Chapter 33 NURSING MANAGEMENT: coronary artery disease and acute coronary syndrome
1. Describe the aetiology and pathophysiology of coronary artery disease, angina and acute coronary syndrome.
2. Identify risk factors for coronary artery disease and the nursing role in the promotion of therapeutic lifestyle changes in patients at risk.
3. Compare and contrast the precipitating factors, clinical manifestations, multidisciplinary care and nursing management of the patient with coronary artery disease and chronic stable angina.
4. Describe the clinical manifestations, complications, diagnostic study results and multidisciplinary care of the patient with acute coronary syndrome.
5. Describe the pathophysiology of myocardial infarction from the onset of injury through the healing process.
6. Identify commonly used drug therapy in treating patients with coronary artery disease and acute coronary syndrome.
7. Identify key issues to include in the rehabilitation of patients recovering from acute coronary syndrome and coronary revascularisation procedures.
8. Describe the precipitating factors, clinical presentation and multidisciplinary care of patients who are at risk of or have experienced sudden cardiac death.
CORONARY ARTERY DISEASE
Cardiovascular disease is the major cause of death in Australia and New Zealand (see Fig 33-1). Coronary artery disease (CAD) is the most common type of cardiovascular disease,1,2 and although the mortality rate from CAD has decreased by 60% in the last few decades due to advances in prevention, assessment and treatment, it remains the leading cause of all cardiovascular disease deaths and thus deaths in general. Patients with CAD can be asymptomatic or develop chronic stable angina. Unstable angina (UA) and myocardial infarction (MI) are more serious manifestations of CAD and are termed acute coronary syndrome (ACS). The Australian Institute of Health & Welfare estimates that 684,800 Australians have CAD;1 and each day approximately 17 New Zealanders die as the result of coronary heart disease—it is the leading single cause of death in New Zealand, accounting for almost one-quarter of all deaths.2
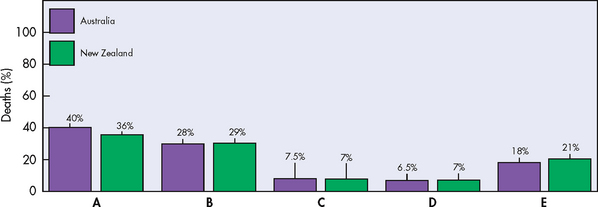
Figure 33-1 Leading causes of death for men and women. A, Total cardiovascular diseases. B, Cancer. C, Respiratory disease. D, Accidents and poisoning. E, All other.
Multiple causal factors contribute to CAD. A number of modifiable risk factors contribute to around 90% of the risk of myocardial infarction observed worldwide:3 blood lipid abnormalities, smoking, hypertension, diabetes mellitus, abdominal obesity, psychosocial factors, physical inactivity and inadequate intake of fruits and vegetables. Given that cardiovascular disease is largely preventable, Australian and New Zealand primary care guidelines2,3 emphasise comprehensive risk assessment to enable the effective management of identified risk factors through lifestyle changes (e.g. weight management, smoking cessation and increasing physical activity) and pharmacological therapy (e.g. anti-platelet agents, blood pressure-lowering agents and lipid-modifying agents).
AETIOLOGY AND PATHOPHYSIOLOGY
Coronary artery disease is a type of blood vessel disorder that is included in the general category of atherosclerosis. The term atherosclerosis is derived from two Greek words: athere, meaning ‘fatty mush’, and skleros, meaning ‘hard’. This combination indicates that atherosclerosis begins as soft deposits of fat that harden with age. Atherosclerosis is often referred to as ‘hardening of the arteries’. Although this condition can occur in any artery in the body, the atheromas (fatty deposits) have a preference for the coronary arteries. Arteriosclerotic heart disease, cardiovascular heart disease, ischaemic heart disease, coronary heart disease and CAD are all terms used to describe this disease process.
Atherosclerosis is the major cause of CAD. It is characterised by a focal deposit of cholesterol and lipids, primarily within the intimal wall of the artery. The genesis of plaque formation is the result of complex interactions between the components of the blood and the elements forming the vascular wall.4,5 Inflammation and endothelial injury play a central role in the development of atherosclerosis.
Intact normal endothelium is more than a simple barrier between the vessel wall and the lumen of the vessel. Normally, it is non-reactive to platelets and leucocytes, as well as coagulation, fibrinolytic and complement factors. However, the endothelial lining can be injured as a result of tobacco use, hyperlipidaemia, hypertension, diabetes mellitus, hyperhomocysteinaemia and infection (e.g. Chlamydia pneumoniae, herpes) causing a local inflammatory response (see Fig 33-2, A).4,5
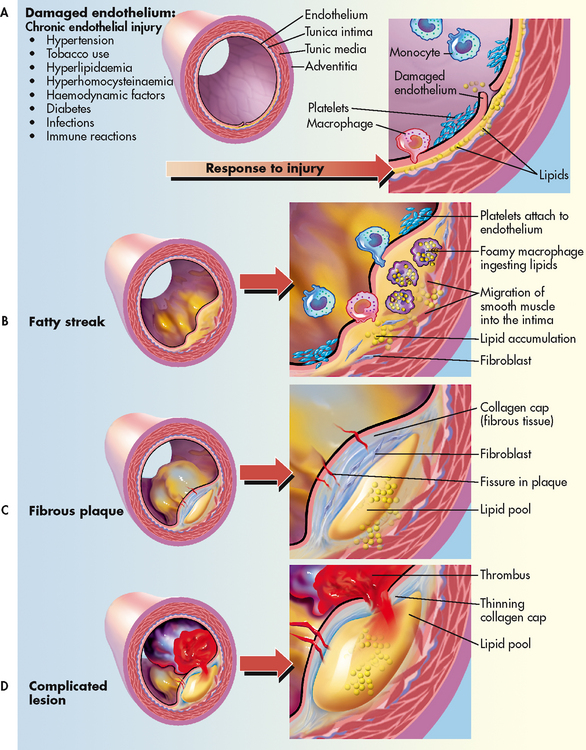
Figure 33-2 Progression of atherosclerosis. A, Damaged endothelium. B, Diagram of fatty streak and lipid core formation. C, Diagram of fibrous plaque. Raised plaques are visible: some are yellow; others are white. D, Diagram of complicated lesion; thrombus is red; collagen is blue. Plaque is complicated by red thrombus deposition.
C-reactive protein (CRP), a non-specific marker of inflammation, is increased in many patients with CAD. Chronic exposure to even minor elevations of CRP can trigger the rupture of plaques and promote the oxidation of low-density lipoprotein (LDL) cholesterol, leading to increased uptake by macrophages in the endothelial lining.6,7
Developmental stages
CAD is a progressive disease that takes many years to develop. When it becomes symptomatic, the disease process is usually well advanced. The stages of development in atherosclerosis are: (1) fatty streak; (2) fibrous plaque resulting from smooth muscle cell proliferation; and (3) complicated lesion.
Fatty streak
Fatty streaks, the earliest lesions of atherosclerosis, are characterised by lipid-filled smooth muscle cells.5 As streaks of fat develop within the smooth muscle cells, a yellow tinge appears. Fatty streaks can be observed in the coronary arteries by age 15 and involve an increasing amount of surface area as the patient ages. It is generally believed that treatment which lowers LDL cholesterol may reverse this process (Fig 33-2, B).4
Fibrous plaque
The fibrous plaque stage is the beginning of progressive changes in the endothelium of the arterial wall. These changes can appear in the coronary arteries by age 30 and increase with age.
Normally the endothelium repairs itself immediately, but in the person with CAD the endothelium is not rapidly replaced, allowing LDLs and growth factors from platelets to stimulate smooth muscle proliferation and thickening of the arterial wall. Once endothelial injury has occurred, lipoproteins (carrier proteins within the bloodstream) transport cholesterol and other lipids into the arterial intima. The fatty streak is eventually covered by collagen, forming a fibrous plaque that appears greyish or whitish.4,5 These plaques can form on one portion of the artery or in a circular fashion involving the entire lumen. The borders can be smooth or irregular with rough, jagged edges.5 The result is a narrowing of the vessel lumen and a reduction in blood flow to the distal tissues (see Fig 33-2, C).
Complicated lesion
The final stage in the development of the atherosclerotic lesion is the most dangerous. As the fibrous plaque grows, continued inflammation can result in plaque instability, ulceration and rupture.5 Once the integrity of the artery’s inner wall has become compromised, platelets accumulate in large numbers, leading to a thrombus. The thrombus may adhere to the wall of the artery, leading to further narrowing or total occlusion of the artery. Activation of the exposed platelets causes expression of glycoprotein IIb/IIIa receptors that bind fibrinogen. This, in turn, leads to further platelet aggregation and adhesion, further enlarging the thrombus. At this stage, the plaque is referred to as a complicated lesion (Fig 33-2, D).5
Collateral circulation
Normally some arterial anastomoses or connections, termed collateral circulation, exist within the coronary circulation. The growth and extent of collateral circulation are attributed to two factors: (1) the inherited predisposition to develop new blood vessels (angiogenesis); and (2) the presence of chronic ischaemia. When an atherosclerotic plaque occludes the normal flow of blood through a coronary artery and the resulting ischaemia is chronic, increased collateral circulation develops (see Fig 33-3). When occlusion of the coronary arteries occurs slowly over a long period, there is a greater chance of adequate collateral circulation developing and the myocardium may still receive an adequate amount of blood and oxygen. However, with rapid-onset CAD (e.g. familial hypercholesterolaemia) or coronary spasm, the time is inadequate for collateral development and a diminished arterial flow results in a more severe ischaemia or infarction.
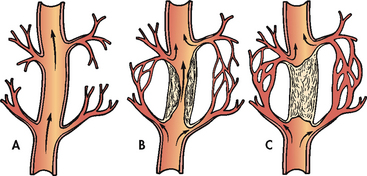
Figure 33-3 Vessel occlusion with collateral circulation. A, Open, functioning coronary artery. B, Partial coronary artery closure with collateral circulation being established. C, Total coronary artery occlusion with collateral circulation bypassing the occlusion to supply blood to the myocardium.
CAD develops over many years and clinical manifestations will not be apparent in the early stages of the disease. Therefore, it becomes extremely important to identify people at risk so that therapeutic lifestyle changes and some treatment strategies can be initiated early.
Risk factors for coronary artery disease
Many risk factors have been associated with CAD. Risk factors in different populations may vary. For example, major risk factors for CAD in Australia and New Zealand, such as high serum cholesterol and hypertension, are more prevalent in Indigenous Australians, Māori, Pacific Islander people and those from the Indian subcontinent.1–3
Risk factors can be categorised as non-modifiable and modifiable (see Table 33-1). Non-modifiable risk factors are age, gender, ethnicity, family history and genetic inheritance. Modifiable risk factors include elevated serum lipids, hypertension, tobacco use, physical inactivity, obesity, diabetes mellitus, metabolic syndrome, psychological states and homocysteine levels.8,9
TABLE 33-1 Risk factors for CAD

HDL, high-density lipoprotein; LDL, low-density lipoprotein
Wellington: New Zealand Guidelines Group; 2009. Australian National
Vascular Disease Prevention Alliance. Guidelines for the assessment of absolute cardiovascular disease risk. 2009.
* Three or more of these risk factors meet the criteria for metabolic syndrome as defined by the national heart lung and blood Institute and American heart Association.
** Alcohol is a risk factor for elevated blood pressure (which itself is a major determinant of risk for atherosclerotic disease), stroke and cardiomyopathy.
Source: Adapted from New Zealand Ministry of health (NZMOH)/New Zealand Guidelines Group. New Zealand cardiovascular guidelines handbook: a summary resource for primary care practitioners. 2nd edn.
Data on risk factors have been obtained in several major studies. In the Framingham study (one of the most widely known), 5209 men and women were observed for 20 years. Over time, it was noted that elevated serum cholesterol (>6.4 mmol/L), elevated systolic blood pressure (BP) (>160 mmHg) and tobacco use (one or more packs per day) were positively correlated with an increased incidence of CAD.2,3 (See Figs 33-4 and 33-5 and Table 33-2 for information about risk assessment.)
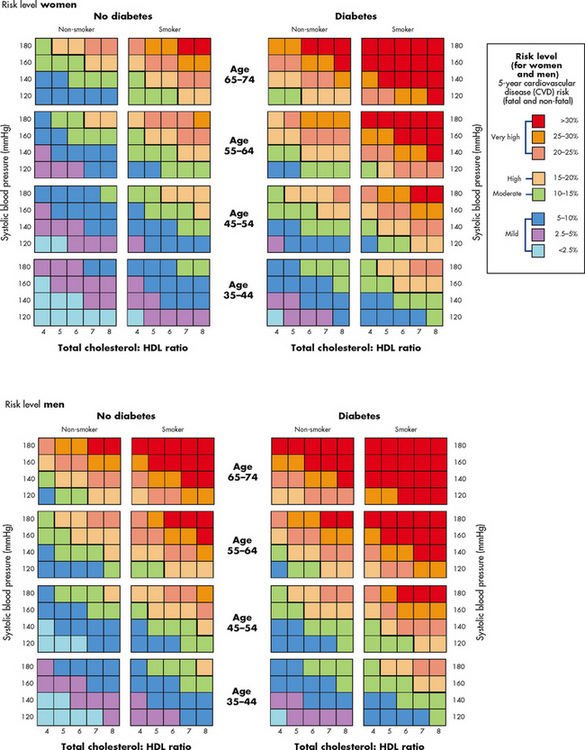
Figure 33-4 New Zealand cardiovascular risk assessment levels.
Source: New Zealand Guidelines Group. www.nzgg.org.nz/guidelines/0154/CVD_handbook_june_2009_update.pdf.
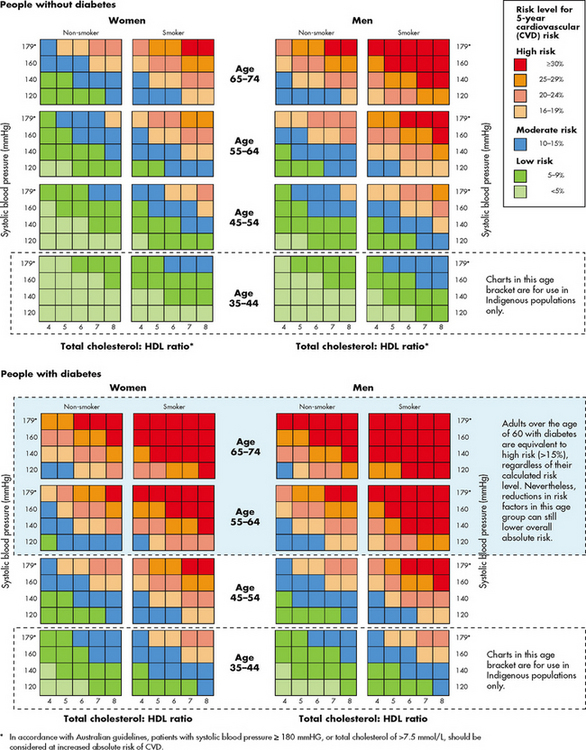
Figure 33-5 Australian cardiovascular risk assessment levels.
Source: Heart Foundation. www.heartfoundation.org.au/SiteCollectionDocu-ments/A_AR_QRG_FINAL%20FOR%20WEB.pdf.
TABLE 33-2 Recommended age to start cardiovascular disease and diabetes risk assessment
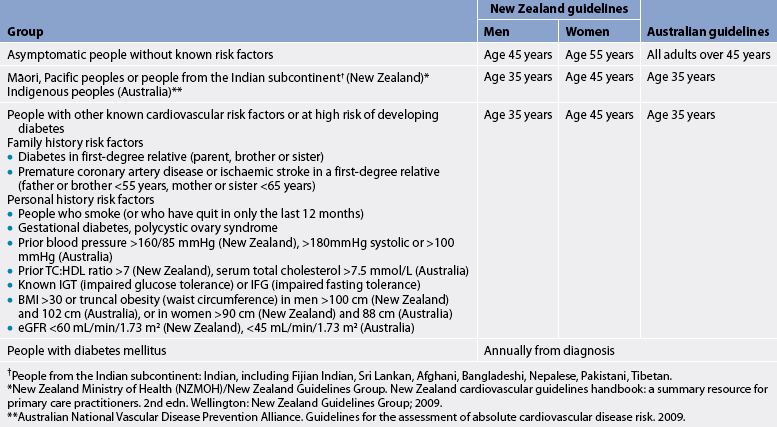
* New Zealand Ministry of Health (NZMOH)/New Zealand Guidelines Group. New Zealand cardiovascular guidelines handbook: a summary resource for primary care practitioners. 2nd edn. Wellington: New Zealand Guidelines Group; 2009.
** Australian National Vascular disease Prevention Alliance. Guidelines for the assessment of absolute cardiovascular disease risk. 2009.
† People from the indian subcontinent: indian, including Fijian Indian, Sri lankan, Afghani, Bangladeshi, Nepalese, Pakistani, Tibetan.
Recently, it has been shown that the degree of coronary artery calcification correlates with the severity of CAD.10 Calcification can be detected by non-invasive means such as electron beam computed tomography (EBCT) (see Ch 32). Measurement of coronary calcification may be useful for predicting adverse cardiovascular events. However, many issues need to be resolved before coronary calcium screening is routinely used in clinical practice. These issues include: (1) optimal coronary calcium thresholds, which can vary according to age, gender and ethnicity; and (2) the cost-effectiveness of using this measure to improve clinical outcomes.
Although LDL cholesterol has been the focal point of risk determination for CAD, many patients who have an MI have normal LDL cholesterol. There has been recent interest in lipoprotein-associated phospholipase A2 (Lp-PLA2), an enzyme produced by macrophages that promotes vascular inflammation. Elevations in Lp-PLA2 have been associated with an increased risk of CAD. Lp-PLA2 levels can be evaluated in conjunction with LDL cholesterol levels and may be ordered as part of the overall clinical evaluation of a patient’s risk of developing CAD. The PLAC test can aid in determining whether a patient with non-elevated LDL cholesterol may be at increased risk of developing CAD. This blood test can be included as part of a normal clinical evaluation to better determine a patient’s risk of developing CAD.
NON-MODIFIABLE RISK FACTORS
Age, gender and ethnicity
The incidence of CAD is almost twice as high among men than women in Australia and New Zealand.1,2 After 65 years, the incidence in men and women equalises, although cardiovascular disease causes more deaths in women than men.1,2,11 Additionally, CAD is present in Australian Indigenous women at rates higher than their non-Indigenous counterparts.1 (See Table 33-2 for the recommended age to start cardiovascular disease and diabetes risk assessment.)
Heart disease kills almost 10 times more women than breast cancer. Even though cardiovascular disease remains the leading cause of death in women and the mortality rate for women with CAD has remained relatively constant in recent years, just 15% of women consider CAD their greatest health risk.11 It is only recently that there has been research focusing on the manifestations and course of CAD in women. Women tend to manifest CAD 10 years later in life than men. This is thought to be related to the loss of the cardioprotective effects of natural oestrogen with the onset of menopause. Most women have symptoms of angina rather than MI when presenting with their initial cardiac event (see the Health disparities box on CAD).
Family history and genetics
Genetic predisposition is an important factor in the occurrence of CAD, although the exact mechanism of inheritance is not fully understood. Some congenital defects in coronary artery walls predispose the person to the formation of plaques. Familial hypercholesterolaemia, an autosomal dominant disorder, has been strongly associated with CAD at early ages (see the Health disparities box on familial hypercholesterolaemia). In most cases, patients presenting with angina or MI can name a parent or sibling who has died of CAD.
HEALTH DISPARITIES
Women
• CAD causes more deaths in women than in men.
• Initial cardiac event for women is more often angina than MI.
• Women with the long QT syndrome have an increased incidence of sudden cardiac death compared to men with the same disorder.
• Before menopause, women have higher HDL cholesterol levels and lower LDL cholesterol levels than men.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; MI, myocardial infarction.
MODIFIABLE MAJOR RISK FACTORS
Elevated serum lipids
An elevated serum lipid level is one of the four most firmly established risk factors for CAD.3,9,12 The various types of serum lipids are presented in Figure 33-6. The risk of CAD is associated with a serum cholesterol level of more than 5.2 mmol/L or a fasting triglyceride level of more than 3.7 mmol/L. (See Table 31-7 for normal serum lipid values.)
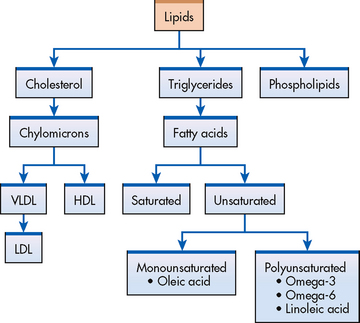
Figure 33-6 Types of serum lipids. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
For lipids to be used and transported by the body, they must become soluble in blood by combining with proteins. Lipids combine with proteins to form lipoproteins. Lipoproteins are vehicles for fat mobilisation and transport. The different types of lipoproteins vary in composition and are classified as high-density lipoproteins (HDLs), LDLs and very-low-density lipoproteins (VLDLs). HDLs contain more protein by weight and fewer lipids than any other lipoprotein. HDLs carry lipids away from arteries and to the liver for metabolism (see Fig 33-7). Therefore high serum HDL levels are desirable and low HDL levels are considered a risk factor for the development of CAD. This process of HDL transport prevents lipid accumulation within the arterial walls. The higher the HDL levels in the blood, the lower the risk of CAD.
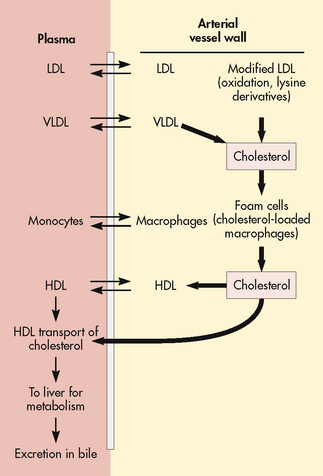
Figure 33-7 Specific types of serum lipoproteins (LDL and VLDL) deliver cholesterol to cells of the blood vessel wall, mostly to form macrophages that become cholesterol foam cells. These are predominant early features of atherosclerotic lesions. HDL is an important cholesterol-transporting carrier, delivering cholesterol to the liver to be excreted in the bile. HDL, high-density lipoprotein; LDL, low-density lipoprotein; VLDL, very-low-density lipoprotein.
There are two types of HDLs: HDL2 and HDL3. They are differentiated by their density and apoprotein composition. Apoproteins are found on lipoproteins and activate enzymes or receptors sites that promote the removal of fat from plasma. Several types of apoproteins exist (e.g. apoprotein A-1, apoprotein A-2, apoprotein B-100, apoprotein C-1, apoprotein E-2). Women produce more apoprotein A-1 than men and premenopausal women have HDL2 levels approximately three times greater than men. This is thought to be related to the protective effects of natural oestrogen. After menopause, their HDL2 levels quickly approximate those of men.
HDL levels can be increased by physical activity, moderate alcohol consumption and oestrogen administration. In general, HDL levels are higher in children and women, decrease with age and are low in persons with CAD. Current research on drug and dietary therapy is focused on strategies to increase HDL levels.8
LDLs contain more cholesterol than any of the other lipoproteins and have an affinity for arterial walls.3,12,13 VLDLs contain both cholesterol and triglycerides and are also thought to deposit cholesterol directly on the walls of arteries. Elevated LDL levels correlate most closely with an increased incidence of atherosclerosis and CAD. Therefore, low serum LDL levels are desirable.14,15
Certain diseases (e.g. type 2 diabetes mellitus, chronic renal failure), drugs (e.g. corticosteroids, hormone replacement therapy) and genetic disorders have been associated with elevated triglyceride levels. Lifestyle factors that can contribute to elevated triglycerides include high alcohol consumption, high intake of refined carbohydrates and simple sugars, and physical inactivity.10,14 When a high triglyceride level is combined with a high LDL level, a smaller denser LDL particle is formed which favours deposition on arterial walls. This pattern is often found in people with insulin resistance.
Lipid metabolism is not completely understood and there is increased interest in the role of the various components of lipoproteins, such as the apoproteins, in the development of CAD.16 A more sophisticated serum screening test can be used to diagnose various lipid disorders involving these other components.17
The current national guidelines for treating elevated LDL cholesterol are based on a person’s 10-year risk of having a non-fatal MI or dying from a coronary event and LDL levels. Risk scores are calculated based on information about the following: (1) age; (2) gender; (3) use of tobacco; (4) systolic BP; (5) use of BP medications; (6) total cholesterol; and (7) HDL cholesterol level.9,12 In general, persons with only 0–1 risk factors are considered low risk for the development of CAD and the LDL goal is less than 4.14 mmol/L. Persons at very high risk have CAD and multiple risk factors. The LDL goal for this group is less than 1.8 mmol/L.12,14,16 Treatment goals and strategies are summarised in Table 33-3.
TABLE 33-3 Treatment decisions for high blood cholesterol based on low-density lipoprotein levels
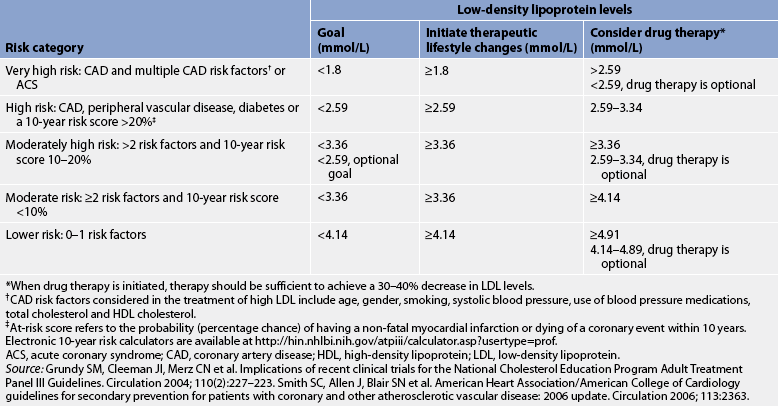
Electronic 10-year risk calculators are available at http://hin.nhlbi.nih.gov/atpiii/calculator.asp?usertype=prof.
ACS, acute coronary syndrome; CAD, coronary artery disease; hDl, high-density lipoprotein; lDl, low-density lipoprotein.
* When drug therapy is initiated, therapy should be sufficient to achieve a 30–40% decrease in LDL levels.
† CAD risk factors considered in the treatment of high LDL include age, gender, smoking, systolic blood pressure, use of blood pressure medications, total cholesterol and HDL cholesterol.
‡ At-risk score refers to the probability (percentage chance) of having a non-fatal myocardial infarction or dying of a coronary event within 10 years.
Source: grundy SM, Cleeman JI, Merz CN et al. Implications of recent clinical trials for the national Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation 2004; 110(2):227–223. Smith SC, Allen J, Blair SN et al. American Heart Association/American College of Cardiology guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation 2006; 113:2363.
Hypertension
The second major risk factor in CAD is hypertension, which is defined as a BP over 140/90 mmHg. Hypertension has been identified as a major risk factor for heart disease beginning with the Framingham study.13 In postmenopausal women, hypertension is associated with a higher incidence of CAD than in men and premenopausal women. Hypertension increases the risk of death from CAD tenfold in all persons.
In 2003, the Heart Foundations of New Zealand and Australia redefined normal BP as below 120 mmHg/80 mmHg, stage 1 hypertension as 140–159 mmHg/90–99 mmHg and stage 2 hypertension as BP over 160 mmHg/100 mmHg.17 The cause of hypertension in 90% of those affected is unknown, but it is usually controllable with diet and/or drugs. Those with stage 1 or 2 hypertension often require more than one drug to reach therapeutic goals (see Table 32-5).17
The stress of a constantly elevated BP increases the rate of atherosclerotic development. This is related to the shearing stress that causes endothelial injury. Atherosclerosis, in turn, causes narrowed, thickened arterial walls and decreases the distensibility and elasticity of vessels. More force is required to pump blood through diseased arterial vasculature and this increased force is reflected in a higher BP. This increased workload is also manifested by left ventricular hypertrophy and decreased stroke volume with each contraction. Salt intake is positively correlated with elevated BP, adding volume and increasing systemic vascular resistance (SVR) to the cardiac workload. (See Ch 32 for a complete discussion of hypertension.)
Tobacco use
A third major risk factor in CAD is tobacco use. The risk of developing CAD is two to six times higher in those who smoke tobacco than in those who do not. Furthermore, tobacco smoking has been linked to a decrease in oestrogen levels, placing premenopausal women at greater risk of CAD. Risk is proportional to the number of cigarettes smoked. Changing to lower-nicotine or filtered cigarettes does not affect risk.
Studies have shown strong evidence that chronic exposure to environmental tobacco (second-hand) smoke also increases the risk of CAD.18 Pipe and cigar smokers, who often do not inhale, have been found to have an increased risk of CAD similar to those exposed to environmental tobacco smoke.
Nicotine in tobacco smoke causes catecholamine (adrenaline and noradrenaline) release. These hormones cause an increased heart rate (HR), peripheral vasoconstriction and increased BP. These changes increase the cardiac workload, necessitating greater myocardial oxygen consumption. Nicotine also increases platelet adhesion, which increases the risk of emboli formation.10,11
Carbon monoxide, a by-product of combustion found in tobacco smoke, affects the oxygen-carrying capacity of haemoglobin by reducing the sites available for oxygen transport. Thus the effects of an increased cardiac workload, combined with the oxygen-depleting effect of carbon monoxide, significantly decrease the oxygen available to the myocardium. There is also some indication that carbon monoxide may be a chemical irritant, thus causing injury to the endothelium.10,13
The benefits of smoking cessation are dramatic and almost immediate: CAD mortality rates drop to those of non-smokers within 12 months.10,13 However, nicotine is highly addictive and often calls for intensive intervention to assist people to quit. In one study, researchers found that time spent with follow-up calls and home counselling increased the chances of quitting over standard care of 30 minutes counselling and educational pamphlets. In addition, it was found that the use of nicotine replacement therapy contributed to successful quitting. Barriers to successful quitting included multiple relapses, stress, weight gain, lack of support and depression.19 (See Ch 10 for information on smoking cessation.)
Physical inactivity
Physical inactivity is the fourth major modifiable risk factor in CAD. Physical inactivity implies a lack of adequate physical exercise on a regular basis. Current recommendations for health-promoting regular physical exercise are defined as brisk walking that occurs at least five or more times a week for at least 30 minutes, causing perspiration and an increase in HR by 30–50 beats per minute.10
The mechanism by which physical inactivity predisposes to CAD is mostly still unknown. Physically active people have increased HDL levels and exercise enhances fibrinolytic activity, thus reducing the risk of clot formation. It is also believed that exercise encourages the development of collateral circulation.
Exercise training for those who are physically inactive decreases the risk of CAD through more efficient lipid metabolism, increased HDL2 production and more efficient oxygen extraction by the working muscle groups, thereby decreasing the cardiac workload. Physically active persons are seldom obese and can achieve a 5–10 mmHg drop in their BP, thus reducing three risk factors in CAD.10
Obesity
The mortality rate from CAD is statistically higher in obese individuals. Obesity is defined as a body mass index (BMI) over 30. BMI is a calculation of body fat based on height and weight (see Chs 39 and 40). The increased risk of CAD is proportional to the degree of obesity. Obese persons are thought to produce increased levels of LDL and triglycerides, which are strongly implicated in atherosclerosis. Obesity is often associated with hypertension, which is three times more likely to develop in an obese person than in a person of normal weight. There is also evidence that individuals who tend to store fat in the abdomen (an ‘apple’ figure) rather than in the hips and buttocks (a ‘pear’ figure) have a higher incidence of CAD.1,10,14 As obesity increases, the heart size grows, causing increased myocardial oxygen consumption. In addition, there is an increase in insulin resistance in obese individuals.20
MODIFIABLE CONTRIBUTING RISK FACTORS
Diabetes mellitus
The incidence of CAD is two to four times greater among persons who have diabetes mellitus, even those with well-controlled blood glucose levels, than in the general population. The patient with diabetes manifests CAD not only more frequently but also at an earlier age. There is no age difference between male or female patients with diabetes in the onset of manifestations of CAD. Diabetes virtually eliminates the lower incidence of CAD in premenopausal women. Diabetic women have a five to seven times higher risk of CAD than non-diabetic women.10,20,21 Undiagnosed diabetes is frequently discovered at the time of MI. Because the person with diabetes has an increased tendency towards connective tissue degeneration and endothelial dysfunction, it is thought that this condition may account for the tendency towards atheroma development seen in the patient with diabetes. Diabetic patients also have alterations in lipid metabolism and tend to have high cholesterol and triglyceride levels.10,20,21
Metabolic syndrome
Metabolic syndrome refers to a cluster of risk factors for CAD whose underlying pathophysiology is thought to be related to insulin resistance. These risk factors include obesity as defined by increased waist circumference, elevated triglycerides, hypertension, abnormal serum lipids and an elevated fasting blood glucose.22,23 These multiple, interrelated risk factors of metabolic origin appear to promote the development of CAD.
Psychological states
The Framingham study provided early evidence that certain behaviours and lifestyles are conducive to the development of CAD.24,25 Several behaviour patterns have been correlated with CAD. However, the study of these behaviours remains controversial and complex. One type of behaviour, referred to as type A, includes perfectionism and a hardworking, driven personality. The type A person often suppresses anger and hostility, has a sense of time urgency, is impatient and often creates stress and tension. This person is more prone to MIs than a type B person, who is more easy going, takes upsets in their stride, knows their personal limitations, takes time to relax and is not an overachiever. Meta-analysis of the type A personality studies has shown that the studies that demonstrated a positive correlation between type A personality and CAD were equal in number to the studies that failed to show a correlation with CAD.24,25
Studies are now focusing on specific negative psychological or behavioural states thought to increase risk of CAD including depression, hopelessness, anxiety, hostility and anger.10,26,27 In one review, depression was supported as a risk factor for both the development and worsening of CAD.28 Depressed patients have elevated levels of circulating catecholamines that may contribute to endothelial injury and inflammation and platelet activation.29 More research on the treatment of depression and other negative psychological states in patients with or at risk of CAD is needed to improve the emotional and physical health of these patients.27
Stressful states have also been correlated with the development of CAD. Sympathetic nervous system (SNS) stimulation and its effect on the heart are generally considered to be the physiological mechanism by which stress predisposes to the development of CAD. SNS stimulation causes an increased release of catecholamines (i.e. adrenaline, noradrenaline). This stimulation increases the HR and intensifies the force of myocardial contraction, resulting in increased myocardial oxygen demand. Also, stress-induced mechanisms can cause elevated lipid and glucose levels and alterations in blood coagulation, which can lead to increased atherogenesis.10
Homocysteine
High blood levels of homocysteine have been linked to an increased risk of CAD and other cardiovascular diseases.30,31 Homocysteine, a sulphur-containing amino acid, is produced by the breakdown of the essential amino acid methionine, which is found in dietary protein. High homocysteine levels (>12–15 μmol/L) possibly contribute to atherosclerosis by: (1) damaging the inner lining of blood vessels; (2) promoting plaque build-up; and (3) altering the clotting mechanism to make clots more likely to occur.
Research is ongoing to determine whether a decline in homocysteine can reduce the risk of heart disease.30 B-complex vitamins (B6, B12, folic acid) have been shown to lower blood levels of homocysteine. Generally, a screening test for homocysteine is not recommended, but is limited to those suspected of having elevated levels such as older patients with pernicious anaemia or people who develop CAD at an early age.
 NURSING AND COLLABORATIVE MANAGEMENT: CORONARY ARTERY DISEASE
NURSING AND COLLABORATIVE MANAGEMENT: CORONARY ARTERY DISEASE
 Health promotion
Health promotion
The appropriate management of risk factors in CAD may prevent, modify or retard the progression of the disease. In Australia and New Zealand during the past 30 years there has been a gradual and persistent decline in coronary deaths, especially in men. The decline can be attributed to the efforts of people to become generally healthier as well as advances in pharmacology and technology to treat CAD. Prevention and early treatment of heart disease must involve a multifactorial approach and need to be ongoing throughout the life span.
 Identification of high-risk persons
Identification of high-risk persons
Regardless of the healthcare setting, nurses are well suited to identify those at risk of CAD. Risk screening involves obtaining personal and family health histories. The patient should be questioned about a family history of heart disease in parents and siblings. The presence of any cardiovascular symptoms should be noted (see Table 32-7). Environmental factors, such as eating habits, type of diet and level of exercise, are assessed to elicit lifestyle patterns. A psychosocial history is included to determine tobacco use, alcohol ingestion, type A behaviours, recent life-stressing events and the presence of any negative psychological states (e.g. anxiety, depression, hopelessness). The place of work and the type of work can provide important information on the kind of activity performed, exposure to pollutants or noxious chemicals and the degree of emotional stress associated with employment.
HEALTH PROMOTION
• Achieve and maintain a healthy weight
• Reduce salt and sodium intake
• Increase level of physical exercise
• Avoid use of all tobacco products
• Limit alcohol intake to small-to-moderate amounts (570 mL beer, 250 mL wine, 60 mL spirits)
• Choose a diet that is low in dietary cholesterol, and total and saturated fat, and high in fruits and vegetables
The nurse should identify the patient’s attitudes and beliefs about health and illness. This information can give some indication of how disease and lifestyle changes may affect the patient and can reveal possible misconceptions about heart disease. Knowledge of the patient’s educational background is helpful in deciding at what level to begin teaching. If the patient is taking medications, it is important to know what they are, when they are taken and what the patient’s compliance and attitudes are regarding the taking of medications.
 Management of high-risk persons
Management of high-risk persons
Once a high-risk person is identified, preventive measures can be taken. Risk factors such as age, gender, ethnicity and genetic inheritance cannot be modified. However, the person with any of these risk factors can still modify the risk of CAD by controlling or changing the additive effects of modifiable risk factors. For example, a young man with a family history of heart disease can decrease his risk of CAD by maintaining an ideal body weight, getting adequate physical exercise, reducing his intake of saturated fats and avoiding tobacco use.
People who have modifiable risk factors should be encouraged and motivated to make lifestyle changes to reduce the risk of CAD. Nurses can play a major role in teaching health-promoting behaviours to those at risk of CAD (see Table 33-4). For highly motivated people, knowing how to reduce this risk may be the only information they need to get started.
TABLE 33-4 Decreasing risk factors for cad
PATIENT & FAMILY TEACHING GUIDE
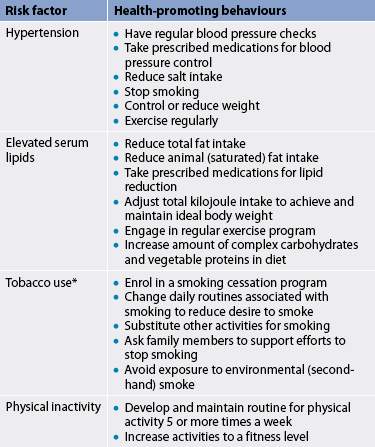
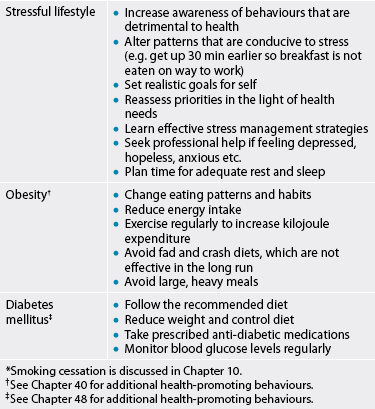
* Smoking cessation is discussed in Chapter 10.
† See Chapter 40 for additional health-promoting behaviours.
‡ See Chapter 48 for additional health-promoting behaviours.
For those who are less motivated to assume responsibility for their health, the idea of risk factor reduction may be so remote that they are unable to perceive a threat of CAD in their life. Few people desire to make lifestyle changes, especially in the absence of symptoms. The nurse should first assist such a person in clarifying personal values. Then, by explaining the risk factors and having the person identify their personal vulnerability to various risks, the nurse may help the person to recognise the susceptibility to CAD. The nurse may also help the person to set realistic goals and choose which risk factor(s) to change first. Some people are reluctant to change until they begin to manifest overt symptoms or actually suffer an MI. Others, having suffered an MI, may find the idea of changing lifelong habits still unacceptable. Nurses must be able to identify such attitudes and respect them.
 Physical activity
Physical activity
A physical activity program should be designed to improve physical fitness by following the FITT formula:
The current recommendation for physical activity is to perform aerobic activity of moderate intensity for at least 30 minutes on 5 or more days a week. Moderate intensity and type can be defined by brisk walking, hiking, cycling and swimming. Many studies have shown the benefit of physical activity in reducing the risk of CAD.10 Regular exercise contributes to weight reduction, reduction of at least 10% in systolic BP and, in some men more than women, an increase in HDL cholesterol.10 Type 2 diabetics experience an improvement in glucose utilisation with exercise leading to better blood glucose control (see Evidence-based practice box).19,20 The Heart Foundations of New Zealand and Australia have programs to encourage people to increase their daily physical activity (see Resources on p 893).
 NUTRITIONAL THERAPY
NUTRITIONAL THERAPY
The Cardiac Society of Australia and New Zealand and the National Heart Foundation of Australia recommend therapeutic lifestyle changes for all people to reduce the risk of CAD by lowering LDL cholesterol. These recommendations provide guidelines that emphasise a decrease in saturated fat and cholesterol intake and an increase in intake of complex carbohydrates (e.g. whole grains, fruit, vegetables).32 (See Tables 33-5 and 33-6.) Fat intake should be about 30% of kilojoule intake, with most coming from monounsaturated fats found in nuts and oils such as olive or canola oil.32 Red meats, eggs and whole milk products are major sources of saturated fat and cholesterol and should be reduced or eliminated from the diet. If the serum triglyceride level is elevated, alcohol intake and intake of simple sugars should be reduced or eliminated.
Therapeutic lifestyle changes diet*
| Nutrient | Recommended intake (% of total daily kilojoules) |
|---|---|
| Total fat† | 25-35% |
| Saturated fat | <7% |
| Polyunsaturated fat | Up to 10% |
| Monounsaturated fat | Up to 20% |
| Carbohydrate | 50-60% |
| Protein | Approximately 15% |
| Cholesterol | <200 mg |
| Sodium | ≤2400 mg |
| Dietary fibre | 20-30 g |
| Total kilojoules‡ | Balance energy intake and expenditure to maintain desirable body weight and prevent weight gain |
Daily food guide available at www.heartfoundation.com.au.
*Therapeutic dietary options for further reduction in low-density lipoproteins include the addition of 2 g per day of plant sterols (e.g. Margarines, nuts, seeds, legumes, vegetable oils and other plant sources) and an additional 10–25 g of soluble dietary fibre.
†This recommendation allows for increased intake of unsaturated fat in place of carbohydrates in people with diabetes mellitus or metabolic syndrome.
‡Daily energy expenditure should include at least moderate physical activity.
TABLE 33-6 Therapeutic lifestyle changes diet menu*
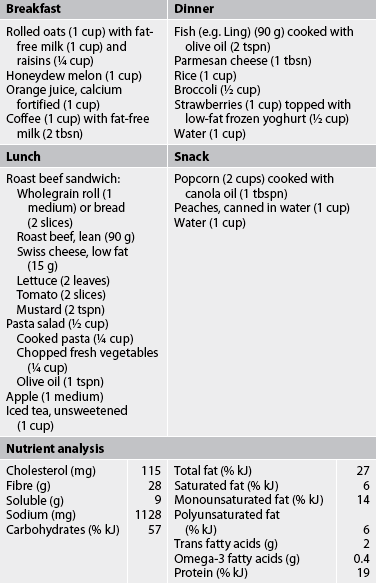
* The sample menu is appropriate for a 25–49-year-old female. No salt is added in recipe preparation or as seasoning. The menu meets or exceeds the Daily reference Intake for nutrients.
Source: Third report of the National Cholesterol Education Program Expert Panel. Detection, evaluation and treatment of high blood cholesterol in adults (Adult Treatment Panel III): final report. National Institutes of health Publication no. 02-5215; 2002. Available at www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf. Gidding SS, Lichtenstein Ah, Faith MS et al. Implementing American Heart Association pediatric and adult nutrition guidelines. Circulation 2009; 119:1161.
Omega-3 fatty acids have been shown to reduce the risks associated with CAD when consumed regularly. For individuals without CAD, the Heart Foundations of Australia and New Zealand recommend eating fatty fish twice a week because fatty fish (e.g. salmon and tuna) contain two types of omega-3 fatty acids: eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Patients with CAD are encouraged to take EPA and DHA supplements with their diet. The Heart Foundations also recommend eating tofu, other forms of soybean, canola, walnut and flaxseed because these products contain alpha-linolenic acid, which becomes omega-3 fatty acid in the body. For more information on the Heart Foundations of Australia and New Zealand nutritional recommendations, see their websites (see Resources on p 893).
Several studies have demonstrated regression in coronary atherosclerosis and reduction in coronary events by lifestyle changes, including a low-saturated-fat diet, avoidance of tobacco and increase in physical activity. Many of these studies included cholesterol-lowering drug therapy as well.12,33,34
 Cholesterol-lowering drug therapy
Cholesterol-lowering drug therapy
An estimated 6.4 million Australians have cholesterol levels over 5.5 mmol/L,1 which equates to about one-quarter of the total population. No equivalent data are readily available for New Zealand, but given the similarity in death rates the incidence is likely to be similar. A complete lipid profile is recommended every 5 years beginning at age 20. Those with a serum cholesterol level greater than 5.5 mmol/L are at high risk of CAD and should be treated. Treatment usually begins with dietary kilojoule restriction (if overweight), decreased dietary fat and cholesterol intake, and exercise instruction. The guidelines for treatment of high cholesterol focus on LDL levels (see Table 33-3). Serum cholesterol levels are reassessed after 6 weeks of diet therapy. If they remain elevated, additional dietary options (see Complementary & alternative therapies box) and drug therapy (see Table 33-7) may be considered.12,14,15,34
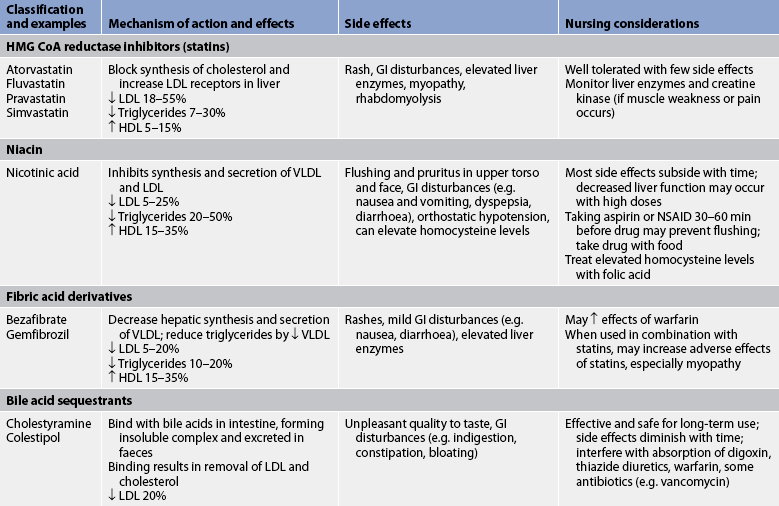
GI, gastrointestinal; HDL, high-density lipoprotein; LDL, low-density lipoprotein; NSAID, non-steroidal anti-inflammatory drug; VLDL, very-low-density lipoprotein.
What is the most effective cardiac rehabilitation program?
EVIDENCE-BASED PRACTICE
Clinical question
In patients with CAD (P), are comprehensive cardiac rehabilitation programs (I) more effective than exercise-only cardiac rehabilitation programs (C) in reducing mortality (O)?
Critical appraisal and synthesis of evidence
• Reduction in all cause mortality
• Reduction in total cardiac mortality
• Despite the reductions in mortality (cardiac and all cause), these programs did not reduce the occurrence of non-fatal myocardial infarction.
Conclusions
• Exercise-based cardiac rehabilitation is effective in reducing cardiac deaths and other causes of mortality in cardiac patients.
• More research is necessary to determine whether exercise only or a comprehensive cardiac rehabilitation intervention is more beneficial.
• A broader sample is needed for subsequent studies that would include more women and is more ethnically diverse.
Implications for nursing practice
• Counsel patients at risk of CAD on the benefits of exercise.
• Include an exercise component in health and wellness programs for patients with CAD and/or those who have had a cardiac event.
P, patient population of interest; I, intervention or area of interest; C, comparison of interest or comparison group; O, outcome(s) of interest.
Natural lipid-lowering agents*
Source: From Ulbricht CE, Basch EM. Natural standard herb and supplement reference: evidence-based clinical reviews. St Louis: Mosby; 2005.
COMPLEMENTARY & ALTERNATIVE THERAPIES
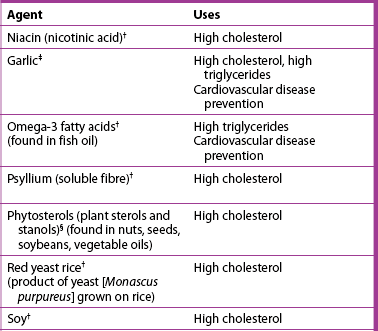
† Strong scientific evidence for its use.
‡ good scientific evidence for its use.
§ Uses based on tradition, theory or limited scientific evidence.
*Cardiovascular disease is a serious health problem. Herbal or other natural therapy should not be initiated without consultation with a healthcare provider. This is especially important when conventional drug therapy for cardiovascular disease is also being used.
 Drugs that restrict lipoprotein production
Drugs that restrict lipoprotein production
The statin drugs are the most widely used and studied lipid-lowering drugs. Examples include pravastatin, simvastatin, fluvastatin and atorvastatin. These drugs inhibit the synthesis of cholesterol in the liver by blocking hydroxy-methyl-glutaryl coenzyme A (HMG-CoA) reductase. An unexplained result of the inhibition of cholesterol synthesis is an increase in hepatic LDL receptors. Consequently, the liver is able to remove more LDLs from the blood. In addition, a small increase in HDLs is also seen with the use of statins.35 Serious adverse effects of these drugs are rare but include liver damage and myopathy that can progress to rhabdomyolysis (breakdown of skeletal muscle). Liver enzymes (e.g. aspartate aminotransferase, alanine aminotransferase) must be monitored regularly and any time dosage is increased. Creatine kinase enzymes are assessed if symptoms of myopathy (e.g. muscle aches, weakness) occur.14,16,35
Niacin (nicotinic acid), a water-soluble B vitamin, is highly effective in lowering LDL and triglyceride levels by interfering with their synthesis. It has been shown to convert patients from a ‘pattern B’, small dense LDL, to a ‘pattern A’, larger, fluffier LDL. These fluffier lipoproteins are less prone to deposition in arterial walls.14,16 Niacin also increases HDL levels better than many other lipid-lowering drugs. Unfortunately, adverse effects of this drug are common and may include severe flushing, pruritus, gastrointestinal (GI) complaints and orthostatic hypotension. Aspirin or a non-steroidal anti-inflammatory drug (NSAID) taken 30–60 minutes before administration may eliminate flushing.
The fibric acid derivatives work by accelerating the elimination of VLDLs and increasing the production of apoproteins A-1 and A-2. They are the most effective drugs for lowering triglycerides and also increasing HDL levels. They have no effect on LDLs. Examples include bezafibrate and gemfibrozil. Although most patients tolerate the drugs well, complaints may include GI irritability. These drugs should be used with caution when combined with statin medications.14,15
 Drugs that increase lipoprotein removal
Drugs that increase lipoprotein removal
The major route of elimination of cholesterol is via conversion to bile acids in the liver. Bile-acid sequestrants increase conversion of cholesterol to bile acids and decrease hepatic cholesterol content. These non-absorbable compounds include cholestyramine and colestipol. The primary effect is a decrease in total cholesterol and LDL.
Administration of these drugs can be associated with complaints related to palatability and a variety of upper and lower GI symptoms, including belching, heartburn, nausea, abdominal pain and constipation. The bile-acid sequestrants have been known to interfere with absorption of other drugs (e.g. warfarin, thiazides, thyroid hormones, β-adrenergic blockers). Separating the time of administration of these drugs from other drugs may decrease this adverse effect.14,15
Drug therapy for hyperlipidaemia is likely to be prolonged, perhaps continuing for a lifetime. It is essential that diet modification be used to minimise the need for drug therapy. The patient must fully understand the rationale and goals of treatment, as well as the safety and side effects of lipid-lowering drug therapy.14,16
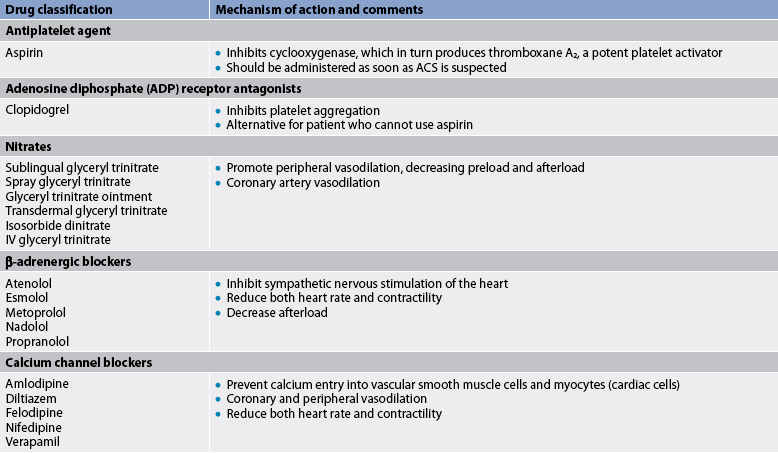
 Antiplatelet therapy
Antiplatelet therapy
Unless contraindicated (e.g. history of GI bleeding), low-dose aspirin is recommended for people at risk of CAD, especially those with a calculated 10-year CAD risk of over 10% (see Table 33-3).1 This recommendation is based on studies that have shown a decrease in first MIs, primarily in men, when taking aspirin.
The Women’s Health Initiative Study demonstrated that women aged 45–65 years do not experience the same benefit from aspirin therapy compared to men. However, the benefit was seen in women over 65 years. Aspirin therapy is not recommended for women with low risk of CAD before 65 years. After 65 years, aspirin is recommended unless contraindicated.36 Common side effects of aspirin include GI upset and bleeding. For people who are aspirin intolerant, clopidogrel can be considered.
Gerontological considerations: coronary artery disease
The incidence of cardiac disease is greatly increased in the elderly and is the leading cause of death in older persons.1 In the older adult, CAD is often a result of the complex interactions of non-modifiable risk factors (e.g. age) and lifelong modifiable risk behaviours (e.g. inactivity, tobacco use). There is evidence that strategies to reduce CAD risk are effective in this age group but are often underprescribed.37
Aggressive treatment of hypertension and hyperlipidaemia will stabilise plaques in the coronary arteries of older adults and cessation of tobacco use helps decrease the risk for CAD at any age.38 Similarly, the older patient should be encouraged to consider a planned exercise program. Activity performance, endurance and ability to tolerate stress can be improved in the older adult with physical training.38 Positive psychological benefits can be derived from a planned exercise program and include increased self-esteem and emotional wellbeing and improved body image. For the older adult who is obese, it is recommended that making modest dietary changes and increasing physical activity (e.g. walking) will result in more positive benefits than aiming for significant weight loss.
When planning an exercise program for older adults, remember the following: (1) longer warm-up periods are needed; (2) longer periods of low-level activity or longer rest periods between sessions are advisable; and (3) heat intolerance may be caused by decreased ability to sweat efficiently. Patients should be taught to avoid exercising in extremes of temperature and to maintain a moderate pace. Older adults should exercise a minimum of 30–40 minutes three or four times a week.
Encouraging older patients to adopt a healthy lifestyle may increase quality of life and reduce the risk of CAD and fatal cardiac events. Older adults face many of the same challenges when it comes to making lifestyle changes. Research has shown that there are two points when the elderly may consider change: when hospitalised and when symptoms (e.g. chest pain) are the result of CAD and not normal ageing.37 The nurse should assess the older adult for readiness to change and then help the patient to select the lifestyle changes most likely to produce the greatest reduction in risk of CAD.
Clinical manifestations
ANGINA
CAD is a progressive disease and patients may be asymptomatic for many years or they may develop chronic stable chest pain syndromes.
AETIOLOGY AND PATHOPHYSIOLOGY
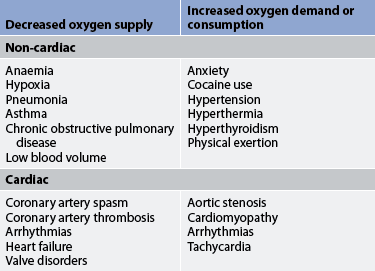
When the demand for myocardial oxygen exceeds the ability of the coronary arteries to supply the heart with oxygen, myocardial ischaemia occurs. Angina, or chest pain, is the clinical manifestation of reversible myocardial ischaemia. Either an increased demand for oxygen or a decreased supply of oxygen can lead to myocardial ischaemia (see Table 33-8). The primary reason for insufficient blood flow is narrowing of coronary arteries by atherosclerosis.38 For ischaemia secondary to atherosclerosis to occur, the artery is usually 75% or more obstructed (stenosed).
On the cellular level, the myocardium becomes hypoxic within the first 10 seconds of coronary occlusion. With total occlusion of the coronary arteries, contractility ceases after several minutes, depriving the myocardial cells of oxygen and glucose for aerobic metabolism. Anaerobic metabolism begins and lactic acid accumulates. Myocardial nerve fibres are irritated by the increased lactic acid and transmit a pain message to the cardiac nerves and upper thoracic posterior nerve roots. This is the reason for referred cardiac pain to the left shoulder and arm. In ischaemic conditions, cardiac cells are viable for approximately 20 minutes. With restoration of blood flow, aerobic metabolism resumes, contractility is restored and cellular repair begins.
CHRONIC STABLE ANGINA
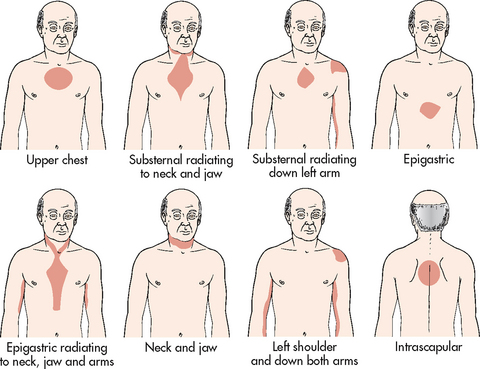
Chronic stable angina refers to chest pain that occurs intermittently over a long period with the same pattern of onset, duration and intensity of symptoms. When questioned (see Table 33-9), some patients may deny feeling pain but will describe a pressure or ache in the chest. It is an unpleasant feeling, often described as a constrictive, squeezing, heavy, choking or suffocating sensation. Angina is rarely sharp or stabbing and it usually does not change with position or breathing. Many people with angina complain of indigestion or burning sensation in the epigastric region. Although most of the pain experienced by people with angina appears substernally, the sensation may occur in the neck or radiate to various locations, including the jaw, shoulders and down the arms (see Fig 33-8). Often people will complain of pain between the shoulder blades and dismiss it as not being related to their heart.
HEALTH DISPARITIES
Women
• Women are older than men when presenting with first MI.
• Once a woman reaches menopause, her risk of an MI quadruples.
• Fewer women than men present with ‘classic’ signs and symptoms of UA or MI.
• Fatigue is often the first symptom of ACS in women.
• Women experience more ‘silent’ MIs compared to men.
• Among those who have an MI, women are more likely than men to suffer a fatal cardiac event within 1 year.
• Women report more disability after a cardiac event than men.
• Women who have coronary artery bypass graft surgery have a higher mortality rate and more complications after surgery than men.
ACS, acute coronary syndrome; CAD, coronary artery disease; MI, myocardial infarction; UA, unstable angina.
The pain usually lasts for only 3–5 minutes and commonly subsides when the precipitating factor is relieved (see Table 33-10). Pain at rest is unusual. An electrocardiogram (ECG) usually reveals transient ST segment depression, indicating ischaemia (see Ch 35).
TABLE 33-10 Precipitating factors of angina
• Increases HR, reducing the time the heart spends in diastole (the time of greatest coronary blood flow), resulting in an increase in myocardial oxygen demand
• Isometric exercise of the arms (e.g. Raking, lifting heavy objects or tree lopping) can cause exertional angina
• Increase workload of the heart
• Blood vessels constrict in response to a cold stimulus
• Blood vessels dilate and blood pools in the skin in response to a hot stimulus
• Stimulate the sympathetic nervous system
• Increase the workload of the heart
• Can increase the workload of the heart
• During the digestive process, blood is diverted to the GI system, reducing blood flow in the coronary arteries
• Nicotine stimulates catecholamine release, causing vasoconstriction and an increased HR
• Diminishes available oxygen by increasing the level of carbon monoxide
• Increases the cardiac workload and sympathetic stimulation
• In a person with CAD, the extra cardiac workload may precipitate angina
Stimulants (e.g. Cocaine, amphetamines)
• Increase HR and subsequent myocardial oxygen demand
• Are related to the occurrence of chronic stable angina, Prinzmetal’s angina, MI and sudden cardiac death
• Manifestations of CAD tend to occur in the early morning after awakening
CAD, coronary artery disease; GI, gastrointestinal; HR, heart rate; MI, myocardial infarction.
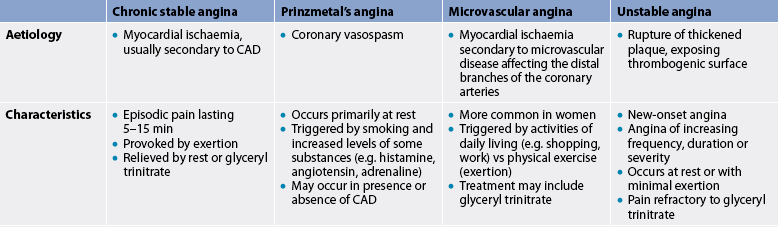
Chronic stable angina can be controlled with medications on an outpatient basis. Because chronic stable angina is often predictable, medications can be timed to provide peak effects during the time of day when angina is likely to occur. For example, if angina occurs when rising, the patient can take medication as soon as they wake up in the morning and wait 30–60 minutes before engaging in activity. (The different types of angina are compared in Table 33-11.)
Silent ischaemia
Silent ischaemia refers to ischaemia that occurs in the absence of any subjective symptoms.39 Patients with diabetes mellitus have an increased prevalence of silent ischaemia, which is thought to be related to diabetic neuropathy affecting the nerves that innervate the cardiovascular system. When patients are monitored (e.g. Holter monitor) and silent ischaemia occurs, ECG changes are revealed. Ischaemia with pain or without pain has the same prognosis.
Nocturnal angina and angina decubitus
Nocturnal angina occurs only at night but not necessarily when the person is in the recumbent position or during sleep. Angina decubitus is chest pain that occurs only while the person is lying down and is usually relieved by standing or sitting.
Prinzmetal’s angina
Prinzmetal’s angina (variant angina) often occurs at rest, usually in response to spasm of a major coronary artery. It is a rare form of angina and is frequently seen in patients with a history of migraine headaches and Raynaud’s phenomenon. The spasm may occur in the absence of CAD, as well as with documented disease. Prinzmetal’s angina is not usually precipitated by increased physical demand. Coronary spasm can be described as a strong contraction of smooth muscle in the coronary artery caused by an increase in intracellular calcium.
Factors that may precipitate coronary artery spasm include increased myocardial oxygen demand and increased levels of certain substances (e.g. histamine, angiotensin, adrenaline, noradrenaline, prostaglandins). When spasm occurs, the patient experiences angina and transient ST segment elevation.5 The pain may occur during rapid eye movement sleep when myocardial oxygen consumption increases. The pain may be relieved by moderate exercise or it may disappear spontaneously. Cyclic, short bursts of pain at a usual time each day may also occur with this type of angina. It is usually treated with calcium channel blockers and/or nitrates.
Microvascular angina
Angina may also occur in the absence of significant coronary atherosclerosis or coronary spasm, especially in women.40 In these patients, chest pain is related to myocardial ischaemia associated with abnormalities of the coronary microcirculation. This is known as coronary microvascular disease (MVD). Coronary MVD affects the small branches of the distal coronary arteries, whereas CAD affects larger coronary arteries. In coronary MVD, plaque can be diffuse and evenly distributed, or develop as blockages in the tiny coronary arteries.40 Coronary MVD is a new concept and may be a cause of heart disease in women. Currently, prevention and treatment of coronary MVD follow the same recommendations as for CAD.41
Multidisciplinary care
CHRONIC STABLE ANGINA
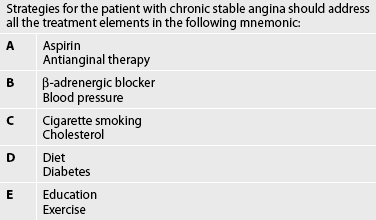
The treatment of chronic stable angina is aimed at decreasing oxygen demand and/or increasing oxygen supply. Continued emphasis on the reduction of risk factors is a priority and should include those strategies discussed for patients with CAD. In addition to antiplatelet and cholesterol-lowering drug therapy, the most common therapeutic intervention for the management of chronic stable angina is the use of nitrate therapy to enhance coronary blood flow (see Table 33-12 and Fig 33-9).41–44
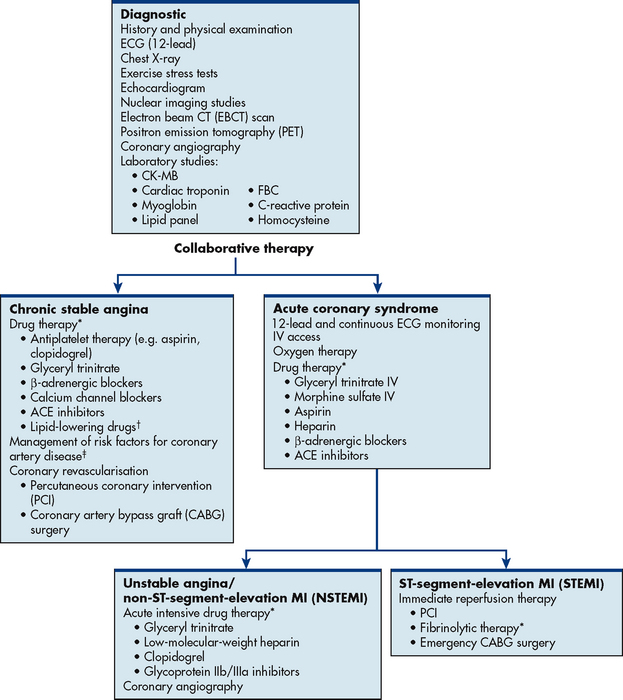
Figure 33-9 Multidisciplinary care: chronic stable angina and acute coronary syndrome. ACE, angiotensin-converting enzyme; CK, creatine kinase; ECG, electrocardiogram; FBC, full blood count; IV, intravenous.
*See Table 33-13.
†See Table 33-7.
‡ See Tables 33-3 and 33-4.
DRUG THERAPY
Drug therapy for chronic stable angina is aimed at preventing MI and death and reducing symptoms. Aspirin (previously discussed) is recommended in the absence of contraindications (see Table 33-13).
Short-acting nitrates
Short-acting nitrates are first-line therapy for the treatment of angina. Nitrates produce their principal effects by the following:
1. Dilating peripheral blood vessels. This results in decreased SVR, venous pooling and decreased venous blood return to the heart. Therefore, myocardial oxygen demand is decreased because of the reduced cardiac workload.
2. Dilating coronary arteries and collateral vessels. This may increase blood flow to the ischaemic areas of the heart. However, when the coronary arteries are severely atherosclerotic, coronary dilation is difficult to achieve.
Sublingual glyceryl trinitrate
Glyceryl trinitrate given sublingually or by translingual spray usually relieves pain in approximately 3 minutes and has a duration of approximately 30–60 minutes. The recommended dose is one tablet taken sublingually (SL) or one metered spray for symptoms of angina. If symptoms are unchanged or worse after 5 minutes, the patient should be instructed to call the emergency medical service (EMS).44
The patient must be instructed in the proper use of glyceryl trinitrate. It should be easily accessible to the patient at all times. For protection from degradation, tablets should be kept in a tightly closed dark glass bottle. The patient should be instructed to place a glyceryl trinitrate tablet under the tongue and allow it to dissolve. If using the spray, it should be directed under the tongue, not inhaled. Glyceryl trinitrate should cause a tingling sensation. If tingling is not felt and chest pain still persists, the patient should call the EMS. The patient should be warned that the HR may increase and a pounding headache, dizziness or flushing may occur. The patient should be cautioned against quickly rising to a standing position because orthostatic hypotension may occur after glyceryl trinitrate use.
Glyceryl trinitrate can be used prophylactically before undertaking an activity that the patient knows may precipitate an anginal attack. In these instances the patient can take a tablet 5–10 minutes before beginning the activity. Any changes in the usual pattern of pain, especially increasing frequency or nocturnal angina, should be reported to the healthcare provider.
Glyceryl trinitrate tablets are marketed in light-resistant bottles with metal caps. Because they tend to lose potency once a bottle has been opened, the patient should be advised to purchase a new supply every 6 months.
Long-acting nitrates
Nitrates, such as isosorbidedinitrate and isosorbidemononitrate, are longer acting than SL or translingual glyceryl trinitrate and can be used to reduce the incidence of anginal attacks.43 Glyceryl trinitrate applied topically using a transdermal patch can also be used prophylactically to prevent angina as it provides controlled, constant delivery. It is not suitable to be used in an acute attack. The patches come in three doses: 5 mg/24 hours, 10 mg/24 hours and 15 mg/24 hours. Generally, the patch is applied once a day to a flat muscular area that is free of hair and/or scars (e.g. upper arm). Patients need to be made aware that tolerance to vasodilation induced by glyceryl trinitrate can develop. It is recommended that patients schedule an 8-hour nitrate-free period every day, usually during the night, unless they experience nocturnal angina.35,42
The predominant side effect of all nitrates is headache from dilation of the cerebral blood vessels. Patients can be advised to take paracetamol with their nitrate to relieve the headache. Over time, headaches may decrease but the principal antianginal effect is still present. Patients should be taught that orthostatic hypotension is also a complication of all nitrates and to be careful when standing suddenly. The nurse should monitor BP after the initial dose as the venous dilation that occurs may cause a drop in BP, especially in volume-depleted patients.
Transdermal controlled-release nitrates
Currently two systems are available for transdermal glyceryl trinitrate drug administration: reservoir and matrix. The reservoir system delivers the drug using a rate-controlled permeable membrane. The matrix system provides for a slow delivery of the drug through a polymer matrix. Both reservoir and matrix delivery systems offer the advantages of steady plasma levels within the therapeutic range during 24 hours, thus making only one application a day necessary. The reservoir system has the disadvantage of dose dumping if the reservoir seal is punctured or broken. An advantage of the matrix system is that there can be no dose dumping. Both systems achieve plasma drug level steady states by 2 hours.
β-adrenergic blockers
β-adrenergic blockers include propranolol, metoprolol, nadolol and atenolol. These drugs decrease myocardial contractility, HR, SVR and BP, all of which reduce the myocardial oxygen demand. β-adrenergic blockers have also been shown to decrease morbidity and mortality in patients with CAD, especially following MI.44
β-adrenergic blockers have many side effects and are sometimes poorly tolerated.43 Side effects may include bradycardia, hypotension, wheezing and GI complaints. Many patients also complain of weight gain, depression and sexual dysfunction. β-adrenergic blockers should be avoided in patients with asthma and used cautiously in patients with diabetes as they mask signs of hypoglycaemia. β-adrenergic blockers should not be discontinued abruptly without medical supervision as this may precipitate an increase in the frequency and intensity of angina attacks.35
Calcium channel blockers
If β-adrenergic blockers are contraindicated, poorly tolerated or do not control anginal symptoms, calcium channel blockers (e.g. nifedipine, verapamil and diltiazem) are used.43 These drugs are also used to manage Prinzmetal’s angina. Most of these agents have sustained-release versions for longer action with the hope of increased patient adherence and stable blood levels of the drug. The three primary effects of calcium channel blockers are: (1) systemic vasodilation with decreased SVR; (2) decreased myocardial contractility; and (3) coronary vasodilation.
Cardiac muscle and vascular smooth muscle cells are more dependent on extracellular calcium than skeletal muscles and are therefore more sensitive to calcium channel blocking agents. Calcium channel blockers cause smooth muscle relaxation and relative vasodilation of coronary and systemic arteries, thus increasing blood flow.
Calcium channel blockers potentiate the action of digoxin by increasing serum digoxin levels during the first week of therapy. Therefore, serum digoxin levels should be closely monitored after starting this therapy. Patients should be taught the signs and symptoms of digoxin toxicity.
Angiotensin-converting enzyme inhibitors
Certain high-risk patients with chronic stable angina may benefit from the addition of an angiotensin-converting enzyme (ACE) inhibitor (e.g. captopril) to the drug regimen.43 These would include patients with diabetes mellitus, significant CAD as determined by coronary angiography (e.g. multi-vessel disease) and/or previous history of MI with left ventricular dysfunction. (ACE inhibitors are discussed on p 881 and in Table 32-6.)
Diagnostic studies
When a patient has a history of CAD or CAD is suspected, the doctor will order a variety of studies (see Fig 33-9). After a detailed health history and physical examination, a chest X-ray is usually taken to look for cardiac enlargement, aortic calcifications and pulmonary congestion. A 12-lead ECG is obtained and compared with an earlier tracing when possible. Certain laboratory tests (e.g. lipid profile) and diagnostic studies (e.g. Holter monitoring, echocardiogram) will be ordered to confirm CAD and identify specific risk factors for CAD.
For patients with known CAD and chronic stable angina, common diagnostic studies include 12-lead ECG, echocardiogram, exercise stress testing, pharmacological nuclear imaging and coronary angiography.43 See Chapter 31 for a discussion of these studies, including nursing considerations. Two of these studies are discussed in further detail here.
EXERCISE STRESS TESTING
Treadmill exercise testing is an important diagnostic test done for the patient with chronic stable angina. ST segment and T wave changes during exercise are an indirect assessment of coronary artery perfusion. Severely abnormal electrocardiograms (ECGs) on exercise testing indicate a significant disease process and may indicate the need for coronary angiography. Unfortunately, the ECG stress test is not always conclusive for CAD. A false-positive test may be found (especially in women) and a false-negative test may be seen if the patient is exercised submaximally or if only one coronary artery is involved. Pharmacological nuclear imaging and echocardiography can complement exercise testing, especially in people with inconclusive results on the exercise testing or who are unable to exercise.
Cardiac catheterisation
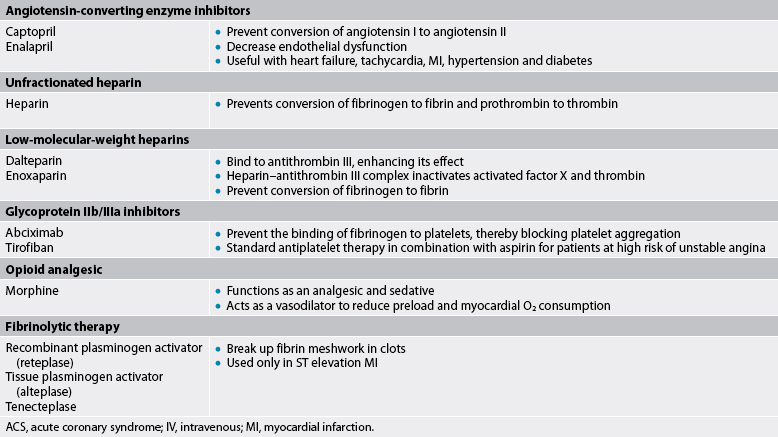
It is not uncommon for a patient with chronic stable angina to undergo a diagnostic cardiac catheterisation and coronary angiography. If a coronary lesion is amenable to an intervention, coronary revascularisation with an elective percutaneous coronary intervention (PCI) may be done.41,42 During this procedure, a catheter equipped with an inflatable balloon tip is inserted into the appropriate coronary artery. When the blockage is located, the catheter is passed through it, the balloon is inflated and the atherosclerotic plaque is compressed, resulting in vessel dilation. This procedure is called balloon angioplasty. Unfractionated heparin (UH) or low-molecular-weight heparin (LMWH) is given in conjunction with PCI to maintain the open vessel.
Intracoronary stents are often inserted in conjunction with balloon angioplasty. Stents are used to treat abrupt or threatened abrupt closure and restenosis following balloon angioplasty. A stent is an expandable mesh-like structure designed to maintain vessel patency by compressing the arterial wall and resisting vasoconstriction (see Figs 33-10 and 33-11). Stents are carefully placed over the angioplasty site to hold the vessel open. Because stents are thrombogenic, the patient is also treated with oral antiplatelet agents such as aspirin or clopidogrel. An IV infusion of a glycoprotein IIb/IIIa inhibitor (e.g. tirofiban) has been found to be beneficial for preventing abrupt closure of the stents. The infusion is initiated during PCI and maintained for 12 hours following the procedure (see Table 33-13).
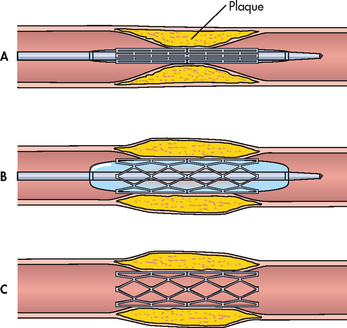
Figure 33-10 Placement of a coronary artery stent. A, The stent is positioned at the site of the lesion. B, The balloon is inflated, expanding the stent. The balloon is then deflated and removed. C, The implanted stent is left in place.
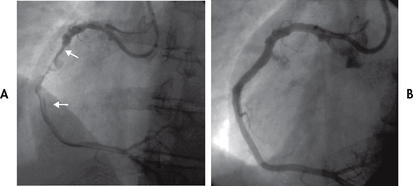
Figure 33-11 A, A thrombotic occlusion of the right coronary artery is shown (arrows). B, The right coronary artery is opened and blood flow restored following angioplasty and placement of a 4-mm stent.
Many stents that are used are drug-eluting stents. This type of stent is coated with a drug (e.g. paclitaxel, sirolimus) that prevents the overgrowth of new intima, the primary cause of stent restenosis.44–46
The most serious complications from stent placement are abrupt closure and vascular injury. Other less common complications include acute MI, stent embolisation, coronary spasm and emergency coronary artery bypass graft (CABG) surgery. The possibility of arrhythmias during and after the procedure is always present. The use of drug-eluting stents and advances in pharmacotherapy have significantly reduced the restenosis rate following PCI.
PCI may not be a feasible option for all patients (e.g. patients with three-vessel CAD and/or significant left main CAD). Coronary revascularisation with CABG surgery may be recommended and is discussed later in the chapter.41
ACUTE CORONARY SYNDROME
When ischaemia is prolonged and not immediately reversible, acute coronary syndrome (ACS) develops and encompasses the spectrum of UA, non-ST segment elevation myocardial infarction (NSTEMI) and ST segment elevation myocardial infarction (STEMI) (see Fig 33-12). Although each remains a distinct diagnosis, this nomenclature (ACS) reflects the relationship between the pathophysiology, diagnosis, prognosis and interventions for these disorders.
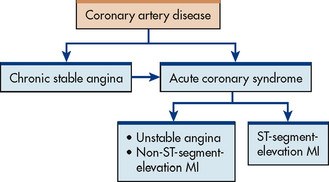
Figure 33-12 The relationships among coronary artery disease, chronic stable angina and acute coronary syndrome. MI, myocardial infarction.
AETIOLOGY AND PATHOPHYSIOLOGY
ACS is associated with deterioration of a once stable atherosclerotic plaque. The once stable plaque ruptures, exposing the intima to blood and stimulating platelet aggregation and local vasoconstriction with thrombus formation. This unstable lesion may be partially occluded by a thrombus (manifesting as unstable angina or NSTEMI) or totally occluded by a thrombus (manifesting as STEMI).44,45 What causes a coronary plaque to suddenly become unstable is not well understood, but systemic inflammation (described earlier) is thought to play a role.9 Patients with suspected ACS require immediate hospitalisation.
Clinical manifestations
UNSTABLE ANGINA
Chest pain that is new in onset, occurs at rest or has a worsening pattern is called unstable angina (UA). The patient with chronic stable angina may develop UA or UA may be the first clinical manifestation of CAD. Unlike chronic stable angina, UA is unpredictable and represents an emergency. The patient with previously diagnosed chronic stable angina will describe a significant change in the pattern of angina. It will occur with increasing frequency and is easily provoked by minimal or no exertion, during sleep or even at rest. The patient without previously diagnosed angina will describe anginal pain that has progressed rapidly in the last few hours, days or weeks, often culminating in pain at rest.42
Women seek medical attention more often with symptoms of UA than men. Studies have shown that women will have prodromal symptoms which are early manifestations of CAD, but because they are not recognised as such, many women present with UA before CAD is diagnosed.47 These symptoms include fatigue, shortness of breath, indigestion and anxiety. Fatigue is the most prominent symptom. Because fatigue can be a symptom of many different diseases and syndromes, a careful history of CAD risk factors should be obtained to identify these women.
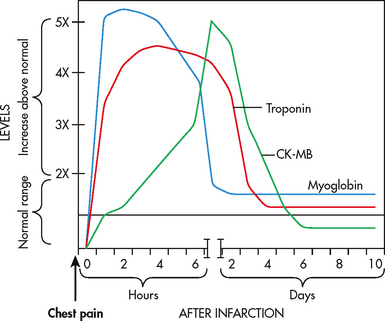
MYOCARDIAL INFARCTION
A myocardial infarction (MI) occurs as a result of sustained ischaemia, causing irreversible myocardial cell death (necrosis) (see Figs 33-13 and 33-14). Approximately 80–90% of all acute MIs are secondary to thrombus formation.43,48 When a thrombus develops, perfusion to the myocardium distal to the occlusion is halted, resulting in necrosis. Contractile function of the heart stops in the necrotic area(s). The degree of altered function depends on the area of the heart involved and the size of the infarction. Most MIs involve some portion of the left ventricle.
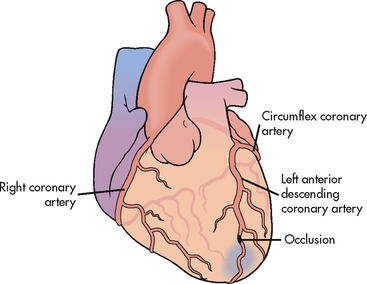
Figure 33-13 Occlusion of the left anterior descending coronary artery, resulting in a myocardial infarction.
Source: Mayo Clinic, Rochester, MN.
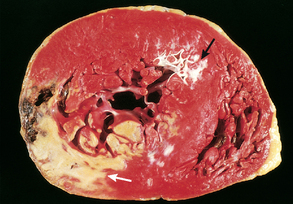
Figure 33-14 Acute myocardial infarction in the posterolateral wall of the left ventricle. This is demonstrated by the absence of staining in the areas of necrosis (white arrow). Note the scarring from a previous anterior wall myocardial infarction (black arrow).
Source: Mayo Clinic, Rochester, MN.
The acute MI process takes time. Cardiac cells can withstand ischaemic conditions for approximately 20 minutes before cellular death begins. The earliest tissue to become ischaemic is the subendocardium (the innermost layer of tissue in the cardiac muscle). If ischaemia persists, it takes approximately 4–6 hours for the entire thickness of the heart muscle to become necrotic (see Fig 33-15).
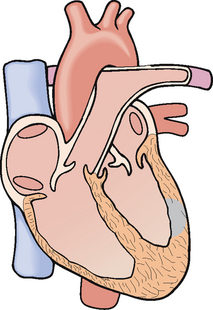
Figure 33-15 Myocardial infarction involving the full thickness of the left ventricular wall.
Source: Mayo Clinic, Rochester, MN.
Infarctions are usually described based on the location of damage (e.g. anterior, inferior, lateral or posterior wall infarction). Damage can occur in more than one location (e.g. anterolateral MI, anteroseptal MI). The location of the infarction correlates with the involved coronary circulation. For example, inferior wall infarctions result from occlusions in the right coronary artery; anterior wall infarctions result from occlusions in the left anterior descending artery. Occlusions in the left circumflex artery usually cause lateral and/or posterior wall MIs.
The degree of pre-established collateral circulation also influences the severity of infarction (see Fig 33-3). In an individual with a history of CAD, collateral circulation may be established that provides the area surrounding the infarction site with a blood supply. This is one explanation why younger people who have an MI are often likely to have a more serious impairment than older people with the same degree of occlusion.
Pain
Severe, immobilising chest pain not relieved by rest, position change or nitrate administration is the hallmark of an MI. Persistent and unlike any other pain, it is usually described as a heaviness, pressure, tightness, burning, constriction or crushing. Common locations are substernal, retrosternal or epigastric areas. The pain may radiate to the neck, jaw and arms or to the back (see Fig 33-8). It may occur while the patient is active, at rest, asleep or awake. However, it commonly occurs in the early morning hours. It usually lasts for 20 minutes or more and is described as more severe than usual anginal pain. When epigastric pain is present, the patient may relate it to indigestion and take antacids without relief.
Not everyone has classic symptoms. Some patients may not experience pain but may have ‘discomfort’, weakness or shortness of breath. Although women and men have more similarities in their symptoms of an acute MI than differences, some women may experience atypical discomfort, shortness of breath or fatigue.48,49 Patients with diabetes mellitus are more likely to experience silent (asymptomatic) MIs due to cardiac neuropathy and present with atypical symptoms (e.g. dyspnoea). Older patients may experience a change in mental status (e.g. confusion), shortness of breath, pulmonary oedema, dizziness or arrhythmia.
Sympathetic nervous system stimulation
During the initial phase of MI, catecholamines (adrenaline and noradrenaline) are released from the ischaemic myocardial cells that normally contain varying quantities of these substances. The increased SNS stimulation results in release of glycogen, diaphoresis and vasoconstriction of peripheral blood vessels. On physical examination, the patient’s skin may be ashen, clammy and cool to touch.
In response to the release of catecholamines, the BP and HR may be elevated initially. Later, the BP may drop because of decreased cardiac output (CO). If severe enough, this may result in decreased renal perfusion and urine output. Crackles may be noted in the lungs, persisting for several hours to several days, suggesting left ventricular dysfunction. Jugular venous distension, hepatic engorgement and peripheral oedema may indicate right ventricular dysfunction.
Cardiac examination may reveal abnormal heart sounds that may seem distant. Careful auscultation may reveal splitting of heart sounds. Other abnormal sounds suggesting ventricular dysfunction are S3 and S4. In addition, a loud holosystolic murmur may develop and may indicate a septal defect or mitral valve dysfunction.
Nausea and vomiting
The patient may be nauseated and vomit. Nausea and vomiting can result from reflex stimulation of the vomiting centre by the severe pain. These symptoms can also result from vasovagal reflexes initiated from the area of the infarcted myocardium.
Fever
The patient’s temperature may increase within the first 24 hours up to 38°C and occasionally to 39°C. The temperature elevation may last for as long as 1 week. This increase in temperature is a systemic manifestation of the inflammatory process caused by myocardial cell death.
Healing process
The body’s response to cell death is the inflammatory process (see Ch 12). Within 24 hours, leucocytes infiltrate the area. Enzymes are released from the dead cardiac cells and are important diagnostic indicators of MI. (See the section on serum cardiac markers on p 878.) The proteolytic enzymes of the neutrophils and macrophages remove all necrotic tissue by the second or third day. During this time, the necrotic muscle wall is thin. The development of collateral circulation improves areas of poor perfusion and may limit the zones of injury and infarction. Once infarction takes place, catecholamine-mediated lipolysis and glycogenolysis occur. These processes allow the increased plasma glucose and free fatty acids to be used by the oxygen-depleted myocardium for anaerobic metabolism. For this reason, serum glucose levels are frequently elevated after MI.47
The necrotic zone is identifiable by ECG changes (e.g. ST segment elevation, pathological Q wave) and by nuclear scanning after the onset of symptoms. At this point, the neutrophils and monocytes have cleared the necrotic debris from the injured area and the collagen matrix that will eventually form scar tissue is laid down.
At 10–14 days after MI, the beginning scar tissue is still weak. The myocardium is considered to be especially vulnerable to increased stress because of the unstable state of the healing heart wall. It is also at this time that the patient’s activity level may be increasing, so special caution and assessment are necessary. By 6 weeks after MI, scar tissue has replaced necrotic tissue. At this time, the injured area is said to be healed. The scarred area is often less compliant than the surrounding fibres. This condition may be manifested by uncoordinated wall motion, ventricular dysfunction or pump failure.47
These changes in the infarcted muscle also cause changes in the unaffected myocardium as well. In an attempt to compensate for the infarcted muscle, the normal myocardium will hypertrophy and dilate. This process is called ventricular remodelling. Remodelling of normal myocardium can lead to the development of late heart failure (HF), especially in the individual with atherosclerosis of other coronary arteries and/or an anterior MI.47
Complications of myocardial infarction
Arrhythmias
The most common complication after an MI is arrhythmias (alteration to the cardiac rhythm), which are present in 80% of MI patients. Arrhythmias are the most common cause of death in patients in the pre-hospital period. Arrhythmias are caused by any condition that affects the myocardial cell’s sensitivity to nerve impulses, such as ischaemia, electrolyte imbalances and sympathetic nervous system stimulation. The intrinsic rhythm of the heartbeat is disrupted, causing a fast HR (tachycardia), a slow HR (bradycardia) or an irregular beat, all of which adversely affect the ischaemic myocardium.
Life-threatening arrhythmias occur most often with anterior wall infarction, HF or shock. Complete heart block is seen in massive infarction. Ventricular fibrillation, a common cause of sudden cardiac death, is a lethal arrhythmia that most often occurs within the first 4 hours after the onset of pain. Premature ventricular contractions may precede ventricular tachycardia and fibrillation. Life-threatening ventricular arrhythmias must be treated immediately. (See Ch 35 for a detailed description of arrhythmias and their management.)
Heart failure
Heart failure is a complication that occurs when the pumping power of the heart has diminished. Depending on the severity and extent of the injury, HF occurs initially with subtle signs such as mild dyspnoea, restlessness, agitation or slight tachycardia. Other signs indicating the onset of HF include pulmonary congestion on chest X-ray, S3 or S4 heart sounds on auscultation, crackles on auscultation of breath sounds and jugular vein distension from right-sided HF. (The treatment of acute decompensated HF is discussed in Ch 34.)
Cardiogenic shock
Cardiogenic shock occurs when inadequate oxygen and nutrients are supplied to the tissues because of severe left ventricular failure. Cardiogenic shock occurs less often since early and rapid treatment of MI with PCI and fibrinolytic therapy. When it does occur, it has a high mortality rate. Cardiogenic shock requires aggressive management, including control of arrhythmias, intraaortic balloon pump (IABP) therapy and support of contractility with the use of vasoactive drugs. The goal of therapy is to maximise oxygen delivery, reduce oxygen demand and prevent complications such as acute renal failure.47 (Cardiogenic shock is discussed in Ch 66.)
Papillary muscle dysfunction
Papillary muscle dysfunction may occur if the infarcted area includes or is adjacent to these structures. Papillary muscle dysfunction causes mitral valve regurgitation, which increases the volume of blood in the left atrium. This condition aggravates an already compromised left ventricle by reducing CO even further. Papillary muscle dysfunction is detected by a systolic murmur at the cardiac apex radiating towards the axilla.
Papillary muscle rupture is a rare but life-threatening complication causing massive mitral valve regurgitation, which results in dyspnoea, pulmonary oedema and decreased CO. The patient experiences rapid clinical deterioration. Treatment consists of rapid afterload reduction with nitroprusside and/or IABP therapy and immediate open heart surgery with mitral valve replacement.47 (See Ch 36 for discussion of valve disorders.)
Ventricular aneurysm
Ventricular aneurysm results when the infarcted myocardial wall becomes thinned and bulges out during contraction. The patient with a ventricular aneurysm may experience refractory HF, arrhythmias and angina. Besides ventricular rupture, which is fatal, ventricular aneurysms harbour thrombi, which can lead to an embolic stroke.
Pericarditis
Acute pericarditis, an inflammation of the visceral and/or parietal pericardium, may result in cardiac compression, decreased ventricular filling and emptying, and HF. It may occur 2–3 days after an acute MI as a common complication of the infarction. Pericarditis is characterised by chest pain, which may vary from mild to severe and is aggravated by inspiration, coughing and movement of the upper body. The pain may be relieved by sitting in a forward position. The pain is usually different from pain associated with an MI.
Assessment of the patient with pericarditis may reveal a friction rub over the pericardium. The sound may be best heard with the diaphragm of the stethoscope at the mid to lower sternal border. It may be persistent or intermittent. Fever may also be present.
Diagnosis of pericarditis can be made with serial 12-lead ECGs. Characteristic ECG changes are diffuse and reflect the inflammation of the pericardium. Treatment may include pain relief by aspirin, corticosteroids or NSAIDs. (Pericarditis is discussed in Ch 36.)
Dressler’s syndrome
Dressler’s syndrome is characterised by pericarditis with effusion and fever that develops 4–6 weeks after MI. It may also occur after open heart surgery. It is thought to be caused by an antigen–antibody reaction to the necrotic myocardium. The patient experiences pericardial pain, fever, a friction rub, pleural effusion and arthralgia. Laboratory findings include an elevated white blood cell count and an elevated sedimentation rate. Short-term corticosteroids are used to treat this condition. (Dressler’s syndrome is discussed in Ch 36.)
Diagnostic studies
In addition to the patient’s history of pain, risk factors and health history, the primary diagnostic studies used to determine whether a person has UA or an MI include an ECG and serum cardiac markers (see Fig 33-9).
ELECTROCARDIOGRAM FINDINGS
The ECG is the primary tool to rule out or confirm UA or an MI. Changes in the QRS complex, ST segment and T wave caused by ischaemia and infarction can develop quickly with UA and MI. For diagnostic and treatment purposes, it is important to distinguish between STEMI and UA or NSTEMI. Patients with STEMI tend to have a more extensive MI that is associated with prolonged and complete coronary occlusion and the development of a pathological Q wave on the ECG. Patients with UA or NSTEMI usually have transient thrombosis or incomplete coronary occlusion and do not develop pathological Q waves. Areas of ischaemia or infarction may be noted on the ECG. Because MI is a dynamic process that evolves with time, the ECG often reveals the time sequence of ischaemia, injury, infarction and resolution of the infarction.
The ECG may also be normal or non-diagnostic when the patient comes to the emergency department (ED) with a complaint of chest pain. Within a few hours, the ECG may change to reflect the infarction process. These changes take place when cellular damage has occurred, interrupting the normal electrical depolarisation of the ventricles. When the initial ECG is non-diagnostic, serial ECGs are done every 2–4 hours.47 (See Ch 35 for discussion of ECG changes associated with ischaemia and MI.)
SERUM CARDIAC MARKERS
Certain proteins, called serum cardiac markers, are released into the blood in large quantities from necrotic heart muscle after an MI. These markers, specifically serum cardiac enzymes and troponin, are important in the diagnosis of MI. When cardiac cells die, their intracellular enzymes are released into circulation. The increase in serum cardiac markers that occurs after cellular death can indicate whether cardiac damage is present and the approximate extent of the damage. Creatine kinase (CK) and troponin are typically measured to diagnose an MI. Figure 33-16 indicates the peak level and duration of these markers in the presence of MI.
CK levels begin to rise approximately 3–12 hours after an MI, peak in 24 hours and return to normal within 2–3 days. The CK enzymes may be fractionated into bands, including the MB band. The CK-MB band is specific to myocardial cells and can help quantify myocardial damage.
Troponin is a myocardial muscle protein released into circulation after myocardial injury. In the heart there are two subtypes: cardiac-specific troponin T (cTnT) and cardiac-specific troponin I (cTnI). These markers are highly specific indicators of MI and have greater sensitivity and specificity for myocardial injury than CK-MB.7,47 Troponin peaks more slowly than CK, but initial results are still available within hours of a myocardial event. It is usually used for diagnostic purposes in conjunction with total CK and the MB fraction.7,47 Serum levels of cTnI and cTnT increase 3–12 hours after the onset of MI, peak at 24–48 hours and return to baseline over 5–14 days.
Myoglobin is released into circulation within a few hours after an MI. Although it is one of the first serum cardiac markers that increase after an MI, it lacks cardiac specificity. In addition, it is rapidly excreted in urine so that blood levels return to normal range within 24 hours after an MI (see Table 31-7).
CORONARY ANGIOGRAPHY
The patient with UA or NSTEMI may undergo coronary angiography to evaluate the extent of the disease and to determine the most appropriate therapeutic modality. If appropriate, a PCI may be performed at this time. Others may be treated with conservative medical management. It remains controversial which is the best management for UA and NSTEMI.41,42 Coronary angiography is the only way to confirm the diagnosis of Prinzmetal’s angina.
OTHER MEASURES
When the ECG and serum cardiac markers do not confirm MI, other measures for diagnosing UA may be considered (see Table 31-7). Exercise stress testing and echocardiograms may be used when a patient has an abnormal but non-diagnostic baseline ECG. A dobutamine stress echocardiogram can be performed in patients unable to exercise. (See Ch 31 for additional information on cardiac assessment.)
Multidisciplinary care
It is extremely important that a patient with ACS is rapidly diagnosed and treated to preserve cardiac muscle. The National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand indicate the importance of collaborative treatment for the rapid treatment of ACS.41 Initial management of the patient with chest pain most often occurs in the ED. Emergency care of the patient with chest pain is presented in Table 33-14. An intravenous (IV) route is established to provide an accessible means for emergency drug therapy. Sublingual glyceryl trinitrate and aspirin (chewable) are given if not given by emergency medical personnel prior to arrival at the ED. Morphine is given IV for pain unrelieved by glyceryl trinitrate. Oxygen is administered by rebreather mask at 10 L per minute. The patient will usually receive ongoing care in a critical care unit or telemetry unit, where continuous ECG monitoring is available. Arrhythmias may be detected and appropriate treatment can be instituted. The multidisciplinary care of ACS is presented in Figure 33-9.
BP, blood pressure; CPR, cardiopulmonary resuscitation; ECG, electrocardiogram; GORD, gastro-oesophageal reflux disease; HR, heart rate; IV, intravenous; PCI, percutaneous coronary intervention.
Vital signs, including pulse oximetry, are monitored frequently during the first few hours after admission and are monitored closely thereafter. Bed rest and limitation of activity are initially ordered for 12–24 hours, with a gradual increase in activity unless contraindicated.
For patients with UA or NSTEMI with negative cardiac markers and ongoing angina, a combination of aspirin, heparin (UH or LMWH) and a glycoprotein IIb/IIIa inhibitor (e.g. abciximab, tirobifan) is recommended. PCI is considered once the patient is stabilised and angina is controlled or if angina returns or increases in severity.41
For patients with STEMI or NSTEMI with positive cardiac markers, reperfusion therapy is initiated (see Fig 33-9). Reperfusion therapy can include emergent PCI or fibrinolytic (thrombolytic) therapy. The goal in the treatment of MI is to salvage as much myocardial muscle as possible.
URGENT/IMMEDIATE PCI
In centres performing at least 200 PCI procedures a year and with trained interventional cardiologists and cardiac surgical capability, urgent/immediate PCI is recommended as the first line of treatment for patients with confirmed MI (i.e. definitive ECG changes and/or positive cardiac markers).44,47 The goal is to open the affected artery within 90 minutes of arrival to the ED. In this situation, the patient will have a cardiac catheterisation to locate the blockage(s), assess the severity of the blockage(s), determine the presence of collateral circulation and evaluate left ventricular function. With actual visualisation of the coronary artery system and left ventricular function, treatment modalities most beneficial to the patient can be selected. Usually PCI with the placement of drug-eluting stent(s) will be performed.46 Patients with severe left ventricular dysfunction may require the addition of IABP therapy and a small percentage of patients may require urgent CABG surgery.
The advantages of PCI are that: (1) it provides an alternative to surgical intervention; (2) it is performed with local anaesthesia; (3) the patient is ambulatory 24 hours after the procedure; (4) the length of hospital stay is approximately 1–3 days compared with the 4–6-day stay of someone having CABG surgery; and (5) there is rapid return to work (approximately 5–7 days after PCI) instead of a 2–8-week convalescence after CABG.
Many advances have been made in PCI during the last decade. Guide wires and catheters with greater flexibility have been developed, enabling cardiologists to manoeuvre the catheters to distal and proximal lesions. Today PCI is more frequently performed than CABG surgery. Reduction of lesion size by greater than 50% occurs in 90% of patients.42 Techniques have been developed to provide blood flow to the distal myocardium during balloon inflation, increasing the safety of the procedure. Dilation may also be done for stenotic grafts from previous CABG surgery.
The most serious complication of PCI is dissection of the newly dilated coronary artery. If the damage is extensive, the coronary artery could rupture, causing cardiac tamponade, ischaemia and infarction, decreased CO and possible death. There is also danger from infarction should the lesion be calcified and a portion of the plaque dislodge and occlude the vessel distal to the catheter. Coronary spasm from the mechanical irritation of the catheter or balloon can occur as well as chemical irritation from the contrast media injection used to visualise the artery. Abrupt closure is a complication that can occur in the first 24 hours after PCI. Restenosis after PCI can also occur and risk is greatest in the first 30 days following the procedure. Nursing care of the patient after PCI is similar to that of cardiac catheterisation (see Table 31-7).
FIBRINOLYTIC THERAPY
Fibrinolytic therapy offers the advantages of availability and rapid administration in facilities that do not have an interventional cardiac catheterisation laboratory or one is too far away to safely transfer the patient. Treatment of MI with fibrinolytic therapy is aimed at stopping the infarction process by dissolving the thrombus in the coronary artery and reperfusing the myocardium. To be of most benefit, fibrinolytic therapy must be given as soon as possible, ideally within the first hour after onset of symptoms and preferably within the first 6 hours after the onset of symptoms. If reperfusion occurs within 6 hours, a 25% reduction in mortality rate has been shown.44,47
Indications and contraindications
All fibrinolytics are given IV (see Table 33-13). The choice of a thrombolytic agent is guided by considerations of availability, efficacy and ease of administration. Although these drugs have different mechanisms of action and different pharmacokinetics, they all produce an open artery by lysis of the thrombus in the coronary artery. The goal for the administration of a fibrinolytic is within 30 minutes of the patient’s arrival to the ED. Optimal outcomes can be achieved if the fibrinolytic is administered within 60 minutes of onset of symptoms.44
Because all the fibrinolytics produce lysis of the clot, they may also lyse other clots (e.g. a postoperative site). Therefore patient selection is important because minor or major bleeding can be a complication of therapy.44 Inclusion criteria for fibrinolytic therapy include: (1) chest pain typical of acute MI for less than 6 hours; (2) 12-lead ECG findings consistent with acute MI; and (3) no absolute contraindications (see Box 33-1). Although patients presenting with chest pain greater than 6 hours in duration and ECG changes indicative of MI may be considered for fibrinolytic therapy, research on the benefit of this therapy is inconclusive.44,47
BOX 33-1 Contraindications for the use of fibrinolytic therapy
Absolute contraindications
• Active internal bleeding or bleeding diathesis (excluding menstruation)
• Known history of cerebral aneurysm or arteriovenous malformation
• Known intracranial neoplasm (primary or metastatic)
• Previous cerebral haemorrhage
• Recent (within past 3 months) ischaemic stroke
Relative contraindications
• Current use of anticoagulants
• Prior ischaemic stroke more than 3 months ago, dementia or known intracranial pathology not covered in absolute contraindications
• Recent (within 3 weeks) surgery (including eye laser surgery) or puncture of non-compressible vessel
• Recent (within 2–4 weeks) internal bleeding
• Serious systemic disease (e.g. advanced/terminal cancer, severe liver/kidney disease)
• Severe uncontrolled hypertension (BP >180/110 mmHg) on presentation or chronic severe poorly controlled hypertension
• Traumatic or prolonged (>10 min) cardiopulmonary resuscitation
Procedure
Each hospital has a protocol to follow for administration of fibrinolytic therapy. However, there are several common factors. Blood for baseline testing is taken, two to three lines for IV therapy are started and all other invasive procedures are done before the fibrinolytic agent is given. This procedure reduces the possibility of bleeding in the patient.
The time at which therapy begins is noted and the patient is monitored during and after the period of time that the fibrinolytic is administered. ECG, vital signs, pulse oximetry and heart and lung assessments are completed frequently to evaluate the patient’s response to therapy. When reperfusion occurs (i.e. the coronary artery that was occluded is opened, blood flow is restored to the myocardium), several clinical markers may change. The most reliable marker is the return of the ST segment to baseline on the ECG. Other markers include a resolution of chest pain and a rapid rise of the CK-MB enzymes within 3 hours of therapy and peaking within 12 hours. The CK-MB levels increase as the necrotic myocardial cells release CK-MB enzymes into the circulation after perfusion has been restored to the area. The presence of reperfusion arrhythmias (e.g. accelerated idioventricular rhythm) is a less reliable marker of reperfusion. These arrhythmias are generally self-limiting and do not require aggressive treatment. (See Ch 35 for management of arrhythmias.)
A major concern with fibrinolytic therapy is reocclusion of the artery. The site of the thrombus is unstable and another clot may form or spasm of the artery may occur. Because of this possibility, most cardiologists begin IV heparin therapy. If another clot develops, the patient will have similar complaints of chest pain and ECG changes will return. The patient will be re-evaluated and may receive a second dose of the fibrinolytic or be transferred to a cardiac catheterisation laboratory for rescue PCI.
The major complication with fibrinolytic therapy is bleeding. Ongoing nursing assessment is essential. Minor bleeding (e.g. surface bleeding from IV sites or gingival bleeding) is expected and can be controlled by applying a pressure dressing or ice packs. If signs and symptoms of major bleeding occur (e.g. drop in BP, an increase in HR, a sudden decrease in the patient’s level of consciousness, blood in the urine or stool) the doctor should be notified and the therapy should be stopped. Depending on the drug selected, therapy may be administered in one IV bolus or over a period of time (30–90 minutes).
DRUG THERAPY
IV glyceryl trinitrate, aspirin, β-adrenergic blockers and systemic anticoagulation with either LMWH given subcutaneously or IV UH are the initial drug treatments of choice for ACS.44,47 IV antiplatelet agents (e.g. glycoprotein IIb/IIIa inhibitor) may also be used if PCI is anticipated. ACE inhibitors are added for select patients following MI (discussed below). Calcium channel blockers or long-acting nitrates can be added if the patient is already on adequate doses of β-adrenergic blockers, cannot tolerate β-adrenergic blockers or has Prinzmetal’s angina.44,47
Drug therapy for patients with ACS is presented in Table 33-13 and Figure 33-9. Specific discussion of a few drugs is presented in this section.
Intravenous glyceryl trinitrate
IV glyceryl trinitrate is used in the initial treatment of the patient with ACS. The goal of therapy is to reduce anginal pain and improve coronary blood flow. It has an immediate onset of action and can be titrated to prevent, treat and stop UA.42,50
IV glyceryl trinitrate is used to decrease preload and afterload while increasing the myocardial oxygen supply. IV glyceryl trinitrate is usually titrated to relieve pain. Because hypotension is a common side effect, BP is closely monitored during this time. Patients who do become hypotensive are often volume depleted and can benefit from an IV fluid bolus.44,47 Tolerance is another side effect of IV nitrate therapy. An effective strategy for this phenomenon is titrating the dose down at night during sleep and titrating the dose up during the day.
Morphine
Morphine is given for chest pain that is unrelieved by glyceryl trinitrate. As a vasodilator, it decreases cardiac workload by lowering myocardial oxygen consumption, reducing contractility and decreasing BP and HR. In addition, morphine can help reduce anxiety and fear. In rare situations, morphine can depress respirations. Patients should be monitored for signs of bradypnoea or hypoxia, a condition to be avoided in myocardial ischaemia and infarction.
β-adrenergic blockers
β-adrenergic blockers are used to decrease myocardial oxygen-demand by reducing HR, BP and contractility. Use of these drugs in the first hours of MI has been shown to reduce the size of the infarction and the incidence of complications. The continuation of β-adrenergic blockers indefinitely is recommended.44 (See Tables 32-5 and 33-13 for a discussion of β-adrenergic blockers.)
ACE inhibitors
ACE inhibitors (e.g. captopril) are recommended following anterior wall MIs or MIs that result in decreased left ventricular function (ejection fraction [EF] <40%) or pulmonary congestion.44,47 The use of ACE inhibitors can help prevent ventricular remodelling and prevent or slow the progression of HF. ACE inhibitors should be continued indefinitely. For patients who cannot tolerate ACE inhibitors, angiotensin receptor blockers (e.g. losartan) should be considered (see Tables 32-5 and 33-13).44
Antiarrhythmia drugs
Arrhythmias are the most common complications after an MI. In general, they are not treated aggressively unless they are life-threatening.48 (The drugs used in the treatment of arrhythmias are discussed in Ch 35.)
Cholesterol-lowering drugs
A fasting lipid panel should be obtained on all patients admitted with ACS. Cholesterol-lowering drugs are recommended for all patients with elevated LDL cholesterol (see Tables 33-3 and 33-7).
Stool softeners
After an MI the patient may be predisposed to constipation as a result of bed rest and opioid administration. Stool softeners such as docusate sodium are given to facilitate and promote the comfort of bowel evacuation. This prevents straining and the resultant vagal stimulation from the Valsalva manoeuvre. Vagal stimulation produces bradycardia and can provoke arrhythmias.
NUTRITIONAL THERAPY
Initially, patients may be nil by mouth except for sips of water until stable (e.g. pain free, nausea resolved). Diet is advanced as tolerated to a low salt, low saturated fat and low cholesterol diet (see Tables 33-5 and 33-6).
CORONARY SURGICAL REVASCULARISATION
Coronary revascularisation with CABG surgery is recommended for patients who: (1) fail medical management; (2) have left main coronary artery or three-vessel disease; (3) are not candidates for PCI (e.g. lesions are long or difficult to access); or (4) have failed PCI with ongoing chest pain.51
Coronary artery bypass graft surgery
CABG surgery consists of the construction of new conduits (vessels to transport blood) between the aorta or other major arteries and the myocardium distal to the obstructed coronary artery (or arteries). The procedure involves one or more grafts using the internal mammary artery, saphenous vein, radial artery, gastroepiploic artery and/or inferior epigastric artery.
CABG surgery requires a sternotomy (opening of the chest cavity) and the use of cardiopulmonary bypass (CPB). CPB involves diverting (bypassing) the patient’s blood from the heart to the CPB machine, where the blood is oxygenated and returned (via a pump) to the patient. In this way, vital organs are perfused while the surgeon operates on a non-beating, bloodless heart.
The internal mammary artery (IMA) is the most common artery used for bypass graft. The left and/or right IMA is left attached to its origin (the subclavian artery) and dissected from the chest wall. It is then anastomosed (connected with sutures) to the coronary artery distal to the stenosis (see Fig 33-17). The long-term patency rate for IMA grafts is 90% after 10 years.51
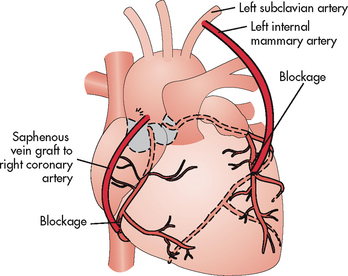
Figure 33-17 The distal end of the left internal mammary artery is grafted below the area of blockage in the left anterior descending artery. The proximal end of the saphenous vein is grafted to the aorta and the distal end is grafted below the area of blockage in the right coronary artery.
The saphenous vein is also used for bypass grafts. It is removed from one or both legs and sections are anastomosed proximally to the ascending aorta and to a coronary artery distal to the blockage. Saphenous vein grafts do develop diffuse intimal hyperplasia, which contributes to future stenosis and graft occlusions. The use of antiplatelet therapy and statins after surgery has improved vein graft patency. Patency rates of these grafts are 66% at 10 years. When vein grafts do become stenosed, stents have been used to open occlusions.51
There has been recent interest in the use of the radial artery as a bypass graft. The radial artery is a thick muscular artery that is prone to spasm when mechanically stimulated. Perioperative calcium channel blockers and long-acting nitrates are used to control this complication. Early studies show patency rates at 5 years to be as high as 84%. There have been no reports of extremity complications (e.g. hand ischaemia, wound infection) following the dissection of this artery.51
For patients with previous CABG surgery, the gastroepiploic or inferior epigastric artery can be used. These arteries are excellent conduits. However, the dissection of the arteries is extensive, increasing the length of surgery and the risk of wound complications at the harvest site, especially in an obese or diabetic patient.51 Because of the number of patients requiring reoperation, research on the use of alternative arteries (e.g. bovine IMAs) and veins (e.g. umbilical veins) and synthetic grafts (e.g. Dacron grafts) will become increasingly more important.51
CABG surgery remains a palliative treatment for CAD and not a cure. Studies have demonstrated improved patient outcomes, quality of life and survival after CABG surgery.51 However, postoperative complications and mortality increase as a function of age. Women have higher operative mortality rates than men. This has been attributed to the late treatment of CAD in women resulting in women presenting at an older age and more ill (e.g. decreased left ventricular function) at surgery. Other possible causes include smaller diameter coronary vessels and the less frequent use of the IMA.51
Minimally invasive direct coronary artery bypass
With recent efforts to reduce cost, length of hospital stay and morbidity, newer approaches to CABG surgery have been developed. Minimally invasive direct coronary artery bypass (MIDCAB) is a technique that offers the patient with single vessel disease (i.e. left anterior descending or right CAD) an approach to surgical treatment that does not involve a sternotomy and CPB.
The technique requires several small incisions between the ribs. A thoracoscope is used to dissect the IMA. The heart is slowed using a β-adrenergic blocker (e.g. esmolol) or stopped temporarily with adenosine, and a mechanical stabiliser is used to immobilise the anastomosis site. The IMA is then sutured to the left anterior descending or right coronary artery. A radial artery or saphenous vein graft may be used if the IMA is not available.
Off-pump coronary artery bypass
The off-pump coronary artery bypass (OPCAB) procedure uses full or partial sternotomy to enable access to all coronary vessels. OPCAB is also performed on a beating heart using mechanical stabilisers and without CPB.
Transmyocardial laser revascularisation
Transmyocardial laser revascularisation (TMR) is an indirect revascularisation procedure used for patients with advanced CAD who are not candidates for traditional bypass surgery and who have persistent angina after maximum medical therapy. The procedure involves the use of a high-energy laser that is triggered electrocardiographically to create channels between the left ventricular cavity and the coronary microcirculation (ventriculocoronary anastomoses). The channels allow blood to flow into ischaemic areas. The procedure may be performed during cardiac catheterisation as a percutaneous TMR or during surgery using a left anterior thoracotomy incision.
Complications following TMR include ventricular arrhythmias, postoperative bleeding and cardiac tamponade, accidental perforation of the great vessels or epicardial coronary arteries, damage to the chordae tendineae and low CO. Although blood flow to the myocardium is improved immediately through the patent channels extending from the outside of the heart to the interior of the left ventricle, optimal results are not seen until new blood vessels form from laser channels and begin to supply the myocardium. Optimal results are seen at about 3–6 months.
 NURSING MANAGEMENT: CHRONIC STABLE ANGINA AND ACUTE CORONARY SYNDROME
NURSING MANAGEMENT: CHRONIC STABLE ANGINA AND ACUTE CORONARY SYNDROME
 Nursing assessment
Nursing assessment
Subjective and objective data that should be obtained from a patient with ACS are presented in Table 33-15.
ACS, acute coronary syndrome; BP, blood pressure; CAD, coronary artery disease; ECG, electrocardiogram; MI, myocardial infarction; WBC, white blood cell.
Source: The assessment and management of cardiovascular risk, 2003. Available at www.nzgg.org.nz. Adapted from Ainsworth bE. The compendium of physical activities tracking guide. University of South Carolina; 2002.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with ACS may include, but are not limited to, those presented in NCP 33-1.
 Planning
Planning
The overall goals for a patient with ACS include: (1) relief of pain; (2) preservation of myocardium; (3) immediate and appropriate treatment; (4) effective coping with illness-associated anxiety; (5) participation in a rehabilitation plan; and (6) reduction of risk factors.
 Nursing implementation: chronic stable angina
Nursing implementation: chronic stable angina
 Acute intervention
Acute intervention
If a nurse is present during an anginal attack, the following measures should be instituted: (1) administration of oxygen; (2) determination of vital signs; (3) prompt pain relief first with a nitrate followed by an opioid analgesic if needed; (4) 12-lead ECG; (5) auscultation of heart sounds; and (6) comfortable positioning of the patient. The patient will most likely appear distressed and have pale, cool, clammy skin. The BP and HR will probably be elevated and an atrial gallop (S4) sound may be heard. If a ventricular gallop (S3) is heard, it may indicate left ventricular dysfunction. A murmur may be heard during an anginal attack secondary to ischaemia of a papillary muscle of the mitral valve. The murmur is likely to be transient and disappear with the cessation of symptoms.
Ask the patient to rate the pain on a scale of 0–10 before and after treatment to evaluate the effectiveness of the interventions. It is important to use the same words that patients use to describe their pain. Some patients may not always verbalise pain. Nurses must be attuned to other manifestations of pain, such as restlessness, elevated HR, respiratory rate or BP, clutching of the bedclothes or other non-verbal cues. Supportive and realistic assurance and a calm, soothing manner will help reduce the patient’s anxiety during an anginal attack.
 Ambulatory and home care
Ambulatory and home care
The patient with a history of angina should be reassured that a long, productive life is possible. Prevention of angina is preferable to its treatment and this is where teaching is important. The patient should be provided information regarding CAD, angina, precipitating factors for angina, risk factor reduction and medications.
Patient teaching can be handled in a variety of ways. One-to-one contact between the nurse and the patient is often the most effective procedure. The time spent in providing daily care is often an ideal teaching period. Teaching tools, such as pamphlets, videotapes, heart models and specially written information are necessary components of patient and family teaching (see Ch 4).
The patient should be assisted in identifying factors that precipitate angina (see Table 33-10) and given instruction on how to avoid or control precipitating factors. For example, the patient should be taught to avoid exposure to extremes of weather and the consumption of large, heavy meals. If a heavy meal is ingested, adequate rest should be planned for 1–2 hours after eating because blood is shunted to the GI tract to aid digestion and absorption.
The patient should be assisted in identifying personal risk factors in CAD. Once these are known, various methods of decreasing any modifiable risk factors should be discussed (see Table 33-4).
Teaching the patient and the family about diets that are low in sodium and reduced in saturated fats may be appropriate (see Tables 33-5 and 33-6). Maintaining ideal body weight is important in controlling angina because weight above this level increases the myocardial workload. Adhering to a regular, individualised exercise program that conditions the heart rather than overstresses the myocardium is important. Most patients can be advised to walk briskly on a flat surface 30 minutes a day at least 5 days a week.10
It is important to teach the patient and family in the proper use of glyceryl trinitrate. Glyceryl trinitrate tablets or ointments may be used prophylactically before an emotionally stressful situation, sexual intercourse or physical exertion (e.g. climbing a long flight of stairs).
Counselling should be provided to assess the psychological adjustment of the patient and family to the diagnosis of CAD and the resulting angina. Many patients feel a threat to their identity and self-esteem, a shock that their body ‘has let them down’ and may be unable to fill their usual roles in society. These emotions are normal and real.
 Nursing implementation: acute coronary syndrome
Nursing implementation: acute coronary syndrome
 Acute intervention
Acute intervention
Priorities for nursing interventions in the initial phase of ACS include pain assessment and relief, physiological monitoring, promotion of rest and comfort, alleviation of stress and anxiety and understanding of the patient’s emotional and behavioural reactions. Research has shown that patients with increased anxiety levels have a greater risk of adverse outcomes such as recurrent ischaemic events and arrhythmias.49 Proper management of these priorities decreases the oxygen needs of a compromised myocardium and reduces the risk of complications. In addition, the nurse should institute measures to avoid the hazards of immobility while encouraging rest.
 Pain
Pain
Glyceryl trinitrate and morphine should be given as needed to eliminate or reduce chest pain. Ongoing evaluation and documentation of the effectiveness of the interventions is important. Once pain is relieved, the nurse may have to deal with denial in a patient who interprets the absence of pain as an absence of cardiac disease.
 Monitoring
Monitoring
Patients have continuous ECG monitoring while in the ED and intensive care unit and usually after transfer to a step-down or general unit. The nurse should be educated in ECG interpretation so that arrhythmias causing further deterioration of the cardiovascular status can be identified and treated. During the initial period after MI, ventricular fibrillation is the most common lethal arrhythmia. In many patients, this arrhythmia is preceded by premature ventricular contractions or ventricular tachycardia. The nurse should also monitor the patient for the presence of silent ischaemia by monitoring the ST segment for shifts above or below the baseline of the ECG. Silent ischaemia occurs without clinical symptoms such as chest pain, but its presence places a patient at higher risk of adverse outcomes and even death.49 If episodes of silent ischaemia are seen on the monitor, the doctor should be notified. (See Ch 35 for a complete discussion of ECG monitoring.)
In addition to frequent vital signs, intake and output should be evaluated at least once a shift and physical assessment should be carried out to detect deviations from the patient’s baseline parameters. Included is an assessment of lung sounds and heart sounds and inspection for evidence of early HF (e.g. dyspnoea, tachycardia, pulmonary congestion, distended neck veins).
Assessment of the patient’s oxygenation status is important, especially if the patient is receiving oxygen. Also, the nares should be checked for irritation or dryness, which can cause considerable discomfort if the nasal route is used for oxygen administration.
 Rest and comfort
Rest and comfort
With a severe insult to the myocardium, as in the case of ACS, it is important for the nurse to promote rest and comfort. Bed rest may be ordered for the first few days after an MI involving a large portion of the ventricle. A patient with an uncomplicated MI (e.g. angina resolved, no signs of complications) may rest in a chair within 8–12 hours after the event. The use of a commode or bedpan is based on patient preference.
When sleeping or resting, the body requires less work from the heart than it does when active. It is important to plan nursing and therapeutic actions to ensure adequate rest periods free from interruption. Comfort measures that can promote rest include frequent oral care, adequate warmth, a quiet atmosphere, use of relaxation therapy (e.g. guided imagery) and assurance that personnel are nearby and responsive to the patient’s needs.
It is important that the patient understand the reasons why activity is limited. However, in spite of this limitation the patient is not completely restricted. Gradually the cardiac workload is increased through more demanding physical tasks so that the patient can achieve a discharge activity level adequate for home care. Phases of cardiac rehabilitation are outlined in Box 33-2.
BOX 33-2 Phases of rehabilitation following acute coronary syndrome
Phase I: hospital
• Occurs while the patient is still hospitalised
• Activity level depends on severity of angina or MI
• Patient may initially sit up in bed or chair, perform range-of-motion exercises and self-care (e.g. washing, shaving) and progress to ambulation in hallway and limited stair climbing
• Attention focuses on management of pain, anxiety, arrhythmias and complications
Phase II: early recovery
• Begins after the patient is discharged
• Usually lasts from 2 to 12 weeks and is conducted in an outpatient facility
• Activity level is gradually increased under the supervision of the cardiac rehabilitation team and with ECG monitoring
• Team may suggest that exercise (e.g. walking) be initiated at home
• Information regarding risk factor reduction is provided at this time
 Anxiety
Anxiety
Anxiety is present in all patients with ACS to various degrees. The nurse’s role is to identify the source of anxiety and assist the patient in reducing it. If the patient is afraid of being alone, a family member should be allowed to sit quietly by the bedside or to check in with the patient frequently. If a source of anxiety is fear of the unknown, the nurse should explore these concerns with the patient and help with appropriate reality testing. If anxiety is caused by lack of information, the nurse should provide teaching appropriate to the patient’s stated need and level. The nurse should answer the patient’s questions with clear, simple explanations sufficient to reduce the patient’s anxiety.
It is important to start teaching at the patient’s level rather than to present a pre-packaged protocol. Frequently the patient is not yet ready to hear about the pathogenesis of CAD. The earliest questions usually relate to how the disease affects perceived control and independence. These questions include the following:
The nurse should advise that a more complete teaching program begins once the patient is feeling stronger. Frequently the patient may not be able to consciously examine the most pervasive concern of ACS patients: am I going to die? Even if a patient denies this concern, it is helpful for the nurse to initiate conversation by remarking that fear of dying is a common concern reported by most patients who have experienced ACS. This gives the patient ‘permission’ to talk about an uncomfortable and fearful topic.
 Emotional and behavioural reactions
Emotional and behavioural reactions
The emotional and behavioural reactions of a patient are varied and frequently follow a predictable response pattern (see Box 33-3). The nurse’s role is to understand what the patient is currently experiencing, to assist the patient in testing reality and to support the use of constructive coping styles. Denial may be a positive coping style in the early phase of recovery.
The nurse has an obligation to maximise and enhance the patient’s social support systems. This entails assessing the support structure of the patient and family and allowing it to function. Often the patient is separated from the most significant support system at the time of hospitalisation. The nurse’s role can include talking with the family, informing them of the patient’s progress, allowing the patient and the family to interact as necessary and supporting the family members who will be able to provide the necessary support to the patient. Open visiting is helpful in decreasing anxiety and increasing support for the patient with ACS. Social isolation has been associated with negative outcomes following MI in both men and women.50 It is important to help the patient identify additional support systems (e.g. spiritual care, local cardiac support groups) that can help the patient after discharge.
 Coronary revascularisation
Coronary revascularisation
Patients with ACS may undergo coronary revascularisation with PCI or CABG surgery. The major nursing responsibilities for the care of the patient following PCI involve monitoring for signs of recurrent angina; frequent assessment of vital signs, including HR and rhythm; evaluation of the groin site for signs of bleeding; and maintenance of bed rest per institution policy.
For patients having CABG surgery, care is provided in the intensive care unit for the first 24–36 hours. Ongoing and intensive monitoring of the patient’s haemodynamic status is critical. The patient will have numerous invasive lines for monitoring cardiac status and other vital organs (see Ch 65). These include a pulmonary artery catheter for measuring cardiac output and other haemodynamic parameters, an intraarterial line for continuous BP monitoring, pleural and mediastinal chest tubes for chest drainage, continuous ECG monitoring to detect arrhythmias, an endotracheal tube to mechanical ventilation, epicardial pacing wires for emergency pacing of the heart, urinary catheter to monitor urine output and nasogastric tube for gastric decompression. Most patients will be extubated within 12 hours and transferred to a step-down unit within 24 hours for continued monitoring of cardiac status.
Many of the postoperative complications that develop after CABG surgery relate to the use of CPB. Major consequences of CPB include bleeding and anaemia from damage to red blood cells and platelets, fluid and electrolyte imbalances and hypothermia as blood is cooled as it passes through the CPB machine. Nursing care is focused on assessing the patient for bleeding (e.g. chest tube drainage, incision sites), monitoring fluid status, replacing electrolytes as needed and restoring temperature (e.g. warming blankets).
Postoperative arrhythmias, specifically atrial arrhythmias, are common in the first 3 days after CABG surgery. Approximately 20–40% of patients develop postoperative atrial fibrillation. Discharge is often delayed in these patients due to the need for anticoagulation. (See Ch 35 for information on treatment of atrial fibrillation.)
Nursing care for the patient with a CABG also involves caring for the surgical sites: the chest, arm, leg and/or abdomen. Care of the radial artery harvest site includes careful observation of the incision site, as well as monitoring sensory and motor function of the distal thumb and fingers. The patient with radial artery harvest should be on a calcium channel blocker for approximately 3 months to decrease the incidence of arterial spasm at the arm or anastomosis site. Care of the leg wound is similar to postoperative care after the stripping of varicose veins (see Ch 37). Management of the chest wound, which involves a sternotomy, is similar to that of other chest surgery (see Ch 27).
Elective CABG is generally well tolerated in older patients. However, the incidence of postoperative complications is high, including arrhythmias, stroke and infection. The nurse caring for older adults must be aware that, although the benefits of treatment may outweigh risks in this population, complications are higher than in younger individuals.
Postoperative nursing care of the patient with a MIDCABG or OPCAB procedure is similar to that for CABG surgery patients. Pain management is essential because patients report higher levels of pain with thoracotomy incisions than a sternotomy incision. The recovery time is somewhat shorter with these procedures and patients often resume routine activities sooner than patients who have CABG surgery.
 Ambulatory and home care
Ambulatory and home care
Cardiac rehabilitation involves restoration of the patient to an optimal state of function in six areas: physiological, psychological, mental, spiritual, economic and vocational. Many patients recover from ACS physically, yet may never attain psychological wellbeing because of misconceptions about the illness or a need to practise illness behaviours. Returning to work and resuming all activities have long been outcome measures of cardiac rehabilitation and are important in terms of the cost-effectiveness of cardiac care and rehabilitation.
In considering rehabilitation, the nurse and patient must recognise that CAD is a chronic disease. It will not be cured, nor will it disappear by itself. Therefore basic changes in lifestyle must be made to promote recovery and health. These changes must frequently be made at a time when a person is middle-aged or older. The patient must also realise that recovery takes time. Resumption of physical activity after ACS or CABG surgery is slow and gradual. However, with appropriate and adequate supportive care, recovery is more likely to occur.
 Patient teaching
Patient teaching
Patient teaching begins with the ED nurse and progresses through the staff nurse to the community health nurse. The purpose of teaching is to give the patient and family the tools they need to make informed decisions about attainment of health. For teaching to be meaningful, the patient must be aware of the need to learn. Careful assessment of the patient’s learning needs helps the nurse to set goals and objectives that are realistic.
The timing of the teaching is important. When patients or families are in crisis (either physiological or psychological), they may not be very interested in learning new information. It is important to remember that early questions should be answered initially in simple, brief terms, without detailed elaboration, and that the answers to these questions often require repetition and elaboration. When the shock and disbelief accompanying a crisis subside, the patient and family are better able to focus on new information.
In addition to teaching the patient and family what they wish to know, several types of information are considered necessary in achieving optimal health. A teaching guide for the patient with ACS is presented in Box 33-4.
PATIENT & FAMILY TEACHING GUIDE
Teach the following to the patient and family:
• Signs and symptoms of angina and MI and reasons they occur*
• Anatomy and physiology of the heart and vessels
• Cause and effect of atherosclerosis
• Definition of terms (e.g. CAD, angina, MI, sudden cardiac death, HF)
• Identification of and decreasing risk factors (see Table 33-4)*
• Rationale for tests and treatments, including ECG, blood tests and angiography and monitoring, rest, diet and medications*
• Appropriate expectations about recovery and rehabilitation (anticipatory guidance)
• Resumption of work, physical activity, sexual activity
• Measures to take to promote recovery and health
• Importance of the gradual, progressive resumption of activity*
CAD, coronary artery disease; ECG, electrocardiogram; EMS, emergency medical system; HF, heart failure; MI, myocardial infarction.
*Identified by patients as most important to learn before discharge.
When medical terminology is used, its meaning should be explained in lay terms. For example, it can be explained that the heart, a four-chambered pump, is a muscle that needs oxygen like all other muscles in order to work properly. When blood vessels supplying the heart muscle with oxygen become narrowed by atherosclerosis, there is less oxygen available to the heart muscle. It is a good idea to have a model of the heart or to use a pad and pencil to sketch what is being explained. Literature written for a non-medical audience is available through the Heart Foundation and is available on its websites (see Resources on p 893). DVDs are also helpful tools that can be used to teach patients.
Anticipatory guidance involves preparing the patient and family for what to expect in the course of recovery and rehabilitation. By learning what to expect during treatment and recovery, the patient gains a sense of control over life. This sense of perceived control allows the patient to consciously consider stressors and thus possibly to promote recovery. The idea of perceived control is operationalised as the process by which the patient exercises choice and makes decisions by cutting back. Cutting back is one way of minimising the psychological and physiological losses after MI (or any other life-changing event). The patient considers what must be cut back (changed), weighs this against what should be cut back and finally determines what will be changed. For example, a middle-aged man who smokes two packs of cigarettes a day, is 10 kg overweight and gets no physical exercise has a seemingly overwhelming task. He may decide that he can live with a weight reduction diet and will get more exercise (although perhaps not daily) but that it is not possible for him to quit smoking. He reasons that because he is modifying two of the three risk factors, he will be safe if he cuts back on tobacco use. Ideally the tobacco risk factor should be a priority for this patient, but if information regarding the risks and effects of tobacco use is not accepted, the patient’s right to choose and to feel in control of life decisions must be respected.
 Physical exercise
Physical exercise
Exercise is an integral part of the rehabilitation program. It is necessary for optimal physiological functioning and psychological wellbeing. Exercise has a direct, positive effect on maximal oxygen uptake, increasing CO, decreasing blood lipids, decreasing BP, increasing blood flow through the coronary arteries, increasing muscle mass and flexibility, improving the psychological state and assisting in weight loss and control. A regular schedule of moderate exercise, even after many years of sedentary living, is beneficial.51
One method used to identify levels of physical activity is through metabolic equivalent (MET) units: 1 MET is the amount of oxygen needed by the body at rest—3.5 mL of oxygen per kilogram per minute or 4.8 kJ/kg of body weight per minute. The MET is used to determine the energy costs of various exercises (see Box 33-5).
BOX 33-5 Metabolic equivalents (METs) for selected activities*
Source: The assessment and management of cardiovascular risk, 2003. Available at www.nzgg.org.nz. Adapted from Ainsworth BE. The compendium of physical activities tracking guide. University of South Carolina; 2002.
*1 MET equals oxygen consumption at rest, which is about 3.5 mL/kg of body weight per minute. An individual exercising at 2 METs is consuming oxygen at twice the resting rate.
*1 MET equals oxygen consumption at rest, which is about 3.5 mL/kg of body weight per minute. An individual exercising at 2 METs is consuming oxygen at twice the resting rate.
In the hospital, the activity level is gradually increased so that by the time of discharge the patient can tolerate moderate-energy activities of 3–6 METs. Many patients with UA that has resolved or an uncomplicated MI are in the hospital for approximately 3–4 days. By day 2, the patient can ambulate in the hallway and begin limited stair climbing (e.g. 3–4 steps). Many doctors order low-level treadmill tests before discharge to assess readiness for discharge, accurate HR for an exercise prescription and potential for ischaemia or reinfarction. If tests are positive (i.e. ischaemia at a low level of energy expenditure), the patient is evaluated for cardiac catheterisation before discharge. If the test is negative, catheterisation may still be done before discharge or several weeks after discharge.41 Because of the short hospital stay, it is critical to give the patient specific guidelines for activity and exercise so that overexertion will not occur. It is important to stress that the patient should ‘listen to what the body is saying’—the most important facet of recovery.
Teaching the patient to check their pulse rate is a nursing responsibility. The patient should be taught the parameters within which to exercise and told the maximum HR that should be present at any point. If the HR exceeds this level or does not return to the rate of the resting pulse within a few minutes, the patient should stop and rest. The patient should also be instructed to stop exercising if angina or dyspnoea occurs.
In a normal, healthy person the minimum threshold for improving cardiorespiratory fitness is 60% of the age-predicted maximum HR (which is calculated by subtracting the person’s age from 220). The ideal training target HR is 80% of maximum HR. Patients who have been physically inactive and are just beginning an exercise program should do so under supervision whenever possible. The more important factor is the patient’s response to exercise in terms of symptoms rather than absolute HR, especially since many patients are on β-adrenergic blockers and may not be able to reach a target HR. This is a point that cannot be overstressed. Basic exercise guidelines for patients following ACS are based on the FITT formula and are presented in Box 33-6.
BOX 33-6 FITT exercise guidelines after ACS
PATIENT & FAMILY TEACHING GUIDE
Warm-up/cool-down
Mild stretching for 3–5 min before the exercise activity and 5 min after the activity is important. Activity should not be started or stopped abruptly.
Intensity
Exercise intensity should be determined by the patient’s HR. If a treadmill test has not been performed, the patient recovering from an MI should not exceed 20 beats/min over the resting HR.
The basic categories of exercise are static (isometric) and dynamic (isotonic). Most daily activities are a mixture of the two. Static exercise involves the development of tension during muscular contraction but produces little or no change in muscle length or joint movement. Lifting, carrying and pushing heavy objects are primarily isometric activities. Because the HR and BP increase rapidly during isometric work, exercise programs involving isometric exercises should be limited. Isotonic exercises involve changes in muscle length and joint movement with rhythmic contractions at relatively low muscular tension. Walking, jogging, swimming, cycling and skipping are examples of activities that are predominantly isotonic. Isotonic exercise can put a safe, steady load on the heart and lungs and may also improve the circulation in many organs.
Many patients are referred to an outpatient cardiac rehabilitation program (see Box 33-2). These programs have been found to be beneficial to patients, but not all patients choose or are able to participate in them. Home-based cardiac rehabilitation programs have been developed as an alternative. Exercise guidelines are developed for the patient and staff maintain ongoing contact with the patient (e.g. via telephone, exercise logs, email).53 Maintaining contact with the patient appears to be the key to the success of these programs.
Research has shown that older women (>65 years) who experience MI have poor adherence to a regular exercise program.52 Often these women describe continued fatigue post-MI that is poorly understood.52 One study found that home-based cardiac rehabilitation programs were more successful for older women compared to traditional outpatient programs.49 Another factor that has been linked to poor adherence to an exercise program after MI is depression.52 Both men and women experience mild to moderate depression post MI that should resolve in 1–4 months.49 If depression persists after this, patients should be referred for appropriate treatment (e.g. counselling, medication).
 Resumption of sexual activity
Resumption of sexual activity
It is important to include sexual counselling for cardiac patients and their partners. This often-neglected area of discussion may be difficult for both patients and healthcare providers to approach. However, the patient’s concern about resumption of sexual activity after hospitalisation for ACS often produces more stress than the physiological act itself. About one-third of men and women either do not resume sexual activity or have a decrease in sexual activity after MI.49 The majority of these patients change their sexual behaviour not because of physical problems, but because they are concerned about sexual inadequacy, death during coitus and impotence. Such misconceptions can be clarified with specific counselling by a concerned and knowledgeable healthcare provider.
Before providing guidelines on the resumption of sexual activity, it is important to know the physiological status of the patient, the physiological effects of sexual activity and the psychological effects of having a heart attack. Sexual activity for middle-aged men and women with their usual partners is no more strenuous than climbing two flights of stairs.
Many nurses are unsure of how and when to begin counselling about resumption of sex. It is helpful to consider sex as a physical activity and to discuss or explore feelings in this area when other physical activities are discussed. One helpful approach is, ‘Many people who have had a heart attack wonder when they will be able to resume sexual activity. Has this been of concern to you?’ Another is, ‘If this has been of concern to you, this information should be helpful’. This type of non-threatening statement raises the topic, allows the patient to explore their personal feelings and gives them an opportunity to raise questions with the nurse or another healthcare provider. Common guidelines are presented in Box 33-7.
BOX 33-7 Sexual activity after ACS
PATIENT & FAMILY TEACHING GUIDE
• Planning of resumption of sexual activity should correspond to sexual activity before hospitalisation for ACS.
• Physical training (exercise) seems to improve the physiological response to coitus; therefore, daily exercise during recovery should be encouraged.
• Consumption of food and alcohol should be reduced before intercourse is anticipated (e.g. waiting 3–4 h after ingesting a large meal before engaging in sexual activity).
• Familiar surroundings and a familiar partner reduce anxiety.
• Masturbation may be a useful sexual outlet and may reassure the patient that sexual activity is still possible.
• Hot or cold showers should be avoided just before and just after intercourse.
• Foreplay is desirable because it allows a gradual increase in heart rate before orgasm.
• Positions during intercourse are a matter of individual choice.
• Orogenital sex places no undue strain on the heart.
• A relaxed atmosphere free of fatigue is optimal.
• Prophylactic use of nitrates is effective in decreasing angina during sexual activity.
• Use of erectile agents (e.g. sildenafil) is contraindicated if taking nitrates in any form.
• Anal intercourse may cause undue cardiac stress because of the possibility of inducing a vasovagal response.
The patient should be counselled that resumption of sex depends on the patient and their partner’s emotional readiness and on the doctor’s assessment of the extent of recovery. It is now known that it is safe to resume sexual activity 7–10 days after an uncomplicated MI.44,47 Some doctors believe that the patient should decide when they are ready to resume sex, while others say that the patient must be able to climb two flights of stairs briskly without dyspnoea or angina before sexual activity can be resumed.
Reading material on resumption of sexual activity may be given to the patient to facilitate discussion. Calmly and matter-of-factly introducing the subject of resumption of sexual activity during teaching about physical activity has positive effects of eliciting questions and concerns that might not have otherwise surfaced. For example, the nurse might begin, ‘Sexual activity is like other forms of activity and should be gradually resumed after MI. If your ability to perform sexually is concerning you, the energy expenditure has been found to be no more than walking briskly or climbing two flights of stairs’. This forms a factual basis for the patient to begin to seek information and explore their personal feelings about resuming sex.
Patients also need to know that inability to perform sexually after MI is common and that sexual dysfunction usually disappears after several attempts. The nurse should reinforce the idea that patience and understanding usually solve the problem. With the availability of drugs to correct erectile dysfunction, many male patients may be interested in using them. However, they should be cautioned that these drugs should not be used with nitrates as severe hypotension and even death have been reported. Encourage patients to discuss the use of these drugs with their doctor or nurse practitioner.53
It is not uncommon for a patient who experiences chest pain on physical exertion to have some angina during sexual stimulation or intercourse. The patient should be instructed to take glyceryl trinitrate prophylactically. It is also helpful to avoid sex soon after a heavy meal or after excessive ingestion of alcohol, when extremely tired or stressed, or with unfamiliar partners. Anal intercourse is to be avoided because of the likelihood of eliciting a vasovagal response.
SUDDEN CARDIAC DEATH
Sudden cardiac death (SCD) is unexpected death from cardiac causes. CAD is the most common cause of SCD and accounts for 80% of all SCDs. The affected person may or may not have a known history of CAD and SCD may be the first sign of illness for 25% of those who die of heart disease.53 Of the patients who survive SCD, only 20% are discharged from hospital without neurological impairment.
AETIOLOGY AND PATHOPHYSIOLOGY
In SCD there is an abrupt disruption in cardiac function, producing an abrupt loss of cardiac output and cerebral blood flow. Death usually occurs within 1 hour of the onset of acute symptoms (e.g. angina, palpitations).
The majority of cases of SCD are caused by acute ventricular arrhythmias (e.g. ventricular tachycardia, ventricular fibrillation). Less commonly, SCD may occur as a result of a primary left ventricular outflow obstruction (e.g. aortic stenosis, hypertrophic cardiomyopathy).
Persons who experience SCD as a result of CAD fall into two groups: (1) those who had an acute MI; and (2) those who did not have an acute MI. The latter group accounts for the majority of cases of SCD.53 In this instance, victims usually have no warning signs or symptoms. Patients who survive are at risk of recurrent SCD due to the continued electrical instability of the myocardium that caused the initial event to occur. For the smaller group who had an MI and suffered SCD, patients usually do have prodromal symptoms, such as chest pain, palpitations and dyspnoea.
It is difficult to predict who is at risk of SCD. However, left ventricular dysfunction (EF <30%) and ventricular arrhythmias following MI have been found to be the strongest predictors.53 Other risk factors for SCD include: (1) male gender; (2) family history of premature atherosclerosis; (3) tobacco use; (4) diabetes mellitus; (5) hypercholesterolaemia; (6) hypertension; and (7) cardiomyopathy.
 NURSING AND COLLABORATIVE MANAGEMENT: SUDDEN CARDIAC DEATH
NURSING AND COLLABORATIVE MANAGEMENT: SUDDEN CARDIAC DEATH
People who have been resuscitated following an episode of SCD generally require a diagnostic examination to determine whether they have had an MI. Thus serial analysis of cardiac markers and ECGs must be obtained and the patient must be treated accordingly. (See the section on multidisciplinary care of ACS on p 878.) In addition, because most people with SCD have CAD, cardiac catheterisation is indicated to determine the possible location and extent of coronary artery occlusion. PCI or CABG surgery may be indicated.
Most SCD patients have a lethal ventricular arrhythmia that is associated with a high incidence of recurrence. Thus it is useful to know when these patients are most likely to have a recurrence and what drug therapy is the most effective treatment. Assessment of arrhythmias includes 24-hour Holter monitoring or other type of event recorder, exercise stress testing, signal-averaged ECG and electrophysiological study (EPS).54 EPS is performed under fluoroscopy; pacing electrodes are placed in selected intracardiac areas and stimuli are selectively used to attempt to produce arrhythmias. The patient’s response to various antiarrhythmic medications can be determined and monitored in a controlled environment. (EPS is discussed in Chs 31 and 35.)
The most common approach to preventing a recurrence is the use of an implantable cardioverter–defibrillator (ICD). Research has shown that an ICD improves survival compared to drug therapy alone.55 (ICDs are discussed in Ch 36.) Drug therapy with amiodarone did not improve survival in clinical trials, but may be used in conjunction with an ICD to decrease episodes of ventricular arrhythmias.54
The nurse caring for a survivor of SCD should be attuned to the patient’s psychosocial adaptation to this sudden ‘brush with death’. Many patients develop a ‘time bomb’ mentality. They fear the recurrence of cardiopulmonary arrest and may become anxious, angry and depressed. Their families are likely to experience the same feelings. This fear often interferes with the resumption of normal activities, such as sexual and recreational activities.54,56 Patients and families also may need to deal with additional issues such as possible driving restrictions and change in occupation. The grief response varies among patients and families. Nurses should be attuned to the specific needs of each patient and family and teach them accordingly while providing appropriate emotional support.
The patient with myocardial infarction
CASE STUDY
Patient profile
Matthew, a 51-year-old successful businessman, was rushed to the hospital by ambulance after experiencing crushing substernal chest pain that radiated down his left arm. He also complained of dizziness and nausea.
Objective data
Multidisciplinary care: emergency department
• Oxygen 2 L/min via nasal cannula
• Glyceryl trinitrate IV, titrate to relieve chest pain; hold for systolic BP <100 mmHg
• Morphine 2–4 mg IV every 5 min as needed for chest pain unrelieved by glyceryl trinitrate
• Metoprolol 5 mg IV every 5 min × 3 doses
• Vital signs, pulse oximetry every 10 min during infusion of reteplase
CRITICAL THINKING QUESTIONS
1. Which coronary artery/ies is/are most likely occluded in this patient’s coronary circulation?
2. Explain the pathogenesis of coronary artery disease. What risk factors may contribute to its development? What risk factors were present in this patient’s life?
3. What is angina? How does chronic stable angina differ from angina associated with myocardial infarction?
4. Explain the pathophysiological basis for the clinical manifestations that this patient exhibited.
5. Explain the significance of the results of the laboratory tests and ECG findings.
6. Provide a rationale for each treatment measure ordered for this patient.
7. Based on the assessment data presented, write one or more priority nursing diagnoses.
1. In teaching a patient about coronary artery disease (CAD), the nurse explains that the changes that occur in this disorder involve:
2. After teaching about ways to decrease risk factors for CAD, the nurse recognises that additional instruction is needed when the patient says:
3. A hospitalised patient with angina tells the nurse that she is having chest pain. The nurse bases her actions on the knowledge that angina:
4. The clinical spectrum of acute coronary syndrome (ACS) includes:
5. In planning activity for the patient recovering from myocardial infarction, the nurse recognises that the healing heart wall is most vulnerable to stress:
6. A patient is admitted to the coronary care unit with chest pain of 24 hours duration, electrocardiogram findings consistent with an acute myocardial infarction and ventricular arrhythmias. The nurse plans care for the patient based on the expectation that the patient will be managed with:
7. Three days after MI, a patient states that he does not understand what the alarm is about because his problem was just a case of ‘bad indigestion’. His reaction is an example of:
8. The most common abnormal finding in individuals at risk for sudden cardiac death is:
1 Australian Institute of Health & Welfare (AIHW). Australia’s health 2010. Canberra: AIHW. Available at www.aihw.gov.au/publications/aus/ah10/11374-c04.pdf. accessed 1 September 2010.
2 New Zealand Ministry of Health (NZMOH)/New Zealand Guidelines Group. New Zealand cardiovascular guidelines handbook: a summary resource for primary care practitioners. 2nd edn. Wellington: New Zealand Guidelines Group; 2009. Available at www.nzgg.org.nz/guidelines/0154/CVD_handbook_june_2009_update.pdf accessed 3 January 2010.
3 Australian National Vascular Disease Prevention Alliance. Guidelines for the assessment of absolute cardiovascular disease risk. Available at www.heartfoundation.org.au/SiteCollectionDocuments/A_AR_Guidelines_FINAL%20FOR%20WEB.pdf, 2009. accessed 3 January 2010.
4 Libby P. The vascular biology of atherosclerosis. In Zipes DP, Libby P, Bonow RO, et al, eds.: Heart disease: a textbook of cardiovascular medicine, 8th edn., Philadelphia: WB Saunders, 2008.
5 Craft J, Gordon C, Tiziani A. Understanding pathophysiology. Sydney: Elsevier, 2011.
6 National Health Foundation. Absolute cardiovascular risk assessment: quick reference guide for health professionals. Available at www.heartfoundation.org.au/SiteCollectionDocuments/A_AR_QRG_FINAL%20FOR%20WEB.pdf. accessed 3 January 2010.
7 Kaski JC, Fernández-Bergés DJ, Consuegra-Sánchez L, Fernández JM, García-Moll X, Mostaza JM, et al. A comparative study of biomarkers for risk prediction in acute coronary syndrome. Results of the SIESTA (Systemic Inflammation Evaluation in non-ST-elevation Acute coronary syndrome) study. Atherosclerosis. 2010;212(2):636–643.
8 Yusuf S, Hawken S, Ounpuu T, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937–952.
9 Spader C. Statins and hsCRP test refine cardio care. Available at http://include.nurse.com/article/20090309/NATIONAL02/103090109, 2009. accessed 3 December 2010.
10 Smith SC, Allen J, Blair SN, et al. American Heart Association/American College of Cardiology guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363.
11 Mosca L, Banka CL, Benjamin EJ, Berra K, Bushnell C, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115:1481–1501.
12 Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239.
13 Ridker PM, Genest J, Libby P. Risk factors for atherosclerotic disease. In Zipes DP, Libby P, Bonow RO, et al, eds.: Heart disease: a textbook of cardiovascular medicine, 8th edn., Philadelphia: WB Saunders, 2008.
14 National Heart Foundation of Australia and Cardiac Society of Australia and New Zealand. Position statement on lipid management. Heart Lung Circ. 2005;14:275–291.
15 Tamam MN, Afonso LC, Ramappa P, Hari P. Primary and secondary prevention of coronary artery disease. Available at http://emedicine.medscape.com/article/164214-overview, Updated 23 November 2010. accessed 3 December 2010.
16 Ridker PM, Genest J, Libby P. Guidelines, diagnosis and treatment of lipid disorders. In Zipes DP, Libby P, Bonow RO, et al, eds.: Heart disease: a textbook of cardiovascular medicine, 8th edn., Philadelphia: WB Saunders, 2008.
17 Heart Foundation of Australia National Blood Pressure Advisory Committee. A guide for managing hypertension, 2008. Canberra: Heart Foundation, 2008.
18 He J, Vupputuri S, Allen K, et al. Passive smoking and the risk of coronary heart disease—a meta-analysis of epidemiologic studies. N Engl J Med. 1999;340(12):920–926.
19 Buchanan LM, El-Banna M, White A, et al. An exploratory study of multicomponent treatment intervention for tobacco dependency. J Nurs Scholarship. 2004;36(4):324–330.
20 Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel KR, Franklin BA. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:e285–e290.
21 Yu PC, Bosnyak Z, Ceriello A. The importance of glycated haemoglobin (HbA(1c)) and postprandial glucose (PPG) control on cardiovascular outcomes in patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89(1):1–9.
22 Alberti KGMM, Donato K, Eckel RH, Fruchart J-C, Grundy S, et al. A Joint Interim Statement of the Association for the Study of Obesity; World Heart Federation; International Atherosclerosis Society; International National Heart, Lung, and Blood Institute; American Heart Association; and International Diabetes Federation Task Force on Epidemiology and Prevention. Harmonizing the metabolic syndrome. Circulation. 2009;120:1640–1645.
23 Grassi G, Seravalle G, Quarti-Trevano F, et al. Metabolic syndrome and cardiometabolic risk: an update. Blood Pressure. 2009;18:7.
24 Futterman LG, Lemberg L. The Framingham Heart Study: a pivotal legacy of the last millennium. Am J Crit Care. 2000;9(2):147–151.
25 Barsky R. Psychiatric and behavioral aspects of cardiovascular disease. In Zipes DP, Libby P, Bonow RO, et al, eds.: Heart disease: a textbook of cardiovascular medicine, 8th edn., Philadelphia: WB Saunders, 2008.
26 Orth-Gomer K, Albus C, Bages N, et al. Psychosocial considerations in the European guidelines for prevention of cardiovascular diseases in clinical practice: third joint task force. Int J Behav Med. 2005;12(3):132–141.
27 Kubzansky LD, Davidson KW, Rozanski A. The clinical impact of negative psychological states: expanding the spectrum of risk for coronary artery disease. Psychosom Med. 2005;67(suppl 1):S10–S14.
28 Frasure-Smith N, Lespérance F. Reflections on depression as a cardiac risk factor. Psychosom Med. 2005;67(suppl 1):S19–S25.
29 Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary artery disease. Psychosom Med. 2005;67(suppl 1):S29–S33.
30 Humphrey LL, Fu R, Rogers K, et al. Homocysteine level and coronary heart disease incidence: a systematic review and meta-analysis. Mayo Clinic Proc. 2008;83(11):1203.
31 Wang G, Mao JM, Wang X, et al. Effect of homocysteine on plaque formation and oxidative stress in patients with acute coronary syndromes. Chin Med J (Engl). 2004;117(11):1650–1654.
32 National Heart Foundation of Australia and the Cardiac Society of Australia and New Zealand: position statement on lipid management—2005. Heart Lung Circ. 2005;14(4):275–291.
33 Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998;280(23):2001–2007.
34 Smith SC, Allen J, Blair SN, et al. American Heart Association/American College of Cardiology guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363.
35 Lehne RA. Pharmacology for nursing care, 7th edn. St Louis: Saunders, 2010.
36 Ridker P, Cook N, Lee I, et al. A randomized control trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–1304.
37 Deaton C. State of the science for the care of older adults with heart disease. Nurs Clin North Am. 2004;39(3):495–528.
38 Fair JM. Cardiovascular risk factor modification: is it effective in older adults? J Cardiovasc Nurs. 2003;18(3):161–168.
39 Gantz P, Gantz W. Coronary blood flow and myocardial ischaemia. In Zipes DP, Libby P, Bonow RO, et al, eds.: Heart disease: a textbook of cardiovascular medicine, 8th edn., Philadelphia: WB Saunders, 2008.
40 Pries A, Habazettl H, Ambrosio G, et al. A review of methods for assessment of microvascular disease in both clinical and experimental settings. Cardiovascular Res. 2008;80:165.
41 Aroney C, Aylward P, Kelly A, et al. Guidelines for the management of acute coronary syndromes: 2007 addendum. Med J Aust. 2008;188(5):302–303.
42 Gluckman TJ, Sachdev M, Schulman SP, et al. A simplified approach to the management of non-ST-segment elevation acute coronary syndromes. JAMA. 2005;293(3):349–357.
43 ACC/AHA 2002 Guideline update for the management of patients with unstable angina and non–ST-segment elevation myocardial infarction. A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. The American College of Cardiology and the American Heart Association. Available at www.courses.ahc.umn.edu/pharmacy/5822/UAUPDATEACCTrial.pdf. accessed 3 December 2010.
44 King SB, Smith SC, Hirshfeld JW. 2007 focused update of the ACC/AHA/SCAI 2005 guideline update for percutaneous coronary intervention. Circulation. 2008;117:261.
45 Libby P, Bonow R, Mann D, et al. Braunwald’s heart disease: a textbook of cardiovascular medicine, 8th edn. Philadelphia: Saunders, 2008.
46 Schampaert E, Cohen EA, Schluter M, et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRUS). J Am Coll Cardiol. 2004;43:1110–1115.
47 McSweeney JC, Cody M, O’Sullivan P, et al. Women’s early warning symptoms of acute myocardial infarction. Circulation. 2003;108(21):2619–2623.
48 Moser D, Riegel B. Care of patients with acute coronary syndrome: ST-segment elevation myocardial infarction. In: Moser D, Riegel B, eds. Cardiac nursing: a companion to Braunwald’s heart disease. St Louis: Saunders Elsevier, 2008.
49 Deaton C, Namasivayam S. Nursing outcomes in coronary heart disease. J Cardiovasc Nurs. 2004;19(5):308–315.
50 McSweeney JC, Coon S. Women’s inhibitors and facilitators associated with making behavioral changes after myocardial infarction. Medsurg Nurs. 2004;13(1):49–54.
51 Lavie CJ, Thomas RJ, Squires RW, Allison TG, Milani RV. Exercise training and cardiac rehabilitation in primary and secondary prevention of coronary heart disease. Mayo Clin Proc. 2009;84(4):373–383.
52 Evangelista O, McLaughlin MA. Review of cardiovascular risk factors in women. Gend Med. 2009;6(suppl 1):17.
53 Cheitlin MD. Sexual activity and cardiovascular disease. Am J Cardiol. 2003;92(9A):3M–8M.
54 Mudawi TO, Albouaini K, Kaye GC. Sudden cardiac death: history, aetiology and management. Br J Hosp Med. 2009;70(2):89.
55 Davis DR, Tang AS. Implantable cardioverter defibrillators: therapy against Canada’s leading killer. CMAJ. 2004;171(9):1037–1038.
56 Dickerson SS, Kennedy M, Wu YW, Underhill M, Othman A. Factors related to quality-of-life pattern changes in recipients of implantable defibrillators. Heart Lung. 2010;39(6):466–476.
Australasian Cardiovascular Nurses College. www.acnc.net.au
Cardiac Society of Australia and New Zealand. www.csanz.edu.au
Heart Foundation of New Zealand. www.nhf.org.nz
Heart Support Australia Ltd. www.heartnet.org.au
National Heart Foundation of Australia. www.heartfoundation.com.au