CHAPTER 6
Skin and Photosensitivity
John S. Ferguson
St John's Institute of Dermatology, Guy's and St. Thomas' Hospitals, London, UK
Ultraviolet radiation
Ultraviolet radiation (UV) comprises 5% of all light penetrating the earth's atmosphere; 95% is visible and infrared (Figure 6.1). Ultraviolet A (UVA) and ultraviolet B (UVB) wavelengths are known to play a role in sun‐induced skin damage. UVC is filtered out by the ozone layer. UV intensity is greatest near the equator and at high altitudes. Other environmental factors such as the season, weather, and time of day also influence intensity. UV is reflected by water (15%), snow (80%), and sand (25%).

Figure 6.1 Non‐ionising, visible, and infrared radiation.
Source: Reprinted with permission (R. Sarkany 2017).
UVA photons have a longer wavelength (315–400 nm) and a lower energy. They can penetrate more deeply into skin. UVA is present all year round and throughout the day at constant levels. UVA can pass through glass and is important in skin tanning responses. UVA can produce ‘reactive oxygen species’ which cause DNA, RNA, amino acid, protein, and lipid damage.
UVB photons have a shorter wavelength (280–315 nm) and carry a high energy. When UVB hits the DNA it causes mutations which can lead to keratinocyte apoptosis. When this occurs in multiple keratinocytes at the same time we call the ensuing inflammation ‘sunburn’. Some UVB‐induced damage will be repaired, but ultimately the accumulated mutations in cutaneous stem cells are the main cause for skin cancer.
UVC (100–280 nm) photons don't occur naturally on the earth's surface but they have a very short wavelength and are highly damaging to all cellular life, including human skin.
UV A and B wavelength light is used therapeutically for many common skin problems such as psoriasis and eczema. This is because of cutaneous immunosuppression caused by UVB and UVA light. UVC is also used in the sterilisation of water and other cleaning techniques.
Skin pigmentation and fitzpatrick skin type
Our skins' physiological tolerance of UV light depends on several factors, including UV intensity, skin colour, and previous skin hardening. Fitzpatrick skin type was developed in 1975 by Thomas Fitzpatrick as a way of categorising the variance in normal cutaneous responses to light among Caucasian and Hispanic patients. It relies upon assessing the related tendencies of patients to burn and tan. Initially, only four lighter skin types were included, but later darker skin types – 5 and 6 – were added. It has been shown that dermatologist assessed Fitzpatrick skin type corresponds to pigmentation in the skin and tendency to burn. Skin type also correlates with skin cancer risk and can be used to determine starting doses for patients having phototherapy. Patients with red hair almost always have skin type 1; skin type 6 is usually a dark shade of brown. The redder pigment, pheomelanin, is less good at absorbing UVB radiation and free radicals than the browner eumelanin. Type 1 skin patients are at a much higher risk of skin cancer than patients with darker skin types. Skin type 6 gives protection from UVB equivalent to a Sun Protection factor (SPF) of 14.
Oculocutaneous albinism
Genetic mutations that control melanin synthesis, distribution, and degradation result in a group of inherited disorders that lead to loss of skin/hair/eye pigment. Oculocutaneous albinism is an autosomal recessive condition characterised by little or absent melanin pigment at birth. Oculocutaneous albinism affects skin, hair, and ocular pigmentation, resulting in sun‐induced skin changes, photophobia, nystagmus, and reduced visual acuity.
Oculocutaneous albinism is traditionally classified into two groups – tyrosinase positive or negative – depending on whether the enzyme is absent or dysfunctional; however, there are numerous subtypes depending on the specific genetic mutation. Affected individuals should be assessed in the neonatal period by a dermatologist and an ophthalmologist. The family may wish to consult a geneticist. Protective clothing and sun avoidance behaviour is vital. A sunscreen blocking UVA and UVB light (broad‐spectrum) should be applied to the skin daily lifelong. Vitamin D replacement will probably be necessary. Skin checks for evidence of sun‐damage or skin cancer should be undertaken regularly.
Other genetic conditions associated with loss of skin pigment include piebaldism, phenylketonuria, tuberous sclerosis, and Waardenburg and Apert syndromes.
The photodermatoses
The photodermatoses comprise photosensitive and photoaggravated conditions. Both can cause abnormal responses to ultraviolet, and sometimes visible, wavelengths of light. Most photosensitive dermatoses are abnormal immune responses triggered by UV and sometimes visible light. They include polymorphous light eruption (PMLE), actinic prurigo, chronic actinic dermatitis (CAD), solar urticaria, and hydroa vacciniforme. Some photosensitive reactions are caused by phototoxicity from, drugs, plants, or metabolites, as in porphyria. Some photodermatoses are caused directly by genetic defects as in xeroderma pigmentosum. Others are photoallergic, as in photo‐contact dermatitis.
Photoaggravated dermatoses are skin conditions that can be made worse by the exposure of skin to UV light. e.g. cutaneous lupus erythematosus, dermatomyositis, herpes simplex, Darier's disease, pellagra, and some cases of rosacea and eczema. Some of these conditions are mentioned elsewhere in this book. Although precancerous and cancerous skin lesions are also caused by UV light these are discussed in detail in other chapters (Chapters 22 and 23).
History and clinical examination
If a patient is suspected of having a photodermatosis it is important to take a history of the problem and its relationship to sun exposure. Patients may not develop symptoms unless they are exposed to intense sun while others may be affected even on days with a low UV index. Ask about seasonal variation and whether they get symptoms through window glass. Try and elicit the time to onset of symptoms following UV exposure, their duration and frequency. It is important to ask whether any scarring or surface change are left behind following the acute phase. Is their skin painful, burning, or itchy? Do they have a background of previous skin disease?
On examination, usually sun‐exposed skin is predominately affected, particularly the face, neck, dorsi of arms and hands and, the lower legs. There is often, but not always, sparing of sun‐protected sites, particularly the buttocks, behind the ears, and under the chin. A classically spared site, suggestive of a photo dermatosis, is beneath a watch strap.
Xeroderma pigmentosum
Xeroderma pigmentosum (XP) comprises a group of autosomal recessive conditions resulting in dysfunctional nucleotide excision repair proteins. These highly conserved genes are present in all living cells. The proteins they code for facilitate the repair of our genetic code.
Xeroderma pigmentosum patients usually present in the first two years of life with delayed onset but persistent and severe sunburn responses. The patients quickly start to show signs of accelerated cutaneous UV damage characterised initially by premature freckling, then later poikiloderma, skin ageing, and early tumour formation. Sometimes skin cancers begin to develop in the first decade of life and as affected patients age they often develop multiple skin cancers, including melanomas, squamous, and basal cell carcinomas. Life expectancy is reduced. Ophthalmic problems include photophobia, keratitis, xerosis, corneal opacification, and pterygium formation. Neurological features can include mental retardation deafness and ataxia. XP is rare, affecting around 1 in 250 000 people.
Management for these patients remains focused on early diagnosis and strict sun avoidance. Photoprotective clothing, broad‐rimmed hats, sunglasses, and broad‐spectrum sunscreens should be regularly used. Support for the patient's parents and advice on how they should engage with schools is vital. The XP website can be a useful source of information and community for XP sufferers (http://xpsupportgroup.org.uk).
In the future, it may be possible to correct DNA damage through gene therapy.
Variant xeroderma pigmentosum
This ‘forme fruste’ of xeroderma pigmentosum presents in later life without exaggerated sunburn responses or neurological involvement. Patients do get multiple skin tumours but develop them much later than in classic type XP. The disease is caused by a mutation in the POLH gene which is not a nucleotide excision repair gene but rather it is a ‘translesion synthesis’ gene. Unlike NER proteins, the POLH protein repairs DNA damaged by UV light during replication.
Cockayne syndrome and trichothiodystrophy also result from autosomal recessive DNA repair defects. Cockayne syndrome is characterised by photosensitivity, short stature learning difficulties, ocular, and nervous system abnormalities. Trichothiodystrophy is associated with chronic photosensitivity and ichthyosis. Their brittle hair shows characteristic ‘tiger tail’ banding on light microscopy.
The porphyrias
The porphyrias are a group of disorders associated with the accumulation of intermediate molecules called porphyrins in the metabolic pathway of heme synthesis. These accumulating porphyrin molecules result in cutaneous, hepatic, and neurovisceral problems. Depending on where in the pathway a defect has developed, symptoms vary. All porphyrias except acute intermittent porphyria show cutaneous involvement. Most are genetic, and present early in life, but the commonest type of porphyria is triggered later by liver damage, although sometimes there is an underlying genetic predisposition. Porphyria patients are sensitive to violet light (400–440 nm): for this reason, conventional sunscreens are ineffective in preventing flares. Most porphyrins fluoresce orange or red, a fact which is exploited to allow discrimination between different types of porphyria in the laboratory.
Porphyria cutanea tarda (PCT) is the commonest form of the disease in Europe. Patients manifest a typical pattern of photosensitivity (Figure 6.2). They develop cutaneous fragility and blisters that heal with scarring, pigment changes, and milia on sun‐exposed skin, particularly the face and dorsi of hands. Patients may develop facial hypertrichosis and onycholysis. Most PCT patients are not aware that their symptoms are caused by light. Men over 40 with excessive alcohol intake are most frequently affected. PCT patients are at increased risk of hepatocellular carcinoma. Urine samples will fluoresce red on examination with a woods lamp.
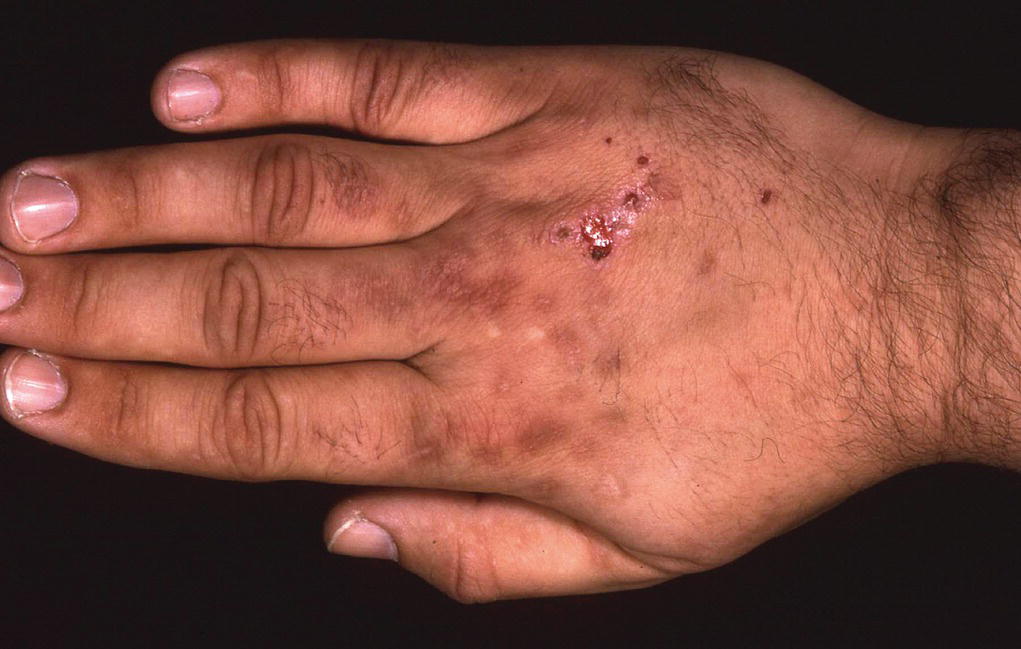
Figure 6.2 Porphyia cutanea tarda.
Erythropoietic protoporphyria (EPP) is the commonest of the inherited forms of the disease. The main feature of this is pain, often a burning or prickling sensation, after just a few minutes of intense sunlight. The pain can be severe. The disease usually presents in childhood with a parental history of a baby crying when exposed to bright light outdoors or through window glass. Over time thickened skin over knuckles and linear scars can develop on patients' cheeks (Figure 6.3).
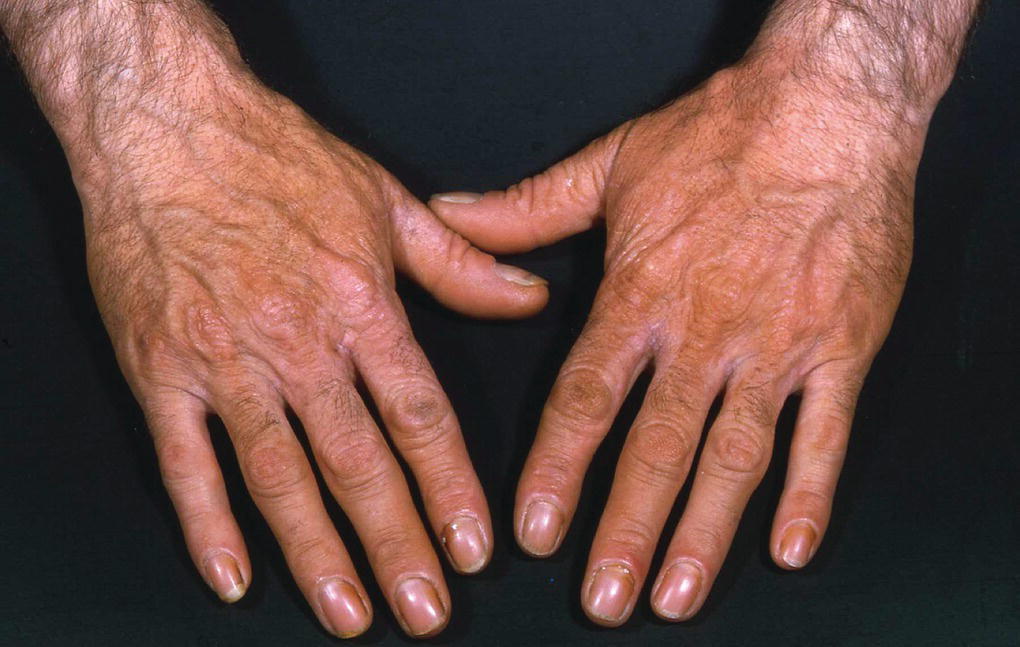
Figure 6.3 Erythropoietic protoporphyria.
All cutaneous porphyria patients should be given sun avoidance and protective clothing advice. Visible light reflecting sunscreens (‘Dundee cream’) and tinted films for windows can be prescribed. For PCT patients it is particularly important to avoid precipitating flares. For haemochromatosis patients with PCT, venesection can be useful; avoiding alcohol and the oral contraceptive pill or hormone replacement therapy (HRT) can be important. In EPP, UVB phototherapy is sometimes used for skin hardening. Melanotide is a new therapy which may prove valuable to EPP patients. Referral of any suspected porphyria patient to a national centre for diagnosis and treatment is worthwhile.
Medications causing photosensitivity
Both topical and systemic medications can lead to photosensitive eruptions (see Chapter 7) (Figure 6.4). The combination of UV or visible light and the chemicals result in a skin reaction. Although most drugs absorb in the ultraviolet range, only a few produce damaging cutaneous effects.

Figure 6.4 Photosensitive drug eruption showing typical ‘phototoxic’ or exaggerated sunburn response with sparing under the chin.
Possible reactions include: phototoxic – resembling sunburn; photoallergic – dermatitis; pseudo porphyria – blistering and skin fragility, subacute lupus erythematosus – erythema and scale; erythema multiforme – targetoid inflammation; lichenoid reaction – erythematous papules and post‐inflammatory pigmentation and pellagra – pigmentation and scale. Of these mechanisms, by far the most common is phototoxicity, which makes up 90% of photosensitive drug reactions. Phototoxicity occurs in many forms and is drug‐specific in terms of wavelength, timing of onset, and clinical appearance. The commonest wavelength implicated in drug phototoxicity is UVA but UVB and visible wavelengths can be responsible.
Photo allergy is an uncommon presentation, usually due to topical preparations applied directly to the skin in the presence of UV light. The most common photo‐allergens are sunscreens and fragrances. A photoallergic reaction is found when a patient develops a positive patch testing response only in the presence of UVA light.
In all cases of severe photosensitive drug reactions, the medication should be stopped if possible and a non‐phototoxic alternative found. Where this is not possible meticulous photoprotection must be adopted (Table 6.1).
Table 6.1 Common photosensitive drugs and associated features.
| Medication | Initial reaction | Notes |
| Thiazides | Exaggerated sunburn | Acute sunburn which develops into a photo‐exposed dermatitis. Lupus‐like reactions and pseudo porphyria can occur |
| Amiodarone | Immediate prickling, delayed erythema | Sometimes resolves into golden brown/slate grey discolouration. Can cause pseudo porphyria |
| Quinine | Exaggerated sunburn | May develop subacute cutaneous lupus (SCLE); depigmentation |
| Non‐steroidal anti‐inflammatory drugs (NSAIDs) | Immediate prickling, delayed erythema | Sometimes patient will develop a pseudo porphyria‐like response, particularly to naproxen |
| Carbamazepine | Exaggerated sunburn | |
| Retinoids | Exaggerated sunburn | Phototoxicity usually normalises after a few weeks |
| Tetracyclines (doxycycline) | Exaggerated sunburn | Can cause grey pigmentation and pseudo porphyria |
| Calcium channel blockers (nifedipine) | Exposed site telangiectasia | |
| Chlorpromazine | Exaggerated sunburn | Can cause slate‐grey pigmentation. Rarely used antipsychotic. |
| BRaf inhibitors (vemurafenib) | Exaggerated sunburn | Photoprotection appropriate or switch to dabrafenib |
| Furosemide | Exaggerated sunburn | Can cause pseudo porphyria |
| Voriconazole | Exaggerated sunburn | Chronic use associated with squamous cell skin cancers |
| Psoralens | Delayed (3–5 d) erythema. Sometimes blisters and pigmentation | Used in ‘PUVA’ phototherapy |
| Photofrin/foscan | Immediate prickling, delayed erythema | Used for photodynamic therapy, visible wavelengths cause an acute pseudoporphyria |
Phytophotodermatitis
Phytophotodermatitis is an acute‐onset blistering rash resulting from photosensitizing plant material in contact with the skin plus UVA light (Figure 6.5). This is a phototoxic reaction not requiring any previous exposure to the plant. Children and agricultural workers are often affected because of their outdoor activities. Implicated plants include meadow grass, common rue, poison ivy/oak, celery, cow parsley, giant hogweed, bergamot, lime, and other citrus fruits. The rash usually appears within hours of exposure to the plant. Patients present with blisters on an erythematous background. The rash usually looks exogenous with bizarre linear streaks on the legs or ‘drip marks’ down the arms. Phytophotodermatitis should be treated with a potent topical steroid and the causative plant material subsequently avoided.
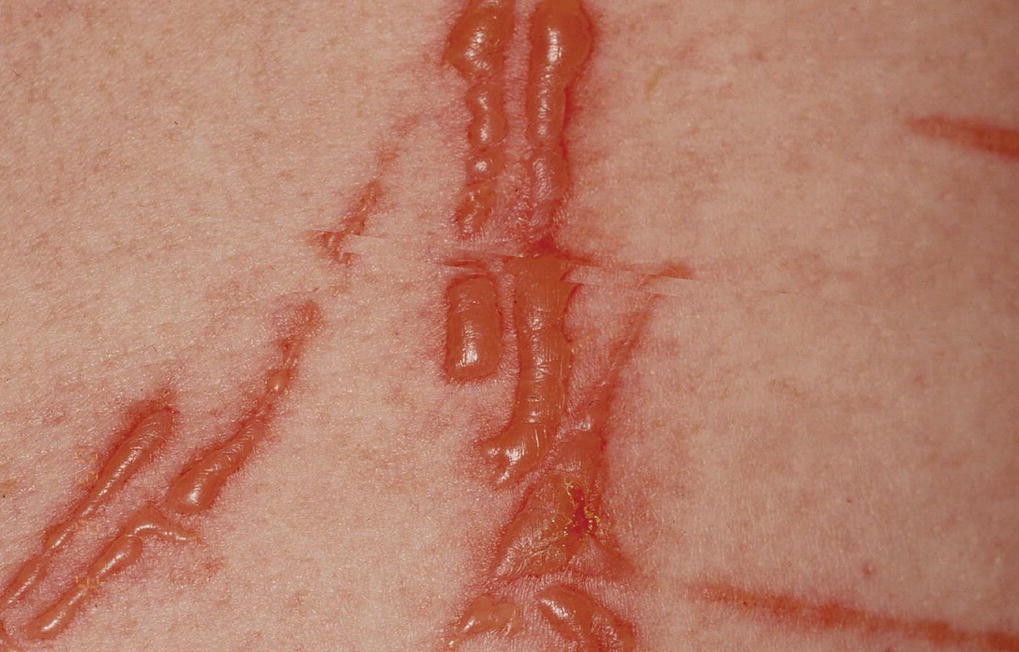
Figure 6.5 Phytophotodermatitis to lime juice.
Photosensitive disorders
Polymorphous light eruption (PMLE)
PMLE presents as a pruritic erythematous papular rash on exposed sites after sunlight exposure in the early spring/summer. The rash is sometimes preceded by a burning sensation and usually affects sites exposed by summer dress such as the neck, forearms, and legs. Except in children, perennially exposed sites are usually spared. Morphology varies but the rash of PMLE can be papular, in plaques, vesicular, oedematous, erythema multiforme‐like, even haemorrhagic (Figure 6.6a, b). The rash develops between 30 minutes to several hours after sun exposure and resolves without scarring over between 1 and 14 days.

Figure 6.6 (a) Polymorphous light eruption (PMLE). (b) PMLE papular and erythematous eruption.
The disorder is commoner in women than men and in the first three decades of life. It is more prevalent in latitudes further from the equator, and it can affect any skin type.
It has been suggested that the mechanism of PMLE may be cutaneous allergy to an endogenous allergen produced in sun‐exposed skin. In normal subjects UVB damage produces a latent immune‐suppressive response as well as a sunburn but in PMLE this immune‐suppressive response is absent. PMLE has an association with lupus erythematosus and some PMLE patients will have a positive antinuclear antibodies (ANAs).
Management includes sun avoidance, sunscreens, and topical or oral corticosteroids when severe. A course of desensitising TL‐01 (narrow‐band UVB) or psoralen with ultraviolet A (PUVA) (oral psoralen plus UVA) prior to sun exposure the following year can be effective.
Solar urticaria
This relatively rare photoallergic skin condition presents with rapid onset of an itchy, stinging erythema and urticaria following between a few seconds to a maximum of around 15 minutes of sun or artificial light exposure. Often patients can recall the exact date and time of the onset of solar urticaria. The rash is transient, resolving within minutes or hours after retreating from the light. UVA and visible wavelengths commonly trigger the reaction but UVB can be responsible too. The reaction is thought to be mediated by a photo‐modified cutaneous antigen. Solar simulator and monochromator light tests confirm the diagnosis. Solar urticaria can be debilitating and difficult to manage. Patients often avoid sunlight and can become reclusive. Sun avoidance, sunscreens with UVA protection, antihistamine, and TL‐01 narrow‐band UVB phototherapy may be helpful. Where conventional treatment has been unsuccessful, anti IgE drugs like omalizumab have been trialled with success.
Chronic actinic dermatitis (CAD)
Middle‐aged or elderly men are most commonly affected by this itchy, flaky, erythematous rash that typically affects the face, posterior and ‘V’ of neck, and the dorsi of hands (Figure 6.7). CAD is worse in summer but often persists throughout winter too. Monochromator testing can implicate UVB, UVA, and visible light. Patch and photo patch testing often identify multiple associated contact allergies, especially to Compositae plants (daisy family), perfumes, sunscreen, and colophony (pine trees and Elastoplast). Except in cases of allergy, sunscreen, and sun avoidance behaviour should be encouraged all year round. Aside from good sun‐protective measures, patients should be managed like eczema, with topical steroids and emollients. Some may require systemic corticosteroids. Methotrexate, azathioprine, and ciclosporin are sometimes useful too.

Figure 6.7 Chronic actinic dermatitis.
Sunprotection behaviour and sunscreens
A patient‐centred approach to sun protection, considering Fitzpatrick skin type, personal skin cancer history, and relevant diagnoses, is crucial. Those with type I and II skin are more at risk of burning and skin cancer than those with type V or VI. Patients with multiple naevi, previous cancers, and photodermatoses need pay even greater attention.
Sun protection is more about behaviour than sunscreens. Good sun protection habits include avoidance of direct sun exposure between 11 a.m. and 3 p.m., sitting in the shade, wearing protective clothing (tighter weaves and darker colours give greater protection), and wearing broad brimmed hats and eye protection. Nevertheless, regular sunscreen use has been shown to reduce the incidence of actinic keratoses, squamous, and melanoma skin cancers in at risk individuals.
On most sunscreen packaging the amount of protection offered against UVB radiation is indicated by the SPF. This is defined as the factor by which the amount of UVB needed to burn an individual's skin is increased by application of 2 mg/cm2 of the sunscreen. For example, SPF30 enables a person to stay 30 times longer in the sun without burning compared to if they were wearing no sunscreen. SPFs are rated on a scale of 2–50. UVA protection is indicated using the ‘star rating’. Protection level is indicated on a scale of 0–5 stars, with 5 providing the highest and 0 the lowest protection. Stars correspond to the percentage of UVA radiation absorbed by the sunscreen in comparison to UVB. A sunscreen with an SPF of 30 and a UVA rating of 4 or 5 stars is sensible.
There are two main types of sunscreen – inorganic and organic. Inorganic sunscreens can look opaque when applied to the skin and are less cosmetically acceptable; however, they are highly effective and are hypoallergenic. Inorganic sunscreens work by reflecting and scattering UV light. They contain minute particles of titanium dioxide for UVB and zinc oxide for UVA. Organic sunscreens are more likely to cause skin irritation and allergy but are less visible. Traditional organic sunscreens often contain combinations of cinnamates and p‐amino benzoic acid (PABA) which absorb predominantly UVB and give a high SPF. Newer, broad‐spectrum organics such as avobenzone (Parsol) and bis‐ethylehexyloxyphenol methoxyphenol triazine (Tinosorb S) absorb both in the UVB and the UVA parts of the spectrum.
Patients should be advised to apply sunscreens thickly and frequently (around two‐hourly is good). Although manufacturers recommend application of around 2 mg/cm2, in practice, most of us apply as little as a quarter of this. Furthermore, we find it hard to apply sunscreen often or evenly enough. Sunscreens can be washed off or wiped off by clothes. Despite their limitations, sunscreens remain part of our defence against the skin cancer epidemic and are vital for patients with photodermatoses. Particularly in at risk individuals, they should be encouraged as an important part of a wider strategy of sun‐protective behaviour.
Vitamin D levels and sun protection
Over the past decade, there has been concern among physicians that Vitamin D3 deficiency may be linked to diseases such as multiple sclerosis, asthma, type I diabetes and malignancies, including melanoma. It is also well known to be associated with rickets in children and osteoporosis in older patients. Some physicians also claim UV may confer other benefits too, such as improved blood pressure. Because 90% of human vitamin D3 is produced in the skin only in the presence of UVB there has been debate about whether dermatologists should rethink their advice on sun protection. How should we balance avoiding the risks of sun burn and skin cancer with ensuring that patients achieve healthy levels of vitamin D?
Although concern about the need to maintain a normal vitamin D is understandable, the data suggest that 25(OH) vitamin D reaches adequate levels after relatively short sun exposures. Cohort studies of patients on holiday in resorts in the Canary Islands and Spain have demonstrated that although sunscreens reduce vitamin D levels, the reduction is not significant and healthy levels are easily reached. Although truly obsessive sun avoidance and sunscreen use can limit vitamin D production, such meticulous behaviour is unusual; indeed, partial, and inconsistent sun protection behaviour is much more common. Although in winter months oral vitamin D3 supplements may be necessary and low vitamin D common, in summer sunscreens are unlikely to impede vitamin D production and should continue to be encouraged as part of good sun protection behaviour. When sun avoidance is imperative (for example in patients with XP), and patients do develop perennial low vitamin D, supplementation is an easy alternative to sun exposure.
Reference
- Sarkany, R. (2017). Sun protection strategies. Medicine 45 (7): 444–447. https://doi.org/10.1016/j.mpmed.2017.04.009.
Further reading
- Collignon, L.N. and Normand, C.B. (2010). Photobiology: Principles, Applications and Effects. New York: Nova Science Publishers.
- Ferguson, J. and Dover, J.S. (2006). Photodermatology. London: Taylor & Francis.
- Lim, H.W., Honigsmann, H., and Hawk, J.L.M. (2007). Photodermatology (Basic and Clinical Dermatology). New York: Informa Healthcare.
- Sarkany, R.P. (2008). Making sense of the porphyrias. Photodermatology, Photoimmunology and Photomedicine 24 (2): 102–108. https://doi.org/10.1111/j.1600‐0781.2008.00336.x.