A neurobiological perspective
Rachel Moseley, Markus Kiefer and Friedemann Pulvermüller
It is by them (our perceptions) also that we think.
(Epicurus in Cicero’s De Finibus, I. 6)
The role of sensory-motor representations for conceptual cognition in ancient philosophy and modern cognitive sciences
Belief in the influence of the physical body and its perceptuomotor systems on psychological and cognitive processes is historically promiscuous. The idea that thought is supported by imagery and modal/perceptual representations may be traced to the discourse of philosophers as far back as the pre-Christian era 300 BC (see Barsalou, 1999, 2008; Machery, 2007). Epicurus (341–270 BC) understood the automatic regeneration of perceptual images (those initially experienced as sensory input) as a fundamental process in human cognition. Jump forward two millennia, and the nature of human thought was still an issue of contention in the battle of rationalism and empiricism, with members of the latter camp arguing the essential sameness of “percepts” and “concepts.” Locke (1632–1704), Berkeley (1685–1753) and Hume (1711–1776) all emphasised the shaping of human thought by perceptual experience, but their remarks were to sink into obscurity.
In response to the total lack of discussion regarding internal imagery and representations endorsed by behaviourism, the cognitive revolution of the mid-20th century, alongside developments in computer technology, evoked a spate of theories that estranged cognition from sensory experience. Cognitivism describes a framework in which cognition involves the manipulation of abstract symbols according to formal, logical and combinatorial rules (Anderson, 2003). Though the external world is experienced through the perceptual systems, experiences of concept referents in the world (e.g., a furry animal, “cat”) are transformed into abstract, amodal symbols, the forms of which are disconnected from their external referents. At the centre of the framework is “an inner arena of disengaged representation, influenced by experience but governed by reasons” (Anderson, 2003, p. 93): amodal cognition divorced from the perceptual systems of the brain (Machery, 2007; Mahon & Caramazza, 2008).
Recent years have seen the emergence of “neo-empiricism” (Machery, 2007), a reimagining of many of the ideas above in the form of what we term “embodiment” or “grounded cognition” (Barsalou, 2008). The central tenets of such a framework include, first, the proposal that representations of concepts are grounded in the very sensory and motor systems through which we experience and interact with our environment. Second, they include the proposal that retrieval and manipulation of these concepts arises from a kind of simulation in which the perceptual or motor states (i.e., neural activation patterns) experienced during concept acquisition are partially reinstated. In other words, “re-enactments or simulations… provide the cognitive-level representations that support memory, language and thought” (Barsalou et al., 2003, p. 85), thus entirely bypassing the abstract level of representation favoured by cognitivists (Anderson, 2003). In his original thesis, Barsalou (1999) suggests that “perceptual states” (or “percepts”), which contain the neural “imprint” of physical/sensory input, are stored in long-term memory where they later act symbolically for that concept in mental manipulation – an idea bearing strong resemblance to previous philosophical viewpoints.
This framework has inspired an influx of research into psychological and cognitive processes, as exemplified by the diversity of this publication. However, we suggest that the major argument in favour of grounded cognition comes from neurobiology, which can explain why and how embodiment might arise within the mind and brain. In this chapter, we will lay out this thesis and underpin it with recent data from neuroimaging and neuropsychology.
“Embodiment” in the brain: The neural basis of conceptual representations
Whilst many current neurocognitive theories discuss the “function” of macroscopic brain parts, cognitive mechanisms are grounded in the interplay between groups of nerve cells or neurons (Pulvermüller, 1999; Palm et al., 2014). One neuromechanistic approach to cognition applies Hebb’s concept of distributed neuronal circuits or cell assemblies, networks of neurons linked by strong synaptic connections that establish them as discrete functional units. Although some cell assemblies may be genetically determined, their formation is thought to be primarily driven by correlated neuronal activation, that is, Hebb’s principle of associative learning:
Any two cells or systems of cells that are repeatedly active at the same time will tend to become “associated,” so that activity in one facilitates activity in the other.
(Hebb, 1949, p. 70)
Hebb’s law, namely that “cells that fire together, wire together,” postulates that synaptic communication between cells is altered by their correlated firing. This enhancement of neural communication is known as long-term potentiation (LTP). It is realised by the development of new dendritic spines and post-synaptic cell receptors (Malenka & Bear, 2004), such that the postsynaptic cell is more sensitive to signals from the presynaptic cell, increasing the efficiency of their neural transmission. These changes are further established when the correlated firing of cell groups is maintained but decline when activity between cells is no longer correlated (long-term depression [LTD]: “neurons out of sync delink”). Synaptic plasticity is such that throughout life, the brain (and, consequently, cognition) is constantly shaped by these two aspects of correlation learning. Because correlated activation can occur in different parts of the brain, and because strong fibre bundles connect distant regions, correlation learning can set up cell assemblies for cognitive processes that are distributed across widespread cortical areas.
Correlations between sensory and motor activations can explain how the arbitrary pairing of word/symbol knowledge and knowledge about actions/objects in the world is mapped in the mind and brain (Pulvermüller, 1999, 2012). During early linguistic development, such “word-world” learning tends to occur in the context of experiencing or interacting with the respective object, action or concept in the real world. A child will generally learn the word for an object when seeing it pointed out by an adult or when exploring its sensory features whilst they are described by a teacher (see Smith et al., 2014, for detailed analysis of this process). Likewise, children generally learn words denoting actions in the context of performing them or alternatively seeing others do so. This transparent labelling of physical objects and visible actions experienced through the perceptual systems is the means through which the majority of early linguistic symbols (words) acquire their meanings and is arguably a prerequisite for all conceptual learning. After a stock of such meaningful symbols has been acquired, further “indirect” semantic learning can be based on context, when novel word forms appear in meaningful contexts that yield reinstatements of previous sensorimotor experience. The necessity of symbol-grounding in actions and perceptions for meaningful language use is illustrated by the famous analogy of the Chinese Room (Searle, 1990): Though both humans and computers could learn to respond to Chinese characters and produce sensible Chinese output through the use of algorithms, programmes and books, neither could be said to understand Chinese if the symbols lack any connection to objects and actions in the real world. This was neatly summarised by Glenberg, et al. (2005):
[L]inguistic symbols… become meaningful only when they are mapped to non-linguistic experiences such as actions and perceptions… no matter how many symbols we look up, if they are only defined in terms of other symbols, the process will not generate any meaning.
(pp. 115–116)
Before semantic learning takes place, a child’s meaningless babble involves simultaneous hearing of the syllable it produces and the resultant coactivation of neurons in articulatory motor and auditory sensory cortex, and may thus drive the formation of distributed cell assemblies mapping knowledge about actions and perceptions. The neuroanatomical connection structure (or topology) of the relevant part of the cortex – the “perisylvian” inferior-frontal and superior-temporal areas – sets up a special type of sensorimotor cell assembly referred to elsewhere as an “action-perception circuit” (Pulvermüller & Fadiga, 2010). These articulatory-auditory circuits are a prerequisite for the repetition of words in the childhood babbling stage. Their formation through simultaneous auditory and motor coactivation has been modelled computationally (Garagnani et al., 2009). The critical predictions of the model, that motor cortex is activated during speech perception and furthermore influences language understanding, are confirmed by experimental evidence (Wilson et al., 2004; D’Ausilio et al., 2009).
These articulatory-auditory action-perception circuits are “semantically extended” due to neuronal correlation brought about by hearing a word and experiencing the referent object, whereby neural populations responsive in object perception (in ventral visual stream) are coactive and therefore incorporated into the circuits representing phonological and articulatory features of spoken word forms (in perisylvian cortex). Action-perception circuits with different cortical distributions may develop for words typically used to speak about different entities. The principle of correlation learning implies that sensory and motor circuits become infused with new properties through linkage with other cell populations and consequently become involved in new cognitive processes whilst retaining their original functional roles, a process called “neural exploitation” (Gallese & Lakoff, 2005) or “information mixing” (Braitenberg & Schüz, 1998). Information mixing by correlation learning also characterises one of the most revolutionary discoveries in contemporary neuroscience outside the language domain, that of mirror neurons involved in motor control of specific actions and equally responsive to sight and sounds of the same actions (Rizzolatti & Craighero, 2004). These cells, first observed to be active during both action execution and observation, are suggested to play a critical role in the representation of action goals and thus action and intention understanding (Rizzolatti & Sinigaglia, 2010). Recent claims about the functional irrelevance of mirror neurons (and, hence, the motor part of action perception circuits) for action and language understanding (Hickok, 2014) have been refuted by the already mentioned results showing that motor systems exert a causal role in language understanding (Schomers et al., 2014).
As a model, this approach is reminiscent of Allport’s sensorimotor semantic theory (1985), which argued that
the same neural elements that are involved in coding the sensory attributes of a (possibly unknown) object presented to eye or hand or ear also make up the elements of the auto-associated activity-patterns that represent familiar object-concepts in “semantic memory.”
(Allport, 1985, p. 53)
Crucially, however, the principles of correlation learning lend new and explanatory power to this view and that of other embodied theorists, revealing how and why this organisation arises. In summary, sensorimotor regions become intimately connected at the synaptic level to the brain regions supporting language and other cognitive operations through a process of associative learning. An account of embodied cognition realised in action-perception circuits makes essentially the same claims as those made by the empiricists and embodiment theorists described at the beginning of this chapter:
- (1) Words should activate the (sensorimotor) brain regions typically involved in experiencing or interacting with that referent in the world.
- (2) As they carry meaning within circuits, sensorimotor cortices should functionally contribute to conceptual cognition.
With the development of increasingly sensitive methods of observing the brain at work, a model of cognition grounded in neurobiology lends itself to hypothesis testing. Thus armed with neurobiological predictions generated by this model and the means for testing them, let us consider the empirical evidence for these two tenets of embodied cognition. We will also address the question of whether the neurobiological perspective offers new answers to old questions, especially those where the empiricist approach appeared to fail.
Concepts activate brain regions for action and perception
Electrophysiological and neurometabolic imaging has demonstrated that nouns describing visual objects activate occipitotemporal brain regions of the object recognition stream (Martin, 2007). Similarly, concepts requiring access to colourand form-related knowledge activate the regions involved in colour and shape perception (Martin et al., 1995; Chao & Martin, 1999; Moscoso Del Prado Martín et al., 2006; Simmons et al., 2007). What, however, of concepts associated with other sensory modalities, such as sound (“bell”), smell (“cinnamon”) or taste (“salt”)? Cutting-edge methodologies in neuroimaging have demonstrated that these, too, activate auditory (Goldberg et al., 2006; Kiefer et al., 2008, 2012); olfactory (González et al., 2006) and gustatory brain regions (Barrós-Loscertales et al., 2012), respectively. Even concepts associated with tactile information, such as “fur,” activate somatosensory regions of the postcentral gyrus; conscious recollection of a concept’s sensory properties evokes activation patterns reminiscent of actual experience with these concepts, just as predicted by theoretical accounts (Goldberg et al., 2006).
The well-established somatotopic organisation of the human motor system allows for great specificity in testing the above hypotheses. Here, again, the neural substrates for performing an action overlap substantially with those activated by mere exposure to action concepts as single words or embedded in sentences: concepts such as “kick” activate dorsal motor regions responsible for locomotion of the legs, whilst “pick” activates hand- and arm-related regions and “lick” activates the cortical motor map representing the tongue and lips (Hauk et al., 2004, 2008; Shtyrov et al., 2004; Pulvermüller et al., 2005; Tettamanti et al., 2005; Aziz-Zadeh & Damasio, 2008; Kana et al., 2012).
The rudimentary formation of such somatotopy for action words has been reported in children 4 to 6 years of age (James & Maouene, 2009), although recent work suggests that the brain manifestation of grounded meaning requires more time (Dekker et al., 2014). Some recent studies addressed the learning of new action words experimentally. Liuzzi et al. (2010) showed that transcranial direct current stimulation (tDCS) of left motor areas had a causal effect on subjects’ ability to learn novel action (but not object) words. Kiefer et al. (2007) showed that a meaningful sensory-motor interaction with a novel object is crucial for grounding action-related concepts and sensorimotor areas. Fargier et al. (2012) had their subjects learn new action words and found a neurophysiological sign of motor cortex involvement (suppressions of μ rhythm activity) for these words after learning; no such effect was seen for object words. These findings demonstrate a neural motor correlate of rapidly learning the semantic link between words and their action-related meanings, as postulated by the action-perception model and related embodiment frameworks. A further implication of embodiment ideas is that semantic mechanisms depend on the experience of the learner (Pulvermüller, 1999), also called the “body-specificity hypothesis” (Casasanto, 2009). This view is supported, for example, by recent work showing differential patterns of lateralisation of semantically related motor activation in left- and right-handers (Willems et al., 2010; Hauk & Pulvermuller, 2011).
Action words are not the only words to evoke activation of action-related knowledge in cortical motor systems. Many objects possess affordances (Gibson, 1977), latent “action possibilities” such as twisting a doorknob or drinking from a mug. Carota et al. (2012) demonstrated that the affordances of items with hand- or face-related uses were manifest in somatotopic motor activation, with tool words such as “fork” activating hand motor regions and food words such as “bread” activating face motor regions. The automatic activation of an object’s affordances on visual presentation of the item itself has been robustly demonstrated (see Fischer & Zwaan, 2008, for review) and interpreted in a context whereby “the motor system spontaneously uses object information to compute possible actions” (p. 831). The mechanical basis of this process might lie in “canonical” motor neurons with visual properties (Rizzolatti & Craighero, 2004), which respond to perception of objects (not to be confused with mirror neurons, motor neurons with visual properties that respond to action observation). The incorporation of these cells in the assembly representing a concept would mean their automatic ignition with the whole network, thus implicitly activating action-related affordance knowledge (Carota et al., 2012). Action priming studies have indeed shown that affordances activate these same frontoparietal mirror regions and facilitate object recognition and naming by modulating activity in ventral visual pathways (Kiefer et al., 2011; Sim et al., 2014) – thus demonstrating a functional role of these regions, as pertains to the discussion below.
As the evidence thus far pertains to embodiment of linguistic material, it is important to mention, briefly, the oppositional suggestion that the representation of visual words within visual systems and action words within motor systems is merely a function of the fact that most of the former are nouns and the latter verbs, which might be categorised differentially due to their grammatical category rather than their semantic association with sensorimotor systems (see Bedny et al., 2008; Mahon & Caramazza, 2008). Reviews (Vigliocco et al., 2011; Kemmerer et al., 2012) as well as recent empirical work with psycholinguistically matched, orthogonalised word categories (Moseley & Pulvermüller, 2014) speak against this interpretation, but in this context it is also important to note that concepts activate sensorimotor systems in nonlinguistic contexts, too. Mental visualisation and actual observation of objects activate occipitotemporal regions overlapping with those activated by object words (see Martin, 2007). Likewise, as noted above, hearing or watching another person’s actions activates motor cells in the observer, a process attributed to the mapping of action concepts (such as “to drink”) onto one’s own motor repertoire in the process of comprehension (Hauk et al., 2006; Rizzolatti & Sinigaglia, 2010). This process is also determined by individual experience, with activation of motor “mirror” systems reflecting the body-specific way that the observer typically achieves an action goal (i.e., drinking) even if they differ from the actor in the means they use to accomplish it (Gazzola et al., 2007).
A case in point: The embodiment of abstract concepts
Thus far, our discussion has been limited to the embodiment of concrete words used to speak about concrete entities, usually objects and actions. How, however, can an embodiment framework, in which sensorimotor regions act as the critical interface for interacting with the world, come to explain abstract words that lack a meaning correlate that could be experienced directly through the senses? It was initially suggested that concepts like “love,” “freedom” and “beauty” necessitate the existence of an amodal, “disembodied” processing system (Mahon & Caramazza, 2008). “Word-world” mapping is indeed insufficient to acquire the meaning of these abstract concepts.
Searle’s Chinese Room illustrates the absolute necessity of some kind of sensorimotor experience as the initial basis for meaning acquisition, but once an individual has acquired a basic stock of concepts through symbol-meaning mapping, there are other mechanisms of conceptual learning. “Word-word learning” describes a process through which words can be learnt through co-occurrence with other, previously learnt linguistic symbols and through defining each other (e.g., “big” to explain “large”) (Pulvermüller, 2012). Additionally, sensorimotor contexts may act as vehicles for relaying the meaning of abstract words when we learn them (Lakoff & Johnson, 1980; Barsalou, 1999, 2008; Lakoff & Nunez, 2000; Gallese & Lakoff, 2005). Lakoff and Johnson (1980), for example, note the association between the concepts of more, increase, and control and an upwards orientation (“My income rose last year” or “I am on top of the situation”), linking it with the perceptual observations that adding to a pile or substance makes it higher and that the victor in a fight will tower over the fallen loser. Behavioural studies would appear to support an association between sensations, actions, bodily states and a range of abstract concepts (Casasanto, 2009). Abstract concepts also activate sensorimotor brain regions (see Kiefer & Pulvermüller, 2012, and Moseley & Pulvermüller, 2014 for review). They might be conceptualised as concepts with covert sensorimotor associations, but the high variability in their usage (a beautiful sunset, flower, dance) leads to weak multiple links to the central features of these instantiations and thus prevents association with sensorimotor systems being as strong as that seen for concrete concepts (see Figure 5.1). In addition to the perceptual and action systems, abstract concepts may also be grounded in the emotional and introspective brain systems (Kiefer & Barsalou, 2013).
For another illustration of the semantic grounding of abstract concepts, we point to abstract emotion words such as “hope” and “fear,” which may be considered as “hidden action words.” Among his many contributions to language philosophy, Wittgenstein (1953) stated that children can only learn the meaning of a novel abstract emotion word if the teacher knows about the child’s inner states, and this is only possible when the child performs emotion-expressing actions such as smiling or crying. Based on this motor manifestation of the “internal” state, adults can teach children their first abstract emotion terms. This view predicts that abstract emotion words elicit action-related activity in the motor cortex along with emotion-related limbic system activation. Functional magnetic resonance imaging (fMRI) does indeed confirm that activity patterns to emotion words extend into the face and arm motor areas involved in affective behaviours and into limbic areas involved in actually experiencing these feelings (Moseley et al., 2012). Like other concepts, comprehension of emotion words is believed to require the partial or full simulation of brain activity associated with the referent emotions (Glenberg et al., 2005). A bold and imaginative study recruited women who had just received cosmetic Botox injections to temporarily paralyse the facial musculature for frowning (Havas et al., 2010). The women took significantly longer to read “angry” sentences (e.g., “The pushy telemarketer won’t let you return to your dinner”) and “sad” sentences (e.g., “You hold back your tears as you enter the funeral home”) than they did reading happy sentences about such topics as fun, achievement and love. Whilst the media overstated these findings with alarmist warnings that “Beware: Botox can lose you your friends” (Metro, 2010), the study bolstered the functional importance of activity in sensorimotor systems for cognitive processing, a topic discussed in the next section. We now return to explore the second central tenet of embodied cognition as realised in the action-perception circuit model.
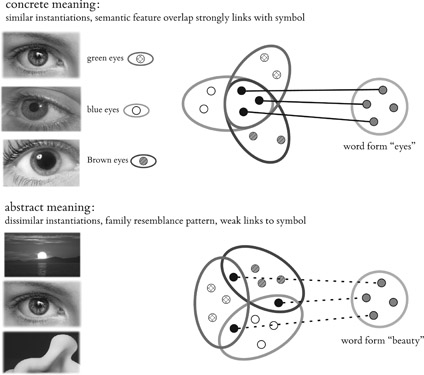
Figure 5.1
Concept grounding by correlation: mechanisms for concrete and abstract semantics. Both concrete and abstract words and constructions can be learnt when they are being used to speak about real-life events, actions and objects or their features. A major difference lies in the variability of the sensorimotor patterns fostering semantic grounding, which is typically low for concrete symbols and high for abstract ones, and results in different neural representation of each. Top panel: Concrete semantics. The concrete word “eye” is used to speak about objects with similar shapes and a range of colours, such that exemplar representations strongly overlap in their sensorimotor semantic feature neurons. The diagram schematically illustrates such sensorimotor semantic overlap (some of which may be carried by visual center-surround cells responding to a circle in one colour on a background of a different one) and feature neurons more specific to individual exemplars (e.g., to specific colour). In concrete semantic learning, neurons of the circuit overlap and frequently occurring, prototypical exemplars strongly interlink with the word form circuit due to high correlation of their activations. Bottom panel: Abstract semantics. The instantiations of abstract words such as “games” or “beauty” are quite variable, exhibiting a “family resemblance” pattern of partial semantic similarity (Wittgenstein, 1953). The diagram schematically shows the putative neural correlate of such family resemblance, where sensorimotor semantic feature neurons are only shared between subsets of exemplar representations of variable instantiations of the concept. The low correlation of activations of neuronal circuits for word forms and for each exemplar representation results in weak links between neural representations of sensorimotor knowledge (in modality-preferential areas) and those of verbal symbols (in the perisylvian cortex). Abstract semantic connections can draw upon partial overlap feature neurons (as shown) and indirect connections by way of neurons in the multimodal cortex that happen to link with several sensorimotor instantiations of an abstract meaning (not shown, see Pulvermüller, 2012).
As they carry meaning within action-perception circuits, sensorimotor cortices should functionally contribute to conceptual cognition
Whilst the literature above demonstrates the presence of sensorimotor activity when participants are exposed to concepts, an intrinsic tenet of embodied cognition is the functional importance of such activity to cognition. According to Barsalou (2008), “simulation provides a core form of computation in the brain” (pp. 618–619); simulations may not identically replicate brain activity as it occurs during perceptual and motor interaction with concepts in the world (Kiefer & Pulvermüller, 2012) but are “cut from the same cloth, running the same systems in a similar manner” (Prinz & Barsalou, 2000, p. 71) and would thus constitute conceptual retrieval. This position is consistent with the suggestion that meaning is represented in neuronal action-perception circuits – but do claims of functional importance for sensorimotor “simulations” hold empirical weight? We present, briefly, three strands of evidence for this argument:
- i) Conceptual activity in sensorimotor systems is instantaneous and automatic.
Recall Epicurus (341–270 BC). In a statement remarkably prescient of the claim above, he opined that “In this moment that the word ‘man’ is spoken, immediately due to the concept… an image is projected in the mind which is related to the sensory input data.” (Muljadi, 2011, p. 6). Much of the neurometabolic fMRI research described above cannot address this issue, as its slow temporal resolution renders it insensitive to the very earliest stages of cognition. Demonstrations of apparently “embodied” activity, for example for leg-, arm- and face-related words (Hauk et al., 2004), might therefore reflect conscious reflection on the meaning of these words (Mahon & Caramazza, 2008). That “doing” and “imagining” share a neural substrate (Goldberg et al., 2006) does not initially appear to present a problem for embodied accounts but implies that the actual retrieval of meaning for these items might be attributed to activity elsewhere in the brain. Sensorimotor activity after the retrieval of a word’s meaning could not be claimed to play an important role in this process.
Fortunately, electrophysiological methodologies such as electroencephalography (EEG) and magnetoencephalography (MEG) allow a window into preconscious processes. Semantically congruent sensorimotor activity for written action and object-related words is in fact evident 150 ms following word presentation (Moseley, Pulvermüller, et al., 2013), precluding the possibility of conscious imagery. Distinctions of even greater specificity in the motor system for spoken foot, mouth and hand words appear from 80 ms (Shtyrov et al., 2014). Sensorimotor semantic activity also occurs when participants are distracted from the stimuli (Shtyrov et al., 2010) and when words are briefly presented and masked so that they are not consciously perceived (Trumpp, Traub, et al., 2013; Trumpp et al., 2014). These findings cannot alone refute the possibility that this activity is auxiliary to semantic processing occurring elsewhere in the brain (Mahon & Caramazza, 2008) but do impressively rule out the possibility that sensory-motor activity during conceptual task simply reflects postconceptual imagery. We may nod to Epicurus after all these years.
- ii) Sensorimotor and cognitive processes are functionally intertwined.
Barsalou’s (1999) original thesis purports that conceptual processing “shares systems with perception at both the cognitive and the neural levels” (p. 577). The neurobiological cell assembly model, too, suggests that cognition, meaning and concepts are built from action and perception mechanisms embodied in action-perception circuits. If higher cognition is driven by sensorimotor neuroanatomical systems, we might predict interesting effects when the situation demands simultaneous cognition and movement/perception. Failure to “multi-task” effectively, in these contexts, lends strong plausibility to the philosophical inseparability of concepts from actions and percepts.
A bidirectional link between sensorimotor and semantic/linguistic systems reveals that these do indeed overlap substantially at the level of brain anatomy (Fischer & Zwaan, 2008; Rueschemeyer et al., 2009). Language processing substantially modulates the activity of cortical motor systems, facilitating or debilitating motor performance in a manner congruent to the semantic nature of the linguistic material being processed (Glenberg & Kaschak, 2003; Buccino et al., 2005), even when that linguistic material is subliminally presented (Boulenger, Silber, et al., 2008). Detailed analysis of stimulus features has revealed that it is indeed the action-semantic properties of words that modulate activity in motor areas, just as the imageability of words modulates activity in temporo-occipital object-processing stream (Hauk, Davis, et al., 2008). Importantly, there are also examples of this relationship in reverse, where modulation of the motor system in an effector-specific manner facilitates processing of words semantically related to actions of the same effector (Pulvermüller, Hauk, et al., 2005; Shebani & Pulvermüller, 2013). Motor interference equally appears during nonlinguistic perceptual processes, with this relationship also occurring in reverse (see Fischer & Zwaan, 2008, for review).
- iii) Sensorimotor damage impairs cognitive processing.
Perhaps the strongest evidence for a functional role of sensorimotor systems in cognition are the cognitive impairments which co-occur with disease, disorder or lesion of the sensorimotor systems. The original evidence refuting a single, unitary semantic system came from patients with a startlingly disproportionate loss of knowledge for one category over others: living things (Warrington & Shallice, 1984). A range of studies in aphasia have linked action word deficits to fronto-motor lesions and object word deficits to temporo-occipital lesions (Kemmerer et al., 2012). Reports of category-specific deficits come from a diverse assortment of other maladies, too: impairments for words evoking action knowledge in amytrophic lateral sclerosis (also known as motor neurone disease: Grossman et al., 2008; Bak & Chandran, 2012), fronto-temporal dementia (Cotelli et al., 2006) and Parkinson’s disease (Boulenger et al., 2008), impairments for sound-related words in a patient with a focal lesion to auditory association cortex (Trumpp, Kliese, et al., 2013). Even in cases of global semantic impairment such as semantic dementia, char-acterised by degeneration beginning in the anterior temporal lobe Hodges and Patterson (2007), a significant degree of category-specificity is evident: such patients exhibit the greatest deficits in the processing of face-related and colour-related words, which draw most heavily on the affected areas (Pulvermüller et al., 2010).
A case in point: “Disembodied” autism
The autism spectrum conditions (ASC) are heritable neurodevelopmental conditions most commonly associated with striking impairments in language and social interaction. Little scientific attention has been directed towards the motor impairments that are also a cardinal feature of ASC (Fournier et al., 2010; McCleery et al., 2013) and possess a neurological correlate in primary motor cortex (Mostofsky et al., 2007). Hypoactivity of the mirror neuron system is also a robust finding in this population (Rizzolatti & Fabbri-Destro, 2010).
Given the breadth of their abnormalities in regions involved in action understanding and “embodiment,” these individuals have been of particular interest in our laboratory. Combined EEG and MEG showed that typical embodiment of action and object concepts is significantly reduced in ASC, particularly for action words (Moseley, Pulvermüller, et al., 2014). This was corroborated with fMRI: We observed specific motor hypoactivity during action word processing which, critical in relation to the functional importance of such activity, correlated with a behavioural deficit for categorising the same words (Moseley, Mohr, et al., 2013). In accordance with our earlier finding of motor activity for emotion words (Moseley et al., 2012), the same population showed striking hypoactivity of motor and limbic systems for emotion words, too (Moseley, Shtyrov, et al., 2014). Though we did not test for a behavioural substrate of this brain abnormality, it is theoretically consistent with the particular problems that people with autism have with understanding emotion and mental state words (Baron-Cohen et al., 1994; Happé, 1994) and with the high prevalence of alexithymia, a difficulty using emotional language to converse and cogitate about emotions, in this population (Hill et al., 2004). This evidence, in turn, is consistent with a functional role for motor systems in emotion understanding as purported by other researchers (Glenberg et al., 2005; Havas et al., 2010).
Interestingly, motor hypoactivity during action word processing, evidence of dysfunction in these motor circuits, correlated not only with a linguistic processing deficit but with additional autistic symptoms relating to social interaction, imagination and behaviour (Moseley, Mohr, et al., 2013). That autistic traits would increase with more severe hypoactivity was an unexpected finding but strongly consistent with a role of sensorimotor systems in a range of cognitive systems. Although it is impossible to infer causality from the correlation that we observed, it is certainly relevant to speculate on the implications of motor dysfunction for other autistic symptoms. A fairly obvious link might be made, first, between mirror neuron deficits and ASC impairments in understanding actions, gestures and imitation (Blake et al., 2003; Dewey et al., 2007; Williams, 2008), processes all putatively associated with the functions of these cells.
More tentative links might be made between motor dysfunction and those most archetypal autistic impairments in social cognition, mentalising (thinking about mental states) and empathy (Baron-Cohen’s [1995] concept of autism as “mind-blindness”). The same author suggests that mentalising may involve “looking inward and projecting or simulating that other person as if we were them… using oneself as a proxy for understanding others.” (Lombardo & Baron-Cohen, 2010, p. 5). Preliminary evidence provides some support for this suggestion in so far that the same circuits, involving sensorimotor cortices, underlie mentalising about oneself and mentalising about others: These had previously been investigated as independent processes. Lombardo et al. (2010) asked participants to make mentalising judgements about either themselves or the British Queen. The primary motor and somatosensory cortices were activated regardless of whether participants were thinking about their own mental processes or those of the Queen, as were a group of other regions. The authors conclude that sensorimotor regions contribute to self/other understanding (as others since then have confirmed (Schippers et al., 2010; Spunt & Lieberman, 2012)), and go so far as to tentatively suggest, from the pattern of their findings, that “higher-level” inference processes might be grounded in “lower-level” sensorimotor simulation. Closely linked with mentalising is the ability to feel empathy for others, another process attributed to the mirror neuron system (Iacoboni, 2009) and robustly abnormal in autism. Sensorimotor systems are involved, at a more basic level, with “feeling another person’s pain,” but the motor systems of individuals with autism do not respond to observing another person in pain (Minio-Paluello et al., 2009).
At a more general level, the body is the brain’s vehicle for learning and experience, and motor development therefore occurs in strong synchrony with development in other domains (Iverson, 2010). The ability to efficiently move the body is followed by an explosion in the variety and number of interactions with people and objects – it follows clearly that those who are unable to do so might be put at significant disadvantage in their linguistic and semantic development. It may thus occur that a fundamental dysfunction in motor systems in ASC derails development in other domains, though causal evidence of this relationship is needed to support this interpretation.
The explanatory power of neurobiological approaches to embodied cognition: Current achievements and future research directions
As a theory realising the principles of embodiment in brain physiology, how well do the findings of empirical neuroscience support the role of action-perception circuits in cognition? We have observed, above, that the model can convincingly ground in brain science the central tenets of embodiment, that of the 1) involvement of and 2) functional importance of sensorimotor systems in cognition. The evidence reviewed above would appear to support both claims, and the theory possesses clear parsimony and economical practicality. There is no need for an amodal processing system for concrete or abstract concepts, no need for any schism between “the arena of symbolic processing and the external world of meaning and action” (Anderson, 2003, pp. 93–94). Since action schemas, for example, are inherently stored in motor systems, abstract models of semantic representation would require the duplication of these schemas (plus any necessary links to motor and premotor cortex to execute them) in another system entirely (Gallese & Lakoff, 2005). There is clearly an element of Ockham’s Razor in embodied models and action-perception theory, which purports the storage of semantic representations in the modal systems (the re-enactment of “perceptual states”) through which we experience these concepts in the world. Rather than simply explaining the existence of category-specificity in the brain, action-perception theory provides precise explanations as to why, developmentally, certain brain regions come to store the meaning of certain types of word. That the Hebbian learning principles purported have been successfully modelled at a computational level (Garagnani et al., 2009) and demonstrated in the human brain in a range of empirical investigations, including learning studies (Kiefer et al., 2007; Liuzzi et al., 2010; Fargier et al., 2012), underpin their relevance in semantic learning.
Realistically, it is probable that any model that is exclusively modal (simulative) or amodal (symbolic) in nature will fail to explain the whole range of neurometabolic, neurophysiological and patient data (Meteyard et al., 2012). The neuroscientific literature certainly supports a functional role in cognition for cell assemblies containing sensorimotor neurons, but it is notable that in the studies discussed above, many patients with motor disorder are still capable of processing these words to some degree (Boulenger et al., 2008; Moseley, Mohr, et al., 2013). Likewise, in the highly celebrated Botox study, women were slower to process angry sentences but still capable of doing so (Havas et al., 2010). Different tasks may require different levels of sensorimotor involvement in semantic processing, and so the influence of task demands on sensorimotor involvement in semantic processing must be investigated (Hauk & Tschentscher, 2013); the varied tasks used in studies on patient populations may not always reveal semantic impairments. In the same vein, context and pragmatics modulate the involvement of sensorimotor systems in conceptual processing (Hoenig et al., 2008; Egorova et al., 2013) and this is not yet fully understood, particularly as it pertains to abstract concepts (Tomasino et al., 2014).
Over and above addressing the question whether meaning is indeed grounded or “embodied,” empirical attention must now turn to the degree to which meaning is embodied (Hauk & Tschentscher, 2013; Pulvermüller, 2013), how situational and task demands might modulate this and the role that other brain regions play in cognition. Distributed circuits reaching into sensorimotor areas appear to carry meaning, but their activation depends on priming, context-induced input and previous neurocognitive states. The grounding of meaning in action perception circuits also implies that it is inadequate to postulate that modality preferential regions such as the premotor cortex are the unique seat of semantic processing. Areas directly linked to more than one sensory or motor area such as the dorso-lateral prefrontal cortex, angular gyrus and temporal pole, may play an important role in understanding the most abstract of concepts and, more generally, as convergence areas receiving sensory and motor activation. These areas cannot meaningfully be labelled as “amodal semantic hubs” because, rather than being “amodal,” they carry converging information from multiple sensorimotor domains (Simmons & Barsalou, 2003; Patterson et al., 2007; Pulvermüller et al., 2010). The involvement of these regions does not refute the importance and role of sensorimotor regions for semantic processing; indeed, such multimodal convergence hubs may be of functional importance for the retrieval of stored concepts or as semantic “power stations” necessary for powering though not necessarily processing per se (Kiefer & Pulvermüller, 2012). The wide distribution of these circuits may, however, explain why small lesions in either sensory or motor or local multimodal areas have but a small effect on semantic processing.
Awaiting further investigation on these issues, we refer back, finally, to Glen-berg et al. (2005), who suggest that “simulating those (emotional) states is a prerequisite for complete and facile understanding of the language about those states” (p. 120, emphasis added). In that it is possible to understand actions that one cannot oneself perform, mirror neuron theorists have also discriminated between “shallow recognition,” which might, for example, allow a nonmusician to recognise that an individual is playing an instrument, from “understanding from the inside,” informed by the observer’s own experiential motor repertoire (Rizzolatti & Sinigaglia, 2010). Musicians and nonmusicians do certainly differ in mirror neuron response during observation (Molnar-Szakacs & Overy, 2006), but a difference in the depth of one’s action understanding may be difficult to capture quantitatively in tasks like those of Boulenger et al. (2008) and Moseley et al. (2013). Nonetheless, we would conclude from the studies above that, in conjunction with other brain regions, sensorimotor involvement in cognition certainly appears to be functionally important, indeed necessary, for optimal performance on a range of cognitive tasks. The evidence rebuts the cognitivist casting of these brain regions in auxiliary roles and instead demonstrates, in line with action-perception theory and the embodiment framework, that early, automatic activation of cell assemblies involving sensorimotor regions plays a functional role in the conceptual retrieval that underlies cognitive processes.
References
Allport, D. A. (1985). Distributed memory, modular subsystems and dysphasia. In S. Newman & R. Epstein (Eds.), Current Perspectives in Dysphasia (pp. 32–60). Edinburgh: Churchill Livingstone.
Anderson, M. L. (2003). Embodied cognition: A field guide. Artificial Intelligence, 149, 91–130.
Aziz-Zadeh, L., & Damasio, A. R. (2008). Embodied semantics for actions: Findings from functional brain imaging. Journal of Physiology, Paris, 102, 35–39.
Bak, T. H., & Chandran, S. (2012). What wires together dies together: Verbs, actions and neurodegeneration in motor neuron disease. Cortex. 48, 936–944.
Baron-Cohen, S. (1995). Mindblindness: An essay on autism and theory of mind. Cambridge, MA: MIT Press.
Baron-Cohen, S., Ring, H., Moriarty, J., Schmitz, B., Costa, D., & Ell, P. (1994). Recognition of mental state terms: Clinical findings in children with autism and a functional neuroimaging study of normal adults. British Journal of Psychiatry, 165, 640–649.
Barrós-Loscertales, A., González, J., Pulvermüller, F., Ventura-Campos, N., Bustamante, J. C., Costumero, V., Parcet, M. A., & Ávila, C. (2012). Reading salt activates gustatory brain regions: FMRI evidence for semantic grounding in a novel sensory modality. Cerebral Cortex, 22, 2554–2563.
Barsalou, L. W. (1999). Perceptual symbol systems. Behavioral Brain Science, 22, 577–660.
Barsalou, L. W. (2008). Grounded cognition. Annual Review of Psychology, 59, 617–645.
Barsalou, L. W., Simmons, W. K., Barbey, A. K., & Wilson, C. D. (2003). Grounding conceptual knowledge in modality-specific systems. Trends in Cognitive Sciences, 7(2), 84–91.
Bedny, M., Caramazza, A., Grossman, E., Pascual-Leone, A., & Saxe, R. (2008). Concepts are more than percepts: The case of action verbs. Journal of Neuroscience, 28, 11347–11353.
Blake, R., Turner, L. M., Smoski, M. J., Pozdol, S. L., & Stone, W. L. (2003). Visual recognition of biological motion is impaired in children with autism. Psychological Science, 14, 151–157.
“Botox can lose you your friends.” (2010). Metro. 11 April.
Boulenger, V., Mechtouff, L., Thobois, S., Broussolle, E., Jeannerod, M., & Nazir, T. A. (2008). Word processing in Parkinson’s disease is impaired for action verbs but not for concrete nouns. Neuropsychologia, 46, 743–756.
Boulenger, V., Silber, B. Y., Roy, A. C., Paulignan, Y., Jeannerod, M., & Nazir, T. A. (2008). Subliminal display of action words interferes with motor planning: A combined EEG and kinematic study. Journal of Physiology, Paris, 102, 130–136.
Braitenberg, V., & Schüz, A. (1998). Cortex: Statistics and geometry of neuronal connectivity (2 ed.). Berlin: Springer.
Carota, F., Moseley, R., & Pulvermüller, F. (2012). Body-part-specific representations of semantic noun categories. Journal of Cognitive Neuroscience, 24, 1492–1509.
Casasanto, D. (2009). Embodiment of abstract concepts: Good and bad in right- and left-handers. Journal of Experimental Psychology: General, 138, 351–367.
Chao, L. L., & Martin, A. (1999). Cortical regions associated with perceiving, naming, and knowing about colors. Journal of Cognitive Neuroscience, 11, 25–35.
Cotelli, M., Borroni, B., Manenti, R., Alberici, A., Calabria, M., Agosti, C., Arévalo, A., Ginex, V., Ortelli, P., Binetti, G., Zanetti, O., Padovani, A., & Cappa, S. F. (2006). Action and object naming in frontotemporal dementia, progressive supranuclear palsy, and corticobasal degeneration. Neuropsychology, 20, 558–565.
D’Ausilio, A., Pulvermüller, F., Salmas, P., Bufalari, I., Begliomini, C., & Fadiga, L. (2009). The motor somatotopy of speech perception. Current Biology, 19, 381–385.
Dekker, T. M., Mareschal, D., Johnson, M. H., & Sereno, M. I. (2014). Picturing words? Sensorimotor cortex activation for printed words in child and adult readers. Brain and Language, 139, 58–67.
Dewey, D., Cantell, M., & Crawford, S. G. (2007). Motor and gestural performance in children with autism spectrum disorders, developmental coordination disorder, and/or attention deficit hyperactivity disorder. Journal of the International Neuropsychological Society, 13, 246–256.
Egorova, N., Shtyrov, Y., & Pulvermüller, F. (2013). Early and parallel processing of pragmatic and semantic information in speech acts: Neurophysiological evidence. Frontiers in Human Neuroscience, 7, 86.
Fargier, R., Paulignan, Y., Boulenger, V., Monaghan, P., Reboul, A., & Nazir, T. A. (2012). Learning to associate novel words with motor actions: Language-induced motor activity following short training. Cortex, 48, 888–899.
Fischer, M. H., & Zwaan, R. A. (2008). Embodied language: A review of the role of the motorsystem in language comprehension. Quarterly Journal of Experimental Psychology, 61, 825–850.
Fournier, K. A., Hass, C. J., Naik, S. K., Lodha, N., & Cauraugh, J. H. (2010). Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. Journal of Autism Development Disorders, 40, 1227–1240.
Gallese, V., & Lakoff, G. (2005). The brain’s concepts: The role of the sensory-motor system in conceptual knowledge. Cognitive Neuropsychology, 22, 455–479.
Garagnani, M., Wennekers, T., & Pulvermüller, F. (2009). Recruitment and consolidation of cell assemblies for words by way of hebbian learning and competition in a multi-layer neural network. Cognitive Computation, 1, 160–176.
Gazzola, V., van der Worp, H., Mulder, T., Wicker, B., Rizzolatti, G., & Keysers, C. (2007). Aplasics born without hands mirror the goal of hand actions with their feet. Current Biology, 17, 1235–1240.
Gibson, J. J. (1977). The theory of affordances. In R. Shaw & J. Bransford (Eds.), Perceiving, acting, and knowing: Toward an ecological psychology (p. 127–142). Hillsdale, NJ: Erlbaum.
Glenberg, A. M., Havas, D., Becker, R., & Rinck, M. (2005). Grounding language in bodily states: The case for emotion. In D. Pecher & R. A. Zwaan (Eds.), The grounding of cognition: The role of perception and action in memory, language, and thinking (pp. 1–17). Cambridge: Cambridge University Press.
Glenberg, A. M., & Kaschak, M. P. (2003). The body’s contribution to language. Psychol Learn Motiv – Adv Res Theory, 43, 93–126.
Goldberg, R. F., Perfetti, C. A., & Schneider, W. (2006). Perceptual knowledge retrieval activates sensory brain regions. Journal of Neuroscience, 26, 4917–4921.
González, J., Barrós-Loscertales, A., Pulvermüller, F., Meseguer, V., Sanjuán, A., Belloch, V., & Ávila, C. (2006). Reading cinnamon activates olfactory brain regions. Neuroimage, 32, 906–912.
Grossman, M., Anderson, C., Khan, A., Avants, B., Elman, L., & McCluskey, L. (2008). Impaired action knowledge in amyotrophic lateral sclerosis. Neurology, 71, 1396–1401.
Happé, F. G. (1994). An advanced test of theory of mind: Understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Developmental Disorders, 24, 129–154.
Hauk, O., Davis, M. H., & Pulvermüller, F. (2008). Modulation of brain activity by multiple lexical and word form variables in visual word recognition: A parametric fMRI study. Neuroimage, 42, 1185–1195.
Hauk, O., Johnsrude, I., & Pulvermüller, F. (2004). Somatotopic representation of action words in human motor and premotor cortex. Neuron, 41, 301–307.
Hauk, O., & Pulvermüller, F. (2011). The lateralization of motor cortex activation to action-words. Frontiers in Human Neuroscience, 29, 5, 149.
Hauk, O., Shtyrov, Y., & Pulvermüller, F. (2006). The sound of actions as reflected by mismatch negativity: Rapid activation of cortical sensory-motor networks by sounds associated with finger and tongue movements. European Journal of Neuroscience, 23, 811–821.
Hauk, O., Shtyrov, Y., & Pulvermüller, F. (2008). The time course of action and action-word comprehension in the human brain as revealed by neurophysiology. Journal of Physiology, Paris, 102, 50–58.
Hauk, O., & Tschentscher, N. (2013). The body of evidence: What can neuroscience tell us about embodied semantics? Frontiers in Psychology, 4, 50.
Havas, D. A., Glenberg, A. M., Gutowski, K. A., Lucarelli, M. J., & Davidson, R. J. (2010). Cosmetic use of botulinum toxin-a affects processing of emotional language. Psychological Science, 21, 895–900.
Hebb, D. O. (1949). The organization of behaviour. New York, NY: Wiley and Sons.
Hickok, G. (2014). Towards an integrated psycholinguistic, neurolinguistic, and sensorimotor framework for speech production. Language, Cognition, and Neuroscience, 29, 52–59.
Hill, E., Berthoz, S., & Frith, U. (2004). Brief report: Cognitive processing of own emotions in individuals with autistic spectrum disorder and in their relatives. Journal of Autism Development Disorders, 34, 229–235.
Hodges, J. R., & Patterson, K. (2007). Semantic dementia: a unique clinicopathological syndrome. The Lancet Neurology, 6(11), 1004–1014.
Hoenig, K., Sim, E.-J., Bochev, V., Herrnberger, B., & Kiefer, M. (2008). Conceptual flexibility in the human brain: Dynamic recruitment of semantic maps from visual, motor, and motion-related areas. Journal of Cognitive Neuroscience, 20, 1799–1814.
Iacoboni, M. (2009). Imitation, empathy, and mirror neurons. Annual Review of Psychology, 60, 653–670.
Iverson, J. M. (2010). Developing language in a developing body: The relationship between motor development and language development. Journal of Child Language, 37, 229–261.
James, K. H, & Maouene, J. (2009). Auditory verb perception recruits motor systems in the developing brain: An fMRI investigation. Developmental Science, 12, 6, F26–34.
Kana, R. K, Blum, E. R., Ladden, S. L, & Ver Hoef, L. W. (2012). “How to do things with words”: Role of motor cortex in semantic representation of action words. Neuropsychologia, 50, 3403–3409.
Kemmerer, D., Rudrauf, D., Manzel, K., & Tranel, D. (2012). Behavioral patterns and lesion sites associated with impaired processing of lexical and conceptual knowledge of actions. Cortex, 48, 826–848.
Kiefer, M., & Barsalou, L. W. (2013). Grounding the human conceptual system in perception, action, and internal states. In W. Prinz, M. Beisert, & A. Herwig (Eds.), Action science: Foundations of an emerging discipline (pp. 381–407). Cambridge: MIT Press.
Kiefer, M., & Pulvermüller, F. (2012). Conceptual representations in mind and brain: Theoretical developments, current evidence and future directions. Cortex, 48, 805–825.
Kiefer, M., Sim, E.-J., Helbig, H., & Graf, M. (2011). Tracking the time course of action priming on object recognition: Evidence for fast and slow influences of action on perception. Journal of Cognitive Neuroscience, 23, 1864–1874.
Kiefer, M., Sim, E.-J., Herrnberger, B., Grothe, J., Hoenig, K. (2008). The sound of concepts: Four markers for a link between auditory and conceptual brain systems. Journal of Neuroscience, 28, 12224–12230.
Kiefer, M., Sim, E.-J., Liebich, S., Hauk, O., Tanaka, J. (2007). Experience-dependent plasticity of conceptual representations in human sensory-motor areas. Journal of Cognitive Neuroscience, 19, 525–542.
Kiefer, M., Trumpp, N., Herrnberger, B., Sim, E. J., Hoenig, K., Pulvermüller, F. (2012). Dissociating the representation of action- and sound-related concepts in middle temporal cortex. Brain and Language, 122, 120–125.
Lakoff, G., & Johnson, M. (1980). Conceptual metaphor in everyday language. Journal of Philosophy, 77, 453–486.
Lakoff, G., & Nunez, R. (2000). Where mathematics comes from: How the embodied mind brings math into being. Santa Fe, NM: Basic Books.
Liuzzi, G., Freundlieb, N., Ridder, V., Hoppe, J., Heise, K., Zimerman, M., Dobel, C., Enriquez-Geppert, S., Gerloff, C., Zwitserlood, P., & Hummel, F. C. (2010). The involvement of the left motor cortex in learning of a novel action word lexicon. Current Biology, 20, 1745–1751.
Lombardo, M. V., & Baron-Cohen, S. (2010). Unraveling the paradox of the autistic self. Wiley Interdisciplinary Reviews: Cognitive Science, 1, 3, 393–403.
Lombardo, M. V, Chakrabarti, B., Bullmore, E. T., Wheelwright, S. J., Sadek, S. A., Suckling, J., & Baron-Cohen, S. (2010). Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience, 22, 1623–1635.
McCleery, J. P., Elliott, N. A., Sampanis, D. S., & Stefanidou, C. A. (2013). Motor development and motor resonance difficulties in autism: relevance to early intervention for language and communication skills. Frontiers in integrative neuroscience, 7.
Machery, E. (2007). Concept empiricism: A methodological critique. Cognition, 104, 19–46.
Mahon, B. Z, & Caramazza, A. (2008). A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. Journal of Physiology, Paris, 102, 59–70.
Malenka, R. C., & Bear, M. F. (2004). LTP and LTD: An embarrassment of riches. Neuron, 44(1), 5–21.
Martin, A. (2007). The representation of object concepts in the brain. Annual Review of Psychology, 58, 25–45.
Martin, A., Haxby, J. V., Lalonde, F. M., Wiggs, C. L., & Ungerleider, L. G. (1995). Discrete cortical regions associated with knowledge of color and knowledge of action. Science, 270, 102–105.
Meteyard, L., Cuadrado, S. R., Bahrami, B., & Vigliocco, G. (2012). Coming of age: A review of embodiment and the neuroscience of semantics. Cortex, 48, 788–804.
Minio-Paluello, I., Baron-Cohen, S., Avenanti, A., Walsh, V., & Aglioti, S. M. (2009). Absence of embodied empathy during pain observation in asperger syndrome. Biological Psychiatry, 65, 55–62.
Molnar-Szakacs, I., & Overy, K. (2006). Music and mirror neurons: From motion to “e”motion. Socio Cognitive Affective Neuroscience, 1, 235–241.
Moscoso Del Prado Martín, F., Hauk, O., & Pulvermüller, F. (2006). Category specificity in the processing of color-related and form-related words: An ERP study. Neuroimage, 29, 29–37.
Moseley, R., Carota, F., Hauk, O., Mohr, B., & Pulvermüller, F. (2012). A role for the motor system in binding abstract emotional meaning. Cerebral Cortex., 22, 1634–1647.
Moseley, R. L., Mohr, B., Lombardo, M. V., Baron-Cohen, S., Hauk, O., Pulvermüller, F. (2013). Brain and behavioral correlates of action semantic deficits in autism. Frontiers in Human Neuroscience, 7, 725.
Moseley, R. L., & Pulvermüller, F. (2014). Nouns, verbs, objects, actions, and abstractions: Local fMRI activity indexes semantics, not lexical categories. Brain and Language, 132, 28–42.
Moseley, R. L., Pulvermüller, F., Mohr, B., Lombardo, M. V., Baron-Cohen, S., Shtyrov, Y. (2014). Brain routes for reading in adults with and without autism: EMEG evidence. Journal of Autism Development Disorders, 44, 137–153.
Moseley, R. L., Pulvermüller, F., & Shtyrov, Y. (2013). Sensorimotor semantics on the spot: Brain activity dissociates between conceptual categories within 150 ms. Science Reports, 3, 1928.
Moseley, R. L., Shtyrov, Y., Mohr, B., Lombardo, M. V., Baron-Cohen, S., & Pulvermüller, F. (2014). Lost for emotion words: What motor and limbic brain activity reveals about autism and semantic theory. Neuroimage, 104, 413–422.
Mostofsky, S. H., Burgess, M. P., & Gidley Larson, J. C. (2007). Increased motor cortex white matter volume predicts motor impairment in autism. Brain, 130, 2117–2122.
Muljadi, P. (2011). Epicureanism: The complete guide. Retrieved from http://books.google.co.uk/books/about/Epicureanism.html?id=dVwamXYyIqcC (Accessed February 3, 2015).
Palm, G., Knoblauch, A., Hauser, F., & Schüz, A. (2014). Cell assemblies in the cerebral cortex. Biological Cybernetics, 108(5), 559–572.
Patterson, K., Nestor, P. J., & Rogers, T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience, 8, 976–987.
Prinz, J. J., & Barsalou, L. W. (2000). Steering a course for embodied representation. In E. Dietrich & A. Markman (Eds.), Cognitive dynamics: Conceptual and representational change in humans and machines (pp. 51–77). Cambridge MA: MIT Press.
Pulvermüller, F. (1999). Words in the brain’s language. Behavioral Brain Science, 22, 253–279; discussion, 280–336.
Pulvermüller, F. (2012). Meaning and the brain: The neurosemantics of referential, interactive, and combinatorial knowledge. Journal of Neurolinguistics., 25, 423–459.
Pulvermüller, F. (2013). How neurons make meaning: Brain mechanisms for embodied and abstract-symbolic semantics. Trends in Cognitive Science, 17 (9), 458–470.
Pulvermüller, F., Cooper-Pye, E., Dine, C., Hauk, O., Nestor, P. J., Patterson, K. (2010). The word processing deficit in semantic dementia: All categories are equal, but some categories are more equal than others. Journal of Cognitive Neuroscience, 22, 2027–2041.
Pulvermüller, F., & Fadiga, L. (2010). Active perception: Sensorimotor circuits as a cortical basis for language. Nature Reviews Neuroscience, 11, 351–360.
Pulvermüller, F., Hauk, O., Nikulin, V. V., & Ilmoniemi, R. J. (2005). Functional links between motor and language systems. European Journal of Neuroscience, 21, 793–797.
Pulvermüller, F., Shtyrov, Y., & Ilmoniemi, R. (2005). Brain signatures of meaning access in action word recognition. Journal of Cognitive Neuroscience, 17 (6), 884–892.
Rizzolatti, G., & Craighero, L. (2004). The mirror-neuron system. Annual Review of Neuroscience, 27, 169–192.
Rizzolatti, G., & Fabbri-Destro, M. (2010). Mirror neurons: From discovery to autism. Experimental Brain Research, 200 (3–4), 223–237.
Rizzolatti, G., & Sinigaglia, C. (2010). The functional role of the parieto-frontal mirror circuit: Interpretations and misinterpretations. Nature Reviews Neuroscience. 11, 264–274.
Rueschemeyer, S. A., Lindermann, O., Van Elk, M., & Bekkering, H. (2009). Embodied cognition: The interplay between automatic resonance and selection-for-action mechanisms. European Journal of Social Psychology, 39, 1180–1187.
Schippers, M. B., Roebroeck, A., Renken, R., Nanetti, L., & Keysers, C. (2010). Mapping the information flow from one brain to another during gestural communication. Proceedings of the National Academy of Science U S A, 107, 9388–9393.
Schomers, M. R, Kirilina, E., Weigand, A., Bajbouj, M., & Pulvermüller, F. (2014). Causal influence of articulatory motor cortex on comprehending single spoken words: TMS evidence. Cerebral Cortex. doi:10.1093/cercor/bhu274/
Searle, J. R. (1990). Is the brain’s mind a computer program? Scientific American, 262, 26–31.
Shebani, Z., & Pulvermüller, F. (2013). Moving the hands and feet specifically impairs working memory for arm- and leg-related action words. Cortex, 49(1), 222–231.
Shtyrov, Y., Butorina, A., Nikolaeva, A., & Stroganova, T. (2014). Automatic ultrarapid activation and inhibition of cortical motor systems in spoken word comprehension. Proceedings of the National Academy of Science U S A, 111, 1918–1923.
Shtyrov, Y., Hauk, O., Pulvermüller, F. (2004). Distributed neuronal networks for encoding category-specific semantic information: The mismatch negativity to action words. European Journal of Neuroscience, 19, 1083–1092.
Shtyrov, Y., Kujala, T., Pulvermüller, F. (2010). Interactions between language and attention systems: Early automatic lexical processing? Journal of Cognitive Neuroscience, 22, 1465–1478.
Sim, E.-J., Helbig, H. B., Graf, M., & Kiefer, M. (2014). When action observation facilitates visual perception: Activation in visuo-motor areas contributes to object recognition. Cerebral Cortex.
Simmons, W. K., Barsalou, L. W. (2003). The similarity-in-topography principle: Reconciling theories of conceptual deficits. Cognitive Neuropsychology, 20, 451–486.
Simmons, W. K., Ramjee, V., Beauchamp, M. S., McRae, K., Martin, A., Barsalou, L. W. (2007). A common neural substrate for perceiving and knowing about color. Neuropsychologia, 45, 2802–2810.
Smith, L. B., Suanda, S. H., & Yu, C. (2014). The unrealized promise of infant statistical word–referent learning. TICS, 18, 251–258.
Spunt, R. P., Lieberman, M. D. (2012). Dissociating modality-specific and supramodal neural systems for action understanding. Journal of Neuroscience.
Tettamanti, M., Buccino, G., Saccuman, M. C., Gallese, V., Danna, M., Scifo, P., Fazio, F., Rizzolatti, G., Cappa, S. F., Perani, D. (2005). Listening to action-related sentences activates fronto-parietal motor circuits. Journal of Cognitive Neuroscience, 17, 273–281.
Tomasino, B., Fabbro, F., & Brambilla, P. (2014). How do conceptual representations interact with processing demands: An fMRI study on action- and abstract-related words. Brain Research, 1591(3), 38–52.
Trumpp, N. M., Kliese, D., Hoenig, K., Haarmeier, T., & Kiefer, M. (2013). Losing the sound of concepts: Damage to auditory association cortex impairs the processing of sound-related concepts. Cortex, 49, 474–486.
Trumpp, N. M., Traub, F., & Kiefer, M. (2013). Masked priming of conceptual features reveals differential brain activation during unconscious access to conceptual action and sound information. PLoS One, 8.
Trumpp, N. M, Traub, F., Pulvermüller, F., & Kiefer, M. (2014). Unconscious automatic brain activation of acoustic and action-related conceptual features during masked repetition priming. Journal of Cognitive Neuroscience, 26, 352–364.
Vigliocco, G., Vinson, D. P., Druks, J., Barber, H., Cappa, S. F. (2011). Nouns and verbs in the brain: A review of behavioural, electrophysiological, neuropsychological and imaging studies. Neuroscience Biobehavioral Reviews, 35(3), 407–426.
Warrington, E. K., & Shallice, T. (1984). Category-specific semantic impairments. Brain, 107, 829–854.
Willems, R. M., Peelen, M. V., Hagoort, P. (2010). Cerebral lateralization of face-selective and body-selective visual areas depends on handedness. Cerebral Cortex, 20, 1719–1725.
Williams, J.H.G. (2008). Self-other relations in social development and autism: Multiple roles for mirror neurons and other brain bases. Autism Research, 1, 73–90.
Wilson, S. M., Saygin, A. P., Sereno, M. I., Iacoboni, M. (2004). Listening to speech activates motor areas involved in speech production. Nature Neuroscience, 7, 701–702.
Wittgenstein, L. (1953). Philosophical investigations. London, UK: Basil and Blackwell.
