Group B Streptococcus
Catherine S. Lachenauer, Michael R. Wessels
Group B streptococcus (GBS ), or Streptococcus agalactiae, is a major cause of neonatal bacterial sepsis in the United States. Although advances in prevention strategies have led to a decline in the incidence of neonatal disease, GBS remains a major pathogen for neonates, pregnant women, and nonpregnant adults.
Etiology
Group B streptococci are facultative anaerobic gram-positive cocci that form chains or diplococci in broth and small, gray-white colonies on solid medium. GBS is definitively identified by demonstration of the Lancefield group B carbohydrate antigen, such as with latex agglutination techniques widely used in clinical laboratories. Presumptive identification can be established on the basis of a narrow zone of β-hemolysis on blood agar, resistance to bacitracin and trimethoprim-sulfamethoxazole (TMP-SMX), lack of hydrolysis of bile esculin, and elaboration of CAMP factor (named for the discoverers, Christie, Atkins, and Munch-Petersen), an extracellular protein that, in the presence of the β toxin of Staphylococcus aureus, produces a zone of enhanced hemolysis on sheep blood agar. Individual GBS strains are serologically classified according to the presence of 1 of the structurally distinct capsular polysaccharides, which are important virulence factors and stimulators of antibody-associated immunity. Ten GBS capsular types have been identified: types Ia, Ib, II, III, IV, V, VI, VII, VIII, and IX.
Epidemiology
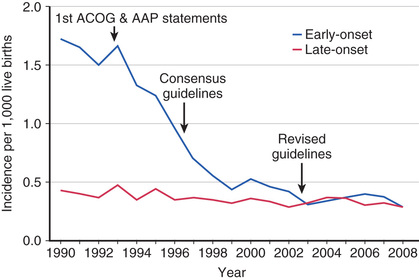
GBS emerged as a prominent neonatal pathogen in the late 1960s. For the next 2 decades, the incidence of neonatal GBS disease remained fairly constant, affecting 1.0-5.4 per 1,000 liveborn infants in the United States. Two patterns of disease were seen: early-onset disease , which presents at <7 days of age, and late-onset disease , which presents at ≥7 days of age. Since the early 1990s, widespread implementation of maternal intrapartum chemoprophylaxis has led to a striking decrease in the incidence of early-onset neonatal GBS disease in the United States, from 1.7 to 0.25 per 1,000 live births in recent years. This strategy has not had a significant effect on the incidence of late-onset disease, which has remained stable at approximately 0.3-0.4 per 1,000 live births (Fig. 211.1 ). The incidence of neonatal GBS disease is higher in premature and low-birthweight infants, although most cases occur in full-term infants. Rates of both early- and late-onset disease are higher in black infants.
Colonization by GBS in healthy adults is common. Vaginal or rectal colonization occurs in up to approximately 30% of pregnant women and is the usual source for GBS transmission to newborn infants. In the absence of maternal chemoprophylaxis, approximately 50% of infants born to colonized women acquire GBS colonization, and 1–2% of infants born to colonized mothers develop early-onset disease. Heavy maternal colonization increases the risk for infant colonization and development of early-onset disease. Additional risk factors for early-onset disease include prolonged rupture of membranes, intrapartum fever, prematurity, maternal bacteriuria during pregnancy, or previous delivery of an infant who developed GBS disease. Risk factors for late-onset disease are less well defined. Whereas late-onset disease may follow vertical transmission, horizontal acquisition from nursery or other community sources (family, healthcare providers, placental capsules) has also been described.
GBS is also an important cause of invasive disease in adults. GBS may cause urinary tract infections, bacteremia, endometritis, chorioamnionitis, and wound infection in pregnant and parturient women. In nonpregnant adults, especially those with underlying medical conditions such as diabetes mellitus, cirrhosis, or malignancy, GBS may cause serious infections such as bacteremia, skin and soft tissue infections, bone and joint infections, endocarditis, pneumonia, and meningitis. In the era of maternal chemoprophylaxis, most invasive GBS infections occur in nonpregnant adults. Unlike neonatal disease, the incidence of invasive GBS disease in adults has increased substantially, doubling between 1990 and 2007.
The serotypes most frequently associated with neonatal GBS disease are types Ia, III, and V; Ib and II are less common. Strains of serotype III are isolated in >50% of cases of late-onset disease and of meningitis associated with early- or late-onset disease. The serotype distribution of colonizing and invasive isolates from pregnant women is similar to that from infected newborns. In Japan, serotypes VI and VIII have been reported as common maternal colonizing serotypes, and case reports indicate that type VIII strains may cause neonatal disease indistinguishable from that caused by other serotypes.
Pathogenesis
A major risk factor for the development of early-onset neonatal GBS infection is maternal vaginal or rectal colonization by GBS. Infants acquire GBS by ascending infection or during passage through the birth canal. Fetal aspiration of infected amniotic fluid may occur. The incidence of early-onset GBS infection increases with the duration of rupture of membranes. Infection may also occur through seemingly intact membranes. In cases of late-onset infection, GBS may be vertically transmitted or acquired later from maternal or nonmaternal sources.
Several bacterial factors are implicated in the pathophysiology of invasive GBS disease, primarily the type-specific capsular polysaccharide . Strains that are associated with invasive disease in humans elaborate more capsular polysaccharide than do colonizing isolates. All GBS capsular polysaccharides are high-molecular-weight polymers composed of repeating oligosaccharide subunits that include a short side chain terminating in N -acetylneuraminic acid (sialic acid ). Studies in type III GBS show that the sialic acid component of the capsular polysaccharide prevents activation of the alternative complement pathway in the absence of type-specific antibody. Sialylated capsular polysaccharide on the GBS surface also interacts with sialic acid–binding lectins or siglecs on human leukocytes to dampen inflammatory gene activation. Thus, the capsular polysaccharide appears to exert a virulence effect by protecting the organism from opsonophagocytosis in the nonimmune host and by downregulating leukocyte activation. In addition, type-specific virulence attributes are suggested by the fact that type III strains are implicated in most cases of late-onset neonatal GBS disease and meningitis. Type III strains are taken up by brain endothelial cells more efficiently in vitro than are strains of other serotypes, although studies using acapsular mutant strains demonstrate that it is not the capsule itself that facilitates cellular invasion. A single clone of type III GBS is highly associated with late-onset disease and meningitis. This clonal group, ST-17, produces a surface-anchored protein called hypervirulent GBS adhesin (HvgA ) that is not present in other GBS isolates. HvgA contributes to GBS adherence to intestinal and endothelial cells and mediates invasion into the central nervous system (CNS) in an experimental infection model in mice. Other putative GBS virulence factors include GBS surface proteins, which may play a role in adhesion to host cells; C5a peptidase, which is postulated to inhibit the recruitment of polymorphonuclear cells into sites of infection; β-hemolysin, which has been associated with cell injury in vitro; and hyaluronidase, which has been postulated to act as a spreading factor in host tissues.
In a classic study of pregnant women colonized with type III GBS, those who gave birth to healthy infants had higher levels of capsular polysaccharide–specific antibody than those who gave birth to infants who developed invasive disease. In addition, there is a high correlation of antibody titer to GBS type III in mother–infant paired sera. These observations indicate that transplacental transfer of maternal antibody is critically involved in neonatal immunity to GBS. Optimal immunity to GBS also requires an intact complement system. The classical complement pathway is an important component of GBS immunity in the absence of specific antibody; in addition, antibody-mediated opsonophagocytosis may proceed by the alternative complement pathway. These and other results indicate that anticapsular antibody can overcome the prevention of C3 deposition on the bacterial surface by the sialic acid component of the type III capsule.
The precise steps between GBS colonization and invasive disease remain unclear. In vitro studies showing GBS entry into alveolar epithelial cells and pulmonary vasculature endothelial cells suggest that GBS may gain access to the bloodstream by invasion from the alveolar space, perhaps following intrapartum aspiration of infected fluid. β-Hemolysin/cytolysin may facilitate GBS entry into the bloodstream following inoculation into the lungs. However, highly encapsulated GBS strains, which enter eukaryotic cells poorly in vitro compared with capsule-deficient organisms, are associated with virulence clinically and in experimental infection models.
GBS induces the release of proinflammatory cytokines. The group B antigen and the peptidoglycan component of the GBS cell wall are potent inducers of tumor necrosis factor-α release in vitro, whereas purified type III capsular polysaccharide is not. Even though the capsule plays a central role in virulence through avoidance of immune clearance, the capsule does not directly contribute to cytokine release and the resultant inflammatory response.
The complete genome sequences of hundreds of GBS strains have been reported, emphasizing a genomic approach to better understanding GBS. Analysis of these sequences shows that GBS is closely related to Streptococcus pyogenes and Streptococcus pneumoniae. Many known and putative GBS virulence genes are clustered in pathogenicity islands that also contain mobile genetic elements, suggesting that interspecies acquisition of genetic material plays an important role in genetic diversity.
Clinical Manifestations
Two syndromes of neonatal GBS disease are distinguishable on the basis of age at presentation, epidemiologic characteristics, and clinical features (Table 211.1 ). Early-onset neonatal GBS disease presents within the 1st 6 days of life and is often associated with maternal obstetric complications, including chorioamnionitis, prolonged rupture of membranes, and premature labor. Infants may appear ill at the time of delivery, and most infants become ill within 24 hr of birth. In utero infection may result in septic abortion or immediate distress after birth. More than 80% of early-onset GBS disease presents as sepsis; pneumonia and meningitis are other common manifestations. Asymptomatic bacteremia is uncommon but can occur. In symptomatic patients, nonspecific signs such as hypothermia or fever, irritability, lethargy, apnea, and bradycardia may be present. Respiratory signs are prominent regardless of the presence of pneumonia and include cyanosis, apnea, tachypnea, grunting, flaring, and retractions. A fulminant course with hemodynamic abnormalities, including tachycardia, acidosis, and shock, may ensue. Persistent fetal circulation may develop. Clinically and radiographically, pneumonia associated with early-onset GBS disease is difficult to distinguish from respiratory distress syndrome . Patients with meningitis often present with nonspecific findings, as described for sepsis or pneumonia, with more specific signs of CNS involvement initially absent.
Table 211.1
Adapted from Schrag SJ, Zywicki S, Farley MM, et al: Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis, N Engl J Med 342:15–20, 2000.
Late-onset neonatal GBS disease presents at ≥7 days of life (may be seen in 1st 2-3 mo) and usually manifests as bacteremia (45–65%) and meningitis (25–35%). Focal infections involving bone and joints, skin and soft tissue, the urinary tract, or lungs may also be seen. Cellulitis and adenitis are often localized to the submandibular or parotid regions. In contrast to early-onset disease, maternal obstetric complications are not risk factors for the development of late-onset GBS disease. Infants with late-onset disease are often less severely ill on presentation than infants with early-onset disease, and the disease is often less fulminant.
Invasive GBS disease in children beyond early infancy is uncommon. Bacteremia without a focus is the most common syndrome associated with childhood GBS disease beyond early infancy. Focal infections may include meningitis, pneumonia, endocarditis, and bone and joint infections.
Diagnosis
A major challenge is distinguishing between respiratory distress syndrome and invasive neonatal GBS infection in preterm infants because the 2 illnesses share clinical and radiographic features. Severe apnea, early onset of shock, abnormalities in the peripheral leukocyte count, and greater lung compliance may be more likely in infants with GBS disease. Other neonatal pathogens, including Escherichia coli and Listeria monocytogenes, may cause illness that is clinically indistinguishable from that caused by GBS.
The diagnosis of invasive GBS disease is established by isolation and identification of the organism from a normally sterile site, such as blood, urine, or cerebrospinal fluid (CSF). Isolation of GBS from gastric or tracheal aspirates or from skin or mucous membranes indicates colonization and is not diagnostic of invasive disease. CSF should be examined in all neonates suspected of having sepsis, because specific CNS signs are often absent in the presence of meningitis, especially in early-onset disease. Antigen detection methods that use group B polysaccharide–specific antiserum, such as latex particle agglutination, are available for testing of urine, blood, and CSF, but these tests are less sensitive than culture. Moreover, antigen is often detected in urine samples collected by bag from otherwise healthy neonates who are colonized with GBS on the perineum or rectum.
Laboratory Findings
Frequently present are abnormalities in the peripheral white blood cell count, including an increased or decreased absolute neutrophil count, elevated band count, increased ratio of bands to total neutrophils, or leukopenia. Elevated C-reactive protein level has been investigated as a potential early marker of GBS sepsis but is unreliable. Findings on chest radiograph are often indistinguishable from those of respiratory distress syndrome and may include reticulogranular patterns, patchy infiltrates, generalized opacification, pleural effusions, or increased interstitial markings.
Treatment
Penicillin G is the treatment of choice of confirmed GBS infection. Empirical therapy of neonatal sepsis that could be caused by GBS generally includes ampicillin and an aminoglycoside, both for the need for broad coverage pending organism identification and for synergistic bactericidal activity. Once GBS has been definitively identified and a good clinical response has occurred, therapy may be completed with penicillin alone. Especially in patients with meningitis, high doses of penicillin (450,000-500,000 units/kg/day) or ampicillin (300 mg/kg/day) are recommended because of the relatively high mean inhibitory concentration (MIC) of penicillin for GBS as well as the potential for a high initial CSF inoculum. The duration of therapy varies according to the site of infection and should be guided by clinical circumstances (Table 211.2 ). Extremely ill near-term patients with respiratory failure have been successfully treated with extracorporeal membrane oxygenation.
Table 211.2
Data from the American Academy of Pediatrics: Group B streptococcal infections. In Kimberlin DW, Brady MT, Jackson MA, Long SS, editors: Red book: 2015 report of the Committee on Infectious Diseases , ed 30. Elk Grove Village, IL, 2015, American Academy of Pediatrics, pp 746–747.
In patients with GBS meningitis, some experts recommend that additional CSF be sampled at 24-48 hr to determine whether sterility has been achieved. Persistent GBS growth may indicate an unsuspected intracranial focus or an insufficient antibiotic dose.
For recurrent neonatal GBS disease , standard intravenous antibiotic therapy followed by attempted eradication of GBS mucosal colonization has been suggested. This suggestion is based on the findings in several studies that invasive isolates from recurrent episodes are usually identical to each other and to colonizing isolates from the affected infant. Rifampin has most frequently been used for this purpose, but one report demonstrates that eradication of GBS colonization in infants is not reliably achieved by rifampin therapy. Optimal management of this uncommon situation remains unclear.
Prognosis
Studies from the 1970s and 1980s showed that up to 30% of infants surviving GBS meningitis had major long-term neurologic sequelae, including developmental delay, spastic quadriplegia, microcephaly, seizure disorder, cortical blindness, or deafness; less severe neurologic complications may be present in other survivors. A study of infants who survived GBS meningitis diagnosed from 1998 through 2006 found that 19% had severe neurologic impairment and 25% had mild to moderate impairment at long-term follow-up. Periventricular leukomalacia and severe developmental delay may result from GBS disease and accompanying shock in premature infants, even in the absence of meningitis. The outcome of focal GBS infections outside the CNS, such as bone or soft tissue infections, is generally favorable.
In the 1990s, the case fatality rates associated with early- and late-onset neonatal GBS disease were 4.7% and 2.8%, respectively. Mortality is higher in premature infants; one study reported a case fatality rate of 30% in infants at gestational age <33 wk and 2% in those ≥37 wk. The case fatality rate in children age 3 mo to 14 yr was 9%, and in nonpregnant adults, 11.5%.
Prevention
Persistent morbidity and mortality from perinatal GBS disease despite advances in neonatal care have spurred intense investigation into modes of prevention. Two basic approaches to GBS prevention have been investigated: elimination of colonization from the mother or infant (chemoprophylaxis) and induction of protective immunity (immunoprophylaxis).
Chemoprophylaxis
Administration of antibiotics to pregnant women before the onset of labor does not reliably eradicate maternal GBS colonization and is not an effective means of preventing neonatal GBS disease. Interruption of neonatal colonization is achievable through administration of antibiotics to the mother during labor . Infants born to GBS-colonized women with premature labor or prolonged rupture of membranes who were given intrapartum chemoprophylaxis had a substantially lower rate of GBS colonization (9% vs 51%) and early-onset disease (0% vs 6%) than did the infants born to women who were not treated. Maternal postpartum febrile illness was also decreased in the treatment group.
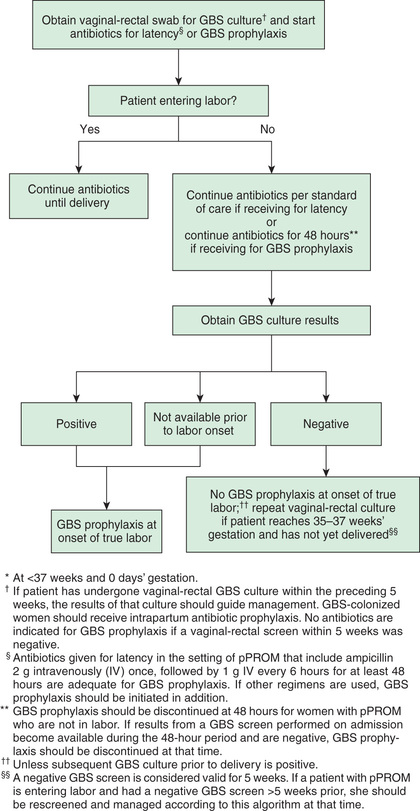
In the mid-1990s, guidelines for chemoprophylaxis were issued that specified administration of intrapartum antibiotics to women identified as high risk by either culture-based or risk factor–based criteria. These guidelines were revised in 2002 after epidemiologic data indicated the superior protective effect of the culture-based approach in the prevention of neonatal GBS disease, and further revised guidelines were issued in 2010. According to current recommendations, vaginorectal GBS screening cultures should be performed for all pregnant women at 35–37 wk gestation, except for those with GBS bacteriuria during the current pregnancy or a previous infant with invasive GBS disease. Any woman with a positive prenatal screening culture, GBS bacteriuria during pregnancy, or a previous infant with invasive GBS disease should receive intrapartum antibiotics. Women whose culture status is unknown (culture not done, incomplete, or results unknown) and who deliver prematurely (<37 wk gestation), experience prolonged rupture of membranes (≥18 hr), experience intrapartum fever (≥38°C [100.4°F]), or have a positive nucleic acid amplification test for GBS should also receive intrapartum chemoprophylaxis (Figs. 211.2 and 211.3 ). Routine intrapartum prophylaxis is not recommended for women with GBS colonization undergoing planned cesarean delivery who have not begun labor or had rupture of membranes.

Penicillin remains the preferred agent for maternal chemoprophylaxis because of its narrow spectrum and the universal penicillin susceptibility of GBS isolates associated with human infection. Ampicillin is an acceptable alternative. If amnionitis is suspected, broad-spectrum antibiotic therapy that includes an agent active against GBS should replace GBS prophylaxis. Occasional GBS isolates have demonstrated reduced in vitro susceptibility to penicillin and other β-lactam antibiotics in association with mutations in penicillin-binding proteins. However, the clinical significance of these higher MIC values is unclear. Because of frequent resistance of GBS to clindamycin (up to 38%), cefazolin should be used in most cases of intrapartum chemoprophylaxis for penicillin-intolerant women. For penicillin-allergic women at high risk for anaphylaxis, clindamycin should be used, if isolates are demonstrated to be susceptible. Vancomycin should be used if isolates are resistant to, or demonstrate inducible resistance to, clindamycin or if clindamycin susceptibility is unknown.
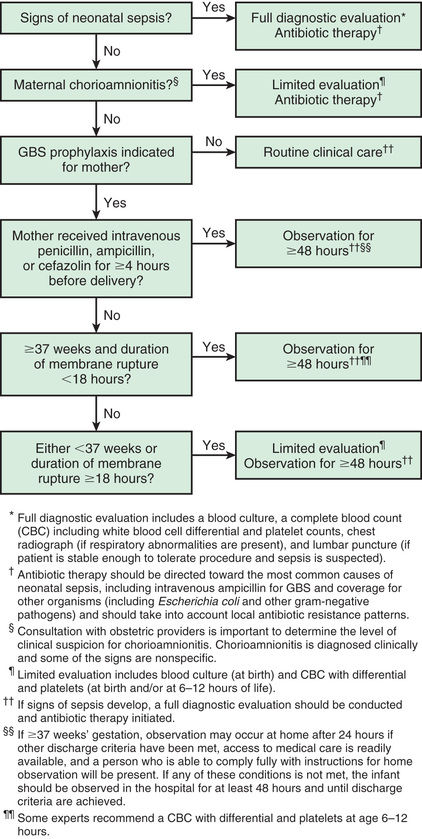
The U.S. Centers for Disease Control and Prevention (CDC) guidelines also provide recommendations for secondary prevention of early-onset GBS disease in newborns (Fig. 211.4 ). Extent of newborn evaluation and decision to institute empirical antibiotics are guided by clinical evaluation of the infant as well as gestational age, maternal risk factors, and receipt of intrapartum prophylaxis. In the era of maternal chemoprophylaxis, most cases of early-onset disease are seen in infants born to women with negative prenatal screening cultures. Data from a large epidemiologic study indicate that the administration of maternal intrapartum antibiotics does not change the clinical spectrum or delay the onset of clinical signs in infants who developed GBS disease despite maternal prophylaxis.
A significant concern with maternal intrapartum chemoprophylaxis has been that large-scale antibiotic use among parturient women might lead to increased rates of antimicrobial resistance or infection in infants with organisms other than GBS, but this has not been borne out. In a population-based study of early-onset neonatal infection from 2005–2014, the incidence of early-onset sepsis both overall and caused by E. coli remained stable. At present, the substantial decline in early-onset neonatal GBS disease favors continued broad-scale intrapartum chemoprophylaxis, but continued surveillance is required.
A limitation of the maternal chemoprophylaxis strategy is that intrapartum antibiotic use is unlikely to have an impact on late-onset neonatal disease, miscarriages, or stillbirths attributed to GBS, or adult GBS disease. In addition, with wider implementation of maternal chemoprophylaxis, an increasing percentage of early-onset neonatal disease has been in patients born to women with negative cultures, that is, false-negative screens.
Maternal Immunization
Human studies demonstrate that transplacental transfer of naturally acquired maternal antibody to the GBS capsular polysaccharide protects newborns from invasive GBS infection, and that efficient transplacental passage of vaccine-induced GBS antibodies occurs. Conjugate vaccines composed of the GBS capsular polysaccharides coupled to carrier proteins have been produced for human use. In early clinical trials, conjugate GBS vaccines were well tolerated and induced levels of functional antibodies well above the range believed to be protective in >90% of recipients. A vaccine containing type III polysaccharide coupled to tetanus toxoid was safely administered to pregnant women and elicited functionally active type-specific antibody that was efficiently transported to the fetus. Vaccines containing GBS surface proteins have been considered as a means to provide protection against strains of multiple serotypes, and availability of whole genome sequencing has enabled identification of vaccine protein candidates.
A successful GBS maternal vaccine administered before or during pregnancy should lead to transplacental passage of vaccine-induced antibody that protects the fetus and newborn against infection by several GBS serotypes. Such a vaccine would eliminate the need for cumbersome cultures during pregnancy, circumvent the various risks associated with large-scale antibiotic prophylaxis, likely have an impact on both early- and late-onset disease, and provide a prevention strategy in middle and low income countries, where maternal chemoprophylaxis may not be feasible. Intrapartum chemoprophylaxis will likely remain an important aspect of prevention, particularly for women in whom opportunities for GBS immunization are missed and for infants born so early that levels of transplacentally acquired antibodies may not be high enough to be protective.