Influenza Viruses*
Fiona P. Havers, Angela J.P. Campbell
Influenza viral infections cause a broad array of respiratory illnesses that are responsible for significant morbidity and mortality in children during seasonal epidemics . Influenza A viruses also have the potential to cause global pandemics , which can happen when a new (novel) influenza A virus emerges and transmits efficiently from person to person.
Etiology
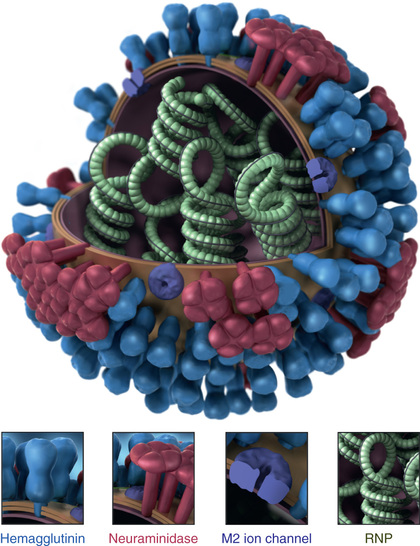
Influenza viruses are large, single-stranded RNA viruses belonging to the family Orthomyxoviridae, which includes three genera (or types): A, B, and C. Influenza A and B viruses are the primary human pathogens causing seasonal epidemics, while influenza virus type C is a sporadic cause of predominantly mild upper respiratory tract illness. Influenza A viruses are further divided into subtypes based on two surface proteins that project as spikes from the lipid envelope, the hemagglutinin (HA) and neuraminidase (NA) proteins (Fig. 285.1 ). Strain variants are identified by antigenic differences in their HA and NA and are designated by the geographic area from which they were originally isolated, isolate number, and year of isolation—for example, influenza A/Victoria/361/2011(H3N2). The HA and NA antigens from influenza B and C viruses do not receive subtype designations, as there is less variation among influenza B and C antigens. However, influenza B viruses can be further broken down into lineages; currently circulating influenza B viruses belong to the B/Yamagata or B/Victoria lineage.
Epidemiology
Influenza has generally been thought to be transmitted primarily via respiratory droplets, but transmission through contact with secretions and small-particle aerosols may also occur. The typical incubation period ranges from 1 to 4 days, with an average of 2 days. Healthy adults are generally considered potentially infectious from a day before symptoms develop until 5-7 days after becoming ill. Children with primary influenza infection have higher influenza viral loads and more prolonged viral shedding than adults; therefore children may be able to infect others for a longer time. Influenza outbreaks occur commonly in schools and childcare settings. Healthcare-associated influenza infections can also occur in healthcare settings, and outbreaks in long-term care facilities and hospitals may cause significant morbidity.
In the United States, seasonal influenza viruses can be detected year round, but circulating viruses are most common during the fall and winter. Transmission through a community is rapid, with the highest incidence of illness occurring within 2-3 wk of introduction.
Antigenic Variation
Influenza A and B viruses contain a genome consisting of 8 single-stranded RNA segments. Minor changes within a subtype continually occur through point mutations during viral replication, particularly in the HA gene, and result in new influenza strains of the same HA type. This phenomenon, termed antigenic drift , occurs in both influenza A and B viruses. Variation in antigenic composition of influenza virus surface proteins occurs almost yearly, which confers a selective advantage to a new strain and contributes to annual epidemics. For this reason, the formulation of the influenza vaccine is reviewed each year and updated as needed.
Less frequent but more dramatic, major changes in virus subtype can occur, resulting in a new influenza A subtype to which most people have little to no immunity. This process is called antigenic shift and can occur through reassortment of viral gene segments when there is simultaneous infection by more than one strain of influenza in a single host, or by direct adaptation of an animal virus to a human host. Antigenic shift occurs in influenza A viruses, which have multiple avian and mammalian hosts acting as reservoirs for diverse strains.
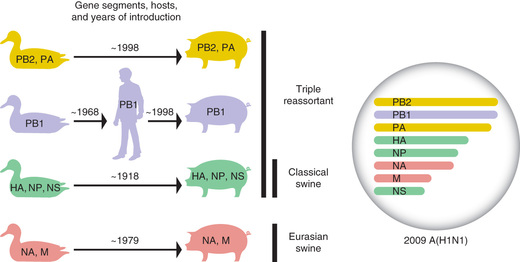
Through the process of reassortment , potentially any of 18 HA and 11 NA proteins currently known to reside in influenza A viruses of nonhuman hosts could be introduced into humans, who may have little existing immunologic cross protection to emerging viruses. A global pandemic can result if an influenza A virus with a novel HA or NA enters a nonimmune human population and acquires the capacity for sustained and efficient transmission between people. Four major global pandemics have occurred since 1900: in 1918 caused by an influenza A(H1N1) virus, 1957 caused by an influenza A(H2N2) virus, 1968 caused by an influenza A(H3N2) virus, and 2009 caused by an influenza A virus designated A(H1N1)pdm09. The most severe pandemic in recorded history occurred in 1918, when the virus was estimated to have killed at least 50 million people. The 1918 pandemic virus was likely the result of direct adaptation of an avian influenza virus to the human host, rather than from reassortment. The 2009 pandemic virus stemmed from reassortment of genes from swine, avian, and human viruses (Fig. 285.2 ). This resulted in the emergence of a novel influenza A(H1N1)pdm09 virus that spread quickly from North America across the globe and replaced the previously circulating seasonal H1N1 viruses.
Several novel influenza viruses, all originating in animals, have also caused outbreaks of human infections. Avian influenza A(H5N1), a virulent avian influenza virus that was first identified in 1997, has caused more than 800 documented cases in 16 countries, with a mortality rate over 50%. Another novel avian influenza, A(H7N9) virus, has caused more than 1,300 documented cases and also appears highly virulent. This virus first caused an outbreak of human infections in China during the spring of 2013, with annual epidemics in China occurring in subsequent years. During the first 4 yearly epidemics, infection was fatal in approximately 40% of documented cases.
In addition, novel influenza A variant viruses have caused human infections (Table 285.1 ). These include H3N2v viruses, which caused 372 confirmed human infections in the United States from 2011 to 2016 and were primarily transmitted through swine contact at agricultural fairs. Influenza viruses that normally circulate in swine are designated variant (“v”) viruses when detected in humans, and H3N2v and other variant viruses, including H1N1v and H1N2v, have sporadically infected humans. In contrast to avian influenza A(H5N1) and A(H7N9) viruses, variant viruses generally cause mild illness and have been primarily detected in children. However, none of these viruses has exhibited sustained, efficient human-to-human transmission.
Table 285.1
Subtypes of Novel Influenza A Viruses and Clinical Syndromes in Human Infections
| LPAI VIRUSES | HPAI VIRUSES | VARIANT VIRUSES* | |
|---|---|---|---|
| Conjunctivitis | H7N2, H7N3, H7N7, H10N7 | H7N3, H7N7 | H1N1v, H3N2v |
| Upper respiratory tract illness | H6N1, H7N2, H7N3, H7N9, H9N2, H10N7 | H5N1, H5N6, H7N7 | H1N1v, H1N2v, H3N2v |
| Lower respiratory tract disease, pneumonia | H7N2, H7N9, H9N2, H10N8 | H5N1, H5N6, H7N7, H7N9 | H1N1v, H3N2v |
| Respiratory failure, acute respiratory distress syndrome | H7N9, H10N8 | H5N1, H5N6, H7N7, H7N9 | H1N1v, H3N2v |
| Multiorgan failure | H7N9, H10N8 | H5N1, H5N6, H7N7, H7N9 | — |
| Encephalopathy or encephalitis | H7N9 | H5N1 | — |
| Fatal outcomes † | H7N9, H9N2, H10N8 | H5N1, H5N6, H7N7, H7N9 | H1N1v, H3N2v |
* Variant viruses of swine origin.
† High mortality in reported cases: about 40% for LPAI H7N9, about 50% for HPAI H5N1, and about 70% for HPAI H5N6.
LPAI, low-pathogenic avian influenza; HPAI, highly pathogenic avian influenza.
From Uyeki TM, Katz JM, Jernigan DB: Novel influenza A viruses and pandemic threats, Lancet 389:2172–2174, 2017.
Seasonal Influenza
An estimated 11,000-45,000 children younger than 18 yr of age are hospitalized annually in the United States as a result of seasonal influenza-associated complications, with approximately 6,000-26,000 hospitalizations in children younger than 5 yr of age. Since 2004, the annual number of reported influenza-associated pediatric deaths in the United States has ranged from 37 to 171 during regular influenza seasons (358 were reported to have occurred during the 2009 H1N1 pandemic). Influenza disproportionately affects children with specific chronic conditions, such as underlying pulmonary, cardiac, or neurologic and neuromuscular disorders. Very young children, especially those younger than 2 yr of age, and children with chronic medical conditions are more likely to develop severe influenza-related complications, including viral and bacterial pneumonia, hospitalization, respiratory failure, and death. However, while children with underlying medical conditions are at higher risk of complications, many healthy children are hospitalized with influenza, and nearly half of pediatric influenza-associated deaths are in children that have no known underlying medical condition.
Influenza also causes a substantial burden of disease in outpatient settings. It contributes to an estimated 600,000 to 2,500,000 outpatient medical visits annually in children younger than 5 yr of age, and has been identified in 10–25% of outpatient visits among all children with respiratory symptoms during influenza season. Influenza may also be underdiagnosed. Many who seek medical care for influenza do not have laboratory testing performed and do not receive a diagnosis of influenza. Every year, 3-4 influenza virus types or subtypes typically co-circulate, including influenza A(H3N2), influenza A(H1N1), and B viruses. Although 1 subtype usually predominates in any given season, it is difficult to predict which will be predominant. Thus, the influenza vaccine varies annually and contains 3 or 4 antigens representing the expected circulating types.
Pathogenesis
Influenza viruses infect the respiratory tract epithelium, primarily the ciliated columnar epithelial cells, by using the HA to attach to sialic acid residues. After viral entry into cells, virus replication occurs usually within 4-6 hr, and new virus particles are assembled and released to infect neighboring cells. With primary infection, virus replication continues for 10-14 days. Influenza virus causes a lytic infection of the respiratory epithelium with loss of ciliary function, decreased mucus production, and desquamation of the epithelial layer. These changes permit secondary bacterial invasion, either directly through the epithelium or, in the case of the middle ear space, through obstruction of the normal drainage through the eustachian tube.
The exact immune mechanisms involved in termination of primary infection and protection against reinfection are complex. Induction of cytokines that inhibit viral replication, such as interferon and tumor necrosis factor, as well as other host defenses, such as cell-mediated immune responses and local and humoral antibody defenses, all likely play a role. Secretory immunoglobulin A antibodies produced by the respiratory mucosa are thought to be an effective and immediate response generated during influenza infection. Serum antibody levels inhibiting HA activity can usually be detected by the second week after infection. These antibodies are also generated by vaccines, and high HA inhibition titers correlate with protection.
Clinical Manifestations
The onset of influenza illness is often abrupt, with a predominance of systemic symptoms including fever, myalgias, chills, headache, malaise, and anorexia. Coryza, pharyngitis, and dry cough are also usually present at the onset of illness but may be less prominent than systemic symptoms. Respiratory manifestations can include isolated upper respiratory tract illness, including croup, or progression to lower tract disease, such as bronchiolitis or pneumonia. More than other respiratory viruses, influenza virus typically causes systemic manifestations such as high temperature, myalgia, malaise, and headache. Less common clinical manifestations can include parotitis and rash.
Abdominal pain, vomiting, and diarrhea may also occur in children; in some studies, diarrhea was reported to be more often associated with influenza A(H1N1)pdm09 compared with influenza A(H3N2) or influenza B viruses. Influenza is a less distinct illness in younger children and infants. The infected young infant or child may be highly febrile and toxic in appearance, prompting a full diagnostic work-up. The typical duration of the febrile illness is 2-4 days. Cough may persist for longer periods, and evidence of small airway dysfunction is often found weeks later. Owing to the high transmissibility of influenza, other family members or close contacts of an infected person often experience a similar illness.
Complications
Otitis media and pneumonia are common complications of influenza in young children. Acute otitis media may be seen in up to 25% of cases of documented influenza. Pneumonia accompanying influenza may be a primary viral process or a secondary bacterial infection (such as with Staphylococcus aureus ) facilitated through damaged respiratory epithelium. Influenza may cause acute myositis or rhabdomyolosis marked by muscle weakness and pain, particularly in the calf muscles, and myoglobinuria. Other extrapulmonary complications include acute renal failure, myocarditis, and sepsis. Central nervous system complications, such as encephalitis, myelitis, and Guillain-Barré syndrome, can occur and are seen more commonly in children than adults. Although it has essentially disappeared in the United States, Reye syndrome can result with the use of salicylates during influenza infection (see Chapter 388 ). Bacterial coinfection may also exacerbate respiratory complications of influenza and lead to sepsis, bacteremia, toxic shock syndrome, and other manifestations.
Influenza is particularly severe in some children, including those with underlying cardiopulmonary disease, including congenital and acquired valvular disease, cardiomyopathy, bronchopulmonary dysplasia, asthma, cystic fibrosis, and neurologic conditions. Pregnant women and adolescent females are at high risk for severe influenza. Children receiving cancer chemotherapy and children with immunodeficiency also have a higher risk of complications and may shed virus for longer periods than immunocompetent children.
Laboratory Findings
The clinical laboratory abnormalities associated with influenza are nonspecific. Chest radiographs may show evidence of atelectasis or infiltrate.
Diagnosis and Differential Diagnosis
The diagnosis of influenza depends on epidemiologic, clinical, and laboratory considerations. In the context of an epidemic, the clinical diagnosis of influenza in a child who has fever, malaise, and respiratory symptoms may be made based on clinical discretion; however, clinical presentation is often indistinguishable from infection with other respiratory viruses, including respiratory syncytial virus, parainfluenza virus, human metapneumovirus, adenovirus, and even rhinovirus. Confirmation of influenza virus infection by diagnostic testing is not required for clinical decisions to prescribe antiviral medications, and prompt suspicion or diagnosis of influenza may allow for early antiviral therapy to be initiated and may reduce inappropriate use of antibiotics.
A number of diagnostic tests may be used for laboratory confirmation of influenza (Table 285.2 ). Although rapid influenza diagnostic tests are often employed because of their ease of use and fast results, they can have suboptimal sensitivity to detect influenza virus infection, particularly for novel influenza viruses. Sensitivities of rapid diagnostic tests are generally 50–70% compared to viral culture or reverse-transcription polymerase chain reaction. Specificities are higher, approximately 95–100%. Therefore false-negative results occur more often than false-positive results, particularly when the prevalence of influenza is high (i.e., during peak influenza activity in the community). The interpretation of negative results should take into account the clinical characteristics and the patient's risk for complications. If there is clinical suspicion for influenza in a patient at high risk for complications (Table 285.3 ), early empiric treatment should be given regardless of a negative rapid diagnostic test result, and another type of test (e.g., reverse-transcription polymerase chain reaction or direct fluorescent antibody testing) may be performed for confirmation.
Table 285.2
Influenza Virus Testing Methods
| METHOD | ACCEPTABLE SPECIMENS | TEST TIME | COMMENTS |
|---|---|---|---|
| Rapid Influenza Diagnostic Tests (antigen detection) | Nasopharyngeal (NP) swab, aspirate or wash, nasal swab, aspirate, or wash, throat swab | <15 min | Rapid turnaround; suboptimal sensitivity |
| Rapid Molecular Assay (influenza nucleic acid amplification) | NP swab, nasal swab | 15-30 min | Rapid turnaround, high sensitivity |
| Immunofluorescence, Direct (DFA) or Indirect (IFA) Fluorescent Antibody Staining (antigen detection) | NP swab or wash, bronchial wash, nasal or endotracheal aspirate | 1-4 hr | Relatively rapid turnaround; requires laboratory expertise and experience |
| RT-PCR (singleplex and multiplex; real-time and other RNA-based) and other molecular assays (influenza nucleic acid amplification) | NP swab, throat swab, NP or bronchial wash, nasal or endotracheal aspirate, sputum | Varies by assay (generally 1-8 hr) | Excellent sensitivity, relatively rapid turnaround compared with conventional methods |
| Rapid cell culture (shell vials, cell mixtures; yields live virus) | NP swab, throat swab, NP or bronchial wash, nasal or endotracheal aspirate, sputum | 1-3 day | Culture isolates important for strain information and antiviral resistance monitoring |
| Viral tissue cell culture (conventional; yields live virus) | NP swab, throat swab, NP or bronchial wash, nasal or endotracheal aspirate, sputum | 3-10 day | Not recommended for routine patient diagnosis |
| Serologic tests (antibody detection) | Paired (appropriately timed) acute and convalescent serum specimens | N/A (not performed during acute infection) | Not recommended for routine patient diagnosis, useful for research studies |
N/A, not applicable; NP, nucleoprotein; RT-PCR, reverse transcription-polymerase chain reaction.
Modified from Centers for Disease Control and Prevention (CDC): Influenza virus testing methods. Available at https://www.cdc.gov/flu/professionals/diagnosis/table-testing-methods.htm in Information for Health Professionals (https://www.cdc.gov/flu/professionals/index.htm ); and from 2018 IDSA Clinical Practice Guidelines.
Table 285.3
Children and Adolescents Who Are at Higher Risk for Influenza Complications for Whom Antiviral Treatment is Recommended*
|
Children younger than 2 yr of age † Persons with chronic pulmonary (including asthma), cardiovascular (except hypertension alone), renal, hepatic, hematologic (including sickle cell disease), and metabolic disorders (including diabetes mellitus); or neurologic and neurodevelopmental conditions (including disorders of the brain, spinal cord, peripheral nerve, and muscle such as cerebral palsy, epilepsy [seizure disorders], stroke, intellectual disability, moderate to severe developmental delay, muscular dystrophy, or spinal cord injury) Persons with immunosuppression, including that caused by medications or by HIV infection Adolescents who are pregnant, or postpartum (within 2 wk after delivery) Persons younger than 19 yr of age who are receiving long-term aspirin- or salicylate-containing medications therapy American Indians/Alaska Natives Persons who are extremely obese (body mass index ≥40) Residents of long-term care facilities Hospitalized patients at high risk for influenza complications |
* Antiviral treatment is recommended for high-risk children with confirmed or suspected influenza; antivirals are also recommended for children who are hospitalized or have severe or progressive disease.
† Although all children younger than 5 yr of age are considered at higher risk for complications from influenza, the highest risk is for those younger than 2 yr of age, with the highest hospitalization and death rates among infants younger than 6 mo of age.
Current for 2018-2019 influenza season.
Adapted from Centers for Disease Control and Prevention (CDC): Influenza antiviral medications: summary for clinicians . Available at https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm . For current details, consult annually updated recommendations at https://www.cdc.gov/flu/professionals/index.htm .
Treatment
Antiviral medications are an important adjunct to influenza vaccination. Three classes of antiviral drugs are licensed for treatment of influenza in children. The neuraminidase inhibitors (NAIs), oral oseltamivir and inhaled zanamivir, may be used for treatment of children from birth and 7 yr, respectively (Table 285.4 ). In December 2012, the U.S. Food and Drug Administration (FDA) approved the use of oseltamivir for the treatment of influenza in infants as young as 2 wk of age, and the Centers for Disease Control and Prevention (CDC), American Academy of Pediatrics, and the Infectious Diseases Society of America recommend its use in infants of any age. A third NAI, peramivir, is given as an intravenous infusion and is approved for treatment in persons 2 yr of age and older.
Table 285.4
Recommended Dosage and Schedule of Influenza Antiviral Medications for Treatment and Chemoprophylaxis in Children for the 2018-2019 Influenza Season: United States
| MEDICATION | TREATMENT DOSING** | CHEMOPROPHYLAXIS DOSING** |
|---|---|---|
| ORAL OSELTAMIVIR * | ||
| Adults | 75 mg twice daily | 75 mg once daily |
| Children ≥12 mo | ||
| Body wt | ||
| ≤15 kg (≤33 lb) | 30 mg twice daily | 30 mg once daily |
| >15-23 kg (33-51 lb) | 45 mg twice daily | 45 mg once daily |
| >23-40 kg (>51-88 lb) | 60 mg twice daily | 60 mg once daily |
| >40 kg (>88 lb) | 75 mg twice daily | 75 mg once daily |
| Infants 0-11 mo † | 3 mg/kg per dose once daily | 3 mg/kg per dose once daily |
| Term infants ages 0-8 mo † | 3 mg/kg per dose twice daily | 3 mg/kg per dose once daily for infants 3-8 mo old; not recommended for infants <3 mo old unless situation judged critical because of limited safety and efficacy data in this age group |
| Preterm infants | See details in footnote ‡ | Not recommended |
| INHALED ZANAMIVIR § | ||
| Adults | 10 mg (two 5 mg inhalations) twice daily | 10 mg (two 5 mg inhalations) once daily |
| Children (≥7 yr old for treatment; ≥5 yr old for chemoprophylaxis) | 10 mg (two 5 mg inhalations) twice daily | 10 mg (two 5 mg inhalations) once daily |
| INTRAVENOUS PERAMIVIR | ||
| Adults | 600 mg intravenous infusion once given over 15-30 min | Not recommended |
| Children (2-12 yr old) | One 12 mg/kg dose, up to 600 mg maximum, via intravenous infusion for 15-30 min | Not recommended |
| Children (13-17 yr old) | One 600 mg dose via intravenous infusion for 15-30 min | Not recommended |
| ORAL BALOXAVIR †† | ||
| Adults | ||
| 40 to <80 kg | One 40 mg dose | Not recommended |
| >80 kg | One 80 mg dose | Not recommended |
| Children | ||
| 2-11 yr | Not recommended | Not recommended |
| 12-17 yr, 40 to <80 kg | One 40 mg dose | Not recommended |
| 12-17 yr, >80 kg | One 80 mg dose | Not recommended |
* Oseltamivir is administered orally without regard to meals, although administration with meals may improve gastrointestinal tolerability. Oseltamivir is available as Tamiflu or as a generic formulation as capsules and as a powder for oral suspension that is reconstituted to provide a final concentration of 6 mg/mL.
† Approved by the FDA for children as young as 2 wk of age. Given preliminary pharmacokinetic data and limited safety data, oseltamivir can be used to treat influenza in both term and preterm infants from birth because benefits of therapy are likely to outweigh possible risks of treatment. CDC and US Food and Drug Administration (FDA)–approved dosing is 3 mg/kg per dose twice daily for children aged 9-11 mo; the American Academy of Pediatrics recommends 3.5 mg/kg per dose twice daily. The dose of 3 mg/kg provides oseltamivir exposure in children similar to that achieved by the approved dose of 75 mg orally twice daily for adults, as shown in two studies of oseltamivir pharmacokinetics in children. The AAP has recommended an oseltamivir treatment dose of 3.5 mg/kg orally twice daily for infants 9-11 mo, on the basis of data that indicated that a higher dose of 3.5 mg/kg was needed to achieve the protocol-defined targeted exposure for this cohort as defined in the CASG 114 study. It is unknown whether this higher dose will improve efficacy or prevent the development of antiviral resistance. However, there is no evidence that the 3.5 mg/kg dose is harmful or causes more adverse events to infants in this age group.
‡ Oseltamivir dosing for preterm infants. The wt-based dosing recommendation for preterm infants is lower than for term infants. Preterm infants may have lower clearance of oseltamivir because of immature renal function, and doses recommended for term infants may lead to high drug concentrations in this age group. Limited data from the National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group provide the basis for dosing preterm infants by using their postmenstrual age (gestational age plus chronological age): 1.0 mg/kg per dose orally twice daily for those <38 wk postmenstrual age; 1.5 mg/kg per dose orally twice daily for those 38-40 wk postmenstrual age; and 3.0 mg/kg per dose orally twice daily for those >40 wk postmenstrual age. For extremely preterm infants (<28 wk), please consult a pediatric infectious diseases physician.
§ Zanamivir is administered by inhalation by using a proprietary Diskhaler device distributed together with the medication. Zanamivir is a dry powder, not an aerosol, and should not be administered by using nebulizers, ventilators, or other devices typically used for administering medications in aerosolized solutions. Zanamivir is not recommended for people with chronic respiratory diseases, such as asthma or chronic obstructive pulmonary disease, which increase the risk of bronchospasm.
** Antiviral treatment duration for uncomplicated influenza is 5 days for oral oseltamivir or inhaled zanamivir, and a single dose for intravenous peramivir or oral baloxavir. Recommended post-exposure chemoprophylaxis with oseltamivir or zanamivir in a non-outbreak setting is 7 days after last known exposure.
†† Oral baloxavir marboxil is approved by the FDA for treatment of acute uncomplicated influenza within 2 days of illness onset in people 12 yr and older. The safety and efficacy of baloxavir for the treatment of influenza have been established in pediatric patients 12 yr and older weighing at least 40 kg. Safety and efficacy in patients <12 yr of age or weighing <40 kg have not been established. Baloxavir efficacy is based on clinical trials in outpatients 12 to 64 yr of age; people with underlying medical conditions and adults >65 yr were not included in the initial published clinical trials (Hayden F et al; Clin Infect Dis 2018). There are no available data for baloxavir treatment of hospitalized patients with influenza.
Adapted from Centers for Disease Control and Prevention (CDC): Influenza antiviral medications: summary for clinicians. Available at https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm . For current details, consult annually updated recommendations at https://www.cdc.gov/flu/professionals/index.htm ; 2018 IDSA Clinical Practice Guidelines; and from Kimberlin DW, Acosta EP, Prichard MN, et al: National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. Oseltamivir pharmacokinetics, dosing, and resistance among children aged <2 yr with influenza, J Infect Dis 207(5):709–720, 2013. For current details, consult annually updated recommendations at https://www.cdc.gov/flu/professionals/index.htm .
The second class of drugs is represented by a new influenza antiviral called baloxavir marboxil that was approved by the FDA in October 2018. Baloxavir is active against both influenza A and B viruses but has a different mechanism of action than neuraminidase inhibitors. Baloxavir is a cap-dependent endonuclease inhibitor that interferes with viral RNA transcription and blocks virus replication. It is approved for treatment of acute uncomplicated influenza in people 12 yr and older.
The third class of drugs, adamantanes, includes oral amantadine and oral rimantadine, which are effective only against influenza A viruses. Genetic mutations have conferred widespread adamantane resistance among circulating influenza A viruses, including seasonal influenza viruses and many H5N1 and H7N9 avian influenza viruses; therefore this class of antivirals is not currently recommended for use.
When initiated early in the course of uncomplicated influenza illness, antiviral agents can reduce the duration of symptoms and the likelihood of complications. Among hospitalized patients, observational studies suggest that early treatment reduces disease severity and mortality. Although most data regarding potential benefit are for adults, a few studies support the use of antiviral agents in children. Antiviral treatment within 2 days of illness onset has been reported to reduce illness duration, the risk of otitis media, and the likelihood of hospitalization in children. Clinical benefit is greatest when antiviral treatment is administered early, especially within 48 hr of influenza illness onset.
CDC recommends treatment as early as possible for (1) hospitalized patients, (2) patients with complicated or progressive illness, and (3) patients at high risk for influenza complications (see Table 285.3 ). Decisions about starting antiviral treatment should not wait for laboratory confirmation of influenza. Although early treatment is desired, treatment even more than 48 hr from onset may be beneficial and is recommended for these 3 categories of patients.
The recommended treatment course for uncomplicated influenza is 1 dose of an oral oseltamivir or inhaled zanamivir given twice daily for 5 days; intravenous peramivir and oral baloxavir are both given as a single dose. Currently, for hospitalized patients and patients with severe or complicated illness, treatment with oral or enterically administered oseltamivir is recommended. The optimal duration and dose are uncertain for severe or complicated influenza and longer courses of treatment (e.g., 10 days of treatment) may be considered.
Clinical judgment, on the basis of the patient's disease severity, age, underlying medical conditions, likelihood of influenza, and time since onset of symptoms, is important when making antiviral treatment decisions for outpatients at high risk for complications. Antiviral treatment can also be considered for any previously healthy, symptomatic outpatient not at high risk with confirmed or suspected influenza on the basis of clinical judgment, if treatment can be initiated within 48 hr of illness onset.
It is possible that some influenza viruses may become resistant during antiviral treatment; this has been reported most often for oseltamivir resistance in influenza A(H1N1) viruses. Following treatment with baloxavir, emergence of viruses with molecular markers associated with reduced susceptibility to baloxavir has been observed in clinical trials. Antiviral resistance and reduced susceptibility can also occasionally occur spontaneously with no known exposure to antiviral drugs. It is important to review annual recommendations and updates published by CDC before prescribing influenza antiviral medications (see https://www.cdc.gov/flu/professionals/antivirals/index.htm ).
Supportive Care
Adequate fluid intake and rest are important in the management of influenza. Bacterial superinfections are relatively common and should be appropriately treated with antibiotic therapy. Bacterial superinfection should be suspected with recrudescence of fever, prolonged fever, or deterioration in clinical status. With uncomplicated influenza, people should usually start to feel better after the first 48-72 hr of symptoms.
Prognosis
The prognosis for recovery from uncomplicated influenza is generally excellent, although full return to normal level of activity and freedom from cough may require weeks rather than days. Fatigue may also persist for weeks. However, severe influenza disease can be associated with hospitalizations and death, even among previously healthy children.
Prevention
Influenza vaccination is the best means of preventing influenza illness. In studies of children who are fully vaccinated, influenza vaccine is 40% to 60% effective in reducing the risk of laboratory-confirmed influenza illness. Vaccine effectiveness can vary from year to year and among different age and risk groups. Recommendations for use of the influenza vaccine have broadened as the impact of influenza is appreciated in such groups as pregnant women and young infants. Starting in the 2008-2009 influenza season, the United States Advisory Committee on Immunization Practices (ACIP) recommended that all children from 6 mo to 18 yr of age be vaccinated for influenza unless they have a specific contraindication to receiving the vaccine. Since the 2010-2011 season, annual flu vaccination is recommended for everyone 6 mo and older, with rare exception. In 2012, the Department of Health in the United Kingdom extended their influenza vaccination program to include all children between the ages of 2 and 17 yr. To protect infants younger than 6 mo who are too young to receive vaccine, household contacts and out-of-home caregivers are groups for whom additional vaccination efforts should be made. Chemoprophylaxis with antiviral medications is a secondary means of prevention and is not a substitute for vaccination.
Vaccines
There are 2 main categories of seasonal influenza vaccines available for children: inactivated influenza vaccine (IIV) and live-attenuated influenza vaccine (LAIV). Previously referred to as the trivalent inactivated vaccine, IIV is given intramuscularly; it uses killed virus components. The LAIV vaccine uses weakened influenza virus and is administered as an intranasal spray. Neither IIV nor LAIV can cause influenza. Although in 2014-2015 ACIP and CDC recommended the use of the LAIV nasal spray vaccine for healthy children 2 through 8 yr of age, this preferential recommendation was removed for the 2015-2016 season, and for the 2016-2017 and 2017-2018 seasons, ACIP and CDC made the interim recommendation that LAIV should not be used. This decision was based on concerns regarding low effectiveness against influenza A(H1N1)pdm09 in the United States noted during the 2013-2014 and 2015-2016 seasons. After review of additional data, LAIV containing an updated influenza A(H1N1)pdm09-like vaccine virus, was again recommended by CDC and ACIP as an option for vaccination for the 2018-2019 season. For the 2018-2019 season, ACIP and CDC made the interim recommendation that LAIV4 may be used.
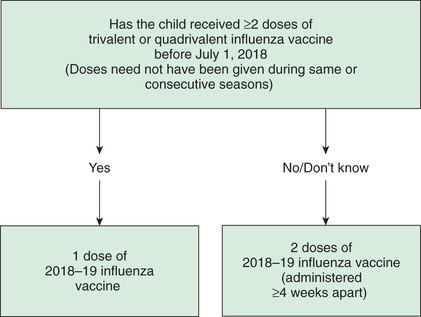
Special vaccination instructions for children 6 mo to 8 yr of age should be followed: children in this age group who have not previously received a total of ≥2 previous doses of trivalent or quadrivalent vaccine require 2 doses (at least 4 weeks apart) of the current season's influenza vaccine to optimize immune response (Fig. 285.3 ). Influenza vaccines have an excellent safety profile, with the most common side effects being soreness, redness, tenderness, or swelling from the injection, and nasal congestion after the nasal spray.
Seasonal influenza vaccines become available in the late summer and early fall each year. The formulation reflects the strains of influenza viruses that are expected to circulate in the coming influenza season. Beginning in the 2013-2014 season, IIVs were available in both trivalent and quadrivalent formulations. The trivalent vaccine (IIV3) contains 2 influenza A strains and 1 influenza B strain; the quadrivalent vaccine (IIV4) contains a second influenza B strain of an antigenically distinct lineage. In addition to IIV and LAIV, a third vaccine category, recombinant hemagglutinin influenza vaccine, became available as a trivalent formulation in the 2013-2014 season but this is not licensed for children.
Ideally, vaccination should be given before the onset of influenza circulation in the community, so that there is time for antibodies to reach protective levels. Healthcare providers should offer vaccination by the end of October, if possible. The ACIP publishes guidelines for vaccine use each year when the vaccines are formulated and released; these should be referred to each season. These guidelines are widely publicized but appear initially in the Morbidity and Mortality Weekly Report published by CDC (https://www.cdc.gov/flu/index.htm ).
Chemoprophylaxis
Routine use of antiviral medications for chemoprophylaxis is not recommended. Examples for which the use of chemoprophylaxis may be considered to prevent influenza after exposure to an infectious person include (1) unvaccinated persons at high risk of influenza complications, (2) persons for whom vaccine is contraindicated or expected to have low effectiveness, and (3) residents/patients in care facilities during institutional influenza outbreaks. Oral oseltamivir or inhaled zanamivir may be used for chemoprophylaxis of influenza; peramivir and baloxavir are not recommended for chemoprophylaxis because of a lack of data, and adamantanes are not currently recommended because of widespread adamantane resistance. Table 285.4 shows the recommendations for dosage and duration of treatment and chemoprophylaxis for the 2018-2019 influenza season, but updated recommendations from the ACIP and CDC should be consulted every season (https://www.cdc.gov/flu/professionals/antivirals/index.htm ).
In general, if chemoprophylaxis can be started within 48 hr of exposure to an infectious person, postexposure chemoprophylaxis for persons at high risk of influenza complications (see Table 285.3 ) is recommended for 7 days after the last known exposure. An alternative to chemoprophylaxis for some persons after a suspected exposure is close monitoring and early initiation of antiviral treatment if symptoms develop. For control of influenza outbreaks among high-risk persons living in institutional settings, such as long-term care facilities, antiviral chemoprophylaxis is recommended for all vaccinated and unvaccinated residents and for unvaccinated healthcare providers. CDC and the Infectious Diseases Society of America recommend antiviral chemoprophylaxis for a minimum of 2 wk and up to 1 wk after the last known case is identified, whichever is longer.