Infectious Complications of Hematopoietic Stem Cell Transplantation
Anna R. Huppler
Hematopoietic stem cell transplantation (HSCT) recipients experience a transient but profound state of immune deficiency. The risk of infection depends on the stage after transplantation (pre- vs postengraftment), ongoing immunosuppression, disruption in barrier functions (indwelling catheters, graft-versus-host disease [GVHD], mucositis) and preexisting infections (Fig. 164.1 ). Management approaches may include the use of prophylactic antimicrobials, preemptive antimicrobials for infection prior to symptomatic disease, or antimicrobial treatment of documented or suspected infection.
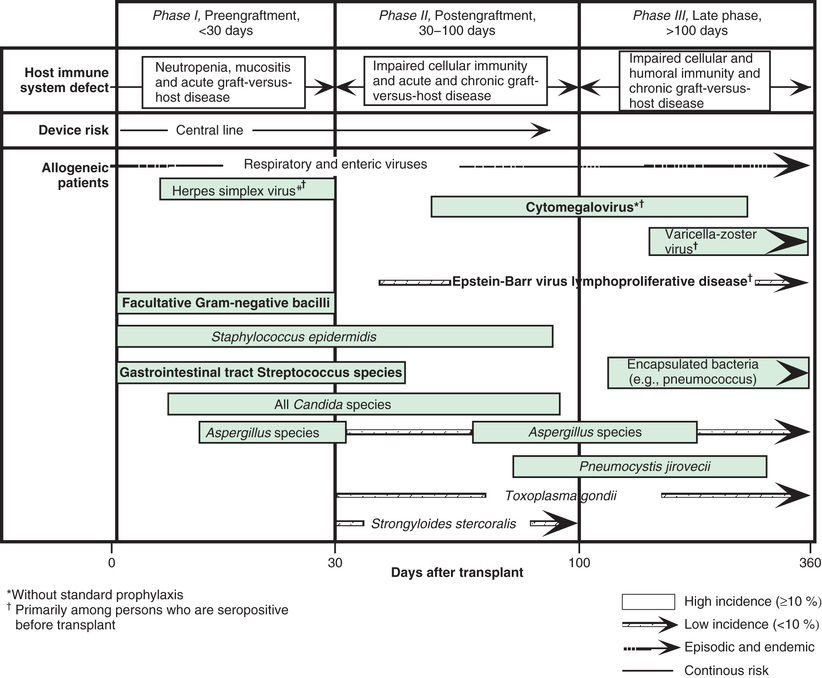
Immediately after transplantation, the absence or paucity of neutrophils (neutropenia ) renders patients susceptible to bacterial and fungal infections. Consequently, most centers start antipseudomonal and antifungal prophylaxis during the conditioning regimen. Despite these prophylactic measures, the majority of patients will develop fever and signs of infection in the early posttransplantation period. The common pathogens include enteric gram-negative bacteria and fungi. An indwelling central venous line, routinely employed in all children given HSCT, is a significant risk factor for infection. Staphylococcal species and Candida are the most frequent pathogens in catheter-related infections (see Chapter 206 ). Multidrug-resistant strains of Pseudomonas aeruginosa and Klebsiella pneumoniae are an emerging problem, with prevalence highly variable among centers. Severe lower respiratory tract disease caused by seasonal respiratory viruses, such as influenza, respiratory syncytial virus (RSV), parainfluenza virus, and human metapneumovirus, can occur during the pre- or postengraftment phase. Published guidelines from the Infectious Disease Society of America and the U.S. Centers for Disease Control and Prevention (CDC) address the management of fever and neutropenia after HSCT.
HSCT recipients remain at increased risk of developing severe infections even after the neutrophil count has normalized because of prolonged depression in T-cell number and function. The manifestations of GVHD, as well as the associated immunosuppressive therapy, are additional risk factors for fungal and viral opportunistic infections. After umbilical cord blood transplant (UCBT), infections are the consequence of both slow neutrophil engraftment and donor T-cell naïveté. In haploidentical transplantation, T-cell depletion results in an increased risk of infection in the first 4-6 mo. Recipients of this type of transplantation, as well as those receiving UBCT, do not have the benefit of adoptive transfer of donor-derived, antigen-experienced T cells. For HSCT recipients after engraftment, invasive fungal disease, herpesviruses, and adenovirus infections represent life-threatening complications that significantly affect outcomes. Additional pathogens to consider include nontuberculous mycobacteria, BK virus, Clostridium difficile, and norovirus.
Invasive fungal disease (IFD) remains a significant cause of infectious morbidity and mortality in allogeneic HSCT recipients. Empirical treatment for IFD is considered for HSCT patients with persistent fever despite 96 hr of broad-spectrum antibiotic treatment. The most common organisms are Aspergillus and Candida species. Infections also occur with non-Aspergillus molds, including Mucor and Rhizopus species (among other agents of mucormycosis), Fusarium, and Scedosporium species. Pneumocystis jiroveci is a unique, noncultivatable cause of fungal pneumonia in immunocompromised patients. Despite prompt and aggressive administration of potent antifungal agents, proven cases of IFD carry case fatality rates of 20–70%. IFD can present early after transplant, although there is a shift toward presentation of infection in the postengraftment period in the presence of GVHD. The risk of developing IFD is mainly influenced by history of previous fungal infection, duration of neutropenia, use of corticosteroid therapy, mucosal tissue damage (GVHD, posttransplant CMV infection, viral respiratory tract infections), and for candidiasis, presence of central venous catheters.
Disseminated candidiasis presents frequently as a central venous catheter–associated infection. However, up to 50% of patients with disseminated candidiasis do not present with positive blood cultures. Patients with and without candidemia can have infection of normally sterile organs, including liver, spleen, kidney, brain, heart, and eye. Mortality rates in pediatric series range from 10–25%. Echinocandins (micafungin, caspofungin) are the initial drugs of choice for candidiasis in immunocompromised patients.
The most common presentation of invasive aspergillosis is pulmonary aspergillosis. The upper airway mucosa (nose and sinuses) can also be a site of initial infection. Infection progresses from lung or sinus sites by direct extension across tissue or angioinvasion resulting in hematogenous dissemination to brain and other organs. The earliest imaging finding is classically 1 or more small pulmonary nodules (Figs. 164.2 and 164.3 ). As a nodule enlarges, the dense central core of infarcted tissue may become surrounded by edema or hemorrhage, forming a hazy rim known as the halo sign. When bone marrow function recovers, the infarcted central core may cavitate, creating the crescent sign. Unfortunately, radiographic signs, including the halo sign, crescent sign, and cavitation, have low sensitivity in pediatric patients. Clinical criteria are used to diagnose proven or probable IFD, requiring direct or indirect microbiologic data. Direct, culture-based diagnosis requires invasive procedures, such as sinus endoscopy or lung biopsy. Indirect measures, known as fungal biomarkers, are used in adult HSCT patients to screen or diagnose for aspergillosis. Fewer data are available for pediatric patients, and currently no major guidelines support routine use of fungal biomarkers to diagnose IFD in immunocompromised children. Galactomannan from serum or bronchoalveolar lavage fluid is a promising adjunct to current diagnostic strategies because of a high negative predictive value for aspergillosis; however, lack of detection of mucormycosis limits its utility as a single diagnostic test. Other tests used in adult patients, such as (1→3)-β-D -glucan, are insufficiently studied for routine use in pediatric patients.
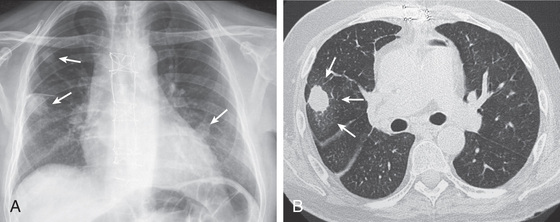
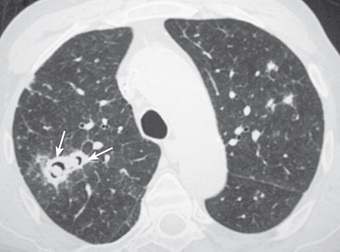
Fungal infection prevention includes isolation of the patient in a laminar airflow or positive pressure room. Universal prophylaxis to prevent Pneumocystis pneumonia is advocated until the return of T-cell function in HSCT patients; the primary agent for prophylaxis is trimethoprim/sulfamethoxazole. Alternative agents are pentamidine, dapsone, and atovaquone. For prevention and treatment of other IFD, liposomal amphotericin B, azole compounds (itraconazole, voriconazole, posaconazole) and echinocandins (caspofungin, micafungin) are used. Voriconazole represents the treatment of choice for adult patients with invasive aspergillosis, but achieving adequate trough levels can be challenging in young children. The agents of mucormycosis are resistant to most azole and echinocandin medications, which makes liposomal amphotericin B the initial drug of choice. IFD often does not respond satisfactorily to antifungal agents alone, and infection may persist until immune function recovers.
Herpesviruses , including cytomegalovirus (CMV), Epstein-Barr virus (EBV), herpes simplex virus (HSV1 and HSV2), and varicella-zoster virus (VZV) are pathogens that can cause significant disease after HSCT. Because herpesviruses can establish latency in the human host, symptomatic infection can occur from viral reactivation as well as acquisition from the donor or de novo infection. Baseline susceptibility to disease and viremia before symptom development can be established with laboratory monitoring (pretransplant donor-recipient serology, posttransplant viral load monitoring) and can inform decisions on prophylactic and preemptive antiviral treatment.
CMV infection remains the most common and potentially severe viral complication in patients receiving allogeneic HSCT. Risk factors for CMV viremia include recipient seropositivity, UCBT, and acute GVHD. The period of maximal risk for CMV disease is 1-4 mo after transplantation. Late presentation of CMV disease is associated with GVHD. Until CMV-specific T-cell responses develop months after transplant, CMV infection may result in a variety of syndromes, including fever, leukopenia, thrombocytopenia, hepatitis, pneumonitis, retinitis, esophagitis, gastritis, and colitis. CMV pneumonia has been reported to occur in up to 15–20% of bone marrow transplant recipients, with a case fatality rate of 85% in the absence of early treatment. Tachypnea, hypoxia, and nonproductive cough signal respiratory involvement. Chest radiography often reveals bilateral interstitial or reticulonodular infiltrates, which begin in the periphery of the lower lobes and spread centrally and superiorly. Gastrointestinal CMV involvement may lead to ulcers of the esophagus, stomach, small intestine, and colon with complications of bleeding or perforation. Fatal CMV infections are often associated with persistent viremia and multiorgan involvement.
CMV disease has largely been prevented through prophylaxis or preemptive approaches. Prophylaxis is based on administration of antiviral drugs to at-risk transplanted patients for a median duration of 3 mo after transplantation. The major drawbacks of this approach are drug toxicity, late CMV disease after withdrawal of prophylaxis, potential unnecessary treatment of patients who would not have reactivated CMV infection, and low cost-effectiveness. Preemptive therapy aims at treating only patients who experience CMV reactivation and thus are at risk of developing overt disease; it starts on detection of CMV in blood but before symptom development. The major drawback of this strategy is the need of serial monitoring of CMV by polymerase chain reaction (PCR) in blood. First-line therapy is usually ganciclovir, with foscarnet as an alternative for resistant strains or ganciclovir intolerance.
EBV-related posttransplant lymphoproliferative disease (PTLD) is a major complication in HSCT and solid-organ transplantation. In patients receiving HSCT, selective procedures of T-cell depletion–sparing B lymphocytes and use of HLA–partially matched family and unrelated donors are risk factors for the development of PTLD. PTLD usually presents in the first 4-6 mo after transplantation as high-grade, diffuse, large-cell B-cell lymphomas that are oligoclonal or monoclonal. High EBV viral loads in blood by PCR predict development of PTLD. Standard treatment of PTLD includes the reduction of immunosuppression, monoclonal antibodies directed against CD20 on B cells (rituximab), or cytotoxic chemotherapy. Prophylactic strategies with rituximab for EBV-positive recipients during conditioning for HSCT have also been employed. Histologic diagnosis of PTLD is required to assess for the emergence of neoplasms in which cells are CD19+ but CD20− , thus eliminating susceptibility to rituximab.
Disseminated adenovirus infection is a life-threatening complication of HSCT recipients. Clinical manifestations include fever, hepatitis, enteritis, meningoencephalitis, and pneumonia. Young children or recipients of donor cells naïve to adenovirus (T-cell–depleted grafts or UCBT) are at particular risk of developing this complication. Diagnosis is based on the demonstration of high viral loads by PCR in blood or recovery of virus in tissue biopsies. Pharmacologic treatment of adenovirus infections is with the antiviral cidofovir, which has significant renal toxicity and limited potency at controlling viral replication. Alternative delivery systems for this drug, such as enterally available prodrugs, are currently being investigated in research settings. Recovery of immune system function is associated with improved survival with disseminated adenovirus infection.
In immunocompromised hosts, severe viral infections, including PTLD and adenovirus infection, originate from a deficiency of virus-specific cytotoxic T lymphocytes (CTLs). This finding provides the rationale for developing strategies of adoptive cell therapy to restore virus-specific immune competence. Multiple protocols are under development and available at some centers for the rapid generation of specific CTL lines of donor or third-party origin.