Megan Kruse, Leticia Varella, Stephanie Valente, Paulette Lebda, Andrew Vassil, and Jame Abraham
INTRODUCTION
Breast cancer is the most common cancer among women worldwide and it accounts for 25% of all cancer diagnosed among women. It is second only to lung cancer as the leading cause of death from cancer in women in North America. When diagnosed early, breast cancer can be treated primarily using surgery, radiation, and systemic therapy. In Western countries at the time of diagnosis more than 90% of patients will have only localized disease. But many other parts of the world, about 60% of patients will have locally advanced or metastatic disease at the time of diagnosis.
EPIDEMIOLOGY
 In the United States, as per American Cancer Society, in 2017, an estimated 252,170 women and 2,470 men will be diagnosed with breast cancer.
In the United States, as per American Cancer Society, in 2017, an estimated 252,170 women and 2,470 men will be diagnosed with breast cancer.
 In addition, about 63,410 new cases of noninvasive (in situ) breast cancer will be diagnosed in 2017.
In addition, about 63,410 new cases of noninvasive (in situ) breast cancer will be diagnosed in 2017.
 In 2017, 40,610 women and 460 men are expected to die from breast cancer in the United States.
In 2017, 40,610 women and 460 men are expected to die from breast cancer in the United States.
 As per International Agency for Cancer Research (IARC) about 1.7 million women will get a diagnosis of breast cancer worldwide in 2017 and about half a million will die globally from breast cancer.
As per International Agency for Cancer Research (IARC) about 1.7 million women will get a diagnosis of breast cancer worldwide in 2017 and about half a million will die globally from breast cancer.
 A U.S woman’s lifetime risk of developing breast cancer is one in eight, or about 12% will develop breast cancer.
A U.S woman’s lifetime risk of developing breast cancer is one in eight, or about 12% will develop breast cancer.
 There are currently more than 3.1 million breast cancer survivors in the United States in 2017.
There are currently more than 3.1 million breast cancer survivors in the United States in 2017.
RISK FACTORS
The risk factors for developing breast cancer in women are listed in Table 12.1. The etiologies of most breast cancers are unknown and sporadic. About 5% to 10% of breast cancers are familial or hereditary.
TABLE 12.1 Risk Factors for Breast Cancer in Women
Increasing age |
Family history of breast cancer at a young age |
Genetic mutations such as BRCA1 or BRCA2 mutations |
Increased mammographic breast density |
Early menarche |
Late menopause |
Nulliparity |
Older age at first child birth |
Increased body mass index (BMI) |
History of atypical lobular hyperplasia, atypical ductal hyperplasia, lobular carcinoma in situ (LCIS), or flat epithelial atypia |
Prior breast biopsies |
Long-term postmenopausal estrogen and progesterone replacement |
Prior thoracic radiation therapy at age under 30 |
Genetics (For More Details Refer to Chapter 44 on Genetics)
 About 5% to 10% of all women with breast cancer may have a specific mutation in a single gene that is responsible for the breast cancer, with the most common mutations occurring in the BRCA1 or BRCA2 genes. Other genes implicated with breast cancer are PTEN (associated with Cowden syndrome), TP53 (associated with Li-Fraumeni syndrome), CDH1 (associated with hereditary diffuse gastric cancer syndrome), STK11, PALB2, CHEK2, and ATM.
About 5% to 10% of all women with breast cancer may have a specific mutation in a single gene that is responsible for the breast cancer, with the most common mutations occurring in the BRCA1 or BRCA2 genes. Other genes implicated with breast cancer are PTEN (associated with Cowden syndrome), TP53 (associated with Li-Fraumeni syndrome), CDH1 (associated with hereditary diffuse gastric cancer syndrome), STK11, PALB2, CHEK2, and ATM.
 Individuals with these hereditary syndromes may develop cancers early in life or multiple cancers, including bilateral breast cancer.
Individuals with these hereditary syndromes may develop cancers early in life or multiple cancers, including bilateral breast cancer.
 Mutations of BRCA1 (chromosome 17q21) and BRCA2 (chromosome 13q12–13q13) are responsible for 85% of hereditary breast cancer. These genes are involved in DNA repair.
Mutations of BRCA1 (chromosome 17q21) and BRCA2 (chromosome 13q12–13q13) are responsible for 85% of hereditary breast cancer. These genes are involved in DNA repair.
 Specific mutations of BRCA1 and BRCA2 are more common in women of Ashkenazi Jewish ancestry.
Specific mutations of BRCA1 and BRCA2 are more common in women of Ashkenazi Jewish ancestry.
 Overall prevalence of disease-related mutation in BRCA1 has been estimated at 1 in 300, while BRCA2 is 1 in 800.
Overall prevalence of disease-related mutation in BRCA1 has been estimated at 1 in 300, while BRCA2 is 1 in 800.
 The cumulative risk estimates for developing breast cancer by age 80 were 72% for BRCA1 carriers and 69% for BRCA2 carriers.
The cumulative risk estimates for developing breast cancer by age 80 were 72% for BRCA1 carriers and 69% for BRCA2 carriers.
 The cumulative risk of a contralateral breast cancer 20 years after a first breast cancer was 40% for BRCA1 mutation carriers and 26% for BRCA2 mutation carriers.
The cumulative risk of a contralateral breast cancer 20 years after a first breast cancer was 40% for BRCA1 mutation carriers and 26% for BRCA2 mutation carriers.
 BRCA-related breast cancer is more likely to be triple negative particularly in the setting of BRCA1 mutations.
BRCA-related breast cancer is more likely to be triple negative particularly in the setting of BRCA1 mutations.
 The cumulative risk estimates for developing ovarian cancer by age 80 were 44% for BRCA1 mutation carriers and 17% for BRCA2 mutation carriers.
The cumulative risk estimates for developing ovarian cancer by age 80 were 44% for BRCA1 mutation carriers and 17% for BRCA2 mutation carriers.
Indications for Genetic Testing
All patients should have a basic assessment for risk of a hereditary breast/ovarian cancer syndrome including documentation of personal and family history (both paternal and maternal sides) of malignancy. All patients with high risk for a hereditary syndrome based on personal/family history and age at diagnosis should undergo genetic counseling before undergoing the genetic test. The genetic counseling visit is an important step in addressing the patient’s goals of testing and is an opportunity to address misconceptions/limitations of genetic testing. There are three possible outcomes of genetic testing for the BRCA mutations: positive, variant of uncertain significance, or negative. A negative result indicates no increased risk of breast cancer due to a germline mutation. A variant of uncertain significance (indeterminate) test result indicates that no conclusive evidence exists to indicate that the mutation does or does not carry an increased risk of the development of breast cancer due to an inherited genetic mutation. A positive result indicates that there exists a mutation in the patient’s genes that has been associated with an inherited risk of developing breast cancer. In general, patients with a history suggestive of a single inherited cancer syndrome should have testing sent for that specific syndrome.
Multigene testing may be cost-effective and efficient if multiple different inherited cancer syndromes could be considered based on history or if single gene testing is negative in a patient with a compelling personal or family history suggestive of an inherited cancer syndrome. One concern with the multigene testing approach is the increased likelihood of detecting a variant of uncertain significance. This also increases the importance of appropriate genetic counseling in conjunction with genetic testing such that results are interpreted in the appropriate manner.
As per NCCN guidelines (accessed in July 2017), patients with breast cancer and one or more of the following features should undergo further genetic risk evaluation:
 Early-age onset breast cancer (age ≤45)
Early-age onset breast cancer (age ≤45)
 Triple negative breast cancer (ER–, PR–, HER-2/neu-) diagnosed age ≤60
Triple negative breast cancer (ER–, PR–, HER-2/neu-) diagnosed age ≤60
 ≥2 breast primaries (with first diagnosed at age ≤50)
≥2 breast primaries (with first diagnosed at age ≤50)
 Diagnosed at age ≤50 with ≥1 close blood relative (first-, second-, or third-degree relative) with breast cancer at any age, pancreatic cancer, or prostate cancer (Gleason score ≥7)
Diagnosed at age ≤50 with ≥1 close blood relative (first-, second-, or third-degree relative) with breast cancer at any age, pancreatic cancer, or prostate cancer (Gleason score ≥7)
 Diagnosed at any age with ≥1 close blood relative with breast cancer diagnosed at age ≤50
Diagnosed at any age with ≥1 close blood relative with breast cancer diagnosed at age ≤50
 Diagnosed at any age with ≥2 close blood relatives with breast cancer, pancreatic cancer, or prostate cancer (Gleason score ≥7) at any age
Diagnosed at any age with ≥2 close blood relatives with breast cancer, pancreatic cancer, or prostate cancer (Gleason score ≥7) at any age
 Personal history of ovarian cancer or ≥1 close blood relative with ovarian cancer, fallopian tube cancer, or primary peritoneal cancers diagnosed at any age
Personal history of ovarian cancer or ≥1 close blood relative with ovarian cancer, fallopian tube cancer, or primary peritoneal cancers diagnosed at any age
 Ashkenazi Jewish descent
Ashkenazi Jewish descent
 Personal history of male breast cancer or male breast cancer in close blood relative at any age
Personal history of male breast cancer or male breast cancer in close blood relative at any age
Management of Patients with Positive BRCA Test
Management recommendations for patients with a known genetic mutation are highly individualized and should be made by an expert. General recommendations include the following:
 Clinical breast examination every 6 to 12 months, starting at age 25
Clinical breast examination every 6 to 12 months, starting at age 25
 Breast magnetic resonance imaging (MRI) with contrast starting at age 25 or earlier based on family history or mammogram if breast MRI is not available
Breast magnetic resonance imaging (MRI) with contrast starting at age 25 or earlier based on family history or mammogram if breast MRI is not available
 Annual mammogram and annual breast MRI with contrast from age 30 to 75
Annual mammogram and annual breast MRI with contrast from age 30 to 75
 Discuss option of bilateral prophylactic mastectomy on a case-by-case basis, since it could prevent breast cancer in >90% of patients with known BRCA1 or BRCA2 mutation
Discuss option of bilateral prophylactic mastectomy on a case-by-case basis, since it could prevent breast cancer in >90% of patients with known BRCA1 or BRCA2 mutation
 Recommend bilateral salpingo-oophorectomy (BSO) ideally between the ages of 35 and 40 or after completion of child bearing. BSO alone will reduce breast cancer risk by about 50%, but it may vary depending upon the specific genes and prevents ovarian cancer by about 95%.
Recommend bilateral salpingo-oophorectomy (BSO) ideally between the ages of 35 and 40 or after completion of child bearing. BSO alone will reduce breast cancer risk by about 50%, but it may vary depending upon the specific genes and prevents ovarian cancer by about 95%.
 Patients who defer BSO may consider concurrent trans-vaginal ultrasound and blood test such as CA-125, although it is not sufficiently sensitive or specific. This can be done at the discretion of the clinician, starting from the age of 30 and 35 years or 5 to 10 years prior to the earliest age of ovarian cancer in family history.
Patients who defer BSO may consider concurrent trans-vaginal ultrasound and blood test such as CA-125, although it is not sufficiently sensitive or specific. This can be done at the discretion of the clinician, starting from the age of 30 and 35 years or 5 to 10 years prior to the earliest age of ovarian cancer in family history.
CHEMOPREVENTION
Risk Assessment
There are many risk models available to assess a women’s risk for sporadic breast cancer, which accounts for 90% of the breast cancer. One of the most commonly used models is the Gail Risk model (https://www.cancer.gov/bcrisktool). It is a statistical model that calculates a woman’s absolute risk of developing breast cancer by using the following criteria:
1.Age
2.Age at menarche
3.Age at first live birth
4.Number of previous biopsies
5.History of atypical ductal hyperplasia (ADH)
6.Number of first-degree relatives with breast cancer
This model is not intended to be used in patients with an existing history of invasive cancer, DCIS, or lobular carcinoma in situ (LCIS). It underestimates the risk of breast cancer in a person with hereditary breast cancer. It is used in calculating the risk in many breast cancer prevention studies including NSABP-P1 and NSABP-P2.
Prevention Studies
The National Surgical Adjuvant Breast and Bowel Project Breast Cancer Prevention Trial (P-1)
The National Surgical Adjuvant Breast and Bowel Project (NSABP) P-1 study showed a 49% reduction in the incidence of invasive breast cancer in high-risk subjects (based upon the Gail Risk Model) who took tamoxifen at a dose of 20 mg daily for 5 years. Women eligible for this trial were at least 35 years old and were assessed to have an absolute risk of at least 1.66% over the period of 5 years using the Gail model or a pathologic diagnosis of LCIS. Twenty-five percent of woman assigned to tamoxifen in this study discontinued the medication compared to 20% in the placebo group. Notable adverse events associated with tamoxifen therapy in this study include increased risk of endometrial cancer (particularly in women age 50 or older), cataracts, and venous thromboembolism (both deep venous thrombosis and pulmonary embolism). An update of results with 7 years of follow-up was published in 2005 showing a continued statistically significant improvement in rate of invasive breast cancer (risk ratio 0.57) and noninvasive breast cancer (risk ratio 0.63) with tamoxifen compared to placebo.
Use of tamoxifen for breast cancer risk reduction should be considered after weighing the risk benefit ratio for each patient. Women with a life expectancy of ≥10 years and no diagnosis/history of breast cancer who are considered at increased risk of breast cancer should receive individualized counseling to decrease breast cancer risk.
NSABP P-2: Study of Tamoxifen and Raloxifene
In the NSABP P-2 study, tamoxifen 20 mg daily was compared with raloxifene 60 mg daily in postmenopausal women with high risk of developing breast cancer (Gail risk model estimate of 5-year breast cancer risk of at least 1.66%). The results of the study revealed that raloxifene was equivalent to tamoxifen in preventing invasive breast cancer (about a 50% reduction). Raloxifene did not reduce the risk of DCIS or LCIS unlike tamoxifen.
Raloxifene has a better side effect profile, which resulted in a lower incidence of uterine hyperplasia, hysterectomy, cataracts, and a lower rate of thromboembolic events. In postmenopausal patients, due to equal efficacy and better side effect profile, raloxifene 60 mg daily could be used instead of tamoxifen for breast cancer prevention. A 2010 update of the NSABP P-2 study after a median follow-up of nearly 7 years confirmed no statistical difference between invasive breast cancer events in the tamoxifen- and raloxifene-treated patients. In addition, significant reductions in risk of endometrial cancer/hyperplasia as well as thromboembolic events were reported with raloxifene compared to tamoxifen.
Aromatase Inhibitors for Risk Reduction
Aromatase inhibitors were shown to decrease the incidence of contralateral breast cancer when used in the adjuvant setting (ATAC, BIG 1-98). These data led to the investigation of AI as chemoprevention for women at high-risk for developing breast cancer.
The MAP.3 trial evaluated the role of exemestane in a risk reduction setting, randomizing women at increased risk for breast cancer (based on age 60 or older, Gail 5-year risk score of at least 1.66%, prior atypical ductal/lobular hyperplasia or LCIS or DCIS status postmastectomy) to either exemestane or placebo. At a median follow-up of 3 years, it was found that exemestane reduced the relative incidence of breast cancers by 65% when compared to placebo. Exemestane was not associated with any significant serious side effects, although hot flashes and arthritis were very common in both the exemestane and placebo groups. Quality of life was minimally impacted by exemestane use with respect to menopausal symptoms.
In the randomized phase III IBIS II trial, postmenopausal women at increased risk of breast cancer (defined as significant family history, history of atypical hyperplasia, or LCIS, nulliparity or age at first birth of ≥ 30) were randomized to receive anastrozole or placebo for 5 years. Results showed a reduction in the risk of developing breast cancer (both invasive and noninvasive) of more than 50% (HR of 0.47) with use of anastrozole compared to placebo. Musculoskeletal events and vasomotor symptoms were significantly more common in patients receiving anastrozole rather than placebo.
In premenopausal women with increased risk of breast cancer as per the Gail risk model, it is reasonable to recommend tamoxifen 20 mg daily for 5 years. In postmenopausal women raloxifene and tamoxifen are equally effective, but raloxifene has been shown to have less side effects. Aromatase inhibitors can also be considered given the data from the MAP.3 and IBIS II trials; however, the FDA has not approved aromatase inhibitors in this setting. Any risk reduction approach should be carefully decided after a detailed risk versus benefit discussion with the patient.
BREAST CANCER SCREENING
Screening Mammograms
 Screening mammography has been shown to decrease breast cancer mortality in women between the ages of 40 and 70 years with an absolute mortality benefit of 1% for women screened annually for 10 years.
Screening mammography has been shown to decrease breast cancer mortality in women between the ages of 40 and 70 years with an absolute mortality benefit of 1% for women screened annually for 10 years.
 Potential harms associated with screening mammography include overdiagnosis and treatment of cancers that would otherwise have been clinically insignificant in a woman’s lifetime as well as the unnecessary anxiety and additional testing that is associated with false positive screening examination.
Potential harms associated with screening mammography include overdiagnosis and treatment of cancers that would otherwise have been clinically insignificant in a woman’s lifetime as well as the unnecessary anxiety and additional testing that is associated with false positive screening examination.
 The American Cancer Society recommends that women ages 40 to 44 should have the choice to start annual mammography screening and women ages 45 to 54 should receive annual mammograms. At age 55, the American Cancer Society suggests that women may switch to having mammograms every other year for breast cancer screening although annual screening may be continued if the patient desires.
The American Cancer Society recommends that women ages 40 to 44 should have the choice to start annual mammography screening and women ages 45 to 54 should receive annual mammograms. At age 55, the American Cancer Society suggests that women may switch to having mammograms every other year for breast cancer screening although annual screening may be continued if the patient desires.
 Women who are at higher than average risk of breast cancer (women with a family history of breast cancer, women with either the BRCA1 or the BRCA2 gene, women with a history of chest irradiation between the ages of 10 and 30, or women with a lifetime risk of breast cancer ≥20%) are recommended to initiate screening mammograms at age 25 to 30 or 10 years earlier than the age of the affected first-degree relative at diagnosis (whichever is later) or 8 years after radiation therapy, as per the American College of Radiology guidelines.
Women who are at higher than average risk of breast cancer (women with a family history of breast cancer, women with either the BRCA1 or the BRCA2 gene, women with a history of chest irradiation between the ages of 10 and 30, or women with a lifetime risk of breast cancer ≥20%) are recommended to initiate screening mammograms at age 25 to 30 or 10 years earlier than the age of the affected first-degree relative at diagnosis (whichever is later) or 8 years after radiation therapy, as per the American College of Radiology guidelines.
 Mammograms should be continued regardless of a woman’s age, as long as she is in good health with an expected life expectancy of at least 10 years. Age alone should not be the reason to stop having regular mammograms. Women with serious health problems or short life expectancies should discuss with their doctors whether to continue having mammograms.
Mammograms should be continued regardless of a woman’s age, as long as she is in good health with an expected life expectancy of at least 10 years. Age alone should not be the reason to stop having regular mammograms. Women with serious health problems or short life expectancies should discuss with their doctors whether to continue having mammograms.
The diagnostic superiority of digital mammography was demonstrated in the Digital Mammographic Imaging Screening Trial (DMIST) published in 2005. This study concluded that the overall accuracy of digital and film mammography was similar however in pre- or peri-menopausal women under the age of 50 or women at any age with dense breasts, digital mammography more accurately detected of breast cancer.
Digital breast tomosynthesis (DBT), commonly referred to as 3-D mammography, is an x-ray technique that uses a finite number of low-dose projections to reconstruct a series of thin-section images of the breast. The STORM (screening with tomosynthesis or standard mammography) trial was a large prospective Italian study that compared conventional screening digital mammography to combined digital mammography and DBT for breast cancer screening in an average risk population. The study demonstrated that detection of breast cancer significantly increased with the addition of DBT to conventional digital mammography. A 2014 study from Friedewald et al. also demonstrated that this technology can decrease the rate of recall for benign findings. As per discussion by Houssami and Skanne, it is important to note that integrated use of 2D mammography and DBT nearly doubles the overall radiation exposure, so careful consideration must be given of how to incorporate DBT to usual 2D screening. However, some DBT vendors have introduced synthetic 2D views which when used in place of conventional 2D images reduces the radiation dose of DBT combination examination.
While breast MRI has been shown to have a higher sensitivity than mammography, the specificity of breast MRI is lower, which can result in more false positives and therefore more biopsies. Patients need to be carefully selected for additional screening with breast MRI. In a high-risk population, the sensitivity of mammography when combined with MRI (92.7%) is higher than the sensitivity of mammography when combined with ultrasound (52%). Therefore, in women in whom supplemental screening is indicated, MRI is recommended when possible. According to the American Cancer Society recommendations, breast MRI can be used as an adjunct to screening mammography in high-risk women, specifically those with BRCA gene mutations (along with their untested first-degree relatives), those who received chest radiation between the ages of 10 and 30, and those whose lifetime risk of breast cancer exceeds 20%.
CLINICAL FEATURES OF BREAST CANCER
Clinical features may include a breast lump, skin thickening or alteration, peau d’orange, dimpling of the skin, nipple inversion or crusting (Paget disease), unilateral nipple discharge, and new onset pain. Patients may instead present with signs and symptoms of metastatic disease.
DIAGNOSIS
1.History and physical examination
2.Bilateral mammogram (80% to 90% accuracy)
3.Biopsy: Any distinct mass should be considered for a biopsy, even if the mammograms are negative
The standard method of diagnosis for palpable lesions is
 Core-needle biopsy
Core-needle biopsy
The options in nonpalpable breast lesions are
 Ultrasound-guided core-needle biopsy
Ultrasound-guided core-needle biopsy
 Stereotactic core-needle biopsy under mammographic localization
Stereotactic core-needle biopsy under mammographic localization
 Needle localization under mammography, followed by surgical excision
Needle localization under mammography, followed by surgical excision
 MRI-guided biopsy
MRI-guided biopsy
4.Laboratory studies
 Complete blood count, liver function tests, and alkaline phosphatase level can be considered depending upon the history and physical.
Complete blood count, liver function tests, and alkaline phosphatase level can be considered depending upon the history and physical.
 Routine use of breast cancer markers such as CA 27:29 and CA 15:3 is not recommended.
Routine use of breast cancer markers such as CA 27:29 and CA 15:3 is not recommended.
5.Pathology and special studies
 Histology and diagnosis (invasive vs. in situ)
Histology and diagnosis (invasive vs. in situ)
 Pathologic grade of the tumor
Pathologic grade of the tumor
 Tumor involvement of the margin
Tumor involvement of the margin
 Tumor size
Tumor size
 Lymphovascular invasion
Lymphovascular invasion
6.Estrogen receptor/progesterone receptor (ER/PR) status should be done in all tumors (both invasive and noninvasive) and biopsies of metastatic or recurrent (patients those who relapsed) lesions.
 As per the ASCO/CAP guidelines (2010), ER/PR is considered as positive if ≥1% of tumor cell nuclei are immunoreactive.
As per the ASCO/CAP guidelines (2010), ER/PR is considered as positive if ≥1% of tumor cell nuclei are immunoreactive.
7.HER-2/neu- testing (as per ASCO/CAP Guidelines 2013)
 Positive for HER-2/neu- is either IHC 3 + (defined as uniform intense membrane staining of more than 30% of invasive tumor cells) or FISH amplified (ratio of HER-2/neu- to CEP17 of ≥2.0 or average HER-2/neu- gene copy number ≥6 signals/nucleus for those test systems without an internal control probe).
Positive for HER-2/neu- is either IHC 3 + (defined as uniform intense membrane staining of more than 30% of invasive tumor cells) or FISH amplified (ratio of HER-2/neu- to CEP17 of ≥2.0 or average HER-2/neu- gene copy number ≥6 signals/nucleus for those test systems without an internal control probe).
 Equivocal for HER-2/neu- is defined as either IHC 2 + or FISH ratio of <2 and average HER-2/neu- gene copy number of ≥ 4.0 and < 6.0 signals/nucleus for test systems without an internal control probe.
Equivocal for HER-2/neu- is defined as either IHC 2 + or FISH ratio of <2 and average HER-2/neu- gene copy number of ≥ 4.0 and < 6.0 signals/nucleus for test systems without an internal control probe.
 Negative for HER-2/neu- is defined as either IHC 0–1 + or FISH ratio < 2 with an average HER-2/neu- gene copy number < 4 signals/nucleus for test systems without an internal control probe.
Negative for HER-2/neu- is defined as either IHC 0–1 + or FISH ratio < 2 with an average HER-2/neu- gene copy number < 4 signals/nucleus for test systems without an internal control probe.
8.Indices of proliferation (e.g., mitotic index, Ki-67, or S phase) can be helpful. Ki-67 can be helpful in distinguishing luminal A versus B in ER/PR-positive lesions. Lack of standardization of Ki-67 testing limits its wide utilization in clinical practice.
9.Radiographic studies are performed on the basis of the findings of the history and physical examination, diagnostic breast imaging and blood tests. Appropriate imaging studies such as CT scan, ultrasound, MRI, or CT/PET scan can be considered as per the clinical indications. They are not routinely recommended for all patients.
 As per the American Society of Clinical Oncology “Choosing Wisely” guidelines, it is not recommended that patients with DCIS or clinical stage I/ II disease receive staging PET, CT, or radionucleotide bone scan as there is no clear evidence indicating benefit, and unnecessary imaging can lead to unnecessary invasive procedures/radiation exposure, overtreatment or misdiagnosis.
As per the American Society of Clinical Oncology “Choosing Wisely” guidelines, it is not recommended that patients with DCIS or clinical stage I/ II disease receive staging PET, CT, or radionucleotide bone scan as there is no clear evidence indicating benefit, and unnecessary imaging can lead to unnecessary invasive procedures/radiation exposure, overtreatment or misdiagnosis.
 The NCCN guidelines recommend that systemic imaging be considered in patients with locally advanced (Stage III) patients and in those with signs or symptoms suggestive of metastatic disease.
The NCCN guidelines recommend that systemic imaging be considered in patients with locally advanced (Stage III) patients and in those with signs or symptoms suggestive of metastatic disease.
10.Breast MRI may be helpful in determining the extent of disease and to facilitate surgical planning in the following patients (as per NCCN guidelines):
 Those with heterogenous and extremely dense mammographic tissue
Those with heterogenous and extremely dense mammographic tissue
 Those with newly diagnosed invasive lobular carcinoma
Those with newly diagnosed invasive lobular carcinoma
 Those with axillary nodal metastasis with unknown primary
Those with axillary nodal metastasis with unknown primary
 Those who are candidates for neoadjuvant chemotherapy and as part of monitoring response to neoadjuvant therapy
Those who are candidates for neoadjuvant chemotherapy and as part of monitoring response to neoadjuvant therapy
 Evaluating the extent of disease in known cancer patients
Evaluating the extent of disease in known cancer patients
•Multifocal and multicentric disease
•Pectoralis and chest wall involvement
 Postlumpectomy patients to evaluate residual disease (close or positive margins)
Postlumpectomy patients to evaluate residual disease (close or positive margins)
 Suspected recurrence of breast cancer
Suspected recurrence of breast cancer
•Inconclusive mammographic/clinical findings
•Reconstruction with tissue flaps or implants
 Lesion characterization
Lesion characterization
 Inconclusive findings on mammogram, ultrasound, and physical examination
Inconclusive findings on mammogram, ultrasound, and physical examination
PATHOLOGY
Infiltrating or invasive ductal cancer is the most common breast cancer histologic type and comprises 70% to 80% of all cases (Table 12.2).
TABLE 12.2 Pathologic Classification of Breast Cancer
Ductal |
Intraductal (in situ) |
Invasive with predominant intraductal component |
Invasive, NOS |
Comedo |
Inflammatory |
Medullary with lymphocytic infiltrate |
Mucinous (colloid) |
Papillary |
Scirrhous |
Tubular |
Other |
Other |
Undifferentiated |
Lobular |
In situ |
Invasive with predominant in situ component |
Invasive |
Nipple |
Paget disease, NOS |
Paget disease with intraductal carcinoma |
Paget disease with invasive ductal carcinoma |
Other types (not typical breast cancer) |
Phyllodes tumor |
Angiosarcoma |
Primary lymphoma |
NOS, not otherwise specified.
STAGING OF BREAST CANCER
For staging of breast cancer the American Joint Committee on Cancer (AJCC) manual, eighth edition, should be followed. This edition of the AJCC manual includes separate anatomic and prognostic staging group systems for breast cancer, reflecting the importance of biomarkers in breast cancer prognosis and treatment decisions. These biomarkers provide a sense of tumor biology. The traditional anatomic stage groups (including only the “TNM” or tumor size, nodal status, and metastasis categories) should now only be used in regions of the world where biomarker tests are not routinely available. The new eighth edition AJCC prognostic staging is the standard for cancer registries in the United States moving forward. This prognostic group staging system includes the TNM categories in addition to
1.Histologic grade
2.HER2 status
3.ER status
4.PR status
5.Oncotype Dx recurrence score (for certain TNM groups only)
Key stage changes that have occurred as a result of the eighth edition of the AJCC staging are summarized in Table 12.3.
Table 12.3Key Stage Changes in AJCC Eighth Edition Breast Cancer Staging
Anatomic TNM Staging | Relevant Biomarkers in AJCC Eighth Edition | AJCC Seventh Edition Stage Group | AJCC Eighth Edition Prognostic Stage Group |
T2N0M0 | Grades 1–3 HER2 negative ER positive PR any Oncotype Dx Recurrence score <11 | IIA | IA |
T2N1M0 | Grade 1 HER2 negative ER positive PR positive | IIB | IB |
T2N1M0 | Grade 2 HER2 positive ER positive PR positive | IIB | IB |
T0-2N2M0 | Grades 1–2 HER-2 positive ER positive PR positive | IIIA | IB |
T3N1-2M0 | Grades 1–2 HER2 positive ER positive PR positive | IIIA | IB |
T1N0M0 | Grades 1–3 HER2 negative ER negative PR negative | IA | IIA |
T1N0M0 | Grade 3 HER2 negative ER positive PR negative | IA | IIA |
T1N0M0 | Grade 3 HER2 negative ER negative PR positive | IA | IIA |
T0-2N2 | Grade 1 HER2 negative ER positive PR positive | IIIA | IIA |
T0-1N1M0 | Grade 2 HER2 negative ER negative PR negative | IIA | IIIA |
T2N0M0 | Grade 2 HER2 negative ER negative PR negative | IIA | IIIA |
Grade 3 HER2 negative ER positive PR negative | IIA | IIIA | |
T2N0M0 | Grade 3 HER2 negative ER negative PR any | IIA | IIIA |
T1-4N3M0 | Grade 1 HER2 negative ER positive PR positive | IIIC | IIIA |
T2N1M0 | Grades 1–2 HER2 negative ER negative PR negative | IIB | IIIB |
T2N1M0 | Grade 3 HER2 negative ER positive PR negative | IIB | IIIB |
T2N1M0 | Grade 3 HER2 negative ER negative PR any | IIB | IIIC |
T0-2N2M0 | Grades 2–3 HER2 negative AND ER/PR negative OR ER positive/PR negative OR ER negative/PR any | IIIA | IIIC |
T3N1-2M0 | Grades 2–3 HER2 negative AND ER/PR negative OR ER positive/PR negative OR ER negative/PR any | IIIA | IIIC |
Prognostic Factors
Anatomic features such as tumor size and lymph node status are important prognostic features. But biologic features of the tumor are equally important or possibly even more important than anatomic features.
1.Number of positive axillary lymph nodes
•This is an important prognostic indicator. Prognosis is worse with increasing number of lymph nodes.
2.Tumor size
•In general, tumors smaller than 1 cm have a good prognosis in patients without lymph node involvement.
3.Histologic or nuclear grade
•Patients with poorly differentiated histology and high nuclear grade have a worse prognosis than others.
•Scarff-Bloom-Richardson grading system and Fisher nuclear grade are commonly used systems. The modified Scarff-Bloom-Richardson grading system assigns a score (1 to 3 points) for features such as size, mitosis, and tubule formation. These scores are added and tumors are labeled low grade (3 to 5 points), intermediate grade (6 to 7 points), or high grade (8 to 9 points).
4.ER/PR status
•ER- and/or PR-positive tumors have better prognosis and these patients are eligible to receive endocrine therapy.
5.Histologic tumor type
•Prognoses of infiltrating ductal and lobular carcinoma are similar.
•Mucinous (colloid) and tubular histologies have better prognosis.
•Inflammatory breast cancer is one of the most aggressive forms of breast cancer.
6.HER-2/neu expression
•HER-2/neu overexpression is a poor prognostic marker and patients with HER-2/neu overexpression are candidates for HER-2/neu-targeted therapies. Availability of effective HER-2/neu-targeted therapies has revolutionized the treatment and outcome of HER-2/neu-positive breast cancer. Because of targeted therapies, for all practical purposes, HER-2/neu positivity can be considered as a good prognostic feature now.
7.Gene expression profiles
•Oncotype DX is a diagnostic genomic assay based on reverse transcription polymerase chain reaction (RT-PCR) on paraffin-embedded tissue (Fig. 12.1). This assay was initially developed to quantify the likelihood of cancer recurrence in women with newly diagnosed, stage I or II, node-negative, ER-positive breast cancer. Patients are divided into low-risk, intermediate-risk, and high-risk groups on the basis of the expression of a panel of 21 genes. The recurrence score determined by this assay is found to be a better predictor of outcome than standard measures such as age, tumor size, and tumor grade.
•The TAILORx study demonstrated excellent overall survival (OS), freedom from recurrence and invasive disease-free survival (DFS) at 5 years in “low risk” patients defined as those with a recurrence score of 0 to 10, all of whom received endocrine therapy only. Patients with recurrence score of 11 to 25 were randomly assigned to receive chemotherapy plus endocrine therapy or endocrine therapy alone, and outcome data on these patients is awaited. Studies have validated the role of Oncotype DX patients with ER-positive node-positive tumors and it can be used in selected settings. Oncotype DX testing has also been studied in DCIS where the resulting “DCIS score” quantifies the ipsilateral breast event risk for both invasive and noninvasive disease after surgical excision without radiation.

FIGURE 12.1 Oncotype DX assay.
•MammaPrint is a DNA microarray assay of 70 genes designed to predict the risk of recurrence of early-stage breast cancer. This testing classifies patients as low risk or high risk. There is no “intermediate” group as there is with the Oncotype. In February 2007, the FDA approved the use of MammaPrint in patients less than the age of 61, with a tumor size less than 5 cm and lymph node negative. The MINDACT study, which was published in 2016, was done to prospectively assess the clinical utility of the MammaPrint in selecting early-stage patients with up to 3 axillary lymph nodes involved for adjuvant chemotherapy. The patients in this study had both genomic and clinical risk defined and those with discordant results (meaning low genomic risk /high clinical risk or high genomic risk/low clinical risk) were randomized to either receive chemotherapy or not. The primary endpoint of the study was survival without distant metastases in patients with high-risk clinical features and low-risk genomic features. It was found that 5-year metastasis-free survival in these patients was similar whether or not chemotherapy was given (absolute difference 1.5%).
An update to the ASCO clinical guidelines regarding use of MammaPrint was released in July 2017 stating that use of the MammaPrint can be considered to assist in decisions regarding adjuvant chemotherapy in ER-positive or PR-positive, HER2-negative, N0 or N1 breast cancer patients with high clinical risk of recurrence as described in the MINDACT study. For those who have low clinical risk of recurrence, use of MammaPrint is not recommended.
 Other genomic assays available for decision making in early breast cancer include the Breast Cancer Index, EndoPredict, PAM50 risk of recurrence score, Mammostrat and Urokinase plasminogen activator, and plasminogen activator inhibitor type 1. Each of these tests is intended to help clinicians identify patients with hormone receptor–positive, HER2-negative early-stage breast cancer who have a low risk of distant recurrence. This information can then be used to aide in making decisions regarding adjuvant systemic therapy.
Other genomic assays available for decision making in early breast cancer include the Breast Cancer Index, EndoPredict, PAM50 risk of recurrence score, Mammostrat and Urokinase plasminogen activator, and plasminogen activator inhibitor type 1. Each of these tests is intended to help clinicians identify patients with hormone receptor–positive, HER2-negative early-stage breast cancer who have a low risk of distant recurrence. This information can then be used to aide in making decisions regarding adjuvant systemic therapy.
 As per the 2016 ASCO clinical practice guideline on “use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer,” there is intermediate quality evidence for use of EndoPredict and Breast Cancer Index in ER/PR-positive, HER2-negative patients with node negative breast cancer to guide decisions on adjuvant systemic therapy. For the PAM50 risk of recurrence score, the evidence is considered high quality and recommendation for use in the above setting is strong.
As per the 2016 ASCO clinical practice guideline on “use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer,” there is intermediate quality evidence for use of EndoPredict and Breast Cancer Index in ER/PR-positive, HER2-negative patients with node negative breast cancer to guide decisions on adjuvant systemic therapy. For the PAM50 risk of recurrence score, the evidence is considered high quality and recommendation for use in the above setting is strong.
Genomic Subtypes of Breast Cancer
Several distinct types of breast cancer are identified by gene expression studies. They differ markedly in prognosis and in the therapeutic targets they express (Table 12.4). The 5 main subtypes, known as the “intrinsic subtypes of breast cancer,” are described here:
TABLE 12.4 Systemic Treatment Recommendations Based upon Subtypes
Luminal A | Endocrine therapy alone |
Luminal B (HER-2/neu-negative) | Endocrine +/− Chemo |
Luminal B (HER-2/neu-positive) | Chemo + anti-HER-2/neu-drugs endocrine therapy |
HER-2/neu-positive (nonluminal) | Chemo + anti-HER-2/neu-drugs |
Triple negative | Chemotherapy |
Special Biologic Subtypes |
|
Endocrine responsive (cribriform, tubular, and mucinous) | Endocrine therapy |
Endocrine nonresponsive (medullary, adenoid, and metaplastic) | Chemotherapy |
 Luminal A and B subtypes: Luminal A and luminal B subtypes express genes associated with luminal epithelial cells of normal breast tissue and overlap with ER-positive breast cancers defined by clinical assays. The luminal A subtype amounts to about 40% to 50% of cancers and has the best prognosis. These tumors are generally ER/PR-positive and HER-2 negative. Approximately 20% of breast cancers are of luminal B subtype, and they have worse prognosis compared to luminal A. The luminal B subtype tends to include tumors that are ER or PR positive and HER2-negative as well as those that are ER, PR, and HER2-positive. Luminal B cancers also tend to be higher grade tumors compared to luminal A cancers. Luminal A cancers are generally responsive to endocrine therapy while luminal B tumors may benefit from a combined approach including chemotherapy and endocrine therapy.
Luminal A and B subtypes: Luminal A and luminal B subtypes express genes associated with luminal epithelial cells of normal breast tissue and overlap with ER-positive breast cancers defined by clinical assays. The luminal A subtype amounts to about 40% to 50% of cancers and has the best prognosis. These tumors are generally ER/PR-positive and HER-2 negative. Approximately 20% of breast cancers are of luminal B subtype, and they have worse prognosis compared to luminal A. The luminal B subtype tends to include tumors that are ER or PR positive and HER2-negative as well as those that are ER, PR, and HER2-positive. Luminal B cancers also tend to be higher grade tumors compared to luminal A cancers. Luminal A cancers are generally responsive to endocrine therapy while luminal B tumors may benefit from a combined approach including chemotherapy and endocrine therapy.
 HER-2-enriched subtype: The HER-2-enriched subtype comprises the majority of clinically HER-2/neu-positive breast cancers. It accounts for 10% to 15% of breast cancers. Not all HER-2/neu-positive tumors are HER-2/neu-enriched. About half of clinical HER-2/neu-positive breast cancers are HER-2/neu- enriched; the other half can include any molecular subtype including HER-2/neu-positive luminal subtypes. Those tumors that are ER/PR-negative and grade 3 tend to fall into the HER2-enriched subtype.
HER-2-enriched subtype: The HER-2-enriched subtype comprises the majority of clinically HER-2/neu-positive breast cancers. It accounts for 10% to 15% of breast cancers. Not all HER-2/neu-positive tumors are HER-2/neu-enriched. About half of clinical HER-2/neu-positive breast cancers are HER-2/neu- enriched; the other half can include any molecular subtype including HER-2/neu-positive luminal subtypes. Those tumors that are ER/PR-negative and grade 3 tend to fall into the HER2-enriched subtype.
 Basal-like subtype: These tumors are usually ER-negative and characterized by low expression of hormone receptor–related genes. Up to 90% of triple negative breast cancers (those that are ER-negative, PR-negative, and HER2-negative) are classified in the basal-like subtype. They have a more aggressive clinical course with higher risk of relapse and derive benefit from chemotherapy.
Basal-like subtype: These tumors are usually ER-negative and characterized by low expression of hormone receptor–related genes. Up to 90% of triple negative breast cancers (those that are ER-negative, PR-negative, and HER2-negative) are classified in the basal-like subtype. They have a more aggressive clinical course with higher risk of relapse and derive benefit from chemotherapy.
 Normal-like subtype: This subtype is represented in a minority of breast cancers and is similar in biomarker profile to luminal A tumors but with a gene profile more consistent with normal breast tissue rather than a luminal A tumor.
Normal-like subtype: This subtype is represented in a minority of breast cancers and is similar in biomarker profile to luminal A tumors but with a gene profile more consistent with normal breast tissue rather than a luminal A tumor.
MANAGEMENT
High-Risk Lesions
Patients with high-risk lesions may be eligible for breast cancer prevention studies. Tamoxifen and raloxifene are two FDA-approved drugs for breast cancer prevention in high-risk settings. As per the MAP.3 study exemestane was found to be effective in breast cancer prevention; however, the drug is not FDA approved for this indication. For breast cancer prevention, in premenopausal patients, tamoxifen is the drug of choice, but in postmenopausal patients, raloxifene or aromatase inhibitors can be used.
Atypical Ductal Hyperplasia (ADH)
 There is a 4- to 5-fold increase in the risk of developing breast cancer in patients with ADH.
There is a 4- to 5-fold increase in the risk of developing breast cancer in patients with ADH.
 There is wide variation in the criteria used in the diagnosis of ADH.
There is wide variation in the criteria used in the diagnosis of ADH.
 If diagnosis is made with core-needle biopsy, the presence of invasive cancer may be missed due to sampling error. As a result, surgical excision of the site of ADH is recommended.
If diagnosis is made with core-needle biopsy, the presence of invasive cancer may be missed due to sampling error. As a result, surgical excision of the site of ADH is recommended.
 Fifteen percent to 30% of cases may be “upgraded” to diagnosis of invasive cancer.
Fifteen percent to 30% of cases may be “upgraded” to diagnosis of invasive cancer.
 Clinical breast examination and mammogram are the preferred screening methods, role of MRI is under investigation.
Clinical breast examination and mammogram are the preferred screening methods, role of MRI is under investigation.
 Tamoxifen 20 mg PO for 5 years: The NSABP P-1 study showed 86% reduction in the risk of developing invasive breast cancer in patients who received tamoxifen.
Tamoxifen 20 mg PO for 5 years: The NSABP P-1 study showed 86% reduction in the risk of developing invasive breast cancer in patients who received tamoxifen.
 The NSABP P-2 study showed similar efficacy for raloxifene 60 mg daily for 5 years, but with fewer adverse effects. Hence, in postmenopausal patients, raloxifene could be considered as the preferred treatment option.
The NSABP P-2 study showed similar efficacy for raloxifene 60 mg daily for 5 years, but with fewer adverse effects. Hence, in postmenopausal patients, raloxifene could be considered as the preferred treatment option.
 LCIS is not considered a form of cancer, but rather a benign lesion that indicates an increased risk of developing invasive breast cancer. In the eighth edition of the AJCC staging system, Tis(LCIS) has been eliminated reflecting the nonmalignant nature of these lesions.
LCIS is not considered a form of cancer, but rather a benign lesion that indicates an increased risk of developing invasive breast cancer. In the eighth edition of the AJCC staging system, Tis(LCIS) has been eliminated reflecting the nonmalignant nature of these lesions.
There is a 24% chance of developing breast cancer in patients within 10 years of developing LCIS.
 Classical LCIS is not managed with surgery; it is managed with close clinical follow-up. If the LCIS is pleomorphic or has necrosis, excision with negative margins can be considered.
Classical LCIS is not managed with surgery; it is managed with close clinical follow-up. If the LCIS is pleomorphic or has necrosis, excision with negative margins can be considered.
 Patients with classical LCIS can be followed up by clinical breast examination every 4 to 12 months and annual mammogram. As per the American Cancer Society, there is insufficient data to recommend regular breast MRIs for all patients with LCIS although this can be considered on an individual basis.
Patients with classical LCIS can be followed up by clinical breast examination every 4 to 12 months and annual mammogram. As per the American Cancer Society, there is insufficient data to recommend regular breast MRIs for all patients with LCIS although this can be considered on an individual basis.
 Tamoxifen or raloxifene (postmenopausal) may be used for prevention of breast cancer (56% reduction in risk as per the NSABP P-1 and P-2 studies).
Tamoxifen or raloxifene (postmenopausal) may be used for prevention of breast cancer (56% reduction in risk as per the NSABP P-1 and P-2 studies).
Noninvasive Breast Cancer
 The extensive use of mammograms has led to the increasing diagnosis of ductal carcinoma in situ (DCIS)
The extensive use of mammograms has led to the increasing diagnosis of ductal carcinoma in situ (DCIS)
 Microcalcification or soft tissue abnormality is seen in the mammogram of DCIS.
Microcalcification or soft tissue abnormality is seen in the mammogram of DCIS.
 DCIS is considered a precursor lesion for invasive breast cancer
DCIS is considered a precursor lesion for invasive breast cancer
 Comedonecrosis and high nuclear grade have been associated with shorter time to recurrence but do not predict higher overall recurrence rates
Comedonecrosis and high nuclear grade have been associated with shorter time to recurrence but do not predict higher overall recurrence rates
In patients with ER positive DCIS, lumpectomy followed by radiation treatment followed by endocrine therapy for 5 years can be considered as the standard treatment approach.
 Based upon NSABP B-24, in premenopausal women with ER positive DCIS treated with lumpectomy, tamoxifen 20 mg daily for 5 years reduced the risk of breast cancer recurrence (ipsilateral and contralateral)
Based upon NSABP B-24, in premenopausal women with ER positive DCIS treated with lumpectomy, tamoxifen 20 mg daily for 5 years reduced the risk of breast cancer recurrence (ipsilateral and contralateral)
 Based upon NSABP B-35, in postmenopausal women with ER positive DCIS, anastrozole 1 mg daily resulted in improvement in breast cancer–free interval for women < 60 years. Based on this data, aromatase inhibitors can be used in postmenopausal patients with ER positive DCIS after lumpectomy.
Based upon NSABP B-35, in postmenopausal women with ER positive DCIS, anastrozole 1 mg daily resulted in improvement in breast cancer–free interval for women < 60 years. Based on this data, aromatase inhibitors can be used in postmenopausal patients with ER positive DCIS after lumpectomy.
 Mastectomy with or without lymph node evaluation can also be considered as a treatment option. In patients who undergo mastectomy, the role of endocrine therapy is limited. In selected patients, endocrine therapy can be considered for contralateral breast cancer prevention.
Mastectomy with or without lymph node evaluation can also be considered as a treatment option. In patients who undergo mastectomy, the role of endocrine therapy is limited. In selected patients, endocrine therapy can be considered for contralateral breast cancer prevention.
 Axillary lymph node evaluation is not recommended in pure DCIS without evidence of invasive cancer. In patients with DCIS on biopsy who are treated with mastectomy, a sentinel lymph node evaluation can be considered at the time of initial surgery as a future sentinel node evaluation would not be possible if invasive disease is found on final surgical pathology.
Axillary lymph node evaluation is not recommended in pure DCIS without evidence of invasive cancer. In patients with DCIS on biopsy who are treated with mastectomy, a sentinel lymph node evaluation can be considered at the time of initial surgery as a future sentinel node evaluation would not be possible if invasive disease is found on final surgical pathology.
 NSABP B-43: study of trastuzumab in HER-2/neu-positive DCIS has completed and waiting for the results.
NSABP B-43: study of trastuzumab in HER-2/neu-positive DCIS has completed and waiting for the results.
Invasive Breast Cancer
A multidisciplinary team should manage breast cancer, with the input from a radiologist, pathologist, breast surgeon, reconstructive surgeon, medical oncologist, and radiation oncologist. Other key members of the multidisciplinary team should include genetic counselors, psychologists, social workers, nurses, and navigators.
After the diagnosis of breast cancer with a core-needle biopsy or fine-needle aspiration cytology, it is important to confirm the histology, prognostic markers, and receptors. Various treatment options should then be discussed with the patient before the treatment plan is finalized.
There are two components to surgical management of breast cancer: removal of the breast cancer and evaluation of lymph nodes.
Patients with DCIS or invasive cancer have two options for removing breast cancer, mastectomy, or lumpectomy. As per NSABP B-06 and EORTC 10801, 20 year local recurrence for mastectomy is 5%, for lumpectomy alone 40%, and lumpectomy with radiation is 14%. Despite these differences in local recurrence, there is no survival difference seen in patients who are treated with mastectomy versus lumpectomy and radiation therapy (breast conservation therapy [BCT]) and therefore both are offered as treatment options. Additionally, for those electing for BCT, adjuvant breast radiation is recommended.
In some cases, despite a desire for BCT, a mastectomy may be recommended. These include a contraindication to receiving radiation, multicentric disease, inflammatory breast cancer, or a large tumor in a small breast where resection would leave a cosmetically unpleasing result.
As per NCCN guidelines, contraindications for breast-conserving therapy requiring radiation therapy include
 Radiation therapy during pregnancy
Radiation therapy during pregnancy
 Widespread disease or calcifications that cannot be incorporated by breast conservation that achieves negative margins with a satisfactory cosmetic result
Widespread disease or calcifications that cannot be incorporated by breast conservation that achieves negative margins with a satisfactory cosmetic result
 Positive pathologic margin (no ink on tumor for invasive cancer, 2 mm margin for DCIS)
Positive pathologic margin (no ink on tumor for invasive cancer, 2 mm margin for DCIS)
 Prior radiation therapy to the breast or chest wall
Prior radiation therapy to the breast or chest wall
 Active connective tissue disease involving the skin (especially scleroderma and lupus)
Active connective tissue disease involving the skin (especially scleroderma and lupus)
 Tumors >5 cm
Tumors >5 cm
Women with a known genetic predisposition to breast cancer such as BRCA 1 or 2 have an increased risk of contralateral breast cancer or ipsilateral breast recurrence with breast-conserving therapy. Prophylactic bilateral mastectomy for risk reduction in these patients may be considered.
The goal of sentinel lymph node biopsy (SLNB) is to provide prognostic information regarding the accurate pathologic staging of breast cancer. This information is used to guide additional management decisions. The SLN is defined as the main lymph node(s) that receives drainage directly from the primary tumor. SLN mapping and resection is the preferred method for staging the clinically negative axilla per NCCN guidelines. SLNB is performed by injection of technetium-labeled sulfur colloid, vital blue dye, or both around the tumor, or the subareolar area is taken up into the breast lymphatic system with a predominant pattern into the axilla. Nodes that contain dye or technetium are identified as the SLN. Identification rates of 92% to 98% of patients are the standard, especially when both techniques are used. If breast cancer were to spread, typically it would spread to the sentinel lymph node (SLN) first before moving to the other lymph nodes. This selective biopsy of potentially positive SLN, and sparing removal of negative lymph nodes decreases pain, sensation loss, and lymphedema compared to traditional axillary lymph node dissection (ALND).
The ACOSOG Z 0011 clinical trial showed that in patients with T1–T2 invasive breast cancer with clinically negative lymph nodes, found to have one to two positive lymph nodes on SLNB, there is no benefit in OS and DFS in performing a complete axillary node dissection. For patients who meet these criteria, a complete ALND can be potentially avoided.
Axillary Lymph Node Dissection
 Among patients with clinically negative axillary lymph nodes, 14% will have positive SLNB and will require additional axillary surgery.
Among patients with clinically negative axillary lymph nodes, 14% will have positive SLNB and will require additional axillary surgery.
 Axillary lymph node dissection (ALND) is complete surgical removal of level I and II axillary lymph nodes. The goal of ALND is to remove axillary burden of disease.
Axillary lymph node dissection (ALND) is complete surgical removal of level I and II axillary lymph nodes. The goal of ALND is to remove axillary burden of disease.
 A complete axillary node dissection is associated with approximately 10% to 25% risk of lymphedema, which can be mild to severe.
A complete axillary node dissection is associated with approximately 10% to 25% risk of lymphedema, which can be mild to severe.
Reconstructive surgery may be used for patients who opt for a mastectomy. It may be done at the time of the mastectomy (immediate reconstruction) or at a later time (delayed reconstruction). Patients diagnosed with early-stage breast cancer and electing to undergo a mastectomy should be offered immediate reconstruction as long as their comorbid conditions do not preclude this intervention. For patients with locally advanced or inflammatory breast cancer, undergoing mastectomy with delayed reconstruction may be the more appropriate management option.
Reconstruction can be done in one of two ways: implant-based (silicone or saline implants) or an autologous tissue graft. Examples of autologous tissue grafts include TRAM (transverse rectus abdominis myocutaneous) flaps, the latissimus dorsi flap, and the DIEP (deep inferior epigastric perforator) flap.
Radiotherapy
 Radiotherapy (RT) is an integral part of breast-conserving treatment (lumpectomy). It is associated with a large reduction in local recurrence and a positive impact on survival.
Radiotherapy (RT) is an integral part of breast-conserving treatment (lumpectomy). It is associated with a large reduction in local recurrence and a positive impact on survival.
 Standard radiation is 45 to 50.4 Gy at 1.8 to 2 Gy per fraction to the whole breast. RT boost to the tumor bed cavity is recommended in patients at higher risk for local failure (based on age, pathology, and margin status). The boost dose is 10 to 16 Gy at 2 Gy per fraction. An alternative hypofractionation schedule is 40 to 42.5 Gy at 2.66 Gy per fraction to the whole breast. This treatment method has been demonstrated to provide comparable cosmetic and oncologic results following breast-conserving surgery in patients with clear surgical margins and negative lymph nodes. Three-dimensional planning, inverse planning for intensity modulation, respiratory control, prone positioning, and proton therapy are techniques employed to minimize cardiac risks for whole breast and postmastectomy chest wall RT in patients with left-sided breast cancer.
Standard radiation is 45 to 50.4 Gy at 1.8 to 2 Gy per fraction to the whole breast. RT boost to the tumor bed cavity is recommended in patients at higher risk for local failure (based on age, pathology, and margin status). The boost dose is 10 to 16 Gy at 2 Gy per fraction. An alternative hypofractionation schedule is 40 to 42.5 Gy at 2.66 Gy per fraction to the whole breast. This treatment method has been demonstrated to provide comparable cosmetic and oncologic results following breast-conserving surgery in patients with clear surgical margins and negative lymph nodes. Three-dimensional planning, inverse planning for intensity modulation, respiratory control, prone positioning, and proton therapy are techniques employed to minimize cardiac risks for whole breast and postmastectomy chest wall RT in patients with left-sided breast cancer.
 RT is usually done after chemotherapy when systemic chemotherapy is indicated.
RT is usually done after chemotherapy when systemic chemotherapy is indicated.
 Postmastectomy radiation treatment to the chest wall, axillary, supraclavicular- and internal mammary lymph node regions decreases the risk of locoregional recurrence and improves survival in patients with multiple positive lymph nodes and patients with T3 or T4 tumors.
Postmastectomy radiation treatment to the chest wall, axillary, supraclavicular- and internal mammary lymph node regions decreases the risk of locoregional recurrence and improves survival in patients with multiple positive lymph nodes and patients with T3 or T4 tumors.
 Two randomized trials showed improvement in OS for postmastectomy radiation in patients with one to three positive lymph nodes, and is being evaluated in more clinical trials. In selected patients, this should be discussed.
Two randomized trials showed improvement in OS for postmastectomy radiation in patients with one to three positive lymph nodes, and is being evaluated in more clinical trials. In selected patients, this should be discussed.
 Other indications that may place patients at risk for local-regional failure and drive the decision for postmastectomy radiation include positive margins, extranodal extension and high-grade disease, young age and high-risk biology (e.g., triple-negative disease), and omission of axillary dissection after positive sentinel lymph node biopsy if sufficient information is present without needing to know if additional axillary lymph nodes are involved. ASCO (American Society of Clinical Oncology), ASTRO (American Society for Radiation Oncology), and SSO (Society of Surgical Oncology) updated guidelines for postmastectomy radiation therapy in 2016.
Other indications that may place patients at risk for local-regional failure and drive the decision for postmastectomy radiation include positive margins, extranodal extension and high-grade disease, young age and high-risk biology (e.g., triple-negative disease), and omission of axillary dissection after positive sentinel lymph node biopsy if sufficient information is present without needing to know if additional axillary lymph nodes are involved. ASCO (American Society of Clinical Oncology), ASTRO (American Society for Radiation Oncology), and SSO (Society of Surgical Oncology) updated guidelines for postmastectomy radiation therapy in 2016.
 For patients receiving neoadjuvant chemotherapy, those who present with clinically node positive, cT3 or cT4 disease, will typically be recommended for postmastectomy radiation regardless of pathologic response outside of a clinical trial. Biopsy is recommended to confirm clinical suspicion of lymph node involvement prior to the initiation of chemotherapy.
For patients receiving neoadjuvant chemotherapy, those who present with clinically node positive, cT3 or cT4 disease, will typically be recommended for postmastectomy radiation regardless of pathologic response outside of a clinical trial. Biopsy is recommended to confirm clinical suspicion of lymph node involvement prior to the initiation of chemotherapy.
Accelerated Partial Breast Irradiation
The primary goal of accelerated partial breast irradiation (APBI) is to shorten the duration of radiation therapy while maintaining adequate local control by targeting the lumpectomy cavity and adjacent at-risk tissue while sparing normal tissues. There are several APBI techniques currently in use and under study, including external beam radiation techniques, intraoperative radiation therapy, and brachytherapy; however, brachytherapy is the most widely used technique. Patients who are clinically felt to be at a lower risk recurrence outside of the lumpectomy site should be selected according to published criteria since the whole breast is not treated. ASTRO and SSO have published guidelines to aid in patient selection, e.g., older patients with Tis or T1 disease, screen-detected, low-intermediate grade, <2.5 cm with margins negative by at least 3 mm. The standard dose for balloon catheter brachytherapy is 34 Gy in 10 fractions delivered twice daily. Phase III data supports the use of intensity modulated external beam radiation therapy delivering 30 Gy in 5 fractions for select patients.
Intraoperative Radiation Therapy (IORT)
Intraoperative radiation therapy (IORT) delivers a concentrated dose of radiation to the tumor bed immediately after the tumor is removed. Two large studies evaluated the role of IORT in women with early-stage breast cancer. In the TARGIT-A study, women ages 45 or older were randomized to receive IORT or whole breast external beam radiation (WBRT) after lumpectomy. Survival rates were similar in both groups but local recurrence was more common in the IORT group. These findings are supported by the ELIOT trial results. In this study, women ages 48 to 75 with tumors ≤ 2.5 cm were randomized to IORT or WBRT, and survival rates were similar in both groups but local recurrence was more common in the IORT group.
Per the 2017 ASTRO/SSO APBI consensus statement update, IORT should be restricted to patients who are also suitable to partial breast radiation, and patients should be counseled that the risk of ipsilateral breast cancer might be higher with IORT.
Adjuvant Systemic Therapy
Adjuvant therapy decisions are made after carefully considering patient-related and tumor-related factors. Patient related factors include, age, comorbid conditions, performance status, patient preference, risk–benefit discussion, and life expectancy. Tumor-related factors are tumor size, lymph node status (stage) ER/PR status, Her-2/neu, grade of the tumor and genomic expression profile (e.g.: Oncotype DX, MammaPrint, etc.) (Fig. 12.2).

FIGURE 12.2 Algorithm for systemic adjuvant therapy.
General Principles of Adjuvant Therapy
1.All patients with breast cancer should be screened for potential clinical trials.
2.ER/PR-positive patients should be considered for antiestrogen therapy.
3.HER-2/neu-positive patients should be considered for HER-2/neu-targeted therapy.
4.Chemotherapy should be considered for the following patients (Table 12.3):
a.ER/PR-negative patients
b.Triple negative patients
c.HER-2/neu-positive patients
d.Node-positive patients
e.High-risk patients based upon Oncotype DX, MammaPrint, or other prognostic classification
Adjuvant Therapy in HER-2/neu-Negative Patients
A variety of adjuvant regimens have been used across the world. Depending upon the biology of the tumor, stage of the disease, patient’s health status, comorbid conditions, and chance of recurrence, an optimal regimen can be chosen (Table 12.5). There is no major difference in efficacy among the regimens.
Estrogen or progesterone receptor positive, HER-2 neu negative patients:
A nonanthracycline-containing regimen such as docetaxel and cyclophosphamide (TC) for four to six cycles can be used in ER positive patients who require systemic chemotherapy. The benefit of anthracycline-containing regimens in receptor positive patients is limited. This was illustrated in the ABC (anthracyclines in early breast cancer) trials (combined analysis of USOR 06-090, NSABP B-46 and NSABP B-49) where women with early-stage breast cancer were randomized to TC for six cycles versus standard anthracycline/taxane/cyclophosphamide based chemotherapy. This trial showed the anthracycline-based chemotherapy improved invasive DFS compared to TC for six cycles overall; however, in subgroup analysis, it was found that the benefit of anthracyclines for ER/PR-positive patients was most substantial for those with four or more lymph nodes involved. In high-risk (such as more than four nodes) ER/PR positive patients, an anthracycline-containing regimen such as dose dense AC followed by dose dense Paclitaxel or TAC regimen should be considered.
Estrogen and progesterone receptor negative, HER-2/neu negative (Triple Negative) patients
These patients are often treated with anthracycline-based chemotherapy in the adjuvant setting; however, the ABC trials showed that the greatest benefit of anthracycline-containing chemotherapy for ER/PR-negative patients occurred when patients had one or more lymph nodes involved. For patients with lymph node negative or small tumors (less than 2 cm), TC chemotherapy for four to six cycles can be considered. In high-risk Triple Negative patients, anthracycline-containing regimens, such as Dose Dense AC followed by Dose Dense Paclitaxel or a TAC regimen should be considered. Role of carboplatin in adjuvant triple negative breast cancer is evaluated in NRG 003 clinical trial.
TABLE 12.5 Nontrastuzumab-Containing Combinations
Commonly Used Regimens |
Dose-dense AC followed by dose-dense paclitaxel chemotherapy
Cycled every 14 d for 4 cycles (All cycles are with filgrastim support) Followed by:
Cycled every 14 d for 4 cycles (All cycles are with filgrastim support) |
Dose-dense AC followed by weekly paclitaxel chemotherapy
Cycled every 14 d for 4 cycles (All cycles are with filgrastim support) Followed by:
|
TC chemotherapy
Cycled every 21 d for 4 -6 cycles |
AC chemotherapy
Cycled every 21 d for 4 cycles |
TAC chemotherapy
Cycled every 21 d for 6 cycles (All cycles are with filgrastim support) |
Other Regimens |
FAC chemotherapy
Cycled every 21 d for 6 cycles |
CAF chemotherapy
Cycled every 28 d for 6 cycles |
CEF chemotherapy
Cycled every 28 d for 6 cycles With cotrimoxazole support |
|
Cycled every 28 d for 6 cycles |
AC followed by docetaxel chemotherapy
Cycled every 21 d for 4 cycles Followed by:
Cycled every 21 d for 4 cycles |
EC chemotherapy
Cycled every 21 d for 8 cycles |
FEC followed by docetaxel
Cycled every 21 d for 3 cycles Followed by:
Cycled every 21 d for 3 cycles |
FEC followed by weekly paclitaxel
Cycled every 21 d for 4 cycles Followed by:
|
FAC followed by weekly paclitaxel
Cycled every 21 d for 4 cycles Followed by:
|
TABLE 12.6 Trastuzumab-Containing Regimens
AC followed by T chemotherapy with trastuzumab
Cycled every 21 d for 4 cycles Followed by:
With:
Followed by:
|
TCH chemotherapy with trastuzumab
Cycled every 21 d for 6 cycles With
Followed by:
|
TCHP chemotherapy followed by trastuzumab (+/- pertuzumab)
Cycled every 21 d for 6 cycles With
Followed by:
|
Dose-dense AC followed by dose-dense paclitaxel chemotherapy with trastuzumab
Cycled every 14 d for 4 cycles Followed by:
Cycled every 14 d for 4 cycles (All cycles are with filgrastim support) With
Followed by:
(Cardiac monitoring is recommended before and during treatment) |
Paclitaxel chemotherapy and trastuzumab
With
Followed by:
|
Adapted from NCCN 2017 Guidelines
Adjuvant Therapy in HER-2/neu-Positive Patients
Incorporation of trastuzumab in the adjuvant therapy is the most important development in the treatment of breast cancer in the past 10 to 15 years. The clinical trials that initially showed benefit for the addition of trastuzumab to standard chemotherapy in treatment of HER2+ breast cancer (NSABP B-31 and NCCTG N9831) have published 10 year of follow-up showing 40% improvement in DFS and 37% improvement in OS. Many trastuzumab-containing regimens have been tested and all are equally effective (Table 12.6). The major difference between regimens is in the cardiac toxicity. Nonanthracycline-containing regimens (such as TCH from the BCIRG 006 trial) and the HERA trial regimens (the majority of which were anthracycline based but used sequential rather than concurrent trastuzumab) had less cardiac toxicity compared to other anthracycline-containing regimens. In the adjuvant setting, trastuzumab has only been tested in combination with chemotherapy.
Based upon the long-term follow-up of BCIRG 006 presented at the San Antonio Breast Cancer Symposium in 2015, a nonanthracycline regimen–containing TCH for six cycles could be considered for most patients with her-2 positive disease given the excellent outcomes. In very high-risk patients (multiple lymph nodes and very young patients), an anthracycline-containing regimen such as AC followed by TH or AC-THP can be considered after discussing about potential side effects such as cardiac toxicity and risk of secondary malignancies (MDS and AML).
The APHINITY study presented and published in June 2017, showed that the addition of adjuvant pertuzumab to standard adjuvant trastuzumab-containing regimen for HER2+ breast cancer resulted in a statistically significant, but a small 1.7% improvement in invasive DFS benefit predominantly in the lymph node positive and hormone receptor–negative patients. A 3.2% improvement in invasive DFS was seen in lymph node positive patients versus 0.5% improvement in lymph node negative patients and 1.6% improvement in invasive DFS improvement was seen in hormone receptor–negative patients compared to 0.5% improvement in hormone receptor–positive patients. Addition of pertuzumab was associated with a greater incidence of diarrhea. Given these results, use of adjuvant pertuzumab for one year in addition to trastuzumab can be considered in selected high-risk patients such as those with positive lymph nodes and those that are ER/PR-negative. The added cost should be considered before recommending pertuzumab in adjuvant setting.
In low-risk patients, especially patients with ER-positive, stage I or II tumors, weekly paclitaxel for 12 cycles with trastuzumab is a very reasonable option as per the APT (adjuvant paclitaxel and trastuzumab in node negative HER2 positive breast cancer) study.
Extended HER2-targeted therapy has also been investigated. The ExteNET study tested the irreversible pan-HER inhibitor neratinib 240 mg PO for 12 months in patients who completed standard trastuzumab-based adjuvant therapy and found a 2.3% benefit in 2-year invasive DFS for patients who received neratinib compared to placebo. Interestingly, pre-specified subgroup analysis showed greater benefit in hormone receptor–positive patients compared to hormone receptor–negative patients. Based on these results, the FDA approved neratinib 240 mg daily for 12 months for extended adjuvant treatment of early-stage HER2-positive breast cancer following adjuvant trastuzumab based therapy in July 2017.
Neoadjuvant or Preoperative Chemotherapy
Neoadjuvant or preoperative chemotherapy can be considered for patients with locally advanced breast cancer (IIB, IIIA, IIIB, IIIC), and inflammatory breast cancer. Response to neoadjuvant chemotherapy is much higher in triple negative and her-2 neu positive patients. In patients with stage III disease or inflammatory breast cancer, neoadjuvant therapy is the treatment of choice.
 Initial surgery is limited to biopsy to confirm the diagnosis and to identify the ER/PR, HER-2/neu- status, and other prognostic features.
Initial surgery is limited to biopsy to confirm the diagnosis and to identify the ER/PR, HER-2/neu- status, and other prognostic features.
 Preoperative evaluation of the breast mass by mammogram, ultrasound, or MRI is recommended.
Preoperative evaluation of the breast mass by mammogram, ultrasound, or MRI is recommended.
 Systemic staging using CT scans or CT/PET scan can be considered for these patients before starting chemotherapy.
Systemic staging using CT scans or CT/PET scan can be considered for these patients before starting chemotherapy.
 Neoadjuvant chemotherapy can potentially reduce the size of the primary tumor so breast-conserving surgery can be performed.
Neoadjuvant chemotherapy can potentially reduce the size of the primary tumor so breast-conserving surgery can be performed.
 Complete pathologic response (pCR) is associated with better outcome compared with residual disease at the time of surgery as demonstrated in the NSABP B-18 and NSABP B-27 trials. These trials showed no difference in DFS or OS between the groups treated with neoadjuvant and adjuvant therapy.
Complete pathologic response (pCR) is associated with better outcome compared with residual disease at the time of surgery as demonstrated in the NSABP B-18 and NSABP B-27 trials. These trials showed no difference in DFS or OS between the groups treated with neoadjuvant and adjuvant therapy.
 HER-2/neu-negative patients:
HER-2/neu-negative patients:
•Usually, a preoperative regimen contains an anthracycline and a taxane. Any adjuvant regimen can be used in a neoadjuvant setting.
•One of the largest neoadjuvant clinical trials is four cycles of AC followed by docetaxel for four cycles given every 3 weeks as per NSABP B-27 trial.
•In the CALGB 40603 trial, the addition of carboplatin to an anthracycline-based neoadjuvant regimen in patients with triple-negative breast cancer was associated with improvement in pCR rates (41% vs. 54%). GeparSixto trials by the German Breast Cancer Group also showed similar high response with carboplatin in neoadjuvant setting.
•In selected high-risk triple negative patients, it is very reasonable to consider adding carboplatin, especially if the tumor is not responding to standard anthracycline and taxane regimens.
 HER-2/neu-positive patients:
HER-2/neu-positive patients:
•Any adjuvant trastuzumab-containing regimen can be used in a neoadjuvant setting.
•Several clinical trials have shown an advantage for dual her-2 blockade in the neoadjuvant setting. Pertuzumab in combination with trastuzumab and docetaxel in the neoadjuvant setting was approved in 2013 based on the results of the phase II NeoSPHERE and TRYPHAENA trials. Lapatinib in combination with trastuzumab and paclitaxel in the neoadjuvant setting was studied in the NeoALTTO trial and was shown to be associated with higher rates of pCR than either anti-HER-2 drug alone.
•In HER-2 positive patients with high-risk features (such as ER/PR negative, stage II and above) they may be treated with pertuzumab in addition to trastuzumab. Commonly used neoadjuvant regimens include TCHP for 6 cycles or AC followed by THP.
Unless there is a contraindication, endocrine therapy should be considered for all patients with ER-positive and/or PR-positive tumors. As per the Oxford overview analysis, tamoxifen can decrease mortality by about 30% and the risk of recurrence by 50% in hormone receptor–positive patients (Fig. 12.3 and Table 12.7).
TABLE 12.7 Endocrine Agents Used in Treatment of Breast Cancer
Selective estrogen-receptor modifier (SERM) with combined estrogen agonist and estrogen antagonist activity |
Tamoxifen (Nolvadex), 20 mg/d PO |
Estrogen receptor downregulator |
Fulvestrant 500 mg intramuscular day 1, day 15 and then once a month |
Aromatase inhibitors |
Anastrozole (Arimidex), 1 mg/d PO |
Letrozole (Femara), 2.5 mg/d PO |
Exemestane (Aromasin), 25 mg/d PO |
CDK4/6 Inhibitors |
Palbociclib 125 mg PO Days 1-21 every 28 days |
Ribociclib 600 mg PO Days 1-21 every 28 days Abemaciclib 150 mg twice a day (continuous dosing) in combination with fulvestrant or 200 mg twice a day as single agent |
LHRH agonist analog in premenopausal women |
Leuprolide (Lupron Depot), 7.5 mg/dose IM monthly, or |
Leuprolide (Lupron Depot), 22.5 mg/dose IM every 3 mo, or |
Leuprolide (Lupron Depot), 30 mg/dose IM every 4 mo |
GnRH agonist analog |
Goserelin (Zoladex), 3.6 mg/dose s.c. implant into the abdominal wall every 28 d or |
Goserelin (Zoladex), 10.8 mg/dose s.c. implant into the abdominal wall every 12 wk |
Used in patients who have tumors that express either ER or PR receptors or both receptors |
LHRH, luteinizing hormone–releasing hormone; GnRH, gonadotropin-releasing hormone; ER, estrogen receptor; PR, progesterone receptor.
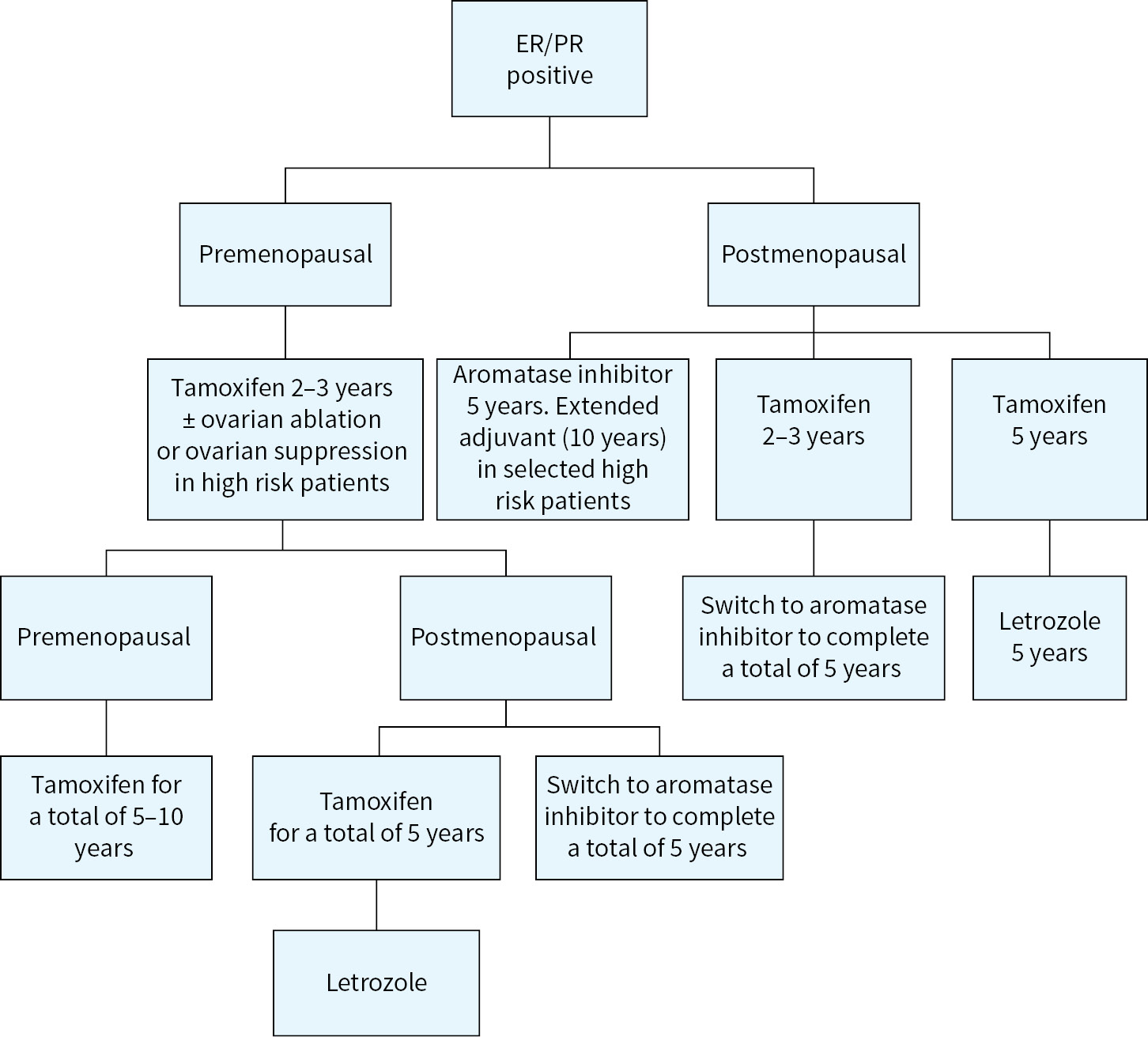
FIGURE 12.3 Adjuvant endocrine therapy.
Several large randomized studies have shown superiority of aromatase inhibitors (AIs) over tamoxifen in the adjuvant setting. If the patient has no contraindication, AIs are the preferred agents in postmenopausal patients. Anastrozole, letrozole, and exemestane are three third-generation AIs approved by the FDA for adjuvant use. The major side effects include arthralgia, osteopenia, osteoporosis, and fractures.
One of the largest adjuvant breast cancer trials (ATAC) compared tamoxifen with anastrozole and combination of both anastrozole and tamoxifen. It was shown that anastrozole is superior to tamoxifen in improving DFS, reducing the incidence of contralateral breast cancer, and has a favorable side-effect profile. For postmenopausal patients, the recommended dose of anastrozole is 1 mg PO daily for 5 years; however, there has been much interest in extending adjuvant therapy to a duration of 10 years as described in the following “Extended Adjuvant Endocrine Therapy” section.
BIG 1-98 showed a similar magnitude of improvement as anastrozole in the ATAC trial in DFS and a reduction of distant metastasis with letrozole. For postmenopausal patients, the recommended dose of letrozole is 2.5 mg PO daily for 5 years. Studies of extended adjuvant therapy with letrozole have been completed and are summarized below:
Switching from Tamoxifen to an Aromatase Inhibitor
In the IES study, exemestane therapy after 2 to 3 years of tamoxifen therapy significantly improved DFS and reduced the incidence of contralateral breast cancer as compared with the standard 5 years of tamoxifen therapy. The FDA has approved exemestane 25 mg daily after 2 to 3 years of tamoxifen in postmenopausal patients (total of 5 years of endocrine therapy).
The Italian tamoxifen anastrozole (ITA) trial, Austrian Breast Colorectal Study Group (ABCSG 8), and Arimidex, Noveldex (ARNO) study have shown an improvement in DFS and OS in patients who were initially treated with 2 to 3 years of tamoxifen and subsequently randomized to 2 to 3 years of anastrozole.
Extended Adjuvant Endocrine Therapy
The MA-17 study showed approximately 43% reduction in recurrence in postmenopausal patients receiving 2.5 mg of letrozole after completing 5 years of tamoxifen (extended adjuvant therapy). The MA.17R trial (an extension of the MA 17 trial) evaluated the role of 10 years of adjuvant letrozole in postmenopausal women. The five additional years of letrozole increased the 5 year DFS by 4% but only decreased the rate of distant recurrence by 1.1%.
The NSABP B-42 randomized phase III trial presented at the 2016 San Antonio Breast Cancer Symposium, however, did not show a DFS benefit with five additional years of adjuvant letrozole. Despite conflicting results of the MA-17R and NSABP B-42 studies, high-risk patients who are tolerating aromatase inhibitor well after 5 years, may consider continuing therapy for five additional years. Various biomarkers such as Breast Cancer Index (BCI) are being developed to aid in selecting patients who will benefit from extended adjuvant therapy.
Endocrine Therapy: Premenopausal Patients
Hormone receptor–positive, premenopausal patients are generally treated with tamoxifen (Fig. 12.3).
Tamoxifen is a selective estrogen-receptor modulator (SERM), with both estrogen agonist and antagonist potential. In premenopausal patients, tamoxifen 20 mg daily is the treatment of choice, unless the patient has any contraindications such as history of thromboembolic disease, stroke, or endometrial cancer. Major adverse effects include a higher incidence of cerebrovascular accidents, thrombosis, endometrial cancer, hot flashes, mood changes, and weight gain.
In general, tamoxifen is recommended for 5 years however many studies support a longer duration of treatment. In the ATLAS study, women who took tamoxifen for a total of 10 years rather than 5 had lower recurrence rate and increased OS. Extended adjuvant use of tamoxifen had little effect on recurrence or mortality rates from 5 to 9 years after diagnosis, but in the second decade following diagnosis, women who had continued tamoxifen treatment beyond 5 years had a 25% lower recurrence rate and a 29% lower breast cancer mortality rate. The results of the aTTom trial also demonstrated improved survival with 10 years of tamoxifen. Prolonged use of tamoxifen is associated with increased side effects (particularly endometrial carcinoma and PE); therefore, the decision to use tamoxifen for 10 years needs to be individualized, depending on the risk of recurrence and potential adverse effects.
Ovarian Ablation or Ovarian Suppression
The Oxford overview and several studies have found that premenopausal patients who stopped having periods after completion of chemotherapy have better survival than those who continued to have periods. Ovarian ablation can be achieved by surgery, radiation, or with LHRH agonists such as leuprolide, GNRH analogues such as goserelin.
The TEXT and SOFT trials, published in 2014, addressed the use of ovarian suppression as adjuvant therapy in premenopausal women with ER-positive breast cancer. In the TEXT trial, women were randomized to receive 5 years of tamoxifen with ovarian suppression or exemestane with ovarian suppression. In the SOFT trial, women were randomized to 5 years of tamoxifen, tamoxifen plus ovarian suppression, or exemestane plus ovarian suppression. DFS was higher in the exemestane plus ovarian suppression group comparing to the tamoxifen plus ovarian suppression group in both trials. In a subgroup analysis of the TEXT trial, younger women (under the age of 35 years) with high-risk disease warranting chemotherapy had higher DFS with exemestane and ovarian suppression compared to tamoxifen alone or tamoxifen with ovarian suppression. Based on these results, it is reasonable to consider exemestane with ovarian suppression for premenopausal women with high-risk disease, particularly those under the age of 35 years.
In general, we recommend use of monthly LHRH agonists such as leuprolide, GNRH analogues such as goserelin or surgical removal of ovaries for those patients who would benefit from ovarian suppression. In patients who are treated with GNRH analogues or LHRH agonists, it is important to make sure that they achieve a complete ovarian suppression by checking the serum estradiol, LH, and FSH although optimal frequency of this monitoring is unknown. Before a young woman decides to undergo bilateral oophorectomy, it is important to make sure that she understands the risks and benefits including its impact on quality of life. In this situation, it may be advisable to use medical ovarian suppression for a period of time, so potential side effects can be reversed with discontinuation of the medication.
Role of Adjuvant BisphosphCnate Therapy in Early Breast Cancer:
In an Oxford overview analysis including data from nearly 19,000 patients treated with adjuvant bisphosphonate therapy, significant reductions in distant breast cancer recurrence (particularly bone recurrence) and breast cancer mortality were found. These effects were limited to women who were postmenopausal when treatment was started. Based on this data, an update to the ASCO clinical practice guidelines occurred in July 2017. The guidelines now recommend that postmenopausal women with breast cancer who are candidates for adjuvant therapy be considered for treatment with either zoledronic acid (4mg IV every 6 months) or clodronate (1,600 mg PO daily). Optimal dosing during and intervals are not known although up to 5 years of treatment can be considered. Definition of menopause in this guideline includes both natural menopause and that induced by ovarian suppression or ablation.
BREAST CANCER IN PREGNANCY
 Breast cancer during pregnancy was initially thought to be more aggressive biologically; however, the overall poor outcome associated with breast cancer in pregnancy is likely related to more advanced stage at the time of diagnosis.
Breast cancer during pregnancy was initially thought to be more aggressive biologically; however, the overall poor outcome associated with breast cancer in pregnancy is likely related to more advanced stage at the time of diagnosis.
 Breast biopsy is safe in all stages of pregnancy and should be done for any mass concerning for cancer.
Breast biopsy is safe in all stages of pregnancy and should be done for any mass concerning for cancer.
Treatment
 Lumpectomy and axillary node dissection can be performed in the third trimester, and radiation therapy can be safely delayed until after delivery.
Lumpectomy and axillary node dissection can be performed in the third trimester, and radiation therapy can be safely delayed until after delivery.
 Modified radical mastectomy is the treatment of choice in the first and second trimesters because radiation treatment is contraindicated during pregnancy.
Modified radical mastectomy is the treatment of choice in the first and second trimesters because radiation treatment is contraindicated during pregnancy.
Chemotherapy
 Chemotherapy should not be administered during the first trimester.
Chemotherapy should not be administered during the first trimester.
 No chemotherapeutic agent has been found to be completely safe during pregnancy.
No chemotherapeutic agent has been found to be completely safe during pregnancy.
 An anthracycline combined with cyclophosphamide (e.g., AC given every 3 weeks for four cycles) has been used safely in the adjuvant or neoadjuvant setting during the second or third trimesters.
An anthracycline combined with cyclophosphamide (e.g., AC given every 3 weeks for four cycles) has been used safely in the adjuvant or neoadjuvant setting during the second or third trimesters.
 Chemotherapy should be scheduled to avoid neutropenia and thrombocytopenia at the time of delivery.
Chemotherapy should be scheduled to avoid neutropenia and thrombocytopenia at the time of delivery.
 Paclitaxel is teratogenic and should not be used during pregnancy.
Paclitaxel is teratogenic and should not be used during pregnancy.
 Growth factors such as filgrastim and pegfilgrastim have been used in pregnancy when necessary; however, data regarding safety of use is limited to case reports and small retrospective series. The FDA considers these drugs as Category C.
Growth factors such as filgrastim and pegfilgrastim have been used in pregnancy when necessary; however, data regarding safety of use is limited to case reports and small retrospective series. The FDA considers these drugs as Category C.
 Her-2 targeted agents such as trastuzumab have been reported to cause oligo/anhydramnios and fetal renal failure, so it should be avoided during pregnancy.
Her-2 targeted agents such as trastuzumab have been reported to cause oligo/anhydramnios and fetal renal failure, so it should be avoided during pregnancy.
 Tamoxifen is teratogenic and should not be used in pregnant women.
Tamoxifen is teratogenic and should not be used in pregnant women.
 Therapeutic abortion does not change the survival rate.
Therapeutic abortion does not change the survival rate.
MALE BREAST CANCER
 Male breast cancer is uncommon.
Male breast cancer is uncommon.
 Risk factors include family history, germline mutation, especially BRCA2, Klinefelter syndrome, and radiation to the chest wall.
Risk factors include family history, germline mutation, especially BRCA2, Klinefelter syndrome, and radiation to the chest wall.
 Presence of gynecomastia is not a risk factor for breast cancer.
Presence of gynecomastia is not a risk factor for breast cancer.
 It may present with a mass beneath the nipple or ulceration.
It may present with a mass beneath the nipple or ulceration.
 The mean age of occurrence is 60 to 70 years.
The mean age of occurrence is 60 to 70 years.
 Eighty percent of male breast cancer is hormone-receptor positive.
Eighty percent of male breast cancer is hormone-receptor positive.
Treatment
 Modified radical mastectomy.
Modified radical mastectomy.
 Lumpectomy is rarely done because it does not offer any cosmetic benefit.
Lumpectomy is rarely done because it does not offer any cosmetic benefit.
 Systemic treatment with chemotherapy and endocrine therapy should follow the general guidelines for female patients.
Systemic treatment with chemotherapy and endocrine therapy should follow the general guidelines for female patients.
 Tamoxifen 20 mg daily is the preferred endocrine therapy agent in male breast cancer.
Tamoxifen 20 mg daily is the preferred endocrine therapy agent in male breast cancer.
 None of the adjuvant treatment modalities have been tested in a randomized clinical trial setting in men.
None of the adjuvant treatment modalities have been tested in a randomized clinical trial setting in men.
Phyllodes Tumor
A phyllodes tumor is clinically suspected when the tumor is growing rapidly and clinical and radiologic features suggestive of fibroadenoma. Phyllodes tumor is classified as benign, borderline, or malignant. It is treated with wide excision without an axillary node dissection. In patients who have recurrent phyllodes tumor, radiation therapy can be considered after wide excision. Role of chemotherapy in phyllodes tumor is limited. Patients with Li-Fraumeni syndrome have an increased risk for phyllodes tumors.
Paget’s Disease of the Nipple
Paget’s may present as bleeding, ulceration, or eczema-like changes of the nipple. Patients should be evaluated for any evidence of invasive or noninvasive breast cancer by appropriate imaging and biopsy as Paget’s disease has been reported to occur with cancer elsewhere in the breast in up to 90% of cases. If the patient has only Paget disease of the nipple areolar complex (NAC), the patient can be treated with mastectomy with axillary lymph node dissection (ALND) or wide excision of the NAC and axillary node surgery with whole-breast radiation. Patients with invasive or noninvasive breast cancer should be managed accordingly.
METASTATIC BREAST CANCER
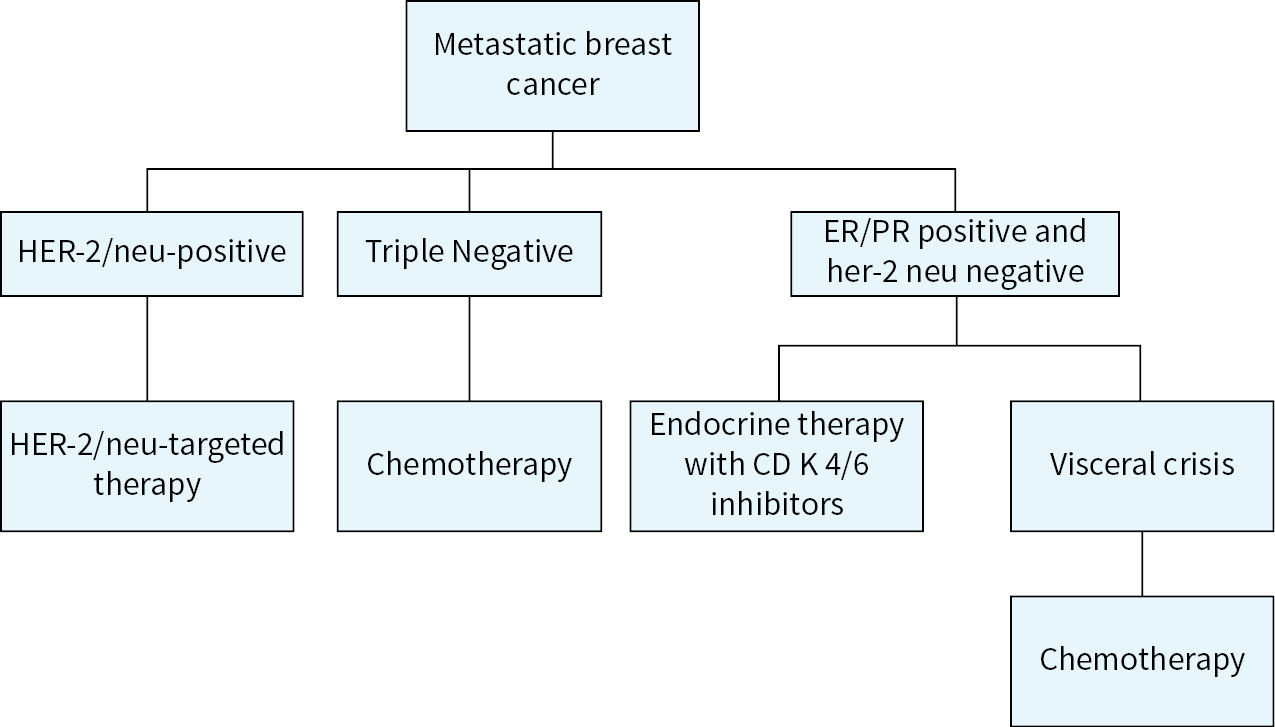
FIGURE 12.4 Algorithm for the management of metastatic breast cancer
Principles of Treatment
1.Repeat biopsy to confirm the diagnosis of recurrent/metastatic breast cancer (Fig. 12.4).
2.Strongly recommend repeating all biomarkers including ER/PR and HER-2/neu.
3.All patients should be considered for clinical trials.
4.Genomic profiling using next generation sequencing can be considered if genomic-based clinical trials or targeted therapy options are available.
5.MSI (microsatellite instability) or MMR (mismatch repair deficient) testing can be considered in selected patients, especially patients with triple negative tumors or those who have limited standard treatment options available.
6.HER-2/neu-positive patients should be treated with HER-2/neu-targeted agents such as trastuzumab, pertuzumab, ado-trastuzumab emtansine (TDM-1), or lapatinib.
7.All ER/PR-positive, HER-2 neu negative patients should be considered for antiestrogen therapy with or without CDK 4/6 inhibitors such as palbociclib or ribociclib.
8.Premenopausal patients with ER-positive disease should be considered for ovarian suppression and endocrine therapy with or without CDK 4/6 inhibitors such as palbociclib or ribociclib.
9.In ER-positive patients, use of chemotherapy should be limited to those with visceral crisis or those who have progressed through various endocrine agents, CDK 4/6 inhibitors, or mTOR inhibitor treatments.
10.Since combination chemotherapy regimens have not shown DFS or OS benefit, patients should be treated with single agents in a sequential manner.
11.All patients with metastatic disease involving the bone should be considered for bone modifying agents such as bisphosphonates (zoledronic acid/pamidronate) or denosumab (RANK ligand inhibitor).
12.Before starting treatment, a detailed assessment of comorbid conditions, performance status, patient preference, toxicities of the treatment, and risk versus benefit discussion should be done with each patient.
13.Goal of treatment should be discussed in detail with the patient, since it is palliative for majority of the patients.
Estrogen Receptor–Positive Metastatic Breast Cancer
 Endocrine therapy is the mainstay of treatment.
Endocrine therapy is the mainstay of treatment.
 Introduction of CDK 4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib has revolutionized the treatment of estrogen receptor–positive metastatic breast cancer.
Introduction of CDK 4/6 inhibitors such as palbociclib, ribociclib, and abemaciclib has revolutionized the treatment of estrogen receptor–positive metastatic breast cancer.
 Selection of endocrine therapy will depend upon the adjuvant or previous endocrine therapy, menopausal status, interval between completion of adjuvant therapy and development of metastatic disease.
Selection of endocrine therapy will depend upon the adjuvant or previous endocrine therapy, menopausal status, interval between completion of adjuvant therapy and development of metastatic disease.
 Premenopausal patients can be treated with tamoxifen or ovarian suppression and an aromatase inhibitor with palbociclib 125 mg daily (day 1 to 21 of a 28-day cycle) or ribociclib 600 mg daily (day 1 to 21 of a 28-day cycle).
Premenopausal patients can be treated with tamoxifen or ovarian suppression and an aromatase inhibitor with palbociclib 125 mg daily (day 1 to 21 of a 28-day cycle) or ribociclib 600 mg daily (day 1 to 21 of a 28-day cycle).
 In postmenopausal patients, aromatase inhibitor with palbociclib or ribociclib should be considered as standard first-line treatment.
In postmenopausal patients, aromatase inhibitor with palbociclib or ribociclib should be considered as standard first-line treatment.
 Second-line options for those who progressed on an aromatase inhibitor or developed metastatic disease in less than 1 year on an AI, include fulvestrant as a single agent, fulvestrant with palbociclib, fulvestrant with abemaciclib, fulvestrant with anastrozole or exemestane with everolimus.
Second-line options for those who progressed on an aromatase inhibitor or developed metastatic disease in less than 1 year on an AI, include fulvestrant as a single agent, fulvestrant with palbociclib, fulvestrant with abemaciclib, fulvestrant with anastrozole or exemestane with everolimus.
 Abemaciclib can be used as single-agent treatment for patients who have had disease progression on prior endocrine therapy and chemotherapy for metastatic disease.
Abemaciclib can be used as single-agent treatment for patients who have had disease progression on prior endocrine therapy and chemotherapy for metastatic disease.
HER-2-Positive Metastatic Breast Cancer
 Based upon the CLEOPATRA study, dual HER-2 blockade with trastuzumab and pertuzumab in combination with a taxane (THP) is considered standard treatment in first-line metastatic setting. The most commonly used regimen is docetaxel 75 mg/m2 with trastuzumab 8 mg/kg loading dose followed by 6 mg/kg and pertuzumab 480 mg loading dose followed by 420 mg given every 3 weeks with growth factor support. Weekly paclitaxel can be used rather than docetaxel in this setting.
Based upon the CLEOPATRA study, dual HER-2 blockade with trastuzumab and pertuzumab in combination with a taxane (THP) is considered standard treatment in first-line metastatic setting. The most commonly used regimen is docetaxel 75 mg/m2 with trastuzumab 8 mg/kg loading dose followed by 6 mg/kg and pertuzumab 480 mg loading dose followed by 420 mg given every 3 weeks with growth factor support. Weekly paclitaxel can be used rather than docetaxel in this setting.
 In the second-line setting, T-DM1 (Ado-trastuzumab emtansine 3.6 mg/kg every 3 weeks is recommended based upon the EMILIA trial.
In the second-line setting, T-DM1 (Ado-trastuzumab emtansine 3.6 mg/kg every 3 weeks is recommended based upon the EMILIA trial.
 In the third-line setting, capecitabine 1000 mg/m2 twice daily for 2 weeks on 1 week off plus lapatinib 1000 mg daily can be considered.
In the third-line setting, capecitabine 1000 mg/m2 twice daily for 2 weeks on 1 week off plus lapatinib 1000 mg daily can be considered.
 Other trastuzumab-containing chemotherapy regimens include combinations of single agent chemotherapy with HER2-targeted therapy such as navelbine and trastuzumab or gemcitabine and trastuzumab.
Other trastuzumab-containing chemotherapy regimens include combinations of single agent chemotherapy with HER2-targeted therapy such as navelbine and trastuzumab or gemcitabine and trastuzumab.
Targeted Therapy
Trastuzumab is a monoclonal antibody directed against HER2/neu, which has been found to be highly effective in the neoadjuvant, adjuvant, and metastatic breast cancer settings. The dose is 4 mg/kg as a loading dose followed by 2 mg/kg weekly. An every 3 week regimen with a loading dose of 8 mg/kg followed by 6 mg/kg is the most commonly used regimen. Addition of 1 year of adjuvant trastuzumab improves DFS and OS among women with HER-2/neu-positive breast cancer. In general, trastuzumab is given in combination with chemotherapy in neoadjuvant, adjuvant, and metastatic settings.
Trastuzumab is well tolerated although, rarely, it can cause infusion reactions and pulmonary toxicity. The major side effect from trastuzumab is cardiac toxicity, particularly when it is used with or after anthracyclines. With anthracycline-containing regimens, the congestive heart failure rate is about 2% to 4%. Nonanthracycline-based regimens such as TCH did not show increased cardiac toxicity. It is important to monitor cardiac function with an echocardiogram or MUGA scan at baseline and every 3 months while patients are receiving trastuzumab.
Pertuzumab is a humanized monoclonal antibody that binds HER-2/neu at a different epitope of the HER-2/neu extracellular domain than that of trastuzumab. It prevents HER-2/neu from dimerizing with HER3. Similar to trastuzumab, pertuzumab causes antibody-dependent, cell-mediated cytotoxicity. Since pertuzumab and trastuzumab bind to different HER-2/neu epitopes and have complementary mechanisms of action, when pertuzumab is combined with trastuzumab, it provides a more comprehensive blockade of HER-2/neu signaling and results in greater antitumor activity in clinical trials. In the CLEOPATRA study, when pertuzumab was given with trastuzumab plus docetaxel, as compared with placebo plus trastuzumab plus docetaxel, in first-line treatment for HER-2/neu-positive metastatic breast cancer, it significantly prolonged progression-free survival (PFS). No additional cardiac toxicity was seen. The FDA-approved dose of pertuzumab is 840 mg, followed by 420 mg every 3 weeks.
Ado-trastuzumab Emtansine (Kadcyla)
Ado-trastuzumab emtansine is an antibody–drug conjugate composed of trastuzumab linked to a highly potent cytotoxic derivative of maytansine (DM1) by a stable linker. DM1 is a microtubule inhibitor. Trastuzumab targets the conjugate to HER-2/neu receptors and the stable linker releases the cytotoxic agent only when the compound is internalized through receptor endocytosis. Ado-trastuzumab emtansine (T-DM1) has been found to be active in trastuzumab- and lapatinib-resistant metastatic breast cancer, as well as in trastuzumab-naïve tumors.
Results of the phase III EMILIA trial that compared trastuzumab emtansine with capecitabine plus lapatinib in advanced HER-2/neu positive breast cancer showed a substantial improvement in PFS and OS with the conjugate, leading to FDA approval of T-DM1 in 2013. Final OS results of the EMILA trial published in 2017 continued to demonstrate an OS advantage of T-DM1 compared to capecitabine plus lapatinib, despite crossover that was allowed from the control group to T-DM1 following initial result reporting.
In the TH3RESA trial, patients with progressive disease after 2 or more anti-HER therapies were randomized to T-DM1 or treatment of physician's choice. The median PFS was significantly longer with T-DM1 (6.2 versus 3.3 months). In an update of OS results published in 2017, a 7 month OS benefit of T-DM1 was found (22.7 vs. 15.8 months) despite nearly 50% of patients crossing over from treatment of physician’s choice to T-DM1.
The dose of ado-trastuzumab emtansine is 3.6 mg/kg IV every 3 weeks and it is extremely well tolerated in clinical trials. Side effects include thrombocytopenia and liver function abnormalities. No significant increase in cardiomyopathy or peripheral neuropathy was seen.
Lapatinib is a potent, small molecule inhibitor of the HER1 and HER2 tyrosine kinases. The inhibitory effects, though reversible, result in blockade of receptor-mediated activation and propagation of downstream signaling involved in regulation of cell proliferation and cell survival. The FDA-approved dose of lapatinib is 1,250 mg daily PO. The side effects include diarrhea and rash.
Neratinib is an irreversible small molecule inhibitor of HER1, 2, and 4, which was approved by the FDA in 2017 for the extended adjuvant treatment of patients with early-stage HER2-amplified breast cancer, following adjuvant trastuzumab-based therapy based on the results of the ExteNET. The FDA-approved dose of neratinib is 240 mg daily continuously for 1 year. The primary side effect of neratinib is diarrhea and antidiarrheal prophylaxis with loperamide is recommended during the first two cycles of treatment. Liver function tests should also be monitored during therapy with dose reduction occurring for severe hepatic impairment. The agent is also being studied for use in metastatic HER2-positive breast cancer in combination with T-DM1 (NSABP FB 10).
CDK 4/6 Inhibitors
Introduction of the novel class of drugs known as CDK 4/6 inhibitors is a major advancement in the treatment of hormone receptor–positive breast cancer. Palbociclib, ribociclib, and abemaciclib are the FDA-approved CKD 4/6 inhibitors for the treatment of hormone receptor–positive metastatic breast cancer. Many ongoing clinical trials are studying the role of palbociclib, ribociclib, and abemaciclib in early breast cancer. Overall CD K4/6 inhibitors are well tolerated. Leukopenia is the most common class–related side effect but the incidence of febrile neutropenia is less than 2%. Abemaciclib causes more diarrhea.
Palbociclib is a cyclin-dependent kinase 4 and 6 inhibitor that has been used in the treatment of hormone-positive metastatic breast cancer in combination with endocrine therapy. In the phase II trial PALOMA-1, patients treated with letrozole and palbociclib had longer PFS as compared to letrozole alone (20.2 versus 10.2 months). These results led to FDA approval of palbociclib with letrozole in the first-line metastatic setting in February 2015. The recently published phase III study (PALOMA-2) confirmed this benefit, showing a median PFS of 24.8 months with letrozole plus palbociclib versus 14.5 months with letrozole alone.
In the PALOMA-3 study, patients with hormone-positive Her-2 negative breast cancer who had progressed during prior endocrine therapy were randomized to receive fulvestrant alone or fulvestrant with palbociclib. The median PFS was 9.5 months in the combination group and 4.6 months in the fulvestrant group. In February 2016, the FDA approved palbociclib in combination with fulvestrant for patients with advanced or metastatic hormone sensitive breast cancer with progression on prior endocrine therapy. The most notable side effect of palbociclib is neutropenia, which occurs in the vast majority of patients, although the neutropenic fever incidence in these studies were less than 2%. FDA-approved dose of palbociclib is 125 mg daily for 3 weeks and one week off, repeating every 4 weeks.
Ribociclib is also a cyclin-dependant kinase 4 and 6 inhibitor, which has been approved for use in combination with an aromatase inhibitor in the initial endocrine treatment of metastatic hormone receptor–positive breast cancer based on the results of the MONALEESA-2 trial. In this trial, postmenopausal woman with HR-positive, HER2-negative advanced, or metastatic breast cancer were treated with letrozole/ribociclib or letrozole/placebo as first-line therapy. It was found that the primary endpoint of PFS was significantly longer in the ribociclib-containing arm (not reached in the ribociclib arm vs. 14.7 months in the placebo arm) with 63% of patients free from progression at 18 months of follow-up versus 42% in the placebo group. The FDA-approved dose of ribociclib is 600 mg daily by mouth 3 weeks on followed by one week off treatment (with continuous aromatase inhibitor therapy). Notable side effects of this drug include neutropenia, Qt interval prolongation, diarrhea, and LFT elevation. Electrocardiogram monitoring is recommended during the first cycle of treatment to assess for Qt interval prolongation.
Abemaciclib is the third cyclin-dependent kinase 4 and 6 inhibitor that is approved for use in advanced breast cancer. It can be used in combination with fulvestrant at a dose of 150 mg twice daily for disease progression following prior endocrine therapy or as a single agent at a dose of 200 mg twice daily for disease progression following prior endocrine therapy and chemotherapy in the metastatic setting. It is unique in its greater specificity for CDK 4 inhibition, which may translate into a different side effect profile than the other drugs in this class.
The phase II MONARCH 1 study, which used abemaciclib 200 mg PO twice a day as a single agent in previously treated hormone receptor–positive metastatic breast cancer, was initially presented at the 2016 American Society of Clinical Oncology meeting and recently published. In this study, a 20% objective response rate was found with over 40% of patients experiencing clinical benefit (including stable disease). In terms of side effects, abemaciclib is associated with less myelosuppression than the other medications in this class but is associated with more diarrhea, nausea, and vomiting.
Subsequently, the phase III MONARCH 2 study, which investigated abemaciclib 150 mg PO twice a day (continuous dosing) plus fulvestrant in HR-positive metastatic breast cancer patients previously treated with endocrine therapy, was published in June 2017 showing a significant improvement in PFS with the addition of abemaciclib to fulvestrant (16.4 vs 9.3 months, p<0.001). The response rate was also improved with the addition of abemaciclib. As seen in the MONARCH 1 study, common adverse events that occurred with abemaciclib included diarrhea, nausea, fatigue, and neutropenia. The MONARCH 3 study of abemaciclib plus a nonsteroidal aromatase inhibitor as initial treatment of HR-positive metastatic breast cancer is expected soon.
Other Agents
Fulvestrant is an ER antagonist (ER down-regulator) and it is indicated in the treatment of hormone receptor–positive metastatic breast cancer in postmenopausal women with disease progression following antiestrogen therapy. Fulvestrant 500 mg should be administered intramuscularly into the buttocks slowly on days 1, 15, and 29 and once monthly thereafter. Side effects are mainly related to pain and injection site reaction.
Everolimus is FDA approved for the treatment of postmenopausal women with advanced hormone receptor–positive, HER-2/neu-negative breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. A randomized phase III study (BOLERO-2) showed everolimus 10 mg per day plus exemestane 25 mg per day improved PFS compared to placebo plus exemestane 25 mg per day. The most common adverse reactions in patients receiving everolimus and exemestane were stomatitis, infections, rash, fatigue, diarrhea, hyperglycemia, and pneumonitis.
Other Chemotherapy Agents Used for Breast Cancer
Capecitabine (Xeloda) is a fluoropyrimidine carbamate and it is an orally administered systemic prodrug of 5′-deoxy-5-fluorouridine (5′-DFUR), which is converted to 5-fluorouracil. It is indicated as monotherapy for metastatic breast cancer. The FDA-approved dose is 1,250 mg/m2 twice a day given for 2 weeks on and 1 week off, repeating every 21 days. For practical purposes most clinicians use 1,000 mg/m2 twice a day 2 weeks on 1 week off. The most common side effects are hand–foot syndrome and diarrhea. Patients should be educated about management of the hand–foot syndrome.
Eribulin mesylate is a nontaxane, tubulin-, and microtubule-targeting chemotherapeutic agent that binds directly with tubulin disrupting mitotic spindles and inhibits microtubule polymerization. A phase III study compared eribulin to treatment of physician’s choice (TPC) in patients with locally recurrent or metastatic breast cancer previously treated with an anthracycline and a taxane. This study showed improvement in PFS and OS with eribulin. The most common side effects were neutropenia and peripheral neuropathy. Eribulin is the only chemotherapy agent that has shown a survival advantage in late lines of therapy for breast cancer. The FDA-approved dose of eribulin is 1.4 mg/m2 administered on days 1 and 8 of a 21-day schedule.
Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a novel paclitaxel formulation that does not require cremophor or polysorbate 80 for solubilization, thus reducing solvent-related toxicity and micelle formation. The FDA-approved dose of nab-paclitaxel is 260 mg/m2 every 3 weeks for the treatment of metastatic breast cancer. The side effects include neutropenia, peripheral neuropathy, nausea, etc. Due to lack of cremophor, nab-paclitaxel does not require premedication with steroids.
This drug belongs to a novel class of drugs called epothilones. Epothilones are nontaxane microtubule-stabilizing agents. The tubulin-polymerizing activity of ixabepilone is stronger than paclitaxel. It has proven efficacy in taxane-resistant settings. Ixabepilone has low susceptibility to tumor resistance mechanisms such as P-glycoprotein (P-gp) and multidrug-resistance protein-1 (MRP1). The FDA-approved ixabepilone in combination with capecitabine in patients with metastatic or locally advanced breast cancer, who are resistant to or refractory to a taxane and anthracycline. Ixabepilone is also approved as monotherapy in patients who are resistant or refractory to taxane, anthracycline, and capecitabine. The dose is 40 mg/m2 administered over 3 hours every 3 weeks. Patients should be premedicated with diphenhydramine and cimetidine an hour prior to the infusion with ixabepilone.
PARP Inhibitors
The poly(ADP-ribose) polymerase (PARP) enzymes function to help repair DNA. Inhibition of the PARP enzymes results in double stranded DNA breaks in dividing cells. In most cells, DNA double strand breaks are able to be repaired through homologous recombination however in BRCA1/2 deficient cells this mechanism is absent. Such cells rely on PARP enzymes for DNA repair and when these enzymes are inhibited, the cells will die. This concept is the foundation for use of PARP inhibitors in BRCA mutation positive–breast cancer patients. Other breast cancers may also be susceptible to PARP inhibition, particularly triple-negative breast cancer where homologous recombination defects may also be present.
Olaparib is FDA approved for treatment of advanced ovarian cancer and primary peritoneal cancer with or without BRCA mutations. It has been studied in a variety of breast cancer settings and findings from the phase III OlympiAD metastatic breast cancer trial were recently published. In this study, metastatic breast cancer patients with germline BRCA mutations were randomized to receive either olaparib 300 mg twice daily or chemotherapy of physician’s choice (capecitabine, eribulin, or vinorelbine). The study showed a statistically significant 3-month improvement in progression-free survival and 42% decrease in risk of disease progression or death with olaparib compared to standard chemotherapy. The response rate was higher in the olaparib-treated patients (60% vs. 29%) and grade 3 or higher adverse events occurred less frequently (37% vs. 50%).
Rucaparib and niraparib are other PARP inhibitors that have been approved for use in advanced ovarian cancer and are currently being studied in breast cancer. Veliparib is another PARP inhibitor that is not yet FDA approved but is actively being studied in breast cancer clinical trials. Talazoparib is a PARP inhibitor that is of particular interest due to its potent activity, thought to be mediated through an increase in “PARP trapping” in addition to PARP inhibition.
Immunotherapy in Breast Cancer
Interest in using immunotherapy, specifically checkpoint inhibitors, in breast cancer treatment has grown significantly in recent years. It is known that PD-1 is expressed in 20% to 30% of breast cancers with more frequent expression in HER2+ and triple-negative disease compared to hormone sensitive disease. Many studies of immunotherapy in breast cancer are ongoing, particularly in the triple-negative subset, and those that have been published to date show only modest activity of PD-1 and PD-L1 in the breast cancer population. In addition, there is no consistent correlation between PD-L1 expression and response to treatment in the available studies.
In the KEYNOTE-012 phase Ib study of pembrolizumab in advanced solid tumors, a response rate of 19% was seen among 27 metastatic triple-negative breast cancer patients enrolled. All patients in this study had PD-1 expression of ≥1%. The subsequent KEYNOTE-086 phase II trial of pembrolizumab, which was presented at the American Society of Clinical Oncology 2017 annual meeting, enrolled 170 previously treated metastatic triple negative breast cancer patients (PD-L1 positive and negative) and demonstrated an objective response rate of 5%.
May 2017 FDA approval of pembrolizumab for patients with unresectable or metastatic, microsatellite instability-high (MSI-H), or mismatch repair deficient (dMMR) solid tumors who have progressed on prior treatment and have no satisfactory treatment options has now raised the question of whether MSI/MMR testing should be done routinely in breast cancer patients. As the triple-negative patients are those breast cancer patients with the fewest satisfactory treatment options, one could make a case to test these patients for MSI/MMR in an attempt to obtain pembrolizumab, although the data for its efficacy in this population is not well defined.
The combination of chemotherapy with immunotherapy has been studied in a phase I trial of atezolizumab in combination with nab-paclitaxel in metastatic triple negative breast cancer with an encouraging response rate of 42% among 24 patients. This combination therapy is currently being evaluated in a global phase III randomized study (the Impassion130 trial). The combination of chemotherapy with immunotherapy has also demonstrated promising results in the neoadjuvant space where pembrolizumab was evaluated with standard neoadjuvant therapy as part of the ISPY-2 trial. In this study, both hormone-receptor positive breast cancers and triple negative breast cancers had an improvement in pathologic complete response with the addition of pembrolizumab to standard chemotherapy. These combinations will now be studied in the larger phase III setting.
Role of Next Generation Sequencing
Next generation sequencing (NGS) has become widely available with the advent of many commercial assays. These tests are often quite expensive and insurance coverage for them is inconsistent. In the metastatic breast cancer setting, NGS may help to identify targets for treatment that are accessible via clinical trial participation or by use of an FDA-approved medication in an off label manner. Despite the availability of these tests and enthusiasm surrounding their promise, evidence supporting the clinical utility of genomic testing in the metastatic breast cancer setting is lacking. When considering sending such testing, providers must counsel the patient on the likelihood of potentially actionable findings and the costs associated with testing.
The SAFIR-01 trial assessed clinical utility of genomic profiling in a metastatic breast cancer population. In this study, investigators profiled metastatic breast tumors prospectively and recorded responses based on treatment decisions guided by genomic analysis. Forty-six percent of patients were found to have actionable mutations and therapy was tailored based on these results for 13% of patients. Of the patients who received a “personalized” therapy, 9% had a partial response and 21% had stable disease for at least 16 weeks.
Multiple ongoing clinical trials, such as the NCI MATCH trial, are designed to pair patients with actionable genomic alterations with biologically rational treatments. As results of such studies become available, the clinical utility of NGS in various tumor types will be elucidated.
Supportive Care Agents
 Bisphosphonates should be used in patients with bony metastatic disease because they prevent progression of lytic lesions, delay skeletal-related events, and decrease pain. However, the optimal frequencies of administration and duration of therapy are not known.
Bisphosphonates should be used in patients with bony metastatic disease because they prevent progression of lytic lesions, delay skeletal-related events, and decrease pain. However, the optimal frequencies of administration and duration of therapy are not known.
 Zoledronic acid (4 mg by 15-minute infusion) and pamidronate (90 mg by 2-hour infusion) are two available biphosphonates approved for bony metastatic disease.
Zoledronic acid (4 mg by 15-minute infusion) and pamidronate (90 mg by 2-hour infusion) are two available biphosphonates approved for bony metastatic disease.
 In the OPTIMIZE-2 trial, metastatic breast cancer patients with bone metastasis were randomized to receive zoledronic acid 4mg IV once every 4 weeks or once every 12 weeks for 1 year. The incidence of skeletal-related events and safety profile was similar for both groups. Based on these results, 12 week interval of dosing for zoledronic acid can be considered noninferior to 4 week interval of dosing.
In the OPTIMIZE-2 trial, metastatic breast cancer patients with bone metastasis were randomized to receive zoledronic acid 4mg IV once every 4 weeks or once every 12 weeks for 1 year. The incidence of skeletal-related events and safety profile was similar for both groups. Based on these results, 12 week interval of dosing for zoledronic acid can be considered noninferior to 4 week interval of dosing.
 Osteonecrosis of the jaw (ONJ) is a very rare but a potential complication of long-term treatment with intravenous bisphosphonates.
Osteonecrosis of the jaw (ONJ) is a very rare but a potential complication of long-term treatment with intravenous bisphosphonates.
The receptor activator of nuclear factor-κB (RANK), the RANK ligand (RANKL), and osteoprotegerin, a decoy receptor for RANK, regulate osteoclastogenesis and may play a key role in bone metastasis. Denosumab (XGEVA), a fully human monoclonal antibody that binds to and neutralizes RANKL, inhibits osteoclast function, prevents generalized bone resorption and local bone destruction, and has become a therapeutic option for preventing or delaying first on-study skeletal-related events in various malignancies.
It is approved for patients with bone metastasis from breast cancer, prostate cancer, and other solid tumors. The dose is 120 mg subcutaneous every 4 weeks. It can cause significant hypocalcemia. So patients should take appropriate calcium replacement. The incidence of osteonecrosis of the jaw is about 2.2% with denosumab. It does not have to be adjusted for renal impairment.
Central Nervous System Metastasis
Central nervous system (CNS) metastasis may consist of either parenchymal or leptomeningeal metastasis. The control of systemic disease is crucial to improving the survival of patients with resectable brain metastasis.
The standard treatment for multiple brain lesions remains whole-brain radiation (WBR) for symptom control, with no associated improvement in survival. The therapy for a single-brain metastasis remains either surgery or radiosurgery (Gamma Knife), with conflicting information as to the benefit of prior WBR. Leptomeningeal metastasis is conventionally treated with intrathecal chemotherapy, and may provide short-term symptom control. The superiority of intrathecal versus systemic chemotherapy in leptomeningeal metastasis is controversial. About 30% of HER-2/neu-positive patients will develop brain metatastic disease, and a lapatinib-containing regimen is an option in these patients as lapatinib is known to cross the blood brain barrier.
LOCALLY RECURRENT BREAST CANCER
After Mastectomy
 Eighty percent of local recurrences occur within 5 years.
Eighty percent of local recurrences occur within 5 years.
 Treatment of choice is surgical excision and radiation therapy.
Treatment of choice is surgical excision and radiation therapy.
 Systemic therapy may be considered based upon ER/PR and HER-2 status. As per the CALOR study, a survival advantage was seen for patients who received systemic therapy after local recurrence. But maximum benefit was in triple negative or high risk patients.
Systemic therapy may be considered based upon ER/PR and HER-2 status. As per the CALOR study, a survival advantage was seen for patients who received systemic therapy after local recurrence. But maximum benefit was in triple negative or high risk patients.
After Lumpectomy
 Mastectomy is the treatment of choice for patients who have only isolated breast cancer recurrence.
Mastectomy is the treatment of choice for patients who have only isolated breast cancer recurrence.
Survivorship
Studies have suggested that up to 50% of cancer survivors experience late effects of cancer treatment. In breast cancer survivors, providers must consider the potential long-term impacts of chemotherapy, surgery, radiation, and endocrine therapy on the patient including risks of cardiac dysfunction, cognitive changes, depression, persistent fatigue, pain, neuropathy, lymphedema, premature menopause, sexual dysfunction, deterioration in bone health, and secondary malignancies.
As per the 2016 Commission on Cancer accreditation standards, a survivorship care plan should be provided to all patients at the completion of curative intent treatment with information including a personalized treatment summary with associated providers identified, guidance of signs of recurrence, information of long-term effects of treatment, guidelines for follow-up care and identification of support services available to the patient. Given the growing body of evidence supporting the importance of a healthy lifestyle, including maintaining an appropriate body weight and incorporating regular physical activity, in decreasing risk of cancer, oncologists should be mindful to ask questions regarding healthy lifestyle behaviors during routine follow-up.
Pregnancy after Breast Cancer
Many patients and oncologists harbor reservations about pregnancy following a breast cancer diagnosis for a variety of reasons. Two of the biggest concerns, particularly for hormone receptor–positive breast cancer survivors, are that pregnancy produces higher levels of estrogen, which could result in breast cancer cell growth and that pregnancy necessitates a gap in adjuvant endocrine treatment.
A large retrospective study presented at the American Society of Clinical Oncology 2017 meeting challenged these concerns by demonstrating that DFS 10 years following diagnosis was no different in survivors who became pregnant compared to those who did not become pregnant. Importantly, this held true when the estrogen receptor-positive cohort was analyzed individually. In secondary analyses, the timing of pregnancy (<2 years after diagnosis or >2 years after diagnosis) and breastfeeding did not affect DFS. The ongoing POSITIVE study will provide additional insight into the impact of interrupting adjuvant endocrine therapy during pregnancy for survivors of ER-positive breast cancer.
FOLLOW-UP FOR PATIENTS WITH OPERABLE BREAST CANCER (BASED ON ASCO GUIDELINES MARCH 2013)
1.History and physical examination every 3 to 6 months for the first 3 years, every 6 to 12 months for the next 2 years, and annually thereafter.
2.Physicians should counsel patients regarding symptoms of recurrence including new lumps, bone pain, chest pain, dyspnea, abdominal pain, and persistent headaches.
3.All women should be counseled to do monthly breast self-examination.
4.Annual mammogram of the contralateral and ipsilateral (remaining breast after lumpectomy) breast.
5.Regular gynecologic follow-up is recommended for all patients. Those who receive tamoxifen should be advised to report any unusual vaginal bleeding to their doctors.
6.Coordination of care: The risk of breast cancer recurrence continues through 15 years after primary treatment and beyond. Continuity of care for patients with breast cancer is recommended and should be performed by a physician experienced in the surveillance of patients with cancer and in breast examination, including the examination of irradiated breasts.
7.Follow-up by a PCP seems to lead to the same health outcomes as specialist follow-up with good patient satisfaction.
8.Routine blood tests including a complete blood count, liver function tests, and alkaline phosphatase levels are not recommended. Serum tumor markers (CA 27-29, and CA 15-3) are not recommended.
9.Chest X ray, ultrasound of the liver, breast MRI, bone scan, and CT scans of the chest, abdomen, pelvis, and brain or PET scans are not recommended routinely, but they are done if symptoms or laboratory abnormalities are present.
Suggested Readings
1.Adams S, Robinson Diamond J, Hamilton EP, et al. Phase Ib trial of atezolizumab in combination with nab-paclitaxel in patients with metastatic triple-negative breast cancer (mTNBC). Abstract presented at: ASCO Annual Meeting. 2016; Chicago, IL. Abstract 1009.
2.Adams S, Schmid P, Rugo H, et al. A phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple negative breast cancer (mTNBC): KEYNOTE-086 cohort A. Abstract presented at: ASCO Annual Meeting. 2017; Chicago, IL. Abstract 1008.
3.Aebi S, Gelber S, Anderson SJ, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomized trial. Lancet Oncol. 2014;15:156–163.
4.Andre F, Bachelot T, Commo F et al. Comparative genomic hybridization array and DNA sequencing to direct treatment of metastatic breast cancer: a multicenter, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014;15(3):267–274.
5.Baselga J, Bradbury I, Eidtmann H, et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2012 Feb 18;379(9816):633–640.
6.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor positive advanced breast cancer. N Engl J Med. 2012;366:520–529.
7.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366:109–119.
8.Blum JL, Flynn PJ, Yothers G, et al. Anthracycles in early breast cancer: the ABC trials- USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49. J Clin Oncol. 2017;35.
9.Chan A, Delaloge S, Holmes FA, et al. Neratinib after trastuzumab-based adjuvant therapy in patients with HER2-positive breast cancer (ExteNET): a multicentre, randomized, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2016;17:367–377.
10.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal AI therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–1670.
11.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14(7):583–589.
12.Coopey SB, Mazzola E, Buckley JM, et al. The role of chemoprevention in modifying the risk of breast cancer in women with atypical breast lesions. Breast Cancer Res Treat. 2012;136:627–633.
13.Cristofanilli M, Turner NC, Bondarenko I, et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016 Apr;17(4):425–439.
14.Cuzick J, Sestak I, Forbes JF, et al. Anastrozole for prevention of breast cancer in high-risk postmenopausal women (IBIS-II): an international, double-blind, randomised placebo-controlled trial. Lancet. 2014 Mar 22;383(9922):1041–1048.
15.Dai X, Li T, Bai Z, et al. Breast cancer intrinsic subtype classification, clinical use and future trends. Am J Cancer Res. 2015;5(10):2929–2943.
16.Davies C, Pan H, Godwin J, et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013 Mar 9;381(9869):805–816.
17.Dhesy-Thind S, Fletcher GG, Blanchette PS, et al. Use of adjuvant bisphosphonates and other bone-modifying agents in breast cancer: a cancer care Ontario and American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2017;35:2062–2081.
18.Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1: a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res. 2017;23(17):5218–5224.
19.Dieras V, Miles D, Verma S, et al. Trastuzumab emtansine versus capecitabine plus lapatinib in patients with previously treated HER2-positive advanced breast cancer (EMILA): a descriptive analysis of final overall survival results from a randomized, open-label, phase 3 trial. Lancet Oncol. 2017;18:732–742.
20.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomized trials. Lancet. 2015;386:1353–1361.
21.Emens LA, Adams S, Loi S, et al. Impassion130: a phase III randomized trial of atezolizumab with nab-paclitaxel for first-line treatment of patients with metastatic triple-negative breast cancer (mTNBC). Abstract presented at: ASCO Annual Meeting. 2016; Chicago, IL. Abstract TPS1104.
22.Fisher B, Costantino, Wickerham DL, et al. Tamoxigen for the prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project p-1 study. J Natl Cancer Inst. 1998; 90:1371–1388.
23.Fisher B, Costantino J, Wickerham DL, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97(22):1652–1662.
24.Finn RS, Crown JP, Lang I, et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015 Jan;16(1):25–35.
25.Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med. 2016 Nov 17;375(20):1925–1936.
26.Francis PA, Regan MM, Fleming GF, et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med. 2015 Jan 29;372(5):436–446.
27.Friedewald SM, Rafferty EA, Rose SL, et al. Breast cancer screening using tomosynthesis in combination with digital mammography. JAMA. 2014;311(24):2499–2507.
28.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER-2/neu-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743.
29.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012 Jan;13(1):25–32.
30.Gnant M, Mlineritsch B, Schippinger W, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–691.
31.Goss P, Ingle J, Ales-Martinez J, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381–2391.
32.Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375:209–219.
33.Gray RG, Rea D, Handley K, et al. aTTom: Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Onco.l 2013;31:(suppl; abstr 5).
34.Harris LN, Ismaila N, McShane LM, et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(10):1134–1150.
35.Hartmann LC, Degnim AC, Santen RJ, et al. Atypical hyperplasia of the breast-risk assessment and management options. N Engl J Med. 2015;372:78–89.
36.Henry NL, Bedard PL, DeMichele A. Standard and Genomic Tools for Decision Support in Breast Cancer Treatment. 2017 ASCO Educational Book. asco.org/edbook
37.Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer-interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: A randomized clinical trial. JAMA. 2017 Jan 3;317(1):48–58.
38.Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med. 2016;375:1738–1748.
39.Hortobagyi GN, Van Poznak C, Harker WG, et al. Continued treatment effect of zoledronic acid dosing every 12 weeks vs 4 weeks in women with breast cancer metastatic to bone. The OPTIMIZE-2 randomized clinical trial. JAMA Oncology. 2017;3(7):906–912.
40.Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast J. 2013;22:101–108.
41.Hu X, Huang W, Fan M. Emerging therapies for breast cancer. J Hematol Oncol. 2017;10:98
42.Jones SE, Savin MA, Holmes FA, et al. Phase III trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–5387.
43.Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician's choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014 Jun;15(7):689–699.
44.Krop IE, Kim SB, Gonzalez Martin A, et al. Trastuzumab emtansine versus treatment of physician’s choice in patients with previously treated HER2-positive metastatic breast cancer (TH3RESA): final overall survival results from a randomised open-label phase 3 trial. Lancet Oncol. 2017:18:743–754.
45.Lambertini M, Kroman N, Ameye L, et al. Safety of pregnancy in patients with history of estrogen receptor positive (ER+) breast cancer: Long-term follow-up analysis from a multicenter study. J Clin Oncol. 2017. 35; suppl abstr LBA 10066.
46.Mainiero MB, Lourenco A, Mahoney MC, Newell MS, Bailey L, Barke LD et al. ACR Appropriateness criteria breast cancer screening. J Am Coll Radiol. 2013;10(1):11–14. doi: 10.1016/j.jacr.2012.09.036.
47.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313.
48.Martin M, Segui M, Anton A, et al. Adjuvant docetaxel for high-risk, node-negative breast cancer. N Engl J Med. 2012;362:2200–2210.
49.Mehta R, Barlow W, Albain K, et al. Combination anastrozole and fulvestrant in metastatic breast cancer. N Engl J Med. 2012;367:435–444.
50.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676.
51.Morrow, M, Schnitt SJ, Norton L. Current management of lesions associated with an increased risk of breast cancer. Nat Rev Clin Oncol. 2015;12:227–238.
52.Moss S, Cuckle H, Evans A, et al. Effect of mammographic screening from age 40 years on breast cancer mortality at 10 years follow-up: a randomized controlled trial. Lancet 2006;368(9552):2053–2060.
53.Muss H, Berry D, Cirrincione C, et al. Adjuvant chemotherapy in older women with early stage breast cancer. N Engl J Med. 2009;360:2055–2065.
54.Nanda R, Chow L, Dees EC, et al. Pembrolizumab in patients with advanced triple-negative breast cancer: phase Ib KEYNOTE-012 study. J Clin Oncol. 2016;34(21):2460–2467.
55.Nanda R, Liu MC, Yau C, et al. Pembrolizumab plus standard neoadjuvant therapy for high-risk breast cancer (BC): Results from I-SPY 2. Abstract presented at: ASCO Annual Meeting. 2017; Chicago, IL. Abstract 506.
56.Pagani O, Regan MM, Walley BA, et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med. 2014 Jul 10;371(2):107–118.
57.Patnaik A, Rosen LS, Tolaney SM, et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non-small cell lung cancer, and other solid tumors. Cancer Discov. 2016;6(7):740–753.
58.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32(33):3744–3753.
59.Piccart-Gebhart MJ, Holmes AP, Baselga J, et al. First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC). J Clin Oncol. 32:5s, 2014 (suppl; abstr LBA4).
60.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER-2/neu-positive breast cancer. N Engl J Med. 2005;353:1659–1672.
61.Pisano ED, Gatsonis C, Hendrick E, et al. Diagnostic performance of digital versus film mammography for breast cancer screening. N Engl J Med. 2005;353: 1773–1793.
62.Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008 Feb 10;26(5):778–785.
63.Ravdin PM, Cronin KA, Howlader N, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007;356:1670–1674.
64.Robson M, Im S-A, Senkus E, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533.
65.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER-2/neu-positive breast cancer. N Engl J Med. 2005;353:1673–1684.
66.Saad A, Abraham J. Role of tumor markers and circulating tumors cells in the management of breast cancer. Oncology (Williston Park). 2008;22:726–731; discussion 734, 739, 743–744.
67.Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013 Sep;24(9):2278–2284.
68.Seidman AD, Berry D, Cirrincione C, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2/neu- overexpressors and random assignment to trastuzumab or not in HER-2/neu- nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–1649.
69.Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015 Jan 1;33(1):13–21.
70.Slamon D, Eiermann W, Robert N, et al. BCIRG 006: 2nd interim analysis phase III randomized trial comparing doxorubicin and cyclophosphamide followed by docetaxel (AC→T) with doxorubicin and cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER-2/neu-positive early breast cancer patients. San Antonio Breast Cancer Symposium; December 14–17, 2006. San Antonio, TX; 2006.
71.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER-2/neu-positive breast cancer. N Engl J Med. 2011;365:1273–1283.
72.Slamon DJ, Eiermann W, Robert NJ, et al. Ten year follow-up of BCIRG-006 comparing doxorubicin plus cyclophosphamide followed by docetaxel (AC→T) with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab (AC→TH) with docetaxel, carboplatin and trastuzumab (TCH) in HER2+ early breast cancer. San Antonio Breast Cancer Symposium; Abstract S5-04, December 11, 2015.
73.Sledge GW, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol. 2017;35:25, 2875–2884.
74.Solin L, Gray R, Baehner F, et al. A multigene expression assay to predict local recurrence risk for ducal carcinoma in situ of the breast. J Natl Cancer Inst. 2013; 105(10):701–710.
75.Sparano J, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671.
76.Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-gene expression assay in breast cancer. N Engl J Med. 2015;373:2005–2014.
77.Swayampakula AK, Dillis C, Abraham J. Role of MRI in screening, diagnosis and management of breast cancer. Expert Rev Anticancer Ther. 2008;8:811–817.
78.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–141.
79.Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2014 Feb 15;383(9917):603–613.
80.Valdivieso M, Kujawa AM, Jones T, Baker LH. Cancer survivors in the United States: a review of the literature and a call to action. Int J Med Sci. 2012;9:163–173.
81.Valente SA, Levine GM, Silverstein MJ, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnestic resonace imaging. Ann Surg Oncol. 2012;19(6):1825–1830.
82.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER-2/neu-positive advanced breast cancer. N Engl J Med. 2012;367:1783–1791.
83.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013 Dec;14(13):1269–1277.
84.Veronesi U, Paganelli G, Viale G et al. A randomized comparison of sentinel-node biopsy with routine axillary dieesction in breast cancer. N Engl J Med. 2003; 349:546–553.
85.Vogel VG, Costantino JP, Wickerham DL, et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP study of tamoxifen and raloxifene (STAR) P-2 trial. JAMA. 2006; 295(23):2727–2741.
86.Vogel VG, Costantino JP, Wickerham DL, et al. Update of the national surgical adjuvant breast and bowel project study of tamoxifen and raloxifene (STAR) P-2 trial: preventing breast cancer. Cancer Prev Res. 2010; 3(6):696–706.
87.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzuman and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122–131.
88.Wang B, Chu D, Feng Y et al. Discovery and characterization of (8S,9R)-5-Fluoro-8-(4-fluorophenyl)-9-(1-methyl-1H-1,2,4-triazol-5-yl)-2,7,8,9-tetrahydro-3H-pyrido[4,3,2-de]phthalazin-3-one (BMN 673, Talazoparib), a Novel, Highly Potent, and Orally Efficacious Poly(ADP-ribose) Polymerase-1/2 Inhibitor, as an Anticancer Agent. J Med Chem. 2016;59(1):335–357.
89.Wimberly H, Brown JR, Schalper K, et al. PD-L1 expression correlates with tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy in breast cancer. Cancer Immunol Res. 2015;3: 326–332.