Chapter 12 NURSING MANAGEMENT: inflammation and wound healing
1. Describe the inflammatory response, including vascular and cellular responses and exudate formation.
2. Explain local and systemic manifestations of inflammation and their physiological bases.
3. Describe the drug therapy, nutrition therapy and nursing management of inflammation.
4. Differentiate among healing by primary, secondary and tertiary intention.
5. Describe the factors that delay wound healing and common complications of wound healing.
6. Describe nursing and collaborative management of wound healing.
7. Explain the aetiology and clinical manifestations of pressure ulcers.
8. Apply a patient risk assessment for pressure ulcers and measures to prevent the development of pressure ulcers.
9. Discuss nursing and collaborative management of the patient with pressure ulcers.
This chapter focuses on inflammation and wound healing. Assessment of risk of pressure ulcers and interventions to prevent and treat pressure ulcers are also described.
Inflammatory response
The inflammatory response is a sequential reaction to cell injury. It neutralises and dilutes the inflammatory agent, removes necrotic materials and establishes an environment suitable for healing and repair. The term inflammation is often but incorrectly used as a synonym for the term infection. Inflammation is always present with infection, but infection is not always present with inflammation. However, a person who is neutropenic may not be able to mount an inflammatory response. An infection involves invasion of tissues or cells by microorganisms, such as bacteria, fungi and viruses. In contrast, inflammation may also be caused by non-living agents, such as heat, radiation, trauma and allergens. If infection is present with inflammation, it is from the invasion of microorganisms.
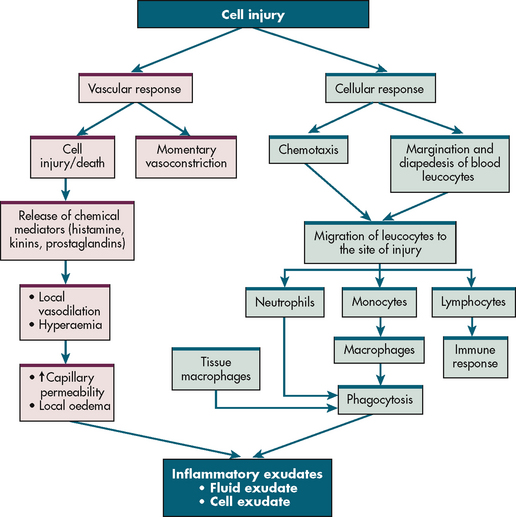
The mechanism of inflammation is basically the same regardless of the injuring agent. The intensity of the response depends on the extent and severity of injury and on the reactive capacity of the injured person. The inflammatory response can be divided into a vascular response, a cellular response, the formation of exudate and healing. Figure 12-1 illustrates the vascular and cellular response to injury.
VASCULAR RESPONSE
After cell injury, local arterioles briefly undergo transient vasoconstriction. Following release of histamine and other chemicals by the injured cells, the vessels dilate. This vasodilation results in increased blood flow, which raises the filtration pressure. Chemical mediators cause increased capillary permeability and facilitate fluid movement from capillaries into tissue spaces. Initially composed of serous fluid, this inflammatory exudate later contains plasma proteins, primarily albumin, and these proteins exert oncotic pressure that further draws fluid from blood vessels. Both vasodilation and increased capillary permeability are responsible for redness, heat and swelling at the site of injury.
As the plasma protein fibrinogen leaves the blood, it is converted to fibrin by the products of the injured cells. Fibrin strengthens a blood clot formed by platelets. In tissue the plug functions to prevent further blood loss, trap bacteria to prevent their spread and serve as a framework for the healing process. Platelets release growth factors that start the healing process.
CELLULAR RESPONSE
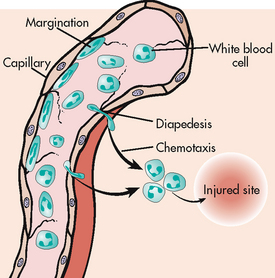
Neutrophils and monocytes move from the circulation to the site of injury (see Fig 12-1). Chemotaxis is the directional migration of white blood cells (WBCs) towards a higher concentration gradient of chemotaxins, which are substances that attract leucocytes to the site of inflammation. Chemotaxis results in an accumulation of neutrophils and monocytes at the focus of injury (see Fig 12-2).
Neutrophils
Neutrophils are the first leucocytes to arrive to the site of injury (usually within 6–12 hours). They phagocytose (engulf) bacteria, other foreign material and damaged cells. With their short life span (they circulate for approximately 7–10 hours before entering the tissues, where they may survive for 24–48 hours), dead neutrophils soon accumulate. In time a mixture of dead neutrophils, digested bacteria and other cell debris accumulates as a creamy substance termed pus.
To keep up with the demand for neutrophils, the bone marrow releases more neutrophils into the circulation. This results in an elevated WBC count, especially the neutrophil count. Sometimes the demand for neutrophils increases to the extent that the bone marrow releases immature forms of neutrophils (bands) into circulation. (Mature neutrophils are classified as segmented neutrophils.) The finding of increased numbers of band neutrophils in circulation is called a shift to the left and is commonly found in patients with acute bacterial infections. (See Ch 29 for a discussion on neutrophils.)
Monocytes
Monocytes are the second type of phagocytic cells that migrate from circulating blood. They are attracted to the site by chemotactic factors and usually arrive at the site within 3–7 days after the onset of inflammation. On entering the tissue spaces, monocytes transform into macrophages. Together with the tissue macrophages, these newly arrived macrophages assist in phagocytosis of the inflammatory debris. The macrophage role is important in cleaning the area before healing can occur. Macrophages have a long life span; they can multiply and may stay in the damaged tissues for weeks. These long-lived cells are important in orchestrating the healing process.
In cases where particles are too large for a single macrophase, they accumulate and fuse to form a multinucleated giant cell. The giant cell is then encapsulated by collagen, leading to the formation of a granuloma. A classic example of this process occurs with the tubercle bacillus in the lung. While the Mycobacteria bacillus is walled off, a chronic state of inflammation exists. The granuloma formed consists of a compact aggregate of active macrophages around the infecting agent and often shows central necrosis.
Lymphocytes
Lymphocytes arrive later at the site of injury. Their primary role is related to humoral and cell-mediated immunity (see Ch 13).
CHEMICAL MEDIATORS
Mediators of the inflammatory response are presented in Table 12-1.
TABLE 12-1 Mediators of inflammation
| Mediator | Source | Mechanisms of action |
|---|---|---|
| Histamine | Stored in granules of basophils, mast cells, platelets | Causes vasodilation and increased vascular permeability by stimulating contraction of endothelial cells and creating widened gaps between cells |
| Serotonin | Stored in platelets, mast cells, enterochromaffin cells of gastrointestinal tract | Same as above; stimulates smooth muscle contraction |
| Kinins (e.g. bradykinin) | Produced from precursor factor kininogen as a result of activation of hageman factor (Xii) of clotting system | Cause contraction of smooth muscle and dilation of blood vessels; result in stimulation of pain |
| Complement components (C3a, C4a, C5a) | Anaphylatoxic agents generated from complement pathway activation | Stimulate histamine release; stimulate chemotaxis |
| Prostaglandins and leukotrienes | Produced from arachidonic acid | PGE1 and PGE2 cause vasodilation; LTB4 stimulates chemotaxis |
| Cytokines | For information on cytokines, see tables 13-5 and 13-6 |
LT, leukotrienes; PG, prostaglandin.
Complement system
The complement system is an enzyme cascade (C1–C9) consisting of pathways to mediate inflammation and destroy invading pathogens (see Fig 12-3). Major functions of the complement system are enhanced phagocytosis, increasing vascular permeability, chemotaxis and cellular lysis. All of these activities are important in the inflammatory response and healing. Cell lysis occurs when the final components create holes in the cell membranes and cause targeted cell death by membrane rupture. In autoimmune disorders, healthy tissue can be damaged by complement activation and the resulting inflammatory response. This process is exemplified in rheumatoid arthritis and systemic lupus erythematosus.
Prostaglandins and leukotrienes
When cells are activated by injury, the arachidonic acid in the cell membrane is rapidly converted to produce prostaglandins, thromboxane and leukotrienes (see Fig 12-4). Prostaglandins are generally considered proinflammatory and are potent vasodilators contributing to increased blood flow and oedema formation. Some subtypes of prostaglandins are formed when platelets are activated and can inhibit platelet and neutrophil aggregation. Prostaglandins also perform a significant role in sensitising pain receptors to arousal by stimuli that would normally be painless. Prostaglandins have a pivotal role as pyrogens when stimulating the temperature-regulating area of the hypothalamus and producing a febrile response.
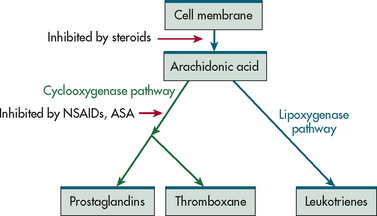
Figure 12-4 Pathway of generation of prostaglandins, thromboxanes and leukotrienes (LTs). Corticosteroids, non-steroidal anti-inflammatory drugs and acetylsalicylic acid act to inhibit various steps in this pathway.
Thromboxane is a powerful vasoconstrictor and platelet-aggregating agent. It causes the brief vasoconstriction and skin pallor at the injury site and promotes clot formation. It has a short half-life and the pallor soon gives way to prostaglandins’ and histamine’s vasodilating effects.
Leukotrienes form the slow-reacting substance of anaphylaxis (SRS-A), which constricts smooth muscles of bronchi, causing narrowing of the airway, and increases capillary permeability, leading to airway oedema.
EXUDATE FORMATION
Exudate consists of fluid and leucocytes that move from the circulation to the site of injury. The nature and quantity of exudate depend on the type and severity of the injury and the tissues involved (see Table 12-2).
TABLE 12-2 Types of inflammatory exudate
| Type | Description | Examples |
|---|---|---|
| Serous | Serous exudate results from outpouring of fluid that has low cell and protein content; it is seen in early stages of inflammation or when injury is mild. | Skin blisters, pleural effusion |
| Catarrhal | Catarrhal exudate is found in tissues where cells produce mucus. Mucus production is accelerated by the inflammatory response. | Runny nose associated with upper respiratory tract infection |
| Fibrinous | Fibrinous exudate occurs with increasing vascular permeability and fibrinogen leakage into interstitial spaces. Excessive amounts of fibrin coating tissue surfaces may cause them to adhere. | Adhesions |
| Purulent (pus) | Purulent exudate consists of white blood cells, microorganisms (dead and alive), liquefied dead cells and other debris. | Furuncle (boil), abscess, cellulitis (diffuse inflammation in connective tissue) |
| Haemorrhagic | Haemorrhagic exudate results from rupture or necrosis of blood vessel walls; it consists of red blood cells that escape into tissue. | Haematoma |
CLINICAL MANIFESTATIONS
The local response to inflammation includes the manifestation of redness, heat, pain, swelling and loss of function (see Table 12-3). Systemic manifestations of inflammation include increased WBCs with a left shift, malaise, nausea and anorexia, increased pulse and respiratory rate, and fever.
TABLE 12-3 Local manifestations of inflammation
| Manifestations | Cause |
|---|---|
| Redness (rubor) | Hyperaemia from vasodilation |
| Heat (colour) | Increased metabolism at inflammatory site |
| Pain (dolour) | Change in ph; change in local ionic concentration; nerve stimulation by chemicals (e.g. histamine, prostaglandins); pressure from fluid exudate |
| Swelling (tumour) | Fluid shift to interstitial spaces; fluid exudate accumulation |
| Loss of function (functio laesa) | Swelling and pain |
Leucocytosis results from the increased release of leucocytes from the bone marrow. An increase in the circulating number of one or more types of leucocytes may be found. Inflammatory reactions are accompanied by the symptoms of malaise, nausea, anorexia and fatigue. The causes of these systemic changes are poorly understood but are probably due to complement activation and the release of cytokines (soluble factors secreted by WBCs and other types of cells that act as intercellular messengers). Some of these cytokines (e.g. interleukins [ILs], tumour necrosis factor [TNF]) are important in causing the systemic manifestations of inflammation, as well as inducing the production of fever. An increase in pulse and respiration follows the rise in metabolism as a result of an increase in body temperature. (Cytokines are discussed in Ch 13.)
Fever
The onset of fever is triggered by the release of cytokines, which cause fever by initiating metabolic changes in the temperature-regulating centre (see Fig 12-5). Prostaglandin synthesis is the most critical metabolic change. Prostaglandins act directly to increase the thermostatic set point. The hypothalamus then activates the autonomic nervous system to stimulate increased muscle tone and shivering and decreased perspiration and blood flow to the periphery. Adrenaline released from the adrenal medulla increases the metabolic rate. The net result is fever.
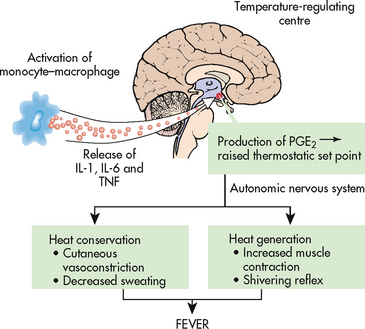
Figure 12-5 Production of fever. When monocytes/macrophages are activated, they secrete cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) and tumour necrosis factor (TNF), which reach the hypothalamic temperature-regulating centre. These cytokines promote the synthesis and secretion of prostaglandin E2 (PGE2) in the anterior hypothalamus. PGE2 increases the thermostatic set point, and the autonomic nervous system is stimulated, resulting in shivering, muscle contraction and peripheral vasoconstriction.
With the physiological thermostat fixed at a higher-than-normal temperature, the rate of heat production is increased until the body temperature reaches the new set point. As the set point is raised, the hypothalamus signals an increase in heat production and conservation to raise the body temperature to the new level. At this point the individual feels chilled and shivers. The shivering response is the body’s method of raising its temperature until the new set point is attained. This seeming paradox is dramatic: the body is hot, yet the individual piles on blankets and may go to bed to get warm. When the circulating body temperature reaches the set point of the core body temperature, the chills and warmth-seeking behaviour cease.
The released cytokines and the fever they trigger activate the body’s defence mechanisms. Beneficial aspects of fever include increased killing of microorganisms, increased phagocytosis by neutrophils and increased proliferation of T cells. Higher body temperatures may also enhance the activity of interferon, the body’s natural virus-fighting substance (see Ch 13).1
Types of inflammation
The basic types of inflammation are acute, subacute and chronic. In acute inflammation the healing occurs in 2–3 weeks and usually leaves no residual damage. Neutrophils are the predominant cell type at the site of inflammation. A subacute inflammation has the features of the acute process but lasts longer. For example, infective endocarditis is a smouldering infection with acute inflammation, but it persists for weeks or months (see Ch 36). Chronic inflammation lasts for weeks, months or even years. The injurious agent persists or repeatedly injures tissue. The predominant cell types present at the site of inflammation are lymphocytes and macrophages. Examples of chronic inflammation include rheumatoid arthritis and osteomyelitis. The prolongation and chronicity of any inflammation may be the result of an alteration in the immune response (e.g. autoimmune disease) and can lead to physical deterioration.
 NURSING AND COLLABORATIVE MANAGEMENT: INFLAMMATION
NURSING AND COLLABORATIVE MANAGEMENT: INFLAMMATION
 Nursing diagnosis
Nursing diagnosis
Nursing diagnoses for the patient with inflammation include, but are not limited to, the following:
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The best management of inflammation is the prevention of infection, trauma, surgery and contact with potentially harmful agents. This is not always possible. A simple mosquito bite causes an inflammatory response. Because occasional injury is inevitable, concerted efforts to minimise inflammation and infection are needed.
Adequate nutrition is essential so that the body has the necessary factors to promote healing when injury occurs. A high fluid intake is needed to replace fluid loss from perspiration: there is a 13% increase for every 1°C increase in temperature above 37.8°C. The increased metabolic rate increases the patient’s need for additional kilojoules.
Early recognition of the manifestations of inflammation is necessary so that appropriate treatment can begin. This may be rest, drug therapy or specific treatment of the injured site. Immediate treatment may prevent the extension and complications of inflammation.
 Acute intervention
Acute intervention
 Observation and vital signs
Observation and vital signs
The ability to recognise the clinical manifestations of inflammation is important. In the individual who is immunosuppressed (e.g. taking corticosteroids or receiving chemotherapy), the classic manifestations of inflammation may be masked. In this individual, early symptoms of inflammation may be malaise or ‘just not feeling well’.
Vital signs are important to note with any inflammation, especially when an infectious process is present. When infection is present, temperature may rise, and pulse and respiration rates may increase.
 Fever
Fever
An important aspect of fever management should be determining its cause. Although fever is usually regarded as harmful, an increase in body temperature is an important host defence mechanism. In the 17th century, Thomas Sydenham noted that ‘fever is a mighty engine which nature brings into the world for the conquest of her enemies’.1 Steps are frequently taken to lower body temperature to relieve the anxiety of the patient and healthcare professionals. Because mild-to-moderate fever usually does little harm, imposes no great discomfort and may benefit host defence mechanisms, antipyretic drugs are rarely essential to patient welfare. Moderate fevers (up to 39.5°C) usually produce few problems in most patients. However, if the patient is very young or very old, is extremely uncomfortable or has a significant medical problem (e.g. severe cardiopulmonary disease, brain injury), the use of antipyretics should be considered.2 Fever in the immunosuppressed patient should be treated rapidly and antibiotic therapy begun because infections can rapidly progress to septicaemia. (Neutropenia is discussed in Ch 30.)
Fever (especially if greater than 40°C) can be damaging to body cells, and delirium and seizures can occur. At temperatures greater than 41°C, regulation by the hypothalamic temperature control centre becomes impaired and damage can occur to many cells, including those in the brain.
Older adults have a blunted febrile response to infection. The body temperature may not rise to the level expected for a younger adult or may be delayed in its onset. The blunted response can delay diagnosis and treatment. By the time fever (as defined for younger adults) is present, the illness may be more severe. Although sponge baths increase evaporative heat loss, they may not decrease the body temperature unless antipyretic drugs have been given to lower the set point. Otherwise, the body will initiate compensatory mechanisms (e.g. shivering) to restore body heat. The same principle applies to the use of cooling blankets; they are most effective in lowering body temperature when the set point has also been lowered.3 A nursing care plan for the patient with a fever is available in NCP 12-1.
 Drug therapy
Drug therapy
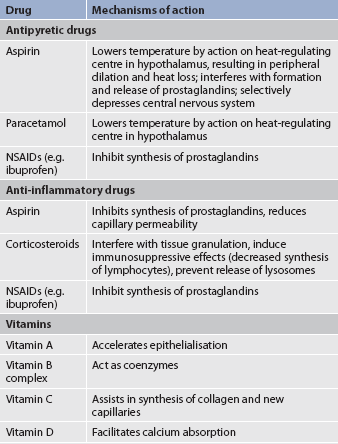
Drugs are used to decrease the inflammatory response and lower the body temperature (see Table 12-4). Aspirin blocks prostaglandin synthesis in the hypothalamus and elsewhere in the body. Acetaminophen acts on the heat-regulating centre in the hypothalamus. Some non-steroidal anti-inflammatory drugs (NSAIDs) (e.g. ibuprofen) have antipyretic effects. Corticosteroids are antipyretic through the dual mechanisms of preventing cytokine production and prostaglandin synthesis. The action of these drugs results in dilation of superficial blood vessels, increased skin temperature and sweating. Antipyretics should be given around the clock to prevent acute swings in temperature. Chills may be evoked or perpetuated by the intermittent administration of antipyretics. These agents cause a sharp decrease in temperature. When the antipyretic wears off, the body may initiate a compensatory involuntary muscular contraction (i.e. chill) to raise the body temperature back up to its previous level. This unpleasant side effect of antipyretic drugs can be prevented by administering these agents regularly at 2- to 4-hour intervals.
Antihistamine drugs may also be used to inhibit the action of histamine. (Antihistamines are discussed in Chs 13 and 26).
 Rest
Rest
Rest helps the body use its nutrients and oxygen for the healing process. The repair process is facilitated by allowing fibrin and collagen to form across the wound edges with little disruption.
 Cold and heat
Cold and heat
Cold application is usually appropriate at the time of the initial trauma to cause vasoconstriction and decrease swelling, pain and congestion from increased metabolism in the area of inflammation. Heat may be used later (e.g. after 24–48 hours) to promote healing by increasing the circulation to the inflamed site and subsequent removal of debris. Heat is also used to localise the inflammatory agents. Warm, moist heat may help debride the wound site if necrotic material is present.
 Compression and immobilisation
Compression and immobilisation
Compression serves to counter the vasodilation effects and development of oedema. Compression by direct pressure over a laceration occludes blood vessels and stops bleeding. Compression bandages provide support to injured joints that have tendons and muscles unable to provide support on their own. The nurse should assess distal pulses and capillary refill before and after application of compression to gauge whether compression would be safe and to evaluate that compression has not compromised circulation.
Immobilisation of the inflamed or injured area promotes healing by decreasing the metabolic needs of the tissues. Immobilisation with a cast or splint supports fractured bones and prevents the possibility of further tissue injury by sharp bone fragments severing nerves or blood vessels and the possibility of haemorrhage. As with compression, the nurse should evaluate the patient’s circulation after application and at intervals in the event swelling occurs within the closed space of a cast that could compromise circulation.
 Elevation
Elevation
Elevating the injured extremity above the level of the heart will reduce oedema at the inflammatory site by increasing venous and lymphatic return. Elevation also helps reduce pain associated with blood engorgement of the injury site. Elevation may be contraindicated in patients with significantly reduced arterial circulation.
Healing process
The final phase of the inflammatory response is healing. Healing includes the two major components of regeneration and repair. Regeneration is the replacement of lost cells and tissues with cells of the same type. Repair is healing as a result of lost cells being replaced by connective tissue. Repair is the more common type of healing and usually results in scar formation.
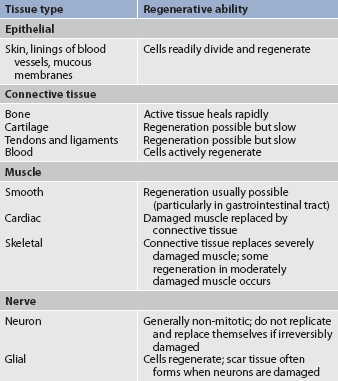
REGENERATION
The ability of cells to regenerate depends on the cell type (see Table 12-5). Labile cells divide constantly. Examples include cells of the skin, lymphoid organs, bone marrow and mucous membranes of the gastrointestinal (GI), urinary and reproductive tracts. Injury to these organs is followed by rapid regeneration.
Stable cells retain their ability to regenerate but do so only if the organ is injured. Examples of stable cells are liver, exocrine pancreas, kidney and bone cells.
Permanent cells do not divide. Examples of these cells are neurons of the central nervous system (CNS), and skeletal and cardiac muscle cells. Damage to CNS neurons or heart or skeletal muscle can lead to permanent loss. If neurons in the CNS are destroyed, the tissue is generally replaced by glial cells. However, recent research demonstrates that minimal neurogenesis may occur from stem cells. Healing of skeletal and cardiac muscle will occur by repair with scar tissue.
REPAIR
Repair is a more complex process than regeneration. Most injuries heal by connective tissue repair. Repair healing occurs by primary, secondary or tertiary intention (see Fig 12-6).
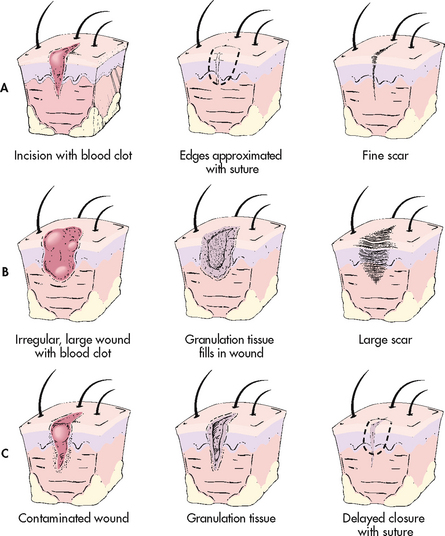
Figure 12-6 Types of wound healing. A, Primary intention. B, Secondary intention. C, Tertiary intention.
Primary intention
Primary intention healing takes place when wound margins are neatly approximated, such as in a surgical incision or a paper cut. A continuum of processes is associated with primary healing (see Table 12-6). These processes include three phases.
TABLE 12-6 Phases in primary intention healing
| Phase | Activity |
|---|---|
| Initial (3–5 days) | Approximation of incision edges; migration of epithelial cells; clot serving as meshwork for starting capillary growth |
| Granulation (5 days–4 weeks) | Migration of fibroblasts; secretion of collagen; abundance of capillary buds; fragility of wound |
| Scar contracture (7 days–several months) | Remodelling of collagen; strengthening of scar |
Initial phase
The initial phase lasts for 3–5 days. The edges of the incision are first aligned and sutured (or stapled) in place. The incision area fills with blood from the cut blood vessels, blood clots form and platelets release growth factors to begin the healing process. This forms a matrix for WBC migration. An acute inflammatory reaction occurs. The area of injury is composed of platelet plugs, erythrocytes, neutrophils (both dead and dying) and other debris. Macrophages ingest and digest cellular debris, fibrin fragments and RBCs. Extracellular enzymes derived from macrophages and neutrophils help digest fibrin. As the wound debris is removed, the fibrin serves as a meshwork for future capillary growth and migration of epithelial cells.
Granulation phase
The granulation (fibroblastic, proliferative, reconstructive) phase is the second step and lasts from 5 days to 3 weeks. The components of granulation tissue include proliferating fibroblasts; proliferating capillary sprouts (angioblasts); various types of WBCs; exudate; and loose, semifluid, ground substance. Fibroblasts are immature connective tissue cells that migrate into the healing site and secrete collagen. In time the collagen is organised and restructured to strengthen the healing site. At this stage it is termed fibrous or scar tissue.
During the granulation phase the wound is pink and vascular. Numerous red granules (young budding capillaries) are present (see Fig 12-7). At this point the wound is friable, at risk of dehiscence and resistant to infection.
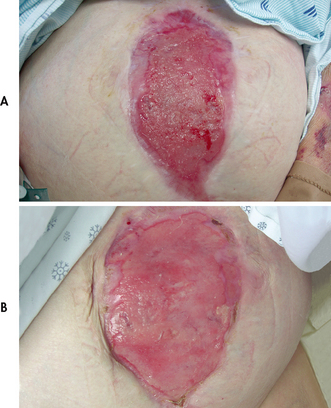
Figure 12-7 A, Wound clean but not granulating (note lack of red cobblestone appearance), suggesting heavy bacterial contamination or other impediments to wound healing. B, Same wound granulating after 1 week of topical antibiotic use (note healthy red cobblestone appearance).
Surface epithelium at the wound edges begins to regenerate. In a few days a thin layer of epithelium migrates across the wound surface. The epithelium thickens and begins to mature, and the wound now closely resembles the adjacent skin. In a superficial wound, re-epithelialisation may take 3–5 days.
Maturation phase and scar contraction
The maturation phase where scar contraction occurs overlaps with the granulation phase. It may begin 7 days after the injury and continue for several months or years. This is the reason abdominal surgery discharge instructions limit lifting for up to 6 weeks. Collagen fibres are further organised and the remodelling process occurs. Fibroblasts disappear as the wound becomes stronger. The active movement of the myofibroblasts causes contraction of the healing area, helping to close the defect and bring the skin edges closer together. A mature scar is then formed. In contrast to granulation tissue, a mature scar is virtually avascular and pale. The scar may be more painful at this phase than in the granulation phase.
Secondary intention
Wounds that occur from trauma, ulceration and infection have large amounts of exudate and wide, irregular wound margins with extensive tissue loss. These wounds may have edges that can be approximated (brought together). The inflammatory reaction may be greater than in primary healing. This results in more debris, cells and exudate. The debris may have to be cleaned away (debrided) before healing can take place.
The process of healing by secondary intention is essentially the same as in primary healing. The major differences are the greater defect and the gaping wound edges. Healing and granulation take place from the edges inwards and from the bottom of the wound upwards until the defect is filled. There is more granulation tissue, and the result is a much larger scar.
Tertiary intention
Tertiary intention (delayed primary intention) healing occurs with delayed suturing of a wound in which two layers of granulation tissue are sutured together. This occurs when a contaminated wound is left open and sutured closed after the infection is controlled. It also occurs when a primary wound becomes infected, is opened, is allowed to granulate and is then sutured. Tertiary intention usually results in a larger and deeper scar than primary or secondary intention.
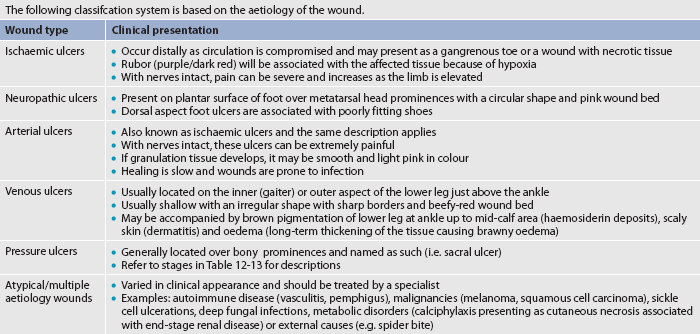
WOUND CLASSIFICATION
Identifying the aetiology of a wound is essential to classifying the wound properly. Wounds can be classified by their cause (surgical or non-surgical; acute or chronic) or depth of tissue affected (superficial, partial-thickness or full-thickness). A superficial wound involves only the epidermis. Partial-thickness wounds extend into the dermis. Full-thickness wounds have the deepest layer of tissue destruction because they involve the subcutaneous tissue and sometimes even extend into the fascia and underlying structures, such as the muscle, tendon or bone. (Wound classification systems are described in Tables 12-7 and 12-8.)
TABLE 12-8 Wound classification systems
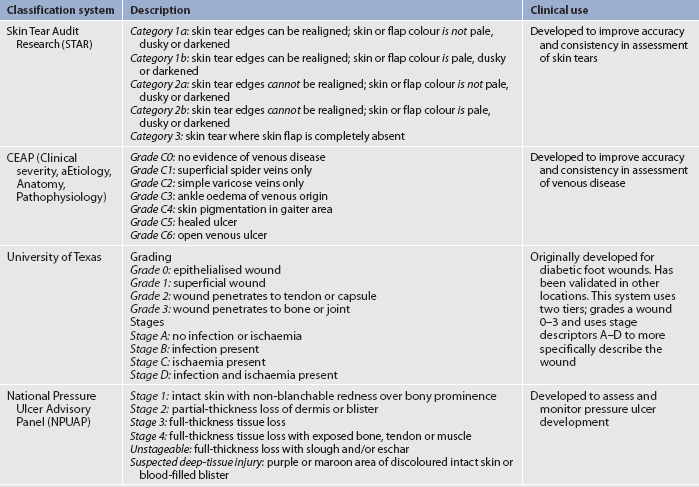
Source: STAR skin tear classification system. Available at www.silverchain.org.au/assets/files/Star-Skin-tear-tool-04022010.pdf, accessed 6 august 2011. CEAP classification of venous disease. Available at www.simondodds.com/Venous/CEAP_classification.htm, accessed 6 august 2011. University of Texas diabetic wound classification. Available at www.fpnotebook.com/surgery/Exam/UnvrstyofTxsDbtcWndClsfctn.htm, accessed 6 August 2011. National Pressure ulcer Advisory Panel. Available at www.npuap.org/pr2.htm, accessed 6 August 2011.
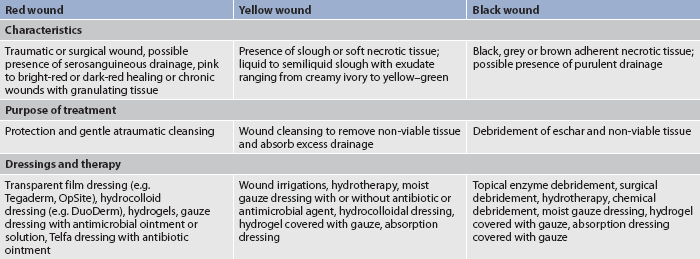
Another system that is sometimes used clinically to classify open wounds is based on the colour of the wound (red, yellow, black) rather than on the depth of tissue destruction (see Table 12-9). The red-yellow-black classification can be applied to any wound allowed to heal by secondary intention, including surgically induced wounds left to heal without skin closure because of a risk of infection. A wound may have two or three colours at the same time. In this situation the wound is classified according to the least desirable colour present.
DELAY OF HEALING
In a healthy person, wounds heal at a normal, predictable rate. However, some factors delay wound healing. These are summarised in Table 12-10.
TABLE 12-10 Factors delaying wound healing
| Factor | Effect on wound healing |
|---|---|
| Nutritional deficiencies | |
| Vitamin C | Delays formation of collagen fibres and capillary development |
| Protein | Decreases supply of amino acids for tissue repair |
| Zinc | Impairs epithelialisation |
| Inadequate blood supply | Decreases supply of nutrients to injured area, decreases removal of exudative debris, inhibits inflammatory response |
| Corticosteroid drugs | Impair phagocytosis by white blood cells, inhibit fibroblast proliferation and function, depress formation of granulation tissue, inhibit wound contraction |
| Infection | Increases inflammatory response and tissue destruction |
| Smoking | Nicotine is a potent vasoconstrictor and impedes blood flow to healing areas |
| Mechanical friction on wound | Destroys granulation tissue, prevents apposition of wound edges |
| Advanced age | Slows collagen synthesis by fibroblasts, impairs circulation, requires longer time for epithelialisation of skin, alters phagocytic and immune responses |
| Obesity | Decreases blood supply in fatty tissue |
| Diabetes mellitus | Decreases collagen synthesis, retards early capillary growth, impairs phagocytosis (result of hyperglycaemia), reduces supply of oxygen and nutrients secondary to vascular disease |
| Poor general health | Causes generalised absence of factors necessary to promote wound healing |
| Anaemia | Supplies less oxygen at tissue level |
COMPLICATIONS OF HEALING
The shape and location of the wound determine how well the wound will heal. Certain factors can interfere with wound healing and lead to complications. These factors may include malnutrition, obesity, decreased blood supply, tissue trauma, smoking, drugs (e.g. corticosteroids, chemotherapy), wound debris such as necrotic tissue, and infection.4 Complications of healing are presented in Box 12-1.
BOX 12-1 Complications of wound healing
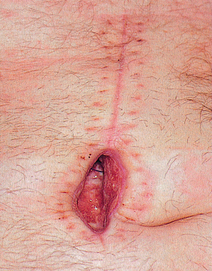
Dehiscence
• Separation and disruption of previously joined wound edges (see Fig 12-8).
• Usually occurs when a primary healing site bursts open.
Haemorrhage
• Bleeding is normal immediately after tissue injury and ceases with clot formation.
• Haemorrhage occurs as abnormal internal or external blood loss caused by suture failure, clotting abnormalities, dislodged clot, infection or erosion of a blood vessel by a foreign object (tubing, drains) or infection process.
Hypertrophic scars
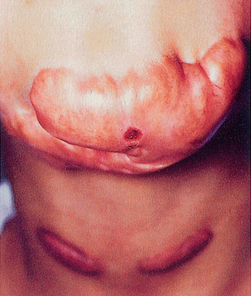
• Occur when an overabundance of collagen is produced during healing (see Fig 12-9).
• Form an inappropriately large, raised red and hard scar that is non–life threatening.
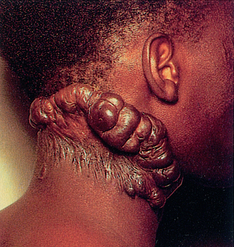
Keloid formation
• Great protrusion of scar tissue that extends beyond wound edges and may form tumour-like masses of scar tissue (see Fig 12-10).
• Permanent without any tendency to subside.
• Patients often complain of tenderness, pain, and hyperparaesthesia, especially in early stages.
• Thought to be a hereditary condition occurring most often in dark-skinned persons.
 NURSING AND COLLABORATIVE MANAGEMENT: WOUND HEALING
NURSING AND COLLABORATIVE MANAGEMENT: WOUND HEALING
 Nursing assessment
Nursing assessment
Observation and recording of wound characteristics are essential. The wound should be thoroughly assessed on admission and on a regular basis thereafter. Deterioration in the wound will require the nurse to assess and document changes more frequently. Various methods exist for measuring wounds.5 One method for measuring wounds is presented in Figure 12-11. It is becoming more common for digital photographs of wounds to be included in a patient’s record to assist with accurate estimation of the healing progress. Nurses should record the consistency, colour and odour of any drainage and report if abnormal for the specific wound situation. Staphylococcus and Pseudomonas species are common organisms that cause purulent, draining wounds.
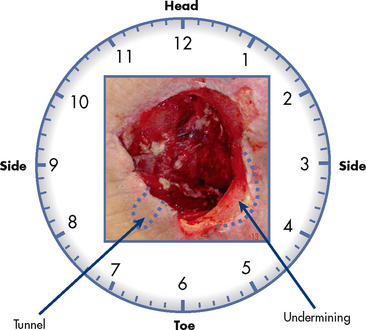
Figure 12-11 Wound measurements are made in centimetres. The first measurement is oriented from head to toe, the second is from side to side and the third is the depth (if any). If there is any tunnelling (when the cotton-tipped applicator is placed in the wound, there is movement) or undermining (when the cotton-tipped applicator is placed in the wound, there is a ‘lip’) around the wound, this is charted in respect to a clock, with 12 o’clock being towards the patient’s head. The wound shown would be charted as a full-thickness red wound, 7 cm × 5 cm × 3 cm, with a 3 cm tunnel at 7 o’clock and 2 cm undermining from 3 o’clock to 5 o’clock.
In healthy people wounds heal at a normal, predictable rate. On admission the nurse needs to identify factors that may delay wound healing and contribute to chronic non-healing wounds (see Table 12-10). Chronic wounds are those that do not heal within the normal time (approximately 3 months). If a wound fails to heal in a timely manner, the nurse should assess and identify factors that may delay healing and refer the patient to a healthcare provider specialising in wound management. Time does not heal all wounds. While caring for patients during the healing process, the nurse needs to continually assess for complications associated with healing (see Box 12-1).
The Wounds West website provides an interactive evidence-based program that addresses the prevention and management of wounds (see the Resources on p 245). The Australian Wound Management Association has developed standards for wound management that provide a framework for clinical practice grounded in theory. These standards aim to provide a foundation for promoting best practice in wound management to maintain and improve quality care outcomes for people with a wound or potential wound (see the Resources on p 245).
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with a wound include, but are not limited to, the following:
 Nursing implementation
Nursing implementation
Nursing and multidisciplinary care for the patient with a tissue injury is highly variable. It depends on the causative agent, the degree of injury and the patient’s condition. Superficial skin injuries may need only cleansing. Adhesive strips or tissue adhesives may be used instead of sutures. The treatment plan can include covering these wounds with a film dressing to provide a moist healing environment and wound protection from trauma. Deeper skin wounds can be closed by suturing the edges together. If the wound is contaminated, it must be converted into a clean wound before healing can occur normally. Debridement of a wound that has multiple fragments or devitalised tissue may be necessary. If the source of inflammation is an internal organ (e.g. appendix, ruptured spleen), surgical removal of the organ is the treatment of choice.
The type of wound management and dressings required depend on the type, extent and characteristics of the wound and the phase of healing.6 The purposes of wound management include: (1) cleaning the wound to remove any dirt and debris from the wound bed; (2) treating infection to prepare the wound for healing; and (3) protecting a clean wound from trauma so that it can heal normally.
Sutures and fibrin sealant are used to facilitate wound closure and create an optimal setting for wound healing. Usually sutures are used to close wounds because suture material provides the mechanical support necessary to sustain closure. A wide variety of suturing materials are available. In contrast, fibrin sealant is a biological tissue adhesive that can function as a useful adjunct to sutures. Fibrin sealant can be used in conjunction with sutures or tape to promote optimal wound integrity, and it can be used independently to seal wound sites where sutures cannot control bleeding or would aggravate bleeding. This adhesive can effectively seal tissue and eliminate potential spaces.
For wounds that heal by primary intention, it is common to cover the incision with a dry, sterile dressing that is removed as soon as the drainage stops or in 2–3 days. Medicated sprays that form a transparent film on the skin may be used for dressings on a clean incision or injury. Transparent film dressings are also commonly used. Sometimes a surgeon will leave a surgical wound uncovered.
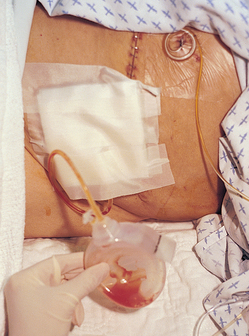
Sometimes drains are inserted into the wound to facilitate removal of fluid. The Jackson-Pratt drainage device is a suction drainage device consisting of a flexible plastic bulb connected to an internal plastic drainage tube (see Fig 12-12).
Topical antimicrobials and antibactericidals (e.g. povidone-iodine, hypochlorite solutions, hydrogen peroxide, chlorhexidine) should be used with caution in wound care because they can damage the new epithelium of healing tissue. They should never be used in a clean granulating wound.
Wound healing management by secondary intention depends on the wound aetiology and type of tissue in the wound. This type of management involves creating an environment to support healing. The red-yellow-black concept of wound care presented in Table 12-9 provides a method of dressing selection based on the wound tissue colour. Examples of types of wound dressings are presented in Table 12-11.
TABLE 12-11 Types of wound dressings
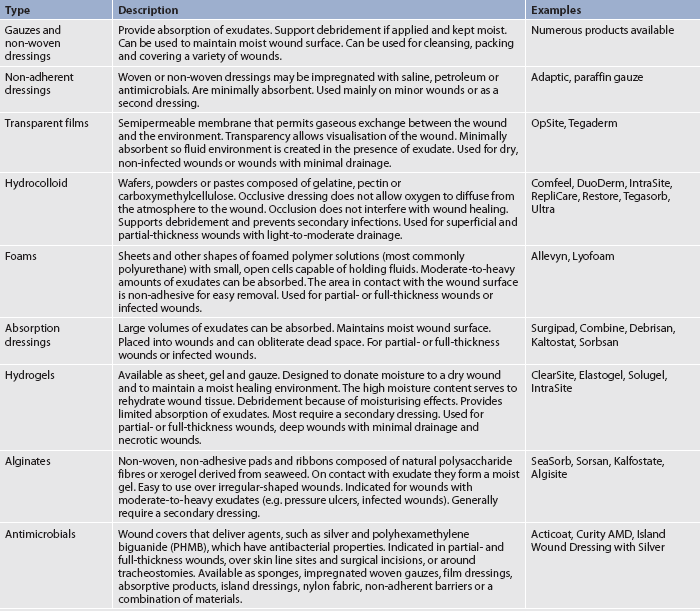
Source: modified from Woundsource. Available at http://www.woundsource.com/product-category/dressings.
 Red, yellow and black wounds
Red, yellow and black wounds
 Red wounds
Red wounds
In red wounds the purpose of treatment is protection of the wound and gentle cleansing (if indicated). Clean wounds that are granulating and re-epithelialising should be kept slightly moist and protected from further trauma until they heal naturally. A dressing material that keeps the wound surface clean and slightly moist is optimal to promote epithelialisation. Transparent film or adhesive semipermeable dressings (e.g. OpSite, Tegaderm) are occlusive dressings that are permeable to oxygen. The wound is then usually covered with a sterile dressing. Unnecessary manipulation during dressing changes may destroy new granulation tissue and break down fibrin formation.
 Yellow wounds
Yellow wounds
A type of dressing used in yellow wounds is an absorption dressing that absorbs exudate and cleanses the wound surface. Absorption dressings work by drawing excess drainage from the wound surface. After these preparations are saturated with exudate, they should be removed by washing with sterile saline or water. The amount of wound secretions determines the number of dressing changes.
Hydrocolloid dressings such as DuoDerm are also used to treat yellow wounds. The inner part of these dressings interacts with the exudate, forming a hydrated gel over the wound. When the dressing is removed, the gel separates and stays over the wound. The wound must be cleansed gently to prevent damage to newly formed tissue. These types of dressings are designed to be left in place for up to 7 days or until leakage occurs around the dressing.
 Black wounds
Black wounds
The immediate treatment of back wounds is debridement of the non-viable eschar tissue. The debridement method used depends on the amount of debris and the condition of the wound tissue (see Table 12-12).7,8
 Negative-pressure wound therapy
Negative-pressure wound therapy
Negative-pressure wound therapy (vacuum-assisted wound closure) is a type of therapy that uses suction to remove drainage and speed wound healing.9 In this therapy, the wound is cleaned and a sponge is cut to the dimensions of the wound. A large occlusive dressing is applied and a small hole is made over the sponge where the tubing is attached. The tubing is connected to a pump, which creates a negative pressure in the wound bed. Wound types suitable for this therapy include acute or traumatic wounds, surgical wounds that have dehisced, pressure ulcers and chronic ulcers. Although the exact mechanism for promoting healing is not known, it is thought that this therapy pulls excess fluid from the wound, reduces bacterial load and encourages blood flow into the wound base. The nurse should monitor the patient’s serum protein levels and fluid and electrolyte balance due to losses from the wound. Additionally, the nurse should maintain vigilance concerning the patient’s coagulation studies (platelet count, prothrombin time [PT], partial thromboplastin time [PTT]).
 Hyperbaric oxygen therapy
Hyperbaric oxygen therapy
Hyperbaric O2 therapy (HBOT) is the delivery of O2 at increased atmospheric pressures.9 It can be given systemically with the patient placed in an enclosed chamber where 100% O2 is administered at 1.5–3 times the normal atmospheric pressure. HBOT allows oxygen to diffuse into the serum, rather than RBCs, and be transported to the tissues. By increasing the O2 content in the serum, it will move past narrowed arteries and capillaries where RBCs cannot go. In addition, elevated O2 levels stimulate angiogenesis (the production of new blood vessels), kill anaerobic bacteria and increase the killing power of WBCs and certain antibiotics (e.g. fluoroquinolones, aminoglycosides). Hyperbaric O2 therapy accelerates granulation tissue formation and wound healing. An alternative approach is to topically administer hyperbaric O2 by creating a chamber around the injured limb. Most systemic treatments last from 90 to 120 minutes, and the number of treatments may vary from 10 to 60, depending on the condition being treated. The topical treatments can last 20 minutes twice daily or 4–6 hours daily. The number of treatments is highly variable.
 Nutritional therapy
Nutritional therapy
Special nutritional measures facilitate wound healing. A high fluid intake is needed to replace fluid loss from sweating and exudate formation. An increased metabolic rate intensifies water loss. Individuals at risk for wound healing problems are those with malabsorption problems (e.g. Crohn’s disease, GI surgery, liver disease), deficient intake or high energy demands (e.g. malignancy, major trauma or surgery, sepsis, fever), and diabetes.10
Undernutrition puts a person at risk of poor healing. A diet high in protein, carbohydrate and vitamins with moderate fat intake is necessary to promote healing. Protein is needed to correct the negative nitrogen balance resulting from the increased metabolic rate. It is also necessary for the synthesis of immune factors, leucocytes, fibroblasts and collagen, which are the building blocks for healing. Carbohydrate is needed for the increased metabolic energy required in inflammation and healing. If there is a carbohydrate deficit, the body will break down protein for the required energy. Vitamin C is needed for capillary synthesis and collagen production by fibroblasts. The B-complex vitamins are necessary as coenzymes for many metabolic reactions. If a vitamin B deficiency develops, a disruption of protein, fat and carbohydrate metabolism will occur. Vitamin A is needed in healing because it aids in the process of epithelialisation. It increases collagen synthesis and tensile strength of the healing wound. Fats are also a necessary component in the diet, as cell membranes are built up of phospholipids, each of which contains fatty acids and triglycerides, which are part of the cellular membrane.
If the patient is unable to eat, enteral feedings and supplements should be the first choice if the GI tract is functional. Parenteral nutrition is indicated when enteral feedings are contraindicated or not tolerated. (Enteral and parenteral nutrition are discussed in Ch 39.)
 Infection prevention and control
Infection prevention and control
The nurse and the patient must scrupulously follow aseptic procedures for keeping the wound free from infection.11 The patient must not touch a recently injured area. The patient’s environment should be as free as possible from contamination from items introduced by roommates and visitors. Antibiotics may be administered prophylactically to some patients. If an infection develops, a culture and sensitivity test should be done to determine the organism and the most effective antibiotic for that specific organism.12 The culture should be taken before the first dose of antibiotic is given. Cultures can be obtained by needle aspiration, tissue culture or swab technique. Medical practitioners will obtain needle and tissue punch biopsy samples; nurses can obtain cultures using the swab technique.13 Concurrent swab specimens are obtained from wounds using: (1) wound exudates; (2) Z-technique; and (3) Levine’s technique. The first technique samples visible wound exudates from the wound bed before cleansing. The Z-technique involves rotating a culture swab over the cleansed wound bed surface in a 10-point Z-track fashion. Levine’s technique involves rotating a culture swab over a cleansed 1 cm2 area near the centre of the wound using sufficient pressure to extract wound fluid from deep tissue layers. Finally, a specimen of viable wound tissue is removed from the centre of the wound using sterile technique.14 When collecting samples, it is important not to use cotton-tipped swabs.
 Psychological implications
Psychological implications
The patient may be distressed at the thought or sight of an incision or wound because of fear of scarring or disfigurement, and drainage and odour from a wound often cause increased alarm. The patient needs to understand the healing process and the normal changes that occur as the wound heals. When changing a dressing, the nurse must be careful not to show inappropriate facial expressions that can alert the patient to problems with the wound or the nurse’s ability to care for it. The nurse should also be careful not to focus on the wound to the extent that the patient is not treated as a total person.
 Patient teaching
Patient teaching
Because patients are being discharged earlier after surgery and many have surgery as outpatients, it is important that the patient or the family, or both, know how to care for the wound and perform dressing changes. Wound healing may not be complete for 4–6 weeks or longer. Adequate rest and good nutrition should be continued throughout this time. Physical and emotional stress should be minimised. Observing the wound for complications such as contractures, adhesions and secondary infection is important. The patient should understand the signs and symptoms of infection. The patient should note changes in wound colour and the amount of drainage. The healthcare provider should be notified of any signs of abnormal wound healing. Medications will often be taken for a period of time after recovery from the acute infection. Medication-specific side effects and adverse effects should be reviewed with the patient, as well as methods to prevent side effects (e.g. taking with food or not). The patient should be instructed to contact the healthcare provider if any of these effects occur. It is also important to teach the patient about the necessity to continue the medications for the specified time. For example, a patient who is instructed to take an antibiotic for 10 days may stop taking it after 5 days because of decreased or absent symptoms. However, the organism may not be entirely eliminated and it may become resistant to the antibiotic if the drug is not continued.
Pressure ulcers
AETIOLOGY AND PATHOPHYSIOLOGY
A pressure ulcer is a localised injury to the skin and/or underlying tissue (usually over a bony prominence) as a result of pressure or pressure in combination with shear and/or friction.15 Pressure ulcers generally fall under the category of healing by secondary intention. The most common site for pressure ulcers is the sacrum, with the heels being the second. Factors that influence the development of pressure ulcers include the amount of pressure (intensity), the length of time the pressure is exerted on the skin (duration) and the ability of the patient’s tissue to tolerate the externally applied pressure. Besides pressure, shearing force (pressure exerted on the skin when it adheres to the bed and the skin layers slide in the direction of body movement), friction (two surfaces rubbing against each other) and excessive moisture contribute to pressure ulcer formation. Factors that put a patient at risk of the development of pressure ulcers are presented in Box 12-2. Individuals at risk include those who are older, incontinent, bed- or wheelchair-bound or recovering from spinal cord injuries.
Since 2007, all public hospitals in Western Australia have taken part in Wounds West’s yearly wound prevalence surveys. In the 2009 survey the prevalence of pressure ulcers was 9% (a 25% decrease from the survey conducted in 2008) and only 6% of patients had 1 or more hospital-acquired pressure ulcers (a 33% decrease from 2008). In the same survey, 56% of patients had a pressure ulcer risk assessment performed within 24 hours of admission and 76% of patients had a pressure-relieving device in situ.16 The incidence of pressure ulcers in nursing home residents is estimated to be about 26%.17
CLINICAL MANIFESTATIONS
The clinical manifestations of pressure ulcers depend on the extent of the tissue involved. Pressure ulcers are graded or staged according to their deepest level of tissue damage or ‘wounding’. Table 12-13 illustrates four pressure ulcer stages based on the National Pressure Ulcer Advisory Panel (NPUAP) guidelines.15,18 When eschar is present, accurate staging of the pressure ulcer is not possible until the eschar is removed by debridement and the ulcer bed can be seen. A pressure ulcer may be unstageable (see Table 12-8). The actual depth of tissue loss is obscured by slough (yellow, tan, grey, green or brown) and/or eschar (tan, brown or black) in the wound bed. A pressure ulcer may also manifest as a blood-filled blister. Until enough slough and/or eschar is removed to expose the base of the wound, the true depth, and therefore stage, cannot be determined. Stable (dry, adherent, intact) eschar on the heels serves as ‘the body’s natural (biological) cover’ and should not be removed.
TABLE 12-13 Staging of pressure ulcers
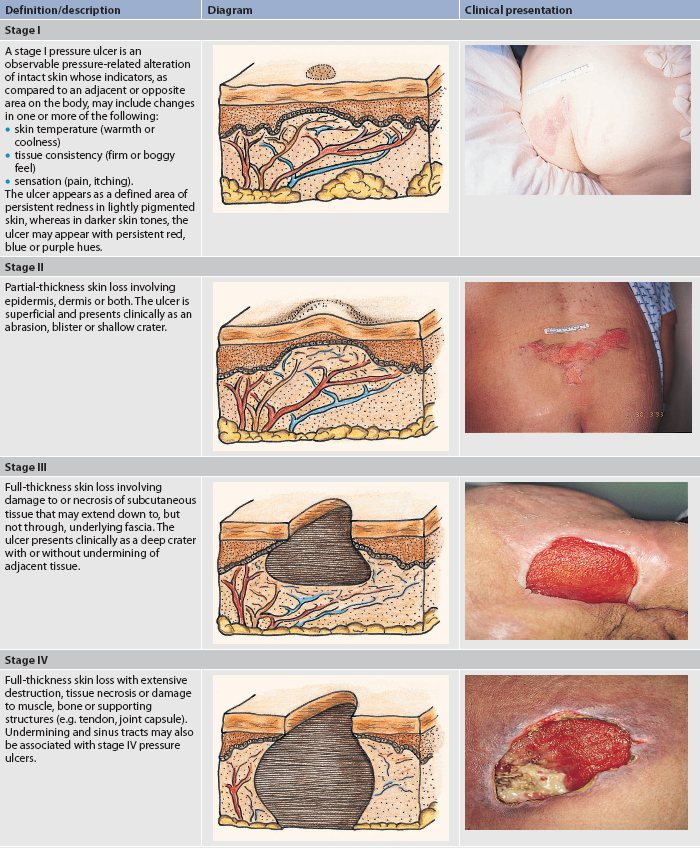
Source: National Pressure Ulcer Advisory Panel. Available at www.npuap.org.
If the pressure ulcer becomes infected, the patient may display signs of infection, such as leucocytosis and fever. In addition, the pressure ulcer may increase in size, odour and drainage; have necrotic tissue; and be indurated, warm and painful. Untreated ulcers may lead to cellulitis, chronic infection or osteomyelitis. The most common complication of a pressure ulcer is recurrence. Therefore, it is important to note the location of previously healed pressure ulcers on an initial admission assessment of a patient.
 NURSING AND COLLABORATIVE MANAGEMENT: PRESSURE ULCERS
NURSING AND COLLABORATIVE MANAGEMENT: PRESSURE ULCERS
Nursing and collaborative management are discussed together because the activities are interrelated. In addition to the nurse, other members of the healthcare team, such as the wound care specialist, plastic surgeon, dietician, physiotherapist and occupational therapist, can provide valuable input into the complex treatment necessary to prevent and treat pressure ulcers.
Specific guidelines for the prediction and management of pressure ulcers were published by the Australian Wound Management Association in 2001.19 These guidelines have been reviewed and the revised guidelines are due to be published in late 2011.20
 Nursing assessment
Nursing assessment
The nurse should conduct a thorough head-to-toe assessment on admission to identify and document a pressure ulcer. Periodic reassessment of skin and wounds should be conducted thereafter based on the individual’s condition and care setting.21 For example, in acute care a patient should be reassessed every 48 hours; in long-term care, a resident should be reassessed weekly for the first 4 weeks after admission and then monthly or quarterly at a minimum; and in home care the client should be reassessed at every nurse visit. Risk assessment should be done using a validated assessment tool, such as the Braden Risk Assessment Scale (see the Resources on p 245). Knowing the level of risk can determine how aggressive preventative measures should be.22
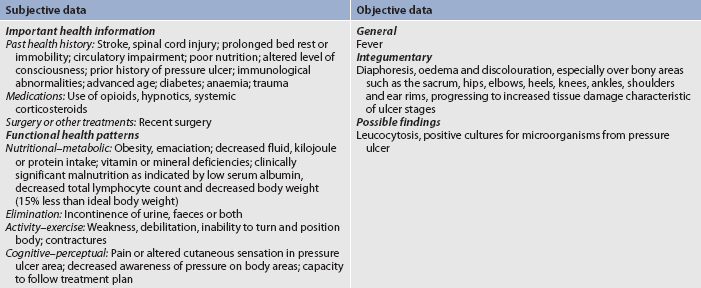
Identification of stage I pressure ulcers may be difficult in patients with dark skin. Box 12-3 presents techniques to help assess darker skin. Subjective and objective data that should be obtained from a patient with or at risk for a pressure ulcer are presented in Table 12-14.
BOX 12-3 Assessing patients with dark skin
• Look for changes in skin colour, such as skin that is darker (purplish, brownish, bluish) than surrounding skin.
• Use natural or halogen light source to assess the skin colour accurately. Fluorescent light casts blue colour, which can make skin assessment difficult.
• Assess the area for the skin temperature using your hand. The area may feel initially warm, then cooler.
• Touch the skin to feel its consistency. Boggy or oedematous feel may indicate a stage I pressure ulcer.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with a pressure ulcer may include, but are not limited to, those presented in NCP 12-2.
 Planning
Planning
The overall goals are that the patient with a pressure ulcer will: (1) have no deterioration of the ulcer stage; (2) reduce or eliminate the factors that led to the pressure ulcer; (3) not develop an infection in the pressure ulcer; (4) have healing of the pressure ulcer; and (5) have no recurrence.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
A primary nursing responsibility is the identification of patients at risk of developing pressure ulcers (see Box 12-2) and implementing pressure ulcer prevention strategies for those identified as being at risk. Once a patient has been identified as being at risk for pressure ulcer development, prevention strategies should be implemented. Prevention remains the best treatment for pressure ulcers.23
• Reposition patients frequently to prevent pressure ulcers.
• Use devices to reduce pressure and shearing force (e.g. alternating pressure mattresses, foam mattresses, wheelchair cushions, padded commode seats, boots [foam, air], lift sheets) as appropriate.
• These devices are not adequate substitutes for frequent repositioning.
 Acute intervention
Acute intervention
Care of the patient with a pressure ulcer requires local care of the wound and support measures of the whole person, such as adequate nutrition, pain management, control of other medical conditions and pressure relief. Both conservative and surgical strategies are used in the treatment of pressure ulcers, depending on the stage and condition of the ulcer. Once a pressure ulcer has developed, the nurse should initiate interventions based on the ulcer characteristics (e.g. stage, size, location, amount of exudate, type of wound, presence of infection or pain) and the patient’s general status (e.g. nutritional state, age, cardiovascular status, level of mobility).24 Careful documentation should be made of the size of the pressure ulcer. A wound-measuring card or tape can be used to note the ulcer’s maximum length and width in centimetres. To find the depth of the ulcer, a sterile cotton-tipped applicator should be placed gently into the deepest part of the ulcer. The length of the portion of the applicator that probed the ulcer can then be measured. Documentation of the healing wound can be done using a pressure ulcer healing tool, such as the Pressure Ulcer Scale of Healing (PUSH) tool (see the Resources on p 245). It is becoming increasingly common for photographs of pressure ulcers to be taken initially and at regular intervals during the course of treatment.
Local care of the pressure ulcer may involve debridement, wound cleaning, application of a dressing and relief of pressure. It is important to select the appropriate pressure-relieving technique (e.g. pad, overlay, mattress, specialty bed) to relieve pressure and keep the patient off the pressure ulcer. A pressure ulcer that has necrotic tissue or eschar (except for dry, stable necrotic feet or heels) must have the tissue removed by surgical, mechanical, enzymatic or autolytic debridement methods. Once the pressure ulcer has been successfully debrided and has a clean granulating base, the goal is to provide an appropriate wound environment that supports moist wound healing and prevents disruption of the newly formed granulation tissue. Reconstruction of the pressure ulcer site by operative repair, including skin grafting, skin flaps, musculocutaneous flaps or free flaps, may be necessary.
Pressure ulcers should be cleaned with non-cytotoxic solutions that do not kill or damage cells, especially fibroblasts. Solutions such as sodium hypochlorite, acetic acid, povidone- iodine and hydrogen peroxide are cytotoxic and therefore should not be used to clean pressure ulcers. It is also important to use enough irrigation pressure to adequately clean the ulcer (27.58–103.42 kPa) without causing trauma or damage to the wound. To obtain this pressure, a 30-mL syringe and a 19-gauge needle can be used.
After the pressure ulcer has been cleansed, it should be covered with an appropriate dressing. The current trend is to keep the pressure ulcer slightly moist, rather than dry, to enhance re-epithelialisation.25 Some factors to consider when selecting a dressing are the maintenance of a moist environment, the prevention of wound desiccation (drying out), the ability to absorb the wound drainage, the location of the wound, the amount of carer time, the cost of the dressing, the presence of infection, clean versus sterile dressings and the care delivery setting. A wet-to-dry dressing should never be used on a clean granulating pressure ulcer; this type of dressing should be used only for mechanical debridement of the wound. (Dressings are discussed in Table 12-11.)
Stage II–IV pressure ulcers are considered to be contaminated or colonised with bacteria.26 It is important to remember that in patients who have chronic wounds or who are immunocompromised, the clinical signs of infection (purulent exudate, odour, erythema, warmth, tenderness, oedema, pain, fever and elevated WBC count) may not be present, even though the pressure ulcer is infected.
Maintaining adequate nutrition is an important nursing responsibility for the patient with a pressure ulcer. Often, the patient is debilitated and has a poor appetite secondary to inactivity. Oral feedings must be adequate in kilojoules, proteins, fluids, vitamins and minerals to meet the patient’s nutritional requirements. The kilojoule intake needed to correct and maintain a nutritional balance may be 125–146 kJ per kilogram per day and 1.25–1.50 g of protein per kilogram per day. Nasogastric or gastrostomy feedings can be used to supplement the oral feedings. If necessary, parenteral nutrition consisting of amino acid and glucose solutions is used when oral and nasogastric feedings are inadequate. (Parenteral and enteral nutrition are discussed in Chapter 39.) NCP 12-2 outlines the care for the patient with a pressure ulcer.
 Ambulatory and home care
Ambulatory and home care
Pressure ulcers affect the quality of life of patients and their carers. Because the recurrence of pressure ulcers is common, educating both the patient and the carer in prevention techniques is extremely important (see Box 12-4). Carers need to know the aetiology of pressure ulcers, prevention techniques, early signs, nutritional support and care techniques for actual pressure ulcers. Because the patient with a pressure ulcer often requires extensive care for other health problems, it is important that the nurse supports the carer through the added responsibility of pressure ulcer treatment.
PATIENT & FAMILY TEACHING GUIDE
1. Identify and explain the risk factors and aetiology of pressure ulcers to the patient and family.
2. Assess all at-risk patients at time of first hospital and/or home visit or whenever the patient’s condition changes, and thereafter at regular intervals based on the care setting (24 h for acute care or every visit in home care).
3. Teach the family care techniques for incontinence. If incontinence occurs, cleanse the skin at the time of soiling, use topical moisture barriers and use pads or briefs that are absorbent.
4. Demonstrate correct positioning to decrease the risk of skin breakdown. Instruct the family to reposition the bed-bound patient at least every 2 h and the chair-bound patient every hour. Never position the patient directly on the pressure ulcer.
5. Assess the resources (i.e. adequacy of carer availability and skill, finances and equipment) of patients requiring pressure ulcer care at home. When selecting ulcer care dressing, consider the cost and the amount of carer time available.
6. Teach the patient and/or family to use clean dressings over sterile dressings using the ‘no touch’ technique when changing dressings. Instruct the family on the disposal of contaminated dressings.
7. Teach the patient and family to inspect the skin daily. Assess and document pressure ulcer status at least weekly; this may require help from the patient and family.
 Evaluation
Evaluation
Expected outcomes for the patient with a pressure ulcer are presented in NCP 12-2.
The patient with inflammation and infection
CASE STUDY
Patient profile
George Costas, a 58-year-old man, was admitted to the hospital emergency department with partial-thickness burns that involved his face, neck and upper trunk. He also had a lacerated right leg. His injuries occurred about 36 hours earlier when he fell out of a tree onto his barbecue (which was lit) while trying to get his cat.
CRITICAL THINKING QUESTIONS
1. What clinical manifestations of inflammation did this patient exhibit, and what are their pathophysiological mechanisms?
2. What type of exudate formation did he develop?
3. What is the basis for the development of the temperature?
4. What is the significance of this patient’s WBC count?
5. Because his wound was deep, primary tissue healing was not possible. How would you expect healing to take place? What complications could he develop?
6. What risk factors does this patient have for the development of a pressure ulcer?
7. Based on the assessment data provided, write one or more appropriate nursing diagnoses. Are there any collaborative problems?
1. A day after having abdominal surgery, a patient has incisional pain, a 37.5°C temperature, slight erythema at the incision margins and 30 mL of serous sanguineous drainage in the Jackson-Pratt drain. Based on these assessment data, what conclusion would the nurse make?
2. A patient is admitted with a chronic leg wound. The nurse assesses local manifestations of erythema and pain at the wound site. What would the nurse anticipate being ordered to assess the patient’s systemic response?
3. A patient is admitted to the medical unit with a 39.8°C temperature. Which intervention would be most effective in restoring normal body temperature?
4. A nurse is caring for a patient who has a pressure ulcer that is treated with debridement, irrigations and moist gauze dressings. How should the nurse anticipate healing to occur?
5. A nurse is caring for a patient with diabetes and a necrotic left great toe who is scheduled for amputation of the affected toe. The patient’s WBC count is 15.0 × 109/L and he has coolness of the lower extremities, weighs 34 kg more than his ideal body weight and smokes two packs of cigarettes per day. Which priority nursing diagnosis addresses the primary factor affecting the patient’s ability to heal?
6. Which of these orders should a nurse question as part of the plan of care for a patient with a stage III pressure ulcer?
7. An 85-year-old patient is assessed to have a score of 16 on the Braden scale. Based on this information, how should the nurse plan for this patient’s care?
8. A 65-year-old stroke patient with limited mobility has a purple area of suspected deep tissue injury on the left greater trochanter. Which of the following nursing diagnoses is most appropriate?
9. An 82-year-old man is being cared for at home by his family. A pressure ulcer on his right buttock measures 1 cm × 2 cm × 0.8 cm in depth, and pink subcutaneous tissue is completely visible on the wound bed. Which stage would the nurse document on the wound assessment form?
1 Atkins E. Fever: its history, cause, and function. Yale J Biol Med. 1982;55:283.
2 Beard R, Day MW. Fever and hyperthermia: learn to beat the heat. Nursing. 2008;37:28.
3 Kiekkas P, Brokalaki H, Theodorakopoulou G, et al. Physical antipyresis in critically ill adults. Am J Nurs. 2008;108:40.
4 Hunter S, Thompson P, Langemo D, et al. Understanding wound dehiscence. Nursing. 2007;37:28.
5 Hanson D, Langemo D, Anderson J, et al. Measuring wounds. Nursing. 2007;37:8.
6 Singer AJ, Dagum AB. Current management of acute cutaneous wounds. N Engl J Med. 2008;359:1037.
7 Caliano C, Jakubek P. Wound bed preparation: the key to success for chronic wounds, part 2. Nursing. 2006;36:76.
8 Caliano C, Jakubek P. Wound bed preparation: the key to success for chronic wounds, part 1. Nursing. 2006;36:70.
9 Takahashi P, Chandra A, Kiemele L, et al. Wound care technologies: emerging evidence for appropriate use in long-term care. Ann Long-Term Care Clin Care Aging. 2008;16(suppl):12.
10 Dorner B, Posthauer ME, Thomas D. National Pressure Ulcer Advisory Panel: the role of nutrition in pressure ulcer prevention and treatment. National Pressure Ulcer Advisory Panel white paper. Available at www.npuap.org/Nutrition%20White%20Paper%20Website%20Version.pdf. accessed 25 March 2011.
11 Slachta PA. Caring for chronic wounds: a knowledge update. Am Nurse Today. 2008;3:27.
12 Sarvis CM. Calling on NERDS for critically colonized wounds. Nursing. 2007;37:26.
13 Rushing J. Obtaining a wound culture specimen. Nursing. 2007;37:18.
14 Gardner SE, Frantz RA, Saltzman CL, et al. Diagnostic validity of three swab techniques for identifying chronic wound infection. Wound Repair Regen. 2006;14(5):548.
15 National Pressure Ulcer Advisory Panel. Revised pressure ulcer stages. Available at www.npuap.org/pr2.htm, 2007. accessed 24 April 2011.
16 Wounds West. Wound West wound survey. Available at www.health.wa.gov.au/woundswest/docs/Wound_survey_2009.pdf, 2009. accessed 24 April 2011.
17 Santamaria N, Carville K, Prentice J, et al. Pressure ulcer prevalence and its relationship to comorbidity in nursing home residents: results from phase 1 of the PRIME Trial. Aust J Wound Manage. 2005;13(3):107. 109–110, 112.
18 Black J, Baharestani M, Cuddigan J, et al. National Pressure Ulcer Advisory Panel’s updated pressure ulcer staging system. Urol Nurs. 2007;27:144.
19 Australian Wound Management Association. Clinical practice guidelines for the prediction and prevention of pressure ulcers. Perth, WA: Cambridge Publishing, 2001.
20 Pan Pacific Pressure Ulcer Forum, Canberra. Available at www.panpacificulcerforums.com.au/index.asp?IntCatId=14, October 2011. accessed 24 April 2011.
21 van Rijswijk L, Lyder C. Pressure ulcers: were they there on admission? Am J Nurs. 2008;108:27.
22 Stotts NA, Gunningberg L. Predicting pressure ulcer risk. Am J Nurs. 2007;107:40.
23 Ayello EA, Lyder CH. Protecting patients from harm: preventing pressure ulcers. Nursing. 2007;37:36.
24 Keast DH, Parslow N, Houghton PE, et al. Best practice recommendations for the prevention and treatment of pressure ulcers: update 2006. Adv Skin Wound Care. 2007;20:447.
25 Bolton L. Operational definition of moist wound healing. J Wound Ostomy Continence Nurs. 2007;34:23.
26 Zulkowski K, Gray-Leach K. Staging pressure ulcers: what’s the buzz in wound care. Am J Nurs. 2009;109:27.
American Society for Microbiology. www.asm.org
Australian Infection Control Association. www.aica.org.au
Australian Society for Microbiology. www.theasm.com.au
Australian Wound Management Association. www.awma.com.au
Braden Risk Assessment Scale. www.bradenscale.com
Centers for Disease Control and Prevention: antibiotic/antimicrobial resistance. www.cdc.gov/drugresistance
National Pressure Ulcer Advisory Panel. www.npuap.org
New Zealand Wound Care Society. www.nzwcs.org.nz
Pressure Ulcer Scale of Healing (PUSH). www.npuap.org/PDF/push3.pdf
Woundcarenet. www.woundcarenet.com
Wound Ostomy and Continence Nurses Society. www.wocn.org
Wounds West. www.health.wa.gov.au/woundswest/home