Chapter 39 NURSING MANAGEMENT: nutritional problems
1. Describe the essential components of a nutritionally sound diet and the relationship of a sound diet to good health.
2. Describe possible adverse interactions between drugs and various foods.
3. Analyse the common aetiological factors, clinical manifestations and nursing management of malnutrition.
4. Explain the indications for use, complications and nursing management of tube feedings.
5. Describe the types of feeding tubes and related nursing management.
6. Define the indications, complications and nursing management related to the use of parenteral nutrition.
7. Compare the aetiological factors, clinical manifestations and nursing management of eating disorders.
This chapter focuses on problems related to nutrition. The primary nutritional problems discussed are malnutrition and eating disorders. Obesity is discussed in Chapter 40.
Nutrition
The nurse in the roles of carer, teacher and resource person can have a strong influence on the nutritional practices of patients and their families. Together with the doctor, the dietician and the pharmacist, the nurse is in an excellent position to assess the dietary practices of patients and provide important information, as well as nutritional resources within and outside institutional settings.
Nutritional problems can occur in all age groups, cultures, ethnic groups and socioeconomic classes. Intelligence and wealth do not preclude the development of poor nutritional habits. However, research findings consistently show that people of lower socioeconomic status generally have poorer nutritional status.1 The nutritional status of a person or a family is influenced by many factors. Attitudes towards food and eating habits are established early. Cultural or religious preferences and requirements are frequently reflected in dietary intake, with culture having been shown to be independent of socioeconomic status as a factor in the poorer health of Indigenous peoples.2 The availability of different food sources also contributes to the individual’s nutritional status.
Normal nutrition
Nutrition is the process by which the body uses food for energy, growth, and maintenance and repair of body tissues. There is a vast body of evidence linking poor nutrition to many diseases. Good nutrition in the absence of any underlying disease process results from the ingestion of a balanced diet. Understanding nutrition is therefore a key nursing skill.
The Healthy Living Pyramid3 (formerly known as the Healthy Eating Pyramid) has been used in Australia and New Zealand for more than 30 years and was introduced as a way of educating people about their diet when the link between diet and disease became more clearly understood. Dietary guidelines in both New Zealand4 and Australia5 encourage people to eat a wide variety of nutritious foods, including plenty of vegetables, legumes, fruits, cereals and lean meat; moderate amounts of milk, yoghurt and cheese; and limited amounts of saturated fat and salt. The Healthy Living Pyramid is a model that can be easily understood by patients because it groups foods together according to their energy content and the nutrients they provide. Figure 39-1 shows the Healthy Living Pyramid and the Healthy Eating Pyramid for lacto-ovo vegetarians. Table 39-1 shows the food groups with nutrients provided. The essential components of the basic food groups are carbohydrates, fats, proteins, vitamins and minerals, and these are described in some detail below. Dietary guidelines for Australia and New Zealand are currently under review and will be republished during 2011.

Figure 39-1 A, The Healthy Living Pyramid. B, The Healthy Eating Pyramid for lacto-ovo vegetarians.
Reproduced with the permission of the Australian Nutrition Foundation Inc. (Nutrition Australia).
TABLE 39-1 Pyramid food groups
| Group | Nutrients provided |
|---|---|
| Bread, cereal, rice, pasta | Thiamine, niacin, iron, protein |
| Vegetable | Vitamins A and C, folic acid |
| Fruit | Vitamins A and C |
| Milk, yoghurt, cheese | Calcium, protein, riboflavin, vitamin B6, vitamin B12 |
| Meat, poultry, fish, dry beans, eggs, nuts | Protein, niacin, thiamine, iron, zinc, vitamin B12, folic acid |
ESSENTIAL NUTRITION COMPONENTS
Carbohydrates
Carbohydrates, which are the body’s primary source of energy, yield approximately 17 kilojoules (kJ) per gram. Carbohydrates are either simple or complex. Simple carbohydrates come in two forms: monosaccharides such as glucose and fructose, which are found in fruits and honey, and disaccharides such as sucrose, maltose and lactose, which are found in substances such as table sugar, malted cereal and milk, respectively. Complex carbohydrates or polysaccharides consist of long chains of sugar units such as glucose and fructose, and commonly appear in the diet as starches, such as cereal grains, potatoes and legumes. Carbohydrates are the chief protein-sparing ingredients in a nutritionally sound diet and comprise approximately 47% of the energy needs of the body. There are currently no recommended levels for carbohydrate intake for Australian and New Zealand adults;5 rather, intake is limited by dietary considerations (i.e. whether or not the person is over- or underweight, has a comorbid condition such as diabetes or heart disease etc.). The recommended intake for dietary fibre is 25–30 g/day.5
Refined carbohydrates, such as white bread and white rice, can be quickly broken down to glucose, the primary fuel for the body. The refining process produces an easily absorbed form of starch but also removes many vitamins, minerals and fibre. These refined carbohydrates increase blood glucose levels more than wholegrains do—for example, eating a boiled potato raises the blood glucose to a higher level than eating the same number of kilojoules from table sugar. Because potatoes are mostly starch they can be rapidly metabolised to glucose. In contrast, table sugar (sucrose) is a disaccharide containing one molecule of glucose and one molecule of fructose. Fructose takes longer to convert to glucose, hence the slower rise in blood glucose. A rapid rise in the blood glucose level stimulates a larger release of insulin (the hormone that directs glucose to the muscles and the liver). As a consequence, the blood glucose level plummets, sometimes to a level below normal baseline levels. High blood levels of glucose and insulin have a negative effect on cardiovascular health. The rapid decline in the blood glucose level can also lead to more hunger after a carbohydrate-rich meal and so contribute to overeating and obesity.6
Glycaemic index
The glycaemic index of food is a ranking of foods based on their immediate effect on blood glucose levels. Carbohydrate foods that break down quickly during digestion have the highest glycaemic index; the blood glucose response is fast and high. Carbohydrates that break down slowly, releasing glucose gradually into the bloodstream, have a low glycaemic index. Low glycaemic index diets mean a smaller rise in the blood glucose level, which can help control established diabetes, help people to lose weight, lower blood lipid levels and improve the body’s sensitivity to insulin. High glycaemic index foods can help refuel carbohydrate stores after exercise.
A person’s daily energy requirements are influenced by body build, age, gender and physical activity. Adjustments in kilojoule intake are necessary depending on changes in health status and daily activity level. An average adult requires an estimated 80–140 kJ per kilogram of body weight per day, leaning towards the higher end if the person is critically ill or very active, and towards the lower end if the person is sedentary.
Fats
Fats are a major source of energy for the body. Despite recommendations that total fat intake should be between 8% and 10% of the diet,7 Australians and New Zealanders are eating in excess of this amount (around 14–16%).8 This level of fat intake is higher than that found in many other societies and is a cause for concern. One gram of fat yields approximately 36 kJ. However, with respect to cardiovascular disease, international comparisons reveal that total fat intake is a poor indicator of heart disease risk; what is important is the type of fat consumed. In regions where saturated fats traditionally make up much of the diet (e.g. eastern Finland), rates of heart disease are much higher than in areas where monounsaturated fats are prevalent (such as the Greek island of Crete). Crete’s Mediterranean diet, based on olive oil, is even better for the heart than the low-fat traditional diet of Japan.
Much of the recent focus in fat consumption has been on trans-fatty acids (TFAs).8 These fats occur naturally in foods such as butter, cheese and meat, but can also be manufactured. Manufactured TFAs form when liquid vegetable oils are partially hydrogenated (hardened) during the manufacturing process, such as when making margarine and shortening for baking. Food Standards Australia New Zealand (FSANZ) reports that TFAs from animal products contribute between 60% and 70% of TFA intake in Australia and New Zealand. TFAs convert to low-density lipoprotein (LDL), which contributes to the incidence of heart disease (see Ch 33). The World Health Organization (WHO) recommends that less than 1% of daily energy intake should consist of TFAs. FSANZ has found that, on average, Australians obtain 0.5% of their daily kilojoules from TFAs and New Zealanders obtain 0.6%.9 This is well below the WHO recommendation and is also below the levels in many other countries.
It is not mandatory to declare TFAs on food labels in Australia and New Zealand, although TFAs must be shown if the manufacturer makes a nutritional claim about cholesterol or saturated, trans, polyunsaturated, monounsaturated, omega-3, omega-6 or omega-9 fatty acids. There has been much discussion in the media about countries that have banned TFAs. For example, the Danish Nutrition Council (DNC) has recommended that TFAs be phased out; however, if the TFA content in the finished food is less than 1 gram per 100 grams of oil or fat, then the DNC considers that food to be free of TFAs. In the US, New York and California have an upper limit of 0.5 g of TFA per standard serve of packaged food or for a restaurant meal. Eating three meals a day with 0.5 g of TFA in each meal will place residents above the 1% limit recommended by WHO and above that which is consumed by most New Zealanders and Australians.
In 2009 FSANZ reviewed the outcome of non-regulatory measures to reduce TFAs in food in Australia and New Zealand.8 It found that intake of TFAs from manufactured sources decreased by 25–45% over a 2-year period. This decline is equivalent to around 0.1% of energy. As a result of these findings, the Australia and New Zealand Food Regulation Ministerial Council has agreed that the non-regulatory approach should continue.
Proteins
Proteins, which are another essential component of a well-balanced diet, are obtained from both animal and plant sources. Ideally, proteins provide 15–20% of daily energy needs.5 The recommended daily protein intake depends on age and varies between 0.9–1.0 g/kg of body weight for adolescents, 0.75–0.84 g/kg of body weight for adults and 1.07 g/kg of body weight for those over 70 years of age.5 One gram of protein yields approximately 16 kJ. Proteins are complex nitrogenous organic compounds, of which amino acids are the fundamental units of structure. The 22 amino acids can be classified as essential and non-essential. The body is capable of synthesising non-essential amino acids if an adequate supply of protein is available. However, essential amino acids cannot be synthesised; their availability depends totally on dietary sources.10 Protein sources containing all the essential amino acids are called complete proteins. Proteins that lack one or more of the essential amino acids are called incomplete proteins. Table 39-2 lists good sources of protein.
Ingested protein undergoes a constant process of degradation and resynthesis. Together with dietary protein, this recycled protein contributes to the nitrogen balance. A positive nitrogen balance occurs whenever there is a net increase in protein content, such as during periods of growth. A negative nitrogen balance exists when dietary intake of protein is insufficient. Proteins are essential for tissue growth, repair and maintenance; body regulatory functions; and energy production.
Vitamins
Vitamins are organic compounds required in small amounts by the body for normal metabolism. All vitamins are enzymes or coenzymes, which function to accelerate the rates of biological reactions in protein, fat and carbohydrate metabolism. The body must rely on a dietary source to meet the requirements for some vitamins, such as vitamin B12 and vitamin C. Vitamins are divided into two categories: water-soluble vitamins (vitamin C and the B-complex vitamins) and fat-soluble vitamins (vitamins A, D, E and K). Table 39-3 shows the recommended daily allowances of vitamins and the symptoms of excess or deficiency. Because the kidneys readily excrete water-soluble vitamins in the urine, toxicity from excessive intake is not common. However, fat-soluble vitamins are not so easily excreted in urine and therefore it is easier to develop toxicity with excessive intake.10
TABLE 39-3 Recommended dietary intake of vitamins and manifestations of imbalance
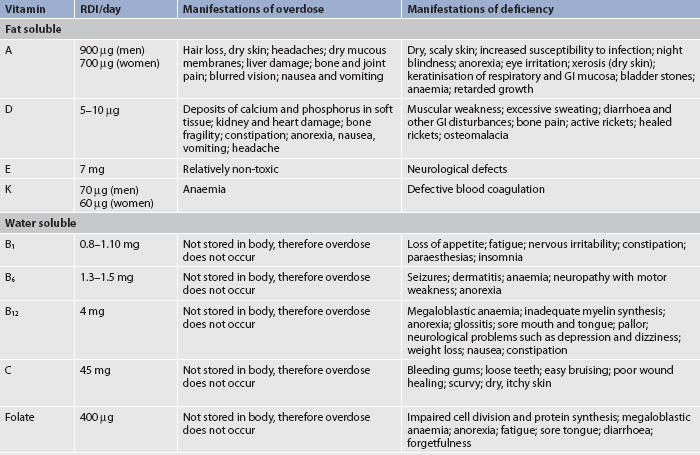
GI, gastrointestinal; RDI, recommended dietary intake (this is the same in both New Zealand and Australia).
Source: Department of Health and Ageing, National Health and Medical research Council, New Zealand Ministry of Health. Nutrient reference values for Australia and New Zealand including recommended dietary intakes. 2006. Available at www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/n35.pdf, accessed February 2011.
Mineral salts
Mineral salts (e.g. magnesium, iron, calcium) make up approximately 4% of total body weight. When minerals are present in minute amounts, they are referred to as trace elements. Minerals required in amounts greater than 100 mg per day are called major minerals. Table 39-4 lists the major minerals and trace elements. Minerals are necessary for the body to build tissues, regulate body fluids and assist in various body functions. Some minerals are stored in the liver in a manner similar to that of the fat-soluble vitamins and can be toxic if taken in excess amounts. Examples include ingestion of excess calcium, which interferes with the body’s ability to use iron, zinc and magnesium, and high dietary copper, which affects zinc absorption and excretion. The amount of minerals needed in the daily diet varies greatly from a few micrograms of trace elements to 1 g or more of the major minerals, such as calcium, phosphorus and sodium.
A well-balanced diet can usually meet the daily requirements of needed minerals; however, deficiency states can occur. Australia and New Zealand have recently developed joint recommendations for nutrient intake for people to keep healthy and reduce their risk of chronic disease (see Table 39-5). Minerals that are thought to be particularly borderline in the Australian and New Zealand populations include folate, calcium and iron for women, as well as iodine and selenium,9 although the revised recommended daily intakes (RDIs) have increased the recommended amount of these minerals to address any deficiencies.4,5 Recent evidence from a number of studies indicated that the iodine status of New Zealanders is declining to the point where intervention is required to ensure that iodine deficiency disorders do not once again widely affect the population.11 These studies provided the evidence for the decision to add iodised salt to commercially prepared bread in New Zealand from September 2009.
TABLE 39-5 Adapting nutrient intake to reduce the risk of chronic disease

Source: Department of Health and Ageing, National Health and Medical research Council, New Zealand Ministry of Health. Nutrient reference values for Australia and New Zealand: executive summary. Available at www.nhmrc.gov.au/publications/synopses/_files/n36.pdf, accessed May 2009.
Probiotics, prebiotics and resistant starches
There is now also a focus on the importance of probiotics, prebiotics and resistant starches in the diet.
Probiotics
The consumption of probiotic bacteria is not new. Many ancient cultures drank fermented milk products. However, it has only been in recent years that these foods have gained acceptance as a way of maintaining digestive health. A probiotic is defined as a mono- or mixed culture of live microorganisms that, applied to a human or animal (e.g. as dried cells or as fermented product), beneficially affects the host by improving the properties of the indigenous microflora.12 Examples are yoghurts and fermented milk products that contain cultures of Lactobacillus GG, Lactobacillus acidophilus and/or Bifidobacteria of various strains.
The generation of immunophysiological regulation in the gastrointestinal tract depends on the establishment of indigenous microflora, which are an important constituent of the mucosal defence barrier. Realisation of this has led to the promotion of the use of probiotics. Probiotic bacteria have been shown to reinforce the different lines of gut defence: immune exclusion, immune elimination and immune regulation. They have been found to enhance humoral immune responses and consequently to promote the intestine’s function as an immunological barrier. The principal target of probiotic therapy is reinforcement of the gut barrier mechanisms.13
Human studies have shown that probiotic bacteria survive passage from the mouth to the terminal ileum, which, coupled with their appearance in faeces, is good evidence to support the idea that they can colonise the large bowel. However, it seems that the colonic microflora of adult humans are very stable and faecal probiotic numbers fall rapidly when consumption of the probiotic bacteria ceases. One way to overcome this problem is to provide substrates so that probiotic growth is enhanced. This is the concept of prebiotics.
Prebiotics
Prebiotics are defined as non-digestible food ingredients that affect the host beneficially by selectively stimulating growth and/or activity of one bacterium, or a limited number of bacteria, in the colon, and therefore improve the health of the host.
Resistant starches
The large bowel is the major site of bacterial colonisation and contains organisms with the potential to be deleterious to human health. Under normal circumstances, these are outnumbered by non-pathogenic bacteria, which metabolise undigested food components, such as resistant starches that have escaped digestion in the small intestine. The resistant starches include monosaccharides (e.g. sorbitol), disaccharides (e.g. lactose), oligosaccharides (e.g. raffinose, stachyose) and polysaccharides (e.g. cellulose, the major component of dietary fibre). Resistant starches are fermented by colonic bacteria to produce carbon dioxide, methane, hydrogen and, most importantly, short chain fatty acids (SCFAs). The three most important SCFAs are acetate, propionate and butyrate. These SCFAs are not simply suppliers of energy for colonic mucosal cells but also seem to mediate some of the effects previously ascribed to dietary fibre. They promote electrolyte and fluid absorption (and so reduce the risk of diarrhoea), enhance blood flow to the large bowel and stimulate colonic muscular contraction. SCFAs appear to slow upper gut transit and thereby increase the efficiency of digestion in the small intestine. Butyrate is of special interest because it is the preferred substrate for normal colon cells and therefore plays a key role in maintaining colonic integrity.
While consumption of some carbohydrates is of benefit in increasing SCFA production, there is considerable individual variation. It appears that some individuals cannot digest certain resistant starches, which limits their potential to improve health. Under these circumstances, consumption of probiotic organisms is one strategy used to modify the large bowel microflora so as to achieve a more favourable population. This approach has been applied successfully to antibiotic-induced diarrhoea.
Genetically modified foods
The genetic make-up of plants and animals has been manipulated for generations using cross-breeding techniques. This involves selecting plants and animals with the most desirable characteristics (e.g. disease resistance, high yield, good meat quality) for breeding the next generation. These days gene technology enables specific characteristics to be identified and then transferred between organisms. Because the resulting plants, animals or microbes have had their genetic material altered in some way, they are commonly referred to as ‘genetically modified’ or ‘GM’ organisms. Foods derived from genetically modified organisms are called ‘GM foods’.14 Examples of GM foods are corn plants with a gene that makes them resistant to insect attack, and soybeans with a modified fatty acid content that makes the oil better suited for frying. Developments are underway to produce plants that are drought tolerant, making them more suitable for changing climatic conditions.14
In Australia, the Office of the Gene Technology Regulator (OGTR) oversees the development and environmental release of GM organisms under the Gene Technology Act 2000. Licences are not issued unless the OGTR is satisfied that any risks posed can be managed in a way that ensures the protection of the health of people and the environment. In New Zealand, similar functions are undertaken by the Environmental Risk Management Authority, under the Hazardous Substances and New Organisms (HSNO) Act 1996. GM foods are regulated by FSANZ under enforceable regulations in both New Zealand and Australia.14 If a GM organism is proposed to be used to produce food, FSANZ will determine whether that food is safe for people to eat.
It is mandatory for GM foods to be identified on food labels in both Australia and New Zealand. Foods on sale in Australia that use GM ingredients (mainly imported) come from six crops: cottonseed oil, soybeans, canola oil (rapeseed oil), corn, potatoes and sugar beet. With the exception of cottonseed oil, none of these crops approved for food use is grown commercially in Australia. In New Zealand no GM crops are grown commercially and no GM fruit, vegetables or meat are sold. Processed foods can contain GM ingredients, but must be labelled accordingly. It is the role of FSANZ to ensure that all food, including GM food, is safe, and FSANZ’s safety guidelines are based on world best-practice standards.
Special diets: vegetarian diet
The common element among all vegetarians is the exclusion of red meat from the diet. Vegetarians have a variety of reasons for following this dietary practice, including religious or cultural beliefs, respect for all living beings, ethical ideals and ecological concerns, and economic factors. Many vegetarians are vegans, who are pure or total vegetarians and eat only plant food, or lacto-ovo vegetarians, who eat plant foods and, sometimes, dairy products and eggs.
Vegetarians can have vitamin or protein deficiencies if their diets are not well planned.3 This is because plant proteins are incomplete proteins. For example, corn has little isoleucine and lysine. Beans have ample isoleucine and lysine but are deficient in tryptophan and methionine. By combining the two, all essential amino acids are supplied. Lacto-ovo vegetarians obtain additional protein sources from dairy products and eggs. Milk made from soybeans is an excellent protein source, especially for true vegans. The primary deficiency of a strict vegan diet is a lack of vitamin B12. This vitamin can be obtained only from animal protein, special supplements or foods that have been fortified with the vitamin. Vegans not taking vitamin B12 supplements are susceptible to the development of megaloblastic anaemia and the neurological signs of vitamin B12 deficiency (see Table 39-3).
Strict vegetarians and lacto-ovo vegetarians are also at risk of iron deficiency. Iron-enriched foods or iron supplements are prescribed during pregnancy, early childhood and adolescence, and after major blood loss. However, caution should be exercised when taking iron supplements as excessive amounts can diminish the absorption of zinc as they compete for the same transport mechanism. The polyphenols in tea and coffee inhibit the absorption of iron from the gastrointestinal tract and so should not be taken simultaneously with iron. Table 39-6 lists examples of foods high in iron. Other deficiencies that may be present in a vegan diet include calcium, zinc, vitamins A and D, and protein.
TABLE 39-6 Foods high in iron*
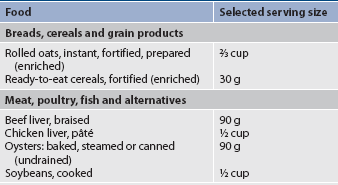
* These foods provide 25–39% of the recommended dietary intake (RDI) of iron.
Malnutrition
Malnutrition is an excess, deficit or imbalance in the essential components of a balanced diet (see Fig 39-2). Terms such as undernutrition and overnutrition are also used to describe malnutrition. Undernutrition describes a state of poor nourishment as a result of inadequate diet or diseases that interfere with normal appetite and assimilation of ingested food. Overnutrition refers to the ingestion of more food than is required for body needs, as in obesity. An example of nutrient imbalance is a vitamin deficiency state such as rickets, which is a bone disorder caused by inadequate vitamin D. Scurvy is a condition characterised by weakness, anaemia and oral ulcerations that is associated with inadequate vitamin C intake.
Malnutrition is most prevalent in developing countries in which adequate food sources do not exist, where there may be poverty and where the inhabitants may not be well educated about their nutritional needs. Economic or political constraints can also preclude the purchase of a balanced diet. Undernutrition does exist in Australia and New Zealand, usually in individuals or groups from a lower socioeconomic background or in individuals who have chronic or acute illnesses. Malnutrition is a risk for all hospitalised patients, with children and the aged being particularly at risk.15 Patients who are nil by mouth (NBM) for an extended period are at high risk of developing protein–energy malnutrition. Patients with conditions that increase protein–energy needs include postoperative patients and those with severe burns, systemic infections and cancer, discussed further below. Patients with conditions that cause defective utilisation of nutrients, such as malabsorption syndrome, short-bowel syndrome and Crohn’s disease (see Ch 42), are also at risk. Protein–energy malnutrition has been found in elderly long-term care residents with a prevalence ranging from 23% to 85%.16
TYPES OF MALNUTRITION
Protein–energy malnutrition
Protein–energy malnutrition (PEM) is the most common form of undernutrition and can result from either primary or secondary factors. (PEM is also known as protein–calorie malnutrition.) Primary PEM is present when nutritional needs are not met as a result of poor eating habits or famine. Secondary PEM is the result of an alteration or defect in ingestion, digestion, absorption or metabolism. In this type of malnutrition, tissue needs are not met even though the dietary intake would be satisfactory under normal conditions. Secondary malnutrition may occur as a result of gastrointestinal (GI) obstruction, surgical procedure, cancer, malabsorption syndromes, drugs or infectious diseases.17
PEM may be due to ingestion of foods that are deficient in complete proteins. In addition to decreased quantities of protein, the diet is generally low in necessary vitamins and minerals. Most malnourished ill patients have this type of combined PEM. Table 39-7 outlines the clinical manifestations of PEM.
Marasmus and kwashiorkor
Marasmus is the result of a concomitant deficiency of both kilojoule and protein intake leading to generalised loss of body fat and muscle. In the severe energy deficiency of marasmus, adaptation is facilitated by high cortisol and growth hormone levels and the depression of insulin and thyroid hormone secretion. Because amino acids are mobilised from muscle to provide the liver with substrate for protein synthesis, plasma protein levels decrease less in cases of marasmus than in kwashiorkor. Patients generally appear ‘wasted’ or emaciated but have normal serum protein levels.
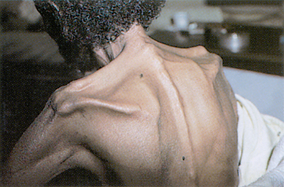
Kwashiorkor is caused by a deficiency of protein intake with the lack of protein being replaced by carbohydrate (starch). Kwashiorkor is an African word meaning ‘first child–second child’. It refers to the observation that the first child develops PEM when the second child is born and replaces the first child at the breast. The weaned child is then fed nutritionally poor food, leading to the development of PEM. These children’s protuberant abdomens give the appearance of being well nourished but they may have very low serum protein levels (hypoalbuminaemic malnutrition).17
Protein deficiency may also occur as a result of a catabolic stress event, such as a GI obstruction, a surgical procedure, cancer, a malabsorption syndrome or an infectious disease. Marasmic infants have gross weight loss, growth retardation and wasting of subcutaneous fat and muscle. Kwashiorkor is characterised by generalised oedema, ‘flaky paint’ dermatosis, thinning, discolouration and reddening of the hair, enlarged fatty liver, retarded growth and apathy.
AETIOLOGY AND PATHOPHYSIOLOGY
Starvation process
Knowledge of the phases of the starvation process is essential to better understand the physiological changes that occur in PEM. Initially, the body selectively uses carbohydrates (glycogen) rather than fat and protein to meet metabolic needs. These carbohydrate stores, found in the liver and muscles, are minimal and may be totally depleted within 18 hours. During this early phase of starvation, the only use of protein is in its obligatory participation in cellular metabolism. However, once carbohydrate stores are depleted, protein begins to be converted to glucose for energy. Alanine and glutamine are the first amino acids to be used by the liver for the formation of glucose in a process termed gluconeogenesis. The resulting available plasma glucose allows the metabolic processes to continue. With these amino acids being used as energy sources, the person may be in negative nitrogen balance (greater nitrogen excretion). However, within 5–9 days, body fat is fully mobilised to supply much of the needed energy.
In prolonged starvation up to 97% of kilojoules are provided by fat, and protein is conserved. Depletion of fat stores depends on the amount available but fat stores are generally used up in 4–6 weeks. Once fat stores have been used, body proteins, including those in internal organs and plasma, can no longer be spared and rapidly decrease because they are the only remaining body source of energy available.
If the malnourished patient has surgery, experiences bodily trauma or has an infection, the stress response with the concomitant increase in energy expenditure is superimposed on the starvation response. These body insults cause an increase in the metabolic rate, with a subsequent increase in energy requirements. Protein stores are no longer spared and are used with increasing frequency for body energy because of the increased metabolic energy needs.
As the protein depletion continues, liver function is impaired and synthesis of proteins is diminished. The plasma oncotic pressure is decreased because of decreased protein synthesis. A major function of plasma proteins, primarily albumin, is the maintenance of the osmotic pressure of the blood. Because of this decreased pressure, a shift in body fluids occurs from the vascular space into the interstitial compartment. As protein ingestion decreases and body stores are depleted, albumin eventually leaks into the interstitial space along with the fluid. Oedema becomes clinically observable. Often the oedema present in the face and legs of the patient masks the muscle wasting that occurs.
As the total blood volume is reduced, the skin appears dry and wrinkled. Along with the shift of fluids to the interstitial space, ions also move. Sodium (a predominant extracellular ion) is found in increased amounts within the cell, and potassium (a predominant intracellular ion) and magnesium are shifted to the extracellular space. The sodium–potassium exchange pump has high-energy needs, using 20–50% of all kilojoules ingested. When the diet is extremely deficient in kilojoules and essential proteins, the pump will fail, leaving sodium inside the cell (along with water), and the cell will expand.
The liver is the body organ that loses the most mass during protein deprivation. It gradually becomes infiltrated with fat secondary to decreased synthesis of lipoproteins. Immediate restoration to a diet of protein and other necessary constituents must be instituted or death will rapidly ensue.
Causes of malnutrition
Factors that contribute to the development of malnutrition include socioeconomic status, famine, war, cultural influences, psychological disorders, medical conditions and medical treatments.17 Awareness of these is the key to identifying those at risk. Box 39-1 lists conditions that increase the risk of malnutrition. Because individuals and families from lower socioeconomic backgrounds spend a greater percentage of their income on food, there is a tendency to seek out cheaper foods as the cost of food increases. These foods may not provide adequate or balanced nutrition. In contrast, some individuals on lower incomes may prefer to select foods that are more expensive but only marginally nutritious because of their prestige value. The nurse and the dietician can assist patients in making food choices that meet nutritional requirements while staying within their limited resources.
BOX 39-1 Conditions that increase the risk of malnutrition
• Excessive dieting to lose weight
• Swallowing disorders (e.g. head and neck cancer)
• Decreased mobility, which limits access to food or its preparation
• Nutrient losses from malabsorption, dialysis, fistulas or wounds
• Drugs with anti-nutrient or catabolic properties, such as corticosteroids and oral antibiotics
• Extreme need for nutrients because of hypermetabolism or stresses such as infection, burns, trauma or fever
• No oral intake and/or receiving standard intravenous solutions (5% dextrose) for 10 days (adults) or for 5 days (older adults)
Patients with physical illnesses
Regardless of the cause of the illness, most sick people have increased nutritional needs. Pathological conditions are frequently aggravated by undernutrition, and an existing deficiency state is likely to become more severe during illness. Malnutrition is not an uncommon consequence of illness, surgery, injury or hospitalisation. Anorexia, nausea, vomiting, diarrhoea, abdominal distension and abdominal cramping may accompany diseases of the GI system. Any combination of these symptoms interferes with normal food consumption and metabolism. In addition, a patient may restrict the dietary intake to a few foods or fluids that may not be nutritionally sound out of fear of aggravating the already disturbed GI function. Figure 65-1 provides guidelines for feeding critically ill patients.
Incomplete diets
Vitamin deficiencies are rare in most developed countries. When vitamin deficiencies are present, several vitamins are usually involved rather than a single vitamin deficiency. The recommended dietary allowances for essential vitamins and minerals can be obtained by eating a diet consisting of foods from the five basic food groups. The same RDI is used in both Australia and New Zealand and is evidence-based.4,5 When vitamin imbalances do occur, they are usually found in people with a pattern of alcohol and drug abuse, people who are chronically ill, the elderly and individuals who follow poor dietary practices. Followers of fad diets or poorly planned vegetarian diets are also subject to a potential deficiency state. Clinical manifestations of vitamin imbalances are most commonly exhibited as neurological manifestations (see Table 39-3). In the growing child, the central nervous system (CNS) is primarily involved, whereas in the adult the peripheral nervous system is most affected.
Malabsorption syndrome is defined as the impaired absorption of nutrients from the GI tract. It may result from decreased amounts of necessary enzymes or a reduced bowel surface area and can quickly lead to a deficiency state. Many drugs may have undesirable GI side effects, as well as altering normal digestive and absorptive processes. For example, antibiotics decrease certain microbiotic bacteria in the colon. These bacteria are responsible for synthesising biotin (an essential vitamin), which is then absorbed across the GI tract wall.
Fever accompanies many illnesses, injuries and infections, with a concomitant increase in the body’s basal metabolic rate (BMR). Careful monitoring is needed because, without an increase in the number of kilojoules ingested in the diet, body protein stores can be used to supply energy, resulting in protein depletion.
The hospitalised patient, especially the older adult, is at risk of becoming malnourished. Prolonged illness, major surgery, sepsis, draining wounds, burns, haemorrhage, fractures and immobilisation can all contribute to malnutrition. The nurse must assume responsibility, along with the healthcare provider and the dietician, for meeting the patient’s nutritional needs. The nurse must also be knowledgeable about the requirements of a patient who is not overtly ill but who is undergoing diagnostic studies. This patient may be nutritionally fit on entering the hospital but can develop nutritional problems because of the dietary restrictions imposed by multiple diagnostic studies.
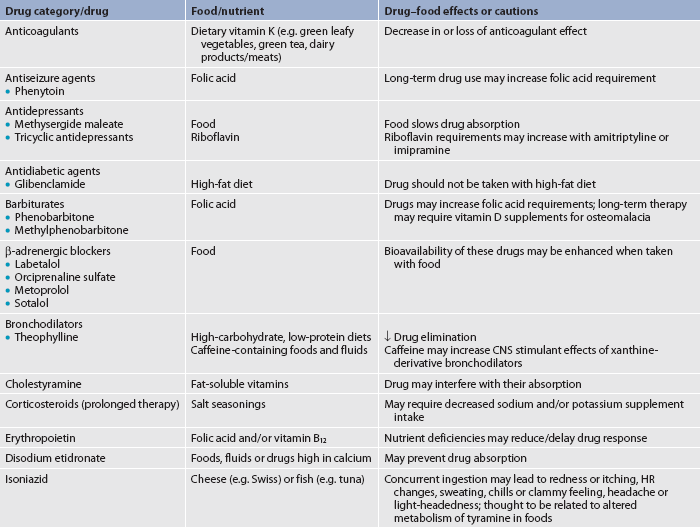
Food–drug interactions
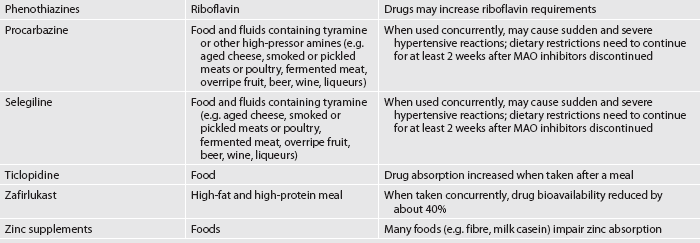
When health conditions require drug therapy, drug and food interactions may not be explored before starting a prescription. Adverse interactions can include incompatibilities, altered drug effectiveness and impaired nutritional status.18 Table 39-8 outlines examples of common drug and food/nutrient interactions. As members of the health team, nurses have a responsibility for monitoring and preventing potential interactions for patients while in the hospital and at home.
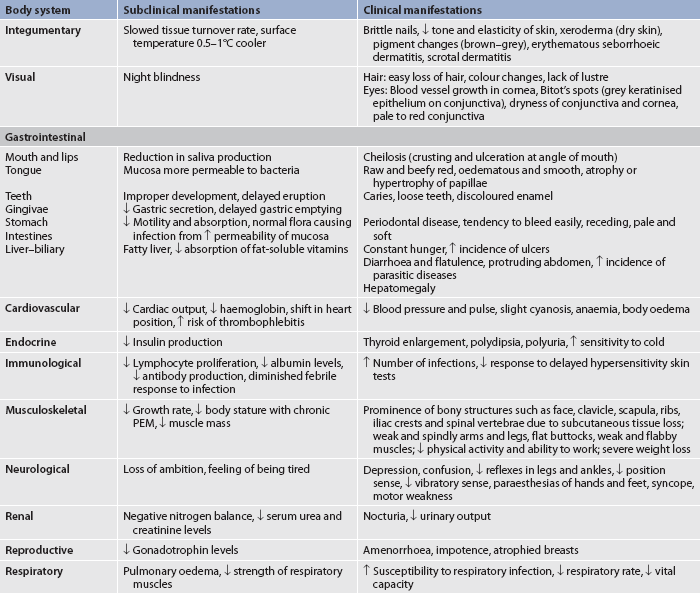
CLINICAL MANIFESTATIONS
The adult who is deprived of adequate protein and energy may have many of the clinical manifestations presented in Table 39-9. The most obvious clinical signs on physical examination are apparent in the skin, eyes, mouth, muscles and CNS. The speed at which the malnutrition develops depends on the quantity and quality of the protein intake, kilojoules, illness and the age of the person.
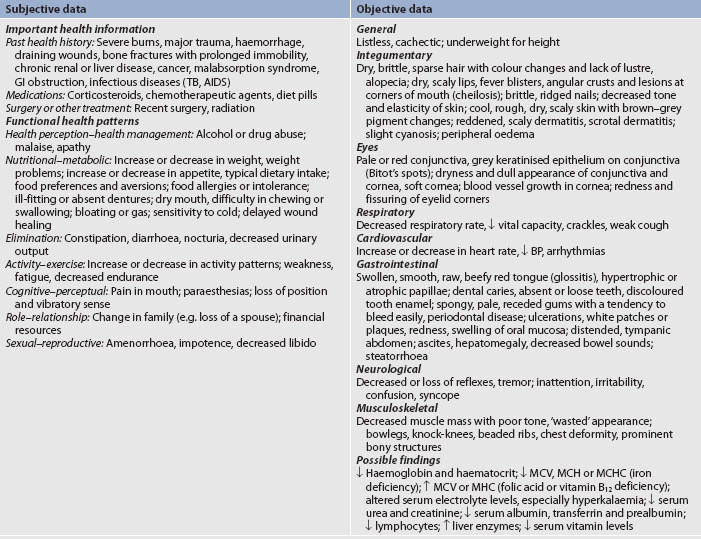
AIDS, acquired immunodeficiency syndrome; B P, blood pressure; GI, gastrointestinal; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; TB, tuberculosis.
Clinical manifestations of malnutrition are the result of numerous interactions occurring at the cellular level. As protein intake is severely reduced, the muscles, which make up the largest reservoir of protein in the body, become wasted and flabby, leading to weakness, fatiguability and decreased endurance. There is decreased protein available for repair and, as a result, wound healing may be delayed. Malnutrition in the hospitalised patient may result in delayed recovery and prolonged hospitalisation. The person is more susceptible to all types of infections. Both humoral and cell-mediated immunity are deficient in PEM. There is a decrease in leucocytes in the peripheral blood. Phagocytosis is altered as a result of the lack of energy (adenosine triphosphate [ATP]) necessary to drive the process. Most malnourished people are anaemic. Anaemia resulting from PEM is usually caused by nutritional deficiencies in iron and folic acid, the necessary building blocks for red blood cells.
The severity of complications from malnutrition ranges from mild to emaciation and death. Major complications include delayed wound healing and increased susceptibility to infection from decreased immune function.
DIAGNOSTIC STUDIES
History and physical examination
A diet history of foods eaten over the past week will reveal a great deal about the patient’s dietary habits and knowledge of good nutrition. In addition to the patient’s height, weight and vital signs, the patient’s physical state should be thoroughly assessed and documented. Each body system should be assessed. Table 39-9 summarises the assessment and findings of the patient with malnutrition.
The diagnosis of PEM can be determined by a variety of laboratory studies used in conjunction with the physical examination. The serum albumin level is somewhat useful in the diagnosis of malnutrition. The degree of protein depletion can be identified with the use of the scale in Box 39-2. Serum albumin has a half-life of approximately 20–22 days. In the absence of marked fluid loss, such as from haemorrhage or burns, the serum albumin value lags behind actual protein changes by more than 2 weeks and therefore is not a good indicator of acute changes in nutritional status. Prealbumin, a protein synthesised by the liver, has a half-life of 2 days and is a better indicator of recent or current nutritional status.10,19 The serum transferrin level is another indicator of protein status. Transferrin, a protein synthesised by the liver and used to transport iron, decreases during states of protein deficiency. Initially, free fatty acid levels rise as fat is released from adipose tissue to provide energy. Blood glucose levels fall and are maintained at a lower level by synthesis of glucose in the liver from amino acids released from muscle. Plasma amino acid levels rise initially as muscle is broken down but then fall as starvation proceeds, with essential amino acids falling more than non-essential amino acids. The plasma insulin level is low.
BOX 39-2 Serum albumin and prealbumin levels
| Albumin | |
| Normal range | 32–45 g/L |
| Mild depletion | 30–31 g/L |
| Moderate depletion | 25–29 g/L |
| Severe depletion | <25 g/L |
| Prealbumin | |
| Normal range | 150–360 mg/L |
| Mild depletion | 100–150 mg/L |
| Moderate depletion | 50–100 mg/L |
| Severe depletion | <50 mg/L |
Serum electrolyte levels reflect changes taking place between the intracellular and extracellular spaces. The serum potassium level is often elevated. Red blood cell count and haemoglobin level indicate the presence and degree of anaemia. The total lymphocyte count decreases during malnutrition states. The total lymphocyte count is calculated by multiplying the percentage of lymphocytes by the total white blood cell count. Liver enzyme levels, a reflection of liver function, may be elevated during malnutrition. Serum levels of both fat-soluble and water-soluble vitamins are usually diminished in malnutrition. The lowered levels of the fat-soluble vitamins correlate with the clinical signs of steatorrhoea (fatty stools).
Anthropometric measurements
Anthropometric measurements, which include gross measures of fat and muscle contents, may be ordered. These measurements tend to be most beneficial in evaluating the long-term effects of malnutrition or responses to nutritional interventions. They consist of measures of skinfold thickness at various sites, which is an indicator of subcutaneous fat stores, and mid-arm muscle circumference, which is an indicator of protein stores. These measurements are then compared with standards for healthy persons of the same age and gender. Training and practice are required to perform these measurements accurately and reliably. To provide information on the patient’s nutritional status in response to treatment, serial measurements are needed. Sites most reflective of body fat are those over the biceps and triceps, below the scapula, above the iliac crest and over the upper thigh. Both skinfold thickness and mid-arm muscle circumference measurements are decreased in chronic PEM and acute protein malnutrition. These measurements may also be influenced by shifts in hydration status. The exact relationship of the mid-arm circumference measure to body composition of functional protein, both muscle and non-muscle, remains to be established.
 NURSING MANAGEMENT: MALNUTRITION
NURSING MANAGEMENT: MALNUTRITION
 Nursing assessment
Nursing assessment
Across all settings of care delivery, the nurse must be aware of the patient’s nutritional status. The recording of the patient’s height and weight is an important component of this assessment. Although the patient’s current weight relative to usual body weight and ideal body weight is important, it is the percentage change in body weight over time that provides information on the degree of weight loss.
In many institutions (acute and long-term care) and in home care, the nurse is responsible for nutritional screening. Nutritional screening identifies individuals who are malnourished or at risk of malnutrition. The purpose of nutrition screening is to determine whether a more detailed nutrition assessment is necessary.19,20 Box 39-3 provides an example of a nursing nutrition screening tool.
BOX 39-3 Admission nutrition screening tool
Source: Adapted from Kovacevich DS, Boney AR, Braunschweig CL et al. Nutrition risk classification: a reproducible and valid tool for nurses. Nutr Clin Pract 1997; 12(1):20–25.
A. Diagnosis
• If the patient has at least one of the following diagnoses, circle and proceed to section D to consider the patient at nutritional risk and stop here.
• Anorexia nervosa/bulimia nervosa
• Malabsorption (coeliac disease, ulcerative colitis, Crohn’s disease, short-bowel syndrome)
• Multiple trauma (closed head injury, penetrating trauma, multiple fractures, burns, thyrotoxicosis)
• Major gastrointestinal surgery within the past year
• Cachexia (temporal wasting, muscle wasting, cancer, cardiac)
B. Nutrition intake history
If the patient has at least one of the following symptoms, circle and proceed to section D to consider the patient at nutritional risk and stop here.
C. Weight history
Any recent unplanned weight loss? No ___ Yes ___
Find percentage of weight loss:
Compare the % weight loss with the chart below and circle appropriate value.
| Length of time | Significant (%) | Severe (%) |
|---|---|---|
| 1 week | 1–2 | >2 |
| 2–3 weeks | 2–3 | >3 |
| 1 month | 4–5 | >5 |
| 3 months | 7–8 | >8 |
| 5+ months | 10 | >10 |
If the patient has experienced a significant or severe weight loss, proceed to section D and consider the patient at nutritional risk.
If the nutrition screening identifies an individual at nutritional risk, a full nutrition assessment is most often warranted. A nutrition assessment is a comprehensive approach to defining nutrition status that uses medical, nutrition and medication histories; physical examination; anthropometric measurements; and laboratory data. Box 39-4 describes the components of a thorough nutrition assessment.
In long-term care, residents need to be regularly assessed for their nutritional status.20 In the community the nurse will need to collect information on diet, oral intake, dental health, swallowing difficulties and any needs for meal assistance. If nutritional problems are identified, referral to a dietician is suggested. Capacity to afford a proper diet will also need to be assessed. Referrals to appropriate community services may be needed if the patient’s poor diet appears to be related to lack of money.
When possible, the patient’s actual height should be measured rather than based on the patient’s self-report. The patient’s current weight relative to usual body weight and ideal body weight should be noted. In addition, the nurse should get a record of the complete diet history from the patient or the family. The patient’s nutritional state may not be the reason medical assistance was sought. However, it may be a contributing factor to the disease, and have an impact on management of and recovery from the disease. The dietician, pharmacist and doctor should also be involved in the assessment and planning of care. However, the nurse, as the first-line healthcare professional dealing with the patient, should take the initiative in determining the severity of any nutritional problems.
Malnutrition can affect the absorption and excretion of some drugs due to the reduction in plasma proteins and protein binding of drugs. It is important that nurses are aware of the action of any drugs that they administer and that they understand where the drugs are metabolised and how they are excreted in order to evaluate the potential impact on patients.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with malnutrition include, but are not limited to, the following:
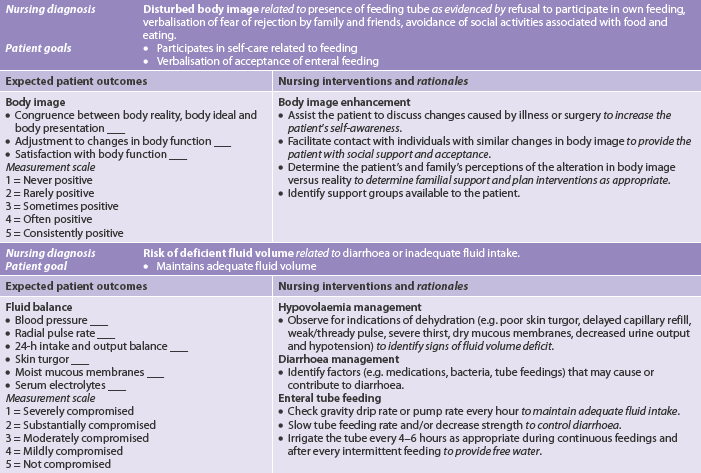
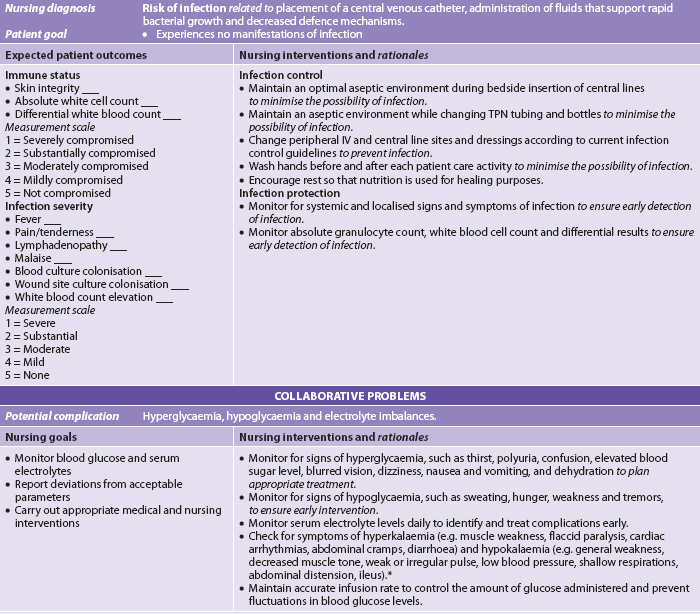
• imbalanced nutrition: less than body requirements related to decreased access, ingestion, digestion or absorption of food or to anorexia or greater body requirements as with burns or sepsis
• feeding self-care deficit related to decreased strength and endurance, fatigue and apathy
• constipation or diarrhoea related to poor eating patterns, immobility or medication effects
• deficient fluid volume related to factors affecting access to or absorption of fluids
• risk of impaired skin integrity related to poor nutritional state
• non-compliance related to alteration in perception, lack of motivation or incompatibility of regimen with lifestyle or resources
• activity intolerance related to weakness, fatigue and inadequate energy intake or iron stores.
 Planning
Planning
The overall goals are that the patient with malnutrition will: (1) achieve weight gain and/or correction of specific nutrient deficiency; (2) consume a specified number of kilojoules per day (with a diet individualised for the patient); and (3) have no adverse consequences related to malnutrition or nutrition therapies.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Nurses are in a good position to teach and reinforce healthy eating habits with individuals and groups of people throughout their life span. The gap between the perceived importance of nutrition and care in selecting foods has widened. To assist in this effort FSANZ-mandated food labels are required on all packaged food. Nutrition Australia’s dietary guidelines offer key recommendations for improving nutrition that are useful points for a teaching program (see Box 39-5).4,5
PATIENT & FAMILY TEACHING GUIDE
The following recommendations apply to most people:
• Choose a diet moderate in sugars.
• Choose a diet moderate in salt and sodium.
• If you drink alcoholic beverages, do so in moderation.
• Choose a diet low in fat, unsaturated fat and cholesterol.
• Choose a diet with plenty of grain products, vegetables and fruits.
• Balance the food you eat with physical activity to maintain or improve your weight.
• Dairy consumption should be limited to 1–2 servings per day.
 Acute intervention
Acute intervention
The nurse must assess the patient’s nutritional state, as well as focusing on the patient’s other physical problems. The nurse must become more aware of who is at risk of malnutrition, why and how to intervene appropriately. In states of increased stress, such as surgery, severe trauma, severe burns and sepsis, more kilojoules and protein are needed. Wound healing requires increased protein synthesis. When fever is present, the metabolic rate is increased and nitrogen loss is accelerated. Even after the body temperature returns to normal, the rate of protein breakdown and resynthesis may be accelerated for several weeks. After major surgery, several weeks of increased protein and kilojoule intake are needed to promote healing and replenish body stores.
The nurse must have a thorough understanding of nutritional support and the rationale for recording the daily weight, intake and output. Daily weights can give an ongoing record of body weight gain or loss. However, rapid gains and losses are usually the result of shifts in fluid balance. The body weight, in conjunction with accurate recording of food and fluid intake, provides a clearer picture of the patient’s fluid and nutritional state. To obtain an accurate weight, the nurse should weigh the patient at the same time each day, on the same scales, with the same type or amount of clothing, and preferably with the bladder recently emptied.
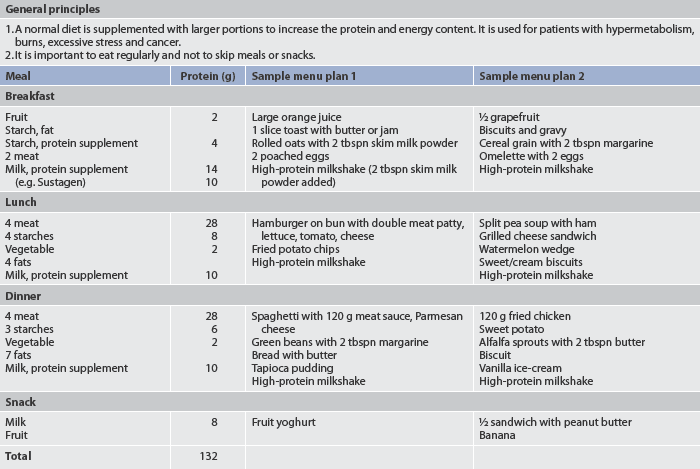
The protein and kilojoule intake required in the malnourished patient depends on the cause of the malnutrition, the treatment being employed and other stressors affecting the patient. If the patient is able to take food by mouth, a daily kilojoule count and diet diary should be kept to give an accurate record of food intake. The nurse and the dietician working with the patient and family can assist in the selection of high-kilojoule and high-protein foods (unless medically contraindicated). Preparation of foods preferred by the patient enhances the daily intake. Discussion with the patient and family about foods that should be eaten to provide high-protein, high-kilojoule content is important. The family can be encouraged to bring the patient’s favourite foods from home while the patient is still hospitalised. Table 39-10 gives examples of a high-kilojoule, high-protein diet.
The undernourished patient usually needs to have between-meal supplements. These may consist of items prepared in the dietary department or commercially prepared products. Commercial preparations are classified as nutritionally complete, elemental, fortified or modular supplements. Nutritionally complete supplements contain the full range of macro- and micronutrients (i.e. they can be used as the sole source of nutrition). Elemental (chemically defined) supplements provide essential nutrients in a readily assimilated form (i.e. they require little or no active digestion and have minimal residue). These are ideal when ‘resting’ of the colon is required. Fortified supplements supplement nutritional intake but will not supply full nutritional requirements. Modular supplements have only one (or occasionally two) macronutrient sources of carbohydrate, protein or fat. Eating modular or fortified supplements between meals increases the total daily intake and provides extra kilojoules, proteins, fluids and nutrients. In addition, multiple small feedings improve the tolerance for food intake by distributing the amount more evenly throughout the day. If the patient is still unable to take in enough kilojoules, tube feedings may be considered using elemental preparations that constitute total nutritional replacement. Total parenteral nutrition (TPN) may be initiated if enteral feedings are not feasible.
 Ambulatory and home care
Ambulatory and home care
With shortened hospital stays, many patients are discharged on a therapeutic diet. Discharge preparation for both the patient and the family is important. They must be carefully instructed on the cause of the undernourished state and ways to avoid the problem in the future. The patient must be made aware that undernourishment, whatever the cause, can recur and that adhering to a diet high in protein and kilojoules for a few weeks cannot fully restore a normal nutritional state. Many months are needed to reach this goal. Diet instruction is usually carried out by the dietician but it is important for the nurse to assess the patient’s understanding and reinforce the information whenever possible, as well as assessing the patient’s capacity to access and afford an adequate diet. The patient’s ability to comply with the dietary instructions must be examined in the light of past eating habits, religious and cultural preferences, age, income, other resources and state of health.
Unless the patient and the family can be convinced of the necessity for dietary change and have the resources to effect change, it is likely that no long-term benefits will be achieved. Ways should be found in which the patient can become actively involved in the recovery process. The need for continuous follow-up care must be strongly emphasised if rehabilitation is to be sustained.
The nurse is in an ideal position to determine the need for community support with meals, especially if the person lives alone. This should be considered as an essential part of discharge planning. Follow-up home visits by the community nurse should also be arranged where appropriate.
Keeping a food diary or a kilojoule count for 3 days at a time is one way to analyse and reinforce healthy eating patterns. These records are also helpful to the healthcare team in the follow-up care. Self-assessment of progress can be encouraged by weighing the patient once or twice a week and by keeping a weight record.
 Evaluation
Evaluation
The expected outcomes are that the patient who is malnourished will: (1) achieve and maintain optimal body weight; (2) consume a well-balanced diet; and (3) experience no adverse outcomes related to malnutrition.
Gerontological considerations: malnutrition
Older adults are at risk of malnutrition with numerous factors influencing their nutritional intake (see Box 39-6). Many of these factors may occur at the same time, thus further increasing the risk of malnutrition. One study of elderly people found that the nutrients most often assessed as being consumed in low amounts were protein, calcium, zinc, folate, vitamin B12 and other B group vitamins, fibre, vitamin D (also absorbed from sunlight), magnesium and vitamin E.21
BOX 39-6 Factors affecting nutritional intake in older adults
GERONTOLOGICAL DIFFERENCES IN ASSESSMENT
The risk factors for malnutrition in the elderly can be identified by nutrition assessment and screening based on the categories listed in Box 39-3. The unique nutritional requirements of an older adult are often overlooked. As a person grows older there are decreases in lean body mass (the metabolically active tissue), BMR and physical activity. Combined, these factors decrease the kilojoule needs for energy. The older person frequently reduces the consumption of needed protein, vitamins and minerals and may take in ‘empty kilojoules’, such as cakes and biscuits. As a group, older adults may be less well informed about what constitutes a well-balanced diet. When these factors are added to already existing medical problems, it is easy to see why poor dietary practices develop. In addition, poor dentition, ill-fitting dentures, anorexia, multiple losses affecting the social setting of meals, low income and medical conditions involving the GI tract contribute to the type and amount of foods that are eaten. The dietary guidelines for older New Zealanders22 and Australians5 recommend, in addition to the general good nutrition guidelines given in Box 39-5, the following: keep active to maintain muscle strength and a healthy body weight; eat at least three meals every day; care for food (prepare and store it correctly); drink adequate amounts of water and/or other fluids; and include foods high in calcium.
Some of the physiological changes associated with ageing affect the nutritional status of older adults. The following changes are of particular interest:
1. changes in the oral cavity (e.g. change in bite surfaces of the teeth, periodontal disease, drying of the mucous membrane of the mouth and tongue, poorly fitting dentures, decreased muscle strength for chewing, decreased number of taste buds, decreased saliva production)
2. changes in digestion and motility (e.g. decreased absorption of vitamin B12, vitamin A and folic acid, and decreased GI motility)
3. changes in the endocrine system (e.g. decreased tolerance to glucose)
4. changes in the musculoskeletal system (e.g. decreased bone density, degenerative joint changes)
5. decrease in vision and hearing (e.g. procurement and preparation of food are more difficult).
Certain illnesses that are more prevalent in the older population are considered diet related. These include atherosclerosis, osteoporosis, diabetes mellitus and diverticulosis. Multiple drugs are often required to treat these and other common chronic illnesses. These drugs can have an adverse effect on the appetite of the older adult, increasing the possibility of inadequate intake caused by anorexia.
To date, with the exception of kilojoules, it has not been determined that older adults have different requirements for specific nutrients from those of middle-aged adults. Generally, kilojoule intake should decrease with age because of the progressive loss of lean body mass and a decrease in the BMR. Therefore, fewer kilojoules are needed to meet nutritional needs. Unless kilojoule intake is decreased by careful attention to food intake, or energy expenditure is increased through greater physical activity and exercise, obesity will result. The use of hydrotherapy has provided a means of increasing exercise in the elderly without the stress on joints that makes many fully weight-bearing exercises unattainable. Heated pools allow exercise programs to be developed that can be carried out on a year-round basis.
Socioeconomic factors are important variables when assessing the nutritional status of older adults. Studies have shown that nearly 20% of elderly Australians are living below the poverty line.23 The number of elderly New Zealanders living below the poverty line is disputed; however, there are still disparities in New Zealand, with Māori 2.5 times more likely to be poor than non-Māori and Pacific Islanders more than 3 times more likely to be poor.24 Obtaining adequate and nutritious food can be an ongoing problem. In many cases people cannot afford to purchase meat, fresh vegetables and fruits that provide many necessary nutrients.
Lifestyle changes, such as retirement or relocation to a nursing home, can have a significant impact on eating habits. Other important considerations that should be assessed include the person’s ethnic background, previous dietary practices, food preferences, knowledge about the characteristics of a proper diet, the availability and accessibility of food shops, transportation and health status. Problems related to any or all of these areas can alert the nurse to the possibility of a nutritional problem.
Malnutrition can occur in an older person when kilojoule requirements, although decreased with age, are not met by the required proportion of protein, carbohydrate and fat. If malnutrition is present, few malnourished older persons are able to ingest enough food to correct the malnourished state. Special strategies, such as adaptive devices (e.g. large-handled eating utensils), are often helpful in increasing dietary intake. Some older people may require nutritional support therapies until their strength and general health are improved.
Many community nutritional programs are available to the older person to make mealtimes a pleasant, social event. Improving the social setting of a meal often improves the dietary intake. Home-delivered meals and meal sites in a central location are popular meal alternatives for many older adults.
Types of specialised nutrition support
ORAL FEEDING
High-kilojoule oral supplements (fortified or modular) may be used for patients whose nutritional intake is deficient. These include milkshakes, puddings and commercially available products (e.g. Ensure, Sustagen). Research suggests that ingestion of such supplements may have a role in improving the nutritional status of elderly patients.25,26 The supplements should not be used as meal substitutes but rather as snacks between meals. In some long-term care facilities, supplements are used instead of water when administering oral medications to increase energy intake. If patients are unable to maintain or achieve adequate nutritional status, nutrition support may be necessary. For a decision-making plan related to nutrition, see the algorithm in Figure 39-3.
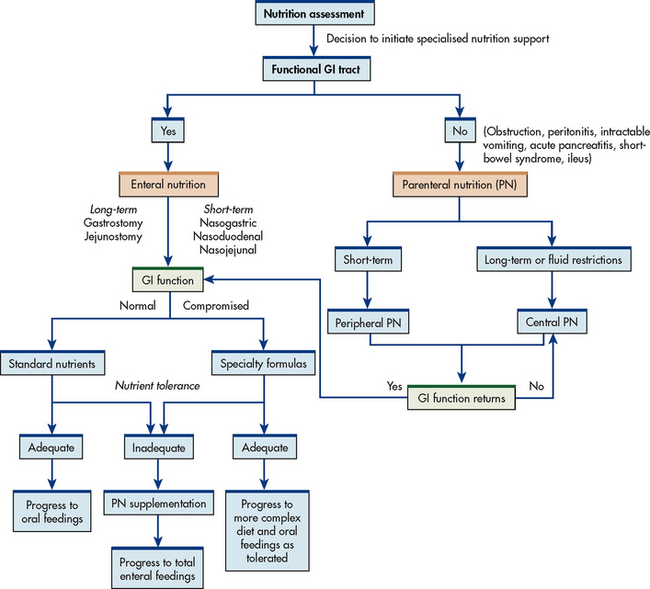
Figure 39-3 Nutrition support algorithm. GI, gastrointestinal.
Source: Adapted with permission of the American Society for Parenteral and Enteral Nutrition (ASPEN). ASPEN Board of Directors: guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. J Parenter Enteral Nutr 2002; 26:8SA.
TUBE FEEDING
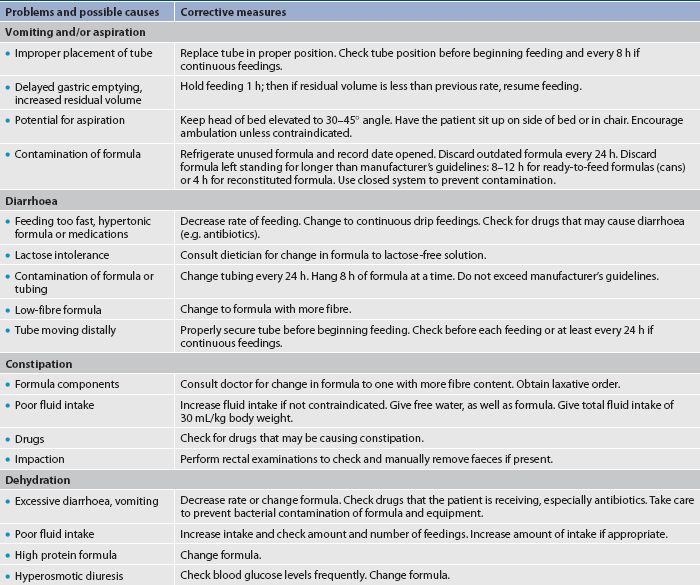
Tube feeding refers to the administration of nutritionally balanced liquefied food or formula through a tube inserted into the stomach, duodenum or jejunum. Tube feeding may be ordered for the patient who has a functioning GI tract but is unable to take any or enough oral nourishment. Specific indications for tube feeding include patients with anorexia, orofacial fractures, head and neck cancer, neurological or psychiatric conditions that prevent oral intake and extensive burns, and those who are receiving chemotherapy or radiation therapy. Tube feeding is considered to be easily administered, safer, more physiologically efficient and definitely less expensive than parenteral nutrition. It is used to provide nutrients by way of the GI tract either alone or as a supplement to oral or parenteral nutrition.
Common delivery options are continuous infusion by pump, intermittent infusion by gravity, intermittent bolus by syringe and cyclic feedings by infusion pump. Continuous infusion is most often used with critically ill patients and when feeding into the small intestine. Intermittent feeding may be preferred as the patient improves or is receiving such feedings at home.26,27
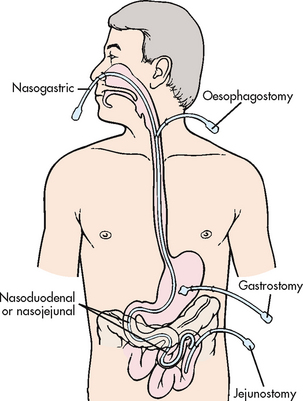
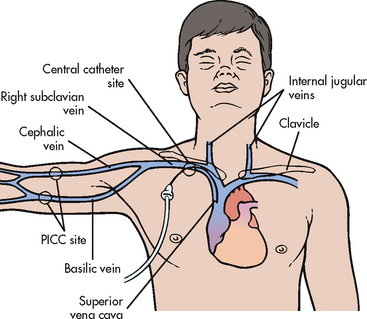
A nasogastric (NG) tube is most commonly used for short-term feeding problems. If feeding is necessary for an extended period of time, other means may be used, such as a gastrostomy tube placed surgically, endoscopically or radiologically, or a jejunostomy tube that empties directly into the jejunum. Transpyloric (nasointestinal) tube placement or placement into the jejunum is used when physiological conditions warrant feeding the patient below the pyloric sphincter (e.g. when oesophageal reflux places a patient at risk of aspiration into the airway). Figure 39-4 shows the location of commonly used enteral feeding tubes.
Nasogastric and nasointestinal tubes
Feeding tubes made of polyurethane, carbothane and silicone materials (some with hydromer grafting for ease of insertion) have added to the comfort level of patients when they need to be used over an extended period of time. These tubes are long, small in diameter, soft and flexible, thereby decreasing the risk of mucosal damage from prolonged placement. Polyvinyl chloride tubes tend to stiffen with time, rendering them unsuitable for long-term use. Polyurethane and silicone tubes are radio-opaque, making their position readily identifiable by X-ray. Many of these tubes have weighted tips, allowing for easier passage of the tube through the pylorus into the duodenum, or have coiled ends, which, when placed in the stomach, are designed such that the coiling promotes propulsion into the duodenum with gastric peristalsis. Placement into the intestine theoretically decreases the likelihood of regurgitation of contents into the oesophagus and subsequent aspiration. With the use of a stylet, these tubes can be placed in a comatose patient because the ability to swallow is not essential during insertion.
Although the smaller feeding tubes have many advantages over wider-lumen tubes, such as the standard decompression NG tube, there are some disadvantages. Because of the small diameter, these tubes are more easily clogged when feedings are thick and they are more difficult to use for checking residual volumes. They are particularly prone to obstruction when oral drugs have not been thoroughly crushed and dissolved in water before administration. They can become dislodged by vomiting or coughing and can also become knotted or kinked in the GI tract. Failure to flush the tubing after both drug administration and residual volume determinations can result in tube clogging. When the tube becomes clogged, removal and insertion of a new tube may be necessary, adding to cost and patient discomfort. It is difficult and, with some brands, contraindicated to aspirate gastric content when using a small-bore tube. The placement of these tubes therefore generally requires radiological confirmation of correct placement before beginning an infusion.
Measurement of the tube length after initial insertion, prior to each feed and once per shift in continuous feeds has been recommended in the literature as an effective technique for reducing the risk of aspiration related to tube migration or inadvertent manipulation.28
Gastrostomy and jejunostomy
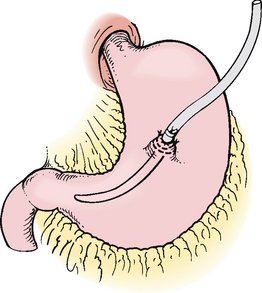
A gastrostomy tube may be used for patients who require nutrition over an extended period of time (see Fig 39-5). Gastrostomy tubes can be placed surgically, radiologically or endoscopically. Figure 39-6 shows the placement procedure of a percutaneous endoscopic gastrostomy [PEG] tube. Contraindications for the placement of a PEG are any condition or drug that increases the risk of bleeding, an inability to transilluminate through the abdominal wall, an inability to indent the gastric wall using digital pressure on the abdomen, hiatus hernia, gastric outlet obstruction, recent myocardial infarction, an inability to tolerate conscious sedation or morbid obesity.29 A PEG or a radiologically placed gastrostomy tube has several advantages, including fewer risks than surgical placement and avoidance of general anaesthesia.
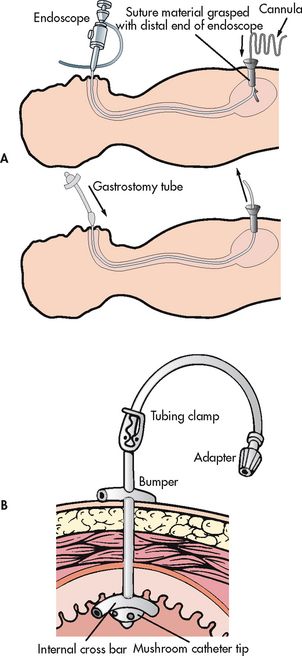
Figure 39-6 Gastrostomy tube placement via percutaneous endoscopy. A, Using endoscopy, a gastrostomy tube is inserted through the oesophagus into the stomach and then pulled through a stab wound made in the abdominal wall. B, A retention disc and bumper secure the tube.
Gastrostomy tube feedings can generally be started when bowel sounds are present, usually within 24 hours after tube placement. Immediately after tube insertion, the tube length from the insertion site to the distal end should be measured and recorded. The tube is then marked at the skin insertion site, although many tubes are pre-marked. At regular intervals the tube insertion length should be rechecked. Often after insertion of a PEG tube the patient gains weight. The external bolster must be adjusted accordingly to ensure there is no tension between the internal and external bolsters, resulting in necrosis of the gastric lining. The tube is most often connected to a pump for continuous feeding. Water may be infused within 2 hours after placement.
Do antibiotics prevent infection from percutaneous endoscopic gastrostomy tubes?
EVIDENCE-BASED PRACTICE
Clinical question
For patients with percutaneous endoscopic gastrostomy (PEG) tubes (P), do prophylactic antibiotics (I) versus placebo or no intervention (C) reduce infections (O) after tube placement (T)?
Implications for nursing practice
• Consult with endoscopist before PEG placement for antibiotic prophylaxis orders.
• Ensure that first dose of antibiotic is administered before skin incision.
P, Patient population of interest; I, intervention or area of interest; C, comparison of interest or comparison group; O, outcome(s) of interest; T, timing.
For the patient with chronic reflux, a jejunostomy tube with continuous feedings may be necessary to reduce the risk of aspiration.29 These can be placed through the PEG tube. Some important nursing implications for the care and feeding of patients with feeding tubes are listed in Box 39-7.
BOX 39-7 Nursing management: feeding tubes
• Check tube placement before feeding and before each drug administration.
• Assess for bowel sounds before feeding.
• Use liquid medications rather than pills.
• If it is necessary to use tablets, be sure to crush drugs to a fine powder to avoid clogging feeding tubes (not for sustained release or enteric coated or microencapsulated forms).
• Follow general principles of tube feeding (e.g. elevating head of bed, checking for residual volumes and flushing tube with water).
• Assess regularly for complications (e.g. aspiration, diarrhoea, abdominal distension, hyperglycaemia, constipation and faecal impaction).
Procedures for tube feeding
The following evidence-based principles are based on the American Society for Parenteral and Enteral Nutrition’s (ASPEN’s) enteral nutrition practice recommendations,28 as the Australasian Society for Parenteral and Enteral Nutrition has not yet published its guidelines for tube feeding.
1. Patient position. The head of the bed should be elevated to a minimum of 30°, but preferably 45°, to prevent aspiration. When a back rest elevation is not tolerated, a reverse Trendelenburg position can be used to elevate the head of the bed, unless contraindicated. If it is necessary to lower the head of the bed for a procedure, the patient should be returned to an elevated position as soon as possible. The nurse should check institution policy for suspending feeding while the patient is supine. If intermittent delivery is used, the patient’s head should remain elevated for 30–60 minutes after feeding.
2. Patency of the tube. The feeding tube should be flushed with 30 mL of water every 4 hours during continuous feeding or before and after intermittent feeding. The feeding tube should also be flushed with 30 mL of water after residual volume measurements. Sterile water is used for tube flushes in immunocompromised or critically ill patients, especially when the safety of tap water cannot be reasonably assumed. Sterile water is also recommended for use before and after medication administration via a feeding tube. If feedings are continuous, it is preferable to use a feeding pump with a built-in alarm that sounds if the tube becomes occluded. If no pump is available, the drip rate should be monitored frequently so that blockage does not occur from the patient lying on the tubing inadvertently or from too slow a drip rate.
3. Tube position. X-ray confirmation should be obtained to determine whether a blindly placed nasogastric or orogastric tube (small-bore or large-bore) is properly positioned in the GI tract before administering feedings or medications. Smaller feeding tubes may be passed directly into the bronchus on insertion or may become dislodged and slip into the bronchus without any obvious respiratory manifestations. The nurse should not rely on the auscultation method to differentiate between gastric and respiratory placement, or to differentiate between gastric and small bowel placement. To determine if a feeding tube has maintained the proper position, the exit site of the feeding tube should be marked at the time of the initial X-ray and the nurse should observe for a change in the external tube length during feedings. If a significant increase in the external length is observed, other bedside tests should be used to help determine whether the tube has become dislocated. When in doubt, an X-ray should be obtained to determine tube location.
4. Aspiration risk. All enterally fed patients should be evaluated for risk of aspiration. Before starting tube feedings, the nurse should ensure that the tube is in the proper position. Head-of-bed elevation should be maintained as described above. Checking gastric residual volumes is important when feedings are administered into the stomach. For example, when the infusion rate is 100 mL/h, after 4 hours the total infused volume of 400 mL may accumulate if gastric emptying is delayed. In addition, gastric secretions can increase the volume beyond this amount. With increased residual volume there is increased risk for aspiration of formula into the pulmonary tract. Gastric residual volumes should be checked every 4 hours during the first 48 hours for gastrically fed patients. After the enteral feeding goal rate is achieved, gastric residual monitoring may be decreased to every 6–8 hours in non–critically ill patients or continued every 4 hours in critically ill patients. (Common problems of patients receiving tube feeding are outlined in Table 39-11.) Feeding tubes may need to be placed below the ligament of Treitz (jejunostomy) when gastric residual volumes consistently measure greater than 500 mL. There is no need to obtain residual volumes for enteral nutrition (EN) delivered through a jejunostomy tube.
5. Formula. When possible, the use of a sterile, liquid EN formula is preferred to powdered or reconstituted formula. Formulas that are reconstituted in advance should be immediately refrigerated and discarded if unused within 24 hours of preparation. Powdered, reconstituted formula and EN with additive should not be up for more than 4 hours. Closed-system EN formulas can stay up for 24–48 hours per the manufacturer’s guidelines. Sterile, decanted formula should be up no longer than 8 hours. When starting a patient on EN, a full-strength isotonic commercial formula is preferred for initial feeding. Diluting the formula with water is not necessary and may increase the risk of diarrhoea from microbial contamination. In the home setting, blended foods from a normal diet may be used as tube feedings, although this is rarely done. Commercial formulas are preferred over blended foods for small-lumen tubes because of the lower risk of tube clogging, the completeness of nutrition and the decreased risk of formula contamination. The feeding should be given at ‘room’ temperature (unless in extreme temperatures such as those of the desert) or body temperature to decrease the likelihood of diarrhoea and other GI complaints. The pleasurable aspects of eating, such as smelling, seeing, tasting and chewing food, are denied the tube-fed patient. If the clinical condition permits, the patient may be allowed to smell, taste and even chew small amounts of food before the feeding, but the chewed food must be spat out.
6. Administration of feeding. The use of disposable gloves is recommended during the administration of EN. Feedings are administered either by the gravity drip method or a feeding pump. Applying pressure to force the feeding can damage the tube. The feeding rate or volume is increased gradually for 24–48 hours to minimise side effects, such as nausea or diarrhoea. Gravity drip methods include bolus and intermittent feedings. Bolus feedings are formula delivered by gravity via a syringe over approximately 15 minutes when the feeding tube is placed in the stomach. A feeding container or bag is used for intermittent feedings and the volume is infused usually over 30–45 minutes either by gravity or with an enteral feeding pump. An enteral infusion pump is required for small-bowel feedings and for continuous feeding. Enteral feeding pumps should be routinely calibrated to ensure accuracy. It is important to remember that the patient still needs water (1 mL per 5 kJ of formula received) and this may be administered with flush water or as additional boluses of water as tolerated.
7. Medication administration. Before giving medications, the enteral feeding formula should be stopped and the tube flushed with at least 15 mL of water. The medication should be diluted as appropriate and administered via the feeding tube using a clean oral syringe (>30 mL in size). The tube should be flushed again with at least 15 mL of water, taking into account the patient’s volume status. Medications should not be directly added to the enteral feeding formula. If giving more than one medication via an enteral feeding tube, each medication should be diluted and administered separately. Liquid dosage forms should be used when available and if appropriate. When liquid dosages are not available, only immediate-release solid dosage forms should be used. Each tablet should be ground to a fine powder and mixed with sterile water before administration. Hard gelatin capsules are opened and the powder/liquid/beads mixed with sterile water. It is important to note that hyperosmolar medications delivered directly into the small intestine can increase the risk of GI distress and diarrhoea.30
8. General nursing considerations. The patient should be weighed daily or several times a week, and the nurse should maintain accurate intake and output records. Initially blood glucose checks to assess glucose tolerance are performed at the bedside. An older patient who has baseline glucose intolerance is particularly at risk for hyperglycaemia. Feedings should be labelled with the date and time that they are initially set up and clearly labelled ‘not for intravenous use’. Administration sets (tubing) should be changed every 24 hours for open-system enteral feedings and per the manufacturer’s guidelines for closed-system feedings.
See NCP 39-1 for care of the patient receiving enteral nutrition.
Complications related to tubes and feedings
The types of problems encountered in patients receiving tube feedings and corrective measures are presented in Table 39-11. When commercial products are used, the concentration, flavour, osmolarity and amounts of protein, sodium and fat vary according to the manufacturer. Most commercial formulas are lactose-free. The concentrations range from 4 to 6 kJ/mL.
The osmolality of the solution is determined by the number and size of particles in solution. With regard to feeding formulas, the more hydrolysed or broken down the nutrients, the greater the osmolality. Many tube feeding formulas are isotonic (i.e. they have the same osmotic pressure as the blood), although some are hypertonic. The more classically dense the formula, the less water it contains. Protein content greater than 16% can lead to dehydration unless the patient is given supplemental water or is sufficiently alert to request additional fluids. The nurse must be aware of this potential problem and must provide extra fluids through the feeding tube or, if permitted, by mouth. Tube feedings with high sodium content are contraindicated in patients with cardiovascular problems, such as congestive heart failure. High fat content is not advocated for patients with short bowel syndrome or ileocaecal resections because of impaired fat absorption. Metabolic abnormalities such as hyperglycaemia, hypokalaemia, hypophosphataemia and hypomagnesaemia must be monitored closely by checking biochemistry results daily.
The dietician can be of considerable assistance to the nursing staff. When close consultation with the nursing staff occurs, existing problems with tube feedings can be quickly and efficiently addressed and resolved. Some institutions have nutrition support teams composed of a doctor, nurse, dietician and pharmacist, whose function is to oversee the nutrition support of select inpatients and outpatients.
In patients receiving gastrostomy or jejunostomy feeding, complications include wound infection or inflammation, peritonitis, septicaemia, peristomal leakage, tube dislodgement, aspiration, bowel perforation and gastrocolic fistula. For patients who are active, agitated or disorientated, or in children, skin level prostheses (buttons) are more suited. Skin care around the tube site is important because the action of the digestive juices is irritating to the skin. The skin around the feeding tube should be assessed daily for signs of redness and maceration. To keep the skin clean and dry, initially it should be rinsed with sterile water and dried, preferably with a hair dryer. Once the site has healed, it can be washed with mild soap and water, with the disc/bumper being moved in order to wash around the site properly. Adaptors and caps should remain attached to the patient during washing. A protective ointment or skin barrier may be used on the skin around the tube if it becomes inflamed. This includes zinc oxide, karaya, Stomahesive, sucralfate powder or Mylanta. In the event that significant leakage problems occur, a stomal therapist can be of great assistance to the nurse. Devices with balloons require a balloon volume check weekly. The contents should be aspirated and discarded, then sterile water should be used for reinflation. Any extension tubes and syringes should be changed daily in hospital and twice weekly if at home. They should then be washed in half strength white vinegar. If the tube becomes clogged, it should be flushed with 30–50 mL of warm water using a flush and aspirate technique. If it still remains blocked, the nurse should massage the tube or instil soda water. The patient and family members can be taught how to care for the feeding tube. Teaching should include skin care, care of the tube and complete information about feeding administration and potential complications.27–29 In the event of a PEG tube falling out, it should be emphasised that a replacement tube, if available, should be reinserted as soon as possible by qualified staff as the stoma begins to close within hours.31
TOTAL PARENTERAL NUTRITION
When the GI tract cannot be used for the ingestion, digestion and absorption of essential nutrients, TPN may be substituted. Parenteral nutrition refers to the administration of nutrients by a route (e.g. bloodstream) other than the GI tract. Total parenteral nutrition is the delivery of a nutritionally adequate hypertonic solution consisting of glucose, protein hydrolysates, minerals and vitamins using an intravenous (IV) route. TPN has become a relatively safe and practical method of delivering total nutritional needs. The goal of using TPN is to meet the patient’s nutritional needs and to allow the growth of new body tissue. Ordinary IV solutions of 5% dextrose (5 g dextrose/100 mL) in water or Hartmann’s solution contain no protein and deliver approximately 700 kJ per litre. The normal adult requires a minimum of 5000–6000 kJ per day to carry out normal physiological functions. Patients who sustain severe injury, surgery or burns and those who are malnourished as a result of medical treatment or disease processes have greatly increased nutritional needs. The volume of normal dextrose solutions needed to meet these high kilojoule requirements would exceed the capacity of the cardiovascular system. Box 39-8 lists common indications for the use of TPN.
Composition
Commercially prepared TPN base solutions are available for both central and peripheral use. These base solutions contain dextrose and protein in the form of amino acids. The pharmacy adds the prescribed electrolytes (e.g. sodium, potassium, chloride, calcium, magnesium, phosphate), vitamins and trace elements (e.g. zinc, copper, chromium, manganese) to customise the solution for the patient. A three-in-one or total nutrient IV admixture containing a fat emulsion, dextrose and amino acids has become widely used, especially in the home setting.
Gerontological considerations: enteral nutrition
Enteral nutrition interventions have a higher risk of complications (especially fluid and electrolyte imbalances) when used in older patients due to the physiological changes associated with ageing. Complications such as diarrhoea can leave patients dehydrated and hypokalaemic. Decreased thirst perception or impaired cognitive function decreases a patient’s ability to seek additional fluids.
With ageing there is a decreased ability to handle glucose loads (glucose intolerance). As a result, older patients may be more susceptible to problems of hyperglycaemia in response to the high carbohydrate load of some enteral feeding formulas. If an older patient has compromised cardiovascular function (e.g. congestive heart failure), there will be a decreased ability to handle large volumes of formula. In this situation the use of more concentrated formulas may be warranted. Older adults are also at increased risk of aspiration caused by gastro-oesophageal reflux disease, hiatus hernia or diminished gag reflex. Physical mobility, fine motor movement and visual system changes associated with ageing may contribute to difficulties in managing enteral nutrition equipment at home. In addition, age-related changes, such as a decrease in lean muscle mass, influence the reliability of measures used for nutritional assessment.
Kilojoules
Kilojoules in TPN are supplied primarily by carbohydrates in the form of dextrose and by fat in the form of fat emulsion. The administration of between 100 g and 150 g of dextrose (1 g provides approximately 14.28 kJ, as opposed to oral carbohydrates, which provide 17 kJ) daily has a protein-sparing effect. Adequate non-protein kilojoules must be provided in the form of glucose and lipids to allow metabolism of amino acids for wound healing and not as energy. However, overfeeding can lead to metabolic complications. To minimise these problems, an energy intake of 100–120 kJ/kg/day in a non-obese patient is often recommended. The provision of both lipid and carbohydrate components meets the energy requirement while minimising the problems of overfeeding. Fat emulsion solutions of 10%, 20% and 30% may be used. These lipid emulsions provide approximately 4.2 kJ/mL (10% solution) or 8.4 kJ/mL (20% solution). The contents of fat emulsions are primarily soybean or safflower triglycerides with egg phospholipids added as an emulsifier. In practice, the conservative approach is IV-administered fat emulsions providing not more than 30% of the total energy to minimise the possible immunosuppressive effects of linoleic acid. The maximum fat emulsion amount should not exceed a dose of 2.5 g/kg per day31 and should be administered slowly over 12–24 hours. Nausea, vomiting and elevated temperature have been reported, especially when lipids are infused quickly. The administration of fat emulsion is contraindicated in patients with a disturbance in fat metabolism. It should also be used with caution in patients who are in danger of fat embolism (e.g. fractured femur) and in patients with an allergy to eggs.
Protein
The normal healthy person of average body size needs approximately 45–65 g of protein daily. In hospital protein should be provided at the rate of 1–1.5 g/kg per day depending on the patient’s needs. In a nutritionally depleted patient under the stress of illness or surgery, requirements can exceed 150 g per day to ensure a positive nitrogen balance. Protein intake levels of 1.5–2 g/kg per day are suggested for most patients with moderate-to-severe illness.31
Electrolytes
The assessment of individual requirements should take place daily at the beginning of therapy and then several times a week as the treatment progresses. The following are ranges for the average daily electrolyte requirements for adult patients without renal or hepatic impairment:
The exact amount needed depends on the patient’s health problem and on electrolyte levels as determined by blood testing.
Trace elements
Zinc, copper, chromium, manganese, selenium, molybdenum and iodine supplements may be added according to the patient’s condition and needs. Levels of these elements are monitored in patients receiving TPN. The doctor may order additional amounts of these elements to be added to the solutions according to the patient’s requirements.
Vitamins
The daily addition of a multivitamin preparation to the TPN generally meets the vitamin requirements. If a multivitamin infusion is used, the vitamin B12 requirement may be met without the need for supplemental injections. It may be necessary for the doctor to order vitamin K separately because it is currently not included in multivitamin preparations.
Methods of administration
Parenteral nutrition may be administered via central or peripheral veins. Central parenteral nutrition is given through a catheter with its tip lying in the superior vena cava. The central venous catheter often originates at the subclavian or jugular vein. More recently, single- or double-lumen peripherally inserted central catheters (PICCs) are being placed, usually into the basilic or cephalic vein, and then advanced into the central circulation. Such catheters are made of soft, flexible material (silicone, polymer) and are 50–60 cm long. Ease of placement, cost and limited complications make this an attractive alternative to a subclavian vein catheter. Central parenteral nutrition is indicated when long-term parenteral support is necessary or when the patient has high protein and energy requirements.
Peripheral parenteral nutrition is administered through a peripherally inserted catheter or vascular access device, which uses a large peripheral vein. It is used when: (1) nutritional support is needed for only a short time; (2) protein and kilojoule requirements are not high; (3) the risk of a central catheter is too great; or (4) parenteral nutrition is used to supplement inadequate oral intake. Both central and peripheral parenteral nutrition are used in patients who are not candidates for enteral support.
Central and peripheral parenteral nutrition differ in tonicity, which is measured in milliosmoles (mOsm; the concentration of particles in a fluid). Blood is isotonic and measures approximately 280 mOsm/L. The standard IV solutions of 5% dextrose and normal saline are essentially isotonic. Central parenteral nutrition solutions are hypertonic, measuring at least 1600 mOsm/L. The high glucose content ranges from 20% to 50%. Central parenteral nutrition must be infused in a large central vein so that rapid dilution can occur; the use of a peripheral vein causes irritation and thrombophlebitis. Nutrients can be infused using smaller volumes than with peripheral parenteral nutrition. Peripheral parenteral nutrition is hypertonic (using as much as 20% glucose), but less so than central parenteral nutrition, and can be safely administered through a large peripheral vein, although phlebitis can occur. Another potential complication of peripheral parenteral nutrition is fluid overload.
In most institutions, commercial preparations of TPN are now used. If TPN solutions are prepared by a pharmacist, strict aseptic technique under a laminar flow hood should be used. Nothing should be added to parenteral nutrition solutions after they are prepared in the pharmacy. The danger of drug incompatibilities and contamination is high. The fewer personnel involved in the preparation and administration of TPN, the lower the risk of infection for the patient. In most hospitals the doctor must order the TPN solution daily. In this way the solution and additives can be adjusted to the patient’s current needs and laboratory results. Each TPN solution label indicates the nutrient content, all additives, the time of mixing and the date and time of expiration. In general, solutions are good for 24 hours and must be refrigerated until half an hour before use. All solutions should be protected from light because of photodegradation of vitamins A and E.18
Catheter placement
The subclavian vein is most commonly used for TPN administration, although the innominate vein or the jugular vein may be used. The procedure is the same as for the insertion of a central venous pressure line and should be done under strict aseptic conditions. A standard isotonic IV solution is infused through the central line until X-ray confirms proper placement of the catheter tip in the superior vena cava and not in the jugular vein. The catheter insertion site is covered with a sterile dressing. The date is marked on the dressing.
Placement of a PICC catheter is done under sterile conditions. A baseline measurement of the upper arm circumference is recommended. A tourniquet is then placed around the upper arm near the axilla to allow examination of the antecubital fossa and selection of a vein. If possible, the patient should be supine with the arm straight and at a 90° angle to the body. Preparation of the insertion site should be done according to institutional policy. The sterile catheter is cut to the predetermined length, depending on the vein selected.
A local anaesthetic is usually used at the insertion site. This site should be cleaned, protected and maintained according to institutional policy. As with the centrally placed line, a chest X-ray is needed to verify proper tip placement before administering any TPN solution. Proper placement of a catheter for central TPN is illustrated in Figure 39-7. Complications frequently associated with catheter placement are haemorrhage, hydrothorax and pneumothorax, haemothorax, air embolus and venous thrombosis. Once established for TPN, a single-lumen central catheter should not be used for the administration of blood or antibiotics, the drawing of blood samples or the monitoring of central venous pressure.
Administration of solution
Because TPN solutions are excellent media for microbial growth, it is essential that proper aseptic techniques be followed. IV tubing is changed every 24 hours. The tubing should be clearly labelled with the date and the time it is put into use. Complications of TPN can be divided into three categories: (1) infections; (2) metabolic problems; and (3) mechanical problems. The major complications of each category are presented in Box 39-9.
 NURSING MANAGEMENT: TOTAL PARENTERAL NUTRITION
NURSING MANAGEMENT: TOTAL PARENTERAL NUTRITION
Vital signs should be monitored every 4–8 hours in the patient receiving TPN. Daily weights give an indication of the patient’s hydration status as therapy progresses. Body weight is considered the sum of the changes in protein, fat and water. On a daily basis, body water fluctuates more than protein or fat. Analysis must be made as to whether gains or losses in weight are caused by fluid gained from oedema, fluid lost through diuresis or an actual increase or decrease in tissue weight. Initial daily monitoring of serum urea, electrolytes, glucose, calcium, magnesium, phosphate, liver function tests, plasma proteins, prothrombin time, plasma and urine osmolality, arterial blood gases and a full blood count is done until stable. Once stable these should be done twice weekly. Assessment of these important values assists the nurse in evaluating the patient’s tolerance of parenteral nutrition. A nursing care plan for the patient receiving parenteral nutrition is presented in NCP 39-2.
Dressings covering the catheter site are changed according to institutional protocol, ranging from every other day to once a week. Some institutions have specially trained nurses from the IV team or the nutritional support team who are responsible for these dressing changes. Otherwise, ward staff do the dressing changes after special instruction. The procedure for changing the dressing is similar to that followed after catheter insertion. The institutional routine should be followed with respect to the appropriate use of solutions for the dressing change. The site is carefully observed for signs of inflammation and infection. Phlebitis can readily occur in the vein as a result of the hypertonic infusion and the area can become infected. The patient receiving parenteral nutrition may be immunosuppressed and thus more susceptible to opportunistic infections. In such patients, signs of inflammation or infection can be subtle, if present at all. Many patients receiving TPN are receiving chemotherapy, corticosteroids or antibiotics, which can mask signs of infection.
If sutures are used to anchor the catheter, they may become infected. If an infection is suspected, a culture specimen of the site and drainage should be sent for analysis and the doctor should be notified immediately. The use of an occlusive dressing protects the wound from contamination.
Hyperglycaemia is a metabolic complication of parenteral nutrition. At the beginning of TPN therapy, the solution is infused at a gradually increasing rate for 24–48 hours. This allows the pancreas to adapt to the increased amount of glucose in the circulation by producing more insulin. Blood glucose levels should be checked at the bedside every 4–6 hours with a glucometer (Ch 48). Some increase in the blood glucose level is expected during the first few days after TPN is started. A sliding scale dose of insulin may be ordered to keep the glucose level between 5.56 and 11.2 mmol/L. In critically ill patients it is recommended that insulin be administered to maintain the blood glucose level between 8.34 and 11.2 mmol/L.28 If no glucometer is available, the urine may be tested for glucose and acetone every 4–6 hours while the patient is receiving TPN. It is important to note that urine testing is not as accurate as blood testing, particularly in the elderly, and thus is not undertaken frequently.
Refeeding syndrome is characterised by fluid retention, electrolyte imbalances (including hypophosphataemia, hypokalaemia and hypomagnesaemia) and hyperglycaemia. Conditions that predispose patients to refeeding syndrome include longstanding malnutrition states such as chronic alcoholism, vomiting and diarrhoea, chemotherapy and major surgery. Hypophosphataemia is the hallmark of refeeding syndrome and is associated with serious outcomes, including cardiac arrhythmias, respiratory arrest and neurological disturbances (e.g. paraesthaesias).28,29,31 In infants, hyperammonaemia can be a problem. Signs include lethargy, twitching and generalised seizures. Correction consists of arginine supplementation at a rate of 0.5–1.0 mmol/kg/day. Metabolic disease, resulting in severe periarticular, lower extremity and back pain in some patients on long-term TPN, is associated with low serum calcium levels. The only treatment is temporary or permanent cessation of TPN.
The nurse must be made aware that speeding up or slowing down the infusion rate is contraindicated. Speeding up the rate results in a large amount of glucose entering the circulation. Endogenous insulin levels are often inadequate to handle this increase in glucose and a hyperglycaemic state results. Conversely, slowing the rate may result in a hypoglycaemic state because it takes time for the pancreatic islet cells to adjust to a reduced glucose level. Checking the amount infused and the rate every 30 minutes to 1 hour is recommended. An infusion pump should be used during the administration of TPN so that the infusion rate can be maintained, and an alarm will sound if the tubing becomes obstructed. Even when an infusion pump is being used, the nurse should periodically check the volume infused because pump malfunctions can alter the rate.
Before setting up and administering TPN, the nurse must check the label and ingredients in the solution to see that they are what the doctor ordered. Solutions must also be examined for signs of contamination, such as a cloudy appearance. If contamination is suspected, the solution should be returned promptly to the pharmacy for replacement. It is the nurse’s responsibility to ensure that the TPN solution is discontinued and replaced with a new solution if the bag is not empty at the end of 24 hours. At room temperature, the solution is an excellent medium for the growth of microorganisms.
Sometimes fat emulsions are infused separately from the parenteral nutrition solution. The preferred delivery method is a continuous low volume such as 20% lipids delivered over 12 hours, depending on patient needs.30 Adverse reactions to lipid emulsions that may occur early are dyspnoea, cutaneous allergic phenomena, nausea, back pain, sweating and dizziness. Temporary hyperlipidaemia may occur and is especially common in renal and hepatic failure. Delayed adverse reactions include hepatomegaly, mild elevation of liver enzymes, splenomegaly, thrombocytopenia, leucopenia and alterations in pulmonary function studies, especially in premature infants with respiratory distress syndrome.29 A major benefit derived from IV fat administration is that a large number of kilojoules can be provided in a relatively small amount of fluid. This is especially beneficial when the patient is at risk of fluid overload.
Catheter-related infection and septicaemia can occur in patients receiving TPN through either peripherally or centrally placed lines.32 Local manifestations of infection include erythema, tenderness and exudate at the catheter insertion site. Systemically the patient may have fever, chills, nausea, vomiting and malaise. If no other causes can be identified, a catheter-related infection should be suspected. Because of the risk of infection, catheters with antibiotic or antiseptic surfaces may be used. To diagnose the presence of infection and to determine the causative organism, cultures are performed of the catheter tip if the catheter has been removed, or of the blood in the catheter if it is still in place. Blood cultures are taken simultaneously from the catheter and a peripheral vein. A chest X-ray is taken to detect changes in pulmonary status. The current TPN solution with tubing should also be cultured and replaced with an entirely new set-up. When the catheter tip is the source of infection, antibiotic therapy may not be necessary because removal of the catheter can eliminate the problem.33 A new central line may be immediately established or replaced by a peripheral route. It is important that a glucose source be maintained to prevent rebound hypoglycaemia.
The same precautions should be followed in weaning from TPN as when therapy is being initiated. The flow rate must be gradually decreased for 1–2 hours, while oral intake is increased. If an emergency situation precludes a slow weaning process, other dextrose or glucose-containing nutrients should be administered. When the catheter is removed, the dressing should be changed daily until the wound heals. Oral nourishment should be encouraged and a careful record of intake should be maintained. Recording of body weight and laboratory analysis of serum electrolyte and glucose levels may continue.
 Home nutrition support
Home nutrition support
Home parenteral or enteral nutrition is an accepted mode of nutritional therapy for patients who do not require hospitalisation but who benefit from continued nutritional support. Some patients have been successfully treated at home for many months and even years. It is important for the nurse to educate the patient or family about catheter or tube care, proper technique in mixing and handling of the solutions and tubing, and side effects.
Home nutrition therapies are expensive, although patients who need parenteral and enteral nutrition are supported under local healthcare schemes. The nurse should ensure that discharge planning personnel are involved early in the admission and that appropriate referral to community services is completed. Home nutrition support may be a burden on the patient and carers and may affect quality of life. The nurse should make the family aware of support groups if available.
Eating disorders
Eating disorders are primarily psychiatric disorders. However, there are a number of nutritional problems associated with these disorders that require the nurse to implement a nutritional plan of care. Eating disorders primarily affect young women. It is estimated that 6% of those with severe eating disorders will die and that only 50% report being cured.34 This means that more than 40% of sufferers are likely to experience chronic nutritional problems.
ANOREXIA NERVOSA
Anorexia nervosa is characterised by a self-imposed weight loss, endocrine dysfunction and a distorted psychopathological attitude towards weight and eating.33 Anorexia nervosa clinically manifests as abnormal weight loss, deliberate self-starvation, intense fear of gaining weight, lanugo (soft, downy hair covering the body except the palms and soles), refusal to eat, continuous dieting, hair loss, sensitivity to cold, compulsive exercise, absent or irregular menstruation, dry skin and constipation. Diagnostic studies often show iron-deficiency anaemia and an elevated serum urea nitrogen level that is reflective of marked intravascular volume depletion and prerenal azotaemia. Lack of potassium in the diet and loss of potassium in the urine lead to potassium deficiency. Manifestations of potassium deficiency include muscle weakness, cardiac arrhythmias and renal failure. If the eating pattern is permitted to continue for a prolonged time, body wasting and signs of severe malnutrition are evident.
Multidisciplinary treatment must involve a combination of nutritional support and psychiatric care. Hospitalisation may be necessary if there are severe physical complications that cannot be managed in an outpatient therapy program. Nutritional replenishment must be closely supervised to ensure consistent and ongoing weight gain. The use of tube or parenteral feedings may be necessary. Improved nutrition, however, is not a cure for anorexia nervosa. The underlying psychiatric problem must be addressed by good psychiatric assessment, followed by individual and family treatment.
BULIMIA NERVOSA
Bulimia nervosa is a disorder characterised by frequent binge eating and self-induced vomiting associated with loss of control over eating and a persistent concern with body image.33,34 Individuals with bulimia may have normal weight for their height or their weight may fluctuate with binging and purging. They may also abuse laxatives, diuretics, exercise or diet drugs. They may have signs of frequent vomiting, such as macerated knuckles, swollen salivary glands, broken blood vessels in the eyes and dental problems.
Bulimia seems to be increasing in incidence and may be even more prevalent than anorexia nervosa, although accurate data are hard to obtain as most people with bulimia do not disclose their condition to family, friends or their healthcare provider. Female high-school students seem to be most susceptible to this syndrome. The cause remains unclear but it is thought to be similar to that of anorexia nervosa. Substance abuse, anxiety, affective disorders and personality disturbances have been reported among persons with bulimia.
Patients with bulimia, similar to patients with anorexia nervosa, go to great lengths to conceal their abnormal eating habits. As the behaviour persists, many problems associated with the condition become increasingly hard to deal with effectively. As with anorexia, a treatment combination of psychological counselling and diet therapy is essential. Education and emotional support for the patient and family are vital.
Culturally competent care: nutrition
People have unique cultural heritages that may affect eating customs and nutritional status. Culture, along with personal preferences, socioeconomic status and religious preferences, can influence food choices. Each culture has its own beliefs and behaviours related to food and the role that food plays in the aetiology and treatment of disease. In addition, culture can dictate what food is considered edible, as well as how it is prepared and when it is eaten. There are a wide array of cultural influences on diet ranging from what foods are selected to when meals are eaten and how and who prepares them. For example, some religions require periods of fasting. Muslims fast every year during the religious month of Ramadan.
The diets of many Indigenous peoples have undergone rapid change from a fibre-rich, high-protein, low-saturated fat ‘traditional’ diet to one in which refined carbohydrates and saturated fats predominate.35,36 Poor nutrition has a major influence on the morbidity and mortality of Indigenous peoples and has been found to contribute about 16% to the burden of chronic disease.36,37 Living in remote communities with little regular access to cheap nutritious food also has an impact (for example, a lettuce can cost up to $10, whereas a bucket of hot chips costs around $3.50). One study that examined the dietary habits of a remote Aboriginal community found that the diet was high in energy-dense, nutrient-poor foods.38 However, research has also shown that improvements in food quality and access to healthy food, along with education about good nutritional practices, lead to marked and sustained improvement in health markers.39
Traditional Māori foods are generally compatible with dietary guidelines, although the addition of fat, sugar, cream and salt to traditional foods has altered this balance. While many Māori have adopted a largely European-style diet, numerous traditional foods and cultural practices have been retained. However, large disparities across a range of risk factors and health outcomes still exist for Māori and Pacific Islander peoples compared to the total New Zealand population, with no change, for example, in the rate of obesity for Māori adults since 1997.40
The nurse should include cultural and ethnic considerations when assessing the patient’s diet history and implementing interventions that require dietary changes. At the same time, the nurse needs to avoid cultural stereotyping by making assumptions or generalisations about diet based on the individual’s cultural background. It is important to know what kind of food the patient normally eats. The nurse should assess their impact on health. For example, traditional foods eaten by some Asian cultures may be low in fat and cholesterol but also low in calcium because of the lack of milk products.
Consideration of cultural beliefs is very important when planning dietary changes and monitoring acceptance of these changes. For example, perception of body weight and size may be influenced by culture (where larger size may denote wealth). Thus, the nurse needs to ask the patient or family about how culture affects dietary choices and weight maintenance.
Teaching related to dietary restrictions and recommended dietary changes should involve the patient’s family. In many situations it is a family member who does the grocery shopping and cooking.
CASE STUDY
CRITICAL THINKING QUESTIONS
1. What are this patient’s risk factors for malnutrition?
2. What is her body mass index?
3. What are contributing factors to this patient developing dysphagia and malnutrition?
4. What should the nurse include in a successful weight gain program for this patient?
5. What possible complications of tube feeding could this patient be at risk of?
6. What is the priority of the nursing care for this patient?
7. Based on the assessment data presented, write one or more appropriate nursing diagnoses.
1. The nurse identifies a need for dietary teaching for a patient whose daily intake of the food groups consists of:
2. In general, nutrient or food interactions with drugs can result in all of the following except:
3. During the first 24 hours of starvation, the order in which the body obtains substrate for energy is:
4. An elderly patient with a recent stroke is exhibiting signs of severe dysphagia. The optimal form of nutrition support at this time would be:
5. A nutritionally stressed patient weighing 60 kg is receiving nil by mouth and total parenteral nutrition. In evaluating the patient’s nutritional intake, the nurse calculates that the daily total parenteral nutrition solution should provide:
6. One advantage of a percutaneous endoscopic gastrostomy tube placement relative to nasogastric feedings for the patient receiving long-term enteral nutrition is that:
7. The nurse recognises that the major goal of treatment for a patient with anorexia nervosa is being met when the patient:
1 Cook JT, Frank DA. Food security, poverty, and human development in the United States. Ann NY Acad Sci. 2008;1136:193.
2 Gilbert PA, Khokhar S. Changing dietary habits of ethnic groups in Europe and implications for health. Nutr Rev. 2008;66:203.
3 Nutrition Australia. About the Healthy Living Pyramid. Available at www.nutritionaustralia.org/national/resource/healthy-living-pyramid. accessed 06 February 2011.
4 New Zealand Ministry of Health. New Zealand food and nutrition guideline statements for healthy adults. Available at www.moh.govt.nz/moh.nsf/indexmh/nutrition-foodandnutritionguidelinestatements, Updated April 2007. accessed February 2011.
5 Department of Health and Ageing, National Health and Medical Research Council, New Zealand Ministry of Health. Nutrient reference values for Australia and New Zealand including recommended dietary intakes. Available at www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/n35.pdf, 2006. accessed February 2011.
6 McKeown NM, Meigs JB, Liu S, et al. Carbohydrate nutrition, insulin resistance and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care. 2004;27:538–546. Seminal paper
7 Queensland Health. Facts on fat. Available at www.health.qld.gov.au/ph/documents/hpu/19383.pdf, 2003. accessed February 2011.
8 Food Standards Australia New Zealand. Review report: trans-fatty acids in the New Zealand and Australian food supply. Available at www.foodstandards.gov.au/_srcfiles/TFAs_Aus_NZ_Food%20_Supply.pdf, July 2009. updated December 2010. accessed February 2011.
9 Food Standards Australia and New Zealand. The prevalence and severity of iodine deficiency in Australia, including Appendix 1: summary of current iodine status in New Zealand, as at. Available at www.foodstandards.gov.au/_srcfiles/Item02 05-Attachment1-Prevalence_and_security_of_iodine_deficiency_in_Australia.pdf, October 2007. accessed February 2011.
10 Craft J, Gordon G. The structure and function of the digestive system. In: Craft J, Gordon G, Tiziani A, eds. Understanding pathophysiology. Sydney: Elsevier, 2011.
11 New Zealand Ministry of Health. Nutrition. Iodine status in New Zealand. Available at www.moh.govt.nz/moh.nsf/indexmh/nutrition-iodine, 2008. accessed February 2011.
12 Sip A, Grajek W. Probiotics and prebiotics. In: Smith J, Charter E, eds. Functional food product development. Oxford: Wiley-Blackwell, 2010.
13 Salminen SJ, Gueimonde M, Isolauri E. Probiotics that modify disease risk, J Nutr. 2005;135(5):1294–1298. Available at http://jn.nutrition.org/content/135/5/1294.full.pdf+html accessed February 2011.
14 Food Standards Australia New Zealand. Genetically modified food. Available at www.foodstandards.gov.au/consumerinformation/gmfoods/, Updated April 2010. accessed February 2011.
15 The Joanna Briggs Institute. Best practice information: effectiveness of interventions for undernourished older inpatients in the hospital setting. Available at www.joannabriggs.edu.au/pdf/BPISEng_11_2.pdf, 2007. accessed February 2011.
16 Woods J, Walker KZ, Iuliano-Burns S, Strauss BJ. Nutrition for the elderly. Asia Pac J Clin Nutr. 2006;15(suppl 3):S78.
17 Norman K, Pichard C, Lochs H, et al. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27:5–15.
18 Boullata JI, Vincent T. Armenti handbook of drug-nutrient interactions, 2nd edn. New York: Humana Press, 2010.
19 Guenter P, Hicks RW, Simmons D, et al. Enteral feeding misconnections: a consortium position statement. Joint communiqué. J Qual Patient Safety. 2008;34:285.
20 Thomas DR. Nutrition assessment in long-term care. Nutr Clin Pract. 2008;23:383.
21 Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27:340.
22 New Zealand Ministry of Health (NZMOH). Food and nutrition guidelines for healthy older people: a background paper. Available at www.moh.govt.nz/moh.nsf/indexmh/food-nutrition-guidelines-for-healthy-older-people-background-paper, 2010. accessed February 2011.
23 Melbourne Institute of Applied Economic and Social Research. Poverty lines Australia. Available at www.melbourneinstitute.com/labour/inequality/poverty/Poverty%20lines%20Australia%20Mar%202010.pdf, March quarter 2010. accessed February 2011.
24 New Zealand Ministry of Health. Reducing inequalities in health. Available at www.moh.govt.nz/moh.nsf/82f4780aa066f8d7cc2570bb006b5d4d/523077dddeed012dcc256c550003938b/$FILE/ReducIneqal.pdf. accessed February 2011.
25 Dechelotte P. Efficacy of a special oral nutritional supplement on nutritional status of malnourished elderly patients. US Institutes for Health Clinical Trials; April 2009. Available at http://clinicaltrialsfeeds.org/clinical-trials/show/NCT00877578 accessed February 2011.
26 Marian M, McGinnis C, et al. Overview of enteral nutrition. In: Gotschlich MM, DeLegge MH, Mattox T, et al, eds. The ASPEN nutrition support core curriculum: a case-based approach—the adult patient. Silver Spring, MD: ASPEN, 2007.
27 Masden H, Frankel EH. The hitchhiker’s guide to parenteral nutrition management for adult patients. Prac Gastroenterol. 2006;30(7):46–68.
28 Enteral Nutrition Practice Recommendations Task Force. Enteral nutrition practice recommendations. J Parenter Enteral Nutr. 2009;33:122.
29 Arora G, Lukens FJ. Percutaneous endoscopic gastrostomy (PEG) tube placement. eMedicine on WebMD. Available at http://emedicine.medscape.com/article/149665-overview, Updated January 2010. accessed February 2011.
30 Yantis MA, Velander R. How to recognise and respond to refeeding syndrome. Nursing. 2008;38:34.
31 Total parenteral nutrition. Merck manual. Available at www.merckmanuals.com/professional/sec01/ch003/ch003c.html. accessed February 2011.
32 Zingg W, Cartier-Fässler V, Walder B. Central venous catheter associated infections. Best Pract Res Clin Anaesthesiol. 2008;22:407.
33 American Dietetic Association. Position of the American Dietetic Association: nutrition intervention in the treatment of anorexia nervosa, bulimia nervosa, and eating disorders not otherwise specified. J Am Diet Assoc. 2006;106(12):2073–2082.
34 Gonzalez A, Kohn MR, Clarke SD. Eating disorders in adolescents. Aust Fam Physician. 2007;36:614.
35 Department of Health and Ageing. National Public Health Partnership. Strategic Inter-Governmental Nutrition Alliance. National Aboriginal and Torres Strait Islander nutrition strategy and action plan, 2000–2010. Canberra: Department of Health and Ageing, 2001.
36 National Health and Medical Research Council. Nutrition in Aboriginal and Torres Strait Islander peoples. An information paper. Available at www.nhmrc.gov.au/publications/_files/n26.pdf, 2000. accessed February 2011.
37 Australian Bureau of Statistics & Australian Institute of Health and Welfare. The health and welfare of Australia’s Aboriginal and Torres Strait Islander Peoples, 2005. Cat. no. 4074.0. Available at www.abs.gov.au/ausstats/abs@.nsf/7884593a92027766ca2568b5007b8617/476d8c4ec4e041c9ca256888002a4904!Open Document. accessed February 2011.
38 Brimblecome JK, O’Dea K. The role of energy costs in food choices for an Aboriginal population in northern Australia. Med J Aust. 2009;190(10):549–551.
39 Lee AJ, Leonard D, Moloney AA, Minniecon DL. Economic interventions to improve access to healthy food. Med J Aust. 2009;190(10):547–548.
40 New Zealand Ministry of Health. A portrait of health. Key results of the 2006/2007 New Zealand Health survey. Available at www.moh.govt.nz/moh.nsf/indexmh/portrait-of-health. accessed February 2011.
Australasian Society for Parenteral and Enteral Nutrition. www.auspen.org.au
Diabetes Australia. www.diabetesaustralia.com.au
Dietitians Association of Australia. www.daa.asn.au
Dietitians New Zealand. www.dietitians.org.nz
Food Standards Australia New Zealand (FSANZ). www.foodstandards.gov.au
Gastrostomy Information and Support Society. www.giss.org.au
National Health and Medical Research Council. www.nhmrc.gov.au
National Heart Foundation of Australia. www.heartfoundation.org.au
New Zealand Food Safety Authority. www.nzfsa.govt.nz
Nutrition Australia. www.nutritionaustralia.org
University of Sydney Glycemic Index. www.glycemicindex.com