Cyanotic Congenital Heart Disease
Evaluation of the Critically Ill Neonate With Cyanosis and Respiratory Distress
Daniel Bernstein
See also Chapter 122 .
A severely ill neonate with cardiorespiratory distress and cyanosis is a diagnostic challenge. The clinician must perform a rapid evaluation to determine whether congenital heart disease is a cause so that potentially lifesaving measures can be instituted. The differential diagnosis of neonatal cyanosis is presented in Table 119.2 (Chapter 119 ).
Cardiac Disease Leading to Cyanosis
Congenital heart disease (CHD) produces cyanosis when obstruction to right ventricular inflow or outflow causes intracardiac right-to-left shunting or when complex anatomic defects cause an admixture of pulmonary (deoxygenated) and systemic (oxygenated) venous return in the heart. Cyanosis from pulmonary edema may also develop in patients with heart failure caused by left-to-right shunts, although the degree is usually less severe. Cyanosis may be caused by persistence of fetal pathways, such as right-to-left shunting across the foramen ovale and ductus arteriosus in the presence of pulmonary outflow tract obstruction or persistent pulmonary hypertension of the newborn (PPHN) (see Chapter 122.9 ).
Differential Diagnosis
The hyperoxia test is one method of distinguishing cyanotic CHD from pulmonary disease. Neonates with cyanotic CHD usually are unable to significantly raise their arterial blood partial pressure of oxygen (PaO 2 ) during administration of 100% oxygen. This test is usually performed using a hood rather than nasal cannula or face mask, to best guarantee delivery of almost 100% oxygen to the patient. False-positive tests can occur if this is not done correctly. If the PaO 2 rises above 150 mm Hg during 100% oxygen administration, an intracardiac right-to-left shunt can usually be excluded. This is not 100% confirmative, however, because some patients with cyanotic CHD may be able to increase their PaO 2 to >150 mm Hg because of favorable intracardiac streaming patterns. In patients with pulmonary disease, PaO 2 generally increases significantly with 100% oxygen as ventilation-perfusion inequalities are overcome. In infants with cyanosis from a central nervous system disorder, the PaO 2 usually normalizes completely during artificial ventilation. Hypoxia in many heart lesions is profound and constant, whereas in respiratory disorders and in PPHN, PaO 2 often varies with time or changes in ventilator management. Hyperventilation may improve the hypoxia in neonates with PPHN and only occasionally in those with cyanotic CHD.
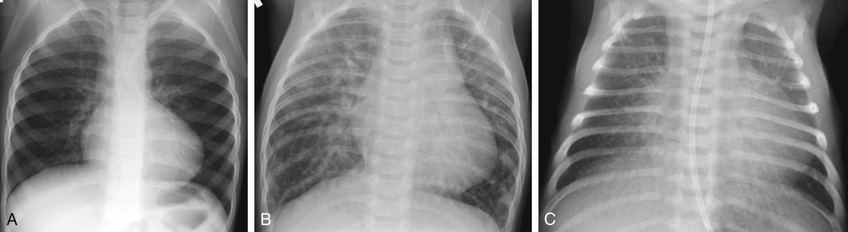
Although a significant heart murmur usually suggests a cardiac basis for the cyanosis, several of the more severe cardiac defects (e.g., transposition of the great vessels) may not initially be associated with a murmur. The chest radiograph may be helpful in the differentiation of pulmonary and cardiac disease; in the latter, it indicates whether pulmonary blood flow is increased, normal, or decreased (Fig. 456.1 ).
Two-dimensional echocardiography with Doppler is the definitive noninvasive test to determine the presence of CHD. Cardiac catheterization is less often used for diagnostic purposes and is usually performed to examine structures that are sometime less well visualized by echocardiography, such as distal branch pulmonary arteries or aortopulmonary collateral arteries in patients with tetralogy of Fallot with pulmonary atresia (see Chapter 457.2 ), or coronary arteries and right ventricular sinusoids in patients with pulmonary atresia and intact ventricular septum (see Chapter 457.3 ). If echocardiography is not immediately available to confirm a diagnosis of cyanotic CHD, the clinician caring for a newborn with possible cyanotic CHD should not hesitate to start a prostaglandin infusion (for a possible ductal-dependent lesion). Because of the risk of hypoventilation associated with prostaglandins, a practitioner skilled in neonatal endotracheal intubation must be available.