The functional mystery of embodying emotions
Martial Mermillod and Johan Lepage
Introduction: The functional mystery of embodying emotions
More than one century ago, William James argued that physiological changes are able to cause specific emotions rather than being their consequence (James, 1890). The so-called James-Lange hypothesis has generated controversy and has been proposed again in different forms and extended in many ways. While the majority of philosophers and scientists of the time held that emotions are a disorder of consciousness that induces instability in the organism, James, in contrast, suggested that neurovegetative manifestations are responsible for the disorder of consciousness. In other words, human beings are thought of as a “reverberation chamber” in which physical changes, however subtle, reverberate until they reach the level of the conscious experience of emotion. This original perspective has given rise to a number of current theoretical approaches. Opposing James’s peripheral approach, Cannon (1927) proposed a more central theory of emotions based on various scientific and experimental facts. For instance, he emphasized that the total separation of the viscera and the central nervous system does not lead to any absolute impairment of emotional behavior. This opposition between a visceral and an abstractive approach to emotions can still be observed in modern theoretical trends: the theory of appraisal and embodiment theory. For example, Damasio and Carvalho (2013) suggested that changes in body state cause automatic physiological reactions as well as mental experience (i.e., feelings) such as hunger, thirst, pain or fear. Similarly, based on theories of embodied simulation (Barsalou, 1999; Niedenthal, 2007, for reviews), Niedenthal et al. (2010) hold that the processing of emotional information is grounded in the brain’s perceptual, affective and sensory-motor systems, which are activated when the emotion is experienced (Wilson-Mendenhall et al., 2013).
For half a century, cognitive psychology and, to a greater extent, cognitive science have extensively explored cognitive and emotional processes as symbolic, abstract and amodal systems (in opposition to the embodied view of cognition previously proposed by William James). However, over the last decade, the embodied view of emotions has once again captured considerable attention in the field of psychology as well as in robotics and computer science (Goldman & Sripada, 2005). Most researchers studying this issue have used noninvasive procedures (McCanne & Anderson, 1987; Strack et al., 1988) to modify subjects’ facial expressions and have found a significant effect on the processing of congruent emotions. Similarly, Havas et al., (2010) used botulinum toxin-A (Botox) injections to show a selective impairment in the processing of emotional sentences or of facial mimicry together with reduced amygdala activity in Botox-injected participants (Hennenlotter et al., 2009). Furthermore, it has been shown that the recognition of emotional facial expressions is influenced by the activation (or deactivation) of facial muscles used to express the same emotion (Beffara et al., 2012; Niedenthal et al., 2010; Pitcher et al., 2008). This latter study provides a plausible mechanism for the changes in emotional processing revealed by the aforementioned experiments: Because the same facial muscles as those used for the expression of an emotion are also involved in its processing in the broad sense (e.g., in feeling or perceiving the emotion), it is possible to change emotional processing by influencing the facial muscles. Nevertheless, one century after the James-Lange hypothesis, while much of the related scientific literature has revealed empirical evidence in support of the embodied view of emotion, the function of the embodiment processes remains largely unknown. More precisely, we have shown in different connectionist simulations that an artificial neural network based on purely bottom-up processes is able to recognize emotional facial expressions as accurately as or even better than human participants on the basis of pure abstract representations (Mermillod, Bonin et al., 2010; Mermillod, Vuilleumier et al., 2009; Mermillod, Vermeulen et al., 2009). More precisely, the categorization processes resulting from a simple artificial neural network including a perceptual layer (based on Gabor filters simulating V1 receptive fields), a hidden layer making it possible to compute nonlinear functions and an output layer that categorizes facial emotional expressions outperform those of humans, who use these evolved and complex loops between the brain and the body. Therefore, the question is: why do biological neural systems not use simpler, parsimonious bottom-up processes from perceptual to cognitive layers in order to recognize emotional stimuli?
The predictive brain hypothesis
One way of addressing the functional role of embodiment could come from another important and innovative field of research that has recently emerged in the cognitive neurosciences, namely, the influence of top-down conceptual or emotional information on low-level perceptual processes. These studies highlight the importance of the orbitofrontal cortex (OFC), which is an important constituent of the frontal cortex involved in embodiment theory, and in particular in the case of emotional processes (Niedenthal et al., 2010) and the predictive brain hypothesis (Bar, 2004). The capacity to anticipate, not only perceptual events, but also the emotional and social environment is one of the most important functions of biological living organisms, in particular in the case of highly social organisms such as humans. To this end, the ability to use previous knowledge from past experiences in order to anticipate future events is vital. Several cognitive neuroscience studies have reported experimental evidence arguing in support of this view (Bullier, 2001; Bar, 2004). According to this theoretical approach, visual recognition is not the result of purely bottom-up processes from the perceptual system (e.g., the retina, the lateral geniculate nucleus and the occipital cortex) to high-level cortical areas dedicated to visual cognition (e.g., identification or categorization of stimuli or events). Instead, an alternative view suggests that there are also top-down processes that are directed from the orbitofrontal cortex (OFC) to low-level perceptual areas (the occipito-temporal pathway) and increase the efficiency of the associated perceptual processes. This perspective has been corroborated by recent data obtained on the basis of fMRI (Kauffmann, Ramanoël & Peyrin, 2014), EEG (Pourtois et al., 2005), magnetoencephalography (MEG) or Dynamic Causal Modelling (DCM) experiments (Bar et al., 2006;, 2008). This latter experiment found that the OFC was activated 50 ms earlier than the temporal areas involved in visual recognition (Figure 12.1). These results were recently confirmed in our psychology department by means of DCM during the recognition of stimuli consisting of natural scenes. Moreover, this neuroimaging data. not only suggests the existence of top-down activity from the OFC to the occipito-temporal cortex (OTC), but it also specifies how this top-down regulation process could occur. More specifically, this neurofunctional model suggests that low spatial frequency information (which is processed very fast by the early perceptual system) constitutes the basis for this top-down neural stream from the OFC to the OTC (or V1). In the lateral genicu-late nucleus, the magnocellular layers act as a high-pass temporal frequency filter and low-pass spatial frequency (LSF) filter, whereas the parvocellular layers correspond to a low-pass temporal filter that processes high spatial frequency (HSF) information. One function of the OFC is therefore to provide feedback focusing on global shapes to the OTC in order to prepare and facilitate the identification of the object. As a result, the OTC benefits from two types of information at the same time: a fine and detailed version of the object that is to be perceived and a framework that is rapidly established by the OFC and makes it possible to guide the processing of visual information. At a behavioral level, as this early activity correlates with correct behavioral recognition, it also suggests that top-down facilitation occurs in the OFC during visual perception. However, although this result illustrates the existence of top-down neural processing during object recognition, the function of these top-down pathways remains unclear, even if the corresponding model assumes that these top-down connections are related to anticipation processes during the recognition of visual objects.
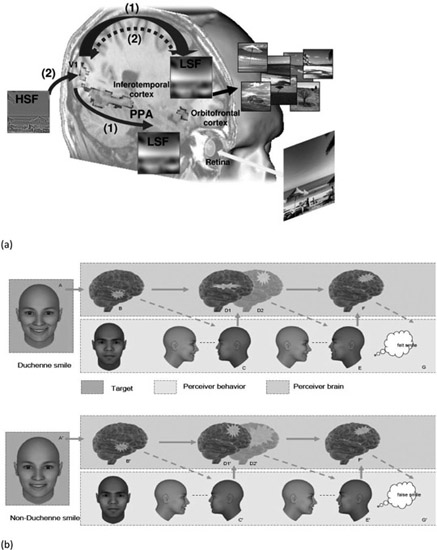
Figure 12.1
(a) Theoretical model of the predictive brain hypothesis based on top-down connectivity from the OFC to the occipito-temporal pathway as revealed by fMRI data. (Source: Kauffmann et al., 2014). (b) The SIMS model specifies the feed-forward and feedback connectivity (between OTC and OFC) involved during embodiment processes in the case of uncertain but relevant information (ambiguous smiles, for instance).
As far as emotional processes are concerned, it has been assumed that this top-down neural stream could be efficient during both the regulation and the anticipation of emotional events (Barrett & Bar, 2009; Kveraga, Ghuman & Bar, 2007). This hypothesis has found support in the work of Kawasaki et al. (2001) who found similarly fast activity in frontal areas (single-cell recording of the ventral prefrontal cortex) during exposure to emotional stimuli. Taken together, those various neuroimaging studies (Pourtois et al., 2005; Vuilleumier et al., 2003), as well as our own studies conducted at the behavioral (Beffara et al., submitted; Mermillod et al., 2010, 2011, 2013) and neurocomputational levels (Mermillod et al., 2009, 2010) point to a preferential link between low spatial frequency information and subsequent emotional processing. Nonetheless, we recently suggested that similar top-down connections from frontal areas to OTC probably underpin the embodiment process (Niedenthal et al., 2010; Figure 12.1). In other words, the recurrent top-down connections set out above from OFC to OTC could have a dual nature, being responsible for: (i) the anticipation of emotional events (Barrett & Bar, 2009) and (ii) the emotional feeling of the emotion through the embodiment process (Niedenthal et al., 2010). This chapter addresses these two complementary hypotheses on the basis of a multidisciplinary approach involving psychology, neural computation and social neuroscience studies.
Filling the gap between the predictive brain hypothesis and embodiment theory
Given the central role of the OFC in anticipating visual and emotional events, on the one hand, and the importance of this structure during mimicry processing on the other (Niedenthal et al., 2010; Kawasaki et al., 2001), it is possible to assume that there is a link, at a functional level, between these two types of processes (i.e., anticipation of events on the basis of mimicry processes). This could, in particular, be the case for the prediction of temporal sequences such as those involved in dynamic events, for example, a predator or a foe running aggressively toward you. It could also be the case in more subtle and more common social situations, for instance, anticipating the trajectory of other individuals in the street or understanding why your partner frowns when you say that you will have to leave the office late because of an important meeting tomorrow afternoon.
Interestingly, the acquisition and prediction of temporal sequences have been extensively explored in the computer science field and more specifically within the theoretical framework of neural computation. This consequently means that we can (i) simulate the importance of top-down connections from associative layers to perceptual layers and (ii) determine whether this process might, at a behavioral level, constitute the root of embodiment theory. Among the different techniques used to process temporal sequence prediction, Simple Recurrent Networks (SRN; Elman, 1990) currently constitutes a type of neural network that uses feedback neural connectivity from a hidden (or “associative”) to an input (or “perceptual”) layer in order to identify and predict temporal sequences in time (Figure 12.2).
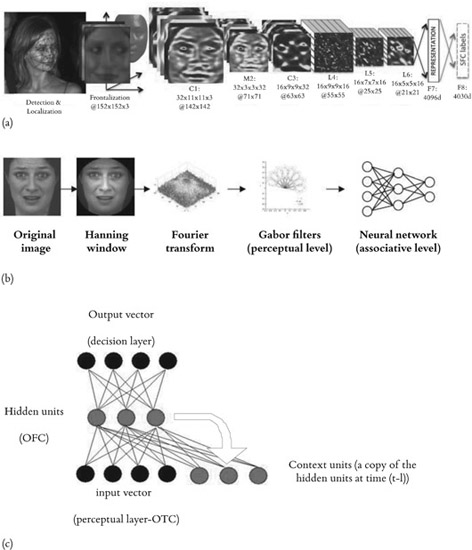
Figure 12.2
(a) Example of a recent neural network that uses a bottom-up approach based on the Deep Belief Network (Source: DeepFace; Taigman et al., 2014); (b) Example of a similar bottom-up approach that we used in previous studies (Source: Mermillod et al., 2010); (c) the top-down recurrent neural network that we will use for the recognition of dynamic emotional expressions (and more general, threatening situations). The neural network presented in (c) will replace the neural network (a simple multilayer perceptron) which is presented in (b). The bottom-up approach has been used in the vast majority of the articles published in the fields of computer vision and neural computation (such as DeepFace, for instance).
Albeit very simplified compared to a biological neural system, this type of artificial neural network is able to learn very complex structures in time (Ans et al., 2004). It is conceivable that this neural computation algorithm could be applied to the anticipation of emotional events. More precisely, if we replace the neural network, a standard multilayer perceptron1 used in Mermillod et al. (2010) or other types of static neural networks (Dailey et al., 2002; Taigman et al., 2014), by an SRN, this latter type of neural network should be able to detect dynamic emotional expressions or more general dynamic threatening events faster and with a better accuracy than a standard multilayer perceptron. Incorporated within the affective psychology framework proposed by Niedenthal et al. (2010), this type of computational modeling approach could neatly fill the gap between, on the one hand, predictive coding, in particular coding within the affective neuroscience framework proposed by Barrett & Bar (2009), and embodiment theory, on the other. In other words, the premotor stage (constituted by the hidden layer), in association with the motor stage (the output layer), could have an indirect top-down influence on the perceptual stage (the input layer). The computational view is supported by both the empirical evidence provided by the embodied cognition theory and the neuroimaging evidence provided by Bar’s model. Goldman & Sripada (2005) have proposed four different theoretical models that might account for the embodiment processes that have been regularly reported at the empirical level. However, to our knowledge, these theoretical models have not so far been implemented in real neural networks in order to permit an assessment of their computational reliability. We suggest here that an SRN could constitute an efficient and parsimonious computational algorithm that might form a bridge between embodiment theory and the neural basis of the predictive brain hypothesis. However, in addition to this “computational hypothesis,” a complementary (or competing) hypothesis emphasizes the social function of embodying emotions. In such a perspective, our capacity to experience other people’s emotions in relation to ourselves “illustrates the social nature of the self, inherently intersubjective” (Decety & Jackson, 2004).
The empathic brain hypothesis
Empathy is broadly defined as a sense of similarity between the feelings one experiences and those expressed by others (e.g., Preston & de Waal, 2002). In line with the embodiment approach, recent brain imaging studies provide evidence for shared affective neuronal networks underlying our ability to empathize. More precisely, brain imaging studies have shown overlapping brain activation patterns when individuals feel their own emotions and observe the same emotions in others (e.g., Decety & Lamm, 2009). Thus, it has been suggested that (i) shared neuronal networks explain how we feel the emotions of others as if they were our own and (ii) these neural networks are activated automatically whenever we observe others displaying emotion (for a critical evaluation of this automaticity, see de Vignemont & Singer, 2006).
Shared neural circuits between self and other
The idea that empathy is an automatic nervous system state that tends to simulate another person’s state is consistent with the notion of embodiment. The simulation models of emotion processing propose that the ability to understand the other’s emotions and mental states is achieved through internally simulating the same psychological state in ourselves (e.g., Goldman & Sripada, 2005). The “direct-matching hypothesis,” derived from the perception–action model of empathy, posits that perceiving another person’s emotions should automatically activate the corresponding representations of these emotions in the observer and that this in turn should activate somatic and autonomic responses (e.g., Decety, 2011). This supposition has been borne out by many behavioral and physiological studies demonstrating that unconscious automatic mimicry of a target generates the autonomic response associated with that bodily state and facial expression in the observer (e.g., Preston & de Waal, 2002).
Facial mimicry and empathy
Mimicry refers to the “tendency for people to show the same expressions as those of the target they are interacting with or observing” (Neumann & Westbury, 2011), and research demonstrates that affective responsiveness relies on involuntary mimicry and somato-sensorimotor resonance between other and self (e.g., Decety, 2011; Dondi, Simion, & Caltran, 1999; Martin and Clark, 1987).
More than a century ago, Lipp (1903) first proposed that people tend to mimic the facial, vocal, or postural expressions of the other’s emotions and that such mimicry evokes the corresponding emotions in the observer via a feedback process. This tendency to automatically mimic the other’s emotional facial expressions, postures and vocalizations, and consequently to converge emotionally, has subsequently been termed “emotional contagion” (Hatfield, Cacioppo & Rapson, 1994), and many researchers consider motor mimicry to be a key component in empathy (e.g., de Wied et al., 2006; Hatfield et al., 1994; Hoffman, 2002; Meltzoff & Decety, 2003; Preston & de Waal, 2002). More precisely, mimicry is believed to constitute a rudimentary form of empathy and to be a key factor in the development of perspective-taking competences (e.g., Hess et al., 1999; Williams et al., 2001). According to the developmental model of empathy defined by Hoffman (2000), motor mimicry constitutes an innate capacity that contributes to the development of empathy in the preverbal years and continues to play a role past childhood (see also Meltzoff & Decety, 2003; Preston & de Waal, 2002). This model integrates research suggesting that the tendency to automatically mirror other people’s emotional expressions manifests itself in the first days of life by reflexive crying in response to other babies’ crying. Several correlational studies have found a relationship between mimicry and empathy, whereas others have failed to demonstrate such a correlation. For example, Sonnby-Borgstrom et al. (2003) assessed participants’ trait-empathy using a questionnaire and found a significant and positive relationship between dispositional empathy and facial mimicry (the high-empathy participants produced greater facial mimicry than the low-empathy participants).
Recent research in the field of social endocrinology provides some empirical support for the idea that mimicry is related to empathy. Testosterone (T) constitutes a biological marker of psychopathy (Stalenheim et al., 1998) and antisocial personality disorder (Virkkunen et al., 1994), and a negative relationship between T levels and empathy has been found (e.g., Harris et al., 1996; Mehta & Beer, 2010). A recent study examined the causality of the relationship between T and empathy through the sublingual administration of a single dose of T within a placebo-controlled, double-blind design (Hermans, Putman & van Honk, 2006). Facial Electromyography (EMG) in response to dynamic facial expressions of happy and angry faces was recorded, and the results revealed that T generally decreased facial mimicry (i.e., attenuation of the mimicry effect in the corrugator supercilii (CS) muscle and, at a nearly significant level, the zygomatic major (ZM) muscle). As noted by the authors, these findings “are consistent with models that assign a critical role to mimicry in the ability to develop and communicate empathy towards conspecifics, and provide a potential causal mechanism of effects of testosterone on empathy.” In a similar vein, Arginine Vasopressin (AVP, a neurohypophysial hormone) has been implicated in male-typical social behaviors, including aggression (e.g., Heinrichs, van Dawans & Domes, 2009). Thompson et al. (2004) examined the effects of intranasal administration of AVP on cognitive, autonomic and somatic responses to emotional facial expressions using a placebo-controlled, double-blind design. The authors found selective enhancements of the CS EMG responses evoked by emotionally neutral facial expressions. In the AVP condition, participants exhibited magnitudes in response to neutral facial expressions that were similar to the magnitudes of placebo participants in response to angry facial expressions. As noted by Heinrichs et al. (2009), “these results suggest that AVP may influence aggression by biasing individuals to respond to emotionally ambiguous social stimuli as if they were threatening or aggressive.” Facial mimicry could constitute a causal mechanism of the effects of AVP on aggression.
Clinical research also provides empirical support for the assumption of a relationship between mimicry and empathy. Several studies have indicated that a lack of empathy is one of the defining characteristics of psychopathy (e.g., Hare et al., 1990), and recent research suggests that a reduced tendency to mimic other people’s emotional facial expressions may represent a physiological marker of psychopathy (e.g., Harrison, Morgan & Critchley, 2010). For instance, depressed individuals display less mimicry of observed happiness (Schwartz et al., 1978; Schwartz et al., 1976). CS responses are also impaired in several conditions characterized by poor empathetic skills, such as autism (Hermans et al., 2009), oppositional defiant disorder and conduct disorder (de Wied et al., 2006), as well as in disorders of social communication such as Asperger’s syndrome (Harrison et al., 2010).
These results suggest a specific relationship between upper (CS) but not lower (ZM) facial mimicry and trait-empathy (see also Harrison et al., 2010). The anatomical connectivity between the lateral-basal amygdala nucleus, the cingu-late motor cortex (M3, M4) and the brainstem facial motor nucleus constitutes a potential neuroanatomical substrate for mimicry (e.g., Morecraft et al., 2001, 2007). The greater anatomical connectivity between amygdala and M3, which codes upper facial muscle representations, could explain the apparently specific relationship between upper facial mimicry (CS) and empathy.
Mirror neurons and empathy
The discovery of mirror neurons (Gallese et al., 1996) provides a physiological mechanism for the direct link between perception and action. Indeed, research on mirror neurons posits that the perception of an emotional facial expression automatically triggers activity in brain regions involved in experiencing similar emotions (e.g., Van der Gaag et al., 2007). Thus, when socially relevant stimuli are involved, “the action of mirror neurons may provide a mechanism by which emotional contagion and empathy can occur” (Neumann & Westbury, 2011; see also Wolf et al., 2001). However, several authors recently produced a critical evaluation of the contribution of the mirror neuron system to empathy (see Blair, 2011; Blair & Fowler, 2008; Decety, 2011). For example, Blairy et al. (1999) found that individuals spontaneously mimic facial expressions and that the decoding of facial expressions is accompanied by shared affects. However, they did not find that emotion recognition accuracy or shared affects are mediated by mimicry (e.g., de Vignemont & Singer, 2006).
While many functions have been attributed to mirror neurons, including empathy and mind-reading, the recent discovery of such cells in the primary motor cortex suggests that mirror neurons constitute a motor system that facilitates acting via learned associations (Hickok, 2009). More recently, several authors have argued that “motor resonance is neither necessary nor a sufficient mechanism for representing another individual’s intentions, especially in a social context” (Decety, 2011; see also Jacob, 2008). Indeed, the contribution of the mirror neurons to affect sharing is a matter of debate. Affect sharing “may simply rely on the activation of the core affect which refers to the automatic discrimination of a stimulus – or features of a stimulus – as appetitive or aversive, hostile or hospitable, pleasant or unpleasant, threatening or nurturing” (Decety, 2011; see also Barret et al., 2007).
Empathy for pain
Recent studies exploring how people respond behaviorally and neurally to the pain of others demonstrate that simply perceiving another individual in pain results in the activation, in the observer, of the neural network involved in the processing of the first-hand experience of pain (e.g., Jackson, Meltzoff & Decety, 2005; Singer et al., 2004). For example, in an fMRI study, participants were exposed (i) to videos of individuals expressing pain as a result of listening to painful sounds and (ii) to the same painful sounds. Overlapping activation between the first-hand experience of pain and second-hand perception of pain in others was found in the aMCC (anterior medial cingulate cortex), SMA (supplementary motor area), AIC (anterior insular cortex), amygdala, and PAG (periaqueductal gray). All in all, brain imaging studies demonstrate that the representation of pain involves somatic sensory features as well as affective-motivational reactions associated with the promotion of protective or recuperative visceromotor and behavioral responses (Akitsuki & Decety, 2009; Jackson et al., 2006; Jackson, Rainville & Decety., 2006; Lamm & Decety, 2008; Lamm, Meltzoff & Decety, 2009; Lamm et al., 2007). Figure 12.3 illustrates this distinction between the sensory-discriminative and the affective-motivational domains.
While fMRI studies have revealed that the same neural circuits are involved in the first-hand experience of pain and the perception of pain in others (see Jackson, Rainville, et al., 2006), several authors have argued that “common activity in the ACC (anterior cingulate cortex) and AIC reflects the operation of distinct but overlapping networks of regions that support perception of self or other people’s pain (Decety, 2011; see also Zaki, Ochsner, Hanelin, Wager & Mackey, 2007). In order to test this hypothesis, Zaki et al. (2007) scanned participants while they received pain stimulation or watched short videos of other people sustaining painful injuries. Connectivity analyses identified clusters in the midbrain and periaqueductal gray with greater connectivity to the AIC during self-pain, while the dorsal medial prefrontal cortex (mPFC) showed greater connectivity to the ACC and AIC during other people’s pain. These results revealed that distinct neural networks were associated with ACC and AIC in response to the first-hand experience of pain and the response to another person’s pain. In line with these findings, recent neurophysiological research on pain suggests the involvement of only partially overlapping neural subpopulations and indicates the involvement of distinct cognitive and affective processes (e.g., Decety & Lamm, 2009). Thus, the extent to which activation during empathy for pain can be attributed to shared neural and mental representations remains an open empirical question.
Moreover, activations in the pain matrix are not necessarily specific to the emotional experience of pain. Indeed, such activations (e.g., amygdala, insula) could also be related to other processes such as negative stimulus evaluation, attention to noxious stimuli or the selection of appropriate skeletomuscular defensive movements (Decety & Lamm, 2009). Thus,
shared neural representations in the affective-motivational as well as in the sensory-discriminative aspects of the pain matrix between perceiving others in pain and experiencing it might be associated with more general survival mechanisms such as the detection and reaction to salient potentially threatening sensory inputs which usually elicit withdrawal reactions when exposed to danger and threat.
(Decety & Svetlova, 2012)
However, suppression of avoidance is an important precondition for empathic concern and the mobilization of helping behavior (e.g., Decety & Moriguchi, 2007). These outcomes require a clear distinction between the self and the other and require the regulation of the emotional consequences of the affective sharing of pain initiated by mimicry processes (Decety & Jackson, 2004), which constitute an “effortful self-regulatory process” (Eisenberg & Eggum, 2009).
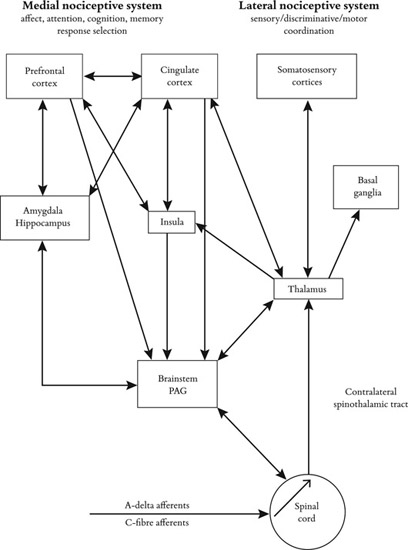
Figure 12.3
Schematic diagram of some of the main anatomical components of the “pain matrix” (e.g., Decety, 2011) and their possible functional significance in the periaqueductal grey matter (PAG). Neurophysiological research on pain processing points out a distinction between the sensory-discriminative and the affective-motivational domains. The primary (SI) and secondary (SII) cortices are involved in the sensory-discriminative aspects of pain (bodily location and intensity of stimulus), while the ACC (anterior cingulate cortex) and anterior insula underpin the affective-motivational component (the evaluation of subjective discomfort and response preparation in the context of painful or aversive stimuli).
Toward an integrative model of empathy for pain
Pain-related affective sharing can provoke either sympathy (an other-oriented motivated response) or personal distress (an aversive, self-oriented motivated response; Decety & Lamm, 2009). While sympathy promotes moral reasoning and altruism, personal distress is associated with the desire to alleviate one’s own but not the other’s distress (Batson et al., 1991). The involvement of the pain matrix may help to explain why observing another individual’s pain does not always result in prosocial behavior (Decety & Lamm, 2009).
While the ability to perceive, share and understand others’ affective states has been broadly defined as empathy-related responding (Eisenberg & Eggum, 2009), empathy constitutes a set of different socioemotional competences. We previously mentioned that empathy is broadly defined as a sense of similarity between the feelings one experiences and those expressed by others (e.g., Preston & de Waal, 2002). Going further, Decety and Jackson (2004) argued that empathy “accounts for the naturally occurring subjective experience of similarity between the feelings expressed by self and others without losing sight of whose feelings belong to whom.” In this perspective, empathy involves “not only the affective experience of the other person’s actual or inferred emotional state but also some minimal recognition and understanding of another’s emotional state” (Decety & Jackson, 2004). The authors propose that empathy and sympathy require several socioemotional competences, which are underpinned by specific neural systems: (i) affective sharing, (ii) self-awareness and (iii) mental flexibility and emotional self-regulation:
- Affective sharing is based on perception-action coupling that leads to shared representations.
- Self-other awareness ensures that even when there is some temporary identification, there is no confusion between self and other. A confusion between the self and the other provokes a more extensive activation of the pain matrix, which is associated with personal distress.
- Mental flexibility and emotional self-regulation make it possible to inhibit self-perspective, to consciously engage in perspective taking and to maintain a clear self-other distinction.
Brain imaging studies have revealed that personal distress is linked to a stronger hemodynamic response in brain areas coding the affective-motivational dimension of pain (e.g., amygdala) as well as the sensory-motor aspects of pain (e.g., middorsal insula, premotor cortex; e.g., Jackson, Brunet et al., 2006). Effortful self-regulatory processes are defined by the efficient direction of executive attention – including the ability to inhibit a dominant response and/or to activate sub-dominant responses, to plan and to detect errors (Rothbart & Bates, 2006). The regulation of cognitive and emotional processes is essential to empathy (Table 12.1) and sympathy (Decety & Jackson, 2004; Eisenberg & Eggum, 2009).
Table 12.1
Key concepts of empathy in social neuroscience.
|
Key concepts
|
|
| • Emotional contagion is an automatic response resulting in a similar emotion being aroused in the observer as a direct result of perceiving the expressed emotion of another. |
| • Empathy is an affective response stemming from the understanding of another's emotional state or of a condition similar to what the other person is feeling or would be expected to feel in the given situation. |
| • Empathic concern (also called sympathy) is an other-oriented emotional response congruent with the perceived welfare of someone in need. |
| • Personal distress is an aversive, self-focused reaction to the expression of another's negative emotion, often leading to avoidance behavior. |
| • Prosocial or helping behavior refers to actions that intend to help or benefit another individual or group of individuals. |
| • Altruism refers to prosocial behaviors that benefit the recipient at a cost to the donor. |
Mental flexibility, which is crucial in order to inhibit one’s own point of view and adopt someone else’s point of view, constitutes an effortful and controlled process. Emotion regulation, which is essential for managing and optimizing inter-subjective transactions between the self and other, is also an effortful and controlled process. Effortful control has been correlated with high levels of sympathy or empathy and low personal distress (Eisenberg et al., 1996). A lack of emotional control would lead to emotional contagion or emotional distress. Research on empathy emphasizes the individual differences in self-regulatory competences (Rothbart & Bates, 2006). Well-regulated people are expected to be the most empathic (Eisenberg et al., 1996), whereas people who are not well-regulated are expected to be biased to experience overarousal followed by personal distress (Eisenberg, Valiente & Champion, 2004).
Social and political issues
Context and similarity
Recent studies have revealed that empathic resonance is modulated early in information processing by how observers conceptualize both the situation and the person who is expressing pain. It seems that empathy is modulated by competitive and cooperative interplay (Englis et al., 1982; Lanzetta & Englis, 1989; Singer et al., 2004). For example, Lanzetta and Englis (1989) provided experimental evidence showing that a competition context generates counter-mimicry (e.g., an expression of pain could trigger a smile) and counter-empathy (e.g., the other’s distress generates positive feelings in the observer). Thus, given the right context, feed-backs from peripheral muscles to the central nervous system could generate an oppositely valenced emotion in the observer from that of the person observed.
While some studies have found that feed-backs from peripheral muscles to the central nervous system vary as a function of the relationship between observer and expresser (e.g., Bourgeois & Hess, 2008), brain imaging studies provide empirical evidence that neural responses to others in pain are also mediated by similarity or familiarity with the target. In a recent fMRI study, less activity was observed in the anterior midcingulate cortex when the participants observed a stigmatized target (vs. nonstigmatized target) in a state of suffering, and the participants also reported both less personal distress and less empathic concern (Decety, Echols & Correll, 2010). Several fMRI studies have found less neural activity in the ACC and inferior insular cortex when participants viewed videos of ethnic out-group members in pain (e.g., Xu et al., 2009) and more neural activity in the medial prefrontal cortex when viewing the suffering of ethnic in-group members (e.g., Mathur et al., 2010). However, Harris and Fiske (2003) found significant mPFC activity in White participants watching Black faces (see also Wheeler & Fiske, 2005). Harris and Fiske (2006) conducted fMRI research on the neural underpinnings of dehumanization based on the hypothesis that only a disgust-evoking, low-low quadrant (groups that are both stereotypically hostile and stereotypically incompetent) would generate dehumanizing prejudice. As expected, the results revealed that only low-low out-groups (e.g., drugs addicts) were associated with the same neural activations as disgusting objects (e.g., vomit) in the form of an activation of the amygdala (fear) and insula (disgust) and an absence of the typical neural signature for social cognition (i.e., activation of the medial prefrontal cortex). These results are consistent with other findings showing that not all out-group members are indiscriminately dehumanized and that suggest that empathic resonance is moderated by how individuals conceptualize the others they perceive in pain.
Because dehumanized groups are believed not to experience complex human emotions (e.g., Leyens et al., 2001, 2003), dehumanizing beliefs could impair embodiment processes. As we noted previously, visual recognition is not the result of pure bottom-up processes from the perceptual system to high-level cortical areas dedicated to visual cognition (e.g., identification or categorization of stimuli): Top-down processes from the OFC to low-level perceptual areas are believed to increase the efficiency of perceptual processes. A possible hypothesis is that dehumanizing beliefs alter object recognition (i.e., the other as human being) through top-down neural processes and might therefore impair embodiment processes (e.g., facial mimicry) and pain-related affective sharing. Interestingly, neuroscience research demonstrates that the OFC enables individuals to experience the emotions that someone else may feel in a specific setting and could therefore facilitate empathy and prosocial behavior (e.g., Bechara, 2004; Mehta & Beer, 2010; Shamay-Tsoory et al., 2010). In contrast, damage to the OFC may underpin some forms of psychopathy. More generally, when the OFC is inhibited or compromised, individuals may behave inappropriately.
All in all, neurophysiological research demonstrates that perceiving or imagining another individual’s pain is associated with hemodynamic responses in the neural network that processes the motivational-affective and the sensory dimensions of pain in oneself. However, recent studies have revealed that this network is modulated by several contextual and dispositional factors, such as social context (Cheng et al., 2007), stigmatization (Decety et al., 2009) or racial bias and dehumanization (e.g., Harris & Fiske, 2006). Thus, “incoming sensory information is constrained by appraisal and reappraisal processing that shapes the emergence of the experience of empathy and behavioral outcomes” (Decety, 2011). The emotional, motivational and behavioral consequences of pain-related affective sharing are also closely related to effortful self-regulatory capacities (e.g., Eisenberg & Eggum, 2009).
From social neuroscience of empathy to political neuroscience
Empathetic overarousal induced by viewing another person’s pain promotes personal distress, whereas optimal levels of arousal promote sympathy and prosocial behavior. While self-regulation makes it possible to inhibit the self-perspective and evaluate the other’s perspective, there are individual differences in these capacities (Rothbart & Bates, 2006). Individuals with low self-regulation abilities are expected to be biased to experience overarousal and, consequently, personal distress (Eisenberg, Valiente & Champion, 2004). Interestingly, recent political and neuroscience research suggests that political orientation reflects individual differences in the functioning of a general mechanism related to cognitive control and emotional self-regulation (e.g., Amodio, Jost, Master & Yee, 2007). More precisely, several studies have revealed that conservatives (i) exhibit more cognitive rigidity (e.g., Amodio et al., 2007) and have a smaller ACC volume than progressives (Kanai et al., 2011); (ii) exhibit more physiological sensitivity to aversive stimuli (e.g., Oxley et al., 2008) and (iii) have a larger right amygdala volume than progressives (Kanai et al., 2011). The ACC is important for both emotion regulation and cognitive control since it controls the level of emotional arousal or response to an emotional event. The ability to maintain low emotional arousal and exercise a high level of cognitive control is crucial for the management of high-conflict emotional situations. The amygdala is part of the limbic system, a brain area involved in emotion processing. Persons with a larger or more active amygdala tend to have stronger physiological and emotional reactions to emotional events.
Conclusion
While recent decades have provided a large amount of evidence in support of an embodied view of cognition and emotion, the purpose of these complex feedbacks from peripheral muscles to the central nervous system remains unclear. On the one hand, the embodiment of emotions could be related to a more computationally oriented function with the goal of anticipating perceptual and, therefore, potentially emotional events. Research in psychology, cognitive neuroscience and neural network modeling may, within the next few decades, provide evidence of the relevance of the embodied view of cognition for the efficient prediction of our perceptual environment (in order to fill the gap between cognitive neuroscience, connectionist modeling and affective psychology theories). However, so far, the question concerning the importance of the embodiment processes for anticipation is as yet unresolved.
On the other hand, emotional embodiment could be related, alternatively or complementarily, to the social nature of the self. This could, in particular, be the case for empathy, which is one of the most complex intersubjective processes (mainly driven by cortical neural areas). Nonetheless, empathy is not only a complex emotional state. At a fundamental level, empathy could also constitute the first step in channeling cognitive neuroscience toward the study of more complex social and political processes such as conservatism, authoritarianism and dehumanization. An important point to note here is that determining the neural basis of social or political phenomena does not necessarily imply the genetic underpinning of the related behaviors and attitudes (e.g., Jost & Amodio, 2012). The brain is outstanding at adapting to its environment, and the functional architecture, or even the volumetric neuroanatomy of different neural areas, could be largely determined by the cognitive or social environment of any specific individual (a large amygdala could be the result of a threatening environment, for instance). Future research will be needed in order to determine the genetic and environmental processes underpinning the most recent data reported by the social and political neurosciences.
Note
References
Akitsuki, Y., & Decety, J. (2009). Social context and perceived agency modulate brain activity in the neural circuits underpinning empathy for pain: An event-related fMRI study. NeuroImage, 47, 722–734.
Amodio, D. M., Jost, J. T., Master, S. L., & Yee, C. M. (2007). Neurocognitive correlates of liberalism and conservatism. Nature Neuroscience, 10, 1246–1247.
Ans, B., Rousset, S., French, R. M., & Musca, S. (2004). Self-refreshing memory in artificial neural networks: Learning temporal sequences without catastrophic forgetting. Connection Science, 16 (2), 71–99.
Bar, M. (2004). Visual objects in context. Nature Reviews Neuroscience, 5 (8), 617–629.
Bar, M., Kassam, K. S., Ghuman, A. S., Boshyan, J., Schmid, A. M., Dale, A. M.,… & Halgren, E. (2006). Top-down facilitation of visual recognition. Proceedings of the National Academy of Sciences of the United States of America, 103 (2), 449–454.
Barrett, L. F., & Bar, M. (2009). See it with feeling: affective predictions during object perception. Philosophical Transactions of the Royal Society B: Biological Sciences, 364 (1521), 1325–1334.
Barrett, L. F., Mesquita, B., Ochsner, K. N., & Gross, J. J. (2007). The experience of emotion. Annual Review of Psychology, 58, 373.
Barsalou, L. W. (1999). Perceptual symbol systems. Behavioral and Brain Sciences, 22 (4), 577–609.
Batson, C. D., Batson, J. G., Singlsby, J. K., Harrell, K. L., Peekna, H. M., & Todd, R. M. (1991). Empathic joy and the empathy-altruism hypothesis. Journal of Personality and Social Psychology, 61, 413–426.
Bechara, A. (2004). The role of emotion in decision-making: Evidence from neurological patients with orbitofrontal damage. Brain and Cognition, 55, 30–40.
Beffara, B., Ouellet, M., Vermeulen, N., Basu, A., Morisseau, T., & Mermillod, M. (2012). Enhanced embodied response following ambiguous emotional processing. Cognitive Processing, 13 (1), 103–106.
Beffara, B., Wicker, B., Vermeulen, N., Ouellet, M., Bret, A., Molina, M.J.F., & Mermillod, M. (2015). Reduction of interference effect by low spatial frequency information priming in an emotional Stroop task. Journal of vision, 15(6), 16. doi:10.1167/15.6.16
Blair, R.J.R. (2011). Should affective arousal be grounded in perception-action coupling? Emotion Review, 3, 109–110.
Blair, R.J.R., & Fowler, K. (2008). Moral emotions and moral reasoning from the perspective of affective cognitive neuroscience: A selective review. European Journal of Developmental Science, 2, 303–323.
Blairy, S., Herrera, P., & Hess, U. (1999). Mimicry and the judgment of emotional facial expressions. Journal of Nonverbal Behavior, 23 (1), 5–41.
Bourgeois, P., & Hess, U. (2008). The impact of social context on mimicry. Biological Psychology, 77, 343–352.
Bullier, J. (2001). Integrated model of visual processing. Brain Research Reviews, 36 (2–3), 96–107.
Cannon, W. B. (1927). The James-Lange theory of emotion: A critical examination and an alternative theory. American Journal of Psychology, 39, 10–124.
Cheng, Y., Lin, C.-P., Liu, H.-L., Hsu, Y.-Y., Lim, K.-E., Hung, D., et al. (2007). Expertise modulates the perception of pain in others. Current Biology, 17, 1708–1713.
Damasio, A. R., & Carvalho, G. B., (2013). The nature of feelings: Evolutionary and neurobiological origins. Nature Review Neuroscience, 14, 143–152.
Dailey, M. N., Cottrell, G. W., Padgett, C., & Adolphs, R. (2002). EMPATH: A neural network that categorizes facial expressions. Journal of Cognitive Neuroscience, 14 (8), 1158–1173.
de Vignemont, F., & Singer, T. (2006). The empathic brain: How, when and why? Trends in Cognitive Sciences, 10, 435–441.
de Wied, M., van Boxtel, A., Zaalberg, R., Goudena, P. P., & Matthys, W. (2006). Facial EMG responses to dynamic emotional facial expressions in boys with disruptive behavior disorders. Journal of Psychiatric Research, 40 (2), 112–121.
Decety, J. (2011). Dissecting the neural mechanisms mediating empathy. Emotion Review, 3, 92–108.
Decety, J., Echols, S., & Correll, J. (2010). The blame game: The effect of responsibility and social stigma on empathy for pain. Journal of Cognitive Neuroscience, 22 (5), 985–997.
Decety, J., & Jackson, P. L. (2004). The functional architecture of human empathy. Behavioral and Cognitive Neuroscience Reviews, 3 (2), 71–100.
Decety, J., & Lamm, C., (2009). Empathy versus personal distress: Recent evidence from social neuroscience. In J. Decety & W. Ickes (Eds.), The social neuroscience of empathy (pp. 198–213). Cambridge: MIT Press.
Decety, J., & Moriguchi, Y. (2007). The empathic brain and its dysfunction in psychiatric populations: Implications for intervention across different clinical conditions. Biopsy-chosocial Medicine, 1, 22.
Decety, J., & Svetlova, M. (2012). Putting together phylogenetic and ontogenetic perspectives on empathy. Developmental Cognitive Neuroscience, 2, 1–24.
Dondi, M., Simion, F., & Caltran, G. (1999). Can newborns discriminate between their own cry and the cry of another newborn infant? Developmental Psychology, 35, 418–426.
Eisenberg, N., & Eggum, N. D. (2009). Empathic responding: Sympathy and personal distress. In J. Decety & W. Ickes (Eds.), The social neuroscience of empathy (pp. 71–83). Cambridge, MA: MIT Press.
Eisenberg, N., Fabes, R. A., Guthrie, I. K., Murphy, B. C., Maszk, P., Holmgren, R., et al. (1996). The relations of regulation and emotionality to problem behavior in elementary school children. Development and Psychopathology, 8, 141–162.
Eisenberg, N., Valiente, C., & Champion, C. (2004). Empathy-related responding: Moral, social, and socialization correlates. In A. G. Miller (Ed.), The social psychology of good and evil: Understanding our capacity for kindness and cruelty. New York: Guilford Press.
Elman, J. L. (1990). Finding structure in time. Cognitive Science, 14 (2), 179–211.
Englis, B. G., Vaughan, K. B., & Lanzetta, J. T. (1982). Conditioning of counter-empathetic responses. Journal of Experimental Social Psychology, 18, 375–391.
Gallese, V., Fadiga, L., Fogassi, & Rizzolatti, G. (1996). Action recognition in the premotor cortex. Brain, 119, 593–609.
Goldman, A., & Sripada, C. (2005). Simulationist models of face-based emotion recognition. Cognition, 94, 193–213.
Hare, R. D., Harpur, T. J., Hakstian, A. R., Forth, A. E., Hart, S. D., & Newman, J. P. (1990). The Revised Psychopathy Checklist: Reliability and factor structure. Journal of Consulting and Clinical Psychology, 2, 338–341.
Harris, L. T., & Fiske, S. T. (2003). BOLD activations to Black and White faces under different social goal conditions. Unpublished raw data.
Harris, L. T, & Fiske, S. T. (2006). Dehumanizing the lowest of the low: Neuroimaging responses to extreme out-groups. Psychological Science, 17, 847–853.
Harris, J. A., Rushton, J., Hampson, E., & Jackson, D. N. (1996). Salivary testosterone and self-report aggressive and prosocial personality characteristics in men and women. Aggressive Behavior, 22, 321–331.
Harrison, N. A., Morgan, R. & Critchley, H. (2010). From facial mimicry to emotional empathy: A role for norepinephrine? Social Neuroscience, 5 (4), 393–400.
Hatfield, E., Cacioppo, J. T., & Rapson, R. L. (1994). Emotional contagion. New York: Cambridge University Press.
Havas, D. A., Glenberg, A. M., Gutowski, K. A., Lucarelli, M. J., & Davidson, R. J. (2010). Cosmetic use of botulinum toxin-A affects processing of emotional language. Psychological Science, 21, 895–900.
Heinrichs, M., von Dawans, B., & Domes, G. (2009). Oxytocin, vasopressin, and human social behavior. Frontiers in Neuroendocrinology, 30, 548–557.
Hennenlotter, A., Dresel, C., Castrop, F., Ceballos-Baumann, A. O., Baumann, A.O.C., Wohlschläger, A. M., & Haslinger, B., (2009). The link between facial feedback and neural activity within central circuitries of emotion—new insights from botulinum toxin-induced denervation of frown muscles. Cerebral Cortex, 19, 537–542.
Hess, U., Philippot, P., & Blairy, S. (1999). Mimicry: Facts and fiction. In P. Philippot, R. Feldman, & E. J. Coats (Eds.), The social context of nonverbal behavior, studies in emotion and social interaction (pp. 213–241). New York: Cambridge University Press.
Hermans, E. J., Putman, P., & van Honk, J. (2006). Testosterone acutely reduces the fear potentiated startle. Biological Psychiatry, 59, 872–874.
Hermans, E. J., van Wingen, G., Bos, P. A., Putman, P., & van Honk, J. (2009). Reduced spontaneous facial mimicry in women with autistic trai ts. Biological Psychology, 80, 348–353.
Hickok, G. (2009). Eight problems for the mirror neuron theory of action understanding in monkeys and human. Journal of Cognitive Neuroscience, 21, 1229–1243.
Hoffman, M. L. (2000). Empathy and moral development. New York: Cambridge University Press.
Hoffman, M. L. (2002) How automatic and representational is empathy, and why. Behavioral and Brain Sciences, 25, 38–39
Jacob, P. (2008). What do mirror neurons contribute to human social cognition? Mind and Language, 23, 190–223.
Jackson, P. L., Brunet, E., Meltzoff, A. N., & Decety, J. (2006). Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia, 44, 752–761.
Jackson, P. L., Meltzoff, A. N., & Decety, J. (2005). How do we perceive the pain of others? A window into the neural processes involved in empathy. NeuroImage, 24, 771–779.
Jackson, P. L., Rainville, P., & Decety, J. (2006). From nociception to empathy: The neural mechanism for the representation of pain in self and in others. Pain, 125, 5–9.
James, W. (1890). The principles of psychology. New York: Dover Publications.
Jost, J. T., & Amodio, D. M. (2012). Political ideology as motivated social cognition: Behavioral and neuroscientific evidence. Motivation and Emotion, 36, 55–64.
Kanai, R., Feilden, T., Firth, C., & Rees, G. (2011). Political orientations are correlated with brain structure in young adults. Current Biology, 21, 677–680.
Kauffmann, L., Ramanoël, S., & Peyrin, C. (2014). The neural bases of spatial frequency processing during scene perception. Frontiers in Integrative Neuroscience, 8.
Kawasaki, H., Kaufman, O., Damasio, H., Damasio, A. R., Granner, M., Bakken, H., Hori, T., Howard, M. A. 3rd, & Adolphs, R. (2001). Single-neuron responses to emotional visual stimuli recorded in human ventral prefrontal cortex. Nature Neuroscience, 4 (1), 15–16.
Kveraga, K., Ghuman, A. S., & Bar, M. (2007). Top-down predictions in the cognitive brain. Brain and Cognition, 65 (2), 145–168.
Lamm, C., & Decety, J. (2008). Is the extrastriate body area (EBA) sensitive to the perception of pain in others? An fMRI investigation. Cerebral Cortex, 18, 2369–2373.
Lamm, C., Meltzoff, A. N., & Decety, J. (2009). How do we empathize with someone who is not like us? Journal of Cognitive Neuroscience, 2, 362–376.
Lamm, C., Nusbaum, H. C., Meltzoff, A. N., & Decety, J. (2007). What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE, 12, e1292.
Lanzetta, J. T., & Englis, B. G. (1989). Expectations of cooperation and competition and their effects on observers’ vicarious emotional responses. Journal of Personality and Social Psychology, 56, 543–554.
Leyens, J.-P, Cortes, B. P., Demoulin, S., Dovidio, J., Fiske, S. T, Gaunt, R., Paladino, M.-R, Rodriguez-Perez, A., Rodriguez-Torres, R., & Vaes, V. (2003). Emotional prejudice, essentialism, and nationalism. European Journal of Social Psychology, 33, 703–718.
Leyens, J.-R, Rodriguez-Perez, A., Rodriguez-Torres, R., Gaunt, R., Paladino, M. R, Vaes, J., & Demoulin, S. (2001). Psychological essentialism and the differential attribution of uniquely human emotions to ingroups and outgroups. European Journal of Social Psychology, 37, 395–411.
Lipps, T. (1903). Kapitel: Die Einfuhlung in Leitfaden der Psychology. Leipzig: Verlag von Wilhem Engelmann.
Martin, G. B., & Clark, R. D. (1987). Distress crying in neonates: Species and peer specificity. Developmental Psychology, 18, 3–9.
Mathur, V. A., Harada, T., Lipke, T., Chiao, J. Y. (2010). Neural basis of extraordinary empathy and altruistic motivation. Neuroimage, 51 (4), 1468–1475.
McCanne, T. R., & Anderson, J. A., (1987). Emotional responding following experimental manipulation of facial electromyographic activity. Journal of Personality and Social Psychology, 52, 759–768.
Meltzoff, A. N., & Decety, J. (2003). What imitation tells us about social cognition: A rapprochement between developmental psychology and cognitive neuroscience. Philosophical Transactions of the Royal Society, London: Biological Sciences, 358, 491–500.
Mermillod, M., Auxiette, C., Chambres, P., Mondillon, L., Galland, F., Jalenques, I., & Durif, F. (2011). Contraintes perceptives et temporelles dans l’exploration du modèle de Ledoux. L’année Psychologique, 111 (3), 465–479.
Mermillod, M., Bonin, P., Mondillon, L., Alleysson, D., & Vermeulen, N. (2010). Coarse scales are sufficient for efficient categorization of emotional facial expressions: Evidence from neural computation. Neurocomputing, 73, 2522–2531.
Mermillod, M., Devaux, D., Derost, P., Rieu, I., Chambres, P., Auxiette, C., Legrand, G., Galland, F., Dalens, H., Coulangeon, L. M., Broussolle, E., Durif, F., & Jalenques, I. (2013). Rapid presentation of emotional expressions reveals new emotional impairments in Tourette’s Syndrome. Frontiers in Human Neuroscience, 7, 149.
Mermillod, M., Droit-Volet, S., Devaux, D., Schaefer, A., & Vermeulen, N. (2010). Are coarse scales sufficient for fast detection of visual threat? Psychological Science, 21 (10), 1429–1437.
Mermillod, M., Vermeulen, N., Lundqvist, D., & Niedenthal, P. M. (2009). Neural computation as a tool to differentiate perceptual from emotional processes: The case of anger superiority effect. Cognition, 110 (3), 346–357.
Mermillod, M., Vuilleumier, P., Peyrin, C., Alleysson, D., & Marendaz, C. (2009). The importance of low spatial frequency information for recognizing fearful facial expressions. Connection Science, 21 (1), 75–83.
Mehta, P., & Beer, J. (2010). Neural mechanisms of the testosterone-aggression relation: The role of the orbitofrontal cortex. Journal of Cognitive Neuroscience, 22, 2357–2368.
Morecraft, R. J., Louie, J. L., Herrick J. L., & Stilwell-Morecraft, K., S. (2001). Cortical innervation of the facial nucleus in the non-human primate: A new interpretation of the effects of stroke and related subtotal brain trauma on the muscles of facial expression. Brain, 124, 176–208.
Morecraft, R. J., McNeal, D. W., Stilwell-Morecraft, K. S., Gedney, M., Ge, J., Schroeder, C. M., & Van Hoesen, G. W. (2007). Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. Journal of Comparative Neurology, 500 (1), 134–165.
Neumann, D. L., & Westbury, H. R. (2011). The psychophysiological measurement of empathy. In D. J. Scapaletti (Ed.), In Psychology of Empathy (pp. 119–142). Hauppauge NY: Nova Science Publishers Inc.
Niedenthal, P. M. (2007). Embodying emotion. Science, 316 (5827), 1002–1005.
Niedenthal, P. M., Mermillod, M., Maringer, M., & Hess, U. (2010). The Simulation of Smiles (SIMS) model: Embodied simulation and the meaning of facial expression. Behavioral and Brain Sciences, 33 (6), 417–433
Oxley, D. R., Smith, K. B., Alford, J. R., Hibbing, M. V., Miller, M. S., Hatemi, P. K., et al. (2008). Political attitudes vary with physiological traits. Science, 321, 1667–1670.
Pitcher, D., Garrido, L., Walsh, V., Duchaine, B. C., (2008). Transcranial magnetic stimulation disrupts the perception and embodiment of facial expressions. Journal of Neuroscience. 28, 8929–8933.
Pourtois, G., Thut, G., Grave de Peralta, R., Michel, C., & Vuilleumier, P. (2005). Two electrophysiological stages of spatial orienting towards fearful faces: Early temporoparietal activation preceding gain control in extrastriate visual cortex. Neuroimage, 26 (1), 149–163.
Preston, S. D., & de Waal, F.B.M. (2002). Empathy: Its ultimate and proximate bases. Behavioral and Brain Sciences, 25, 1–72.
Rothbart, M. K., & Bates, J. E. (2006). Temperament. In W. Damon, R. Lerner, & N. Eisenberg (Eds.), Handbook of child psychology: Vol. 3. Social, emotional, and personality development (6th ed., pp. 99–166). New York: Wiley.
Rudrauf, D., David, O., Lachaux, J. P., Kovach, C. K., Martinerie, J., Renault, B., & Damasio, A. R. (2008). Rapid interactions between the ventral visual stream and emotion-related structures rely on a two-pathway architecture. The Journal of Neuroscience, 28 (11), 2793–2803.
Schwartz, G. E., Fair, P. L., Mandel, M. R., Salt, P., Mieske, M., & Klerman, G. L. (1978). Facial electromyography in the assessment of improvement in depression. Psychosomatic Medicine, 40, 355–360.
Schwartz, G. E., Fair, P. L., Salt, P., Mandel, M. R., & Klerman, G. L. (1976). Facial muscle patterning to affective imagery in depressed and nondepressed subjects. Science, 192, 489–491.
Shamay-Tsoory, S. G., Harari, H., Aharon-Peretz, J., & Levkovitz, Y. (2010). The role of the orbitofrontal cortex in affective theory of mind in criminal offenders with psychopathic tendencies. Cortex, 46, 668–677.
Singer, T., Seymour, B., O’Doherty, J., et al. (2004). Empathy for pain involves the affective but not the sensory components of pain. Science, 303, 1157–1161.
Sonnby-Borgstrom, M., Jonsson, P., & Svensson, O. (2003). Emotional empathy as related to mimicry reactions at different levels of information processing. Journal of Nonverbal Behavior, 27, 3–23.
Stalheim, E. G., Eriksson, E., Von Knorring, L., & L. Wide (1998). Testosterone as a biological marker in psychopathy and alcoholism. Psychiatry Research, 77, 79–88.
Strack, F., Martin, L., & Stepper, S., (1988). Inhibiting and facilitating conditions of the human smile: A nonobtrusive test of the facial feedback hypothesis. Journal of Personality and Social Psychology, 54, 768–777.
Taigman, Y., Yang, M., Ranzato, M. A., & Wolf, L. (2014). DeepFace: Closing the gap to human-level performance in face verification. Proceedings of Computer Vision and Pattern Recognition Conference (CVPR 2014), Columbus.
Thompson, R., Gupta, S., Miller, K., Mills, S. & Orr, S. (2004). The effects of vasopressin on human facial responses related to social communication. Psychoneuroendocrinology, 29, 35–48.
Van der Gaag, C., Minderaa, R. B., & Keysers, C. (2007). Facial expressions: What the mirror neuron system can and cannot tell us. Social Neuroscience, 2 (3), 179–222.
Virkkunen, M., Rawlings, R., Tokola, R., Poland, R. E., Guidotti, A., Nemeroff, C., Bissette, G., Kalogeras, K., Karonen, S. L., & Linnoila, M. (1994). CSF biochemistries, glucose metabolism, and diurnal activity rhythms in alcoholic, violent offenders, fire setters, and healthy volunteers. Archive of General Psychiatry, 51, 20–27.
Vuilleumier, P., Armony, J. L., Driver, J., & Dolan, R. J. (2003). Distinct spatial frequency sensitivities for processing faces and emotional expressions. Nature Neuroscience, 6 (6), 624–631.
Wheeler, M. E., & Fiske, S. T. (2005). Controlling racial prejudice: Social-cognitive goals affect amygdala and stereotype activation. Psychological Science, 16, 56–63.
Williams, J.H.G., Whiten, A., Suddendorf, T., & Perrett, D. I. (2001). Imitation, mirror neurons and autism. Neuroscience and Biobehavioural Reviews, 25, 287–295.
Wilson-Mendenhall, C. D., Barrett, L. F., & Barsalou, L. W. (2013). Situating emotional experience. Frontiers in Human Neuroscience, 7 (164), 1–16.
Wolf, N. S., Gales, M. E., Shane, E., & Shane, M. (2001). The developmental trajectory from amodal perception to empathy and communication: The role of mirror neurons in this process. Psychoanalytic Inquiry, 21 (2), 94–112.
Xu, X., Zuo, X., Wang, X., & Han, S. (2009). Do you feel my pain? Racial group membership modulates empathic neural responses. The Journal of Neuroscience, 29, 8525–8529.
Zaki, J., Ochsner, K. N., Hanelin, H., Wager, T. D., & Mackey, S. (2007). Different circuits for different pain: Patterns of functional connectivity reveal distinct networks for processing pain in self and others. Social Neuroscience, 2, 276–291.


