TABLE 15-1. DIAGNOSTIC TESTS USED IN DIFFERENTIAL DIAGNOSIS OF ARF
KNOWLEDGE COMPETENCIES
1. Describe the etiology, pathophysiology, clinical presentation, patient needs, and principles of management of acute renal failure (ARF).
2. Differentiate between the three types of ARF:
• Prerenal
• Intrarenal
• Postrenal
3. Compare and contrast the pathophysiology, clinical presentation, patient needs, and management approaches of life-threatening electrolyte imbalances:
• Sodium (Na+)
• Potassium (K+)
• Calcium (Ca++)
• Magnesium (Mg++)
• Phosphorus (PO4––)
4. Differentiate between the indications for and the efficacy of the different types of renal replacement therapies.
5. Describe the nursing interventions for patients undergoing renal replacement therapy.
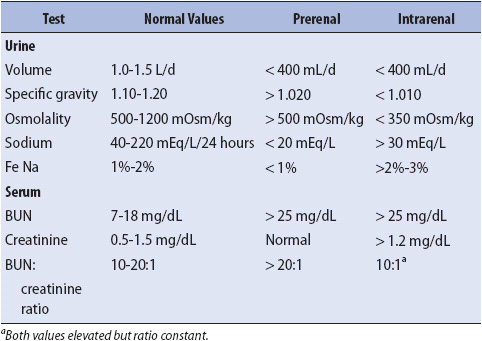
There are a wide variety of diagnostic tests available for use in determining the cause and location of renal dysfunction. The creatinine and blood urea nitrogen (BUN) levels are monitored closely, because these levels and their relationship to each other (BUN:creatinine ratio) provide valuable information about the kidney’s filtering ability. The BUN level provides valuable information about the state of renal perfusion, whereas the creatinine level is more precise in evaluating actual tubular function. Creatinine clearance is useful for assessing the glomerular filtration rate. Urine Na+ values vary as the kidneys attempt to retain or excrete water. Urine volume, specific gravity (SG), and osmolality are useful in identifying the kidney’s ability to excrete and concentrate fluid. Comparisons of these test values as found in prerenal and intrarenal failure are shown in Table 15-1. These tests help establish a firm diagnosis.
TABLE 15-1. DIAGNOSTIC TESTS USED IN DIFFERENTIAL DIAGNOSIS OF ARF

Radiologic tests also give important information about the kidneys. A kidney ultrasound may be used to look for stones or injuries. Both CT and MRI are able to identify tumors, hemorrhage, trauma, and perfusion of the kidney. An arteriogram identifies the vascular system of the kidney and renal artery stenosis.
Physical assessment related to the kidneys includes monitoring of the intake and output, daily weights, and noting a positive or negative fluid balance. Observation of the patient’s urine for color, clarity, and odor adds to the assessment. Signs of volume overload may include pulmonary crackles, peripheral edema, jugular venous distention, or an S3 heart sound. Volume deficit may be denoted by the presence of dry mucus membranes and weak peripheral pulses. The kidneys help control the internal environment of the body, therefore, when kidney function decreases, the progressive care nurse may see changes in most, if not all, body systems.
The most common renal problem seen in acutely ill patients is the development of acute renal failure (ARF), also now called acute kidney injury (AKI). ARF/AKI is the abrupt decrease of renal function with progressive retention of metabolic waste products (eg, creatinine and urea). Oliguria, urine output of less than 400 mL/day, is a common finding in ARF. The development of ARF in the acutely ill patient has an estimated mortality of 30%-80%. Patients who develop ARF due to sepsis have a higher mortality. A history of chronic renal failure (CRF) complicates the clinical course of any illness.
A classification system (Figure 15-1) for the AKI spectrum is Risk of renal dysfunction, Injury to the kidney, Failure of kidney function, Loss of kidney function, and End-stage kidney disease (RIFLE). This system allows patients to be classified by changes in serum creatinine and/or urine output.
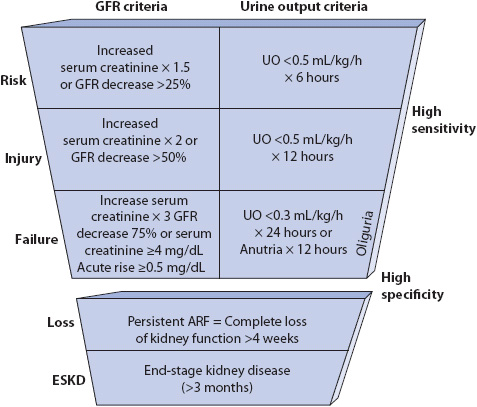
Figure 15-1. Defining characteristics of RIFLE criteria. (From: Bellomo R, Ronco C, Kellum JA, et al. Acute Renal Failure-Definition, outcome measures, animal models, fluid therapy and information technology needs. Avail able at: http://ccforum.com/content/8/4/R206. Page R206. Accessed April 1, 2013.)
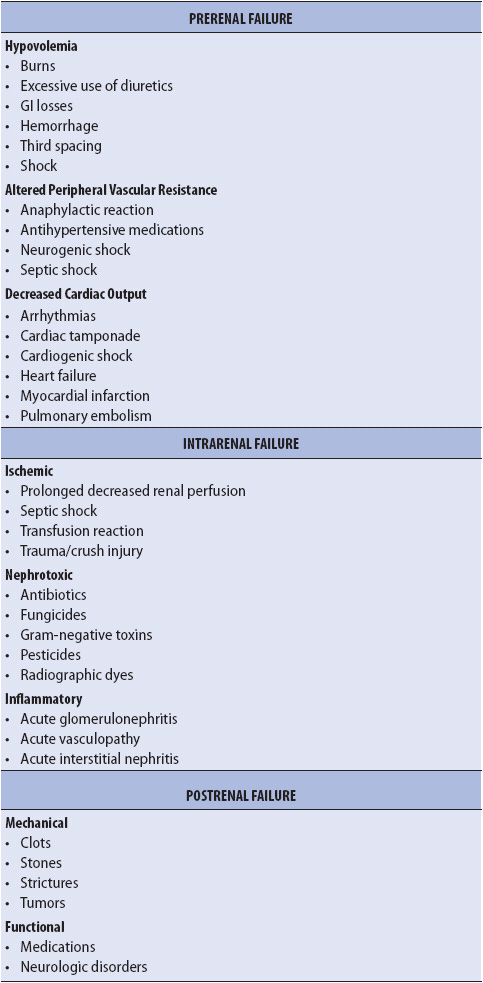
Acute renal failure is best understood when the condition is considered in terms of the location of damage to the renal system: prerenal, intrarenal, or postrenal causes of failure. Each type of ARF has different etiologies, pathophysiology, laboratory findings, and clinical presentation.
Physiologic conditions that lead to decreased perfusion of the kidneys, without intrinsic damage to the renal tubules, are identified as prerenal failure (Table 15-2). The decrease in renal arterial perfusion causes a decrease in the rate of filtration of blood through the glomerulus. When perfusion pressure falls less than 70 mm Hg, the protection of autoregulation is lost, further decreasing glomerular filtration.
TABLE 15-2. CAUSES OF ARF
Renal tubular function, at this point, is still completely normal. As a result of the decreased glomerular filtration rate (GFR), the kidneys are unable to adequately filter waste products from the blood. Consequently, more Na+ and water are reabsorbed by the kidneys, resulting in oliguria. If the decreased perfusion state persists, irreversible damage to the renal tubules may occur, resulting in intrarenal failure. Most forms of prerenal failure are easily reversed by treating the cause and increasing renal perfusion.
Physiologic conditions that damage the renal tubule, nephron, or renal blood vessels are identified as intrarenal failure (see Table 15-2). Following prolonged decreases in renal perfusion, the kidneys gradually suffer damage that is not readily reversed with the restoration of renal perfusion. Acute tubular necrosis is the most common form of intrarenal failure.
When the insult to the kidney is nephrotoxic (from drugs or substances that cause direct damage to the kidney), the nephron damage occurs primarily at the epithelial layer. Because this layer has the ability to regenerate, rapid healing often occurs following nephrotoxic insults. When the insult is ischemic or inflammatory, the nephron’s basement membrane is also damaged and regeneration is not possible. Ischemic and inflammatory insults are more likely to cause CRF than nephrotoxic insults.
The underlying pathophysiologic abnormality in intrarenal failure is renal cellular damage. In healthy kidneys, the glomerulus normally acts as a filter, preventing the passage of large molecules into the glomerular filtrate. Damage to the glomerulus allows protein and cellular debris to enter the renal tubules, leading to intraluminal obstruction.
Contrast-induced nephropathy (CIN) is seen in about 10% of patients receiving contrast media. It is thought that the contrast media causes renal vasoconstriction. CIN is defined as a 25% increase in creatinine or an absolute increase of 0.5 mg/dL. Patients who are at increased risk for CIN include diabetics, the elderly, or those with underlying renal insufficiency. Also, using greater amounts of the media increases the risk of CIN. The rise in creatinine occurs within 24 hours but frequently resolves in 3-5 days. The patient may be oliguric or may have no decrease in urine output.
Physiologic conditions that partially or completely obstruct urine flow from the kidney to the urethral meatus can cause postrenal failure (see Table 15-2). Partial obstruction increases renal interstitial pressure, which in turn increases Bowman capsule pressure and opposes glomerular filtration. Complete obstruction leads to urine backup into the kidney, eventually compressing the kidney. With complete obstruction, there is no urine output from the affected kidney. Postrenal failure is an uncommon cause of ARF in acutely ill patients. The treatment for postrenal failure is focused on removing the obstruction.
There are three clinical phases of ARF, seen primarily in intrarenal failure. The first, the oliguric phase, begins within 48 hours of the insult to the kidney. In intrarenal failure, the oliguric phase is accompanied by a significant rise in BUN and creatinine. The degree of elevation of these waste products is less pronounced in prerenal failure. The most common complications seen in this phase of renal failure are fluid overload and acute hyperkalemia. The oliguric phase may last from a few days to several weeks. The longer the oliguric phase continues, the poorer the patient’s prognosis.
The diuretic phase follows the oliguric phase. During this phase, there is a gradual return of renal function. Although the BUN and creatinine continue to rise, there is an increase in urine output. The patient’s state of hydration prior to the diuretic phase determines the amount of urine output. A patient who is fluid overloaded may excrete up to 5 L of urine a day and have marked Na+ wasting. The average time in this phase is 7 to 10 days. Patients must be observed carefully for risk of complications from fluid and electrolyte deficits. If the patient receives dialysis during the oliguric phase, the diuretic phase may be decreased or absent.
The recovery phase marks the stabilization of laboratory values and can last 3 to 12 months. Some degree of residual renal insufficiency is common following ARF. Some patients never recover renal function and progress to CRF.
The diverse causes of renal failure determine the clinical presentation of the patient. Renal failure can cause multiple organ dysfunction and, therefore, manifests itself in a variety of ways. Uremia describes the clinical syndrome that accompanies the detrimental effects of renal dysfunction on the other organ systems. The clinical presentation of the patient in uremia reflects the degree of nephron loss and, correspondingly, the loss of renal function.
Signs and Symptoms
• Oliguria (< 400 mL/day) or anuria (< 100 mL/day)
• Tachycardia
• Hypotension (prerenal)
• Hypertension (intrarenal)
• Flat neck veins (prerenal)
• Distended neck veins (intrarenal)
• Dry mucous membranes
• Cool, clammy skin
• Lethargy
• Deep, rapid respirations
• Vomiting
• Nausea
• Confusion
Laboratory tests are extremely important in diagnosing and evaluating the effectiveness of interventions in the ARF patient. Table 15-1 presents the usual laboratory values seen in prerenal and intrarenal failure.
A collaborative approach of the healthcare team to the treatment of patients in renal failure begins with early recognition of patients at risk for renal failure. The focus is on maintaining adequate renal perfusion and avoiding renal compromise.
Much has changed in the prevention and treatment of ARF over the past several decades. These advances have focused on prompt correction of hypotension and the early use of renal replacement therapies (RRTs) before the development of uremia. Once the patient develops ARF, the goal is to quickly reestablish homeostasis by elimination of the underlying cause. Management of ARF also includes correction of fluid imbalance, prevention and correction of life-threatening electrolyte imbalances, treatment of acidosis, prevention of further renal damage, prevention and treatment of infection, and the improvement of nutritional status.
Prevention of CIN involves the use of extensive hydration before and after any procedure using contrast media. There is debate about whether IV normal saline or sodium bicarbonate is best for hydration. Patients who are considered to be at risk for CIN may also receive oral acetylcysteine with their hydration. Diuretics should be avoided during this time. Also, stopping nephrotoxic drugs such as aminoglycoside antibiotics, non-steroidal anti-inflammatory drugs, and chemotherapeutic agents prior to the procedure may be helpful.
Maintaining fluid balance in the renal failure patient is a challenge. A fine balance must be achieved in providing the fluid necessary for adequate renal perfusion while preventing fluid overload. It is often difficult to assess if the patient is volume depleted or overloaded. In these cases the patient may be transferred to a critical care unit for placement of a pulmonary artery catheter to assess the patient’s fluid status.
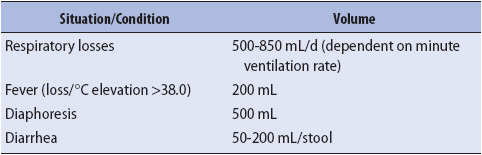
1. Calculate daily fluid needs. In prerenal disease, fluid replacement must be matched with fluid loss, both in amount and composition. Insensible fluid losses must be considered in this calculation (Table 15-3). Normal saline volume loading (before a potential insult) of the patient at risk for renal dysfunction is a widely accepted practice. Additionally, volume expansion is beneficial in preventing a volume-depleted patient from progressing to intrarenal failure. In contrast, oliguric patients can rarely tolerate more than 1000 mL of fluid per day. It is often necessary to place constraints on other therapies (eg, IV drug administration, nutritional support) during this phase. During the diuretic phase, the patient may require 1 to 4 L of fluid per day to prevent hypovolemia. The patient is frequently allowed to lose more fluid than is replaced in an effort to facilitate fluid movement from the interstitial and intracellular spaces into the vascular space.
2. Obtain accurate intake and output measurements. All insensible losses should be included in the measurements. Fluid therapy decisions are often based on the patient’s output.
3. Obtain daily weights. Body weight should be allowed to decrease by 0.2 to 0.3 kg/day as a result of catabolism. If the patient’s weight is stable or increasing, volume expansion is suspected. If weight loss exceeds these recommendations, volume depletion or hyper-catabolism is investigated.
4. Administer diuretics when the patient’s response is hypervolemic only. Increasing dosages may be used in an attempt to determine the optimal dose. Once the diagnosis of renal failure is established, diuretics may be used to avoid fluid overload and to potentiate the effects of antihypertensive medications. Potassium-sparing diuretics are typically avoided because K+ elimination is diminished in renal failure. Two commonly used diuretics are mannitol and furosemide. Mannitol, an osmotic diuretic, is used in an attempt to prevent ARF. It causes vasodilation of the renal vessels and expands vascular volume by enhancing movement of fluid from the interstitial space. The benefit of using mannitol once ARF is established is unclear. Mannitol can contribute to fluid overload without excretory renal function and should be used cautiously. Furosemide, a loop diuretic, is the most common diuretic used in ARF. It works by blocking Na+ reabsorption in the renal tubules, thereby enhancing excretion of Na+ and water. It is often used to reduce fluid overload and dialysis frequency in ARF. Furosemide is used cautiously in patients receiving aminoglycoside antibiotics because it potentiates the nephrotoxic effects of these medications.
5. Institute RRT as needed. There are three types of RRT available. These include intermittent hemodialysis (IHD), peritoneal dialysis (PD), or the newer continuous renal replacement therapy (CRRT), which includes several varieties of therapy. These are discussed later in this chapter. Sustained low efficiency dialysis (SLED) is considered one of the continuous therapies. These newer therapies may be better tolerated in hemodynamically-unstable patients than peritoneal dialysis or hemodialysis but generally are only done in the critical care unit because of the intensity of monitoring required. However, because some progressive care units (PCUs) may use these therapies, they are included in this chapter.
TABLE 15-3. MINIMAL VOLUMES OF FLUID ASSOCIATED WITH INSENSIBLE FLUID LOSSES
There are a number of electrolyte imbalances that can occur in renal failure, the most common being hyperkalemia, hypocalcemia, hypermagnesemia, and hyperphosphatemia. In ARF, the electrolyte status guides decisions about the type of fluid replacement and RRT. The management of these electrolyte disorders is detailed later in this chapter.
Renal failure patients often develop metabolic acidosis, with mild respiratory alkalosis compensation.
1. Administer sodium bicarbonate (NaHCO3) as indicated. Treatment is usually not instituted until the serum bicarbonate level drops to less than 15 mEq/L. Even then, replacement of only half the base deficit is made to avoid overcorrection of the pH. Excessive administration of NaHCO3 can cause metabolic alkalosis, tetany, and pulmonary edema.
2. If a patient is being dialyzed, using a dialysate-containing bicarbonate will facilitate buffering of the patient’s acidotic state. Dialysates containing bicarbonate are preferred to those with lactate.
In ARF, drugs metabolized or excreted by the kidney require adjustment to avoid excessive blood levels and potential nephrotoxicity. Particular attention must be given to medication scheduling related to RRT schedules. Medications may be eliminated or have their actions potentiated by these therapies. As a result, selected medications, such as antibiotics, are often monitored with peak and trough levels. A clinical pharmacist is a helpful resource on appropriate medication selection, dosing, and monitoring during ARF.
1. Modify medication dosing. Because many medications are eliminated by the kidney, drug administration (dose and schedule) must be altered in the patient with renal failure. Medication dose and schedule decisions are based on the drug and the patient’s degree of renal dysfunction. The phase of renal failure and other concomitant treatments help determine the appropriate dose of medication.
2. Administer antihypertensive agents as needed. Hypertension is a major problem for many renal failure patients, often requiring concomitant use of several antihypertensive agents. Most antihypertensive agents are not removed by RRT. During hemodialysis, it is important to adjust the dosage schedule of antihypertensive agents to avoid hypotensive episodes during dialysis. Some antihypertensive agents, however, are eliminated by the kidney. Therefore, dialysis patients receiving these medications require alterations in their dose or dosing schedule.
Renal failure patients are at high risk for infection and are commonly treated with antimicrobial agents. These antimicrobial agents need to be carefully selected and monitored, and often require dosage adjustment. Careful monitoring of both renal function and drug levels during antimicrobial therapy is necessary to avoid further renal damage. Assessment of surgical and line placement sites for signs of inflammation is imperative.
The challenge in the management of the renal failure patient’s nutritional status is to provide a balance between sufficient calories and protein to prevent catabolism, yet not create problems, such as fluid and electrolyte imbalances or increase the requirement for RRT. The clinical dietician is an important resource for the healthcare team. The typical renal failure patient is hypermetabolic, with caloric needs potentially twice normal. Additional stresses, related to being ill, can further elevate caloric requirements. Nausea and vomiting, common in uremia, further decrease oral caloric intake. Adequate nutrition is also important in preventing infection by helping maintain the integrity of the immune system. Hyperglycemia should be avoided in the patient aiming for a target plasma glucose of 110-149 mg/dL.
1. Restrict the patient’s fluid, K+, Na+, and protein intake. Because these patients cannot eliminate wastes, fluid, or electrolytes, their dietary intake of these substances is typically restricted. The degree of restriction depends on the cause and severity of their disease; for example, the level of Na+ restriction is determined by the cause of the renal failure and the serum Na+ level. Some causes lead to Na+ wasting and others to Na+ retention. Phosphorus may need to be restricted and Ca++ supplemented if the Ca++ level is low in conjunction with normal PO4–– levels.
2. Administer necessary vitamin supplementation. Supplementation of folic acid, pyridoxine, and the water-soluble vitamins is most frequently necessary.
3. Consult a dietitian for a diet plan. Dietary requirements change for patients depending on their renal status and the severity of their underlying condition. Although the precise role of nutrition in ARF is controversial, malnutrition is thought to increase morbidity and mortality. Nutrition, enteral or parenteral, used in conjunction with daily RRT, is thought to improve survival and promote healing of renal tubular cells though enteral nutrition is the preferred route.
The usual approach to hypercatabolic states is to provide adequate proteins and carbohydrates to provide for resynthesis of damaged or lost tissue elements. Protein requirements may range initially from 0.8 to 1 g/kg/day and increase with RRT to 1 to 1.5 g/kg/day to a maximum of 1.7 g/kg/day for patients on CRRT. Nonprotein calories, usually in the form of fat, are given for nonanabolic metabolic needs.
The kidneys play a major role in the regulation of fluid and electrolyte balance in the body. Regulation of body fluids and electrolytes helps ensure a stable internal environment, resulting in maximal intracellular function. Any renal dysfunction results in abnormalities in both fluid and electrolyte balance.
For all of the electrolyte disorders, the indications for treatment vary from patient to patient. The signs and symptoms of any electrolyte imbalance are not necessarily determined by the degree of abnormality. Rather, the signs and symptoms are determined by the cause of the condition, as well as the magnitude and rapidity of onset. For many of the electrolyte imbalances, it is difficult to determine at precisely what level signs or symptoms may occur.
Serum osmolality, a measure of the number of particles in a unit of blood volume, is an important indicator of fluid status. Because serum osmolality is determined primarily by the serum Na+ level, evaluation of Na+ levels provides valuable information on serum osmolality and potential excesses or deficits of total body water. A quick estimate of serum osmolality can be calculated by simply doubling the serum Na+ value. Normal serum osmolality values are 285 to 295 mOsm/kg. Abnormal serum Na+ levels are classified as disorders of osmolality, with hyperosmolality referring to high Na+ levels, which may be indicative of water deficit, or hypo-osmolality referring to low sodium levels, which may be indicative of water excess.
Acutely ill patients often are at risk for disorders of osmolality, with children and the elderly at highest risk. As a person ages, the hypothalamus becomes less sensitive to changes in osmolality and is, therefore, less able to alert the body to abnormalities through normal mechanisms. Additionally, the neurologic signs indicative of osmolality disorders are often ignored or assessed as being related to age rather than to a physiologic abnormality.
Hyperosmolar disorders are the result of a deficit of water. The causes of hyperosmolality include inadequate intake of water, excessive loss of water, or conditions that cause an inhibition of antidiuretic hormone (ADH). In acutely ill patients, hyperosmolar disorders develop because of inadequate intake, usually related to loss of consciousness or endotracheal intubation, and ADH inhibition, as manifested by diabetes insipidus in a patient with a head injury. The signs and symptoms seen are the result of the ensuing cerebral dehydration. Water is pulled from the intracellular space to enhance intravascular volume, leaving the cells dehydrated.
Signs and Symptoms
• Lethargy
• Restlessness
• Disorientation
• Delusions
• Seizures
• Oliguria
• Hypotension
• Thirst
• Dry mucous membranes
• Coma
Diagnostic Tests
• Serum Na+ > 145 mEq/L
• Serum osmolality > 295 mOsm/kg
• Urine SG > 1.030
Hypo-osmolality disorders are the result of an excess of water. The causes of hypo-osmolality include excess intake or impaired secretion of water, excess ADH as in the syndrome of inappropriate ADH, replacement of volume loss with pure water, and salt-wasting disorders. Hypo-osmolar disorders are extremely common in acutely ill patients, most often related to the use of D5W IV solutions. Because these patients have often lost some volume, balanced fluid replacement is extremely important. The signs and symptoms seen with hypo-osmolar disorders are related to cerebral intracellular swelling, as water moves from the intravascular to the intracellular spaces.
Signs and Symptoms
• Confusion
• Delirium
• Seizures
• Muscle twitching
• Nausea
• Weight gain
• Headache
• Personality changes
• Coma
• Anorexia
• Vomiting
Diagnostic Tests
• Serum Na+ < 135 mEq/L
• Serum osmolality < 280 mOsm/kg
• Urine SG < 1.010
There are three primary causes of hyperkalemia: increased intake, decreased excretion, and redistribution of K+ from intracellular to extracellular fluid. Rarely is increased intake a sole cause of hyperkalemia, but it is commonly found in combination with decreased K+ excretion. The most common causes of hyperkalemia in the acutely ill are ARF, cellular destruction (eg, from crush injuries), and excess supplementation. Because cardiac tissue is sensitive to K+ levels, hyperkalemia often manifests first as changes in the electrical conduction, demonstrated by changes on ECG tracings. Elevated serum K+ levels alter the conduction of electrical impulses, particularly in cardiac and muscle tissue. These conduction abnormalities can lead to serious cardiac arrhythmias and death.
Because K+ impacts normal neuromuscular and cardiac function, these systems are carefully evaluated when hyperkalemia is suspected. It is important to note that a patient may be experiencing hyperkalemia and may have no ECG or rhythm changes.
Signs and Symptoms
• Vague muscle weakness
• Decreased deep tendon reflexes
• Flaccid paralysis
• Mental confusion
• Nausea
• Diarrhea
• Cramping
ECG Changes
• Tall, tented T waves
• QT interval may shorten
• Intraventricular conduction is slowed
• Widened QRS
• Wide P waves
• Bradycardia
• First-degree atrioventricular (AV) block
• Advanced AV block with ventricular escape rhythms, ventricular fibrillation, or asystole
Diagnostic Tests
• Serum K+ > 5.5 mEq/L
The causes of hypokalemia include decreased intake, increased excretion or impaired conservation of potassium, excess or abnormal loss, and increased movement of K+ into the cells. In the acutely ill patient, hypokalemia is often related to the use of diuretics and excess losses through the gastrointestinal tract. Muscle weakness, including cardiac muscle, is the hallmark sign of hypokalemia. Asystole can result from severe hypokalemia. Depressed levels of serum K+ lead to increased irritability of cardiac muscle and neuromuscular cells. Serious cardiac arrhythmias, and death, may result from hypokalemia.
Signs and Symptoms
• Weakness
• Respiratory muscle weakness, hypoventilation
• Paralytic ileus
• Abdominal distention
• Cramping
• Confusion, irritability
• Lethargy
• Ventricular ectopy and flat, inverted T waves
• QT interval prolongation
• U-wave development
• ST-segment shortening and depression
Diagnostic Tests
• Serum K+ < 3.5 mEq/L
The causes of hypercalcemia are threefold: increased Ca++ release from the bone, increased Ca++ absorption from the gastrointestinal tract, and decreased Ca++ excretion.
Signs and Symptoms
• Somnolence
• Stupor
• Nausea
• Anorexia
• Polyuria
• Lethargy
• Coma
• Vomiting
• Constipation
• Renal calculi
ECG Changes
• Arrhythmias
• Shortened QT interval
• Shortened ST segment
• Flat, inverted T waves
Diagnostic Tests
• Serum Ca++ > 10.5 mg/dL
True hypocalcemia is rare. The causes of hypocalcemia are classified into three categories: decreased absorption of Ca++, increased loss of Ca++, and decreased amounts of physiologically active Ca++. Acutely ill patients may develop hypocalcemia most often related to either gastrointestinal losses or malabsorption. The low Ca++ levels result in muscle contraction, seen as tetany, and bronchospasm.
Signs and Symptoms
• Positive Chvostek sign (twitching of the upper lip in response to tapping of the facial nerve)
• Positive Trousseau sign (carpopedal spasm in response to occlusion of circulation to the extremity for 3 minutes)
• Tetany
• Seizures
• Respiratory arrest
• Bronchospasms
• Stridor
• Wheezing
• Paralytic ileus
• Diarrhea
ECG Changes
• Arrhythmias
• Lengthened QT interval
• ST-segment sagging and prolongation
• T-wave inversion
Diagnostic Tests
• Serum Ca++ < 8.5 mg/Dl
Hypermagnesemia is most commonly seen in renal failure patients with an inability to excrete Mg++ or with increased intake of Mg++ from antacid. ARF is the most common etiology of hypermagnesemia in acutely ill patients. Both neuromuscular and cardiac depressions are observed. Hypermagnesemia may also develop in nonrenal failure situations when Mg++ intake is increased, excretion is decreased, or adrenal insufficiency or hyperparathyroidism causes increased Mg++.
Signs and Symptoms
• Respiratory depression
• Hypotension
• Diminished deep tendon reflexes
• Flaccid paralysis
• Drowsiness
• Lethargy
ECG Changes
• Cardiac arrest
• Prolonged PR and QT intervals
• Widened QRS
• Increased T-wave amplitude
• Bradycardia
Diagnostic Tests
• Serum Mg++> 2.1 mEq/L
Hypomagnesemia frequently occurs in alcoholic and acutely ill patients and is often associated with hypocalcemia and hypokalemia. Hypomagnesemia can be caused by decreased intake, increased excretion, such as with diuretic therapy, and excessive loss of body fluids. The hypomagnesemia seen in the acutely ill is most often the manifestation of a compromised nutritional status, secondary to starvation and malabsorption.
Signs and Symptoms
• Hyperreflexia
• Positive Chvostek and Trousseau signs
• Nystagmus
• Seizures
• Tetany
ECG Changes
• Prolonged PR and QT intervals
• Broad, flat T waves
• Ventricular arrhythmias
Diagnostic Tests
• Serum Mg++ < 1.3 mEq/L
The most common cause of hyperphosphatemia in all patients, including the acutely ill, is renal failure; the regulation of phosphate in the body is done by the kidneys. Hyperphosphatemia is also seen in hypoparathyroidism, excessive intake of alkali or vitamin D, Addison disease, and with bone tumors or fractures. Hyperphosphatemia is often associated with hypocalcemia.
Signs and Symptoms
• Muscle cramps
• Joint pain
• Seizures
Diagnostic Tests
• Serum phosphate > 4.5 mg/dL
Hypophosphatemia is caused by hyperparathyroidism, hyperinsulinism, administration of IV glucose, and conditions that cause bone deterioration, such as osteomalacia. This condition is not often seen in acutely ill patients. When seen, it is frequently in conjunction with hypercalcemia.
Signs and Symptoms
• Muscle weakness and wasting
• Fatigue
• Confusion
• Oliguria
• Tachycardia
• Anorexia
• Dyspnea
• Cool skin
Diagnostic Tests
• Serum phosphate < 3.0 mg/dL
1. Administer free water. Fluid replacement can be given orally, if feasible, or with intravenous administration of D5W. The goal is to normalize the serum Na+ level over a 48- to 72-hour period. A gradual return to normal avoids cellular overhydration.
2. Monitor Na+ and serum osmolality level frequently. Care must be taken to correct the Na+ and osmolality level gradually. Correcting these levels too quickly may precipitate hypo-osmolar conditions and seizures.
3. Administer desmopressin (nasally) or vasopressin (IV, IM, subcutaneously) in diabetes insipidus. These medications inhibit the action of ADH.
1. Restrict water intake. Mild, asymptomatic hyponatremia often is not treated or is treated only with a water restriction.
2. Institute RRT. RRT is indicated for severe fluid overload in the presence of renal failure.
3. Administer hypertonic saline. Hypertonic saline may be needed to correct Na+ levels less than 115 mEq/L when the patient is symptomatic. Careful, slow administration of hypertonic saline is important to avoid sudden shifts in serum osmolality and subsequent hyperosmolality.
4. Monitor Na+ and serum osmolality levels frequently. Care must be taken to correct these levels gradually. Rapid correction can precipitate hyperosmolar conditions and seizures.
Of all the potential electrolyte disorders, hyperkalemia is considered the most life-threatening because of potassium’s profound impact on the electrophysiology of the heart. Hyperkalemia is also the most common reason for initiation of dialysis in the ARF patient.
1. Initiate cardiac monitoring. Because hyperkalemia does affect cardiac tissue, continuous ECG monitoring assists in recognizing cardiac manifestations of altered K+ levels.
2. Restrict dietary intake of K+ to 40 mEq/d. A dietary restriction is considered conservative management and is usually instituted in conjunction with other therapies aimed at removing K+ from the body.
3. Administer cation-exchange resins. Sodium polystyrene sulfonate (Kayexalate) is used to increase K+ excretion and is administered by mouth or enema with sorbitol. Sorbitol acts to draw fluid into the bowel where the polystyrene causes an exchange between Na+ and K+ ions. The K+ is then eliminated from the body through feces.
4. Administer hypertonic (50%) glucose and regular insulin. Insulin acts to drive K+ into the cells on a temporary basis, thereby protecting the heart from the effect of the elevated serum (extracellular) K+ level.
5. Administer NaHCO3. Its administration causes movement of K+ temporarily into the cell, encouraging the exchange of hydrogen (H+) ion inside the cells with the excess K+ ion outside the cell.
6. Administer calcium salts, such as calcium gluconate. Calcium elevates the stimulation threshold, protecting the patient from the negative myocardial effects of hyperkalemia. The administration of calcium does not change the level of K+ in the extracellular fluid.
7. Institute RRT. Hemodialysis may be necessary for rapidly removing K+ when the patient’s K+ level cannot be controlled by other methods.
1. Administer K+ supplementation. Depending on the severity of the deficit, oral or IV replacements can be utilized. Ideally, IV supplementation of K+ is given through a central line due to the irritating nature of K+ to the tissues. Potassium replacement is given in at least 50 mL of fluid with no more than 20 mEq replaced per hour. It is common for patients to be unable to tolerate more than 10 mEq/h if the supplementation is given peripherally. Because K+ is primarily an intracellular cation, allow at least 1 hour after administration for the movement of the K+ into the cells before evaluating the serum K+ level. A level obtained too quickly after supplementation is completed may reflect an artificially high serum value. Also, hemolysis may occur during blood draws resulting in an artificially high level.
2. Evaluate the patient’s diuretic therapy.
1. Administer normal saline IV and diuretics. In the presence of normal renal function, normal saline infusions given with diuretics increase the GFR and enhance Ca++ excretion from the kidneys.
2. Administer corticosteroids. Corticosteroids decrease absorption of Ca++ from the gastrointestinal tract.
3. Administer plicamycin. Plicamycin increases the bone uptake and storage of Ca++.
4. Administer oral phosphate (PO4––) supplementation. PO4–– binds Ca++ so that it is excreted in stool.
1. Administer Ca++ supplementation. Calcium-containing antacids may be used. Often Ca++ supplementation is done concurrently with the administration of PO4–– binders, such as aluminum hydroxide. There is a reciprocal relationship between Ca++ and PO4–– levels in the body. Calcium may be given orally in the form of antacids or intravenously as calcium gluconate or calcium chloride when symptoms are serious.
2. Administer vitamin D supplementation. Vitamin D is necessary for Ca++ to be absorbed from the gastrointestinal tract.
3. Institute seizure precautions. Patients with hypocalcemia are at risk for developing tetany and seizures.
1. Institute RRT. See later under Hyperphosphatemia.
2. Discontinue use of Mg++-containing antacids.
3. Administer normal saline and diuretics. If the patient has normal renal function, the administration of saline and diuretics increases GFR and enhances excretion of Mg++.
4. Administer calcium gluconate intravenously.
1. Administer Mg++ supplementation. Oral administration or Mg++ sulfate IM or IV may be used. IV Mg++ should not be given faster than 150 mg/min. Total daily replacement should not exceed 30 to 40 g.
2. Reduce auditory, pressure, and visual stimuli.
1. Administer aluminum hydroxide-binding gels. These gels bind with phosphate in the intestine, limiting the absorption, promoting excretion, and decreasing the serum level.
2. Institute RRT. If the patient is symptomatic, hemodialysis is the most effective choice to rapidly decrease the serum levels.
3. Administer acetazolamide. Acetazolamide increases the urinary excretion of phosphate.
1. Administer phosphate supplementation. Supplementation can be administered by mouth or IV.
2. Discontinue use of phosphate-binding gels.
For many years, hemodialysis and peritoneal dialysis were the only therapies available to manage renal failure or situations in which the patient becomes volume overloaded. Many acutely ill patients cannot tolerate the rapid fluid and electrolyte shifts associated with traditional hemodialysis because of hemodynamic instability and cardiac arrhythmias. Peritoneal dialysis, an option for patients who cannot tolerate the hemodynamic changes associated with hemodialysis, is limited to patients without recent abdominal incisions, respiratory distress, or bowel perforations.
Several alternative therapies to manage acute fluid and electrolyte problems have been introduced during the past 25 years, beginning with continuous arteriovenous hemofiltration (CAVH). A number of additional CRRTs have been introduced, offering more treatment options for the acutely ill patient with renal failure or fluid overload. These therapies include using a double-lumen venous access and a pump for continuous venovenous hemofiltration (CVVH) and the addition of dialysate for continuous venovenous hemodialysis (CVVHD). Continuous venovenous hemodiafiltration (CVVHDF) combines the principles of CVVH and CVVHD. Some patients may benefit from high-volume hemofiltration to promote even higher clearances of substances from the bloodstream. Using CRRT, many of the desirable outcomes of hemodialysis can be accomplished without the associated hemodynamic instability. Slow low efficiency dialysis (SLED) involves using hemodialysis at lower flow rates usually over a 12-hour period at night. This therapy decreases the large fluid shifts problematic for the hemodynamically-unstable patient.
The goal of any type of RRT is the removal of excess fluid and uremic toxins and correction of electrolyte imbalances. Each of the RRT methods is able to accomplish that goal, with varying levels of success. These homeostatic corrections are accomplished through the processes of diffusion, osmosis, filtration, or convection. Diffusion, the process by which substrates move from an area of high concentration to one of a lesser concentration, provides for movement of fluids and electrolytes from the body into the filtrate. Through osmosis, water from an area of lesser solute concentration moves to an area of greater solute concentration, becoming part of the filtrate. Filtration also occurs, allowing for movement of water and solute as a result of a difference in hydrostatic pressure. Convection involves the movement of fluids and solutes being pushed through a membrane by pressure and creating a drag, which pulls larger particles along with the fluid.
Renal replacement therapies are grouped into three general categories: those requiring arteriovenous access, those requiring venous access only, or those requiring a peritoneal access (Table 15-4). RRT is applied for periods of 4 hours or more, with some requiring continuous use. Except for peritoneal dialysis, all of the RRT devices require extracorporeal blood flow. This flow is accomplished through the use of two catheters, one arterial and one venous, or through a single venous catheter with two lumens. Filtration and dialysis occur as the blood moves through a dialyzer or hemofilter.
TABLE 15-4. SUMMARY OF RENAL REPLACEMENT THERAPIES
Before any type of RRT can be performed, access to the bloodstream or peritoneum is necessary. The type of access is determined by the reason for initiation and method of renal replacement. It can be either temporary or permanent.
Permanent access is achieved by placement of either an arteriovenous fistula or graft. A fistula is a surgically created anastomosis between an artery, usually the radial, brachial, or femoral, and an adjacent vein. This anastomosis allows arterial blood to flow through the vein, causing venous enlargement and engorgement. Permanent access is necessary for patients requiring chronic dialysis.
Arteriovenous grafts are placed in patients who do not have adequate vessels to create a fistula. A prosthetic graft is implanted subcutaneously and used to anastomose an artery to a vein. A period of maturation, usually 2 to 3 weeks, is necessary before the access can be used. This maturation time allows for the venous side to dilate and the vessel wall to thicken, permitting repeated insertion of dialysis needles.
Temporary access to the bloodstream is obtained through cannulation of an artery and/or a large-diameter vein, with a large-bore, double- or single-lumen catheter specifically designed for dialysis. These catheters are inserted and maintained similar to other arterial and central venous devices, but are generally larger and used primarily for dialysis treatments. A single double-lumen catheter is more commonly used than a single-lumen, single-vessel catheter to maximize the filtration and dialysis capabilities of the renal replacement devices. These catheters can be used for extended periods of time with meticulous attention to sterile technique. The location for catheter placement is chosen to maximize blood flow and prevent kinking of the catheter with patient movement. To initiate hemofiltration (CVVH), hemodialysis (CVVHD), or hemodiafiltration (CVVHDF), a single 14- to 16-gauge double-lumen catheter is placed in the subclavian, jugular, or femoral vein. The jugular is the preferred site and the subclavian is the least preferred site.
Peritoneal catheters are made of silastic tubing, with multiple perforations to allow for fluid exchange, and an attached cuff, soft disk, or balloon to anchor the catheter. When peritoneal dialysis needs to be initiated immediately, a rigid stylet, designed for single acute use only, is inserted. Both types of catheters are inserted through small incisions in the abdomen and threaded into the peritoneal space.
There are a variety of dialyzers and hemofilters available for use. The type of dialyzer or hemofilter chosen is determined by the patient’s condition and desired outcomes of the RRT. All dialyzers have a blood and dialysate compartment, separated by a semipermeable membrane. The dialyzer has two inlet ports and two outlet ports, one each for blood and dialysate. During dialysis, blood and dialysate are pumped through the dialyzer in opposite directions.
Hemofilters are made of highly permeable hollow fibers or plates. These fibers or plates are surrounded by an ultra-filtrate space and have arterial and venous blood ports. Plasma water and certain solutes are separated from the blood by the hemofilter and drain into a collection device.
Dialysate solution, used in any therapy that has dialysis as a component, is specifically designed to create concentration gradients so that optimal removal of wastes, acid-base and electrolyte balance, and maintenance of extracellular fluid balance can be achieved. The specific solution is determined by the patient’s condition and desired outcomes. Although standard solutions may initially be used, they can be tailored to meet the individual patient’s needs and contain varying concentrations of Na+, K+, Mg+, Ca++, Cl–, glucose, and buffers.
Initiation of hemodialysis or SLED through a temporary access is accomplished using a procedure called coupling. During coupling, the dialysis catheter and the dialysis circuitry are connected, using sterile technique. To initiate dialysis through a permanent access, two 14- or 16-gauge needles are inserted into the dilated vein of the fistula or the graft portion of the synthetic graft. One needle is considered arterial, used for blood outflow, and the other is considered venous, used for blood return.
The basic components of a hemodialysis system are shown in Figure 15-2. Blood, leaving the patient through the arterial needle, is pumped through the circuitry and returned to the patient through the venous needle. A blood pump moves the blood through the dialysis circuitry and dialyzer, allowing for different flow rates. Both arterial and venous pressures are monitored in the circuitry.
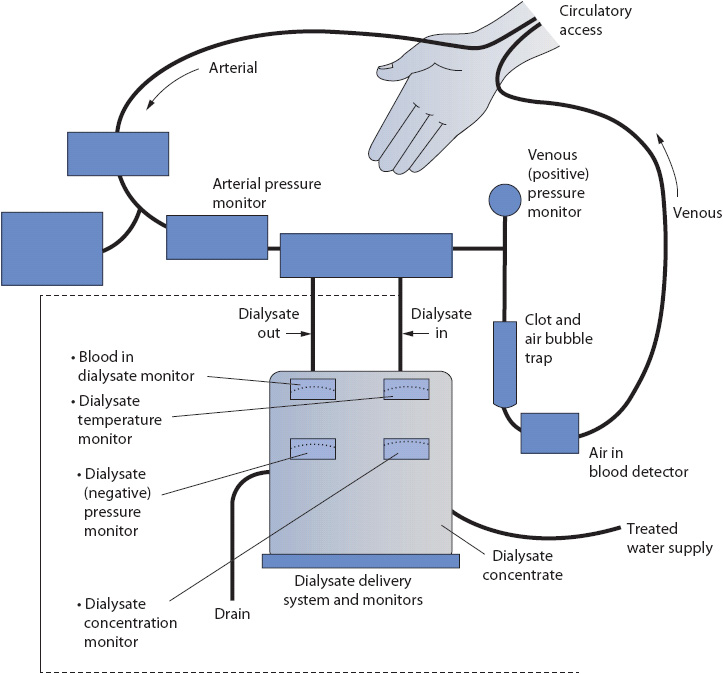
Figure 15-2. Components of a hemodialysis system. (Reprinted from Thompson JM, McFarland GK, Hirsch JE, et al, eds. Mosby’s Manual of Clinical Nursing. St Louis, MO: Mosby; 1989:592, with permission from Elsevier.)
Peritoneal dialysis is accomplished through a series of cycles or exchanges. The dialysate, administered into the peritoneal cavity, remains in the cavity for a preset amount of time (dwell time) and then is drained. Each set of these activities is called a cycle or exchange. Dialysate flows into the peritoneal cavity by gravity, taking approximately 10 minutes for 2 L of fluid to infuse. During the dwell time, diffusion, osmosis, and ultrafiltration occur. Dwell times are based on patient need. With an optimally functioning catheter, it takes 10 minutes for 2 L of fluid to drain from the abdomen. Other forms of peritoneal dialysis include continuous ambulatory peritoneal dialysis (CAPD) and continuous cyclic peritoneal dialysis (CCPD), although these forms are generally not used in the PCU. However, if the patient uses this type of therapy at home for CRF, it is possible that it will be continued in the PCU.
In CRRT, the blood lines are primed with a saline solution with or without unfractionated heparin as an anticoagulant and then attached to the appropriate vascular access catheter arm (one for outflow and one for inflow). Blood is pumped from the outflow side and passes through the hemofilter. The use of anticoagulation (ie, unfractionated heparin or citrate) assists with blood flow and prolongs the filter life. The blood returns to the body via the inflow tubing after fluid and electrolytes are moved into the ultrafiltrate. The ultrafiltrate is collected in a bag after removal. In CVVHD, blood leaves the patient through the outflow catheter and is pumped through a dialyzer rather than a hemofilter. Wastes and fluid are removed and drained into an ultrafiltrate bag. The blood is then returned to the body through the inflow catheter. The dialysate is pumped through the dialyzer countercurrent to blood flow. Figure 15-3 shows the basic setup of CVVHD. In CVVHDF, both dialysis fluid and replacement fluids are used to make the system more efficient.
Figure 15-3. Components of a CVVHD system. (Used with permission from Strohschein BL, Caruso DM, Greene KA. Continuous venovenous hemodialysis. Am J Crit Care. 1994;3:95.)
Each type of RRT is indicated for different clinical situations and achieves different goals. The goals of therapy are clearly delineated before selection of the type of therapy.
Hemodialysis is implemented when aggressive therapy is indicated in acute situations. Hemodialysis is contraindicated in patients with hemodynamic instability (although hypotension may be a relative contraindication), hypovolemia, coagulation disorders, or vascular access problems.
Considered the gold standard for the treatment of ARF and CRF, hemodialysis is the most effective of all of the RRTs. Fluid and uremic wastes can be eliminated from the body during a 4- to 6-hour treatment. Approximately 200 mL of blood is utilized in the circuit, which can add to a patient’s unstable condition.
Today, peritoneal dialysis is rarely used for acutely ill patients who need dialysis but are unable to tolerate the hemodynamic changes associated with hemodialysis. CRRT is used instead. Peritoneal dialysis may be performed in a PCU for a patient who is on chronic peritoneal dialysis and presently hospitalized with an acute illness. Utilizing the peritoneal membrane as the dialyzer, effective elimination of fluid and waste products can be achieved. Peritoneal dialysis is slower and less effective than hemodialysis.
Peritoneal dialysis is contraindicated in patients who have had recent or extensive abdominal surgery; who have abdominal adhesions, peritonitis, or respiratory distress; or who are pregnant.
Patients appropriate for CRRT are chosen after evaluating their clinical diagnosis, hemodynamic parameters, and metabolic status. The specific type of CRRT is selected after considering the patient’s fluid and electrolyte status, metabolic needs, and severity of uremia. The most commonly used forms of CRRT are CVVH, CVVHD, or CVVHDF.
When a slow, continuous ultrafiltration is desired, slow continuous ultrafiltration (SCUF) is the therapy of choice. This therapy is primarily for use in patients with a fluid volume excess and some degree of renal function. Because fluid removal is the primary goal, this procedure is performed without simultaneous fluid replacement. There is a minimal impact on the urea and creatinine levels.
The main objective of CVVH is fluid removal. Although large changes in blood chemistries are not expected, it is possible for a patient to achieve and maintain a stable volume and composition of electrolytes in his or her extracellular fluid. The higher the blood flow rate achieved in CVVH, the more solutes that can be removed. Because large volumes of fluid can be removed, the healthcare team has more flexibility in treating patients. Nutrition, a problem in many acutely ill patients, can often be enhanced in these patients because nutrition (even total parenteral nutrition when enteral cannot be tolerated) can be provided without fear of fluid overload.
Continuous venovenous hemofiltration, in some institutions, has become the treatment of choice when patients have contraindications to hemodialysis or peritoneal dialysis. Fluid shifts in CVVH are less rapid than with hemodialysis, making the therapy attractive when persistent hemodynamic instability, especially hypotension, is present. Other patients who may benefit from CVVH are patients with uncontrolled heart failure, pulmonary edema, or hepatorenal syndrome. Patients can be maintained on CVVH for several weeks until either long-term hemodialysis can be initiated or there is return of renal function. There are no absolute contraindications for CVVH. Unfortunately, the therapy has to be discontinued for transportation off the unit such as for selected diagnostic tests (eg, computed tomographic scans) and the continuous nature of the therapy limits mobility, particularly if a femoral access is used (eg, out of bed to chair).
Continuous venovenous hemodialysis combines the principles of hemofiltration with a slow form of dialysis (see Figure 15-3). More aggressive removal of fluid and solute is possible than with CVVH. Dialysate is infused through a dialyzer, countercurrent to the patient’s blood flow.
The indications for CVVHD are similar to those for hemodialysis. Selection of CVVHD is generally made because a patient is unstable and not able to tolerate the rapid fluid and electrolyte shifts that occur with hemodialysis. CVVHD provides an avenue for these hemodynamically unstable patients to achieve a stable fluid and electrolyte balance without further compromise of their status. There are no absolute contraindications for CVVHD. Maintaining patency of the dialyzer is key to successful CVVHD. Patients with coagulopathies require special monitoring.
The frequency of RRT as a therapy in PCUs is on the rise. Some practitioners feel CRRT will replace hemodialysis as the therapy of choice for ARF in the more unstable patient.
Although each therapy has unique characteristics, all require similar interventions. Careful observations and interventions are essential, as is accurate fluid management. Close monitoring of mean arterial pressure, urine output, central venous pressure, daily weights, and state of anticoagulation are critical. Careful monitoring of acid-base and serum chemistries is mandatory. The progressive care nurse assumes a primary responsibility for early recognition and initial interventions for patient and system problems.
Alspach JG, ed. Core Curriculum for Critical Care Nursing. 6th ed. Philadelphia, PA: Saunders; 2006.
Candela L, Yucha C. Renal regulation of extracellular fluid volume and osmolality. Nephrol Nurs J. 2004;31(4):397-406.
Hinkle C. Electrolye disorders in the cardiac patient. Crit Care Nurs Clin North Am. 2011;23(4): 635-643.
McCance K, Huether S, ed. Pathophysiology: The Biologic Basis for Disease in Adults and Children. 6th ed. Maryland Heights, MO: Mosby Elsevier; 2010.
Molzhan A, Butera E, eds. Contemporary Nephrology Nursing: Principles and Practice. 2nd ed. Pittman, NJ: American Nephrology Nursing Association; 2007.
Morton P, Fontaine, D, eds. Critical Care Nursing: A Holistic Approach. 9th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2009.
Schrier RW, ed. Renal and Electrolyte Disorders. 7th ed. Philadelphia, PA: Wolters Kluwer/Lippincott Williams & Wilkins; 2010.
Urden LD, Stacy KM, Lough ME, ed. Thelan’s Critical Care Nursing: Diagnosis and Management. 5th ed. St Louis, MO: Mosby; 2006.
Yucha C. Renal regulation of acid-base balance. Nephrol Nurs J. 2004;31(2):201-208.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative Work Group. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204-R212.
Broden CC. Acute renal failure and mechanical ventilation: reality or myth? Crit Care Nurse. 2009;29(2):62-76.
Cotton AB. Medical nutrition therapy in acute kidney injury. Nephrol Nurs J. 2007;34(4):444-445.
Dirkes S. Acute kidney injury: not just acute renal failure anymore? Crit Care Nurse. 2011;31(1):37-49.
Druml W. Nutritional management of acute renal failure. J Ren Nutr. 2005;15(1):63-70.
Isaac S. Contrast-induced nephropathy: nursing implications. Crit Care Nurse. 2012;32(3):41-48.
KDIGO clinical practice guideline for acute kidney injury: acute kidney injury work group. Kidney Int Suppl. 2012;2(1):1-138.
Russell T. Acute renal failure related to rhabdomyolysis: pathophysiology, diagnosis and collaborative management. Nephrol Nurs J. 2005;32(4):409-417.
Uchino S, Kellum JA, Bellomo R, et al. Beginning and ending supportive therapy for the kidney investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-838.
Wood S. Contrast-induced nephropathy in critical care. Crit Care Nurse. 2012;32(6):15-23.
Acute Dialysis Quality Initiative. Guidelines for Practice. Crit Care. 2004;8:204-212. www.adqi.net. 2004.
ANNA. Continuous Renal Replacement Therapy: Nephrology Nursing Guidelines for Care. Pittman, NJ: Anthony Janeppi, Inc; 2005.
Bernardini J. Peritoneal dialysis: myths, barriers and achieving optimal outcomes. Nephrol Nurs J. 2004;31(5):494-498.
Dirkes S, Hodge K. Continuous renal replacement therapy in the adult intensive care unit: history and current trends. Crit Care Nurse. 2007;27(2):61-81.
Golestaneh L, Richter B, Amato-Hayes, M. Logistics of renal replacement therapy: relevant issues for critical care nurses. AJCC. 2012;21(2):126-130.
Kelman E, Watson D. Preventing and managing complications of peritoneal dialysis. Nephrol Nurs J. 2006;33(6):647-657.
Oudemans-van Straaten HM, Wester JP, de Pont AC, et al. Anticoagulation strategies in continuous renal replacement therapy: Can the choice be evidence based? Intensive Care Med. 2006;32:188-202.
Palevsky PM, Baldwin I, Davenport A, Goldstein S, Paganini E. Renal replacement therapy and the kidney: minimizing the impact of renal replacement therapy on the recovery of acute renal failure. Curr Opin Crit Care. 2005;11:548-554.
Wooley JA, Btaiche I, Good KL. Metabolic and nutritional aspects of acute renal failure in critically ill patients requiring continuous renal replacement therapy. Nut Clin Practice. 2005;20(2):176-191.
American Society of Nephrology (www.asn-online.org). Accessed April 1, 2013.
National Institute of Diabetes, Digestive, and Kidney Diseases (www.niddk.nih.gov). Accessed April 1, 2013.
U.S. Renal Data Systems (www.usrds.org). Accessed April 1, 2013.