Diphtheria (Corynebacterium diphtheriae )
Amruta Padhye, Stephanie A. Fritz
Diphtheria is an acute toxic infection caused by Corynebacterium species, typically Corynebacterium diphtheriae and, less often, toxigenic strains of Corynebacterium ulcerans. Although diphtheria was reduced from a major cause of childhood death to a medical rarity in the Western hemisphere in the early 20th century, recurring reminders of the fragility of this success, particularly in conflict areas, emphasize the need to continue vigorous promotion of those same control principles across the global community.
Etiology
Corynebacteria are aerobic, nonencapsulated, non–spore-forming, mostly nonmotile, pleomorphic, gram-positive bacilli. C. diphtheriae is by far the most frequently isolated agent of diphtheria. C. ulcerans is more often isolated from animal sources and can cause human disease similar to C. diphtheriae . Selective medium (e.g., cystine-tellurite blood agar or Tinsdale agar) that inhibits growth of competing organisms is required for isolation and, when reduced by C. diphtheriae, renders colonies gray-black. Differentiation of C. diphtheriae from C. ulcerans is based on urease activity; C. ulcerans is urease-positive. Four C. diphtheriae biotypes (mitis, intermedius, belfanti, gravis ) are capable of causing diphtheria and are differentiated by colony morphology, hemolysis, and fermentation reactions. The ability to produce diphtheritic toxin results from acquisition of a lysogenic corynebacteriophage by either C. diphtheriae or C. ulcerans, which encodes the diphtheritic toxin gene and confers diphtheria-producing potential on these strains. Thus, indigenous nontoxigenic C. diphtheriae can be rendered toxigenic and disease-producing after importation of a toxigenic C. diphtheriae . Demonstration of diphtheritic toxin production by the modified Elek test, an agar immunoprecipitin technique, alone or in conjunction with polymerase chain reaction (PCR) testing for carriage of the toxin gene, is necessary to confirm disease. Toxigenic and nontoxigenic strains are indistinguishable by colony type, microscopic features, or biochemical test results.
Epidemiology
Unlike other diphtheroids (coryneform bacteria), which are ubiquitous in nature, C. diphtheriae is an exclusive inhabitant of human mucous membranes and skin. Spread is primarily by airborne respiratory droplets, direct contact with respiratory secretions of symptomatic individuals, or exudate from infected skin lesions. Asymptomatic respiratory tract carriage is important in transmission. In areas where diphtheria is endemic, 3–5% of healthy individuals can carry toxigenic organisms, but carriage is exceedingly rare when diphtheria is rare. Skin infection and skin carriage are silent reservoirs of C. diphtheriae, and organisms can remain viable in dust or on fomites for up to 6 mo. Transmission through contaminated milk and through an infected food handler has been proved or suspected.
In the 1920s, >125,000 diphtheria cases, with 10,000 deaths, were reported annually in the United States, with the highest fatality rates among very young and elderly persons. The incidence then began to decrease and, with widespread use of diphtheria toxoid in the United States after World War II, declined steadily through the late 1970s. Since then, ≤5 cases have occurred annually in the United States, with no epidemics of respiratory tract diphtheria. Similar decreases occurred in Europe. Despite the worldwide decrease in disease incidence, diphtheria remains endemic in many developing countries with poor immunization rates against diphtheria.
When diphtheria was endemic, it primarily affected children <15 yr old. Since the introduction of toxoid immunization, the disease has shifted to adults who lack natural exposure to toxigenic C. diphtheriae in the vaccine era and have low rates of booster immunization. In the 27 sporadic cases of respiratory tract diphtheria reported in the United States in the 1980s, 70% occurred among persons >25 yr old. The largest outbreak of diphtheria in the developed world since the 1960s occurred from 1990–1996 in the newly independent countries of the former Soviet Union, involving >150,000 cases in 14 countries. Of these, >60% of cases occurred in individuals >14 yr old. Case fatality rates ranged from 3–23% by country. Factors contributing to the epidemic included a large population of underimmunized adults, decreased childhood immunization rates, population migration, crowding, and failure to respond aggressively during early phases of the epidemic. Cases of diphtheria among travelers from these endemic areas were transported to many countries in Europe.
Most proven cases of respiratory tract diphtheria in the United States in the 1990s were associated with importation of toxigenic C. diphtheriae, although clonally related toxigenic C. diphtheriae has persisted in this country and Canada for at least 25 yr. World Health Organization (WHO) surveillance reports indicate that most cases of diphtheria worldwide occur in the Southeast Asia and Africa regions. In Europe, increasing reports of respiratory and systemic infections have been attributed to C ulcerans ; animal contact is the predominant risk factor.
Cutaneous diphtheria, a curiosity when diphtheria was common, accounted for more than 50% of reported C. diphtheriae isolates in the United States by 1975. This indolent local infection, compared with mucosal infection, is associated with more prolonged bacterial shedding, greater contamination of the environment, and increased transmission to the pharynx and skin of close contacts. Outbreaks are associated with homelessness, crowding, poverty, alcoholism, poor hygiene, contaminated fomites, underlying dermatosis, and introduction of new strains from exogenous sources. It is no longer a tropical or subtropical disease; 1,100 C. diphtheriae infections were documented in a neighborhood in Seattle (site of the last major U.S. outbreak), from 1971–1982; 86% were cutaneous, and 40% involved toxigenic strains. Cutaneous diphtheria is an important source for toxigenic C. diphtheriae in the United States, and its importation is frequently the source for subsequent sporadic cases of respiratory tract diphtheria. Cutaneous diphtheria caused by C. ulcerans from travel to tropical countries or animal contact has been increasingly reported.
Pathogenesis
Both toxigenic and nontoxigenic C. diphtheriae cause skin and mucosal infection and can rarely cause focal infection after bacteremia. The organism usually remains in the superficial layers of skin lesions or respiratory tract mucosa, inducing local inflammatory reaction. The major virulence of the organism lies in its ability to produce a potent polypeptide exotoxin, which inhibits protein synthesis and causes local tissue necrosis and resultant local inflammatory response. Within the first few days of respiratory tract infection (usually in the pharynx), a dense necrotic coagulum of organisms, epithelial cells, fibrin, leukocytes, and erythrocytes forms, initially white and advancing to become a gray-brown, leather-like adherent pseudomembrane (diphtheria is Greek for leather). Removal is difficult and reveals a bleeding edematous submucosa. Paralysis of the palate and hypopharynx is an early local effect of diphtheria toxin. Toxin absorption can lead to systemic manifestations: kidney tubule necrosis, thrombocytopenia, cardiomyopathy, and demyelination of nerves. Because the latter 2 complications can occur 2-10 wk after mucocutaneous infection, the pathophysiology in some cases is suspected to be immunologically mediated.
Clinical Manifestations
The manifestations of C. diphtheriae infection are influenced by the anatomic site of infection, the immune status of the host, and the production and systemic distribution of toxin.
Respiratory Tract Diphtheria
In a classic description of 1,400 cases of diphtheria in California (1954), the primary focus of infection was the tonsils or pharynx (94%), with the nose and larynx the next 2 most common sites. After an average incubation period of 2-4 days (range 1-10 days), local signs and symptoms of inflammation develop. Infection of the anterior nares is more common among infants and causes serosanguineous, purulent, erosive rhinitis with membrane formation. Shallow ulceration of the external nares and upper lip is characteristic. In tonsillar and pharyngeal diphtheria , sore throat is the universal early symptom. Only half of patients have fever, and fewer have dysphagia, hoarseness, malaise, or headache. Mild pharyngeal injection is followed by unilateral or bilateral tonsillar membrane formation, which can extend to involve the uvula (which may cause toxin-mediated paralysis), soft palate, posterior oropharynx, hypopharynx, or glottic areas (Fig. 214.1 ). Underlying soft tissue edema and enlarged lymph nodes can cause a bull-neck appearance. The degree of local extension correlates directly with profound prostration, bull-neck appearance, and fatality due to airway compromise or toxin-mediated complications (Fig. 214.2 ).
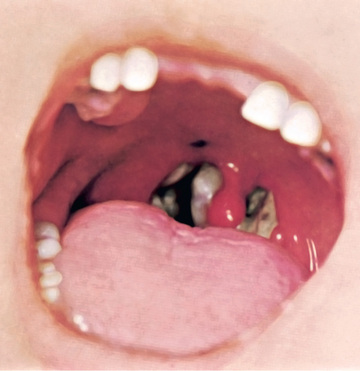

The characteristic adherent membrane, extension beyond the faucial area, dysphagia, and relative lack of fever help differentiate diphtheria from exudative pharyngitis caused by Streptococcus pyogenes or Epstein-Barr virus. Vincent angina, infective phlebitis with thrombosis of the jugular veins (Lemierre syndrome), and mucositis in patients undergoing cancer chemotherapy are usually differentiated by the clinical setting. Infection of the larynx, trachea, and bronchi can be primary or a secondary extension from the pharyngeal infection. Hoarseness, stridor, dyspnea, and croupy cough are clues. Differentiation from bacterial epiglottitis, severe viral laryngotracheobronchitis, and staphylococcal or streptococcal tracheitis hinges partially on the relative paucity of other signs and symptoms in patients with diphtheria and primarily on visualization of the adherent pseudomembrane at laryngoscopy and intubation.
Patients with laryngeal diphtheria are at significant risk for suffocation because of local soft tissue edema and airway obstruction by the diphtheria membrane, a dense cast of respiratory epithelium, and necrotic coagulum. Establishment of an artificial airway and resection of the pseudomembrane can be lifesaving, but further obstructive complications are common, and systemic toxic complications are inevitable.
Cutaneous Diphtheria
Classic cutaneous diphtheria is an indolent, nonprogressive infection characterized by a superficial, ecthyma-like, nonhealing ulcer with a gray-brown membrane. Diphtheria skin infections cannot always be differentiated from streptococcal or staphylococcal impetigo, and these conditions frequently coexist. In most cases, a primary process, such as dermatosis, laceration, burn, bite, or impetigo, becomes secondarily infected with C. diphtheriae. Extremities are more often affected than the trunk or head. Pain, tenderness, erythema, and exudate are typical. Local hyperesthesia or hypesthesia is unusual. Respiratory tract colonization or symptomatic infection with toxic complications occurs in the minority of patients with cutaneous diphtheria. Among infected adults in the Seattle outbreak, 3% with cutaneous infections and 21% with symptomatic nasopharyngeal infection, with or without skin involvement, demonstrated toxic myocarditis, neuropathy, or obstructive respiratory tract complications. All had received at least 20,000 units of equine antitoxin at the time of hospitalization.
Infection at Other Sites
C. diphtheriae occasionally causes mucocutaneous infections at other sites, such as the ear (otitis externa), the eye (purulent and ulcerative conjunctivitis), and the genital tract (purulent and ulcerative vulvovaginitis). The clinical setting, ulceration, membrane formation, and submucosal bleeding help differentiate diphtheria from other bacterial and viral causes. Rare cases of septicemia are described and are universally fatal. Sporadic cases of endocarditis occur, and clusters among intravenous drug users have been reported in several countries; skin was the probable portal of entry, and almost all strains were nontoxigenic. Sporadic cases of pyogenic arthritis, mainly from nontoxigenic strains, have been reported in adults and children. Diphtheroids isolated from sterile body sites should not be routinely dismissed as contaminants without careful consideration of the clinical setting.
Diagnosis
Specimens for culture should be obtained from the nose and throat and any other mucocutaneous lesion. A portion of membrane should be removed and submitted for culture along with underlying exudate. The laboratory must be notified to use selective medium. C. diphtheriae survives drying. If obtained in a remote area, a dry swab specimen can be placed in a silica gel pack and sent to the laboratory. Evaluation of a direct smear using Gram stain or specific fluorescent antibody is unreliable. Culture isolates of coryneform organisms should be identified to the species level, and toxigenicity and antimicrobial susceptibility tests should be performed for C. diphtheriae isolates. It is recommended that all isolates be sent to a reference laboratory. In the United States, the Centers for Disease Control and Prevention (CDC) Pertussis and Diphtheria Laboratory provides support to local and state health departments needing assistance with isolation, identification, and subtyping of C. diphtheriae and C. ulcerans.
Complications
Respiratory tract obstruction by pseudomembranes may require bronchoscopy or intubation and mechanical ventilation. Two other tissues usually remote from sites of C. diphtheriae infection can be significantly affected by diphtheritic toxin : the heart and the nervous system.
Toxic Cardiomyopathy
Toxic cardiomyopathy occurs in 10–25% of patients with respiratory diphtheria and is responsible for 50–60% of deaths. Subtle signs of myocarditis can be detected in most patients, especially the elderly, but the risk for significant complications correlates directly with the extent and severity of exudative local oropharyngeal disease, as well as delay in administration of antitoxin. The first evidence of cardiac toxicity characteristically occurs during the 2nd and 3rd wk of illness as the pharyngeal disease improves, but can appear acutely as early as the 1st wk of illness, a poor prognostic sign, or insidiously as late as the 6th wk. Tachycardia disproportionate to fever is common and may be evidence of cardiac toxicity or autonomic nervous system dysfunction. A prolonged P-R interval and changes in the ST-T wave on an electrocardiographic tracing are relatively frequent findings; dilated and hypertrophic cardiomyopathy detected by echocardiogram has been described. Single or progressive cardiac dysrhythmias can occur, including first-, second-, and third-degree heart block. Temporary transvenous pacing may improve outcomes. Atrioventricular dissociation and ventricular tachycardia are also described, the latter having a high associated mortality. Heart failure may appear insidiously or acutely. Elevation of the serum aspartate transaminase concentration closely parallels the severity of myonecrosis. Severe dysrhythmia portends death. Histologic postmortem findings are variable: little or diffuse myonecrosis with acute inflammatory response. Recovery from toxic myocardiopathy is usually complete, although survivors of more severe dysrhythmias can have permanent conduction defects.
Toxic Neuropathy
Neurologic complications parallel the severity of primary infection and are multiphasic in onset. Acutely or 2-3 wk after onset of oropharyngeal inflammation, hypesthesia and local paralysis of the soft palate typically occur. Weakness of the posterior pharyngeal, laryngeal, and facial nerves may follow, causing a nasal quality in the voice, difficulty in swallowing, and risk for aspiration. Cranial neuropathies characteristically occur in the 5th wk, leading to oculomotor and ciliary paralysis, which can cause strabismus, blurred vision, or difficulty with accommodation. Symmetric demyelinating polyneuropathy has onset 10 days to 3 mo after oropharyngeal infection and causes principally motor deficits with diminished deep tendon reflexes. Nerve conduction velocity studies and cerebrospinal fluid findings in diphtheritic polyneuropathy are indistinguishable from those of Guillain-Barré syndrome. Paralysis of the diaphragm may ensue. Complete neurologic recovery is likely, but rarely vasomotor center dysfunction 2-3 wk after onset of illness can cause hypotension or cardiac failure.
Recovery from myocarditis and neuritis is often slow but usually complete. Corticosteroids do not diminish these complications and are not recommended.
Treatment
Specific antitoxin is the mainstay of therapy and should be administered on the basis of clinical diagnosis. Because it neutralizes only free toxin, antitoxin efficacy diminishes with elapsed time after the onset of mucocutaneous symptoms. Equine diphtheria antitoxin is available in the United States only from the CDC. Physicians treating a case of suspected diphtheria should contact the CDC Emergency Operations Center (770-488-7100 at all times). Antitoxin is administered as a single empirical dose of 20,000-100,000 units based on the degree of toxicity, site and size of the membrane, and duration of illness. Skin testing must be performed before administration of antitoxin. Patients with positive sensitivity testing or with a history of hypersensitivity reaction to horse equine protein should be desensitized. Antitoxin is probably of no value for local manifestations of cutaneous diphtheria, but its use is prudent because toxic sequelae can occur. Commercially available intravenous immunoglobulin preparations contain low titers of antibodies to diphtheria toxin; their use for therapy of diphtheria is not proved or approved. Antitoxin is not recommended for asymptomatic carriers.
The role of antimicrobial therapy is to halt toxin production, treat localized infection, and prevent transmission of the organism to contacts. C. diphtheriae is usually susceptible to various agents in vitro, including penicillins, erythromycin, clindamycin, rifampin, and tetracycline. Resistance to erythromycin is common in populations if the drug has been used broadly, and resistance to penicillin has also been reported. Only erythromycin or penicillin is recommended; erythromycin is marginally superior to penicillin for eradication of nasopharyngeal carriage. Appropriate therapy is erythromycin (40-50 mg/kg/day divided every 6 hr by mouth [PO] or intravenously [IV]; maximum 2 g/day), aqueous crystalline penicillin G (100,000-150,000 units/kg/day divided every 6 hr IV or intramuscularly [IM]), or procaine penicillin (300,000 units every 12 hr IM for those ≤10 kg in weight; 600,000 units every 12 hr IM for those >10 kg in weight) for 14 days. Once oral medications are tolerated, oral penicillin V (250 mg four times daily) may be used. Antibiotic therapy is not a substitute for antitoxin therapy. Some patients with cutaneous diphtheria have been treated for 7-10 days. Elimination of the organism should be documented by negative results of at least 2 successive cultures of specimens from the nose and throat (or skin) obtained 24 hr apart after completion of therapy. Treatment with erythromycin is repeated if either culture yields C. diphtheriae.
Supportive Care
Droplet precautions are instituted for patients with pharyngeal diphtheria; for patients with cutaneous diphtheria, contact precautions are observed until the results of cultures of specimens taken after cessation of therapy are negative. Cutaneous wounds are cleaned thoroughly with soap and water. Bed rest is essential during the acute phase of disease, usually for ≥2 wk until the risk for symptomatic cardiac damage has passed, with return to physical activity guided by the degree of toxicity and cardiac involvement.
Prognosis
The prognosis for patients with diphtheria depends on the virulence of the organism (subspecies gravis has the highest fatality rate), patient age, immunization status, site of infection, and speed of administration of the antitoxin. Mechanical obstruction from laryngeal diphtheria or bull-neck diphtheria and the complications of myocarditis account for most diphtheria-related deaths. The case fatality rate of almost 10% for respiratory tract diphtheria has not changed in 50 yr; the rate was 8% in a Vietnamese series described in 2004. At recovery, administration of diphtheria toxoid is indicated to complete the primary series or booster doses of immunization, because not all patients develop antibodies to diphtheria toxin after infection.
Prevention
Protection against serious disease caused by imported or indigenously acquired C. diphtheriae depends on immunization. In the absence of a precisely determined minimum protective level for diphtheria antitoxin, the presumed minimum is 0.01-0.10 IU/mL. In outbreaks, 90% of individuals with clinical disease have had antibody values <0.01 IU/mL, and 92% of asymptomatic carriers have had values >0.1 IU/mL. In serosurveys in the United States and Western Europe, where almost universal immunization during childhood has been achieved, 25% to >60% of adults lack protective antitoxin levels, with typically very low levels in elderly persons.
All suspected diphtheria cases should be reported to local and state health departments. Investigation is aimed at preventing secondary cases in exposed individuals and at determining the source and carriers to halt spread to unexposed individuals. Reported rates of carriage in household contacts of case patients are 0–25%. The risk for development of diphtheria after household exposure to a case is approximately 2%, and the risk after similar exposure to a carrier is 0.3%.
Asymptomatic Case Contacts
All household contacts and people who have had intimate respiratory or habitual physical contact with a patient are closely monitored for illness for 7 days. Cultures of the nose, throat, and any cutaneous lesions are performed. Antimicrobial prophylaxis is presumed effective and is administered regardless of immunization status, using a single injection of benzathine penicillin G (600,000 units IM for patients <6 yr old, or 1,200,000 units IM for patients >6 yr old) or erythromycin (40-50 mg/kg/day divided qid PO for 10 days; max 2 g/day). Diphtheria toxoid vaccine, in age-appropriate form, is given to immunized individuals who have not received a booster dose within 5 yr. Children who have not received their 4th dose should be vaccinated. Those who have received fewer than 3 doses of diphtheria toxoid or who have uncertain immunization status should be immunized with an age-appropriate preparation on a primary schedule.
Asymptomatic Carriers
When an asymptomatic carrier is identified, antimicrobial prophylaxis is given for 10-14 days and an age-appropriate preparation of diphtheria toxoid is administered immediately if a booster has not been given within 1 yr. Droplet precautions (respiratory tract colonization) or contact precautions (cutaneous colonization only) are observed until at least 2 subsequent cultures obtained 24 hr apart after cessation of therapy have negative results.
Repeat cultures are performed about 2 wk after completion of therapy for cases and carriers; if results are positive, an additional 10-day course of oral erythromycin should be given and follow-up cultures performed. Susceptibility testing of isolates should be performed, as erythromycin resistance is reported. Neither antimicrobial agent eradicates carriage in 100% of individuals. In one report, a single course of therapy failed in 21% of carriers. Transmission of diphtheria in modern hospitals is rare. Only those who have an unusual contact with respiratory or oral secretions should be managed as contacts. Investigation of the casual contacts of patients and carriers or persons in the community without known exposure has yielded extremely low carriage rates and is not routinely recommended.
Vaccine
Universal immunization with diphtheria toxoid throughout life, to provide constant protective antitoxin levels and to reduce severity of C. diphtheriae disease, is the only effective control measure. Although immunization does not preclude subsequent respiratory or cutaneous carriage of toxigenic C. diphtheriae, it decreases local tissue spread, prevents toxic complications, diminishes transmission of the organism, and provides herd immunity when at least 70–80% of a population is immunized.
Diphtheria toxoid is prepared by formaldehyde treatment of toxin, standardized for potency, and adsorbed to aluminum salts, enhancing immunogenicity. Two preparations of diphtheria toxoids are formulated according to the limit of flocculation (Lf) content, a measure of the quantity of toxoid. The pediatric (6 mo to 6 yr) preparations (i.e., DTaP [diphtheria and tetanus toxoids with acellular pertussis vaccine], DT [diphtheria and tetanus toxoids vaccine]) contain 6.7-25.0 Lf units of diphtheria toxoid per 0.5 mL dose; the adult preparation (Td; 10% of pediatric diphtheria toxoid dose, Tdap [diphtheria and tetanus toxoids with acellular pertussis vaccine]) contain no more than 2-2.5 Lf units of toxoid per 0.5 mL dose. The higher-potency (D) formulation of toxoid is used for primary series and booster doses for children through 6 yr of age because of superior immunogenicity and minimal reactogenicity. For individuals ≥7 yr old, Td is recommended for the primary series and booster doses because the lower concentration of diphtheria toxoid is adequately immunogenic and increasing the content of diphtheria toxoid heightens reactogenicity with increasing age.
For children 6 wk to 6 yr of age, five 0.5 mL doses of diphtheria-containing (D) vaccine (DTaP preferred) are given in the primary series, including doses at 2, 4, and 6 mo of age, and a 4th dose, an integral part of the primary series, at 15-18 mo. A booster dose is given at 4-6 yr of age (unless the 4th primary dose was administered at ≥4 yr). For persons ≥7 yr old not previously immunized for diphtheria, three 0.5 mL doses of lower-level diphtheria-containing (d) vaccine are given in a primary series of 2 doses at least 4 wk apart and a 3rd dose 6 mo after the 2nd dose. The 1st dose should be Tdap, and subsequent doses should be Td. The only contraindication to tetanus and diphtheria toxoid is a history of neurologic or severe hypersensitivity reaction after a prior dose. For children <7 yr old in whom pertussis immunization is contraindicated, DT is used. Those whose immunization is begun with DTaP or DT before 1 yr of age should have a total of five 0.5 mL doses of diphtheria-containing (D) vaccines by 6 yr of age. For those whose immunization is begun at around 1 yr old, the primary series is three 0.5 mL doses of diphtheria-containing (D) vaccine, with a booster given at 4-6 yr, unless the 3rd dose was given after the 4th birthday.
A booster dose, consisting of the adult preparation of Tdap, is recommended at 11-12 yr of age. Adolescents 13-18 yr old who missed the Td or Tdap booster dose at 11-12 yr or in whom it has been ≥5 yr since the Td booster dose also should receive a single dose of Tdap if they have completed the DTP/DTaP series.
There is no association of DT or Td with convulsions. Local adverse effects alone do not preclude continued use. The rare patient who experiences an Arthus-type hypersensitivity reaction or a temperature >39.4°C (103°F) after a dose of Td usually has high serum tetanus antitoxin levels and should not be given Td more frequently than every 10 yr, even if the patient sustains a significant tetanus-prone injury. The DT or Td preparation can be given concurrently with other vaccines. Haemophilus influenzae type b (Hib), meningococcal, and pneumococcal conjugate vaccines containing diphtheria toxoid (PRP-D) or the variant of diphtheria toxin, CRM197 protein, are not substitutes for diphtheria toxoid immunization and do not affect reactogenicity.