Kawasaki Disease
Mary Beth F. Son, Jane W. Newburger
Kawasaki disease (KD), formerly known as mucocutaneous lymph node syndrome and infantile polyarteritis nodosa , is an acute febrile illness of childhood seen worldwide, with the highest incidence occurring in Asian children. KD is a systemic inflammatory disorder manifesting as a vasculitis with a predilection for the coronary arteries. Approximately 20–25% of untreated children develop coronary artery abnormalities (CAA) including aneurysms, whereas <5% of children treated with intravenous immune globulin (IVIG) develop CAA. Nonetheless, KD is the leading cause of acquired heart disease in children in most developed countries, including the United States and Japan.
Etiology
The cause of KD remains unknown. Certain epidemiologic and clinical features support an infectious origin, including the young age-group affected, epidemics with wavelike geographic spread of illness, the self-limited nature of the acute febrile illness, and the clinical features of fever, rash, enanthem, conjunctival injection, and cervical lymphadenopathy. Further evidence of an infectious trigger includes the infrequent occurrence of the illness in infants <3 mo old, possibly the result of maternal antibodies, and the rarity of cases in adults, possibly the result of prior exposures with subsequent immunity. However, there are features that are not consistent with an infectious origin. For example, it is unusual to have multiple cases present at the same time within a family or daycare center. Furthermore, no single infectious etiologic agent has been successfully identified, despite a comprehensive search.
A genetic role in the pathogenesis of KD seems likely, as evidenced by the higher risk of KD in Asian children regardless of country of residence and in siblings and children of individuals with a history of KD. Furthermore, linkage studies and genome-wide association studies (GWAS) have identified significant potential associations between polymorphisms in the ITPKC gene, a T-cell regulator, with increased susceptibility to KD and more severe disease. Other candidate genes for KD identified by GWAS include CASP3, BLK, and FCGR2A. Lastly, associations of single nucleotide polymorphisms (SNPs) in the human leukocyte antigen class II region (HLA-DQB2 and HLA-DOB) with KD have been reported. The concordance rate among identical twins, however, is approximately 13%.
Epidemiology
For the majority of patients, KD is a disease of early childhood, and nearly all epidemiologic studies show a higher susceptibility to KD in boys. Data from the Kids Inpatient Database to study trends in KD hospitalizations in 2003, 2006, 2009, and 2012 reported that U.S. hospitalizations for KD seemed to decline significantly over the study period, with 6.68 per 100,000 children hospitalized for KD in 2006 vs 6.11 per 100,000 in 2012. Children age <5 yr had the highest annual hospitalization rates, and children of Asian and Pacific Islander ancestry had the highest rates among all racial groups. In other countries, such as the United Kingdom, South Korea, and Japan, the rate of KD seems to be increasing.
In Japan, nationwide surveys have been administered every 2 yr to monitor trends in KD incidence. In 2012 the highest recorded rate thus far of 264.8 per 100,000 children ages 0-4 yr was described, with the highest rate in young children ages 9-11 mo. Fortunately, the proportion of Japanese patients with coronary aneurysm and myocardial infarction has decreased over time, at 2.8% in the most recent survey.
Several risk stratification models have been constructed to determine which patients with KD are at highest risk for CAA. Predictors of poor outcome across several studies include young age, male gender, persistent fever, poor response to IVIG, and laboratory abnormalities, including neutrophilia, thrombocytopenia, transaminitis, hyponatremia, hypoalbuminemia, elevated levels of N-terminal–brain natriuretic protein and elevated C-reactive protein (CRP) levels. Asian and Pacific Islander race and Hispanic ethnicity are also risk factors for CAA. Three specific risk scores have been constructed by Japanese researchers; of these, the Kobayashi score is the most widely used and has high sensitivity and specificity. Unfortunately, application of these risk scores in non-Japanese populations does not appear to accurately identify all children at risk for IVIG resistance and CAA. Body surface area (BSA)–adjusted coronary artery dimensions on baseline echocardiography in the 1st 10 days of illness appear to be good predictors of involvement during follow-up. Accordingly, baseline z scores may provide a useful imaging biomarker.
Pathology
KD is a vasculitis that predominantly affects the medium-size arteries. The coronary arteries are most often involved, although other arteries (e.g., axillary, subclavian, femoral, popliteal, brachial) can also develop dilation. A 3-phase process to the arteriopathy of KD has been described. The 1st phase is a neutrophilic necrotizing arteritis occurring in the 1st 2 wk of illness that begins in the endothelium and moves through the coronary wall. Saccular aneurysms may form from this arteritis. The 2nd phase is a subacute/chronic vasculitis driven by lymphocytes, plasma cells, and eosinophils, which may last weeks to years and results in fusiform aneurysms. The vessels affected by the subacute/chronic vasculitis then develop smooth muscle cell myofibroblasts, which cause progressive stenosis in the 3rd phase. Thrombi may form in the lumen and obstruct blood flow (Fig. 191.1 ).

Clinical Manifestations
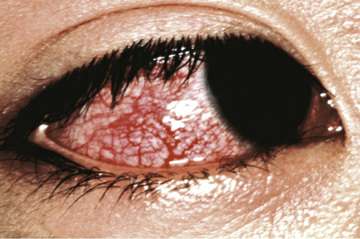
Fever is characteristically high spiking (≥38.3°C [101°F]), remitting, and unresponsive to antipyretics. The duration of fever without treatment is generally 1-2 wk but may be as short as 5 days or may persist for 3-4 wk. In addition to fever, the 5 principal clinical criteria of KD are (1) bilateral nonexudative conjunctival injection with limbal sparing; (2) erythema of the oral and pharyngeal mucosa with strawberry tongue and red, cracked lips; (3) edema (induration) and erythema of the hands and feet; (4) rash of various forms (maculopapular, erythema multiforme, scarlatiniform or less often psoriatic-like, urticarial or micropustular); and (5) nonsuppurative cervical lymphadenopathy, usually unilateral, with node size >1.5 cm (Table 191.1 and Figs. 191.2 to 191.5 ). Perineal desquamation is common in the acute phase. Periungual desquamation of the fingers and toes begins 2-3 wk after the onset of illness and may progress to involve the entire hand and foot (Fig. 191.6 ).
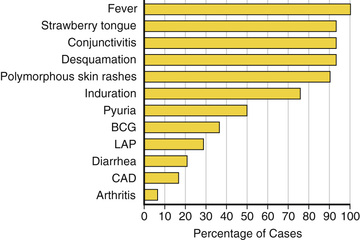



Symptoms other than the principal clinical criteria are common in the 10 days before diagnosis of KD, which may be explained in part by the finding that up to a third of patients with KD have confirmed, concurrent infections. Gastrointestinal (GI) symptoms (vomiting, diarrhea, or abdominal pain) occur in >60% of patients, and at least 1 respiratory symptom (rhinorrhea or cough) occurs in 35%. Other clinical findings include significant irritability that is especially prominent in infants and likely caused by aseptic meningitis, mild hepatitis, hydrops of the gallbladder, urethritis and meatitis with sterile pyuria, and arthritis. Arthritis may occur early in the illness or may develop in the 2nd or 3rd wk. Small or large joints may be affected, and the arthralgias may persist for several weeks. Clinical features that are not consistent with KD include exudative conjunctivitis, exudative pharyngitis, generalized lymphadenopathy, discrete oral lesions (ulceration or exudative pharyngitis), splenomegaly, and bullous, petechial, or vesicular rashes.
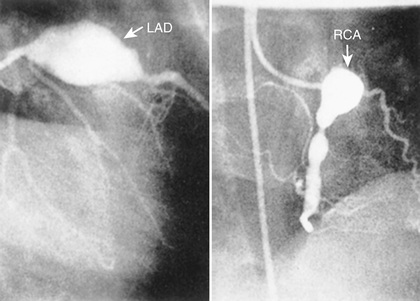
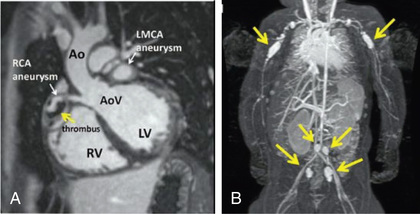
Cardiac involvement is the most important manifestation of KD. Myocarditis occurs in most patients with acute KD and manifests as tachycardia disproportionate to fever, along with diminished left ventricular systolic function. Occasionally, patients with KD present in cardiogenic shock (KD shock syndrome ), with greatly diminished left ventricular function. Case series of KD shock syndrome indicate that these patients may be at higher risk for coronary artery dilation. Pericarditis with a small pericardial effusion can also occur during the acute illness. Mitral regurgitation of at least mild severity is evident on echocardiography in 10–25% of patients at presentation but diminishes over time, except among rare patients with coronary aneurysms and ischemic heart disease. Up to 25% of untreated patients develop CAA in the 2nd to 3rd week of illness; initially these are usually asymptomatic and detected by echocardiography. Almost all the morbidity and mortality in KD occur in patients with large or giant coronary artery aneurysms , defined by the 2017 American Heart Association (AHA) scientific statement on the diagnosis and treatment of KD as having a z score ≥10 or an absolute dimension of ≥8 mm. Specifically, large or giant aneurysms are associated with the greatest risk of thrombosis or stenosis, angina, and myocardial infarction (Figs. 191.7 and 191.8A ). Rupture of a giant aneurysm is a rare complication that generally occurs in the 1st months after illness onset and may present as hemopericardium with tamponade. Axillary, popliteal, iliac, or other arteries may also become aneurysmal, but always in the setting of giant coronary aneurysms (Fig. 191.8B ).
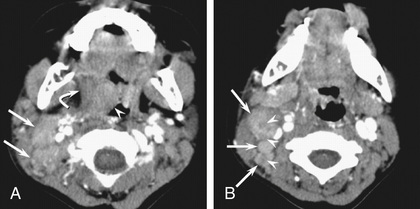
Occasionally KD initially presents with only fever and lymphadenopathy (node-first KD ). This presentation may be confused with bacterial or viral cervical lymphadenopathy or lymphadenitis and may delay the diagnosis of KD. Persistence of high fever, lack of response to antibiotics, and subsequent development of other signs of KD suggest the diagnosis. Children with node-first KD tend to be older (4 vs 2 yr) and have more days of fever and higher CRP levels. In addition to cervical adenopathy, many had retropharyngeal and peritonsillar inflammation on CT scans (Fig. 191.9 ).
KD can be divided into 3 clinical phases. The acute febrile phase is characterized by fever and the other acute signs of illness and usually lasts 1-2 wk. The subacute phase is associated with desquamation, thrombocytosis, development of CAA, and the highest risk of sudden death in patients who develop aneurysms; it generally lasts 3 wk. The convalescent phase begins when all clinical signs of illness have disappeared and continues until the erythrocyte sedimentation rate (ESR) returns to normal, typically 6-8 wk after the onset of illness.
Laboratory and Radiology Findings
There is no diagnostic test for KD, but patients usually have characteristic laboratory findings. The leukocyte count is often elevated, with a predominance of neutrophils and immature forms. Normocytic, normochromic anemia is common. The platelet count is generally normal in the 1st wk of illness and rapidly increases by the 2nd to 3rd wk of illness, sometimes exceeding 1 million/mm3 . An elevated ESR or CRP value is universally present in the acute phase of illness. The ESR may remain elevated for weeks, in part from the effect of IVIG. Sterile pyuria, mild elevations of the hepatic transaminases, hyperbilirubinemia, and cerebrospinal fluid pleocytosis may also be present. KD is unlikely if the ESR, CRP, and platelet counts are normal after 7 days of fever.
Two-dimensional echocardiography is the most useful test to monitor for development of CAA. Although frank aneurysms are rarely detected in the 1st week of illness, coronary arteries are commonly ectatic. Moreover, coronary artery dimensions, adjusted for BSA (z scores), may be increased in the 1st 5 wk after presentation, and as previously noted, baseline z scores may offer prognostic information regarding ultimate coronary artery dimensions. Children with non-KD febrile illnesses also have mildly increased z scores compared with nonfebrile controls, but not to the same degree as patients with KD. Aneurysms have been defined with use of absolute dimensions by the Japanese Ministry of Health and are classified as small (≤4 mm internal diameter [ID]), medium (>4 to ≤8 mm ID), or giant (>8 mm ID). Some experts believe that a z -score–based system for classification of aneurysm size may be more discriminating, because it adjusts the coronary dimension for BSA. The AHA z -score classification system is as follows:
- 1. No involvement: always <2
- 2. Dilation only: 2 to <2.5; or if initially <2, a decrease in z score during follow-up ≥1
- 3. Small aneurysm: ≥2.5 to <5
- 4. Medium aneurysm: ≥5 to <10, and absolute dimension <8 mm
- 5. Large or giant aneurysm: ≥10, or absolute dimension ≥8 mm
Echocardiography should be performed at diagnosis and again after 1-2 wk of illness. If the results are normal, a repeat study should be performed 6-8 wk after onset of illness. If results of either of the initial studies are abnormal or the patient has recurrent fever or symptoms, more frequent echocardiography or other studies may be necessary. In patients without CCA at any time during the illness, echocardiography and a lipid profile are recommended 1 yr later. After this time, periodic evaluation for preventive cardiology counseling is warranted, and some experts recommend cardiologic follow-up every 5 yr. For patients with CAA, the type of testing and the frequency of cardiology follow-up visits are tailored to the patient's coronary status.
Diagnosis
The diagnosis of KD is based on the presence of characteristic clinical signs. For classic KD the diagnostic criteria require the presence of fever for at least 4 days and at least 4 of 5 of the other principal characteristics of the illness (see Table 191.1 ). The diagnosis of KD should be made within 10 days, and ideally within 7 days, of fever onset to improve coronary artery outcomes. In atypical or incomplete KD , patients have persistent fever but <4 of the 5 characteristic clinical signs. In these patients, laboratory and echocardiographic data can assist in the diagnosis (Fig. 191.10 ). Incomplete cases occur most frequently in infants, who also have the highest likelihood of development of CAA. Ambiguous cases should be referred to a center with experience in the diagnosis of KD. Establishing the diagnosis with prompt institution of treatment is essential to prevent potentially devastating coronary artery disease. For this reason, it is recommended that any infant age ≤6 mo with fever for ≥7 days without explanation undergo echocardiography to assess the coronary arteries.

Differential Diagnosis
Adenovirus, measles, and scarlet fever lead the list of common childhood infections that mimic KD (Table 191.2 ). Children with adenovirus typically have exudative pharyngitis and exudative conjunctivitis, allowing differentiation from KD. A common clinical problem is the differentiation of scarlet fever from KD in a child who is a group A streptococcal carrier. Patients with scarlet fever typically have a rapid clinical response to appropriate antibiotic therapy. Such treatment for 24-48 hr with clinical reassessment generally clarifies the diagnosis. Furthermore, ocular findings are quite rare in group A streptococcal pharyngitis and may assist in the diagnosis of KD.
Features of measles that distinguish it from KD include exudative conjunctivitis, Koplik spots, rash that begins on the face and hairline and behind the ears, and leukopenia. Cervical lymphadenitis can be the initial diagnosis in children who are ultimately recognized to have KD. Less common infections such as Rocky Mountain spotted fever and leptospirosis are occasionally confused with KD. Rocky Mountain spotted fever is a potentially lethal bacterial infection, and appropriate antibiotics should not be withheld if the diagnosis is under consideration. Its distinguishing features include pronounced myalgias and headache at onset, centripedal rash, and petechiae on the palms and soles. Leptospirosis can also be an illness of considerable severity. Risk factors include exposure to water contaminated with urine from infected animals. The classic description of leptospirosis is of a biphasic illness with a few asymptomatic days between an initial period of fever and headache and a late phase with renal and hepatic failure. In contrast, patients with KD have consecutive days of fever at diagnosis and rarely have renal or hepatic failure.
Children with KD and pronounced myocarditis may demonstrate hypotension with a clinical picture similar to that of toxic shock syndrome. Features of toxic shock syndrome that are not usually seen in KD include renal insufficiency, coagulopathy, pancytopenia, and myositis. Drug hypersensitivity reactions, including Stevens-Johnson syndrome, share some characteristics with KD. Drug reaction features such as the presence of periorbital edema, oral ulcerations, and a normal or minimally elevated ESR are not seen in KD. Systemic-onset juvenile idiopathic arthritis (sJIA) is also characterized by fever and rash, but physical findings include diffuse lymphadenopathy and hepatosplenomegaly. Arthritis is required to develop at some point in the disease course to make the diagnosis, but may not be present in the 1st few wk of illness. Laboratory findings may include coagulopathy, elevated fibrin degradation product values, and hyperferritinemia. Interestingly, there are reports of children with sJIA who have echocardiographic evidence of CAA. Coronary aneurysms have also been reported in Behçet disease, primary cytomegalovirus infection, and meningococcemia.
Treatment
Patients with acute KD should be treated with 2 g/kg of IVIG as a single infusion, usually administered over 10-12 hr within 10 days of disease onset, and ideally as soon as possible after diagnosis (Table 191.3 ). In addition, moderate (30-50 mg/kg/day divided every 6 hr) to high-dose aspirin (80-100 mg/kg/day divided every 6 hr) should be administered until the patient is afebrile, then lowered to antiplatelet doses. Other NSAIDs should not be given during therapy with aspirin because they may block the action of aspirin. The mechanism of action of IVIG in KD is unknown, but treatment results in defervescence and resolution of clinical signs of illness in approximately 85% of patients. The prevalence of coronary disease, in 20–25% in children treated with aspirin alone, is <5% in those treated with IVIG and aspirin within the 1st 10 days of illness. Strong consideration should be given to treating patients with persistent fever, abnormal dimensions of the coronary arteries, or signs of systemic inflammation who are diagnosed after the 10th day of fever. The dose of aspirin is usually decreased from antiinflammatory to antithrombotic doses (3-5 mg/kg/day as a single dose) after the patient has been afebrile for 48 hr. Aspirin is continued for its antithrombotic effect until 6-8 wk after illness onset and is then discontinued in patients who have had normal echocardiography findings throughout the course of their illness. Patients with CAA continue with aspirin therapy and may require anticoagulation, depending on the degree of coronary dilation (see later).
Corticosteroids have been used as primary therapy with the 1st dose of IVIG in hopes of improving coronary outcomes. A North American trial using a single pulse dose of intravenous methylprednisolone (30 mg/kg) with IVIG as primary therapy did not improve coronary outcomes. However, a trial in Japan utilizing the Kobayashi score to identify high-risk children demonstrated improved coronary outcomes with a regimen of prednisolone (2 mg/kg) plus IVIG as primary therapy. Furthermore, a systematic review and meta-analysis of 16 comparative studies demonstrated that early treatment with corticosteroids improved coronary artery outcomes in children with KD. Despite these promising results, administration of corticosteroids as primary therapy to all children with KD awaits the development of a risk score that identifies high-risk children in a multiracial population.
IVIG-resistant KD occurs in approximately 15% of patients and is defined by persistent or recrudescent fever 36 hr after completion of the initial IVIG infusion. Patients with IVIG resistance are at increased risk for CAA. Therapeutic options for the child with IVIG resistance include a 2nd dose of IVIG (2 g/kg), a tapering course of corticosteroids, and/or infliximab (Table 191.4 ). For the most severely affected patients with enlarging coronary aneurysms, additional therapies such as cyclosporine or cyclophosphamide may be administered, with consultation from specialists in pediatric rheumatology and cardiology.
Table 191.4
Treatment Options for IVIG-Resistant Patients With Kawasaki Disease*
| AGENT | DESCRIPTION | DOSE |
|---|---|---|
| MOST FREQUENTLY ADMINISTERED | ||
| IVIG: 2nd infusion | Pooled polyclonal IG | 2 g/kg IV |
| IVIG + prednisolone | IVIG + corticosteroid | IVIG: 2 g/kg IV + prednisolone 2 mg⋅kg−1 ⋅d−1 IV divided every 8 hr until afebrile, then prednisone orally until CRP normalized, then taper over 2-3 wk |
| Infliximab | Monoclonal antibody against TNF-α | Single infusion: 5 mg/kg IV given over 2 hr |
| ALTERNATIVE TREATMENTS | ||
| Cyclosporine | Inhibitor of calcineurin-NFAT pathway |
IV: 3 mg⋅kg−1 ⋅d−1 divided every 12 hr PO: 4-8 mg⋅kg−1 ⋅d−1 divided every 12 hr Adjust dose to achieve trough 50-150 ng/mL; 2 hr peak level 300-600 ng/mL |
| Anakinra | Recombinant IL-1β receptor antagonist | 2-6 mg⋅kg−1 ⋅d−1 given by subcutaneous injection |
| Cyclophosphamide | Alkylating agent blocks DNA replication | 2 mg⋅kg−1 ⋅d−1 IV |
| Plasma exchange | Replaces plasma with albumin | Not applicable |
* IVIG resistance is defined as persistent or recrudescent fever at least 36 hours and <7 days after completion of 1st IVIG infusion. The top 3 treatments have been most frequently used, although no comparative effectiveness trial has been performed. Pulsed high-dose corticosteroid treatment is not recommended. The alternative treatments have been used in a limited number of patients with KD.
CRP, C-reactive protein; IG, immunoglobulin; IL, interleukin; IV, intravenous(ly); IVIG, intravenous immune globulin; NFAT, nuclear factor of activated T cells; PO, oral; TNF, tumor necrosis factor.
Complications
Patients with KD and aneurysms may experience myocardial infarction, angina, and sudden death. For this reason, antithrombotic medications are the cornerstone of therapy for the child with coronary disease. Aspirin is continued indefinitely in children with coronary aneurysms. When aneurysms are moderate sized, dual-antiplatelet therapy is sometimes administered. For those with large or giant aneurysms, anticoagulation with warfarin or low-molecular-weight heparin is added to aspirin. For acute thrombosis that occasionally occurs in an aneurysmal or stenotic coronary artery, thrombolytic therapy may be lifesaving.
Long-term follow-up of patients with coronary artery aneurysms is tailored to the past (i.e., worst-ever) and current coronary status, with a schedule of testing recommended in the 2017 AHA scientific statement on KD. Testing may include echocardiography, assessment for inducible ischemia, advanced imaging (CT, MRI, or invasive angiography), physical activity counseling, and cardiovascular risk factor assessment and management. Patients with coronary artery stenosis and inducible ischemia may be managed with coronary artery bypass grafting (CABG) or catheter interventions, including percutaneous transluminal coronary rotational ablation, directional coronary atherectomy, and stent implantation.
Patients undergoing long-term aspirin therapy should receive annual influenza vaccination to reduce the risk of Reye syndrome. A different antiplatelet agent can be substituted for aspirin during the 6 wk after varicella vaccination. IVIG may interfere with the immune response to live-virus vaccines as a result of specific antiviral antibody, so the measles-mumps-rubella and varicella vaccinations should generally be deferred until 11 mo after IVIG administration. Nonlive vaccinations do not need to be delayed.
Prognosis
The vast majority of patients with KD return to normal health; timely treatment reduces the risk of coronary aneurysms to <5%. Acute KD recurs in 1–3% of cases. The prognosis for patients with CCA depends on the severity of coronary disease; therefore, recommendations for follow-up and management are stratified according to coronary artery status. Published fatality rates are very low, generally <1.0%. Overall, 50% of coronary artery aneurysms regress to normal lumen diameter by 1-2 yr after the illness, with smaller aneurysms being more likely to regress. Intravascular ultrasonography has demonstrated that regressed aneurysms are associated with marked myointimal thickening and abnormal functional behavior of the vessel wall. Giant aneurysms are less likely to regress to normal lumen diameter and are most likely to lead to thrombosis or stenosis. CABG may be required if there is inducible ischemia; it is best accomplished with the use of arterial grafts, which grow with the child and are more likely than venous grafts to remain patent over the long-term. Heart transplantation has been required in rare cases where revascularization is not feasible because of distal coronary stenoses, distal aneurysms, or severe ischemic cardiomyopathy. A study from Japan reported outcomes in adult patients with a history of KD and giant aneurysms. These patients required multiple cardiac and surgical procedures, but the 30-yr survival rate approached 90%.
Whether children who have had KD and normal echocardiography findings throughout their course are at higher risk for the development of atherosclerotic heart disease in adulthood remains unclear. Studies of endothelial dysfunction in children with a history of KD and normal coronary dimensions have produced conflicting results. However, reassuring data suggest that the standardized mortality ratio among adults in Japan who had KD in childhood without aneurysms is indistinguishable from that of the general population. All children with a history of KD should be counseled regarding a heart-healthy diet, adequate amounts of exercise, tobacco avoidance, and intermittent lipid monitoring. Among children with coronary aneurysms, the AHA recommends treatment thresholds for risk factors for atherosclerotic heart disease that are lower than those for the normal population.