist will report an upper limit that is three times greater than the “noise level” in the experiment (a “three sigma” upper limit), though sometimes an upper limit is reported that is only twice the noise level.
Let’s return from Mars for a moment so we may better understand upper limits and noise levels. Imagine that you are traveling on a bus from Nashville, Tennessee, to Denver, Colorado, with your pet Great Dane, Fido. While on the bus, you use an old-fashioned bathroom scale to measure the weight of Fido. Your measurement reveals that Fido weighs 150 pounds. How certain are you that Fido weighs 150 pounds and not 152 or 147 pounds?
In order to be sure, you repeat this measurement over and over again and get a different answer every time, with your answers ranging, seemingly randomly, from 145 pounds to 155 pounds. Your confidence in your ability to ever know Fido’s exact weight is shaken. Obviously, weighing a Great Dane on an old scale on a bus while moving at high speed on an interstate highway yields an answer with limited accuracy. The limit in accuracy is due partly to the equipment (is the scale calibrated correctly? does the scale work the same way every time?) and partly to variables that you cannot control (bumps on the highway; Fido squirming; Fido’s food and water intake (and deposits); your poor eyesight in reading the line toward which the arrow on the scale points). All of these factors contribute to the “noise” in the data. Nothing you do will allow you to get an exact answer, but the measurements you made allow you to calculate both an average value for Fido’s weight as well as the uncertainty, i.e., the noise, on that measurement.
If you did this experiment with Fido by making 100 measurements and then calculating your answer, you might have found that Fido’s weight was 151 ± 2 pounds (read as: 151 plus or minus 2). This answer means that if you were to put Fido on a scale and measure his weight for the 101st time, the likelihood that your answer would be more than 149 and less than 153 (within “one sigma” of the average) is 68 percent; you also can be confident that when you measure Fido’s weight for the 101st time, you have a 95 percent chance that your answer will be greater than 147 pounds and less than 155 pounds (within two sigma); finally, 99.7 percent of the time when you make that 101st measurement, you will get a weight in between 145 and 157 pounds (within three sigma). You still don’t know for sure exactly what Fido weighs, but you have an incredibly high degree of certainty that Fido’s weight is within a known 12-pound range. In the real world, knowing a most likely value and having a quantitative measure of the accuracy with which we know that value is the best we can do.
On your next interstate bus trip, you travel with and weigh—100 times—your sister’s plump pet guinea pig, Sassafras. The noise level is dependably the same, ± 2 pounds (the noise has nothing to do with the weight of Sassafras and everything to do with the measuring equipment and the measuring environment), and you find that Sassafras weighs in (the average value of your measurements) at a robust 2.8 pounds. You know that guinea pigs normally don’t weigh this much, though Sassafras might. How confident can you be that Sassafras weighs 2.8 pounds? From the statistics of your measurements, you can be 65 percent certain that her weight is between 0.8 and 4.8 pounds, 95 percent certain that her weight is between 0 and 6.8 pounds (you have 100 percent confidence that her weight is not below zero), and 99.7 percent certain that Sassafras weighs less than 8.8 pounds. If you are honest with yourself and understand your data, you would acknowledge that you have no idea if Sassafras weighs 2.8 pounds, since 2.8 pounds is barely greater than the noise in the data. All you know, with great confidence, is that Sassafras weighs less than 8.8 pounds and probably weighs less than 6.8 pounds.
What if you tried this same experiment with your pet gerbil, Orion? Since a typical gerbil weighs only a small fraction of one pound, if you use the same scale on the same bus on the same highway as you used to measure the weight of Fido and Sassafras, all you’ll learn is that Orion doesn’t weigh enough to register on the scale; however, because of the bounciness of the bus, you still get the same level of noise, ± 2 pounds. You could then, with 99.7 percent confidence, report Orion’s weight as “less than 6 pounds.” In this case, “6 pounds,” which is three times greater than your noise level, would be your “three sigma” upper limit. That’s all you know for certain. If Orion weighs 0.2 or 4 or 7 or 12 or 29 ounces, you would get the same answer—less than 6 pounds—when measuring Orion’s weight with this scale.
Pimentel and the Mariner 7 team eventually concluded that all they knew about methane in the atmosphere of Mars is that they had 95 percent confidence (their upper limit of 3.7 parts per million was twice their noise level) that the abundance of methane was very small. With 95 percent confidence, out of every million molecules in the atmosphere, fewer than four might be methane, and with 99.7 percent confidence, fewer than six might be methane. Keep in mind that “fewer than 6” could be 5 or 2 or 1 or even 0. Mars might have a very little bit of methane or absolutely no methane at all. Despite the initial publicity fuss, in their robust final analysis the Mariner 7 IRS experiment did not detect methane.
In August 1969, at the first Mariner 7 press conference, Pimentel perhaps should have had the wisdom to step more cautiously. After all, he was one of the team of Berkeley chemists who, in 1965, had shown that heavy water in Earth’s atmosphere, not algae on Mars, was likely responsible for the Sinton bands. However, the oppressive historical burden shouldered by anyone studying Mars tended to steer investigators very easily toward incautiousness.
The Mariner 7 methane fiasco was an example of science done poorly by premature press conference, for which NASA, the Jet Propulsion Laboratory, the University of California at Berkeley, the involved scientists, and the media all share responsibility. The news of the retraction made page 3 of the Los Angeles Times and page 8 of the Wall Street Journal,15 the latter of which noted, quite fairly, that “the mistaken identification last month of methane and ammonia was a perfect example of the hazards of quick conclusions.” The New York Times did not report on Pimentel’s retraction, which must not have fit within the category of “all the news that’s fit to print.”
mariner 9
Mariner 9 was the second of the last pair of Mars missions launched in NASA’s Mariner mission series, and the imaging results of Mariner 9 were revolutionary. While Mariner 8 failed on launch, Mariner 9 was launched successfully on May 30, 1971, and was inserted into orbit around Mars on November 13, 1971, thereby becoming the first spacecraft to orbit another planet. Just achieving that goal was a spectacular accomplishment.
When Mariner 9 reached Mars, the surface of the planet was obscured by a lingering global dust storm. After about a month, however, the view from this orbiting spacecraft to the surface of Mars became clear. The primary mission of Mariner 9 was to image the entire surface of the planet. When these images were transmitted to Earth, Mars metamorphosed from a distant, unknown planet with a handful of large, lunar-like craters into a well-known world, complete with gigantic volcanoes, a canyon—Valles Marineris—grander by far than our own Grand Canyon, ancient riverbeds, outflow channels, and vast crater-strewn regions. Rather than being a bigger version of Earth’s Moon, Mars had the biggest volcanoes, the longest and deepest canyons, and the widest, highest-volume, water-carved valleys and channels in the solar system. Suddenly, Mars was a grand and beautiful world, with a rich areological history waiting to be unveiled.
The initial data from Mariner 9’s surveillance of the Martian surface yielded enormous surprises that led science team members to speculate fervently about Martian life. Harold Masursky, of the U.S. Geological Survey and leader of the television examination team, speculated intensely about the origin of the ancient river valleys imaged by the spacecraft camera. Clearly, large volumes of water once flowed on the Martian surface. On that conclusion, his guess about Martian history was reasonable. He then continued, noting that these discoveries “increase the likelihood that future missions to land on the planet might find some signs of life, or at least fossils of past life.” On that point, the historical burden of Mars, and perhaps the interests of the press, had led Masursky up to, if not over, the edge. Project team member Rudolph Hanel, of the Goddard Space Flight Center, reported, “We have not found any signs of life on Mars. We can’t expect to. But we have not seen anything that would exclude life.” The pendulum of hope for finding life on Mars was swinging back in the favor of “yes.”16
The infrared interferometer (IRIS) on Mariner 9 was used from late 1971 through much of 1972 to collect an extensive library of spectra of the Martian atmosphere. The most important goals for the IRIS science team were to measure the temperature profile of the atmosphere—that is, how the temperature changes from the surface to the top of the atmosphere—and the temperature and pressure at the Martian surface. The design of IRIS also allowed the Mariner 9 team to measure the abundances of minor atmospheric constituents, including some molecules like methane that could have biological implications.
The world had to wait until 1977 to learn about the Mariner 9 measurements of methane. That year, William C. Maguire, of the Laboratory for Planetary Atmospheres at the Goddard Space Flight Center, published a paper in a refereed scientific journal, without an accompanying press conference, in which he presented his analysis of a carefully selected subset of the Mariner 9 IRIS spectra. Maguire analyzed and averaged 1,747 spectra, selected from the total data set to include only spectra taken after the 100th orbit of the spacecraft. This very reasonable culling of the data was made because the global dust storm on Mars that was present when Mariner 9 arrived at Mars had ended by the 100th orbit, and the dust entrained in the atmosphere had mostly settled back to the surface. Since the dust in the atmosphere decreased the appearance in the spectra of weak atmospheric gases, the spectra taken during the first one hundred orbits were of little scientific value.
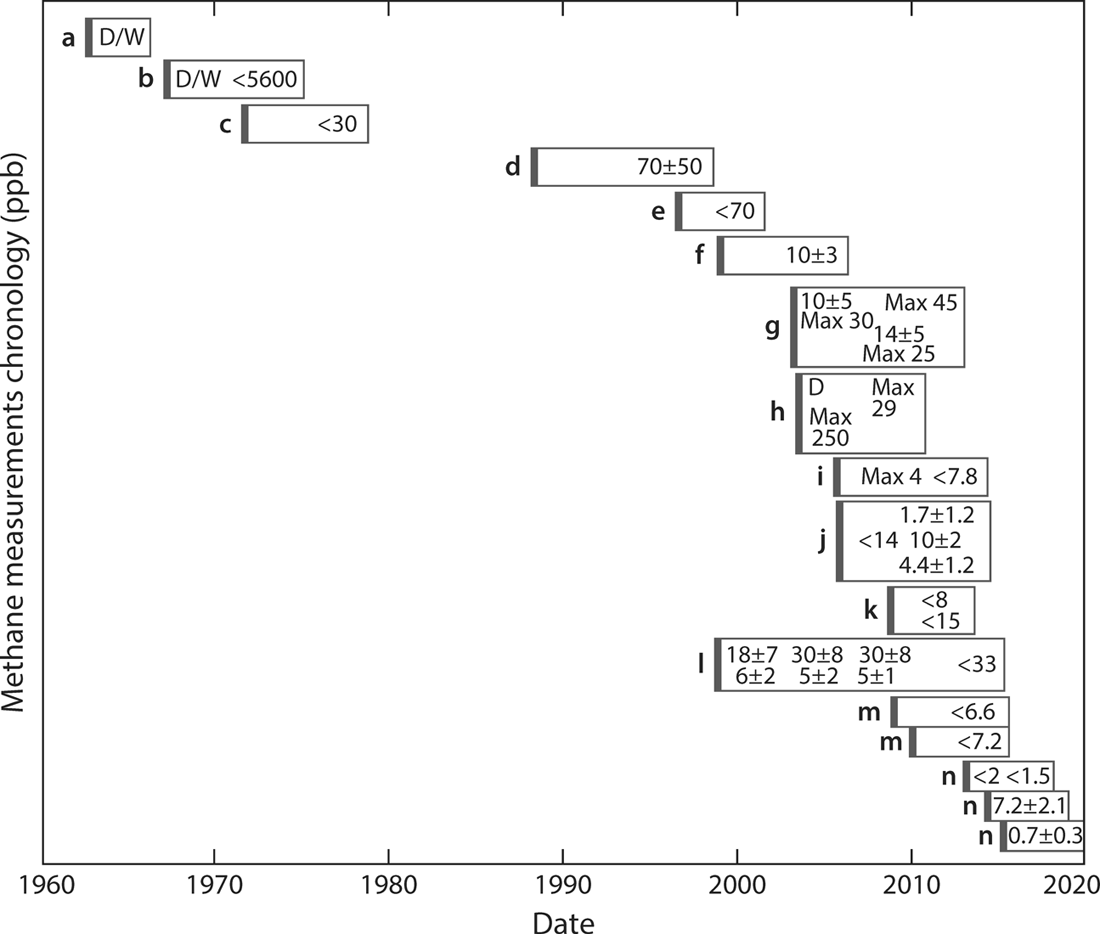
Figure 12.5. A fifty-year chronology of measurements of Martian methane. With one exception (“l”), each box represents one experimental measurement. The left side of each box marks, approximately, the date the measurement was made. The numbers within each box, which indicate the amount of methane reported, are placed approximately at the date(s) publicly reported. When more than one number appears (with the exception of box “l”), that measurement has been reevaluated and the reported answer changed. For example, for “h,” the initial report simply noted a detection, a later report gave quantitative values with the range extending up to 250 parts per billion, and a yet later report reduced the upper limit of the range to 29 parts per billion. A detection with no numerical value reported is noted with a “D.” If that detection was later withdrawn, retracted, or never published, this final conclusion (or lack thereof) is noted with a “W.” All nondetection values (identified with the “<” symbol) are three-sigma upper limits. In box “l,” the first set of numbers was for the Martian summer (higher value) and winter (lower value) of 1998–2000, the second set for 2000–2002, the third set for 2002–2004; the final upper limit was a revision that applies to all three of these sets of numbers. Multiple boxes for the same source (“m” and “n”) indicate the same observing team made measurements on different dates that are reported separately in this plot. Data are presented in this figure for observations discussed in later chapters.
a: Kaplan, Connes, and Connes (data 1964–65; reported 1965–66)
b: Mariner 7 (data 1969; published 1972)
c: Mariner 9 (data 1972; published 1977)
d: Krasnopolsky, Kitt Peak National Observatory (data 1988; published 1997)
e: Infrared Space Observatory (data 1997; published 2000)
f: Krasnopolsky, Canada-France-Hawai’i Telescope (data 1999; published 2004)
g: Mars Express (data 2004; published 2004, 2008, 2011)
h: Mumma, 2003 (data 2003; reported 2004, 2006; published 2009)
i: Mumma, Infrared Telescope Facility (data 2006; published 2009, 2013)
j: Krasnopolsky, Infrared Telescope Facility (data 2006; published 2009, 2012)
k: Krasnopolsky, Infrared Telescope Facility (data 2009; published 2011)
l: Mars Global Surveyor (data 1998–2000, 2000–2002, 2002–2004; published 2010, 2015)
m: Villanueva, multiple telescopes (data 2009, 2010; published 2013)
n: Mars Curiosity Rover (data 2013, early 2014, mid-2014; published 2013, 2015)
In his study, Maguire reported that he was unable to detect any methane. His final result was an upper limit (taken by Maguire to mean a value of twice the noise level) of 20 parts per billion (ppb),17 a result nearly 200 times lower than the two-sigma upper limit of 3,700 parts per billion reported in 1972 as the final answer from the Mariner 7 mission data. This answer means, very simply, that the best we can say, based on the Mariner 9 IRIS measurements, is that we are 95 percent certain that the abundance of methane in the Martian atmosphere is less than 20 methane molecules out of every billion molecules and 99.7 percent certain that methane abundance is below 30 molecules per billion. Some methane might exist in the atmosphere of Mars, but not much.
Methane was merely one of several minor atmospheric constituents for which Maguire obtained upper limits, and he offered no commentary on his methane measurements. Methane in the Martian atmosphere, or the absence thereof, was of virtually no interest to astronomers anymore.
a Pimentel Hall on the UC Berkeley campus is now named in his honor. In addition, on May 15, 2017, the American Chemical Society established this building as a Historic Chemical Landmark because the IRS was created and developed here. (http://www.berkeleyside.com/2017/05/24/mars-work-uc-berkeleys-george-pimentel-recognized-national-historic-landmark/)