RADIATION- AND CHEMOTHERAPY-ASSOCIATED EMETIC SYMPTOMS
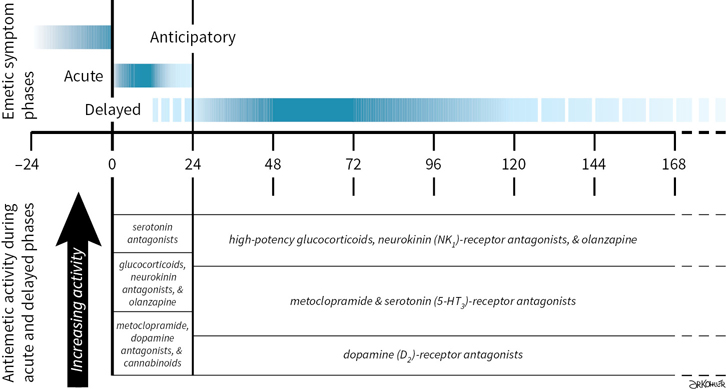
Radiation- and chemotherapy-associated emetic symptoms are labeled as “acute” or “delayed” by their temporal relationship with the start of emetogenic treatments (Fig. 38.1). Although the terms are useful for describing clinical events and approaches to symptom management, the assignment of symptoms onset and duration to fixed periods predated identification of principal neural mechanisms that elicit acute- and delayed-phase symptoms, and remain an oversimplification of physiological events that occur when emetogenic treatments are repeated within the span of a single day or on two or more consecutive days.
Acute-phase Symptoms
Emetic symptoms that occur within 24 hours after treatment are identified as acute-phase symptoms (Table 38.1). Acute-phase symptoms have been shown to correlate with serotonin (5-hydroxytryptamine, 5-HT) release from enterochromaffin cells. Emetic signals are propagated at local serotonin (5-HT3 subtype) receptors and transmitted along afferent vagus nerve fibers. They activate a diffuse series of effector nuclei in the medulla oblongata (the so-called “vomiting center”), which integrates afferent emetic signals and subsequently activates and coordinates motor nuclei that produce the physiologic changes associated with vomiting.
 In general, the greatest incidence of acute symptoms occurs within 2 to 6 hours after treatment.
In general, the greatest incidence of acute symptoms occurs within 2 to 6 hours after treatment.
 Onset is generally within 1 to 3 hours after commencing chemotherapy. Notable exceptions include the following:
Onset is generally within 1 to 3 hours after commencing chemotherapy. Notable exceptions include the following:
•Mechlorethamine (nitrogen mustard), which generally induces rapid symptom onset (≤1 hour)
•Cyclophosphamide, after intravenous administration, and carboplatin have long latency periods before acute-phase onset, and symptoms may persist or intermittently recur for ≥12 hours after treatment.
Delayed-Phase Symptoms
Delayed-phase symptoms are defined as those that occur >24 hours after treatment (Table 38.1) and are associated with central activation of neurokinin type 1 (NK1) receptors, for which substance P is the natural ligand. Drugs with high emetogenic potential and, in some cases, drugs with moderate emetic risk may cause delayed-phase symptoms (Table 38.2). Symptoms may commence as early as 16 to 18 hours after emetogenic treatment, with a period of greatest incidence between 24 and 96 hours after treatment. Delayed emesis may occur in patients who do not experience symptoms acutely, but incidence characteristically decreases in patients who achieve complete emetic control during the acute phase. Although emesis is typically less severe during the delayed phase than during the acute phase, the reported severity of nausea is similar during both phases.
Anticipatory Events
Anticipatory nausea or vomiting describes emetic symptoms occurring before repeated exposure to emetogenic treatment that develop as an aversive conditioned response as a consequence of poor emetic control during prior therapy with nausea reported to occur more commonly than anticipatory vomiting. The risk of developing anticipatory symptoms has been shown to increase with repeated courses of emetogenic treatment, particularly in patients who experience incomplete emetic control during treatments they previously received, but emetic symptoms during pregnancy and motion sickness have been identified as contributing risk factors. Although anxiolytic amnestic drugs are helpful in preventing and delaying anticipatory symptoms, complete control throughout all antineoplastic treatments is the best preventive strategy against developing symptoms. Behavioral therapies such as relaxation techniques and systematic desensitization may be useful if symptoms occur. After symptoms develop, medical interventions for anticipatory symptoms during subsequent emetogenic treatment are limited to preventing the reinforcement of conditioned stimulae, which may exacerbate symptoms.
EMETOGENIC (EMETIC) POTENTIAL
Emetic potential or risk and symptom patterns vary among medications used in antineoplastic chemotherapy and radiation therapy techniques.
Chemotherapy
Intrinsic emetogenicity (Table 38.3) is an antineoplastic drug’s propensity for causing emetic symptoms. Drug dose or dosage is often the second most significant factor affecting emetogenic potential and the duration for which symptoms persist.
The number of emetogenic drugs used in combination, administration schedule, treatment duration, and route of administration are also mitigating factors. Emetic potential may be lessened or eliminated by protracted drug delivery over hours or days, and increased by rapid drug administration, repeated emetogenic treatments, and brief intervals between repeated doses (Table 38.3). When emetogenic treatment is given on more than one day, physiological processes associated with acute and delayed phase symptoms may overlap and both should be considered in designing effective antiemetic prophylaxis. The potential and duration for delayed symptoms depends upon the sequence in which emetogenic drugs are administered and the emetogenic risk each drug presents.
Radiation
The emetic potential of ionizing radiation correlates directly with the amount of radiation given per dose or fraction, the total dose administered, and the rate at which it is administered. Largetreatment volumes (>400 cm2); fields including the upper abdomen, upper hemithorax, and whole body; and a history of poor emetic control with chemotherapy are risk factors for severe emesis. Emetic potential increases when radiation and chemotherapy are administered concomitantly (“radiochemotherapy”).
PATIENT RISK FACTORS
Patients at greatest risk for emetic symptoms include the following:
 Female sex, particularly women with a history of persistent and/or severe emetic symptoms during pregnancy
Female sex, particularly women with a history of persistent and/or severe emetic symptoms during pregnancy
 Children and young adults
Children and young adults
 Patients with a history of acute- and/or delayed-phase emetic symptoms during prior treatments are at great risk for poor emetic control during subsequent treatments
Patients with a history of acute- and/or delayed-phase emetic symptoms during prior treatments are at great risk for poor emetic control during subsequent treatments
 Patients with low performance status and a predisposition to motion sickness.
Patients with low performance status and a predisposition to motion sickness.
 Nondrinkers are at greater risk than patients with a history of chronic alcohol consumption (>100 g ethanol daily for several years)
Nondrinkers are at greater risk than patients with a history of chronic alcohol consumption (>100 g ethanol daily for several years)
 Patients with intercurrent pathologies, such as gastrointestinal (GI) inflammation, compromised GI motility or obstruction, constipation, brain metastases, metabolic abnormalities (hypovolemia, hypercalcemia, hypoadrenalism, uremia), visceral organs invaded by tumor, and concurrent medical treatment (opioids, bronchodilators, aspirin, nonsteroidal anti-inflammatory drugs), may predispose and exacerbate emetic symptoms during treatment and complicate good emetic control
Patients with intercurrent pathologies, such as gastrointestinal (GI) inflammation, compromised GI motility or obstruction, constipation, brain metastases, metabolic abnormalities (hypovolemia, hypercalcemia, hypoadrenalism, uremia), visceral organs invaded by tumor, and concurrent medical treatment (opioids, bronchodilators, aspirin, nonsteroidal anti-inflammatory drugs), may predispose and exacerbate emetic symptoms during treatment and complicate good emetic control
PRIMARY ANTIEMETIC PROPHYLAXIS
Primary prophylaxis is indicated for all patients whose antineoplastic treatment presents at least a low risk of producing emetic symptoms; that is, when >10% of persons receiving similar chemotherapy or radiation therapy without antiemetic prophylaxis are predicted to experience emetic symptoms (Table 38.3).
 Planning effective antiemetic primary prophylaxis
Planning effective antiemetic primary prophylaxis
•Evaluate the emetic potential for each drug included in treatment, which includes the severity, onset, and duration of symptoms associated with individual drugs (Table 38.1), and how drug dose or dosage, schedule, and route of administration may affect those factors.
•Patients who receive combination chemotherapy should receive antiemetic prophylaxis based on the most emetogenic component of treatment.
•Include primary prophylaxis against acute-phase symptoms for all treatments with low, moderate, or high emetic potential, and delayed-phase prophylaxis for treatments with moderate or high emetic potential.
•For patients who receive antineoplastic chemotherapy and radiation concomitantly, antiemetic prophylaxis is selected based on the chemotherapy component that presents the greatest emetogenic potential, unless the emetic risk from radiation is greater.
•Patients who receive moderately or highly emetogenic treatment for more than one day should receive antiemetic prophylaxis appropriate for the drug with greatest emetogenic potential on each day of treatment.
•If antineoplastic treatment is associated with delayed emetic symptoms, continue antiemetic prophylaxis:
•For at least three days after highly emetogenic treatment is completed.
•For at least two days after moderately emetogenic treatment is completed.
•Treatment-appropriate antiemetic prophylaxis should precede each emetogenic treatment and proceed on a fixed schedule. Patients should not be expected to recognize symptom prodromes and to rely on unscheduled (i.e., as needed) antiemetics.
•Antiemetics should be given at the lowest effective doses.
•Patients’ responses to antiemetic prophylaxis and treatment should be serially monitored and documented with standardized validated tools.
•Health care providers historically underestimate the incidence and severity of emetic symptoms associated with chemotherapy and radiation therapy, particularly nausea.
•Patient input is essential to capture information about
•Events that health-care providers cannot observe due to patient location and the subjective nature of nausea.
•Conditions and interventions that modulate a patient’s emetic symptoms.
•Changes in a patient’s response to antiemetic prophylaxis through a succession of treatment cycles or courses.
•The MASCC has developed and makes available online a standardized eight-item questionnaire that can be used to document the number of vomiting episodes and the number and severity of episodes of nausea both acutely and within the four days (24 to 120 hours) following the day on which emetogenic treatment was given.
•The MASCC Antiemesis Tool (MAT), a guide for using the tool, and Patient Outcomes Score Sheets are available in 17 languages in digital formats for downloading, and in an application for handheld devices.
•Non-profit entities may use the MAT without incurring charges. Commercial companies are required to obtain written approval from MASCC, and will incur a nominal fee prior to using the MAT.
•Information about gaining approval for using the MAT is available online at http://www.mascc.org/index.php?option=com_content&view=article&id=352:MAT&catid=24:guidelines-and-assessment-tools [Accessed 4 December 2017].
Figure 38.2 integrates evidence-based guidelines for treatment-appropriate antiemetic prophylaxis recommended by the National Comprehensive Cancer Network, the Multinational Association of Supportive Care in Cancer and European Society for Medical Oncology, the American Society of Clinical Oncology, current labeling for medications that have received U.S. Food and Drug Administration (FDA) approval for commercial use, and the consensus of experts in oncology. Recommendations are based on the assessment of emetic risk and generally apply to adult patients, but may not be appropriatein all clinical situations. Drug selection and utilization should be tempered by professional judgment, including an assessment of patient-specific risk factors and circumstances, and recognition of available resources.
Clinicians may expect to encounter a minority of patients who do not respond to treatment-appropriate antiemetic prophylaxis recommended by oncology specialty organizations’ guidelines. Suboptimal antiemetic prophylaxis places patients at risk for breakthrough and refractory emetic symptoms and debilitating morbidity, which may adversely affect patient safety, comfort, and quality of life, and complicate their care.
For patients who respond sub-optimally to initial antiemetic prophylaxis, re-evaluate factors that may cause or contribute to emetic symptoms, and those that may compromise the effectiveness of pharmacological prophylaxis, including
 The emetogenic risk associated with treatment.
The emetogenic risk associated with treatment.
•The appropriateness of initial antiemetic prophylaxis for the emetogenic challenge presented by treatment.
•Selection of drugs, doses/dosages, and administration routes and schedules for use.
 Healthcare provider adherence in prescribing and patient compliance in using planned antiemetic prophylaxis.
Healthcare provider adherence in prescribing and patient compliance in using planned antiemetic prophylaxis.
 Disease status.
Disease status.
 Co-morbid conditions (electrolyte abnormalities, renal failure, sepsis, constipation, tumor infiltrating or obstructing the gastrointestinal tract, intracranial disease, vestibular dysfunction).
Co-morbid conditions (electrolyte abnormalities, renal failure, sepsis, constipation, tumor infiltrating or obstructing the gastrointestinal tract, intracranial disease, vestibular dysfunction).
 Whether medications concomitantly administered with emetogenic drugs and antiemetics may potentially compromise antiemetic effectiveness:
Whether medications concomitantly administered with emetogenic drugs and antiemetics may potentially compromise antiemetic effectiveness:
•Using medications with intrinsic emetogenic potential unrelated to antineoplastic treatment that nevertheless increase the cumulative emetogenic burden.
•By altering the pharmacokinetics of emetogenic drugs that result in exposures greater in magnitude or duration than would otherwise occur.
•By altering the pharmacokinetics of agents used in antiemetic prophylaxis or treatment.
Empiric secondary prophylaxis and treatment for patients who demonstrate sub-optimal antiemetic control should follow a rational approach. In general, pharmacological interventions typically include drugs presumed to mediate antiemetic effects through an interaction with one or more neurotransmitter receptors implicated in either provoking or mitigating emesis, and through mechanisms not exploited by antiemetics already in use. Unfortunately, drugs used empirically often are less safe at effective or clinically useful doses and schedules (e.g., dopaminergic and cannabinoid receptor antagonists) than agents recommended for primary prophylaxis. Whether used adjunctively or as replacement for initial prophylaxis, second-line alternatives may increase treatment costs and the risk of overtreatment and adverse effects.
BREAKTHROUGH SYMPTOMS
Primary antiemetic prophylaxis recommended by oncology specialty organizations’ guidelines are associated with complete control (no emesis) during the acute phase in ≥80% of patients who receive highly emetogenic treatments and even greater complete control rates in the setting of moderately emetogenic treatment; however, more than 50% of patients who receive moderately or highly emetogenic therapy still may experience delayed or breakthrough nausea or emesis in spite of good control achieved acutely. In general, it is more difficult to arrest emetic symptoms after they develop than it is to prevent them from occurring. Breakthrough symptoms require rapid intervention. All patients who receive moderately or highly emetogenic treatment should from the outset of treatment have access to antiemetic medications for treating breakthrough symptoms, whether through orders for treatment during a visit or admission to a healthcare facility, or, for outpatients, a supply of antiemetic medication and clear instructions about how to use it in supplementing or modifying their initial antiemetic regimen. If needed and once begun, breakthrough treatment should be administered at scheduled intervals and continued at least until after emetogenic treatment is completed and symptoms abate.
In general, nausea may still occur and often is more prevalent than vomiting in patients who achieve overall good or better control of emesis during the acute and delayed symptoms phases.
Sub-optimal Control
Sub-optimal control of emetic symptoms with antiemetic prophylaxis raises the following questions:
 Was the prophylactic strategy given an adequate trial (time of initiation relative to the start of emetogenic treatment and duration of use)?
Was the prophylactic strategy given an adequate trial (time of initiation relative to the start of emetogenic treatment and duration of use)?
 Were the antiemetics selected and the doses and administration schedules prescribed appropriate for the emetogenic challenge?
Were the antiemetics selected and the doses and administration schedules prescribed appropriate for the emetogenic challenge?
 Did the patient understand and comply with instructions for antiemetic use?
Did the patient understand and comply with instructions for antiemetic use?
 Would increased doses or shorter administration intervals improve antiemetic effectiveness without causing or exacerbating adverse effects associated with the antiemetics utilized?
Would increased doses or shorter administration intervals improve antiemetic effectiveness without causing or exacerbating adverse effects associated with the antiemetics utilized?
Rescue Interventions
If it becomes necessary to “rescue” a patient from a suboptimal response
 Assess a symptomatic patient’s state of hydration and serum/plasma electrolytes for abnormal results.
Assess a symptomatic patient’s state of hydration and serum/plasma electrolytes for abnormal results.
•Replace fluids and electrolytes as needed.
 Add antiemetic agents that act through mechanisms different from antiemetics already in use.
Add antiemetic agents that act through mechanisms different from antiemetics already in use.
•It may be necessary to use more than one additional drug to establish antiemetic control.
 Give scheduled doses around-the-clock at least until emetogenic treatment is completed, and at doses and on a schedule appropriate for the medication.
Give scheduled doses around-the-clock at least until emetogenic treatment is completed, and at doses and on a schedule appropriate for the medication.
•Do not rely on as needed administration to achieve or maintain control of emetic symptoms.
 Consider replacing ineffective drugs with a more potent or longer-acting agent from the same pharmacologic class.
Consider replacing ineffective drugs with a more potent or longer-acting agent from the same pharmacologic class.
 Consider replacing an antiemetic medication that requires ingestion and absorption from the gastrointestinal tract or percutaneous absorption with the same or a different drug given by a different administration route (disintegrating tablets and soluble films for oral administration, injectable formulations).
Consider replacing an antiemetic medication that requires ingestion and absorption from the gastrointestinal tract or percutaneous absorption with the same or a different drug given by a different administration route (disintegrating tablets and soluble films for oral administration, injectable formulations).
•Emetic symptoms may impair GI motility and drug absorption from the gut.
•Some patients may be too ill to swallow and retain oral medications.
•Rectal suppositories are a practical alternative for patients who cannot ingest medications, but the rate and extent of absorption varies among drugs and patients.
•Clinicians should query and ascertain patients’ willingness to comply with rectal administration.
•Sustained- and extended-release formulations (oral, transdermal patches, and injectable sustained- or extended-release products) should not be used to initially bring ongoing symptoms under control.
 Replace drugs associated with unacceptable adverse effects with one or more drugs from the same or a different pharmacologic class without a potential for the same toxicity, or for which particular adverse effects are less likely to occur.
Replace drugs associated with unacceptable adverse effects with one or more drugs from the same or a different pharmacologic class without a potential for the same toxicity, or for which particular adverse effects are less likely to occur.
These strategies may be utilized during cyclical treatment or to intervene when response to prophylaxis is unsatisfactory.
Secondary Antiemetic Prophylaxis and Treatment
When antiemetic treatment is needed for breakthrough symptoms, re-evaluate the prophylactic regimen that failed to provide adequate antiemetic control before repeating cycles of emetogenic treatment. Consider alternative antiemetic prophylaxis strategies during subsequent emetogenic treatments, including
 Consider escalating antiemetic prophylaxis to a regimen appropriate for the next greater level of emetic risk.
Consider escalating antiemetic prophylaxis to a regimen appropriate for the next greater level of emetic risk.
 Add additional scheduled antiemetics at appropriate doses and administration intervals.
Add additional scheduled antiemetics at appropriate doses and administration intervals.
•Consider drugs that previously proved of value in controlling breakthrough symptoms or another drug that acts through the same pharmacological mechanism.
 For regimens that included a 5-HT3 antagonist, consider switching to a different 5-HT3 antagonist.
For regimens that included a 5-HT3 antagonist, consider switching to a different 5-HT3 antagonist.
•Not all patients achieve the same measure of antiemetic control with every 5-HT3 antagonist.
 Consider adding an anxiolytic drug to the patient’s regimen.
Consider adding an anxiolytic drug to the patient’s regimen.
 Consider adding a NK1-receptor antagonist to antiemetic prophylaxis if its potential for pharmacokinetic interactions will not adversely affect concomitantly administered medications.
Consider adding a NK1-receptor antagonist to antiemetic prophylaxis if its potential for pharmacokinetic interactions will not adversely affect concomitantly administered medications.
 If alternative treatment for a patient’s neoplastic disease exists, consider a different regimen with which similar therapeutic benefit may be achieved without greater adverse outcomes.
If alternative treatment for a patient’s neoplastic disease exists, consider a different regimen with which similar therapeutic benefit may be achieved without greater adverse outcomes.
•Perhaps worth considering only if the goal of treatment is not curative.
NON-PHARMACOLOGICAL INTERVENTIONS
 Guidance for patients that may preserve nutritional status and alleviate emetic symptoms, include
Guidance for patients that may preserve nutritional status and alleviate emetic symptoms, include
•Eat small frequent meals low in fat content, especially for patients with anorexia or early satiety.
•Choose healthful foods.
•Eat soft, bland, easily digested foods served at room temperature.
•Eat dry foods; for example, crackers, toasted bread, and dry cereals.
•Avoid foods and beverages known or found to produce nausea.
•Advise patients to avoid favorite foods to prevent developing conditioned aversions to those foods, particularly at times when emetic symptoms are anticipated to occur.
•Avoid sweet, fatty, highly salted and spicy foods, dairy products, and foods with strong odors.
•For patients who are nauseated by the smell of food:
•Let someone else do the cooking. Leave areas when and where cooking smells are present.
•Avoid foods and beverages that provoke nausea.
•Patients may experience sensitivities to food odors, appearance, taste, textures (“mouth feel”).
•Greasy and fried foods and brewing coffee may provoke symptoms.
•Suggest prepared foods that can be warmed at a low temperature or a meal that does not need to be cooked.
 Acupressure or acupuncture
Acupressure or acupuncture
•Stimulation of the ventral side of the wrist where the median nerve is closest to the surface of the skin, an acupuncture point referred to as pericardium-6 (P-6) or Neiguan point may be of benefit in some patients.
ANTIEMETIC DRUGS
Serotonin (5-HT3 subtype)-Receptor Antagonists
 Among 5-HT3-receptor antagonists that have received FDA approval for commercial use, dolasetron, granisetron, and ondansetron comprise the first-generation agent, palonosetron is a second-generation agent.
Among 5-HT3-receptor antagonists that have received FDA approval for commercial use, dolasetron, granisetron, and ondansetron comprise the first-generation agent, palonosetron is a second-generation agent.
Acute Phase
 5-HT3-receptor antagonists are safer and more effective against acute-phase symptoms than other pharmacological classes of medications with clinically useful antiemetic activity.
5-HT3-receptor antagonists are safer and more effective against acute-phase symptoms than other pharmacological classes of medications with clinically useful antiemetic activity.
 Administering 5-HT3-receptor antagonists at doses/dosages greater than those shown to be maximally effective do not substantially improve emetic control.
Administering 5-HT3-receptor antagonists at doses/dosages greater than those shown to be maximally effective do not substantially improve emetic control.
 Single-dose prophylaxis is preferred for acute-phase symptoms.
Single-dose prophylaxis is preferred for acute-phase symptoms.
•After administration of a single maximally effective dose, additional doses of 5-HT3-receptor antagonists within the first 24 hours after emetogenic treatment have not been shown to improve emetic control.
 Dolasetron, granisetron, ondansetron, and palonosetron have excellent oral bioavailability, and, when given at maximally effective doses, each agent provides equivalent antiemetic protection whether given orally or parenterally.
Dolasetron, granisetron, ondansetron, and palonosetron have excellent oral bioavailability, and, when given at maximally effective doses, each agent provides equivalent antiemetic protection whether given orally or parenterally.
Delayed Phase
 Metoclopramide and prochlorperazine are less expensive, and as effective as dolasetron, granisetron, and ondansetron at controlling emetic symptoms.
Metoclopramide and prochlorperazine are less expensive, and as effective as dolasetron, granisetron, and ondansetron at controlling emetic symptoms.
 Palonosetron has the longest half-life among 5-HT3-receptor antagonists currently marketed in the USA, and has additional pharmacological characteristics not shared by first-generation 5-HT3-receptor antagonists.
Palonosetron has the longest half-life among 5-HT3-receptor antagonists currently marketed in the USA, and has additional pharmacological characteristics not shared by first-generation 5-HT3-receptor antagonists.
•A single dose of palonosetron 0.5 mg orally, or 0.25 mg intravenously, is recommended before starting chemotherapy, but other doses and schedules have proven safe (see below).
Potential Side Effects
 Side effects common to all 5-HT3-receptor antagonists include
Side effects common to all 5-HT3-receptor antagonists include
•Headache
•Constipation
•Diarrhea
•Transiently increased hepatic transaminase concentrations
•Transient effects on cardiac electrophysiology, decreased cardiac rate, and cardiovascular adverse effects (see drug-specific comments below)
•Serotonin syndrome, most often associated with concomitant use of drugs that affect serotonin neurotransmission and/or reuptake
5-HT3-Receptor Antagonists and Pharmacogenomics
 Pharmacogenomic evaluation may help to identify patients at risk for sub-optimal and adverse responses to 5-HT3-receptor antagonists that are substrates for catabolism by cytochrome P450 (CYP) enzymes (Table 38.4).
Pharmacogenomic evaluation may help to identify patients at risk for sub-optimal and adverse responses to 5-HT3-receptor antagonists that are substrates for catabolism by cytochrome P450 (CYP) enzymes (Table 38.4).
 CYP2D6 is polymorphically expressed among human populations.
CYP2D6 is polymorphically expressed among human populations.
•Persons with >2 functionally competent (wild type) CYP2D6 alleles may have increased metabolic capacity (characterized as ultra-rapid metabolizers), which has been associated with diminished emetic control in patients who received 5-HT3-receptor antagonists for which CYP2D6 metabolism predominates.
•Patients who lack one or both CYP2D6 alleles or express one or more variant alleles with reduced function generally have altered functional capacity for CYP2D6 substrates (poor and intermediate metabolizers) and may have high concentrations and attenuated elimination of 5-HT3-receptor antagonist substrates for which CYP2D6 metabolism predominates.
 Patients who express genetic polymorphism for 5-HT3 receptors or the ABCB1 (P-glycoprotein, MDR1) transporter may experience suboptimal antiemetic responses with 5-HT3-receptor antagonists.
Patients who express genetic polymorphism for 5-HT3 receptors or the ABCB1 (P-glycoprotein, MDR1) transporter may experience suboptimal antiemetic responses with 5-HT3-receptor antagonists.
Dolasetron
 The oral tablet formulation is approved for use in antiemetic prophylaxis in initial and repeat courses of moderately emetogenic chemotherapy in patients ≥2 years of age.
The oral tablet formulation is approved for use in antiemetic prophylaxis in initial and repeat courses of moderately emetogenic chemotherapy in patients ≥2 years of age.
 The frequency and magnitude of adverse effects associated with dolasetron use are related to serum concentrations of hydrodolasetron, its active metabolite.
The frequency and magnitude of adverse effects associated with dolasetron use are related to serum concentrations of hydrodolasetron, its active metabolite.
•On December 17, 2010, the U.S. FDA announced the removal of the indication for dolasetron mesylate injection for preventing nausea and vomiting associated with initial and repeated courses of emetogenic chemotherapy, and the addition to product labeling of a contraindication against this use in pediatric and adult patients. The FDA Communication explained dolasetron mesylate causes a dose-dependent prolongation in cardiac QT, PR, and QRS intervals that can increase the risk of developing torsade de pointes, which may be fatal [FDA Drug Safety Communication: Abnormal heart rhythms associated with the use of Anzemet (dolasetron mesylate). URL: https://www.fda.gov/drugs/drugsafety/ucm237081.htm [Accessed 4 December 2017].
•Risk factors for serious abnormal arrhythmias include
•Underlying structural heart disease and preexisting conduction system abnormalities; for example, patients with congenital long-QT syndrome, complete heart block, or those at risk for complete heart block
•Elderly individuals
•Sick sinus syndrome, atrial fibrillation with slow ventricular response, myocardial ischemia, persons receiving drugs known to prolong the PR interval (e.g., verapamil) and QRS interval (e.g., flecainide, quinidine)
•Hypokalemia or hypomagnesemia
•Serum potassium and magnesium concentrations should be evaluated, and, if abnormal, corrected before initiating treatment with dolasetron.
•Potassium and magnesium concentrations should be monitored after dolasetron administration as clinically indicated.
•Patients at risk for developing hypokalemia or hypomagnesemia while receiving dolasetron should be monitored with ECG.
•The FDA also recommended ECG monitoring in patients with congestive heart failure, bradycardia, underlying heart disease, and in the elderly and patients with renal impairment who receive dolasetron.
•Dolasetron mesylate tablets may still be used in antiemetic prophylaxis for emetogenic chemotherapy, because the risk of developing aberrant cardiac conduction with the oral formulation is considered less than what has been observed with the dolasetron injection.
•Dolasetron mesylate injection also retained FDA approval for the prevention and treatment of postoperative nausea and vomiting patients whose age is ≥2 y, because dosages for that indication (intravenously, a single dose of 0.35 mg/kg up to a maximum of 12.5 mg/dose) are less than those used in antiemetic prophylaxis for chemotherapy (1.8 mg/kg·dose up to a maximum of 100 mg/dose), and therefore, are less likely to adversely affect cardiac electrophysiology.
Granisetron
 Indicated for use in antiemetic prophylaxis in initial and repeated courses of emetogenic cancer therapies, including high-dose cisplatin and radiation therapy.
Indicated for use in antiemetic prophylaxis in initial and repeated courses of emetogenic cancer therapies, including high-dose cisplatin and radiation therapy.
•Granisetron Injection has received FDA approval for use in patients ≥2 years of age.
•Injectable products may contain benzyl alcohol, which has been associated with serious adverse reactions, including death in neonates.
•Injectable formulations without preservatives are currently marketed.
•Oral formulations (tablets and solution) have not received FDA approval for use in pediatric patients.
•Product labeling for granisetron tablets and solution for oral administration include descriptions of use in patients 2–16 years of age, but, in contrast with labeling for injectable formulations, stipulate safety and effectiveness in pediatric patients have not been established.
 Granisetron transdermal patch (Sancuso®; Kyowa Kirin, Inc., Bedminster, NJ), an adhesive-backed patch (52 cm2), contains 34.3 mg of granisetron and delivers an average daily dose of 3.1 mg granisetron for up to 7 days.
Granisetron transdermal patch (Sancuso®; Kyowa Kirin, Inc., Bedminster, NJ), an adhesive-backed patch (52 cm2), contains 34.3 mg of granisetron and delivers an average daily dose of 3.1 mg granisetron for up to 7 days.
•The patch is indicated for the prevention of nausea and vomiting in patients receiving moderately and/or highly emetogenic chemotherapy regimens of up to 5 consecutive days duration.
•Safety and effectiveness in pediatric patients have not been established.
•A patch is applied to clean, dry, intact skin on the outer upper arm a minimum of 24 hours (up to 48 hours) before emetogenic chemotherapy administration and remains in place ≥24 hours after chemotherapy is completed.
•The duration of application should not exceed 7 days.
•Patches should not be cut into pieces.
•Patches should not be reapplied or reused after being removed from an application site.
•During clinical development, patients who received granisetron 3.1 mg/day transdermally for up to 7 days experienced a comparatively greater incidence of constipation than patients who received granisetron 2 mg/day orally for 1–5 days (5.4% vs. 3%, respectively) and a lesser incidence of headache (0.7% vs. 3%, respectively).
•Continuous transdermal administration of granisetron may increase the potential for 5-HT3-receptor antagonists to mask progressive ileus and gastric distention attributable to malignancy or another pathology.
•Granisetron may degrade with exposure to natural or artificial sunlight (e.g., sun lamps, tanning beds), and results of an in vitro study suggested a potential for photogenotoxicity. Patients must be instructed to keep the transdermal patch covered with clothing at all times, and to keep the application site protected from light exposure for 10 days after a patch is removed.
•Application of a heating pad (average temperature 42°C [107.6°]) over a granisetron transdermal patch for four hours daily during five consecutive days of wear resulted in increased concentrations of granisetron in plasma during the period when heat was applied. Heating pads and other heat sources should not be applied over or near a site where a granisetron transdermal patch is applied.
•Adverse effects unique to the product and associated with the route of administration, such as
•Skin reactions at or around the site of patch application, including the following:
•Patch non-adhesion
•Erythema
•Irritation, pain
•Hypersensitivity reactions (erythematous macular or papular rashes, pruritis, urticaria)
•Vesicle formation, burn
 Granisetron extended-release injection for subcutaneous use (SUSTOL®; Heron Therapeutics, Redwood City, CA). Each prefilled syringe contains 10 mg granisetron incorporated in an extended-release polymer vehicle comprising one dose.
Granisetron extended-release injection for subcutaneous use (SUSTOL®; Heron Therapeutics, Redwood City, CA). Each prefilled syringe contains 10 mg granisetron incorporated in an extended-release polymer vehicle comprising one dose.
•Indicated in combination with other antiemetics for prophylaxis against acute and delayed emetic symptoms during initial and repeated courses of moderately emetogenic cancer therapies or anthracycline + cyclophosphamide-containing regimens.
•Each subcutaneously administered dose continuously delivers granisetron for an extended period of time.
•Measurable granisetron levels can be detected in serum for up to seven days after administration.
•Safety and effectiveness in patients <18 years of age have not been established.
•Preparation and administration
•SUSTOL® is intended ONLY for subcutaneous injection administered by a health care provider.
•The product is supplied as a kit stored under refrigeration (2°–8°C [35.6°–46.4°F]) and protected from light.
•Each kit contains a single-use, amber, glass syringe pre-filled with the granisetron/polymer matrix product, a special thin-walled administration needle, two pouches for warming the syringe, and a needle protection device.
•None of the kit components should be substituted with other materials or devices.
•Preparation for use entails:
•Kit removal from refrigeration at least 60 minutes before administration.
•Warming the kit contents to room temperature.
•Activating a syringe warming pouch and wrapping the syringe pre-filled with medication in the pouch for 5–6 minutes.
•After warming the product syringe to body temperature, the drug is administered as a single subcutaneous injection in the skin of the back of the upper arm or the abdomen at least one inch from the umbilicus, avoiding sites where skin is burned, hardened, inflamed, swollen, or otherwise compromised.
•A topical anesthetic may be used at the injection site prior to administration.
•The drug product is a viscous liquid and should be administered subcutaneously, slowly by sustained pressure over 20 to 30 seconds.
•Health care providers are advised depressing the syringe plunger forcefully will NOT hasten administration.
•Initial and repeated administration is constrained by renal function:
•Not more frequently than every 7 days in patients whose creatinine clearance (Clcr) is ≥60 mL/min (≥1 mL/s)
•Not more frequently than every 14 days in patients with Clcr = 30 to 59 mL/min (0.5–0.98 mL/s)
•AVOID use in patients with Clcr <30 mL/min (<0.5 mL/s)
•Adverse effects unique to the product and associated with the route of administration, include
•Infections at the injection site.
•Bleeding at the injection site.
•Bruising/hematomas at the injection site with median onset of 2 days; delayed onset ≥5 days in 15% of patients.
•Pain and tenderness at the injection site with median duration of 5 days, but persisting for >7 days in 6% of patients.
•Nodule formation at the injection site that may persist for >3 weeks after administration.
 ECG abnormalities are rare with granisetron use at FDA-approved dosages and schedules.
ECG abnormalities are rare with granisetron use at FDA-approved dosages and schedules.
Ondansetron
 Used in antiemetic prophylaxis in initial and repeat courses of emetogenic cancer therapies:
Used in antiemetic prophylaxis in initial and repeat courses of emetogenic cancer therapies:
•Ondansetron injection has received FDA approval for use in patients ≥6 months of age for the prevention of emetic symptoms associated with highly emetogenic chemotherapy.
•Oral formulations (tablets, orally disintegrating tablets, film, and solution) have received FDA approval for use in patients ≥4 years of age receiving moderately emetogenic chemotherapy.
•The safety and effectiveness of ondansetron formulations for oral administration have not received FDA approval for use in pediatric patients for the prevention of emetic symptoms associated with highly emetogenic cancer chemotherapy or radiation therapy.
 The risk of adverse effects is low at FDA-approved dosages and schedules.
The risk of adverse effects is low at FDA-approved dosages and schedules.
•The risk of ECG abnormalities associated with use has been shown to vary directly with the dose administered.
 On June 29 2012, the FDA announced preliminary results from a clinical study conducted by GlaxoSmithKline showed ondansetron prolongs the cardiac QT interval in a dose-dependent manner, which could pre-dispose patients to develop an abnormal and potentially fatal ventricular tachyarrhythmia known as torsades de pointes [FDA Drug Safety Communication: New information regarding QT prolongation with ondansetron (Zofran) at https://www.fda.gov/drugs/drugsafety/ucm310190.htm Last accessed 4 December 2017].
On June 29 2012, the FDA announced preliminary results from a clinical study conducted by GlaxoSmithKline showed ondansetron prolongs the cardiac QT interval in a dose-dependent manner, which could pre-dispose patients to develop an abnormal and potentially fatal ventricular tachyarrhythmia known as torsades de pointes [FDA Drug Safety Communication: New information regarding QT prolongation with ondansetron (Zofran) at https://www.fda.gov/drugs/drugsafety/ucm310190.htm Last accessed 4 December 2017].
•Risk factors for developing QT prolongation with ondansetron include the following:
•Underlying heart conditions, such as congenital long QT syndrome, congestive heart failure, or bradyarrhythmias
•Hypokalemia and hypomagnesemia
•Concomitant use of medications that also are associated with QT prolongation
•A comparison between single intravenous doses of ondansetron 32 mg and 8 mg revealed the maximum mean difference in QTcF (the QT interval measurement corrected by the Fridericia formula) from placebo after baseline-correction was 20 msec and 6 msec, respectively.
•Consequently, product labeling was amended to state ondansetron 0.15 mg/kg administered intravenously over 15 minutes every 4 hours for three doses may continue to be used in adults and children with chemotherapy-induced nausea and vomiting, but no single intravenous dose should exceed 16 mg.
•Ondansetron product labeling includes warnings against using the drug in patients with congenital long QT syndrome and recommends ECG monitoring in patients with uncorrected electrolyte abnormalities such as hypokalemia or hypomagnesemia, congestive heart failure, bradyarrhythmias, and in patients concomitantly using other medications that can prolong the QT interval.
•Patients should be advised to contact a healthcare professional immediately if they experience signs and symptoms of an abnormal heart rate or rhythm while they are taking ondansetron.
•Recommendations for a single, oral, 24-mg ondansetron dose in prophylaxis against chemotherapy-induced nausea and vomiting were not affected.
Palonosetron
 Palonosetron is a second-generation 5-HT3-receptor antagonist with a substantially longer elimination half-life (approximately 40 hours) than dolasetron, granisetron, or ondansetron, and additional characteristics that, in contrast with first-generation 5-HT3-receptor antagonists, suggest pharmacological advantages, including
Palonosetron is a second-generation 5-HT3-receptor antagonist with a substantially longer elimination half-life (approximately 40 hours) than dolasetron, granisetron, or ondansetron, and additional characteristics that, in contrast with first-generation 5-HT3-receptor antagonists, suggest pharmacological advantages, including
•Allosteric binding that produces a conformational change in 5-HT3 receptors with increased binding affinity between the receptor and palonosetron, which may be the result of at least one more molecule binding to the same receptor (suggests positive cooperativity).
•In contrast, granisetron and ondansetron exhibit simple competitive binding with 5-HT3 receptors.
•Binding to 5-HT3-receptor that results in receptor internalization, and, consequently, a persistent inhibition of receptor function.
•Evidence indicating palonosetron bound to internalized NK1-receptors diminishes signalling (crosstalk) between NK1 and substance P receptors.
 Palonostron is currently available as an injectable product, and in a product for oral administration formulated in combination with the NK1-receptor antagonist, netupitant.
Palonostron is currently available as an injectable product, and in a product for oral administration formulated in combination with the NK1-receptor antagonist, netupitant.
•Palonosetron injection received FDA approval for use in adult patients in antiemetic prophylaxis for
•Acute and delayed nausea and vomiting in initial and repeat courses of moderately emetogenic chemotherapy.
•Acute nausea and vomiting in initial and repeat courses of highly emetogenic chemotherapy.
•Palonosetron injection also received FDA approval for use in pediatric patients from 1 month to 17 years of age in antiemetic prophylaxis for initial and repeat courses of emetogenic chemotherapy including highly emetogenic chemotherapy.
 Palonosetron is one among 5-HT3-receptor antagonists, or the only drug preferentially recommended for antiemetic prophylaxis by the following:
Palonosetron is one among 5-HT3-receptor antagonists, or the only drug preferentially recommended for antiemetic prophylaxis by the following:
•National Comprehensive Cancer Network® guidelines for patients who receive moderately emetogenic intravenously administered chemotherapy without concomitant use of a NK1-receptor antagonist antiemetic
•Multinational Association of Supportive Care in Cancer/European Society for Medical Oncology guidelines for patients treated with chemotherapy regimens containing an anthracycline and cyclophosphamide (an “AC” regimen) when a NK1-receptor antagonist cannot be used in combination with dexamethasone and a 5-HT3-receptor antagonist.
 There is a low risk of adverse effects at dosages and schedules currently approved by the U.S. Food and Drug Administration.
There is a low risk of adverse effects at dosages and schedules currently approved by the U.S. Food and Drug Administration.
•The risk of ECG abnormalities associated with palonosetron use, including QTc prolongation, has been shown less than that associated with dolasetron and ondansetron.
•FDA-approved product labeling indicates single doses of palonosetron 0.25, 0.75, or 2.25 mg in 221 healthy adult men and women in a double blind, randomized, parallel, placebo, and positive (moxifloxacin) controlled trial demonstrated no significant effect on any ECG interval including QTc interval duration.
•Product labeling for palonosetron injection indicates a single dose before starting chemotherapy, but safety has been demonstrated with other doses and schedules:
•10 mcg/kg single dose (healthy subjects)
•0.75 mg single dose before chemotherapy
•0.25 mg/dose every second day for 3 doses with dexamethasone before chemotherapy
•0.25 mg/day for 3 consecutive days (healthy subjects)
•0.25 mg/day for 1, 2, or 3 consecutive days (prior to high-dose chemotherapy)
•No differences were observed in control of vomiting over a 7-day evaluation period among patients who received 1, 2, or 3 doses.
•Only about 8% of patients who received one dose and about 20% of patients who received two or three doses were without emesis and did not receive rescue medications.
•Palonosetron 0.25 mg IV followed at least 72 h after the initial dose by a second 0.25 mg dose for breakthrough symptoms was effective in 67% of patients who experienced nausea or vomiting.
Neurokinin (NK1 Subtype)-Receptor Antagonists
 NK1-receptor antagonists have demonstrated activity against acute phase emetic symptoms, but are more effective against delayed phase emesis than other pharmacological classes of antiemetics currently available.
NK1-receptor antagonists have demonstrated activity against acute phase emetic symptoms, but are more effective against delayed phase emesis than other pharmacological classes of antiemetics currently available.
 International guidelines recommend using a 5-HT3-receptor antagonist, dexamethasone, and a NK1-receptor antagonist in antiemetic primary prophylaxis for patients receiving highly emetogenic and high-dose chemotherapy regimens, and as an option for moderately emetogenic chemotherapy.
International guidelines recommend using a 5-HT3-receptor antagonist, dexamethasone, and a NK1-receptor antagonist in antiemetic primary prophylaxis for patients receiving highly emetogenic and high-dose chemotherapy regimens, and as an option for moderately emetogenic chemotherapy.
 Currently, three NK1-receptor antagonists in formulations for oral administration have received FDA approval for commercial use in the USA, including aprepitant, rolapitant, and netupitant. Fosaprepitant dimeglumine is a pro-drug for aprepitant formulated for intravenous administration.
Currently, three NK1-receptor antagonists in formulations for oral administration have received FDA approval for commercial use in the USA, including aprepitant, rolapitant, and netupitant. Fosaprepitant dimeglumine is a pro-drug for aprepitant formulated for intravenous administration.
 Safe use of NK1-receptor antagonists with other medications prudently requires health care providers to recognize the potential for drug-drug interactions during concomitant use.
Safe use of NK1-receptor antagonists with other medications prudently requires health care providers to recognize the potential for drug-drug interactions during concomitant use.
•Table 38.5 identifies pharmacokinetic characteristics that become important when NK1-receptor antagonists are used concomitantly with other medications.
Aprepitant and Fosaprepitant
 Aprepitant is currently FDA approved for use in preventing acute and delayed nausea and vomiting associated with initial and repeat courses of moderately or highly emetogenic cancer chemotherapy in patients ≥6 months of age (≥6 kg).
Aprepitant is currently FDA approved for use in preventing acute and delayed nausea and vomiting associated with initial and repeat courses of moderately or highly emetogenic cancer chemotherapy in patients ≥6 months of age (≥6 kg).
 Commercially available products for oral administration include the following:
Commercially available products for oral administration include the following:
•Aprepitant capsules for patients ≥12 years of age.
•Aprepitant liquid (suspension) for patients ≥6 months of age (≥6 kg) who are not able to swallow capsules.
•Fosaprepitant for injection (lyophilized powder): safety and effectiveness is not established in pediatric patients.
 Approval was based on studies with emetogenic chemotherapy given on a single day.
Approval was based on studies with emetogenic chemotherapy given on a single day.
•Aprepitant and fosaprepitant utilization in prophylaxis for emetogenic chemotherapy given on a single day (Day 1)a:
Regimen | Day 1 | Days 2 and 3b |
Oral only | Aprepitant 125 mg orally, 1 hour before starting chemotherapy | Aprepitant 80 mg/day orally in the morning for 2 daysb |
IV only | Fosaprepitant 150 mg intravenously, 30 min before starting chemotherapy | No additional doses |
 Potential drug interactions with aprepitant and fosaprepitant
Potential drug interactions with aprepitant and fosaprepitant
•Both aprepitant and fosaprepitant share the liability of potential interactions with concomitantly administered medications
•Aprepitant is a substrate and moderate inhibitor of the cytochrome P450 (CYP) enzyme CYP3A4, and a moderate inducer of CYP3A4 and CYP2C9. Inhibition may occur after a single dose; induction occurs after repeated doses.
•Aprepitant inhibits CYP3A4 in the gut and liver.
•The potential for interaction with many CYP3A4 substrates is unknown.
 Aprepitant increases the bioavailability of concomitantly administered dexamethasone and methylprednisolone.
Aprepitant increases the bioavailability of concomitantly administered dexamethasone and methylprednisolone.
•When either dexamethasone or methylprednisolone is used in combination with aprepitant 125- or 80-mg doses for antiemetic prophylaxis, decrease orally administered glucocorticoid doses by 50% and intravenously administered glucocorticoid doses by 25%.
•Do not modify the doses of steroids used as components of a chemotherapy regimen.
 Aprepitant metabolism and elimination may be adversely affected by drugs that inhibit or induce CYP3A4.
Aprepitant metabolism and elimination may be adversely affected by drugs that inhibit or induce CYP3A4.
•Common side effects of aprepitant in combination with a 5-HT3-receptor antagonist and high-potency glucocorticoids include the following:
•Abdominal pain, epigastric discomfort
•Dyspepsia
•Hiccups
•Anorexia
•Dizziness
•Fatigue, asthenia
Netupitant
 Netupitant received FDA approval for commercial use, but only in combination with palonosetron in a product formulated for oral administration with a fixed strength ratio of netupitant 300 mg with 0.5 mg palonosetron (AKYNZEO® capsules; Distributed and marketed by Eisai Inc., Woodcliff Lake, NJ, under license of Helsinn Healthcare SA, Switzerland).
Netupitant received FDA approval for commercial use, but only in combination with palonosetron in a product formulated for oral administration with a fixed strength ratio of netupitant 300 mg with 0.5 mg palonosetron (AKYNZEO® capsules; Distributed and marketed by Eisai Inc., Woodcliff Lake, NJ, under license of Helsinn Healthcare SA, Switzerland).
•AKYNZEO® is indicated for the prevention of acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
 Recommendations for AKYNZEO® (netupitant with palonosetron) use in antiemetic primary prophylaxis with emetogenic chemotherapy given on a single day (Day 1) for patients ≥18 years of age:
Recommendations for AKYNZEO® (netupitant with palonosetron) use in antiemetic primary prophylaxis with emetogenic chemotherapy given on a single day (Day 1) for patients ≥18 years of age:
Day 1 | Days 2–4 |
Highly emetogenic chemotherapy: |
AKYNZEO® one capsule orally, approximately 1 h before starting chemotherapy, plus Dexamethasone 12 mg orally, 30 min before starting chemotherapy | Dexamethasone 8 mg orally, once daily for 4 consecutive days |
Chemotherapy not considered highly emetogenic, including anthracyclines and cyclophosphamide-based chemotherapies: |
AKYNZEO® one capsule orally, approximately 1 h before starting chemotherapy, plus Dexamethasone 12 mg orally, 30 min before starting chemotherapy | No additional prophylaxis necessary |
 Potential drug interactions with netupitant
Potential drug interactions with netupitant
•Netupitant is a substrate for metabolism and a moderate inhibitor of CYP3A4.
•Avoid concomitant use of CYP3A4 substrates for one week, if feasible. If concomitant use of CYP3A4 substrates during 7 days after Akynzeo® use is not avoidable, consider reducing the doses of CYP3A4 substrates.
•The potential for interaction with many CYP3A4 substrates is unknown.
•Netupitant increases the bioavailability of concomitantly administered dexamethasone.
•Dexamethasone doses should be decreased when used in combination with netupitant for antiemetic prophylaxis.
•Do not modify the doses of steroids used as components of a chemotherapy regimen.
 Adverse reactions associated with the use of netupitant-plus-palonosetron fixed combination product:
Adverse reactions associated with the use of netupitant-plus-palonosetron fixed combination product:
•Headache
•Asthenia
•Fatigue
•Dyspepsia
•Constipation
Rolapitant
 Rolapitant is indicated for use in patients ages ≥18 years in prevention against acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
Rolapitant is indicated for use in patients ages ≥18 years in prevention against acute and delayed nausea and vomiting associated with initial and repeat courses of cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
 Rolapitant is administered orally, one-to-two hours before emetogenic chemotherapy.
Rolapitant is administered orally, one-to-two hours before emetogenic chemotherapy.
 Adverse reactions associated with the use of rolapitant in combination with dexamethasone and a 5-HT3-receptor antagonist as antiemetic prophylaxis for highly or moderately emetogenic chemotherapy regimens, include
Adverse reactions associated with the use of rolapitant in combination with dexamethasone and a 5-HT3-receptor antagonist as antiemetic prophylaxis for highly or moderately emetogenic chemotherapy regimens, include
•Fatigue
•Constipation
•Headache
•Hiccups
•Abdominal pain
•Dizziness
•Dyspepsia
 Potential drug interactions with rolapitant:
Potential drug interactions with rolapitant:
•There is no drug interaction between rolapitant and dexamethasone. No dose adjustment for dexamethasone is required when used concomitantly with rolapitant.
•After a single dose or rolapitant CYP2D6 inhibition lasts at least 7 days and may last longer.
•Avoid concomitant use of rolapitant and pimozide (CYP2D6 substrate). The resulting increase in concentrations of pimozide in plasma may result in QT/QTc prolongation.
•Monitor for adverse reactions if rolapitant use concomitant with other CYP2D6 substrates with a low or narrow therapeutic index cannot be avoided.
•Rolapitant had no significant effects on the pharmacokinetics of ondansetron, a CYP2D6 substrate.
•Rolapitant inhibits intracellular efflux transport of substrates for efflux transport by the Breast Cancer Resistance Protein (BCRP, ABCG2, MXR1) transport.
•Be wary of concomitant use of rolapitant and BCRP substrates that have a low or narrow therapeutic index (e.g., daunorubicin, doxorubicin, epirubicin, irinotecan, methotrexate, mitoxantrone, rosuvastatin, topotecan).
•Monitor for adverse reactions related to BCRP substrates if concomitant use with rolapitant cannot be avoided.
•Use the lowest effective dose of rosuvastatin if it is used concomitantly with rolapitant.
•Rolapitant inhibits intracellular efflux transport of P-glycoprotein (P-gp, MDR1, ABCB1) substrates.
•Monitor for adverse reactions related to P-gp substrates if concomitant use with rolapitant cannot be avoided.
Glucocorticoids
 High-potency glucocorticoids such as dexamethasone and methylprednisolone are effective as single agents against mild to moderate acute-phase symptoms.
High-potency glucocorticoids such as dexamethasone and methylprednisolone are effective as single agents against mild to moderate acute-phase symptoms.
 Dexamethasone and methylprednisolone are active against both acute- and delayed-phase symptoms.
Dexamethasone and methylprednisolone are active against both acute- and delayed-phase symptoms.
•At clinically useful doses, dexamethasone and methylprednisolone are equally effective after either intravenous or oral administration.
Both dexamethasone and methylprednisolone enhance the antiemetic effectiveness of 5-HT3 and NK1-receptor antagonists when used concomitantly.
 Prophylaxis and treatment are empirically based; safety and efficacy comparisons are lacking.
Prophylaxis and treatment are empirically based; safety and efficacy comparisons are lacking.
 In antiemetic prophylaxis for emetogenic treatment given on a single day, single doses of dexamethasone and methylprednisolone are as effective as multiple-dose schedules.
In antiemetic prophylaxis for emetogenic treatment given on a single day, single doses of dexamethasone and methylprednisolone are as effective as multiple-dose schedules.
•Optimal dosages and schedules have not been determined, but there is no evidence that single doses of dexamethasone >20 mg improves antiemetic response.
 Potential for adverse effects after a single dose is generally low and limited to GI upset and activating psychogenic effects such as anxiety, insomnia, and sleep disturbances.
Potential for adverse effects after a single dose is generally low and limited to GI upset and activating psychogenic effects such as anxiety, insomnia, and sleep disturbances.
•Co-administration with drugs that decrease gastric acid production (histamine H2-receptor antagonists or proton pump inhibitors) is recommended to prevent GI irritation.
•Administering steroids early in a patient’s waking cycle may minimize adverse effects on sleep.
 Adrenocortical suppression is generally not a problem when high-potency glucocorticoids are used for brief periods.
Adrenocortical suppression is generally not a problem when high-potency glucocorticoids are used for brief periods.
 Glycemic control may be a problem in patients with incipient or frank diabetes.
Glycemic control may be a problem in patients with incipient or frank diabetes.
Dopamine (D2 subtype)-Receptor Antagonists
 Optimal doses and schedules have not been established.
Optimal doses and schedules have not been established.
 Overall, antiemetic activity varies directly with D2-receptor antagonism.
Overall, antiemetic activity varies directly with D2-receptor antagonism.
 Adverse effects correlate with dose and frequency of administration, and include the following:
Adverse effects correlate with dose and frequency of administration, and include the following:
•Sedation
•Extrapyramidal reactions (dystonias, akathisia, dyskinesia)
•Anticholinergic effects
•ECG changes (haloperidol, droperidol)
•Hypotension with rapid intravenous administration (phenothiazines)
 Anecdotal evidence supports the use of D2-receptor antagonists with 5-HT3 antagonists ± steroids for acute-phase symptoms, and with steroids, metoclopramide, or lorazepam for delayed-phase symptoms.
Anecdotal evidence supports the use of D2-receptor antagonists with 5-HT3 antagonists ± steroids for acute-phase symptoms, and with steroids, metoclopramide, or lorazepam for delayed-phase symptoms.
Metoclopramide
 Metoclopramide has affinity for several neurotransmitter receptors associated with antiemetic activity, but is often categorized among D2-receptor antagonists, and, at high doses, becomes a competitive antagonist at vagal and central 5-HT3 receptors.
Metoclopramide has affinity for several neurotransmitter receptors associated with antiemetic activity, but is often categorized among D2-receptor antagonists, and, at high doses, becomes a competitive antagonist at vagal and central 5-HT3 receptors.
 Activity against delayed-phase symptoms is equivalent to that of ondansetron.
Activity against delayed-phase symptoms is equivalent to that of ondansetron.
 Gastrointestinal prokinetic effects may benefit patients with intercurrent GI motility disorders or gastroesophageal reflux disease.
Gastrointestinal prokinetic effects may benefit patients with intercurrent GI motility disorders or gastroesophageal reflux disease.
 Long-term use has been associated with developing dyskinesias, tardive dyskinesia may be irreversible.
Long-term use has been associated with developing dyskinesias, tardive dyskinesia may be irreversible.
Benzodiazepines
 Benzodiazepines are important adjuncts to antiemetics for their anxiolytic and anterograde amnestic effects.
Benzodiazepines are important adjuncts to antiemetics for their anxiolytic and anterograde amnestic effects.
•Irrespective of its cause, anxiety may be a factor in developing or exacerbating emetic symptoms prior, during, and after completing emetogenic treatments.
Benzodiazepines are clinically useful for mitigating akathisias associated with D2-receptor antagonists.
 Available products:
Available products:
•Lorazepam, midazolam, diazepam are available in oral and injectable formulations.
Alprazolam is available in solid formulations for oral administration.
•Lorazepam and alprazolam tablets are rapidly absorbed after sublingual administration.
 Primary liability is dose-related sedation.
Primary liability is dose-related sedation.
 Pharmacodynamic effects are exaggerated in elderly patients.
Pharmacodynamic effects are exaggerated in elderly patients.
Cannabinoids
 Commercially available cannabinoids are agonists at endocannabinoid (CB1 subtype) receptors.
Commercially available cannabinoids are agonists at endocannabinoid (CB1 subtype) receptors.
•Dronabinol is an oral formulation of Δ9-tetrahydrocannabinol (Δ9-THC) with antiemetic activity similar to low doses of prochlorperazine.
•Nabilone is a synthetic CB1-receptor agonist formulated for oral administration.
•Both dronabinol and nabilone are controlled substances (Schedule II) in the United States
 Antiemetic benefit may be achieved without producing psychotropic effects. Cannabinoid use is empiric since optimal doses and administration schedules have not been determined.
Antiemetic benefit may be achieved without producing psychotropic effects. Cannabinoid use is empiric since optimal doses and administration schedules have not been determined.
 The incidence of adverse effects associated with dronabinol and nabilone is greater than with phenothiazines at doses and schedules that produce comparable antiemetic effects.
The incidence of adverse effects associated with dronabinol and nabilone is greater than with phenothiazines at doses and schedules that produce comparable antiemetic effects.
 Adverse effects occur within the range of clinically useful doses; incidence and severity vary with dose and correlate inversely with the interval between successive doses. Potential adverse effects include the following:
Adverse effects occur within the range of clinically useful doses; incidence and severity vary with dose and correlate inversely with the interval between successive doses. Potential adverse effects include the following:
•Sedation
•Confusion/decreased cognition
•Dizziness
•Short-term memory loss
•Euphoria/dysphoria
•Ataxia
•Dry mouth
•Orthostatic hypotension ± increased heart rate
Anticholinergic (Antimuscarinic) Agents and Histamine (H1)-Receptor Antagonists
 Utility in preventing and treating emetic symptoms is not defined.
Utility in preventing and treating emetic symptoms is not defined.
 Anticholinergics may be most effective in prophylaxis; less effective after emetic symptoms develop.
Anticholinergics may be most effective in prophylaxis; less effective after emetic symptoms develop.
 Anticholinergics are useful in prophylaxis and treatment for patients whose emetic symptoms are referable to movement.
Anticholinergics are useful in prophylaxis and treatment for patients whose emetic symptoms are referable to movement.
 Individual agents have in different proportions affinities for histaminic and cholinergic neuronal receptors, and, in some cases, agonistic and antagonistic activities at adrenergic, dopaminergic, and other neuroreceptors.
Individual agents have in different proportions affinities for histaminic and cholinergic neuronal receptors, and, in some cases, agonistic and antagonistic activities at adrenergic, dopaminergic, and other neuroreceptors.
 Adverse effects correlate directly with dose and frequency of administration, and include the following:
Adverse effects correlate directly with dose and frequency of administration, and include the following:
•Sedation
•Dry mouth
•Loss of visual accommodation/blurred vision
•Deceased GI motility with constipation or diarrhea
•Urinary retention or frequency
•Mydriasis ± photophobia
•Increased heart rate
Olanzapine
 Olanzapine, an atypical neuroleptic or antipsychotic, is a potent antagonist at multiple neurotransmitter receptors, including muscarinic (m1 > m2–4), serotonergic (5-HT2A, 5-HT2C, 5-HT3, 5-HT6), alpha adrenergic (α1), dopaminergic (D1, D2, D4), and histaminergic receptors (H1).
Olanzapine, an atypical neuroleptic or antipsychotic, is a potent antagonist at multiple neurotransmitter receptors, including muscarinic (m1 > m2–4), serotonergic (5-HT2A, 5-HT2C, 5-HT3, 5-HT6), alpha adrenergic (α1), dopaminergic (D1, D2, D4), and histaminergic receptors (H1).
 Olanzapine 10 mg/day orally for four consecutive days or placebo in combination with dexamethasone, aprepitant or fosaprepitant, and a 5-HT3-receptor antagonist demonstrated significantly better control of nausea than the comparator arm during the acute, delayed, and overall (0–120 h) periods after treatment-naïve patients’ initial cycle of cisplatin ≥70 mg/m2 ± other antineoplastics or cyclophosphamide 600 mg/m2 + doxorubicin 60 mg/m2 regimens.
Olanzapine 10 mg/day orally for four consecutive days or placebo in combination with dexamethasone, aprepitant or fosaprepitant, and a 5-HT3-receptor antagonist demonstrated significantly better control of nausea than the comparator arm during the acute, delayed, and overall (0–120 h) periods after treatment-naïve patients’ initial cycle of cisplatin ≥70 mg/m2 ± other antineoplastics or cyclophosphamide 600 mg/m2 + doxorubicin 60 mg/m2 regimens.
•Olanzapine is a substrate for direct glucuronidation catalyzed by uridine diphosphate glucuronosyltransferase (UGT) enzymes, UGT1A4 and UGT2B10, and for oxidation catalyzed primarily by CYP1A2 and flavin-containing monooxygenase-3, and to a lesser extent by CYP2D6 and CYP3A4.
•Olanzapine’s pharmacokinetic behavior is susceptible to drugs and substances that induce and inhibit CYP1A2 (e.g., carbamazepine, fluvoxamine, tobacco)
•Olanzapine is a substrate with moderate affinity for P-glycoprotein (P-gp, MDR1, ABCB1), and has been shown to inhibit P-gp at concentrations achieved during therapeutic use
 Adverse effects associated with olanzapine include the following:
Adverse effects associated with olanzapine include the following:
•Sedation, insomnia, fatigue
•Nervousness, agitation, cognitive impairment
•Headache
•Dizziness and orthostatic hypotension
•Increased appetite
•Weight gain, new-onset diabetes, hyperlipidemia, and increased serum alanine aminotransferase with prolonged use
•CAUTION: Product labeling for olanzapine includes a boxed warning about its use in elderly patients. In clinical trials, elderly patients (≥65 y) with dementia-related psychosis experienced an increased incidence of death and adverse cerebrovascular events including stroke.
•Olanzapine shares with other atypical neuroleptics a potential for inhibiting human ether à go-go-related (hERG) gene-encoded potassium channels, but has demonstrated a low potential for inhibiting ventricular repolarization, and, therefore, for causing torsades de pointes; however, effects on cardiac electrophysiological similar to class III anti-arrhythmic drugs were observed at concentrations that can be measured under conditions of impaired olanzapine elimination, during concomitant use of CYP1A2 competitive substrates or inhibitors, and under conditions of overdose.
STRATEGIES FOR COMBINING ANTIEMETICS
Antiemetics in combination can be more effective than single agents by targeting two or more operative neural pathways.
 Numerous studies have demonstrated control of acute-phase emetic symptoms improves significantly with the combination of 5-HT3-receptor antagonists and high-potency glucocorticoids. Acute-phase symptom control is further augmented when aprepitant is used in combination with a 5-HT3-receptor antagonist and a glucocorticoid.
Numerous studies have demonstrated control of acute-phase emetic symptoms improves significantly with the combination of 5-HT3-receptor antagonists and high-potency glucocorticoids. Acute-phase symptom control is further augmented when aprepitant is used in combination with a 5-HT3-receptor antagonist and a glucocorticoid.
 Delayed-phase symptom control is improved by the combination of high-potency glucocorticoids and NK1-receptor antagonists. However, NK1-receptor antagonists may compromise the safety of concomitantly administered medications due to potential inhibition or inductive effects on cytochrome P450 metabolizing enzymes.
Delayed-phase symptom control is improved by the combination of high-potency glucocorticoids and NK1-receptor antagonists. However, NK1-receptor antagonists may compromise the safety of concomitantly administered medications due to potential inhibition or inductive effects on cytochrome P450 metabolizing enzymes.
•In cases where prophylaxis against delayed-phase symptoms is indicated but concurrent medications make the use of a NK1-receptor antagonist problematic, glucocorticoids alone or in combination with either metoclopramide or a 5-HT3 or D2-receptor antagonist may improve control of symptoms.
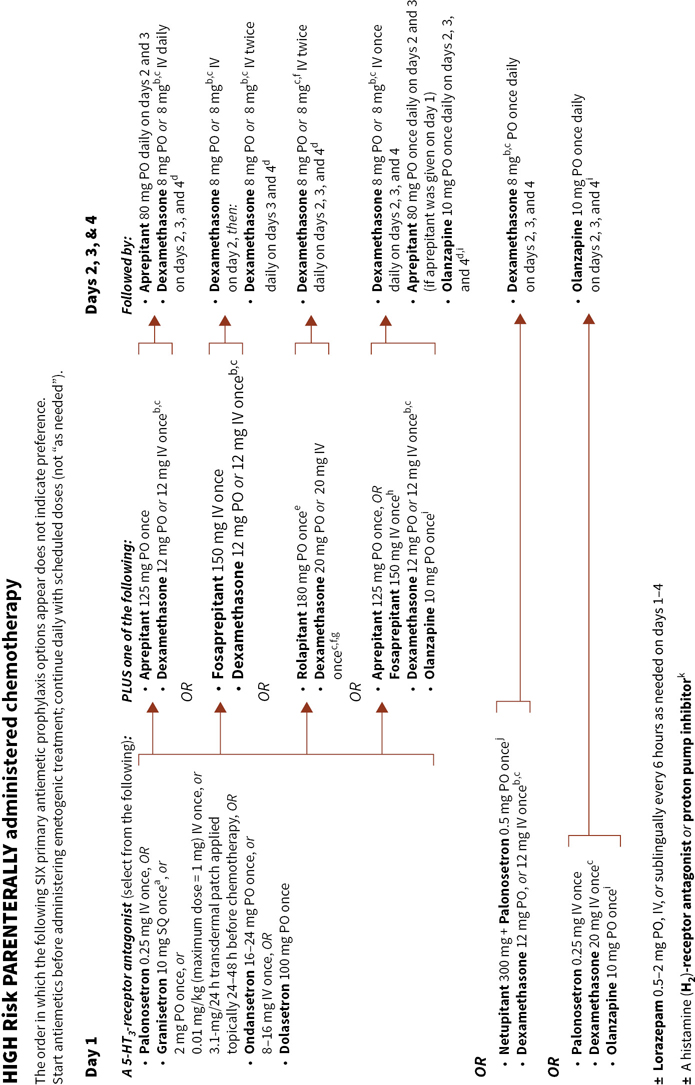




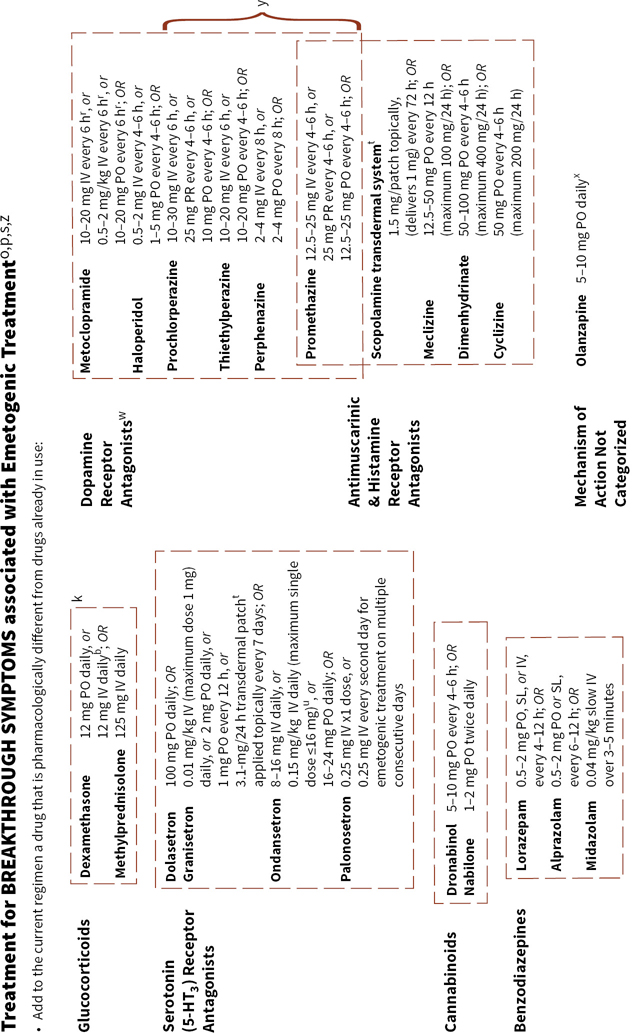
FIGURE 38.2Algorithms for antiemetic prophylaxis and treatment for parenterally and orally administered emetogenic drugs and ionizing radiation (external beam).
a Granisetron extended- release injection is a unique formulation of granisetron incorporated into a polymer-based drug delivery system. The formulation is administered only by SUBCUTaneous injection and is not interchangeable with granisetron formulated for intraVENous or intraMUSCular administration. Granisetron extended-release injection has an extended elimination half-life (~24 h) and should not be administered at intervals <1 wk.
b If NK1-receptor antagonists are not given on day 1, recommend dexamethasone 20 mg either PO or IV once on day 1, followed by 8 mg twice daily either PO or IV on days 2, 3, and 4.
c Dexamethasone dose and schedule recommendations are based on those used in clinical trials or in FDA-approved product labeling; however, dexamethasone doses may be individualized: lower doses, frequency of use, and omission on the second and subsequent days after starting emetogenic treatment may be acceptable based on patient characteristics.
d NCCN Guidelines® indicate some NCCN member institutions use a 5-HT3-receptor antagonist on days 2, 3, and 4 in addition to a steroid and a NK1-receptor antagonist except when palonosetron, granisetron extended-release injection, or granisetron transdermal patch were given on day 1 (Antiemesis, V.1.2017—February 22, 2017. National Comprehensive Cancer Network, Inc., 2017. Accessed March 9, 2017, at http://www.nccn.org).
e Rolapitant has an extended elimination half-life (169–183 h) and should not be administered at intervals <2 wk.
f VARUBI™ (rolapitant) tablets, for oral use; September 2015, product label. TESARO Inc., Waltham MA.
g The recommendation for dexamethasone dose and schedule is contrary to the recommendation that appears in NCCN Guidelines®. Dexamethasone bioavailability is not increased by concomitant use with rolapitant; thus, FDA approved product labeling for VARUBI™ stipulates a 20-mg dexamethasone dose on day 1 and dexamethasone 8 mg twice daily on days 2– 4.
h Consider escalating to this option when emesis occurred during a previous cycle of chemotherapy using an olanzapine regimen without a NK1-receptor antagonist or a NK1-receptor antagonist-containing regimen without olanzapine.
i Consider decreasing the olanzapine dose to 5 mg/ day for elderly or overly sedated patients.
j Available in the USA only as a fixed-dose combination product containing netupitant 300 mg and palonosetron 0.5 mg per capsule for oral administration.
k Consider administering a histamine (H2 subtype)-receptor antagonist (other than cimetidine) OR a proton pump inhibitor concurrently with dexamethasone to mitigate gastrointestinal irritation.
l When used in combination with a NK1-receptor antagonist, there is no preferred 5-HT3-receptor antagonist.
m Consider adding a NK1-receptor antagonist to dexamethasone and a 5-HT3-receptor antagonist as with prophylaxis for high emetic risk regimens for select patients with additional risk factors, or those who previously experienced suboptimal emetic control with the combination of a steroid and 5-HT3-receptor antagonist.
n No additional primary prophylaxis is recommended if palonosetron or granisetron extended-release injection was given, or granisetron transdermal system (patch) was applied 24–48 h prior to starting emetogenic treatment.
o Antiemetic prophylaxis should be repeated each day emetogenic treatment is administered.
p Oral prophylaxis should begin one hour before commencing cytotoxic treatment. IV prophylaxis may be given minutes before emetogenic treatment.
q When =2 orally administered emetogenic drugs are used concomitantly, the emetic risk potential may be increased and require more aggressive prophylaxis.
r In July 2013, the European Medicines Agency’s Committee on Medicinal Products for Human Use recommended changes to the use of metoclopramidecontaining medicines in the European Union, including restricting the dose and duration of metoclopramide use to minimize known risks of potentially serious neurological adverse effects. For adults and children the maximum recommended dose during a 24-hour period is 0.5 mg/ kg body weight; in adults, the recommended dose is 10 mg given up to three times daily (i.e., a maximum daily dose of 30 mg) by all routes of administration. References: European Medicines Agency recommends changes to the use of metoclopramide; 26 July 2013, EMA/ 443003/ 2013 and ibid. Changes aim mainly to reduce the risk of neurological side effects; 20 December 2013, EMA/ 13239/ 2014 Corr. 1. At: www.ema.europa.eu Last accessed 17 March 2017
s Medications are not listed in order of preference. Groups of pharmacologically similar alternatives are circumscribed by a broken line.
t Avoid using sustained-, delayed-and extended-release formulations (oral, transdermal patches, and injectable products) to establish control of ongoing symptoms.
u Product labeling for ondansetron hydrochloride injection approved by the U.S. FDA recommends single intravenously administered doses should not exceed 16 mg. [ZOFRAN® (ondansetron hydrochloride) injection, for intravenous or intramuscular use; March 2017 product label. GlaxoSmithKline, Research Triangle Park, NC]
v Dexamethasone 12 mg is the only dexamethasone dose tested in combination with aprepitant in large randomized trials. w Generally, regimens containing D2-receptor antagonists and metoclopramide doses =20 mg should include primary prophylaxis with anticholinergic agents against acute dystonic extrapyramidal reactions; e.g., diphenhydramine 25 – 50 mg PO or IV every 6 hours. Benztropine and trihexyphenidyl are alternatives. Parenteral administration is preferred for prompt treatment of extrapyramidal symptoms, as well as interrupting or discontinuing the drug which provoked the adverse reaction.
x When olanzapine is not part of antiemetic prophylaxis already in use.
y When administered IV, phenothiazines should be given over 30 minutes to prevent hypotension.
z Medications identified for breakthrough symptoms should be added to a patient’s primary antiemetic prophylaxis regimen without replacing drugs used in primary prophylaxis unless drugs used to treat breakthrough symptoms duplicate mechanistically those used in primary prophylaxis or a patient has experienced unacceptable adverse effects attributable to a component of primary prophylaxis. 5-HT3, serotonin receptor [5-HT3 subtype], ASCO, American Society of Clinical Oncology, D2, dopamine receptor [D2 subtype], IM, intramuscularly, IV, intravenously, NCCN, National Comprehensive Cancer Network®, MASCC/ ESMO (Multinational Association of Supportive Care in Cancer/ European Society for Medical Oncology), PO (orally), PR (rectally), q. (every), RT (radiation therapy), SL (sublingually), SQ (SUBCUTaneously).
TABLE 38.1Onset and Duration of Emesis with Selected Chemotherapy Agents
Drug | Onset of Emesis (h) | Duration of Emesis (h) |
Aldesleukin | 0–6 | — |
Altretamine | 3–6 | — |
Asparaginase | 1–3 | — |
Bleomycin | 3–6 | — |
Carboplatin | 6–8 | >24 |
Carmustine | 2–6 | 4–24 |
Chlorambucil | 48–72 | — |
Cisplatin | 1–6 | 24–48+ |
Cyclophosphamide | 6–18 | 6–24+ |
Cytarabine | 6–12 | 3–5 |
Dacarbazine | 1–5 | 1–24 |
Dactinomycin | 2–6 | 12–24 |
Daunorubicin | 2–6 | 24 |
Doxorubicin | 4–6 | 6–24+ |
Etoposide | 3–8 | 6–12 |
Fluorouracil | 3–6 | 3–4 |
Hydroxyurea | 6–12 | — |
Ifosfamide | 1–6 | 6–12 |
Irinotecan | 2–6 | 6–12 |
Lomustine | 2–6 | 4–12 |
Mechlorethamine | 0.5–2 | 1–24 |
Melphalan | 6–12 | — |
Mercaptopurine | 4–8 | — |
Methotrexate | 4–12 | 3–12 |
Mitomycin | 1–6 | 3–12 |
Mitotane | Long latency | Persistent |
Paclitaxel | 3–8 | 3–8 |
Pentostatin | Long latency | Persistent (>24) |
Plicamycin | 4–6 | 12–24 |
Procarbazine | 24–27 | Variable |
Streptozocin | 1–4 | 12–24 |
Teniposide | 3–8 | 6–12 |
Thioguanine | 4–8 | — |
Thiotepa | 6–12 | Variable |
Vinblastine | 4–8 | — |
Vincristine | 4–8 | — |
Vinorelbine | 4–8 | — |
TABLE 38.2Antineoplastic Drugs Implicated in Delayed Emesis
Drug | Dosages | Combinations that Exacerbate Developing Symptoms |
Carboplatin | ≥300 mg/m2 | ± other cytotoxic agents |
Cisplatin | ≥50 mg/m2 |
|
Cyclophosphamide | ≥600 mg/m2 | + anthracycline combinations ± other cytotoxic agents |
Doxorubicin | ≥50 mg/m2 |
|
Oxaliplatin | ≥85 mg/m2 | ± other cytotoxic agents |
TABLE 38.3Emetic Risk for Single Agents as a Function of Drug, Dosage or Dose, and Route of Administration
|
| Acute Phase Emetic Potential (incidencea) |
|
| High (>90%), Moderate (30%–90%), Low (10%–30%), Minimal (<10%) |
Drugs and Drug Combinations | MASCC/ESMO | NCCN | ASCO | Current Product Labeling |
ado-Trastuzumab emtansine | Lowb | Lowc |
|
|
Afatenib (orally) | Lowb | Minimal–lowc |
|
|
Aldesleukin |
| Moderatec (>12–15 million international units/m2); Lowc (≤12 million international units/m2) |
|
|
Alectinib (orally) |
| Minimal–lowc |
|
|
Alemtuzumab | Moderateb | Minimalc | Moderated |
|
Altretamine (orally) | Highb | Moderate–highc |
|
|
Amifostine |
| Moderatec (>300 mg/m2); Lowc (≤300 mg/m2) |
|
|
Arsenic trioxide |
| Moderatec |
|
|
Asparaginase |
| Minimalc |
|
|
Atezolizumab |
| Lowc |
|
|
Avelumab |
|
|
| Lowe,f |
Axitinib (orally) | Lowb | Minimal–lowc |
|
|
Azacitidine | Moderateb | Moderatec | Moderated |
|
Belinostat | Lowb | Lowc |
|
|
Bendamustine | Moderateb | Moderatec | Moderated |
|
Bevacizumab | Minimalb | Minimalc | Minimald |
|
Bexarotene (orally) |
| Minimal–lowc |
|
|
Bleomycin | Minimalb | Minimalc | Minimald |
|
Blinatumomab | Lowb | Lowc |
|
|
Bortezomib | Lowb | Minimalc | Lowd |
|
Bosutinib (orally) | Moderateb | Minimal–lowc |
|
|
Brentuximab vedotin | Lowb | Lowc |
|
|
Busulfan | Minimalb | Moderatec | Minimald |
|
Busulfan (orally) |
| Moderate–highc (≥4 mg/d); Minimal–lowc (<4 mg/d) |
|
|
Cabazitaxel | Lowb | Lowc | Lowd |
|
Cabozantinib (orally) |
| Minimal–lowc |
|
|
Capecitabine (orally) | Lowb | Minimal–lowc |
|
|
Carboplatin | Moderateb | High (AUC ≥4 mg/mL·min)c; Moderate (AUC <4 mg/mL·min)c,i | Moderated |
|
Carfilzomib | Lowb | Lowc |
|
|
Carmustine | Highb | High (>250 mg/m2)c; Moderate (≤250 mg/m2)c,i | Highd |
|
Catumaxomabh | Lowb |
| Lowd | Moderatee,g |
Ceritinib (orally) | Moderateb | Moderate–highc |
|
|
Cetuximab | Lowb | Minimalc | Minimald |
|
Chlorambucil (orally) | Minimalb | Minimal–lowc |
|
|
Cisplatin | Highb | Highc | Highd |
|
Cladribine | Minimalb | Minimalc | Minimald |
|
Clofarabine | Moderateb | Moderatec | Moderated |
|
Cobimetinib (orally) |
| Minimal–lowc |
|
|
Crizotinib (orally) | Moderateb | Moderate–highc |
|
|
Cyclophosphamide | High (≥1,500 mg/m2)b; Moderate (<1,500 mg/m2)b | High (>1,500 mg/m2)c; Moderatec (≤1,500 mg/m2) | Highd (≥1,500 mg/m2); Moderated (<1,500 mg/m2) |
|
Cyclophosphamide (orally) | Moderateb | Moderate–highc (≥100 mg/m2 per d); Minimal–lowc (<100 mg/m2 per d) |
|
|
Cyclophosphamide + an Anthracycline, including: daunorubicin, doxorubicin, epirubicin, or idarubicin; an “AC” regimen | Highb | Highc (only doxorubicin and epirubicin are included in the definition of an “AC” regimen) | Highd |
|
Cytarabine | Moderate (>1,000 mg/m2)b; Low (≤1,000 mg/m2)b | Moderatec (>200 mg/m2); Lowc (100–200 mg/m2); Minimalc (<100 mg/m2) | Moderated (>1,000 mg/m2); Lowd (≤1,000 mg/m2) |
|
Dabrafenib (orally) | Lowb | Minimal–lowc |
|
|
Dacarbazine | Highb | Highc | Highd |
|
Dactinomycin |
| Moderatec,i | Highd |
|
Daratumumab |
| Minimalc |
|
|
Dasatinib (orally) | Lowb | Minimal–lowc |
|
|
Daunorubicin | Moderateb | Moderatec,i | Moderated |
|
Daunorubicin, liposomalh |
|
|
| Lowe,j |
Decitabine |
| Minimalc |
|
|
Denileukin diftitox |
| Minimalc |
|
|
Dexrazoxane |
| Minimalc |
|
|
Dinutuximab |
| Moderatec |
|
|
Docetaxel | Lowb | Lowc | Lowd |
|
Doxorubicin | Moderateb | High (≥60 mg/m2)c; Moderatec,i (<60 mg/m2) | Moderated |
|
Doxorubicin, liposomal | Lowb | Lowc | Lowd |
|
Elotuzumab |
| Minimalc |
|
|
Epirubicin | Moderateb | High (>90 mg/m2)c; Moderatec,i (≤90 mg/m2) | Moderated |
|
Eribulin mesylate | Lowb | Lowc |
|
|
Erlotinib (orally) | Minimalb | Minimal–lowc |
|
|
Estramustine |
| Moderate–highc |
|
|
Etoposide | Lowb | Lowc | Lowd |
|
Etoposide (orally) | Lowb | Moderate–highc |
|
|
Everolimus (orally) | Lowb | Minimal–lowc |
|
|
Fludarabine | Minimalb | Minimalc | Minimald |
|
Fludarabine (orally)k | Lowb | Minimal–lowc |
|
|
Floxuridine |
| Lowc |
|
|
Fluorouracil | Lowb | Lowc | Lowd |
|
Gefitinib (orally)l | Minimalb | Minimal–lowc |
|
|
Gemcitabine | Lowb | Lowc | Lowd |
|
Hydroxyurea (orally) | Minimalb | Minimal–lowc |
|
|
Ibrutinib (orally) | Lowb | Minimal–lowc |
|
|
Idarubicin | Moderateb | Moderatec | Moderated |
|
Idelalisib (orally) | Lowb | Minimal–lowc |
|
|
Ifosfamide | Moderateb | High (≥2,000 mg/m2 per dose)c; Moderatec,i (<2,000 mg/m2 per dose) | Moderated |
|
Imatinib (orally) | Moderateb | Minimal–lowc |
|
|
Interferon alfa |
| Moderatec (≥10 million international units/m2); Lowc (>5 to <10 million international units/m2); Minimalc (≤5 million international units/m2) |
|
|
Ipilimumab | Lowb | Minimalc |
|
|
Irinotecan | Moderateb | Moderatec,i | Moderated |
|
Irinotecan, liposomal |
| Lowc |
|
|
Ixabepilone | Lowb | Lowc | Lowd |
|
Ixazomib (orally) |
| Minimal–lowc |
|
|
Lapatinib (orally) | Lowb | Minimal–lowc |
|
|
Lenalidomide (orally) | Lowb | Minimal–lowc |
|
|
Lenvatinib (orally) |
| Moderate–highc |
|
|
Lomustine (orally) |
| Moderate–highc (single day) |
|
|
Mechlorethamine | Highb | Highc | Highd |
|
Melphalan |
| Moderatec |
|
|
Melphalan (orally) | Minimalb | Minimal–lowc |
|
|
Mercaptopurine (orally) |
| Minimal–lowc |
|
|
Methotrexate | Lowb | Moderatec,i (≥250 mg/m2); Lowc (>50 to <250 mg/m2); Minimalc (≤50 mg/m2) | Lowd |
|
Methotrexate (orally) | Minimalb | Minimal–lowc |
|
|
Mitomycin | Lowb | Lowc | Lowd |
|
Mitotane (orally) |
| Moderate–highc |
|
|
Mitoxantrone | Lowb | Lowc | Lowd |
|
Necitumumab |
| Lowc |
|
|
Nelarabine |
| Minimalc |
|
|
Nilotinib (orally) | Lowb | Minimal–lowc |
|
|
Niraparib (orally) |
|
|
| Lowe,m |
Nivolumab | Minimalb | Minimalc |
|
|
Obinutuzumab |
| Minimalc |
|
|
Ofatumumab | Minimalb | Minimalc |
|
|
Olaparib (orally) | Lowb | Moderate–highc |
|
|
Omacetaxine mepesuccinate |
| Lowc |
|
|
Osimertinib (orally) |
| Minimal–lowc |
|
|
Oxaliplatin | Moderateb | Moderatec,i | Moderated |
|
Paclitaxel | Lowb | Lowc | Lowd |
|
Paclitaxel protein (albumin-)-bound particles (nab-paclitaxel) | Lowb | Lowc |
|
|
Palbociclib (orally) |
| Minimal–lowc |
|
|
Panitumumab | Lowb | Minimalc | Lowd |
|
Panobinostat (orally) |
| Moderate–highc |
|
|
Pazopanib (orally) | Lowb | Minimal–lowc |
|
|
Pegaspargase |
| Minimalc |
|
|
Peginterferon alfa |
| Minimalc |
|
|
Pembrolizumab | Minimalb | Minimalc |
|
|
Pemetrexed | Lowb | Lowc | Lowd |
|
Pentostatin |
| Lowc |
|
|
Pertuzumab | Lowb | Minimalc |
|
|
Pixantrone dimaleateh | Minimalb |
|
|
|
Plicamycinh |
|
|
| Low– moderatee,n,o |
Pomalidomide (orally) | Minimalb | Minimal–lowc |
|
|
Ponatinib (orally) | Lowb | Minimal–lowc |
|
|
Pralatrexate | Minimalb | Lowc | Minimald |
|
Procarbazine (orally) | Highb | Moderate–highc |
|
|
Ramucirumab |
| Minimalc |
|
|
Regorafenib (orally) | Lowb | Minimal–lowc |
|
|
Ribociclib (orally) |
|
|
| Lowe,p |
Rituximab | Minimalb | Minimalc | Minimald |
|
Romidepsin | Moderateb | Lowc |
|
|
Rucaparib (orally) |
| Moderate–highc |
|
|
Ruxolitinib (orally) | Minimalb | Minimal–lowc |
|
|
Siltuximab |
| Minimalc |
|
|
Sonidegib (orally) |
| Minimal–lowc |
|
|
Sorafenib (orally) | Minimalb | Minimal–lowc |
|
|
Streptozocin | Highb | Highc | Highd |
|
Sunitinib (orally) | Lowb | Minimal–lowc |
|
|
Talimogene laherparepvec |
| Lowc |
|
|
Tegafur uracilh (orally) | Lowb |
|
|
|
Temozolomide (injection) | Moderateb | Moderatec |
|
|
Temozolomide (orally) | Moderateb | Moderate – highc (>75 mg/m2·day); Moderatec (≤75 mg/m2·day if given concurrently with radiation therapy); Minimal – lowc (≤75 mg/m2·day) |
|
|
Temsirolimus | Lowb | Minimalc | Lowd |
|
Teniposide |
|
|
| Low– moderatee,q |
Thalidomide (orally) | Lowb | Minimal–lowc |
|
|
Thioguanine (orally) | Minimalb | Minimal–lowc |
|
|
Thiotepa | Moderateb | Lowc |
|
|
Topotecan | Lowb | Lowc | Lowd |
|
Topotecan (orally) |
| Minimal–lowc |
|
|
Trabectedin | Moderateb | Moderatec,i |
|
|
Trametinib (orally) |
| Minimal–lowc |
|
|
Trastuzumab | Minimalb | Minimalc | Lowd |
|
Tretinoin (orally) |
| Minimal–lowc |
|
|
Trifluridine/tipiracil (orally) |
| Moderate–highc |
|
|
Valrubicin |
| Minimalc |
|
|
Vandetanib (orally) | Lowb | Minimal–lowc |
|
|
Vemurafenib (orally) | Minimalb | Minimal–lowc |
|
|
Venetoclax (orally) |
| Minimal–lowc |
|
|
Vinblastine | Minimalb | Minimalc | Minimald |
|
Vincristine | Minimalb | Minimalc | Minimald |
|
Vincristine, liposomal |
| Minimalc |
|
|
Vinflunine ditartrateh | Lowb |
|
|
|
Vinorelbine | Minimalb | Minimalc | Minimald |
|
Vinorelbine (orally)h | Moderateb |
|
|
|
Vismodegib (orally) | Minimalb | Minimal–lowc |
|
|
Vorinostat (orally) | Lowb | Minimal–lowc |
|
|
ziv-Aflibercept | Lowb | Lowc |
|
|
TABLE 38.4Catabolism of 5-HT3-receptor antagonists by cytochrome P450 (CYP) enzymes.
5-HT3-receptor antagonists | Catalysts for metabolism | Metabolites (activity vs. parent drug) |
Dolasetron | Stereoselectively by carbonyl reductase | Reduction to hydrodolasetron (~50-times more active than dolasetron) |
Hydrodolasetron | Primarily CYP2D6, also CYP3A subfamily and flavin monooxygenase | Hydroxylation (inactive) |
Granisetron | Primarily CYP3A subfamily. Also, CYP1A1 | Oxidation, then conjugation (inactive) |
Ondansetron | Primarily CYP3A4. Also, CYP2D6 and CYP1A2 | Hydroxylation, then conjugation (inactive) |
Palonosetron | Primarily CYP2D6, also CYP3A4 and CYP1A2 | N-oxide-palonosetron (<1% parent drug activity), 6-S-hydroxypalonosetron (<1% parent drug activity) |
TABLE 38.5Selected Pharmacokinetic Characteristics for NK1-Receptor Antagonists
Drug | Half-lifea | Plasma protein binding | Metabolic Catalysts | | Transporters |
Substrate for | Inhibits | Induces | Substrate for | Inhibits | Induces |
Aprepitant | 9–13 h | 95% | CYP3A4 > CYP1A2 & CYP2C19 | CYP3A4, weakly (40-mg dose) to moderately (125/80-mg doses) | CYP3A4, CYP2C9 (moderately) | P-gp (unlikely to interact with other P-gp substrates) |
|
|
Fosaprepitant dimeglumine | approximately 11 h (for aprepitant) |
|
| CYP3A4, weakly | — b |
|
|
|
Rolapitant | 169–183 h | 99.8% | CYP3A4 | CYP2D6, moderately (may persist for ≥7 days after a single dose) |
|
| BCRP P-gp |
|
M19 (active C4-pyrrolidine-hydroxylated rolapitant) | 158 h |
|
|
|
|
|
|
|
Netupitant (formulated with palonosetron) | 80 ±29 h | >99.5% | CYP3A4 > CYP2C9 & CYP2D6 | CYP3A4, moderatelyc,d |
|
| BCRPc P-gpc |
|
Netupitant active metabolites: |
M1 (N-demethyl netupitant) |
| >97% (concentrations 100–2000 ng/mL) |
| CYP3A4c |
|
|
|
|
M2 (netupitant N-oxide) |
|
|
|
| P-gp |
|
|
M3 (monohydroxy netupitant) |
|
|
|
|
|
|
|
Suggested Readings
1.Aapro M. Methodological issues in antiemetic studies. Invest New Drugs. 1993;11(4):243–253.
2.Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006;17(9):1441–1449.
3.Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2005;13(2):117–121.
4.Antiemesis, V.1.2017 — February 22, 2017. National Comprehensive Cancer Network, Inc., 2017. Available at: http://www.nccn.org [Accessed March 9, 2017]
5.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–4198.
6.Borison HL, McCarthy LE. Neuropharmacology of chemotherapy-induced emesis. Drugs. 1983;25(Suppl 1):8–17.
7.Botrel TE, Clark OA, Clark L, Paladini L, Faleiros E, Pegoretti B. Efficacy of palonosetron (PAL) compared to other serotonin inhibitors (5-HT3R) in preventing chemotherapy-induced nausea and vomiting (CINV) in patients receiving moderately or highly emetogenic (MoHE) treatment: systematic review and meta-analysis. Support Care Cancer. 2011;19(6):823–832.
8.Bubalo JS, Cherala G, McCune JS, Munar MY, Tse S, Maziarz R. Aprepitant pharmacokinetics and assessing the impact of aprepitant on cyclophosphamide metabolism in cancer patients undergoing hematopoietic stem cell transplantation. J Clin Pharmacol. 2012;52(4):586–594.
9.Celio L, Frustaci S, Denaro A, et al. Palonosetron in combination with 1-day versus 3-day dexamethasone for prevention of nausea and vomiting following moderately emetogenic chemotherapy: a randomized, multicenter, phase III trial. Support Care Cancer. 2011;19(8):1217–1225.
10.D’Acquisito R, Tyson LB, Gralla RJ, et al. The influence of a chronic high alcohol intake on chemotherapy-induced nausea and vomiting [abstract]. Proc Am Soc Clin Oncol. 1986;5:257.
11.de Wit R, Herrstedt J, Rapoport B, et al. Addition of the oral NK1 antagonist aprepitant to standard antiemetics provides protection against nausea and vomiting during multiple cycles of cisplatin-based chemotherapy [see comments]. J Clin Oncol. 2003;21(22):4105–4111. Comment in: J Clin Oncol. 2003;21:4077–4080.
12.Feyer PC, Maranzano E, Molassiotis A, Roila F, Clark-Snow RA, Jordan K. Radiotherapy-induced nausea and vomiting (RINV): MASCC/ESMO guideline for antiemetics in radiotherapy: update 2009. Support Care Cancer. 2011;(19 Suppl 1):S5–14.
13.Gralla R, Lichinitser M, Van Der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003;14(10):1570–1577.
14.Hesketh PJ, Bohlke K, Lyman GH, et al. Antiemetics: American Society of Clinical Oncology focused guideline update. J Clin Oncol. 2015;34:381–386.
15.Hesketh PJ, Rossi G, Rizzi G, et al. A randomized phase Ill study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann Oncol. 2014;25:1340–1346.
16.Hickok JT, Roscoe JA, Morrow GR, et al. 5-Hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial. Lancet Oncol. 2005;6(10):765–772.
17.Hunt TL, Gallagher SC, Cullen MR Jr, Shah AK. Evaluation of safety and pharmacokinetics of consecutive multiple-day dosing of palonosetron in healthy subjects. J Clin Pharmacol. 2005;45(5):589–596.
18.Italian Group for Antiemetic Research. Cisplatin-induced delayed emesis: pattern and prognostic factors during three subsequent cycles. Ann Oncol. 1994;5(7):585–589.
19.Kaiser R, Sezer O, Papies A, et al. Patient-tailored antiemetic treatment with 5-hydroxytryptamine type 3 receptor antagonists according to cytochrome P-450 2D6 genotypes [see comments]. J Clin Oncol. 2002;20(12):2805–2811. Comment in: J Clin Oncol. 2002;20:2765–2767.
20.Kris MG, Gralla RJ, Clark RA, et al. Incidence, course, and severity of delayed nausea and vomiting following the administration of high-dose cisplatin. J Clin Oncol. 1985;3(10):1379–1384.
21.Kris MG, Roila F, De Mulder PH, Marty M. Delayed emesis following anticancer chemotherapy. Support Care Cancer. 1998;6(3):228–232.
22.Molassiotis A, Coventry PA, Stricker CT, et al. Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage. 2007;34(2):148–159.
23.Morrow GR, Hickok JT, Burish TG, Rosenthal SN. Frequency and clinical implications of delayed nausea and delayed emesis. Am J Clin Oncol. 1996;19(2):199–203.
24.Navari RM, Gray SE, Kerr AC. Olanzapine versus aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a randomized phase Ill trial. J Support Oncol. 2011;9(5):188–195.
25.Navari RM, Qin R, Ruddy KJ, et al. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting [see comment]. N Engl J Med. 2016;375(2):134–142. Comment in: N Engl J Med. 2016;375(14):1395–1396.
26.Rapoport BL, Chasen MR, Gridelli C, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of cisplatin-based highly emetogenic chemotherapy in patients with cancer: two randomised, active-controlled, double-blind, phase 3 trials. Lancet Oncol. 2015;16(9):1079–1089.
27.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–43.
28.Roila F, Molassiotis A, Herrstedt J, et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann Oncol. 2016;27(Suppl 5):v119–v33.
29.Rojas C, Slusher BS. Mechanisms and latest clinical studies of new NK1 receptor antagonists for chemotherapy-induced nausea and vomiting: rolapitant and NEPA (netupitant/palonosetron). Cancer Treat Rev. 2015;41(10):904–913.
30.Rojas C, Slusher BS. Pharmacological mechanisms of 5-HT3 and tachykinin NK1 receptor antagonism to prevent chemotherapy-induced nausea and vomiting. Eur J Pharmacol. 2012;684(1–3):1–7.
31.Roscoe JA, Morrow GR, Aapro MS, Molassiotis A, Olver I. Anticipatory nausea and vomiting. Support Care Cancer. 2011;19(10):1533–1538.
32.Ruhlmann C, Herrstedt J. Palonosetron hydrochloride for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther. 2010;10(2):137–148.
33.Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009;10(2):115–124.
34.Schwartzberg LS, Modiano MR, Rapoport BL, et al. Safety and efficacy of rolapitant for prevention of chemotherapy-induced nausea and vomiting after administration of moderately emetogenic chemotherapy or anthracycline and cyclophosphamide regimens in patients with cancer: a randomised, active-controlled, double-blind, phase 3 trial. Lancet Oncol. 2015;16(9):1071–1079.
35.Sullivan JR, Leyden MJ, Bell R. Decreased cisplatin-induced nausea and vomiting with chronic alcohol ingestion. N Engl J Med. 1983;309(13):796.
36.Tremblay PB, Kaiser R, Sezer O, et al. Variations in the 5-hydroxytryptamine type 3B receptor gene as predictors of the efficacy of antiemetic treatment in cancer patients. J Clin Oncol. 2003;21(11):2147–2155.
 In general, the greatest incidence of acute symptoms occurs within 2 to 6 hours after treatment.
In general, the greatest incidence of acute symptoms occurs within 2 to 6 hours after treatment. Onset is generally within 1 to 3 hours after commencing chemotherapy. Notable exceptions include the following:
Onset is generally within 1 to 3 hours after commencing chemotherapy. Notable exceptions include the following: Female sex, particularly women with a history of persistent and/or severe emetic symptoms during pregnancy
Female sex, particularly women with a history of persistent and/or severe emetic symptoms during pregnancy Children and young adults
Children and young adults Patients with a history of acute- and/or delayed-phase emetic symptoms during prior treatments are at great risk for poor emetic control during subsequent treatments
Patients with a history of acute- and/or delayed-phase emetic symptoms during prior treatments are at great risk for poor emetic control during subsequent treatments Patients with low performance status and a predisposition to motion sickness.
Patients with low performance status and a predisposition to motion sickness. Nondrinkers are at greater risk than patients with a history of chronic alcohol consumption (>100 g ethanol daily for several years)
Nondrinkers are at greater risk than patients with a history of chronic alcohol consumption (>100 g ethanol daily for several years) Patients with intercurrent pathologies, such as gastrointestinal (GI) inflammation, compromised GI motility or obstruction, constipation, brain metastases, metabolic abnormalities (hypovolemia, hypercalcemia, hypoadrenalism, uremia), visceral organs invaded by tumor, and concurrent medical treatment (opioids, bronchodilators, aspirin, nonsteroidal anti-inflammatory drugs), may predispose and exacerbate emetic symptoms during treatment and complicate good emetic control
Patients with intercurrent pathologies, such as gastrointestinal (GI) inflammation, compromised GI motility or obstruction, constipation, brain metastases, metabolic abnormalities (hypovolemia, hypercalcemia, hypoadrenalism, uremia), visceral organs invaded by tumor, and concurrent medical treatment (opioids, bronchodilators, aspirin, nonsteroidal anti-inflammatory drugs), may predispose and exacerbate emetic symptoms during treatment and complicate good emetic control Planning effective antiemetic primary prophylaxis
Planning effective antiemetic primary prophylaxis The emetogenic risk associated with treatment.
The emetogenic risk associated with treatment. Healthcare provider adherence in prescribing and patient compliance in using planned antiemetic prophylaxis.
Healthcare provider adherence in prescribing and patient compliance in using planned antiemetic prophylaxis. Disease status.
Disease status. Co-morbid conditions (electrolyte abnormalities, renal failure, sepsis, constipation, tumor infiltrating or obstructing the gastrointestinal tract, intracranial disease, vestibular dysfunction).
Co-morbid conditions (electrolyte abnormalities, renal failure, sepsis, constipation, tumor infiltrating or obstructing the gastrointestinal tract, intracranial disease, vestibular dysfunction). Whether medications concomitantly administered with emetogenic drugs and antiemetics may potentially compromise antiemetic effectiveness:
Whether medications concomitantly administered with emetogenic drugs and antiemetics may potentially compromise antiemetic effectiveness: Was the prophylactic strategy given an adequate trial (time of initiation relative to the start of emetogenic treatment and duration of use)?
Was the prophylactic strategy given an adequate trial (time of initiation relative to the start of emetogenic treatment and duration of use)? Were the antiemetics selected and the doses and administration schedules prescribed appropriate for the emetogenic challenge?
Were the antiemetics selected and the doses and administration schedules prescribed appropriate for the emetogenic challenge? Did the patient understand and comply with instructions for antiemetic use?
Did the patient understand and comply with instructions for antiemetic use? Would increased doses or shorter administration intervals improve antiemetic effectiveness without causing or exacerbating adverse effects associated with the antiemetics utilized?
Would increased doses or shorter administration intervals improve antiemetic effectiveness without causing or exacerbating adverse effects associated with the antiemetics utilized? Assess a symptomatic patient’s state of hydration and serum/plasma electrolytes for abnormal results.
Assess a symptomatic patient’s state of hydration and serum/plasma electrolytes for abnormal results. Add antiemetic agents that act through mechanisms different from antiemetics already in use.
Add antiemetic agents that act through mechanisms different from antiemetics already in use. Give scheduled doses around-the-clock at least until emetogenic treatment is completed, and at doses and on a schedule appropriate for the medication.
Give scheduled doses around-the-clock at least until emetogenic treatment is completed, and at doses and on a schedule appropriate for the medication. Consider replacing ineffective drugs with a more potent or longer-acting agent from the same pharmacologic class.
Consider replacing ineffective drugs with a more potent or longer-acting agent from the same pharmacologic class. Consider replacing an antiemetic medication that requires ingestion and absorption from the gastrointestinal tract or percutaneous absorption with the same or a different drug given by a different administration route (disintegrating tablets and soluble films for oral administration, injectable formulations).
Consider replacing an antiemetic medication that requires ingestion and absorption from the gastrointestinal tract or percutaneous absorption with the same or a different drug given by a different administration route (disintegrating tablets and soluble films for oral administration, injectable formulations). Replace drugs associated with unacceptable adverse effects with one or more drugs from the same or a different pharmacologic class without a potential for the same toxicity, or for which particular adverse effects are less likely to occur.
Replace drugs associated with unacceptable adverse effects with one or more drugs from the same or a different pharmacologic class without a potential for the same toxicity, or for which particular adverse effects are less likely to occur. Consider escalating antiemetic prophylaxis to a regimen appropriate for the next greater level of emetic risk.
Consider escalating antiemetic prophylaxis to a regimen appropriate for the next greater level of emetic risk. Add additional scheduled antiemetics at appropriate doses and administration intervals.
Add additional scheduled antiemetics at appropriate doses and administration intervals. For regimens that included a 5-HT3 antagonist, consider switching to a different 5-HT3 antagonist.
For regimens that included a 5-HT3 antagonist, consider switching to a different 5-HT3 antagonist. Consider adding an anxiolytic drug to the patient’s regimen.
Consider adding an anxiolytic drug to the patient’s regimen. Consider adding a NK1-receptor antagonist to antiemetic prophylaxis if its potential for pharmacokinetic interactions will not adversely affect concomitantly administered medications.
Consider adding a NK1-receptor antagonist to antiemetic prophylaxis if its potential for pharmacokinetic interactions will not adversely affect concomitantly administered medications. If alternative treatment for a patient’s neoplastic disease exists, consider a different regimen with which similar therapeutic benefit may be achieved without greater adverse outcomes.
If alternative treatment for a patient’s neoplastic disease exists, consider a different regimen with which similar therapeutic benefit may be achieved without greater adverse outcomes. Guidance for patients that may preserve nutritional status and alleviate emetic symptoms, include
Guidance for patients that may preserve nutritional status and alleviate emetic symptoms, include Acupressure or acupuncture
Acupressure or acupuncture Among 5-HT3-receptor antagonists that have received FDA approval for commercial use, dolasetron, granisetron, and ondansetron comprise the first-generation agent, palonosetron is a second-generation agent.
Among 5-HT3-receptor antagonists that have received FDA approval for commercial use, dolasetron, granisetron, and ondansetron comprise the first-generation agent, palonosetron is a second-generation agent. 5-HT3-receptor antagonists are safer and more effective against acute-phase symptoms than other pharmacological classes of medications with clinically useful antiemetic activity.
5-HT3-receptor antagonists are safer and more effective against acute-phase symptoms than other pharmacological classes of medications with clinically useful antiemetic activity. Administering 5-HT3-receptor antagonists at doses/dosages greater than those shown to be maximally effective do not substantially improve emetic control.
Administering 5-HT3-receptor antagonists at doses/dosages greater than those shown to be maximally effective do not substantially improve emetic control. Single-dose prophylaxis is preferred for acute-phase symptoms.
Single-dose prophylaxis is preferred for acute-phase symptoms. Dolasetron, granisetron, ondansetron, and palonosetron have excellent oral bioavailability, and, when given at maximally effective doses, each agent provides equivalent antiemetic protection whether given orally or parenterally.
Dolasetron, granisetron, ondansetron, and palonosetron have excellent oral bioavailability, and, when given at maximally effective doses, each agent provides equivalent antiemetic protection whether given orally or parenterally. Metoclopramide and prochlorperazine are less expensive, and as effective as dolasetron, granisetron, and ondansetron at controlling emetic symptoms.
Metoclopramide and prochlorperazine are less expensive, and as effective as dolasetron, granisetron, and ondansetron at controlling emetic symptoms. Palonosetron has the longest half-life among 5-HT3-receptor antagonists currently marketed in the USA, and has additional pharmacological characteristics not shared by first-generation 5-HT3-receptor antagonists.
Palonosetron has the longest half-life among 5-HT3-receptor antagonists currently marketed in the USA, and has additional pharmacological characteristics not shared by first-generation 5-HT3-receptor antagonists. Side effects common to all 5-HT3-receptor antagonists include
Side effects common to all 5-HT3-receptor antagonists include Pharmacogenomic evaluation may help to identify patients at risk for sub-optimal and adverse responses to 5-HT3-receptor antagonists that are substrates for catabolism by cytochrome P450 (CYP) enzymes (Table 38.4).
Pharmacogenomic evaluation may help to identify patients at risk for sub-optimal and adverse responses to 5-HT3-receptor antagonists that are substrates for catabolism by cytochrome P450 (CYP) enzymes (Table 38.4). CYP2D6 is polymorphically expressed among human populations.
CYP2D6 is polymorphically expressed among human populations. Patients who express genetic polymorphism for 5-HT3 receptors or the ABCB1 (P-glycoprotein, MDR1) transporter may experience suboptimal antiemetic responses with 5-HT3-receptor antagonists.
Patients who express genetic polymorphism for 5-HT3 receptors or the ABCB1 (P-glycoprotein, MDR1) transporter may experience suboptimal antiemetic responses with 5-HT3-receptor antagonists. The oral tablet formulation is approved for use in antiemetic prophylaxis in initial and repeat courses of moderately emetogenic chemotherapy in patients ≥2 years of age.
The oral tablet formulation is approved for use in antiemetic prophylaxis in initial and repeat courses of moderately emetogenic chemotherapy in patients ≥2 years of age. The frequency and magnitude of adverse effects associated with dolasetron use are related to serum concentrations of hydrodolasetron, its active metabolite.
The frequency and magnitude of adverse effects associated with dolasetron use are related to serum concentrations of hydrodolasetron, its active metabolite. Indicated for use in antiemetic prophylaxis in initial and repeated courses of emetogenic cancer therapies, including high-dose cisplatin and radiation therapy.
Indicated for use in antiemetic prophylaxis in initial and repeated courses of emetogenic cancer therapies, including high-dose cisplatin and radiation therapy. Granisetron transdermal patch (Sancuso®; Kyowa Kirin, Inc., Bedminster, NJ), an adhesive-backed patch (52 cm2), contains 34.3 mg of granisetron and delivers an average daily dose of 3.1 mg granisetron for up to 7 days.
Granisetron transdermal patch (Sancuso®; Kyowa Kirin, Inc., Bedminster, NJ), an adhesive-backed patch (52 cm2), contains 34.3 mg of granisetron and delivers an average daily dose of 3.1 mg granisetron for up to 7 days. Granisetron extended-release injection for subcutaneous use (SUSTOL®; Heron Therapeutics, Redwood City, CA). Each prefilled syringe contains 10 mg granisetron incorporated in an extended-release polymer vehicle comprising one dose.
Granisetron extended-release injection for subcutaneous use (SUSTOL®; Heron Therapeutics, Redwood City, CA). Each prefilled syringe contains 10 mg granisetron incorporated in an extended-release polymer vehicle comprising one dose. ECG abnormalities are rare with granisetron use at FDA-approved dosages and schedules.
ECG abnormalities are rare with granisetron use at FDA-approved dosages and schedules. Used in antiemetic prophylaxis in initial and repeat courses of emetogenic cancer therapies:
Used in antiemetic prophylaxis in initial and repeat courses of emetogenic cancer therapies: The risk of adverse effects is low at FDA-approved dosages and schedules.
The risk of adverse effects is low at FDA-approved dosages and schedules. On June 29 2012, the FDA announced preliminary results from a clinical study conducted by GlaxoSmithKline showed ondansetron prolongs the cardiac QT interval in a dose-dependent manner, which could pre-dispose patients to develop an abnormal and potentially fatal ventricular tachyarrhythmia known as torsades de pointes [FDA Drug Safety Communication: New information regarding QT prolongation with ondansetron (Zofran) at https://www.fda.gov/drugs/drugsafety/ucm310190.htm Last accessed 4 December 2017].
On June 29 2012, the FDA announced preliminary results from a clinical study conducted by GlaxoSmithKline showed ondansetron prolongs the cardiac QT interval in a dose-dependent manner, which could pre-dispose patients to develop an abnormal and potentially fatal ventricular tachyarrhythmia known as torsades de pointes [FDA Drug Safety Communication: New information regarding QT prolongation with ondansetron (Zofran) at https://www.fda.gov/drugs/drugsafety/ucm310190.htm Last accessed 4 December 2017].