Wheezing, Bronchiolitis, and Bronchitis
Wheezing in Infants: Bronchiolitis
Samantha A. House, Shawn L. Ralston
General Pathophysiology of Wheezing in Infants
Wheezing, the production of a musical continuous sound that originates in narrowed airways, is heard on expiration as a result of airway obstruction. Infants are more likely to wheeze than are older children, as a result of differing lung mechanics. Obstruction of airflow is affected by both airway size and compliance of the infant lung. Resistance to airflow through a tube is inversely related to the radius of the tube to the 4th power. In children younger than 5 yr, small-caliber peripheral airways can contribute up to 50% of the total airway resistance. Marginal additional narrowing, such as that caused by inflammation related to viral infection, is then more likely to result in wheezing.
Infant chest wall compliance is also quite high, thus the inward pressure produced in normal expiration subjects the intrathoracic airways to collapse. Differences in tracheal cartilage and airway smooth muscle tone increase the collapsibility of the infant airways in comparison with older children. These mechanisms combine to make the infant more susceptible to airway obstruction, increased resistance, and subsequent wheezing. The mechanical portion of the infant propensity to wheeze resolves with normal growth and muscular development.
Although wheezing in infants most frequently results from inflammation due to acute viral infections, there are many potential causes of wheezing (Table 418.1 ).
Table 418.1
Differential Diagnosis of Wheezing in Infancy
| INFECTION |
| Viral |
| Other |
| ASTHMA |
| ANATOMIC ABNORMALITIES |
| Central Airway Abnormalities |
| Extrinsic Airway Anomalies Resulting in Airway Compression |
| Intrinsic Airway Anomalies |
| Immunodeficiency States |
| MUCOCILIARY CLEARANCE DISORDERS |
| ASPIRATION SYNDROMES |
| OTHER |
Acute Bronchiolitis
Acute bronchiolitis is a diagnostic term used to describe the clinical picture produced by several different viral lower respiratory tract infections in infants and very young children. The respiratory findings observed in bronchiolitis include tachypnea, wheezing, crackles, and rhonchi which result from inflammation of the small airways (Fig. 418.1 ). Despite its commonality, a universal set of diagnostic criteria for bronchiolitis does not exist, with significant disagreement about the upper age limit for appropriate use of the diagnosis. Some clinicians restrict the term to children younger than 1 yr, and others extend it to the age of 2 yr or beyond.
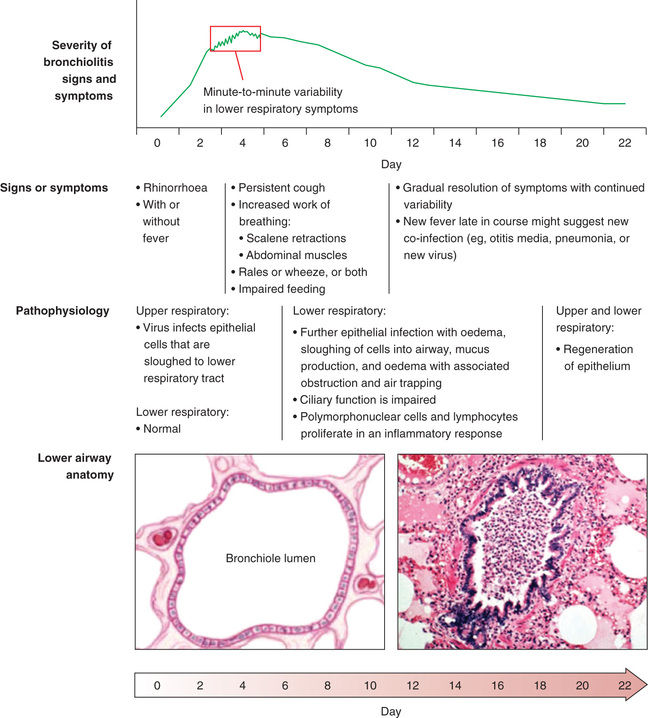
The pathophysiology of acute bronchiolitis is characterized by bronchiolar obstruction with edema, mucus, and cellular debris (see Fig. 418.1 ). Resistance in the small air passages is increased during both inspiration and exhalation, but because the radius of an airway is smaller during expiration, the resultant respiratory obstruction leads to expiratory wheezing, air trapping, and lung hyperinflation. If obstruction becomes complete, trapped distal air will be resorbed and the child will develop atelectasis. Hypoxemia is a consequence of ventilation-perfusion mismatch. With severe obstructive disease hypercapnia can develop.
Respiratory syncytial virus (RSV) is responsible for more than 50% of cases of bronchiolitis in most reports. Other agents include human metapneumovirus, rhinovirus, parainfluenza, influenza, bocavirus, and adenovirus. Viral coinfection is reported to impact severity and clinical manifestations, although its significance remains contested. Respiratory viruses can be identified in more than one third of asymptomatic patients younger than the age of 1 yr, calling into question the specificity of current tests for active infection. Although bacterial pneumonia is sometimes confused clinically with bronchiolitis, viral bronchiolitis is rarely followed by bacterial superinfection.
Well over 100,000 young children are hospitalized annually in the United States with the diagnosis of bronchiolitis, making it the most common diagnosis resulting in hospitalization for children younger than 1 yr of age in the United States over the past several decades. The increasing rates of hospitalization for bronchiolitis observed from 1980 to 1996 (thought to reflect increased attendance of infants in daycare centers, changes in criteria for hospital admission linked to pulse oximetry use, and/or improved survival of premature infants and other children at risk for severe disease) have not continued. Hospitalization rates have been stable in subsequent years despite introduction and routine use of RSV immunoprophylaxis in high-risk populations.
Bronchiolitis is more common in males, those exposed to second-hand tobacco smoke, those who have not been breastfed, and those living in crowded conditions. Risk is also higher for infants with mothers who smoked during pregnancy. Older family members, including older siblings, are a common source of infection; they might experience only minor upper respiratory symptoms (colds) given that bronchiolar edema may be less clinically apparent as airway size increases.
Asthma (see Chapter 169 ) is another important cause of wheezing, and the possibility of this diagnosis complicates the treatment of young children with bronchiolitis, although accurate diagnosis of asthma in the very young can be difficult. In prospective, longitudinal population cohort studies of infants, up to half of the cohort experienced a wheezing illness prior to school age, although when followed into adulthood only about 5–8% of patients prove to have asthma. In the largest U.S. cohort, 3 patterns of infant wheezing were proposed: transient early wheezing, comprising about 20% of the cohort, characterized by lower lung function at birth which improves with growth resulting in resolution of wheezing by age 3; persistent wheezing, comprising about 14% of the cohort, characterized by declining lung function and wheezing before and after age 3; and late-onset wheezing, comprising 15% of the cohort, characterized by relatively stable lung function and wheezing that does not begin until after age 3. The remaining 50% of the population did not suffer a wheezing illness. Following the cohort into adulthood revealed continued declines in the rates of persistent symptoms. Similar patterns are also seen in birth cohort studies in other countries.
Multiple studies attempting to predict which infants suffering from early wheezing illnesses will go on to have asthma in later life have failed to achieve discriminant validity. Interestingly, in both U.S. and U.K. prospective cohorts, wheezing with an onset after the first 18-36 mo of life is one of the strongest predictors of eventual asthma in both cohorts. Other proposed risk factors for persistent wheezing include parental history of asthma and allergies, maternal smoking, persistent rhinitis (apart from acute upper respiratory tract infections), allergen sensitization, eczema, and peripheral eosinophilia, although no single factor is strongly discriminative. Despite several randomized trials, there is no evidence that early administration of inhaled corticosteroids to high-risk populations can prevent the development of asthma.
Clinical Manifestations
History and Physical Examination
The initial history of a wheezing infant should describe the recent event including onset, duration, and associated factors (Table 418.2 ). Birth history includes weeks of gestation, neonatal complications including history of intubation or oxygen requirement, maternal complications, and prenatal smoke exposure. Past medical history includes any comorbid conditions. Family history of cystic fibrosis, immunodeficiencies, asthma in a first-degree relative, or any other recurrent respiratory conditions in children should be obtained. Social history should include any second-hand tobacco or other smoke exposure, daycare exposure, number of siblings, pets, and concerns regarding home environment (e.g., dust mites, construction dust, heating and cooling techniques, mold, cockroaches). The patient's growth chart should be reviewed for signs of failure to thrive.
Table 418.2
Pertinent Medical History in the Wheezing Infant
Acute bronchiolitis is usually preceded by exposure to contacts with a minor respiratory illness within the previous week (see Fig. 418.1 ). The infant first develops signs of upper respiratory tract infection with sneezing and clear rhinorrhea. This may be accompanied by diminished appetite and fever. Gradually, respiratory distress ensues, with paroxysmal cough, dyspnea, and irritability. The infant is often tachypneic, which can interfere with feeding. Apnea may precede lower respiratory signs early in the disease, particularly with very young infants. Term infants at a postconceptual age of <44 wk and preterm infants at postconceptual age <48 wk are at highest risk for apneic events.
On physical examination, evaluation of the patient's vital signs with special attention to the respiratory rate and oxygen saturation is an important initial step. The exam is often dominated by wheezing and crackles. Expiratory time may be prolonged. Work of breathing may be markedly increased, with nasal flaring and retractions. Complete obstruction to airflow can eliminate the turbulence that causes wheezing; thus the lack of audible wheezing is not reassuring if the infant shows other signs of respiratory distress. Poorly audible breath sounds suggest severe disease with nearly complete bronchiolar obstruction.
Diagnostic Evaluation
Evaluation of wheezing in infancy and early childhood depends on suspected etiology. The diagnosis of acute bronchiolitis is clinical, particularly in a previously healthy infant presenting with a first episode of wheezing following a period of upper respiratory symptoms. Chest radiography is not routinely indicated in children with suspected bronchiolitis. Areas of atelectasis associated with bronchiolitis are often observed on chest radiographs and may be difficult to distinguish from bacterial pneumonia; as a result, obtaining chest radiography in a patient whose clinical course and exam are consistent with bronchiolitis may encourage unnecessary antibiotic use. Laboratory testing is also not routinely indicated; the white blood cell and differential counts are usually normal and are not predictive of bacterial superinfection. Viral testing (polymerase chain reaction, or rapid immunofluorescence) is not routinely recommended in the diagnosis of bronchiolitis but may be helpful if such testing prevents more invasive evaluations. Concurrent serious bacterial infection (sepsis, pneumonia, meningitis) is unlikely, although confirmation of viral bronchiolitis may obviate the need for a sepsis evaluation in the young febrile infant. Otitis media may complicate bronchiolitis.
For young children with wheezing in whom the presentation does not clinically fit with the diagnosis of bronchiolitis, including those without other signs of viral infection, with very severe presentation, or complicated clinical course, further workup should be considered and should be dictated by individual clinical context. Children with recurrent or refractory episodes of wheezing in infancy, particularly if associated with failure to thrive, may require evaluation for chronic disorders such as cystic fibrosis or immunodeficiency.
Treatment
The treatment of children with viral bronchiolitis is supportive management. Those who are experiencing respiratory distress (hypoxia, inability to feed, apnea, extreme tachypnea) should be hospitalized. Risk factors for severe disease include younger age, preterm birth, or underlying comorbidity such as cardiovascular, pulmonary, neurologic, or immunologic disease. Hypoxemic children should receive supplemental oxygen. There is a developing consensus surrounding target oxygen saturations; national guidelines in the United States propose a threshold of 90%. Oxygen can be administered via a number of delivery devices, and some children with severe disease may require positive pressure ventilation. High-flow nasal cannula is a noninvasive mode of oxygen delivery capable of providing some positive end expiratory pressure, particularly in young children. Some use high flow as rescue therapy in patients who do not respond to standard care. The utility of high-flow nasal cannula in avoiding intubation in some children and reducing the duration of required supplemental oxygen is being actively explored because current data are mixed.
Some children may also require support with supplemental hydration. Fluid can be administered intravenously or enterally via nasogastric tube, with some preference given to the latter due to an association between better outcomes and continued provision of enteral nutrition. If intravenous fluids are administered, care should be taken to use isotonic fluids due to risk of hyponatremia. Frequent suctioning of nasal and oral secretions often provides relief of distress and improves work of breathing and ability to feed, although this should be limited to the nares or oropharynx because deep tracheal suctioning does not provide additional benefit. Chest physiotherapy has been extensively evaluated and provides no benefit to children with bronchiolitis.
Pharmacologic agents have largely proven ineffective in the management of bronchiolitis. Cochrane reviews have failed to demonstrate any impact on clinical outcomes with use of albuterol or corticosteroids in bronchiolitis; neither are currently recommended for management. Response to bronchodilators is unlikely and unpredictable in children younger than 1 yr, and there is no validated method of assessing response in the clinical setting. The use of inhaled or oral steroids in very young children with wheezing has not been shown to prevent the progression of childhood wheezing or development of asthma. There is debate over the use of hypertonic saline in children with bronchiolitis, although most studies and meta-analyses fail to demonstrate any benefit. Racemic epinephrine has not been found to improve length of stay or clinical outcomes among inpatients with bronchiolitis, although there is some evidence to suggest that it may reduce risk of hospitalization when used in the outpatient setting. Ribavirin, the only currently available antiviral medication targeting RSV, is also not currently recommended, because of minimal impact on disease outcomes, and because it is costly, difficulty to administer, and associated with important toxicities.
Prognosis
Infants with acute bronchiolitis are at highest risk for further respiratory compromise in the first 72 hours after onset of cough and dyspnea. The case fatality rate is <1% in developed countries, with death attributable to respiratory arrest and/or failure or severe dehydration and electrolyte disturbances. A majority of deaths due to bronchiolitis occur in children with complex medical conditions or comorbidities such as bronchopulmonary dysplasia, congenital heart disease, or immunodeficiency. The median duration of symptoms in ambulatory patients is approximately 14 days; 10% may be symptomatic for 3 wk. Severe lower respiratory tract infection at an early age has been identified as a possible risk factor for the development of asthma, although most children with early childhood wheezing will not go on to suffer from asthma. It is unclear whether viral infections causing bronchiolitis incite an immune response that manifests as asthma later in life or whether those infants have an inherent predilection for asthma that is first manifested as viral bronchiolitis.
Prevention
Meticulous hand hygiene is the best measure to prevent transmission of the viruses responsible for bronchiolitis. For high-risk populations, palivizumab , an intramuscular monoclonal antibody to the RSV F protein, may be given as a prophylactic agent. Palivizumab has been demonstrated to reduce risk of hospitalization due to RSV bronchiolitis in certain populations. It has not been shown to decrease mortality and does not protect against bronchiolitis caused by other viruses and is also quite costly. As a result, there is some controversy surrounding which populations should receive palivizumab. U.S. guidelines suggest use for children born at <29-wk completed gestation or those with significant heart disease or chronic lung disease of prematurity, through the 1st or 2nd (for those with persistent chronic lung disease of prematurity) yr of life. Prophylaxis may be considered in infants with neuromuscular disease and immunocompromised states. The development of an effective preventive strategy available at a lower cost would be particularly advantageous in developing nations, where access to care and intervention for severe bronchiolitis are more limited.
Bibliography
Alansari K, Toaimah FH, Khalafalla H, et al. Caffeine for the treatment of apnea in bronchiolitis: a randomized trial. J Pediatr . 2016;177:204–2011.
American Academy of Pediatrics. Updated guidance for palivizumab prophylaxis among infants and young children at increased risk of hospitalization for respiratory syncytial virus infection. Pediatrics . 2014;134(2):415–418.
Badgett RG, Vindhyal M, Stirnaman JT, et al. A living systematic review of nebulized hypertonic saline for acute bronchiolitis in infants. JAMA pediatrics . 2015;169(8):788–789.
Byington CL, Wilkes J, Korgenski K, Sheng X. Respiratory syncytial virus-associated mortality in hospitalized infants and young children. Pediatrics . 2015;135(1):e24–e31.
Cunningham S, Nair H, Campbell H. Deciphering clinical phenotypes in acute viral lower respiratory tract infection: bronchiolitis is not an island. Thorax . 2016;71(8):679–680.
Cunningham S, Rodriguez A, Adams T, et al. Oxygen saturation targets in infants with bronchiolitis (BIDS): a double-blind, randomised, equivalence trial. Lancet . 2015;386:1041–1048.
Essouri S, Baudin F, Chevret L, et al. Variability of care in infants with severe bronchiolitis: less-invasive respiratory management leads to similar outcomes. J Pediatr . 2017;188:156–162.
Fernandes RM, Andrade MG, Constant C, et al. Acute viral bronchiolitis: physician perspectives on definition and clinically important outcomes. Pediatr Pulmonol . 2016;51(7):724–732.
Fernandes RM, Bialy LM, Vandermeer B, et al. Glucocorticoids for acute viral bronchiolitis in infants and young children. Cochrane Database Syst Rev . 2013;(6) [CD004878].
Florin TA, Plint PC, Zorc JJ. Viral bronchiolitis. Lancet . 2017;389:211–224.
Franklin D, Babl FE, Schlapbach LJ, et al. A randomized trial of high-flow oxygen therapy in infants with bronchiolitis. N Engl J Med . 2018;378(12):1121–1130.
Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev . 2014;(6) [CD001266].
Hall CB, Weinberg GA, Blumkin AK, et al. Respiratory syncytial virus-associated hospitalizations among children less than 24 months of age. Pediatrics . 2013;132(2):e341–e348.
Hartling L, Bialy LM, Vandermeer B, et al. Epinephrine for bronchiolitis (review). Cochrane Database Syst Rev . 2011;(12) [CD003123].
Hasegawa K, Mansbach JM, Camargo CA Jr. Infectious pathogens and bronchiolitis outcomes. Expert Rev Anti Infect Ther . 2014;12(7):817–828.
Hasegawa K, Tsugawa Y, Brown DF, et al. Trends in bronchiolitis hospitalizations in the United States, 2000-2009. Pediatrics . 2013;132(1):28–36.
Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax . 2008;63(11):974–980.
Kepreotes E, Whitehead B, Attia J, et al. Hihg-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet . 2017;389(930):939.
Mahant S, Parkin PC. Apnea in bronchiolitis: challenges of studying an uncommon complication of a common condition. J Pediatr . 2016;177:11–12.
Mansbach JM, Clark S, Teach SJ, et al. Children hospitalized with rhinovirus bronchiolitis have asthma-like characteristics. J Pediatr . 2016;172:202–204.
Mansbach JM, Piedra PA, Teach SJ, et al. Prospective multicenter study of viral etiology and hospital length of stay in children with severe bronchiolitis. Arch Pediatr Adolesc Med . 2012;166:700–706.
McCulloh R, Koster M, Ralston S, et al. Use of intermittent vs continuous pulse oximetry for nonhypoxemic infants and young children hospitalized for bronchiolitis. JAMA Pediatr . 2015;169(10):898–904.
Meissner HC. Viral bronchiolitis in children. N Engl J Med . 2016;374(1):62–72.
Pignotti MS, Carmela Leo M, Pugi A, et al. Consensus conference on the appropriateness of palivizumab prophylaxis in respiratory syncytial virus disease. Pediatr Pulmonol . 2016;51(10):1088–1096.
Principi T, Coates AL, Parkin PC, et al. Effect of oxygen desaturations on subsequent medical visits in infants discharged from the emergency department with bronchiolitis. JAMA Pediatr . 2016;170(6):602–608.
Ralston SL, Lieberthal AS, Meissner HC, et al. Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics . 2014;134:e1474–e1502.
Ren CL, Esther CR Jr, Debley JS, et al. Official American thoracic society clinical practice guidelines: diagnostic evaluation of infants with recurrent or persistent wheezing. Am J Respir Crit Care Med . 2016;194(3):356–373.
Stern DA, Morgan WJ, Halonen M, et al. Wheezing and bronchial hyper-responsiveness in early childhood as predictors of newly diagnosed asthma in early adulthood: a longitudinal birth-cohort study. Lancet . 2008;372(9643):1058–1064.
Thompson M, Vodicka TA, Blair PS, et al. Duration of symptoms of respiratory tract infections in children: systematic review. BMJ . 2013;347:f7072.
Zhang L, Mendoza-Sassi PA, Klassen TP, Wainwright C. Nebulized hypertonic saline for acute bronchiolitis: a systematic review. Pediatrics . 2015;136(4):687–701.
Bronchitis
Lauren E. Camarda, Denise M. Goodman
Nonspecific bronchial inflammation is termed bronchitis and occurs in multiple childhood conditions. Acute bronchitis is a syndrome, usually viral in origin, with cough as a prominent feature.
Acute tracheobronchitis is a term used when the trachea is prominently involved. Nasopharyngitis may also be present, and a variety of viral and bacterial agents, such as those causing influenza, pertussis, and diphtheria, may be responsible. Isolation of common bacteria such as Staphylococcus aureus and Streptococcus pneumoniae from the sputum might not imply a bacterial cause that requires antibiotic therapy.
Acute Bronchitis
Clinical Manifestations
Acute bronchitis often follows a viral upper respiratory tract infection. It is more common in the winter when respiratory viral syndromes predominate. The tracheobronchial epithelium is invaded by the infectious agent, leading to activation of inflammatory cells and release of cytokines. Constitutional symptoms including fever and malaise follow. The tracheobronchial epithelium can become significantly damaged or hypersensitized, leading to a protracted cough lasting 1-3 wk.
The child first presents with nonspecific upper respiratory infectious symptoms, such as rhinitis. Three to 4 days later, a frequent, dry, hacking cough develops, which may or may not be productive. After several days, the sputum can become purulent, indicating leukocyte migration but not necessarily bacterial infection. Many children swallow their sputum which can produce emesis. Chest pain may be a prominent complaint in older children and is exacerbated by coughing. The mucus gradually thins, usually within 5-10 days, and then the cough gradually abates. The entire episode usually lasts about 2 wk and seldom longer than 3 wk.
Findings on physical examination vary with the age of the patient and stage of the disease. Early findings include no or low-grade fever and upper respiratory signs such as nasopharyngitis, conjunctivitis, and rhinitis. Auscultation of the chest may be unremarkable at this early phase. As the syndrome progresses and cough worsens, breath sounds become coarse, with coarse and fine crackles and scattered high-pitched wheezing. Chest radiographs are normal or can have increased bronchial markings.
The principal objective of the clinician is to exclude pertussis and pneumonia, which is more likely caused by bacterial agents requiring antibiotic therapy. Absence of abnormality of vital signs (tachycardia, tachypnea, fever) and a normal physical examination of the chest reduce the likelihood of pneumonia.
Differential Diagnosis
Persistent or recurrent symptoms should lead the clinician to consider entities other than acute bronchitis. Many entities manifest with cough as a prominent symptom (Table 418.3 ).
Table 418.3
Treatment
There is no specific therapy for acute bronchitis. The disease is self-limited, and antibiotics, although often prescribed, do not hasten improvement. Frequent shifts in position can facilitate pulmonary drainage in infants. Older children are sometimes more comfortable with humidity, but this does not shorten the disease course. Cough suppressants can relieve symptoms but can also increase the risk of suppuration and inspissated secretions and therefore should be used judiciously. Antihistamines dry secretions and are not helpful; expectorants are likewise not indicated. Nonprescription cough and cold medicines should not be used in children younger than 4 yr of age, and their use is cautioned in children age 4-11 yr.
Chronic Bronchitis
Chronic bronchitis is well recognized in adults, formally defined as 3 mo or longer of productive cough each year for 2 or more yr. The disease can develop insidiously, with episodes of acute obstruction alternating with quiescent periods. Some predisposing conditions can lead to progression of airflow obstruction or chronic obstructive pulmonary disease, with smoking as the major factor (up to 80% of patients have a smoking history). Other conditions include air pollution, occupational exposures, and repeated infections. In children, cystic fibrosis, bronchopulmonary dysplasia, and bronchiectasis must be ruled out.
The applicability of this definition to children is unclear. The existence of chronic bronchitis as a distinct entity in children is controversial. Like adults, children with chronic inflammatory diseases or those with toxic exposures can develop damaged pulmonary epithelium. Thus chronic or recurring cough in children should lead the clinician to search for underlying pulmonary or systemic disorders (see Table 418.3 ). One proposed entity that shares characteristics with asthma and other forms of suppurative lung disease is persistent or protracted bacterial bronchitis. Protracted bacterial bronchitis is defined as a chronic (>3 wk) wet cough, characterized by bacterial counts of 104 colony-forming units/mL or greater from bronchoalveolar lavage and resolution of cough within 2 wk of treatment with antimicrobial therapy.
Cigarette Smoking and Air Pollution
Exposure to environmental irritants, such as tobacco smoke and air pollution, can incite or aggravate cough. There is a well-established association between tobacco exposure and pulmonary disease, including bronchitis and wheezing. This can occur through cigarette smoking or by exposure to passive smoke. Marijuana smoke and inhalants are other irritants sometimes overlooked when eliciting a history.
A number of pollutants compromise lung development and likely precipitate lung disease, including particulate matter, ozone, acid vapor, and nitrogen dioxide. Proximity to motor vehicle traffic is an important source of these pollutants. Because these substances coexist in the atmosphere, the relative contribution of any one to pulmonary symptoms is difficult to discern.
Bibliography
Craven V, Everard ML. Protracted bacterial bronchitis: reinventing an old disease. Arch Dis Child . 2013;98:72–76.
Jones LL, Hashim A, McKeever T, et al. Parental and household smoking and the increased risk of bronchitis, bronchiolitis and other lower respiratory infections in infancy: systematic review and meta-analysis. Respir Res . 2011;12:5.
Shields MD, Bush A, Everard ML, et al. On behalf of the British thoracic society cough guideline group. Recommendations for the assessment and management of cough in children. Thorax . 2008;63:iii1–iii15.
Zgherea D, Pagala S, Mendiratta M, et al. Bronchoscopic findings in children with chronic wet cough. Pediatrics . 2012;129(2):e364–e369.