Antonio Stecco, Stefano Casadei, Alessandro Pedrelli, Julie Ann Day, Carla Stecco
Introduction
Myofascial tissue is gaining increasing attention in the field of medicine and manual therapy. Its anatomy, physiology and biomechanical behavior have been the object of numerous research papers that are influencing the development of treatment modalities for musculoskeletal dysfunctions.
Studies concerning myofasciae have primarily focused on the anatomy and pathology of specific areas, such as the abdominal fascia, the Achilles tendon enthesis organ, plantar fascia or the iliotibial tract. While these studies are important, they do not provide a vision of the human fascial system as an interrelated, tensional network of connective tissue.
Fascia is the connecting element that unites all parts of the musculoskeletal system. It is continuous with ligaments, joint capsules and the outer layer of the periosteum. Whilst these structures vary in their denomination and composition, in terms of percentage of collagen or elastic fibers, fiber alignment and number of collagen fiber sublayers, together they form the so-called soft tissues.
While more and more authors are now associating the causes of many pathological conditions to a lack of balance within the tridimensional myofascial system, specific indications for treatment are not always provided.
Low back pain is one problem that clinicians address on a daily basis. The Fascial Manipulation
®
approach to low back pain will be discussed in this chapter.
The Fascial Manipulation® method
Luigi Stecco, physiotherapist and author of the Fascial Manipulation
®
method (Stecco L 2004, Stecco L & Stecco A 2017), has focused on the relationship between muscles, deep fascia and its components (epimysium, perimysium and endomysium – see
Ch. 1
) to develop a new approach to musculoskeletal dysfunctions that takes into account movement limitation, weakness and distribution of pain. Based on the idea of fascia as the uniting element between the various body segments, this author also considers fascia, with its innervation, relationship with muscle spindles and Golgi tendon organs and the presence of conspicuous myotendinous expansions that link adjacent segments together, as having a potential role as a coordinating component for motor units of unidirectional muscle chains.
Fascial Manipulation
®
aims at interpreting the passage of tensional compensation from one segment to another and the evolution from an initial, segmental disturbance to a more generalized dysfunction
. ‘Manus sapiens potens est’
(
Fig. 9.1
), the logo coined for this method, means ‘A knowledgeable hand is powerful’, suggesting that only by understanding the origin of a problem can manual therapies resolve them rapidly and efficiently. Numerous histological, biomechanical, and functional studies have been undertaken to verify some of the hypotheses proposed by Stecco, focusing on the anatomy of the superficial (Lancerotto et al. 2011) and deep fascia (Stecco C et al. 2009), innervation (Stecco C et al. 2007), and the possible mechanisms of action of the manual technique itself.
The thoracolumbar fascia (TLF) region is one area of fascia that has been closely examined and its implications in low back pain have been considered (Langevin et al. 2009, Schilder et al. 2014). The TLF is a large, diamond-shaped sheet that forms part of the deep fascia. While descriptions vary amongst different authors, according to C. Stecco (2015), the TLF consists of two macroscopic layers, anterior and posterior, the latter having three sublayers, a feature of aponeurotic-type deep fascia (Benetazzo et al. 2011). These three sublayers have different fiber characteristics, thickness and innervation, with the outermost layer being the most innervated. The multilayer posterior lamina connects latissimus dorsi and the contralateral gluteus maximus and joins with part of the external oblique and trapezius, via their fasciae. This lamina also fuses with the serrati posterior fascia and the erector spinae aponeurosis. Distally, it attaches to the posterior superior iliac spine, the iliac crests and the long dorsal sacroiliac ligament, as well as to the supraspinal ligament and spinous processes to the L4 level. The anterior layer of the TLF attaches medially to the transverse processes of the lumbar vertebrae and laterally to the internal oblique and transversus abdominis muscles. The different fiber directions give the TLF a crosshatched appearance.

Figure 9.1
Fascial Manipulation® logo.
Therefore, the TLF links with most of the body. It connects with:
•
the deep structures of the spine by extending down to the spinal muscles, spinal ligaments, vertebral column and spinal canal
•
the upper limbs, head and neck by virtue of its links with the trapezius and latissimus dorsi
•
the lower limbs by melding with the fascia of the gluteus maximus; the fascia of the gluteus maximus muscle is continuous with the posterior lamina of the TLF and, distally, it continues with the fascia lata and the iliotibial tract (Stecco A et al. 2013a). By connecting the two halves of the body with the upper and lower limbs, it permits contralateral force transmission during walking and running
•
the midline of the abdomen due to the fusion of the above-mentioned anterior and posterior laminae in the lateral trunk region to form the lateral raphe, a weave of connective tissue that joins with the transversus abdominis and internal oblique muscles. These muscles wrap around to the front of the body, surrounding the rectus abdominis and merging at the linea alba.
The TLF is clearly a part of an extensive network of interrelated structures that merit consideration as a whole. Unbalanced tension between any of these related structures could lead to the development of low back pain.
Stecco’s biomechanical model
Luigi and Antonio Stecco (2009) divide the body into 14 functional segments: the head (subdivided into three: eye, ear, jaw); neck; thorax; lumbar; pelvis; scapula; humerus; elbow; wrist; fingers; hip; knee; ankle and foot.
Each functional segment is composed of portions of muscles (mono- and biarticular fibers), that tense or move their fascia (deep and epimysial) and the associated joint components (tendons, ligaments, capsule). Thus, several components form each segment:
•
active components (muscular fibers)
•
passive components (the joint and its components)
•
and a force transmitting element (fascia).
Latin terms are used to distinguish these 14 segments from simple joints (
Fig. 9.2
).
The myofascial unit
Six myofascial units (MFUs) govern each functional segment, controlling its movements on the three spatial planes (sagittal – also known as median; frontal – also known as coronal; and horizontal) (Day et al. 2012).
A MFU is a functional unit formed by:
•
motor units innervating monoarticular and biarticular muscle fibers
•
the joint moved in only one direction when these fibers contract
•
the fascia that connects these fibers to the articular and periarticular components (menisci, ligaments, tendons, joint capsule)
•
the nerve components involved in this contraction.
Thus, different vectors act on each segment. Between the two principal vectors of every MFU (monoarticular fibers and biarticular fibers), there are many smaller vectors formed by single muscle fibers situated some distance apart. If we analyze all of these fibers, we can see how the monoarticular fibers are deeper and more voluminous than the biarticular ones. Partially free to slide in their fascial sheaths, the deep monoarticular muscle fibers transfer their tension to the superficial fascial layers via the continuity of the endomysium, perimysium and epimysium. The monoarticular fibers can exert strength and stability during movement while the biarticular fibers transmit tension between adjoining segments. While regional specializations in fascial structures do exist (Stecco A
et al. 2009a), the same ‘architecture’ can be found in every MFU.
Figure 9.2
Fourteen body segments with associated Latin names and abbreviations.
Due to this multiplication of vectors, each MFU has a fine control over a specific movement. The continuity of endomysium with perimysium and epimysium permits harmonious synchronization of all these tensional forces.
Each MFU has two functionally different areas. The first is on the deep fascia covering the muscle belly and can be considered as the active component of the MFU. Known as the center of coordination (CC), according to the Fascial Manipulation
®
model, the forces involved in muscle fiber contraction converge in this small area. The second area is a passive component. It is situated around the joint that is moved by MFU muscle fiber contraction. Called the center of perception (CP), this is where the patient feels the movement resulting from MFU activity (in a physiological situation) or pain (in a pathological situation).
Center of coordination
The term center of coordination (CC) infers that the fascia is potentially involved in feedback concerning movement of body segments due to its connections to muscle spindles, Golgi tendon organs and other mechanoreceptors. The CC is a small area of deep fascia where muscular forces of a MFU, generated by the contraction of mono- and biarticular fibers, converge.
This myofascial tension or force converges at a CC because:
•
part of the epimysial fascia is free to slide over the underlying muscle fibers
•
part of the fascia is anchored to the bone, so stretch can converge in one point
•
part of the fascia is inserted onto the bone, partially separating the tensioning of one MFU from the successive one.
CCs are usually situated within the deep fascia overlying a muscle belly and are not close to the joint. Receptors embedded in these areas are not normally stretched or irritated by movement, therefore these areas are rarely spontaneously symptomatic.
Center of perception
The center of perception (CP) is where traction produced by MFU muscle-fiber activity is perceived. The CP of each MFU is located in a circumscribed area over the joint capsule, tendons and ligaments. This is the area commonly indicated by the patient as being symptomatic.
According to Stecco A et al. (2013b), impeded sliding between collagen fiber layers within the fascia of a malfunctioning MFU could produce atypical afferent information from mechanoreceptors embedded within the fascial component of the MFU. Furthermore, given that the connective tissue capsule of muscle spindles is embedded in endomysium and it is continuous with the perimysium, altered sliding of these fascial tissues could affect correct muscle spindle firing (Stecco L 2016). Consequent anomalies in motor unit recruitment result in unaligned joint movement. Over time, joint conflict, inflammation of periarticular soft tissues, pain or joint instability can develop, with symptoms arising in the corresponding CP.
New terminology for movement
To simplify the interpretation of myofascial dysfunctions, Stecco describes movement in terms of directions on the three spatial planes. In classical terminology, the hip joint flexes when the femur moves forward but at the knee, the same direction of movement is called extension. In Fascial Manipulation
®
terminology, all forward movements on a sagittal (medial) plane are called antemotion and all backward movements retromotion. Adduction and abduction are substituted by lateromotion (outwards from the center) and mediomotion (inwards from the periphery), and intrarotation and extrarotation are used every time a segment moves on the horizontal plane. Thus, movements on the sagittal plane are governed by the MFUs of ante- and retromotion, movements on the frontal (coronal) plane by the MFUs of latero- and mediomotion and movements on the horizontal plane by the MFUs of intra- and extrarotation (
Table 9.1
).
Table 9.1
Terminology used in Fascial Manipulation®
to describe movement on the three spatial planes
|
Sagittal plane
|
Frontal plane
|
Horizontal plane
|
|
Antemotion (AN)
|
Mediomotion (ME)
|
Intrarotation (IR)
|
|
Retromotion (RE)
|
Lateromotion (LA)
|
Extrarotation (ER)
|
Due to its rich innervation, directional afferents originating from the fascial part of each MFU could contribute to proprioceptive information (Stecco C et al. 2010). Consequently, the deep fascia component is considered as being potentially active in movement coordination and peripheral motor control.
Fascial mediation of agonist–antagonist interaction
Agonists are a group of muscles that contract to provide the force required to produce a particular movement. Antagonists are the muscles that oppose the action of the agonists. In Stecco’s model, agonist and antagonist MFU interaction is important for myofascial force transmission and the coordination of movement, as also demonstrated by other studies (Huijing & Baan 2003). As the agonist MFU is activated during movement (albeit forwards, backwards or sideways), the antagonist MFU adapts according to the angle of inclination of the body part (reciprocal inhibition).
In almost every MFU, many monoarticular fibers insert onto the intermuscular septum that separates two antagonist MFUs. The intermuscular septa, together with the epimysial sheaths, could play a direct role in the regulation of the muscular fibers of the two MFUs.
For example, the MFU for elbow extension has its own antagonist myofascial unit that coordinates elbow flexion (
Fig. 9.3
). When the elbow extends, the
monoarticular fibers contract and the intermuscular septum (where they insert) is stretched. The monoarticular fibers of the antagonist MFU (elbow flexion) insert on the other side of this same septum meaning that during extension, its monoarticular components are stretched a little too, causing embedded stretch receptors to fire. Therefore, fascia can be considered as an active component in agonist–antagonist activity.
Figure 9.3
Fascial connections among synergic muscles in the anterior region of the upper limb.
The myofascial sequences
Stabilization by monoarticular vectors and synchronization between adjacent segments by biarticular muscular fibers allows for precision and stability of each segment during movement. The biarticular muscle fibers composing each MFU connect unidirectional MFUs responsible for movement in only one direction to form myofascial sequences (MFS). Part of the biarticular fibers from each MFU also insert onto the deep fascia of adjacent segments forming myotendinous expansions that link one segment to the next (Stecco C et al. 2008, Stecco A et al. 2009b) and providing the anatomical substratum of the MFS.
For example, the anatomical continuity of the MFS on the sagittal plane in the lower limb can be traced from antemotion talus (dorsiflexion of the foot), which is carried out by tibialis anterior, extensor digitorum longus and extensor hallucis longus muscles. These muscles originate from the condyles of the tibia and the fibula, from the intermuscular septa and the overlying fascia. The tendinous expansion of the quadriceps tensions the anterior fascia of the leg proximally, whereas the previously listed muscles traction it distally. The fascia lata connects with the anterior fascia of the leg, and is tensioned distally by its insertions into the intermuscular septa of the vastus medialis and lateralis. Moreover, it is continuous with the ileopsoas fascia, which inserts onto the vastus medialis that tensions it distally, together with the sartorius. Ileopsoas fascia continues above over the fascia of the iliacus and psoas minor muscles. The iliacus fascia is involved in antemotion of the pelvis (retroversion of the pelvis) together with the inferior rectus abdominis, which is surrounded by the lateral raphe. This weave of connective tissue joins the abdominal muscles and the anterior and posterior layers of the TLF.
In the antagonist sequence, retromotion of the foot is activated just prior to the push-off, or toe-off, phase of the gait cycle. In this phase, the foot is slightly supinated, bringing the lateral compartment, comprising the abductor digiti minimi, in contact with the ground. Abductor digiti minimi originates from the plantar aponeurosis, which is the continuation of the Achilles tendon of triceps surae (
Fig. 9.4
).
The contraction of the triceps surae stretches the popliteal fascia and the fibers of the gastrocnemius inserted into it. A few fibers of the biceps femoris, as well as some fibers of the semitendinosus and semimembranosus, tension the popliteal fascia and the fascia of the leg proximally. These latter muscles participate both in retromotion genu (knee flexion) and retromotion coxa (hip extension); therefore, they not only tension the popliteal fascia but also the sacrotuberous ligament. This myofascial concatenation is tensioned during the push-off phase of each step. The sacrotuberous ligament continues proximally with the TLF. The erector spinae muscles originate from the TLF and during
retromotion lumbi
(extension of the spine) they become tensors of the retromotion sequence of the lower limb.
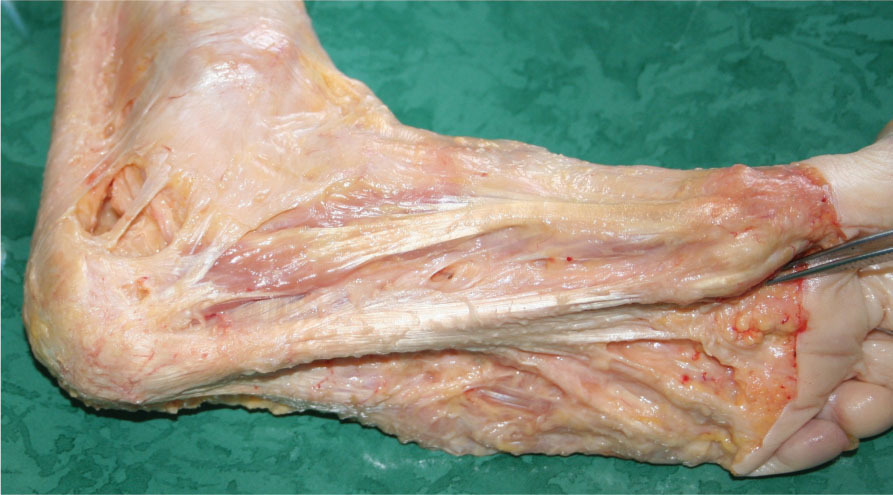
Figure 9.4
Dissection of the plantar aponeurosis. While a longitudinal continuity along the main axis of the foot is evident, the plantar aponeurosis also continues with the medial and lateral fasciae of the foot (fascia of the abductor hallucis and abductor digiti minimi muscles, respectively).
Similar anatomical continuity is found in each MFS. Sequences on the same spatial plane (sagittal, frontal, or horizontal) are reciprocal antagonists; therefore, altered areas along the fascia of one MFS can potentially compromise the entire spatial plane related to that MFS.
Key Point
In English, densification
is a neologism coined by Stecco to describe the palpable sensation of lack of sliding between interfascial and intrafascial layers. From a histological viewpoint, it mainly concerns changes in density of the extracellular matrix of the loose connective tissue layers found between the different fascial layers and interfaces.
Clinical reasoning
In cases of low back pain, clinicians apply Stecco’s biomechanical model to interpret the spread of tensional compensations from one segment to another. A tensile alteration in any given MFU causes a counter tension in another MFU along the same sequence, as a means of preserving the fascia’s basal tension. Such tensile adjustments are a remedy that often creates acute pain because the free nerve endings, or mechanoreceptors, in this segment of fascia are subjected to excessive, abnormal traction. The body then compensates this tension as a means of re-establishing equilibrium. Compensatory tension can be symmetrical (in the antagonist sequence) but, with reference to the MFU where the compensation originated, it could be localized in a proximal or distal segment. In the trunk, each MFU of antemotion and retromotion can act with the contralateral side of the body. For example, it is possible to find that a hypertonic left-sided component of the antemotion MFU in the lumbar region is compensated by a right-sided component of retromotion.
In summary, if the basal tension of the fascia is altered by the formation of a ‘
densification
’, then tensional compensations can develop either in the symmetrically antagonist MFU or along the ipsilateral sequence, or in the contralateral sequence.
Low back pain is always approached through an accurate examination of each individual case in order to identify the densified CCs that are involved in compensations. While both descending and ascending compensations are common, some examples of ascending compensations that originate in the foot and spread upwards on the three planes are described below:
•
On the sagittal plane, a restriction within a toe extensor tendon (e.g. hammer toe) can provoke contraction of the triceps surae causing the knee to hyperextend in compensation for the increase in the angle of the ankle joint. The hyperextended knee (
genu recurvatum
) causes anteversion of the pelvis with consequent shortening of the iliopsoas muscle. An exaggerated lumbar lordosis results, leading to a dorsal kyphosis and, in an attempt to neutralize the other curves, an exaggerated cervical lordosis may form.
•
In the frontal plane, lowered arches (flat feet) often produce a medial deviation of the knee (
genu valgus
). Hip abduction (frontal plane) ensues, with a lowered iliac crest due to a restriction of the tensor fascia lata. This variation in pelvic alignment initially inclines the vertebral column to the same side and, in time, produces compensation in the opposite side.
•
On the horizontal plane, misalignment can begin with a unilateral hallux valgus. This alteration can be bilateral but to simplify the analysis only one limb is considered here. Intrarotation of the forefoot (
hallux valgus
) is compensated by eversion of the talus due to contraction of the peronei muscles (
talus valgus
). The knee and the hip intrarotate, bringing the pelvis forward on the same side and causing contralateral compensation in the trunk.
Therefore, it can be seen that the biomechanics of the lumbar spine are strictly connected to the correct functioning of the ankle and knee because of the fascial continuity between these three anatomical segments.
For example, it is common to find the origin of low back pain in a now ‘silent’ knee or ankle that suffered a strain or tendinitis years ago (see Case Example). That is because the fascial system is a complex network that runs the entire length of the body and connects all of its parts. It responds to mechanical traction induced by muscular activity in different regions and plays a relevant role in epimuscular force transmission in every spatial plane and trajectory of movement.
Analysis might reveal that:
•
Various painful areas are distributed along a sequence; for example, if pain is localized in the medial part of the thigh, knee and ankle then one could hypothesize a dysfunction of the mediomotion sequence of the lower limb.
•
Various painful areas are distributed on one plane: if pain is localized in the right side of the lumbar region plus lateral right thigh and medial lower leg, then one could hypothesize a dysfunction on the frontal plane.
Clinicians aim to elaborate a valid hypothesis that explains the compensatory pathways chosen by the body to counterbalance one or more fascial densifications. This hypothesis is fundamental in developing a correct therapeutic plan and for validation during on-going assessments.
Assessment
Key Point
Silent CCs
are asymptomatic areas of deep fascia that can be deduced from the patient’s symptoms and located by using knowledge of the continuity of the sequences on the three planes.
Application of the Fascial Manipulation® method involves compilation of a specific assessment chart that assists the selection of CCs to treat, and provides concise documentation of treatment sessions. Patient’s personal data and history, an abbreviated description of the presenting symptoms, including pain location, characteristics, and chronicity, and identifiable painful movements are all noted.
Patients are encouraged to report any concomitant pain because even minor painful areas are useful in indicating the disturbed sequence or plane.
The following questions are taken into consideration:

In which plane have the various tensional compensations developed?
In which plane have the various tensional compensations developed?

What could have been the initial trauma that determined these compensations?
What could have been the initial trauma that determined these compensations?

Are they ascending or descending compensations?
Are they ascending or descending compensations?

Are there any hidden compensatory strategies (
silent CCs
)?
Are there any hidden compensatory strategies (
silent CCs
)?
Multisegmental problems are approached through the analysis of chronological events involved in each individual case. Asymptomatic previous disturbances are frequently the cause of presenting pain
because fascia often repairs a trauma initially with densification and, subsequently, with a buildup of an excess of collagen fibers, neutralizing the lack of elasticity due to established densifications by extending tension along the same sequence. While heat applied to an initial densification can resolve changes in the extracellular matrix of the muscular fascia, any excess fibers will not be spontaneously removed by the body’s physiological reparative mechanisms because they are normal collagen fibers and are not recognized as being inappropriate.
Once the clinician has formulated a hypothesis, movement and palpation assessments are then performed.
Movement assessment
Clinicians evaluate the mobility of two or more joint segments on all three planes by using standardized movement tests (
Fig. 9.5
) to determine the more compromised plane of movement. The most painful or limited movement in the majority of segments suggests which MFS or plane may be implicated in the dysfunction.
As mentioned above, pain in the pelvis or the lumbar region may be merely the latest counter compensation, the cause being a now silent knee segment or a previous ankle problem.
Palpation assessment
Once altered movements are noted, palpatory tests guide therapists in selecting the combination of densified CCs to be treated (
Fig. 9.6
). All six CCs of the implicated segments are palpated and compared. CCs that are painful, densified and from which a referred pain expands towards the CP are the most likely candidates for treatment.
Treatment
Treatment consists of a deep friction over the CCs. Therapists use the olecranon of their elbow, their knuckle, or fingertips over densified CCs, creating localized hyperemia through friction.
Figure 9.5
Example of movement assessment of the LA-LU myofascial units (MFU): the right side stretches during an eccentric contraction; the therapist notes the amplitude and whether lateral flexion is harmonious or whether other compensations appear. The patient may report pain or tension.
Figure 9.6
Example of palpatory (sensitivity) test of RE-LU center of coordination (CC) located in the erector spinae’s deep fascia, lateral to L1.
Treatment is directed toward the fascia because of its particular characteristics:
•
Elasticity: fascia is an elastic tissue with established limits, which permits involvement in motor coordination and perception of motion. Different studies do suggest that fascia is richly innervated. Nerves passing through the deep fascia are surrounded by loose connective tissue. Therefore, they are subjected to traction when the fascia lengthens. However, when these nerves terminate in neuroreceptors (e.g. free nerve endings) they insert directly into the collagen fibers. The abundance of free and encapsulated nerve endings could activate specific patterns of proprioceptors, potentially providing directional and spatial afferent information.
•
Plasticity: external stress, such as repeated mechanical stimuli, thermal stress and chemical or metabolic dysfunctions, can alter fascia. External mechanical stimuli stimulate protein turnover and fibroblast activity within fascia, altering the mechanical properties of its extracellular matrix. These characteristics and the reported abundant innervation of deep fascia indicate that it could have the capacity to perceive mechanosensitive signals.
•
Malleability: ‘densification’ is not a permanent and irreparable pathological condition. Fascial tissue is easily accessible and it possesses a strong capacity for repairing and regenerating itself. Just as stressful stimuli can modify fascial consistency, manipulation of specific areas can restore its physiological condition.
In general, if a traumatic stimulus causes local inflammation, rest and physiological movement induce reorganization of collagen fibers along the lines of traction and a normal healing process of the damaged area ensues. Repeated inflammation (overuse, repetitive strains) can affect the extracellular matrix, or ground substance, of the loose connective tissue layer interposed between the multilayers of collagen fibers present in the deep fascia (
Table 9.2
). In chronic situations, this can lead to increases in the number of collagen fibers resulting in a chaotic redistribution of these fibers at the CCs. Trauma, disuse with consequent diminished circulation, repetitive motion and poor posture can cause the ground substance to transform from a sol to gel state, resulting in lack of sliding.
Table 9.2
Physiological and pathological reactions of fascia to stress
|
Repeated mechanical stimuli
|
Chronic dysfunction
|
|
Inflammation
|
Repeated inflammation
|
|
Repair
|
Ground substance densification
|
|
Reorganization of collagen fibers
|
Collagen fiber dysplasia
|
|
Healing
|
Fibrosis
|
Key Point
In Fascial Manipulation® treatment of deep muscular fascia, the term friction refers to the force required to modify the increased viscosity of the extracellular matrix in the loose connective tissue located between intrafascial collagen fiber layers. The manual technique is referred to as a deep friction because it involves a perpendicular pressure together with a tangential oscillation applied to densified tissue. This technique is a potentially effective method for influencing flow characteristics of hyaluronan (HA) in the extracellular matrix (Roman et al. 2013). Furthermore, deep friction applied to such tissue causes an increase in local temperature, setting off a chain reaction that modifies HA viscosity (Cowman et al. 2015).
Densification of a CC may cause hypertonicity within a MFU, which can be responsible for incorrect joint movement. If the soft tissues surrounding a joint (CP) do not stretch according to physiological lines, then multimodal receptors embedded in these tissues can transform from mechanoreceptors to nociceptors and signal the dysfunction as pain.
Under physiological conditions, CCs are not hypersensitive nor do they produce referred pain when stimulated. Normally, the elasticity of the fascia allows it to adapt to compression without straining embedded nerve endings. Densified CCs increase overall tension, lowering the pain threshold of the free nerve endings, and minimal compression can then be sufficient to set off local as well as referred pain. This process can also involve the CP of the implicated MFU, the CP of the antagonist MFU, or the entire MFS, which can explain the irradiation of pain along this structure. These stresses can apparently resolve spontaneously through tensional compensations and a densified CC often becomes silent (similar to latent trigger points) and pain is no longer felt at the CP. This seemingly balanced condition is unstable and, whenever the body is no longer capable of maintaining these compensations, chronic fascial densifications become active, and pain presents again.
The alteration of a CC can cause joint pain (in the CP) as well as a joint blockage. In the latter case, if it is a recent lesion then it is possible to intervene directly with joint mobilizations. Freeing the articulation reduces the painful afferent and eliminates MFU hypertonicity. However, if the chronicity of the problem has created a true ‘densification’ of the CC or, in more chronic conditions, a fibrotic state with collagen fiber dysplasia, then manipulation applied directly to the CC is required (Pedrelli et al. 2009, Pavan et al. 2014).
Considerable amounts of hyaluronan (HA), previously referred to as hyaluronic acid, are found in the ground substance of the loose connective tissue layer between the deep fascia and the surface of muscle and in the intrafascial layers (Stecco C et al. 2011). HA normally acts as a lubricant but, under pathological conditions, it aggregates, increasing ground substance viscoelasticity, resulting in densification (Stecco A et al. 2013b). Manipulation is required to act on a densified CC for a sufficient amount of time for the friction against the fascia to produce heat (Borgini et al. 2010). This heat is needed to modify the consistency of the ground
substance and to initiate the inflammatory process required for healing.
If a tensional balance in the fascia is restored, physiological movements can align new collagen fibers along the normal lines of force. It is therefore important not to focus treatment at the sites of pain, which is often merely the consequence of the dysfunction. The aim is always to trace back to initial disturbances.
Treatment begins once the assessment has defined the CCs to be treated. Treatment of the sequences is characterized by the fact that the selected CCs must be part of a plan for restoring global postural equilibrium.
It is imperative to:
•
Choose a proximal CC and a distal one to release fascial tension.
•
Choose one or more CCs of the antagonist sequence.
After having manipulated two points then it is useful to re-assess movement. If symptoms have improved, then it can be taken as an indication to continue with that sequence or plane; otherwise it is best to re-elaborate the treatment plan.
Treatment can vary in its intensity or depth for the following reasons:
•
Whenever disturbances of the deep fascia have extended to include the superficial fascia then more superficial maneuvers using a larger contact surface are employed.
•
Static compression or stretch is used when there is a localized swelling.
•
Deep friction is used when either densification or fibrosis of the fascial tissue is present. Here the intention is to bypass the hypodermis layer, which includes the superficial fascia, to act on the deep fascia.
Case Example Fascial Manipulation® applied to low back pain
LR is a 50-year-old male who has been suffering from low back pain for 1 year. Symptoms were resistant to treatment of any kind.
He had never suffered from backache prior to this and a CAT scan has excluded a prolapsed disc. Pain, localized along the right posterior side of the lumbar region and pelvis, had begun 1 year ago after a tennis match and since then it has never completely disappeared but it has varied in intensity. Symptoms are accentuated with running and bending forward.
Two segments (LU, PV) are indicated as the site of pain distributed in the posterior part (RE) of both segments. This could indicate the sequence of retromotion but this would mean considering only the present situation without considering other potential causes. Certainly, by treating the CC of RE-LU and RE-PV it is likely that the patient will have some relief but at the first attempt at running it may well recur. When asked ‘Have you ever had pain in the past in any part of your body? Did you ever suffer from fractures or ankle sprains? Did something happen to your knees in the past?’
the patient recalls having a right first metatarsal fracture when aged 40 (10 years ago) and he remembers rupturing his right Achilles tendon 1 year later (9 years ago).
From this data it was possible to hypothesize that the spasm in the retromotion sequence (RE-TA, RE-LU, RE-PV) may have been determined by a compensation created by the antagonist antemotion sequence (AN-PE) that had not caused any problems for 10 years. It required an intense physical stress, such as a long tennis match, to decompensate the tensile equilibrium.
Movement assessment revealed pain along the posterior leg during the test for antemotion talus (ankle) and pes (foot). Palpation assessment revealed densification of five CCs (RE-LU, AN-PV, RE-TA, AN-TA and AN-PE).
The AN-PE and RE-TA CCs were treated first. Post-treatment movement assessment demonstrated that the pain in the lumbar region had decreased from 8 to 3 on the VAS scale. The other three CCs were treated in order to complete the tensile balance (
Fig. 9.7
).
The patient was pain-free after the first treatment; therefore, the following advice was given: ‘When the treated points are no longer tender you can recommence running. The first run should be brief and you should stop if any pain reappears. Try again after 2 days and if the pain is felt again then ask for a second appointment.’
The athlete called after 10 days to say that he had some pain during the first run but during the following run, no disturbance had been felt.

Figure 9.7
Anatomical location of centers of coordination (RE-LU, AN-PV, RE-TA, AN-TA and AN-PE) treated in the Case Example.
As treatment is usually at a distance from the site of pain or the inflamed area, this technique can be applied during the acute phase of a dysfunction.
A study of 24 subjects with chronic non-specific low back pain (Branchini et al. 2016), has shown that Fascial Manipulation
®
associated with a physiotherapy program compared to a physiotherapy program alone gave statistically and clinically significant improvements in the short and medium term for all outcomes, including pain, function and quality of life.
Conclusion
The Fascial Manipulation®
method indicates a new manner for comprehending the possible connections between different sites of pain, introducing interesting perspectives for clinicians involved in the manual treatment of musculoskeletal dysfunctions such as low back pain.
Acknowledgment
The authors are grateful to Luigi Stecco for his unfailing support.
References
Benetazzo L et al 2011 3D reconstruction of the crural and thoracolumbar fasciae. Surg Radiol Anat 33(10):855–862
Borgini E, Antonio S, Julie Ann D, Stecco C 2010 How much time is required to modify a fascial fibrosis? J Bodyw Mov Ther 14:318–325
Branchini M et al 2016 Fascial Manipulation® for chronic aspecific low back pain: a single blinded randomized controlled trial. F1000Res 4:1208
Cowman MK, Schmidt TA, Raghavan P, Stecco A 2015 Viscoelastic properties of hyaluronan in physiological conditions. F1000Res 4:622.
Day JA, Copetti L, Rucli G 2012 From clinical experience to a model for the human fascial system. J Bodyw Mov Ther 16 (3):372–380
Huijing PA, Baan GC 2003 Myofascial force transmission: muscle relative position and length determine agonist and synergist muscle force. J Appl Physiol. 94:1092–1107
Lancerotto L et al 2011 Layers of the abdominal wall: anatomical investigation of subcutaneous tissue and superficial fascia. Surg Radiol Anat 33(10):835–842
Langevin HM et al 2009 Ultrasound evidence of altered lumbar connective tissue structure in human subjects with chronic low back pain. BMC Musculoskelet Disord 10:151
Pavan PG, Stecco A, Stern R, Stecco C 2014 Painful connections: densification versus fibrosis of fascia. Curr Pain Headache Rep 18(8):441
Pedrelli A, Stecco C, Day JA 2009 Treating patellar tendinopathy with Fascial Manipulation. J Bodyw Mov Ther 13:73–80
Roman M, Chaudhry H, Bukiet B et al 2013 Mathematical analysis of the flow of hyaluronic acid around fascia during manual therapy motions. J Am Osteopath Assoc 113(8):600–610
Schilder A et al 2014 Sensory findings after stimulation of the thoracolumbar fascia with hypertonic saline suggest its contribution to low back pain. Pain 155:222–231
Stecco A et al 2009a Pectoral and femoral fasciae: common aspects and regional specializations. Surg Radiol Anat 31:35–42
Stecco A et al 2009b Anatomical study of myofascial continuity in the anterior region of the upper limb. J Bodyw Mov Ther 13:53–62
Stecco A et al 2013a The anatomical and functional relation between gluteus maximus and fascia lata. J Bodyw Mov Ther 17: 512–517
Stecco A, Gesi M, Stecco C, Stern R 2013b Fascial component of the myofascial pain syndrome. Curr Pain Headache Rep 17(8):352
Stecco A et al 2014 Ultrasonography in myofascial neck pain: randomized clinical trial for diagnosis and follow-up. Surg Radiol Anat 36(3):243–253
Stecco C et al 2007 Anatomy of the deep fascia of the upper limb. Second part: study of innervation. Morphologie 91:38–43
Stecco C et al 2008 The expansions of the pectoral girdle muscles onto the brachial fascia: morphological aspects and spatial disposition. Cells Tissues Organs 188:320–329
Stecco C et al 2009 Mechanics of crural fascia: from anatomy to constitutive modelling. Surg Radiol Anat 31:523–529
Stecco C et al 2010 The ankle retinacula: morphological evidence of the proprioceptive role of the fascial system. Cells Tissues Organs 192:200–10
Stecco C et al 2011 Hyaluronan within fascia in the etiology of myofascial pain. Surg Radiol Anat 33(10):891–896
Stecco C 2015 Functional atlas of the human fascial system. Churchill Livingstone/Elsevier, United Kingdom.
Stecco L 2004 Fascial manipulation for musculoskeletal pain. Piccin, Padova
Stecco L, Stecco A 2017 Fascial manipulation for musculoskeletal pain – Theoretical part, 2nd edn. Piccin, Padova
Stecco L 2016 Atlas of Physiology of the Muscular Fascia. Piccin, Padova
Stecco L, Stecco C 2009 Fascial Manipulation, Practical part. Piccin, Padova






In which plane have the various tensional compensations developed?
In which plane have the various tensional compensations developed?

What could have been the initial trauma that determined these compensations?
What could have been the initial trauma that determined these compensations?

Are they ascending or descending compensations?
Are they ascending or descending compensations?

Are there any hidden compensatory strategies (
silent CCs
)?
Are there any hidden compensatory strategies (
silent CCs
)?


