Nontuberculous Mycobacteria
Ericka V. Hayes
Nontuberculous mycobacteria (NTM ), also referred to as atypical mycobacteria and mycobacteria other than tuberculosis (MOTT), are all members of the genus Mycobacterium and include species other than Mycobacterium tuberculosis complex and Mycobacterium leprae . The NTM constitute a highly diverse group of bacteria that differ from M. tuberculosis complex bacteria in their pathogenicity, interhuman transmissibility, nutritional requirements, ability to produce pigments, enzymatic activity, and drug susceptibility. In contrast to the M. tuberculosis complex, NTM are acquired from environmental sources and not by person-to-person spread, although the latter is under debate, especially in patients with cystic fibrosis. Their omnipresence in the environment means that the clinical relevance of NTM isolation from clinical specimens is sometimes unclear; a positive culture might reflect occasional presence or contamination rather than true NTM disease. NTM are associated with pediatric lymphadenitis, otomastoiditis, serious lung infections, and, rarely, disseminated disease. Treatment is long-term and cumbersome and often requires adjunctive surgical intervention. Comprehensive guidelines on diagnosis and treatment are provided by the American Thoracic Society (ATS) and British Thoracic Society (BTS).
Etiology
NTM are ubiquitous in the environment all over the world, existing as saprophytes in soil and water (including municipal water supplies, tap water, hot tubs, and shower heads), environmental niches that are the supposed sources of human infections. With the introduction of molecular identification tools such as 16S recombinant DNA gene sequencing, the number of identified NTM species has grown to more than 150; the clinical relevance (i.e., percentage of isolates that are causative agents of true NTM disease, rather than occasional contaminants) differs significantly by species.
Mycobacterium avium complex (MAC ; i.e., M. avium , Mycobacterium intracellulare, and several closely related but rarer species) and Mycobacterium kansasii are most often isolated from clinical samples, yet the isolation frequency of these species differs significantly by geographic area. MAC bacteria have been frequently isolated from natural and synthetic environments, and cases of MAC disease have been successfully linked to home exposure to shower and tap water. Although the designation M. avium suggests that human infections are acquired from birds (Latin avium ), molecular typing has established that M. avium strains that cause pediatric lymphadenitis and adult pulmonary disease represent the M. avium hominis suis subgrouping, mainly found in humans and pigs and not in birds.
Some NTM have well-defined ecologic niches that help explain infection patterns. The natural reservoir for Mycobacterium marinum is fish and other cold-blooded animals, and the fish tank granuloma , a localized skin infection caused by M. marinum, follows skin injury in an aquatic environment. Mycobacterium fortuitum complex bacteria and Mycobacterium chelonae are ubiquitous in water and have caused clusters of nosocomial surgical wound and venous catheter–related infections. Mycobacterium ulcerans is associated with severe, chronic skin infections (Buruli ulcer disease ) and is endemic mainly in West Africa and Australia, although other foci exist. Its incidence is highest in children <15 yr old. M. ulcerans had been detected in environmental samples by polymerase chain reaction (PCR) but was only recently recovered by culture from a water strider (an insect of the Gerris genus) from Benin.
Epidemiology
Humans are exposed to NTM on a daily basis. In rural U.S. counties, where M. avium is common in swamps, the prevalence of asymptomatic infections with M. avium complex, as measured by skin test sensitization, approaches 70% by adulthood. Still, the incidence and prevalence of the various NTM disease types remain largely unknown, especially for pediatric NTM disease. In Australian children the overall incidence of NTM infection is 0.84 per 100,000, with lymphadenitis accounting for two thirds of cases. The incidence of pediatric NTM disease in the Netherlands is estimated at 0.77 infections per 100,000 children per year, with lymphadenitis making up 92% of all infections.
In comparison, estimations of the prevalence of NTM from respiratory samples in adults are 5-15 per 100,000 persons per year, with important differences between countries or regions. Because pulmonary NTM disease progresses slowly, over years rather than months, and usually takes several years to cure, the prevalence of pulmonary NTM disease is much higher than incidence rates would suggest.
The paradigm that NTM disease is a rare entity limited to developed countries is changing. In recent studies in African countries with a high prevalence of HIV infection, it has been found that NTM might play a much larger role as a cause of tuberculosis-like disease of children and adults than previously assumed and thus confuse the diagnosis of tuberculosis.
Although it is generally believed that NTM infections are contracted from environmental sources, recent whole genome sequence analysis of Mycobacterium abscessus strains of patients in a cystic fibrosis (CF) clinic in the United Kingdom has raised the possibility of nosocomial transmission among CF patients.
Pathogenesis
The histologic appearances of lesions caused by M. tuberculosis and NTM are often indistinguishable. The classic pathologic lesion consists of caseating granulomas. Compared to M. tuberculosis infections, NTM infections are more likely to result in granulomas that are noncaseating, poorly defined (nonpalisading), irregular or serpiginous or even absent, with only chronic inflammatory changes observed. The histology likely reflects the immune status of the patient.
In patients with AIDS and disseminated NTM infection, the inflammatory reaction is usually scant, and tissues are filled with large numbers of histiocytes packed with acid-fast bacilli (AFB). These disseminated NTM infections typically occur only after the number of CD4 T lymphocytes has fallen below 50/µL, suggesting that specific T-cell products or activities are required for immunity to mycobacteria.
The pivotal roles of interferon (IFN)-γ, interleukin (IL)-12, and tumor necrosis factor (TNF)-α in disease pathogenesis are demonstrated by the high incidence of mostly disseminated NTM disease in children with IFN-γ and IL-12 pathway deficiencies and in persons treated with agents that neutralize TNF-α.
Observed differences in pathogenicity, clinical relevance, and spectrum of clinical disease associated with the various NTM species emphasize the importance of bacterial factors in the pathogenesis of NTM disease, although exact virulence factors remain largely unknown.
Clinical Manifestations
Lymphadenitis of the superior anterior cervical or submandibular lymph nodes is the most common manifestation of NTM infection in children (Table 244.1 ). Preauricular, posterior cervical, axillary, and inguinal nodes are involved occasionally. Lymphadenitis is most common in children 1-5 yr of age and has been related to soil exposure (e.g., playing in sandboxes) and teething, although exact predisposing conditions have not been found. Given the constant environmental exposure to NTM, the occurrence of these infections might also reflect an atypical immune response of a subset of the infected children during or after their first contact with NTM. However, in healthy children with isolated NTM lymphadenitis, immunodeficiency is very rare.
Table 244.1
| SYNDROME | MOST COMMON CAUSES | LESS FREQUENT CAUSES* |
|---|---|---|
| Chronic nodular disease (adults with bronchiectasis; cystic fibrosis) | MAC (M. intracellulare, M. avium ), M. kansasii, M. abscessus | M. xenopi, M. malmoense, M. szulgai, M. smegmatis, M. celatum, M. simiae, M. goodii, M. asiaticum, M. heckeshornense, M. branderi, M. lentiflavum, M. triplex, M. fortuitum, M. arupense, M. abscessus subsp. bolletii, M. phocaicum, M. aubagnense, M. florentinum, M. abscessus subsp. massiliense, M. nebraskense, M. saskatchewanense, M. seoulense, M. senuense, M. paraseoulense, M. europaeum, M. sherrisii, M. kyorinense, M. noviomagense, M. mantenii, M. shinjukuense, M. koreense, M. heraklionense, M. parascrofulaceum, M. arosiense |
| Cervical or other lymphadenitis (especially children) | MAC | M. scrofulaceum, M. malmoense (northern Europe), M. abscessus, M. fortuitum, M. lentiflavum, M. tusciae, M. palustre, M. interjectum, M. elephantis, M. heidelbergense, M. parmense, M. bohemicum, M. haemophilum, M. europaeum, M. florentinum, M. triplex, M. asiaticum, M. kansasii, M. heckeshornense |
| Skin and soft tissue disease | M. fortuitum group, M. chelonae, M. abscessus, M. marinum, M. ulcerans (Australia, tropical countries only) | M. kansasii, M. haemophilum, M. porcinum, M. smegmatis, M. genavense, M. lacus, M. novocastrense, M. houstonense, M. goodii, M. immunogenum, M. mageritense, M. abscessus subsp. massiliense, M. arupense, M. monacense, M. bohemicum, M. branderi, M. shigaense, M. szulgai, M. asiaticum, M. xenopi, M. kumamotense, M. setense, M. montefiorense (eels), M. pseudoshottsii (fish), M. shottsii (fish) |
| Skeletal (bone, joint, tendon) infection | M. marinum, MAC, M. kansasii, M. fortuitum group, M. abscessus, M. chelonae | M. haemophilum, M. scrofulaceum, M. heckeshornense, M. smegmatis, M. terrae/chromogenicum complex, M. wolinskyi, M. goodii, M. arupense, M. xenopi, M. triplex, M. lacus, M. arosiense |
| Disseminated infection | M. genavense, M. haemophilum, M. xenopi | |
| HIV-seropositive host | M. avium, M. kansasii | M. marinum, M. simiae, M. intracellulare, M. scrofulaceum, M. fortuitum, M. conspicuum, M. celatum, M. lentiflavum, M. triplex, M. colombiense, M. sherrisii, M. heckeshornense |
| HIV-seronegative host | M. abscessus, M. chelonae | M. marinum, M. kansasii, M. haemophilum, M. chimaera, M. conspicuum, M. shottsii (fish), M. pseudoshottsii (fish) |
| Catheter-related infections | M. fortuitum, M. abscessus, M. chelonae | M. mucogenicum, M. immunogenum, M. mageritense, M. septicum, M. porcinum, M. bacteremicum, M. brumae |
| Hypersensitivity pneumonitis | Metal workers; hot tub | M. immunogenum M. avium |
* The available information is sparse for selected pathogens such as M. xenopi, M. malmoense, M. szulgai, M. celatum, and M. asiaticum and the newly described species.
HIV, Human immunodeficiency virus; MAC, Mycobacterium avium complex.
From Brown-Elliott BA, Wallace Jr. RJ: Infections caused by nontuberculous mycobacteria other than Mycobacterium avium complex. In Bennett JF, Dolin R, Blaser MJ, editors: Mandell, Douglas, and Bennett's principles and practice of infectious diseases, ed 8, Philadelphia, 2015, Elsevier (Table 254-1).
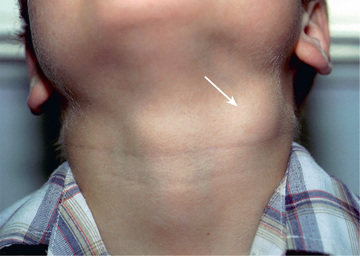
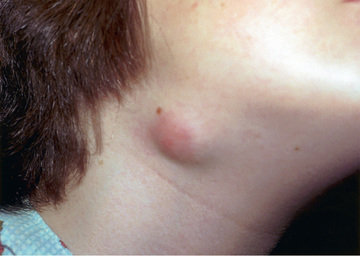
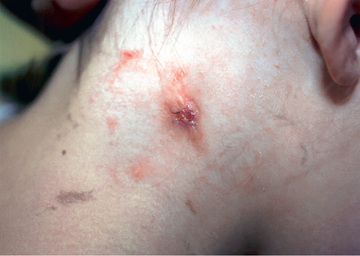
Affected children usually lack constitutional symptoms and present with a unilateral subacute and slowly enlarging lymph node or group of closely approximated nodes >1.5 cm in diameter that are firm, painless, freely movable, and not erythematous (Fig. 244.1 ). The involved nodes occasionally resolve without treatment, but most undergo rapid suppuration after several weeks (Fig. 244.2 ). The center of the node becomes fluctuant, and the overlying skin thins and becomes erythematous and often even violaceous. Eventually, the nodes rupture and can form cutaneous sinus tracts that can drain persistently, reminiscent of scrofula from tuberculosis (Fig. 244.3 ).
In the United States and Western Europe, M. avium complex accounts for approximately 80% of NTM lymphadenitis in children. M. kansasii accounts for most other cases of lymphadenitis in the United States. Mycobacterium malmoense and Mycobacterium haemophilum have also been described as causative agents of lymphadenitis. M. malmoense is only common in Northwestern Europe. For M. haemophilum , underestimation of its importance is likely because the bacteria require specific culture conditions (hemin-enriched media, low incubation temperatures). On the basis of PCR analysis of lymph node samples from lymphadenitis cases in The Netherlands, M. haemophilum is the 2nd most common cause of this infection, after M. avium complex. One study suggests that children with M. avium complex lymphadenitis are significantly younger than those infected by M. haemophilum , possibly related to age-specific environmental exposures. Mycobacterium lentiflavum is also an emerging NTM associated with lymphadenitis.
Cutaneous disease caused by NTM is rare in children (see Table 244.1 ). Infection usually follows percutaneous inoculation with fresh or salt water contaminated by M. marinum. Within 2-6 wk after exposure, an erythematous papule develops at the site of minor abrasions on the elbows, knees, or feet (swimming pool granuloma ) and on the hands and fingers of fish tank owners, mostly inflicted during tank cleaning (fish tank granuloma ). These lesions are usually nontender and enlarge over 3-5 wk to form violaceous plaques. Nodules or pustules can develop and occasionally will ulcerate, resulting in a serosanguineous discharge. The lesions sometimes resemble sporotrichosis, with satellite lesions near the site of entry, extending along the superficial lymphatics. Lymphadenopathy is usually absent. Although most infections remain localized to the skin, penetrating M. marinum infections can result in tenosynovitis, bursitis, osteomyelitis, or arthritis.
M. ulcerans infection is the 3rd most common mycobacterial infection in immunocompetent patients, after M. tuberculosis and M. leprae infection, and causes cutaneous disease in children living in tropical regions of Africa, South America, Asia, and parts of Australia. In some communities in West Africa, up to 16% of people have been affected. Children <15 yr old are particularly affected in rural tropical counties, accounting for 48% of infected individuals in Africa. Infection follows percutaneous inoculation from minor trauma, such as pricks and cuts from plants or insect bites. After an incubation period of approximately 3 mo, lesions appear as an erythematous nodule, usually on legs or arms. The lesion undergoes central necrosis and ulceration. The lesion, often called a Buruli ulcer after the region in Uganda where a large case series was reported, has a characteristic undermined edge, expands over several weeks, and can result in extensive, deep soft tissue destruction or bone involvement. Lesions are typically painless, and constitutional symptoms are unusual. Lesions might heal slowly over 6-9 mo or might continue to spread, leading to deformities, contractures, and disability.
Skin and soft tissue infections caused by rapidly growing mycobacteria , such as M. fortuitum, M. chelonae, or M. abscessus, are rare in children and usually follow percutaneous inoculation from puncture or surgical wounds, minor abrasions, or tattooing. There has been a large outbreak of M. fortuitum furunculosis related to nail salon footbaths. Clinical disease usually arises after a 4-6 wk incubation period and manifests as localized cellulitis, painful nodules, or a draining abscess. M. haemophilum can cause painful subcutaneous nodules, which often ulcerate and suppurate in immunocompromised patients, particularly after kidney transplantation.
NTM are an uncommon cause of catheter-associated infections but are becoming increasingly recognized in this respect. Infections caused by M. fortuitum, M. chelonae, or M. abscessus can manifest as bacteremia or localized catheter tunnel infections.
Otomastoiditis , or chronic otitis media, is a rare extrapulmonary NTM disease type that specifically affects children with tympanostomy tubes and a history of topical antibiotic or steroid use. M. abscessus is the most common causative agent, followed by M. avium complex (see Table 244.1 ). Patients present with painless, chronic otorrhea resistant to antibiotic therapy. CT can reveal destruction of the mastoid bone with mucosal swelling (Fig. 244.4 ).
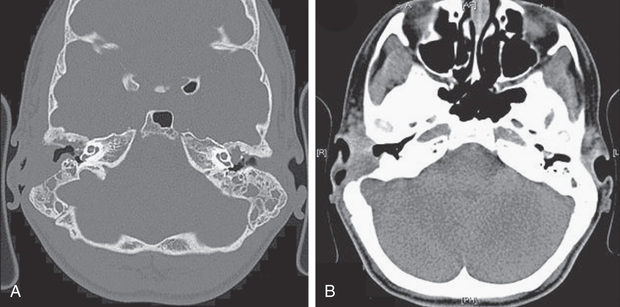
Delayed or unsuccessful treatment can result in permanent hearing loss. In unusual circumstances, NTM causes other bone and joint infections that are indistinguishable from those produced by M. tuberculosis or other bacterial agents. Such infections usually result from operative incision or accidental puncture wounds. M. fortuitum infections from puncture wounds of the foot resemble infections caused by Pseudomonas aeruginosa and Staphylococcus aureus .
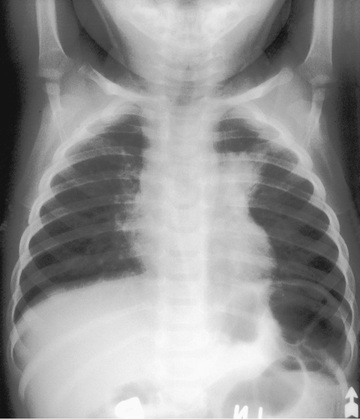
Pulmonary infections are the most common form of NTM illness in adults but are rare in children. M. avium complex bacteria, the most commonly identified organisms (see Table 244.1 ), are capable of causing acute pneumonitis, chronic cough, or wheezing associated with paratracheal or peribronchial lymphadenitis and airway compression in normal children. Associated constitutional symptoms such as fever, anorexia, and weight loss occur in 60% of these children. Chest radiographic findings are very similar to those for primary tuberculosis, with unilateral infiltrates and hilar lymphadenopathy (Fig. 244.5 ). Pleural effusion is uncommon. Rare cases of progression to endobronchial granulation tissue have been reported.
Pulmonary infections usually occur in adults with underlying chronic lung disease. The onset is insidious and consists of cough and fatigue, progressing to weight loss, night sweats, low-grade fever, and generalized malaise in severe cases. Thin-walled cavities with minimal surrounding parenchymal infiltrates are characteristic, but radiographic findings can resemble those of tuberculosis. A separate disease manifestation occurs in postmenopausal women and is radiologically characterized by bronchiectasis and nodular lesions, often affecting the middle lobe and lingula.
Chronic pulmonary infections specifically affect children with CF and are generally caused by M. abscessus and M. avium complex. M. abscessus primarily affects children, and M. avium complex is most common among adults. The percentage of CF patients with at least 1 sputum culture positive for NTM is 6–8.1% overall and increases with age; in CF patients <12 yr old, a prevalence of 3.9% has been reported. The strong representation of M. abscessus in these patients is remarkable, because this bacterium is an uncommon isolate in other categories of patients. There are indications that NTM infections in CF patients further accelerate the decline in lung function; antimycobacterial therapy can result in weight gain and improved lung function in affected patients.
Disseminated disease is usually associated with M. avium complex infection and occurs in immunocompromised children. The 1st category of patients with disseminated disease includes persons with mutations in genes coding for the interferon-γ receptor (IFNGR) or the IL-12 receptor, or for IL-12 production. Patients with complete IFNGR deficiency have severe, difficult-to-treat disease. Those with partial IFNGR deficiency or IL-12 pathway mutations have milder disease that can respond to IFN-γ and antimycobacterial therapy. Multifocal osteomyelitis is particularly prevalent in persons with the IFNGR1 818del4 mutation. Recurrences, even years after a course of treatment, and multiple infections are well documented. The 2nd category of patients affected by disseminated disease is patients with acquired immunodeficiency syndrome (AIDS ). Disseminated NTM disease in patients with AIDS usually appears when CD4 cell counts are <50 cells/µL; in younger children, especially those <2 yr old, these infections occur at higher CD4 cell counts. The most recent estimate of the incidence of disseminated NTM disease is 0.14-0.2 episodes per 100 person-years, a 10-fold decrease from its incidence before highly active antiretroviral therapy (HAART) was available.
Colonization of the respiratory or gastrointestinal (GI) tract probably precedes disseminated M. avium complex infections, but screening studies of respiratory secretions or stool samples are not useful to predict dissemination. Continuous high-grade bacteremia is common, and multiple organs are infected, typically including lymph nodes, liver, spleen, bone marrow, and GI tract. Thyroid, pancreas, adrenal gland, kidney, muscle, and brain can also be involved. The most common signs and symptoms of disseminated M. avium complex infections in patients with AIDS are fever, night sweats, chills, anorexia, marked weight loss, wasting, weakness, generalized lymphadenopathy, and hepatosplenomegaly. Jaundice, elevated alkaline phosphatase or lactate dehydrogenase levels, anemia, and neutropenia can occur. Imaging studies usually demonstrate massive lymphadenopathy of hilar, mediastinal, mesenteric, or retroperitoneal nodes. The survival in children with AIDS has improved considerably with the availability of HAART.
Disseminated disease in children without any apparent immunodeficiency is exceedingly rare.
Diagnosis
For infections of lymph nodes, skin, bone, and soft tissues, isolation of the causative NTM bacteria by Mycobacterium culture, preferably with histologic confirmation of granulomatous inflammation, normally suffices for diagnosis (Table 244.2 ). The differential diagnosis of NTM lymphadenitis includes acute bacterial lymphadenitis, tuberculosis, cat-scratch disease (Bartonella henselae) , mononucleosis, toxoplasmosis, brucellosis, tularemia, and malignancies, especially lymphomas. Differentiation between NTM and M. tuberculosis may be difficult, but children with NTM lymphadenitis usually have a Mantoux tuberculin skin test reaction of <15 mm induration, unilateral anterior cervical node involvement, a normal chest radiograph, and no history of exposure to adult tuberculosis. Definitive diagnosis requires excision of the involved nodes for culture and histology. Fine-needle aspiration for PCR and culture can enable earlier diagnosis, before excisional biopsy.
Table 244.2
American Thoracic Society Diagnostic Criteria for Nontuberculous Mycobacteria (NTM) Lung Disease
AFB, Acid-fast bacilli; HRCT, high-resolution computed tomography.
Data from Griffith DE, Aksamit T, Brown-Elliott BA, et al: An official ATS/IDSA statement: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases, Am J Respir Crit Care Med 175:367–416, 2007.
From Brown-Elliott BA, Wallace Jr. RJ: Infections caused by nontuberculous mycobacteria other than Mycobacterium avium complex. In Bennett JF, Dolin R, Blaser MJ, editors: Mandell, Douglas, and Bennett's principles and practice of infectious diseases, ed 8, Philadelphia, 2015, Elsevier (Table 254-3).
The diagnosis of pulmonary NTM infection in children is difficult because many species of NTM, including M. avium complex, are omnipresent in our environment and can contaminate clinical samples or be present but not causative of disease. As a result, isolation of these bacteria from nonsterile specimens (respiratory and digestive tract) does not necessarily reflect true disease. To determine the clinical relevance of isolation of NTM, the ATS/BTS diagnostic criteria are an important support. These criteria take into consideration clinical features and radiologic, pathologic, and microbiologic findings. Their hallmark is the need for multiple positive cultures yielding the same NTM species to make a definitive diagnosis of pulmonary NTM disease. In children, definitive diagnosis often requires invasive procedures such as bronchoscopy and pulmonary or endobronchial biopsy; in CF patients, more aggressive sample pretreatment is necessary to prevent overgrowth by other species, especially Pseudomonas . The chance of NTM isolation being clinically relevant differs significantly by species; some species are more likely causative agents of true pulmonary disease (M. avium , M. kansasii , M. abscessus , M. malmoense ), whereas others are more likely contaminants (Mycobacterium gordonae , M. fortuitum , M. chelonae ).
Blood cultures are 90–95% sensitive in AIDS patients with disseminated infection. M. avium complex may be detected within 7-10 days of inoculation in almost all patients by automated blood culture systems. In adults, some studies have shown that liver biopsy cultures and stains are more sensitive than blood culture or bone marrow biopsy workup. Commercially available DNA probes differentiate NTM from M. tuberculosis. If DNA probes cannot identify the causative mycobacteria, DNA sequencing of bacterial housekeeping genes will always yield a clue to the identity of these NTM. Identification of histiocytes containing numerous AFB from bone marrow and other biopsy tissues provides a rapid presumptive diagnosis of disseminated mycobacterial infection.
Treatment
Therapy for NTM infections is long-term and cumbersome; expert consultation is advised. Therapy involves medical, surgical, or combined treatment (see Chapter 241 , Table 241.3 ). Isolation of the infecting strain followed by drug-susceptibility testing is ideal, because it provides a baseline for drug susceptibility. Important discrepancies exist between in vitro drug susceptibility and in vivo response to treatment, explained in part by synergism, mainly among first-line antituberculosis drugs. In vitro, slow growers (M. kansasii, M. marinum, Mycobacterium xenopi, M. ulcerans, M. malmoense ) are usually susceptible to the first-line antituberculosis drugs rifampicin and ethambutol ; M. avium complex bacteria are often resistant to these drugs alone but susceptible to the combination and have variable susceptibility to other antibiotics, most importantly the macrolides. Rapid growers (M. fortuitum, M. chelonae, M. abscessus ) are highly resistant to antituberculosis drugs and often have inducible macrolide-resistance mechanisms. Susceptibility to macrolides, aminoglycosides, carbapenems, tetracyclines, and glycylcyclines are most relevant for therapy guidance. In all NTM infections, multidrug therapy (MDT) is essential to avoid development of resistance.
The preferred treatment of NTM lymphadenitis is complete surgical excision. Clinical trials revealed that surgery is more effective than antibiotic treatment (see Table 241.3 ). Nodes should be removed while still firm and encapsulated. Excision is more difficult if extensive caseation with extension to surrounding tissue has occurred, and complications of facial nerve damage or recurrent infection are more likely in such cases. Incomplete surgical excision is not advised, because chronic drainage can develop. If there are concerns or risk factors for possible M. tuberculosis infection, therapy with isoniazid, rifampin, ethambutol, and pyrazinamide should be administered until cultures confirm the cause to be NTM (see Chapter 242 ). If surgery of NTM lymphadenitis cannot be performed for some reason, or removal of infected tissue is incomplete, or recurrence or chronic drainage develops, a 3 mo trial of chemotherapy is warranted. Clarithromycin or azithromycin combined with rifabutin or ethambutol are the most common therapy regimens reported (see Table 241.3 ). Suppuration may still occur on antibiotic therapy. In select patients, a wait-and-see approach can be chosen because the disease can resolve spontaneously, although resolution can take several months.
Posttraumatic cutaneous NTM lesions in immunocompetent patients usually heal spontaneously after incision and drainage without other therapy (see Table 241.3 ). M. marinum is susceptible to rifampin, amikacin, ethambutol, sulfonamides, trimethoprim-sulfamethoxazole, and tetracycline. Therapy with a combination of these drugs, particularly clarithromycin and ethambutol, may be given until 1 mo after the lesion has disappeared. Corticosteroid injections should not be used. Superficial infections with M. fortuitum or M. chelonae usually resolve after surgical incision and open drainage, but deep-seated or catheter-related infections require removal of infected central lines and therapy with parenteral amikacin plus cefoxitin, ciprofloxacin, or clarithromycin.
Some localized forms of M. ulcerans skin disease (Buruli ulcer) can heal spontaneously; for most forms, excisional surgery with primary closure or skin grafting is recommended. Provisional guidelines by the World Health Organization recommend treatment with rifampin and streptomycin, with or without surgery. Currently, all-oral regimens of rifampicin and fluoroquinolones or macrolides are being tested in clinical trials. In clinical experience, a drug treatment duration of 8 wk generally leads to low recurrence levels. Physiotherapy after surgery is essential to prevent contractures and functional disabilities.
Pulmonary infections should be treated initially with isoniazid, rifampin, ethambutol, and pyrazinamide pending culture identification and drug-susceptibility testing particularly if their is high suspicion for tuberculosis. For slow-growing NTM, a combination of rifampin or rifabutin, ethambutol, and clarithromycin (or azithromycin) is recommended; exceptions are M. kansasii , for which a regimen of isoniazid, rifampicin, and ethambutol is advised, and M. simiae , for which no effective regimen is known, and regimens are usually designed on the basis of in vitro drug susceptibilities. After culture conversion, treatment should be continued for at least 1 yr. For pulmonary disease caused by rapidly growing NTM, a combination of macrolides, fluoroquinolones, aminoglycosides, cefoxitin, and carbapenems is the optimal therapy; 3- or 4-drug regimens are selected on drug-susceptibility testing results. In patients with CF, inhaled antibiotics may have a role.
Patients with disseminated M. avium complex and IL-12 pathway defects or IFNGR deficiency should be treated for at least 12 mo with clarithromycin or azithromycin combined with rifampin or rifabutin and ethambutol. In vitro susceptibility testing for clarithromycin is important to guide therapy. Once the clinical illness has resolved, lifelong daily prophylaxis with azithromycin or clarithromycin is advisable to prevent recurrent disease. The use of interferon adjunctive therapy is determined by the specific genetic defect.
In children with AIDS, prophylaxis with azithromycin or clarithromycin is indicated to prevent infection with M. avium complex. Although few pediatric studies exist, the U.S. Public Health Service recommends either azithromycin (20 mg/kg once weekly PO, maximum 1,200 mg/dose; or 5 mg/kg once daily PO, maximum 250 mg/dose in patients intolerant of larger dose) or clarithromycin (7.5 mg/kg/dose twice daily PO; maximum 500 mg/dose) for HIV-infected children with significant immune deficiency, as defined by the CD4 count (children ≥6 yr old, CD4 count <50 cells/µL; 2-6 yr old, <75/µL; 1-2 yr old, <500/µL; <1 yr old, <750/µL). Primary prophylaxis may be safely discontinued in children >2 yr old receiving stable HAART for >6 mo and experiencing sustained (>3 mo) CD4 cell recovery well above the age-specific target for initiation of prophylaxis: >100 cells/µL for children ≥6 yr old and >200/µL for children 2-5 yr old. For children <2 yr old, no specific recommendations for discontinuing MAC prophylaxis exist.