Chlamydia pneumoniae
Stephan A. Kohlhoff, Margaret R. Hammerschlag
Chlamydia pneumoniae is a common cause of lower respiratory tract diseases, including pneumonia in children and bronchitis and pneumonia in adults.
Etiology
Chlamydiae are obligate intracellular pathogens that have established a unique niche in host cells. Chlamydiae cause a variety of diseases in animal species at virtually all phylogenic levels. The most significant human pathogens are C. pneumoniae and Chlamydia trachomatis (see Chapter 253 ). Chlamydia psittaci is the cause of psittacosis, an important zoonosis (see Chapter 254 ). There are now 9 recognized chlamydial species.
Chlamydiae have a gram-negative envelope without detectable peptidoglycan, although recent genomic analysis has revealed that both C. pneumoniae and C. trachomatis encode proteins forming a nearly complete pathway for synthesis of peptidoglycan, including penicillin-binding proteins. Chlamydiae also share a group-specific lipopolysaccharide antigen and use host adenosine triphosphate for the synthesis of chlamydial proteins. Although chlamydiae are auxotrophic for 3 of 4 nucleoside triphosphates, they encode functional glucose-catabolizing enzymes that can be used to generate adenosine triphosphate. As with peptidoglycan synthesis, for some reason these genes are turned off. All chlamydiae also encode an abundant surface-exposed protein called the major outer membrane protein. The major outer membrane protein is the major determinant of the serologic classification of C. trachomatis and C. psittaci isolates.
Epidemiology
C. pneumoniae is primarily a human respiratory pathogen. The organism has also been isolated from nonhuman species, including horses, koalas, reptiles, and amphibians, where it also causes respiratory infection, although the role that these infections might play in transmission to humans is unknown. C. pneumoniae appears to affect individuals of all ages. The proportion of community-acquired pneumonias associated with C. pneumoniae infection is 2–19%, varying with geographic location, the age group examined, and the diagnostic methods used. Several studies of the role of C. pneumoniae in lower respiratory tract infection in pediatric populations have found evidence of infection in 0–18% of patients based on serology or culture for diagnosis. In 1 study, almost 20% of the children with C. pneumoniae infection were coinfected with Mycoplasma pneumoniae. C. pneumoniae may also be responsible for 10–20% of episodes of acute chest syndrome in children with sickle cell disease, up to 10% of asthma exacerbations, 10% of episodes of bronchitis, and 5–10% of episodes of pharyngitis in children. Asymptomatic infection appears to be common based on epidemiologic studies.
Transmission probably occurs from person to person through respiratory droplets. Spread of the infection appears to be enhanced by close proximity, as is evident from localized outbreaks in enclosed populations, such as military recruits and in nursing homes.
Pathogenesis
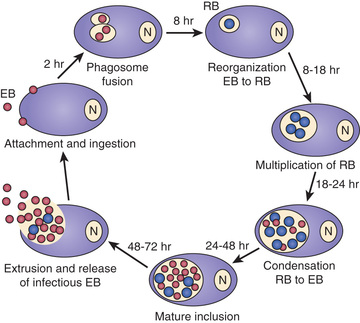
Chlamydiae are characterized by a unique developmental cycle (Fig. 252.1 ) with morphologically distinct infectious and reproductive forms: the elementary body (EB) and reticulate body (RB). Following infection, the infectious EBs, which are 200-400 µm in diameter, attach to the host cell by a process of electrostatic binding and are taken into the cell by endocytosis that does not depend on the microtubule system. Within the host cell, the EB remains within a membrane-lined phagosome. The phagosome does not fuse with the host cell lysosome. The inclusion membrane is devoid of host cell markers, but lipid markers traffic to the inclusion, which suggests a functional interaction with the Golgi apparatus. The EBs then differentiate into RBs that undergo binary fission. After approximately 36 hr, the RBs differentiate into EBs. At approximately 48 hr, release can occur by cytolysis or by a process of exocytosis or extrusion of the whole inclusion, leaving the host cell intact. Chlamydiae can also enter a persistent state after treatment with certain cytokines such as interferon-γ, treatment with antibiotics, or restriction of certain nutrients. While chlamydiae are in the persistent state, metabolic activity is reduced. The ability to cause prolonged, often subclinical, infection is one of the major characteristics of chlamydiae.
Clinical Manifestations
Infections caused by C. pneumoniae cannot be readily differentiated from those caused by other respiratory pathogens, especially M. pneumoniae. The pneumonia usually occurs as a classic atypical (or nonbacterial) pneumonia characterized by mild to moderate constitutional symptoms, including fever, malaise, headache, cough, and often pharyngitis. Severe pneumonia with pleural effusions and empyema has been described. Milder respiratory infections have been described, manifesting as a pertussis-like illness.
C. pneumoniae can serve as an infectious trigger for asthma, can cause pulmonary exacerbations in patients with cystic fibrosis, and can produce acute chest syndrome in patients with sickle cell anemia. C. pneumoniae has been isolated from middle ear aspirates of children with acute otitis media, most of the time as co-infection with other bacteria. Asymptomatic respiratory infection has been documented in 2–5% of adults and children and can persist for 1 yr or longer.
Diagnosis
It is not possible to differentiate C. pneumoniae from other causes of atypical pneumonia on the basis of clinical findings. Auscultation reveals the presence of rales and often wheezing. The chest radiograph often appears worse than the patient's clinical status would indicate and can show mild, diffuse involvement or lobar infiltrates with small pleural effusions. The complete blood count may be elevated with a left shift but is usually unremarkable.
Specific diagnosis of C. pneumoniae infection has been based on isolation of the organism in tissue culture. C. pneumoniae grows best in cycloheximide-treated HEp-2 and HL cells. The optimum site for culture is the posterior nasopharynx; the specimen is collected with wire-shafted swabs in the same manner as that used for C. trachomatis. The organism can be isolated from sputum, throat cultures, bronchoalveolar lavage fluid, and pleural fluid, but few laboratories perform such cultures because of technical difficulties. A multiplexed nucleic acid amplification testing assay (Film Array, Biofire Diagnostics, Salt Lake City, UT) received FDA clearance in 2012 for the detection of 17 viruses and C. pneumoniae , M. pneumoniae, and Bordetella pertussis. The Film Array system combines nucleic acid extraction, nested polymerase chain reaction, detection, and data analysis.
Serologic diagnosis can be accomplished using the microimmunofluorescence (MIF) or the complement fixation tests. The complement fixation test is genus specific and is also used for diagnosis of lymphogranuloma venereum (see Chapter 253.4 ) and psittacosis (see Chapter 254 ). Its sensitivity in hospitalized patients with C. pneumoniae infection and children is variable. The Centers for Disease Control and Prevention (CDC) has proposed modifications in the serologic criteria for diagnosis. Although the MIF test was considered to be the only currently acceptable serologic test, the criteria were made significantly more stringent. Acute infection, using the MIF test, was defined by a 4-fold increase in immunoglobulin (Ig) G titer or an IgM titer of ≥16; use of a single elevated IgG titer was discouraged. An IgG titer of ≥16 was thought to indicate past exposure, but neither elevated IgA titers nor any other serologic marker was thought to be a valid indicator of persistent or chronic infection. Because diagnosis would require paired sera, this would be a retrospective diagnosis. The CDC did not recommend the use of any enzyme-linked immune assay for detection of antibody to C. pneumoniae because of concern about the inconsistent correlation of these results with culture results. Studies of C. pneumoniae infection in children with pneumonia and asthma show that more than 50% of children with culture-documented infection have no detectable MIF antibody.
Treatment
The optimum dose and duration of antimicrobial therapy for C. pneumoniae infections remain uncertain. Most treatment studies have used only serology for diagnosis, and thus microbiologic efficacy cannot be assessed. Prolonged therapy for 2 wk or longer is required for some patients, because recrudescent symptoms and persistent positive cultures have been described following 2 wk of erythromycin and 30 days of tetracycline or doxycycline.
Tetracyclines, macrolides (erythromycin, azithromycin, and clarithromycin), and quinolones show in vitro activity. Like C. psittaci, C. pneumoniae is resistant to sulfonamides. The results of treatment studies have shown that erythromycin (40 mg/kg/day PO divided twice a day for 10 days), clarithromycin (15 mg/kg/day PO divided twice a day for 10 days), and azithromycin (10 mg/kg PO on day 1, and then 5 mg/kg/day PO on days 2-5) are effective for eradication of C. pneumoniae from the nasopharynx of children with pneumonia in approximately 80% of cases.
Prognosis
Clinical response to antibiotic therapy varies. Coughing often persists for several weeks even after therapy.