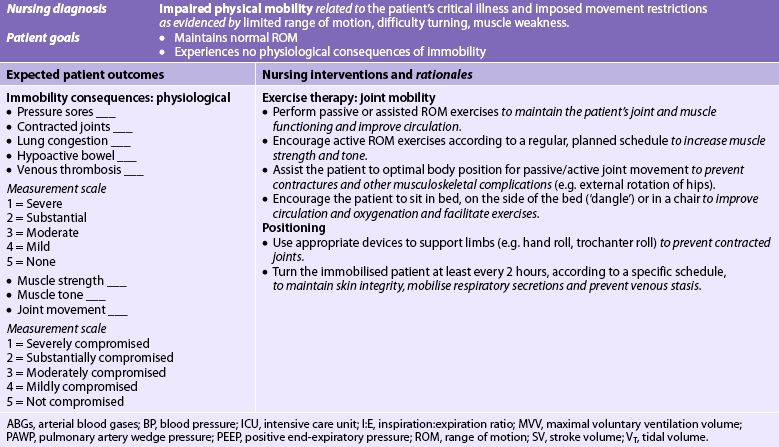
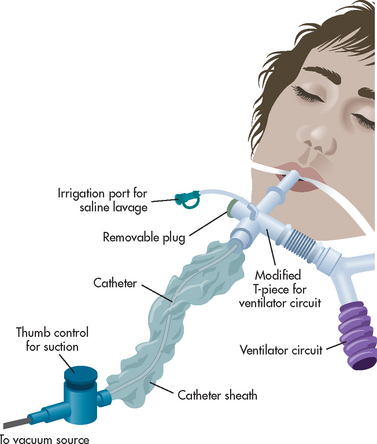
Chapter 65 NURSING MANAGEMENT: critical care environment
1. Select appropriate nursing interventions to manage the needs and common problems of critically ill patients.
2. Develop effective strategies to manage issues related to the families of critically ill patients.
3. Discuss the principles of haemodynamic monitoring and related multidisciplinary care of critically ill patients.
4. Describe the purpose, indications and function of circulatory assist devices and related multidisciplinary care.
5. Examine types of artificial airways and appropriate nursing interventions to manage the care of a patient requiring respiratory support.
6. Differentiate between the indications for, and modes of, mechanical ventilation.
7. Describe the principles of mechanical ventilation and related multidisciplinary care of critically ill patients.
assist-control ventilation (ACV),
bilevel positive airway pressure (BiPAP),
circulatory assist devices (CADs),
closed-suction technique (CST),
continuous positive airway pressure (CPAP),
controlled mandatory ventilation (CMV),
high-frequency ventilation (HFV),
intraaortic balloon pump (IABP),
negative pressure ventilation,
positive end-expiratory pressure (PEEP),
positive pressure ventilation (PPV),
pressure support ventilation (PSV),
synchronised intermittent mandatory ventilation (SIMV),
Critical care nursing
CRITICAL CARE UNITS
Critical care units (CCUs) or intensive care units (ICUs) are designed to meet the health needs of acute and critically ill patients. As far back as the 1800s Florence Nightingale developed the concept of clustering the most acutely ill patients.1 During the poliomyelitis and tuberculosis pandemics in the middle of the 20th century, special units staffed by specialised health personnel were established. These units were equipped with technical equipment to manage patients’ airways and ventilate patients. Lessons learned from World War II and the Korean War solidified the concepts of triage and specialty nursing units and by the late 1950s these concepts were being incorporated into hospital systems.2,3
In the 1960s technological developments allowed for more accessible monitoring of the electrocardiogram (ECG), arterial and central venous pressures, and arterial blood gases (ABGs). This led to the development of coronary care units for patients with acute myocardial infarction. In these units patients’ cardiac rhythms were continually monitored. Nurses followed protocols to manage arrhythmias aggressively. By the 1970s the ICU was a standard unit in most Western-style general hospitals worldwide. Since that time, technical advances have continued at a rapid pace, bringing improved monitoring capabilities and new strategies to manage life-threatening problems.3
In some acute care settings, the concept of ICU care has expanded from delivering care within the unit to offering assessment and care to acutely or critically ill patients within wards in the same hospital or emergency departments in smaller regional hospitals. The concept of the virtual ICU is designed to augment the care given at the bedside by viewing patient data and giving expert recommendations for care from a remote location. Another development is the critical care outreach service, which usually comprises an experienced critical care nurse or clinical nurse consultant who monitors discharged ICU patients in the hospital ward and liaises with ward-based nurses and doctors about patient progress and care needs. The aim of this role is to augment patient recovery and minimise the chance of patients requiring readmission to ICU.4
The term critical care nursing is often used interchangeably with the term intensive care nursing but it is not exclusively restricted to that specialty area. The critical care nurse is responsible for assessing life-threatening conditions, instituting appropriate interventions and evaluating treatment outcomes while concurrently addressing the psychosocial needs of the patient and family. The technology available in critical care areas is extensive and continually evolving. The capability exists to continuously monitor ECG levels, blood pressure, oxygen levels, respiratory function, central venous pressure, intracranial pressure and core body temperature. More advanced monitoring devices allow for the measurement of pressures in the pulmonary artery (PA)—known as PA pressures—cardiac output, end-tidal carbon dioxide (CO2) tension and even tissue oxygen consumption. Patients may be receiving continual support from mechanical ventilators, intraaortic balloon pumps or dialysis machines.
The common abbreviations used in critical care nursing are listed in Table 65-1.
TABLE 65-1 Abbreviations commonly used in the intensive care unit
| Abbreviation | Term |
|---|---|
| CI | Cardiac index |
| CO | Cardiac output |
| CVP | Central venous pressure |
| FIO2 | Fraction of inspired oxygen |
| IABP | Intraaortic balloon pump |
| MAP | Mean arterial pressure |
| PA | Pulmonary artery |
| PAS, PAD | PA systolic (pressure), PA diastolic (pressure) |
| PAWP | Pulmonary artery wedge pressure |
| PVR | Pulmonary vascular resistance |
| SPO2 | Percentage oxygen saturation of haemoglobin measured by pulse oximetry |
| SvO2 | Percentage oxygen saturation of haemoglobin in mixed venous blood (e.g. in the PA) |
| SI | Stroke index |
| SV | Stroke volume |
| SVR | Systemic vascular resistance |
| VAD | Ventricular assist device |
CRITICAL CARE NURSE
The critical care nurse cares for patients with acute and unstable physiological problems in an environment equipped for technically advanced methods of assessing and managing patient problems. In both Australia and New Zealand there are diverse types of critical care settings and practice can vary markedly depending on unit size, specialty and resources.5,6 Critical care nursing requires in-depth knowledge of anatomy, physiology, pathophysiology and pharmacology, and advanced assessment skills, as well as the ability to use advanced medical technology. The critical care nurse provides ongoing assessment and early recognition and management of complications while fostering healing and recovery. Apart from taking patients’ observations, the critical care nurse, in consultation with intensivists (specialised medical practitioners), provides respiratory care and undertakes routine ventilator setting manipulations, manages continuous renal replacement therapy, titrates vasoactive medications and monitors patients’ ECGs.7,8 Critical care nurses who have undertaken advanced life support (ALS) certification initiate treatment during a cardiac arrest. The nurse also provides all the personal care required by the patient, as well as giving psychosocial support to the patient and family. The critical care nurse is a pivotal member of the healthcare team who communicates and collaborates with other healthcare providers (e.g. intensivists, medical specialists, physiotherapists, pharmacists, social workers, dieticians).
Nursing practice in the ICU often follows a one nurse to one or two patient allocation model, depending on patient acuity and staffing and unit practice.5 The ICU nurse spends most of their working hours near the patient’s bedside ensuring that the patient is monitored continually. Entry to the specialisation of ICU nursing occurs over a long period, beginning with supervised clinical orientation and regular and frequent informal or formal educational/professional development programs. These can occur within the hospital or health service area or as formal postgraduate qualifications.
The staff mix in any unit usually comprises nurses who have beginning, intermediate and extensive experience in the specialty. The specialist critical care nurse usually has postgraduate or post-registration specialist qualifications and significant experience within their specialty. It is recommended that at least 50% of the nursing staff in any critical care unit have specialty post-registration qualifications.5 Critical care nurse specialists, as defined by the Australian College of Critical Care Nurses (ACCCN), are those who provide competent, accountable and holistic care for critically ill patients through the integration of advanced knowledge, skills and humanistic values, within a sound ethical and legal framework.5,7 ACCCN provides the opportunity for nurse specialists to gain credentials in adult or paediatric critical care nursing. This designation requires extensive practice experience in critical care nursing and successful submission of a comprehensive portfolio. Australian Clinical Nurse Consultants (CNCs), New Zealand Nurse Consultants (NCs) or Nurse Practitioners (NPs) function at a more advanced or extended practice level, advising and coordinating patient management, as well as often participating in research related to nursing practice. These nurses usually have higher degree qualifications (master’s or doctorate).
CRITICAL CARE PATIENT
Patients are generally admitted to a critical care area for one of three reasons. First, the patient has the potential to be, or is already, critically ill and therefore physiologically unstable, requiring advanced and sophisticated clinical judgements by the nurse or medical team. Second, the patient may be at risk of serious complications and require frequent and often invasive assessment. Third, the patient may require intensive and complicated nursing support related to the use of intravenous (IV) polypharmacy (e.g. sedative or neuromuscular blockade, thrombolytics or drugs requiring titration) and advanced medical technological interventions (e.g. mechanical ventilation, intracranial pressure monitoring, continuous renal replacement therapy and haemodynamic monitoring).
ICU patients can be clustered by disease condition (e.g. neurological, cardiovascular, respiratory) or by age group (e.g. neonatal, paediatrics, adult). Patients commonly treated in the ICU include those with medically focused problems (e.g. respiratory distress, myocardial ischaemia or acute neurological impairment) or those receiving care and close monitoring after major surgery or organ transplantation. Common admission medical diagnoses include respiratory failure, myocardial ischaemia or infarction, acute head injury, postoperative cardiac surgery, trauma, burns, spinal injuries, sepsis, diabetic ketoacidosis, drug overdoses, poisonings and thyroid, adrenal or haematological crises. Patients with multiple comorbidities may also be monitored in the ICU while receiving care for unrelated conditions. Patients who are not expected to recover from an illness are usually not admitted to an ICU. For example, the ICU is not usually the place to manage patients in a persistent coma or purely to prolong the natural process of dying.
Despite the emphasis on caring for the patient who is expected to survive, the incidence of death is higher in ICU patients than in non-ICU patients. This suggests a need for caution and coordination of care when discharging patients from ICUs to hospital wards. In general, non-survivors are older, have pre-existing health problems and experience longer ICU stays.9
High-dependency units (HDUs), or step-down units, have been established to offer an intermediate level of care between the ICU and the hospital ward. Generally, patients in HDUs are at risk of serious complications but their risk is lower or they require less invasive support than that of ICU patients. Examples of patients frequently managed in HDUs include patients scheduled for interventional cardiac procedures (e.g. intracoronary stent or cardiac pacemaker implantation), those awaiting heart transplant and patients requiring vasoactive IV drugs but not requiring invasive ventilation. Patients in these units can be monitored for alterations to cardiac rhythm, arterial blood pressure, oxygen saturation and end-tidal CO2. The use of HDUs provides specialised nursing care for an at-risk patient population in a less intensive and therefore more cost-effective environment.
Common problems of critical care patients
The patient admitted to the ICU is at risk of developing numerous complications and specific problems. Critically ill patients are usually unable to mobilise out of or far from their bed and are at risk of developing skin problems. The use of multiple, invasive devices predisposes the patient to iatrogenic infections, resulting in sepsis and/or multiple organ dysfunction syndrome (MODS; see Ch 66). Adequate nourishment for the critically ill patient is paramount but may be challenging in some circumstances as it is not without complications. Other special problems for ICU patients relate to impaired communication, pain, anxiety, sensory–perception problems and sleep disorders.
Nutrition
Frequently, patients are admitted to ICU with conditions that result in either hypermetabolic states (e.g. burns, trauma, sepsis) or catabolic states (e.g. acute kidney injury). Some patients may be admitted in severely malnourished states, such as those that occur with chronic cardiac, pulmonary or liver diseases. In general, malnutrition has been linked to increases in mortality and morbidity in critical illness. Determining who to feed, what to feed, when to feed and how to feed (e.g. route of administration) are crucial questions that must be considered when caring for a critically ill patient.10 The critical care nurse must collaborate with the intensivist and the dietician to determine how best to meet the nutritional needs of critically ill patients. An additional factor contributing to underfeeding patients in ICU is the frequent interruptions in enteral feedings due to medication administration and essential invasive diagnostic tests and procedures.
Nutritional support in critically ill patients
EVIDENCE-BASED PRACTICE
Clinical question
What is the relationship between nutritional support and outcomes for critical care patients?
Evidence
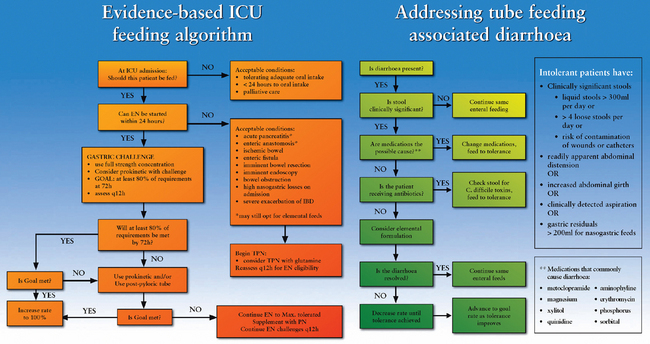
Published meta-analyses show enteral nutrition (EN) started within 24 h of injury or ICU admission may reduce infectious complications and save lives. If EN cannot be initiated within 24 h of ICU admission, a published meta-analysis shows parenteral nutrition (PN) started within 24 h of injury or ICU admission may save lives.
Critical appraisal and synthesis of evidence
• Nutritional support (EN or PN) is indicated when a critically ill patient is expected to remain in the ICU for at least 2 days and is not expected to commence oral intake within 24 h.
• EN should be commenced within 24 h of injury or ICU admission.
• If EN cannot be commenced within 24 h of injury or ICU admission, PN should be commenced within 24 h of injury or ICU admission.
• If a patient who is commenced on EN is not achieving at least 80% of their calculated goals by 72 h, consider post-pyloric feeding, use of prokinetics or supplementing intake with PN.
Heighes PT, Doig GS, Sweetman EA, Simpson F. An overview of evidence from systematic reviews evaluating early enteral nutrition in critically ill patients: more convincing evidence is needed. Anaesthesia and Intensive Care. 2010;38(1):167–174.
Doig GS, Heighes PT, Simpson F, Sweetman EA. Early enteral nutrition reduces mortality in trauma patients requiring intensive care: a meta-analysis of randomised controlled trials. Injury. 2010;12(2):1363–1950.
Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Enteral nutrition within 24 h of ICU admission significantly reduces mortality: a meta-analysis of RCTs. Intensive Care Med. 2009;35(12):2018–2027.
Simpson F, Doig GS. Parenteral vs enteral nutrition in the critically ill patient: a meta-analysis of trials using the intention to treat principle. Intensive Care Med. 2005;31(1):12–23.
Doig GS, Simpson F, Finfer S, Delaney A, Davies AR, Mitchell I, Dobb G, for the Nutrition Guidelines Investigators of the ANZICS Clinical Trials Group. Effect of evidence-based feeding guidelines on mortality of critically ill patients: a cluster randomized controlled trial. JAMA. 2008;300(23):2731–2741.
The primary goal of nutritional support is to prevent or correct nutritional deficiencies. This is usually accomplished by the provision of enteral nutrition (i.e. delivery of kilojoules via the gastrointestinal [GI] tract) or parenteral nutrition (i.e. delivery of kilojoules intravenously). Enteral nutrition is thought to preserve the structure and function of the gut mucosa and to help prevent translocation of gut bacteria. In addition, enteral nutrition may be associated with fewer complications.11,12 Parenteral nutrition should be considered only when the enteral route is unsuccessful in providing adequate nutrition or is contraindicated. Examples of these conditions are paralytic ileus, diffuse peritonitis, intestinal obstruction, pancreatitis, GI ischaemia, intractable vomiting and severe diarrhoea.12 Figure 65-1 outlines the Australia and New Zealand nutrition guidelines for ICU patients.
Anxiety
Symptoms of anxiety are frequently reported by both patients and their loved ones during and after time spent in ICU.13 The primary sources of anxiety for patients include the perceived or anticipated threat to physical health, actual loss of control of body functions and an environment that is foreign. Many patients and families feel uncomfortable in the ICU environment with its complex equipment, high noise and light levels and sometimes isolation from family and friends, as well as lack of privacy at times. Pain and sleeplessness enhance anxiety, as do immobilisation, loss of control and impaired communication.13 One recent study reported that at hospital discharge there was a significant association between patients’ perceptions of their intensive care experience and their levels of anxiety, depression, avoidance and intrusion symptoms at hospital discharge.14
To help reduce anxiety, the nurse should encourage patients and families to express concerns, ask questions and state their needs. Nurses and doctors should include the patient and family in conversations when possible or appropriate and explain the purpose of equipment and procedures. The nurse should also structure the patient’s surrounding environment in a way that may decrease anxiety. For example, family members can be encouraged to bring in photographs and personal items. Judicious use of anti-anxiety drugs (e.g. lorazepam) and complementary therapies (e.g. imagery, music, massage) may reduce the stress response that can be triggered by anxiety and should be considered.15
Pain
The control of pain in the ICU patient is paramount but can be challenging for the critical care nurse. Pain is a common and treatable condition among intensive care patients. However, a recent study that explored nurses’ knowledge and barriers regarding pain management in intensive care units found that knowledge about correct management of pain was poor, highlighting the need for the ICU nurse to engage in continuing education regarding best practice in pain management.16
For some critically ill patients, continuous IV sedation (e.g. fentanyl or morphine combined with midazolam) is a practical and effective strategy for pain control, and to relieve anxiety and manage agitation.15 However, patients receiving deep sedation can become unresponsive, and this prevents the nurse and other healthcare providers from properly assessing the patient’s neurological status. Additionally, administering sedative and analgesic medications can lead to unintended consequences, including delayed recovery from critical illness and slower weaning from mechanical ventilation. To address these limitations, guidelines that include a daily, scheduled interruption of sedation are being considered in practice. Daily sedative interruption allows the patient to awaken and the healthcare provider to conduct a neurological examination and assess readiness for weaning.17–19 One study indicated that the use of structured approaches to sedation management, including guidelines, protocols and algorithms, can reduce variation in clinical practice, reduce the likelihood of unnecessary or prolonged sedation and result in shorter duration of mechanical ventilation and ICU length of stay.19 However, this was not supported in a recent Australian study,20 indicating the need for further research in this area. The use of a sedation scoring instrument (e.g. Ramsay Motor Activity Assessment Scale [MAAS]) and accompanying protocol to titrate the IV infusion aims to keep patients adequately sedated and pain-free yet rousable.21 In some ICUs, diazepam combined with morphine infusions are often used with a pain scale to assess efficacy. One benefit with this regimen is that patient neurological assessments can potentially be performed with greater accuracy.
Impaired communication
Inability to communicate can be a distressing problem for the patient who may be unable to speak because of the use of sedative and paralysing drugs or an endotracheal tube.15 As part of any procedure the nurse should explain what will happen or is happening to the patient. When the patient cannot speak, the nurse should explore alternative methods of communication, including the use of devices such as picture boards, notepads, magic slates or computer keyboards. When speaking with the patient, the nurse should look directly at the patient and use hand gestures when appropriate. For patients who do not speak English, the use of an accredited hospital interpreter or family member is essential to facilitate communication.
Non-verbal communication is important. High levels of procedure-related touch and decreased levels of affection-related or comfort-related touch may characterise the ICU patient experience. Patients have different levels of tolerance for being touched, often related to cultural background and personal preference. It may be appropriate to provide comforting touch but with ongoing evaluation of the patient’s response. Often, the ICU nurse encourages the family to touch and talk to the patient, even if the patient is unresponsive or comatose.
Sensory–perception problems
Acute and reversible sensory–perception changes are common in ICU patients.22 The combination of alterations in mentation (e.g. delusions, short attention span, loss of recent memory), psychomotor behaviour (e.g. restlessness, lethargy) and sleep–wake cycle (e.g. daytime sleepiness, night-time agitation) has been inappropriately labelled ICU psychosis. The patient experiencing these alterations is not psychotic but is suffering from delirium. In a review of six Australian and New Zealand ICUs, the risk of developing delirium was up to 45% in patients who required ventilation support for more than 36 hours.23 Demographic factors predisposing patients to delirium include advanced age, pre-existing cerebral illnesses (e.g. dementia), use of medications that block rapid eye movement (REM) sleep (e.g. narcotics) and a history of drug or alcohol abuse. Environmental factors that can contribute to delirium include sleep deprivation, anxiety, sensory overload and immobilisation. Physical conditions, such as haemodynamic instability, hypoxaemia, electrolyte disturbances and severe infections, can precipitate delirium. Additionally, some drugs (e.g. sedatives [benzodiazepines], frusemide, antimicrobials [aminoglycosides]) have been associated with the development of delirium. Patients with delirium are likely to remain in hospital longer and have lower 6-month survival than do patients without delirium. These patients may also have persistent cognitive impairment following discharge.24
The challenge for the ICU nurse is to identify all predisposing factors and attempt to improve the patient’s mental clarity and cooperation with therapy. It is imperative that physiological factors be addressed (e.g. correction of oxygenation, perfusion and electrolyte problems). The use of clocks and calendars may help the patient to remain oriented. If the patient demonstrates unsafe behaviour, hyperactivity, insomnia or delusions, symptoms may be managed pharmacologically with neuroleptic drugs (e.g. haloperidol).15 In addition, the presence of family members may help reorient the patient and reduce agitation.
Sensory overload can also contribute to patient distress and anxiety. Environmental noise levels are particularly high in the ICU.25 The nurse can limit noise and assist the patient in understanding noises that cannot be prevented. Conversation is a particularly stressful noise, especially when the discussion concerns the patient and is conducted in the presence of, but without participation from, the patient. The nurse can eliminate this source of stress by identifying suitable places for patient-related discussions and, whenever possible, including the patient in the discussion. The nurse can also limit noise levels directly by muting telephones, setting monitor alarms appropriate to the patient’s condition and eliminating unnecessary alarms. For example, the nurse should silence the blood pressure alarms while manipulating invasive lines and then reactivate the alarms when the procedures are complete. Similarly, ventilator alarms should be transiently silenced during procedures such as endotracheal suctioning. Overhead paging and other unnecessary noise should be limited in patient care areas.
Sleep problems
Nearly all ICU patients experience sleep disturbances. Patients may have difficulty falling asleep or have disrupted sleep because of noise, anxiety, pain, frequent monitoring or treatment procedures.26 Drugs such as sedatives and hypnotics may result in disturbed sleep patterns, including reductions in slow wave and REM sleep.27 Sleep disturbance is a significant stressor in the ICU, contributing to delirium and possibly affecting recovery. The ICU nurse can structure the environment to promote the patient’s sleep–wake cycle. Strategies include clustering activities, scheduling rest periods, dimming lights at night-time, opening curtains during the daytime, obtaining physiological measurements without physically moving or disrupting the patient, limiting noise and providing comfort measures (e.g. touch or massage). If necessary, and not contraindicated, benzodiazepines (e.g. temazepam) or similar medications can be considered to induce and maintain sleep.
ISSUES RELATED TO FAMILIES
When someone becomes critically ill, care must be extended beyond the patient to the patient’s family because they are intimately connected. Family members play a valuable role in the patient’s recovery and should be considered members of the healthcare team. They can contribute to the patient’s wellbeing by:
1. providing a link to the patient’s personal life (e.g. news of friends, family and job)
2. advising the patient in healthcare decisions or functioning as the decision maker when the patient cannot
3. helping with activities of daily living (e.g. bathing, oral suctioning)
To be effective in caring for their loved one, family members need guidance and support from the nurse. The experience of having a friend or family member in the ICU is physically and emotionally difficult. Anxiety regarding the patient’s condition and prognosis and concerns about the patient’s pain and other discomfort are some of the issues families confront. They may question the quality of care that the patient is receiving. In addition, some families may experience anxiety about financial issues related to the provision of care during a critical illness. Consulting a social worker may be helpful.
The family will typically be experiencing disruption of their daily routines to support the patient. They may be far from their own home and supportive friends and family members. Ultimately, families of the critically ill patient are considered to be in crisis and family-centred care is imperative. To provide family-centred care effectively, the nurse should be mindful of family dynamics and interactions. The nurse can help the family to cope with this new situation through active listening, reducing anxiety through information giving and supporting those who become upset or angry.28 The family’s feelings should be acknowledged and accepted and placed within the context of the situation. Other healthcare team members, such as chaplains, pastoral care workers, social workers and psychologists, may be helpful in assisting the family to adjust and should be consulted as necessary. The extent to which family-centred care is provided will, in turn, affect the patient’s clinical course in the ICU.
Families of critically ill patients need information, reassurance and ready access to their loved one.28 Lack of information is a major source of anxiety for the family/significant others. The nurse should assess the family’s understanding of the patient’s status, treatment plan and prognosis, and provide information as appropriate. The nurse should also provide information to the family when the patient’s condition changes. It is recommended that a spokesperson for the family be identified so that information between the healthcare team and the family can be coordinated.
The family needs reassurance about the way in which the patient’s care is managed and decisions are made. The family should have the opportunity to be involved in decision making. If the patient has an advance directive or living will, the family will need to see that the patient’s wishes are understood and respected. When patients are incapable of making their own healthcare decisions, they may have designated a durable power of attorney, and this person should be involved in the patient’s plan of care.29 The family should also be invited to meet other healthcare team members, including intensive care doctors, physiotherapists, social workers and the chaplain. The nurse should evaluate the appropriateness of including family members in multidisciplinary care conferences. It can help family members to accept and cope with problems if they observe that healthcare providers are hopeful, caring and competent, decisions are deliberate and they have the opportunity to help shape the course of care.
Visiting policies in Australian and New Zealand ICUs are usually open and flexible and often tailored to the patient’s health status. Nurses assess the patient’s and family members’ needs and preferences and incorporate these into the plan of care. When the family members first visit the patient in ICU, it is important for the nurse to prepare them for the experience by briefly describing the patient’s appearance and the physical environment (e.g. equipment, noise). It is helpful if the nurse can accompany the family members as they enter the room. They should be encouraged to speak to and touch the patient. Family members participating in patient care is a contentious issue. The desires and rights of the patient should be considered first before encouraging this practice. The nurse should observe the responses of both the patient and family at these times. In some ICUs in New Zealand and Australia, visiting has been expanded to include the family pet. The positive benefits of pets visiting (e.g. decrease in agitation and anxiety30) far outweigh the risks (e.g. transmission of infection from pet to patient) and should be considered as part of the visiting policy when feasible.
In addition to traditional family visiting, research has demonstrated that family members of patients undergoing invasive procedures, including cardiopulmonary resuscitation (CPR), should be given the option of being present at the bedside during these events. Even when the outcomes are not favourable, being present can help family members remove doubts about the patient’s condition, decrease their anxiety and fear, facilitate the need to be together and to support their loved one, and facilitate the grief process when death occurs.31
Culturally competent care: critical care patients
Providing culturally competent care to critically ill patients and families is challenging. Often, the nurse is focused on meeting the physiological needs of the patient and may not appreciate the influence of the patient’s culture on the illness experience. At a minimum, the cultural dimensions of the meaning of sickness and health, pain, dying and death, and grief should be explored when caring for critically ill patients and their families.
Cultural perspectives on dying and death are complex. Informing some patients that they are dying as a way of letting them prepare for death is considered by some as an infringement on the role of the family.29
Customs surrounding dying and death vary widely, from leaving a window open to allow the spirit of the deceased to leave to providing the final bath for the deceased. The nurse caring for the dying patient must make every attempt to understand and accommodate the family’s cultural traditions. The expressions of grief that follow the loss of a loved one are highly individualised and influenced by several variables, including: (1) the relationship between the grieving person and the person lost; (2) whether or not the loss is sudden or anticipated; (3) the support systems available to the grieving person; (4) past experiences with loss; and (5) the person’s religious and cultural beliefs.29 It is of utmost importance that the critical care nurse proceeds cautiously when approaching patients facing death and their families. Asking patients, ‘What do you want to know?’ and ‘Who do you want with you when discussing options?’ is a good starting point for what can be very difficult conversations.
Haemodynamic monitoring
Haemodynamic monitoring refers to the measurement of pressure and flow (and, by extension, oxygenation) within the cardiovascular system. Both invasive (internally placed devices) and non-invasive (external devices) haemodynamic measurements are made in the ICU. Values commonly measured include systemic and pulmonary arterial pressures, central venous pressure (CVP), PA pressure and pulmonary capillary wedge pressure (PAWP), cardiac output/index, stroke volume/index and oxygen saturation of the haemoglobin of arterial blood (SaO2) and mixed venous blood (SvO2). In patients with underlying mitral or tricuspid valve disease, these measures will be altered and need to be taken into account. From these measurements the clinician calculates several values, including the resistance of the systemic and pulmonary arterial vasculature (and oxygen content, delivery and consumption). When these data are integrated with clinical assessment data, the nurse can derive a picture of the patient’s haemodynamic status, oxygen delivery and consumption and the effect of therapy on these parameters. It is important that all measures be made with attention to technical aspects. False or inaccurate data are potentially misleading and may result in unnecessary or inappropriate treatment.
HAEMODYNAMIC TERMINOLOGY
Cardiac output and cardiac index
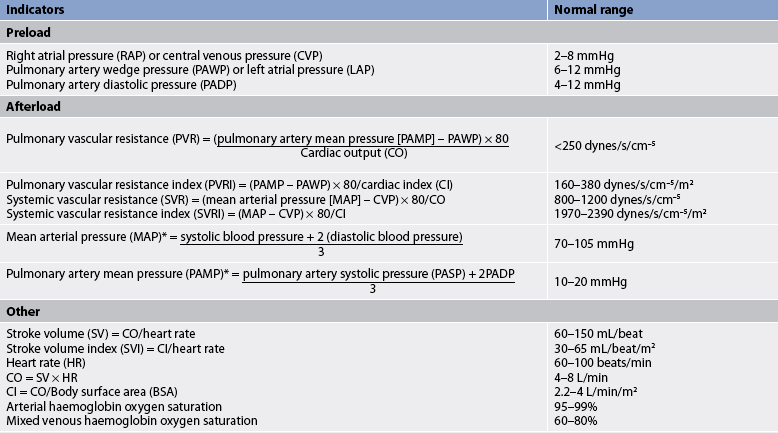
The volume pumped by the left ventricle with each heartbeat is called the stroke volume (SV). Cardiac output (CO) is the volume of blood pumped by the heart in 1 minute and is therefore calculated by multiplying SV by heart rate. Cardiac index (CI) is the measurement of the CO adjusted for body size; it is a more precise measurement of the efficiency of the pumping action of the heart individualised to the patient. Although minor beat-to-beat changes may occur, generally the left and right ventricles pump the same volume. Like CI, the stroke volume index (SVI) is the measurement of SV adjusted for body size. CO and the forces opposing blood flow determine blood pressure, the force exerted by blood on the vessel wall. The opposing resistance to blood flow by the systemic circulation vessels is called systemic vascular resistance (SVR) or, in the case of the pulmonary vessels, pulmonary vascular resistance (PVR). Preload, afterload and contractility determine SV (and thus CO and blood pressure). Understanding these concepts and relationships is essential for the critical care nurse. (See Ch 31 for discussion of assessment of the cardiovascular system.) The formulas and normal values for common haemodynamic parameters are given in Table 65-2.
Preload
Preload is the load (or stretch) imposed by the initial fibre length of the cardiac muscle before contraction (i.e. at the end of diastole). The primary determinant of preload is the amount of blood filling the ventricle during diastole. Left ventricular preload is called left ventricular end-diastolic pressure. PAWP, a measure of pulmonary capillary pressure that is measured with a catheter occluding the pulmonary artery for a brief period, reflects left ventricular end-diastolic pressure under normal conditions (i.e. when there is no mitral valve disease, intracardiac defect or arrhythmia). CVP, measured in the right atrium or in the vena cava close to the heart, is considered reflective of right ventricular preload or right ventricular end-diastolic pressure when there is no tricuspid valve disease, intracardiac defect or arrhythmia.
The effects of preload are explained by Starling’s law, which states that the more a myocardial fibre is stretched during filling, the more it shortens during systole and the greater the force of the contraction. As preload increases, force generated in the following contraction increases, and thus SV and CO increase. The greater the preload, the greater the myocardial (heart muscle) stretch and the greater the oxygen requirement of the myocardium. Hence, increases in CO, via increased preload, require increased delivery of oxygen to the myocardium. It should be remembered that the change in SV with preload comes about because of stretching of the heart muscle. However, the clinical measurement made is not a direct measurement of the muscle length; the measurement made is the pressure at the time of the peak stretch (end diastole) (see Table 65-2). This pressure indirectly reflects the amount of myocardial stretch and is related to some extent to volume present. This pressure is also important because it indicates pressure in the blood vessels of the lung or in the blood returning to the heart. Preload can be increased by fluid administration and decreased by diuresis or intravascular fluid losses.
Afterload
Afterload refers to the forces opposing ventricular ejection. These forces include systemic arterial pressure, the resistance offered by the aortic valve, and the mass and density of the blood to be moved. Clinically, although the measures fail to include all the components of afterload, SVR and arterial pressure are indices of left ventricular afterload. Similarly, PVR and pulmonary arterial pressure are indices of right ventricular afterload. Increased afterload often results in a decreased CO. CO can be restored by decreasing afterload (i.e. decreasing forces opposing contraction). When afterload is reduced, myocardial oxygen needs are decreased. Thus, CO is increased and myocardial oxygen requirements are decreased. Drug therapy directed at reducing afterload (e.g. glyceryl trinitrate) is often used in the management of heart failure (see Ch 34). Drug therapy directed at increasing afterload (e.g. noradrenaline) is often used in the management of septic shock when SVR may be reduced.
Vascular resistance
SVR is the resistance of the systemic vascular bed. PVR is the resistance of the pulmonary vascular bed. Both of these measures reflect afterload as described earlier and are adjusted for body size (see Table 65-2).
Contractility
Contractility describes the strength of myocardial contraction. Contractility is said to increase when preload is unchanged yet the heart contracts more forcefully. Adrenaline, noradrenaline, isoprenaline, dopamine, dobutamine, digoxin, calcium and milrinone increase contractility. These agents are termed positive inotropes. Contractility is diminished during times of metabolic acidosis or by drugs that exert negative inotropic actions, such as barbiturates, alcohol, procainamide, calcium channel blockers and β-adrenergic blockers. Increased contractility results in increased SV, as long as there is adequate intravascular volume and increased myocardial oxygen requirements. There are no direct clinical measures of cardiac contractility. To determine contractility indirectly, the nurse measures the patient’s preload (CVP and PAWP) and CO and graphs the results. Contractility is diminished in the failing heart.
PRINCIPLES OF INVASIVE PRESSURE MONITORING
Invasive lines are commonly used in the ICU to measure systemic and pulmonary blood pressures. Components of a typical invasive arterial pressure monitoring system are illustrated in Figure 65-2. The catheter, pressure tubing, flush system and transducer are usually disposable.
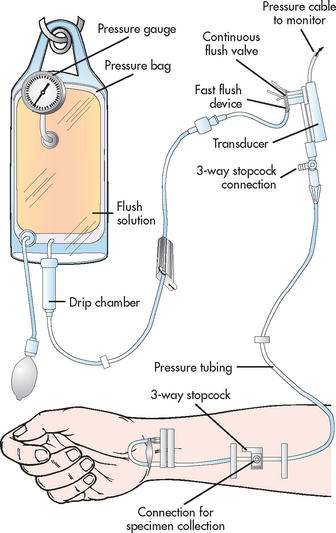
Figure 65-2 Components of a pressure monitoring system. The cannula, shown entering the radial artery, is connected via pressure (non-distensible) tubing to the transducer. The transducer converts the pressure wave into an electronic signal. The transducer is wired to the electronic monitoring system, which amplifies, conditions, displays and records the signal. Stopcocks are inserted into the line for specimen withdrawal and for referencing and zeroing procedures. A flush system, consisting of a pressurised bag of intravenous fluid, tubing and a flush device, is inserted into the line. The flush system provides continuous slow (approximately 3 mL hourly) flushing and provides a mechanism for fast flushing of lines. All items, except the electronic monitoring system, are commonly disposable equipment.
To measure pressure accurately, equipment must be levelled to a reference point on the patient and zero balanced (to atmospheric pressure), and dynamic response characteristics optimised. Levelling or referencing means positioning the transducer so that the zero reference point is at the level of the atria of the heart.32,33 The stopcock nearest the transducer is usually the zero reference for the transducer. To place this level with the atria, the nurse uses an external landmark, the phlebostatic axis. To identify the phlebostatic axis, two imaginary lines are drawn with the patient supine (see Fig 65-3, A). The first line, a horizontal line, is drawn through the mid-chest, halfway between the outermost anterior and posterior surfaces. The second line, a vertical line, is drawn through the fourth intercostal space at the sternum. The phlebostatic axis is the intersection of the two imaginary lines. Once the phlebostatic axis is identified, it may be marked on the patient’s chest with a permanent marker. The port of the stopcock nearest the transducer must be positioned level with the phlebostatic axis. It is recommended that the transducer be taped to the patient’s chest at the phlebostatic axis or mounted on a bedside pole at the height.32,33 If mounted on a bedside pole, the transducer level should be realigned after patient repositioning.
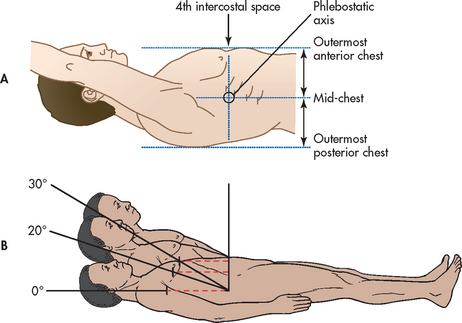
Figure 65-3 Identification of the phlebostatic axis. A, The phlebostatic axis is an external landmark used to identify the level of the atria in the supine patient. The phlebostatic axis is defined as the intersection of two imaginary lines: one drawn vertically through the fourth intercostal space at the sternum and another drawn horizontally through the mid-chest, halfway between the outermost anterior and outermost posterior points of the chest. B, As the backrest of the supine patient is elevated, the phlebostatic axis remains at the same anatomical location, becoming progressively elevated from the floor. The zero reference point must be repositioned with changes in backrest elevation to keep it at the phlebostatic level.
Zeroing confirms that when pressure within the system is zero, the monitor reads zero, thus negating the influence of atmospheric pressure on readings. This is accomplished by opening the reference stopcock to room air, pressing the zero button on the relevant pressure module and observing the monitor for a reading of zero. Most transducers in current use are disposable and have little zero drift. Zeroing the transducer is recommended during initial set up, immediately after insertion of the arterial line, when the transducer has been disconnected from the pressure cable or the pressure cable has been disconnected from the monitor, and when the accuracy of the measurements is questioned, and it should be done according to the manufacturer’s guidelines.33
Optimising dynamic response characteristics involves checking that the equipment reproduces, without distortion, a signal that changes rapidly. A dynamic response test (square wave test) is performed every 8–12 hours and when the system is opened to air or the accuracy of the measurements is questioned. It involves checking that the equipment reproduces a distortion-free signal (see Fig 65-4).34
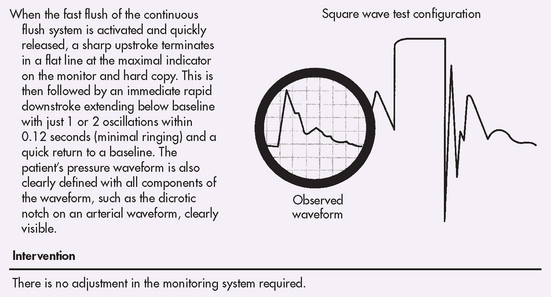
Figure 65-4 Optimally damped system. Dynamic response test (square wave test) using the fast flush system: normal response.
Steps in obtaining blood pressure measurements with an invasive line are given in Box 65-1. Pressure measurements can be obtained from both digital and printed analogue outputs but accurate readings are best obtained from a printed pressure tracing, most often at the end of expiration. Initial readings are made with the patient supine. Unless the patient’s blood pressure is extremely sensitive to orthostatic changes, values at modest degrees of backrest elevation (up to 45°) are generally equivalent to measurements with the patient supine. It is not necessary to reposition the patient for each pressure reading. However, it is necessary to move the zero reference stopcock to keep it positioned at the phlebostatic axis (see Fig 65-3, B).
BOX 65-1 Measurement of blood pressure with invasive lines
1. Explain the procedure to the patient.
2. Position the patient supine and flat or, if appropriate, elevated up to 45° or prone.
3. Confirm that the zero reference (port of the stopcock nearest the transducer) is placed at the level of the phlebostatic axis (see Fig 65-3). If the reference stopcock is not taped to the patient’s chest, a levelling device should be used to position the stopcock on a bedside pole at the point level with the phlebostatic axis.
4. Observe the monitor tracing and assess the quality of the tracing. If necessary, perform a dynamic response test (see Fig 65-4).
5. Obtain an analogue printout, if available, and measure the systolic and diastolic pressures at end expiration (see Fig 65-5). If no printout is available, freeze the tracing on the oscilloscope screen and use the cursor to measure the pressures at end expiration.
6. Record the pressure measurements promptly, including (if available) the printout marked to identify the points read.
TYPES OF INVASIVE PRESSURE MONITORING
Arterial blood pressure
Continuous arterial pressure monitoring is indicated for patients in many situations, including acute hypertension and hypotension, respiratory failure, shock, neurological injury, coronary interventional procedures, continuous infusion of vasoactive drugs (sodium nitroprusside) and frequent ABG sampling. A 20-gauge, 5-cm, non-tapered Teflon cannula over the needle is typically used to cannulate a peripheral artery, such as the radial, brachial or femoral arteries, using a percutaneous approach. After insertion, the catheter is firmly secured and covered with an occlusive dressing.35,36 It is important that the insertion site be immobilised so that the catheter line is not dislodged and lines are not kinked.
Measurements
The nurse can use the arterial line to obtain systolic, diastolic and mean blood pressures (see Fig 65-5). High- and low-pressure alarms should be set based on the patient’s current status and activated. In heart failure, the systolic upstroke may be slower. In volume depletion, systolic pressure varies greatly with mechanical ventilation, diminishing during inspiration. In severe congestive heart failure, systolic amplitude does not vary with ventilation. With arrhythmias it is useful to observe simultaneous ECG and pressure tracings. Arrhythmias that significantly diminish arterial pressure are more urgent than those that cause only a slight decrease in systolic amplitude.
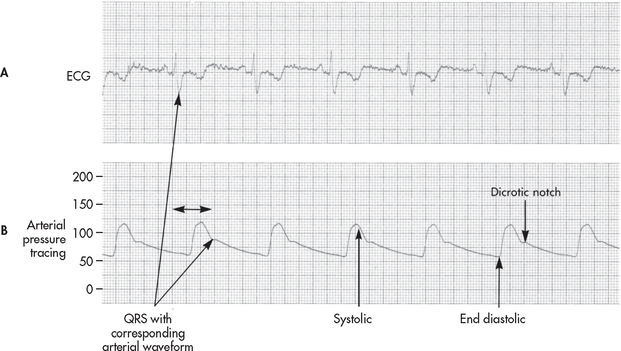
Figure 65-5 Simultaneously recorded A, electrocardiogram (ECG) tracing and B, system arterial pressure tracing. Systolic pressure is the peak pressure. The dicrotic notch indicates aortic valve closure. Diastolic pressure is the lowest value before contraction. Mean pressure is the average pressure over time calculated by the monitoring equipment.
Complications
Arterial lines carry the risk of haemorrhage, infection, thrombus formation and neurovascular impairment.35,36 Haemorrhage is most likely to occur when the catheter becomes dislodged or the line becomes disconnected. To avoid this serious complication, the nurse uses Luer-Lock connections and always checks the arterial waveform and that the alarms are activated. If the pressure in the line falls (e.g. when the line is disconnected), the low-pressure alarm sounds immediately, allowing prompt correction of the problem. Pressure is always monitored when an arterial line is in place, even if the line was placed for ABG sampling.
Infection is a risk with any invasive line. The nurse should inspect the insertion site for local signs of inflammation and monitor the patient for signs of systemic infection. To limit the risk of contamination and catheter-related infection, the catheter site, pressure tubing, flush bag and transducer are usually changed approximately every 96 hours.36 When infection is suspected, the catheter should be removed and the equipment changed. Additionally, disruption to invasive catheters for procedures should be minimised as each time lines are accessed this increases the risk of infection.
Circulatory impairment can result from formation of a thrombus around the catheter, release of an embolus, spasm or occlusion of the circulation by the catheter. Before inserting a line into the radial artery, an Allen test should be performed to confirm that ulnar circulation is sufficient to sustain the hand. In this test, pressure is applied to the radial and ulnar arteries simultaneously. The patient is instructed to open and close the hand repeatedly. The hand should blanch. Pressure on the ulnar artery is released while compressing the radial artery. If pinkness fails to return within 6 seconds, the ulnar artery is insufficient, indicating that the radial artery should not be used for line insertion.
To help maintain line patency and limit thrombus formation, the nurse should assess the continuous flush irrigation system every 1–4 hours to determine that the pressure bag is inflated to 300 mmHg (or the recommended pressure for the system) and that the flush bag contains fluid, so that the system is delivering approximately 3 mL per hour. Currently a solution of non-heparinised 0.9% normal saline is used for the flush solution unless otherwise indicated.
Once the catheter is inserted, the nurse should evaluate the neurovascular status distal to the arterial insertion site hourly. The limb with compromised arterial flow will appear cool and pale, with capillary refill greater than 3 seconds. There may be symptoms of neurological impairment, such as tingling or paraesthesia. Neurovascular impairment can result in loss of a limb and is an emergency.
Multiple lumen central venous catheters
Multiple lumen central venous catheters are widely used in the critical care setting, not only for measurement of CVPs, but also for administration of IV medications either by bolus or continuous infusion. Central vein access is essential because drugs such as adrenaline or noradrenaline, in particular, have vasoconstrictor properties which, if given peripherally, may compromise delivery and cause local tissue irritation and ischaemia.
Pulmonary artery flow-directed catheter
PA pressure monitoring is used to guide acute-phase management of patients with complicated cardiac, pulmonary and intravascular volume problems (see Box 65-2). In addition to CVP, PA diastolic (PAD) pressure and PAWP are sensitive indicators of fluid volume status and cardiac function. PAD pressure and PAWP are increased in fluid volume overload and heart failure. They are decreased with volume deficit. Fluid therapy based on the PA pressure allows restoration of fluid balance while avoiding overcorrection of the problem. Monitoring PA pressures can allow precise therapeutic manipulation of preload, contractility (indirectly) and afterload.
BOX 65-2 Clinical indications for pulmonary artery catheterisation*
Acute respiratory distress syndrome
Acute respiratory failure in patients with chronic obstructive pulmonary disease
Evaluation of circulatory syndromes (e.g. heart failure, mitral valve regurgitation, intraventricular shunts)
Hypotension unresponsive to fluid resuscitation
Intraaortic balloon pump therapy
Myocardial infarction with complications (e.g. heart failure, cardiogenic shock, ventricular septal rupture)
Perioperative fluid imbalance in high-risk patients (e.g. cardiac history)
*List is not exhaustive
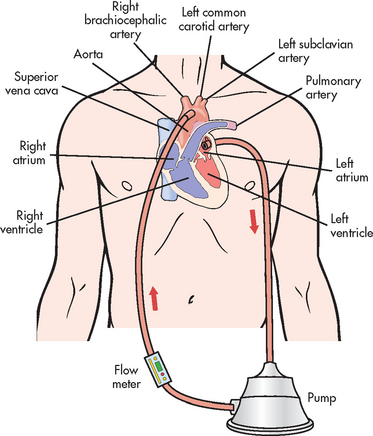
A PA flow-directed catheter (e.g. Swan-Ganz) is used to measure CVP and PA pressures, including PAWP. The standard PA catheter is number 7.5 French gauge, 110 cm long, with four or five lumens (see Fig 65-6). When properly positioned, the distal lumen port (catheter tip) is within the PA (see Fig 65-7). This port is used to monitor PA pressures and withdraw mixed venous blood specimens (e.g. to evaluate oxygen saturation). A balloon connected to an external valve via the second lumen surrounds the distal lumen port. Balloon inflation has two purposes: (1) to allow moving blood to float the catheter forwards; and (2) to allow PAWP measurement. There will be one or two proximal lumens, with exit ports in the right atrium (if only one) or right atrium and right ventricle (if two). The right atrium port is used for measurement of right atrial pressure (or CVP), injection of fluid for CO determination and withdrawal of blood specimens. If a second proximal port is available, it is used for infusion of fluids and drugs or blood sampling. A thermistor lumen port located near the distal tip is wired to an external connector. This port is used for monitoring blood or core temperature and for the thermodilution method of measuring CO.
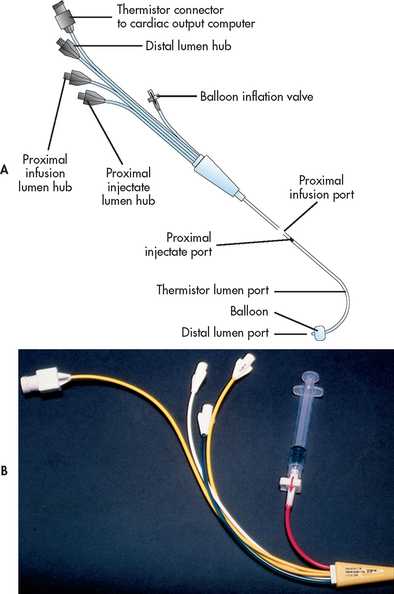
Figure 65-6 Pulmonary artery (PA) catheter. A, The illustrated catheter has five lumens. When properly positioned, the distal lumen exit port is in the PA, and the proximal lumen ports are in the right atrium and right ventricle. The distal and one of the proximal ports are used to measure PA and central venous pressures, respectively. A balloon surrounds the catheter near the distal end. The balloon inflation valve is used to inflate the balloon with air to allow reading of the pulmonary artery wedge pressure. A thermistor located near the distal tip senses PA temperature and is used to measure thermodilution cardiac output when a solution cooler than the body temperature is injected into a proximal port. B, An actual catheter.
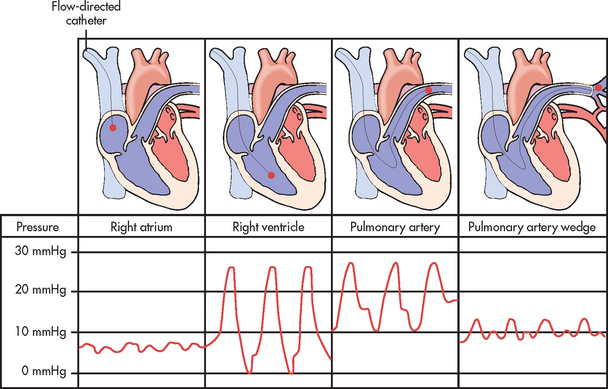
Figure 65-7 Position of the pulmonary artery flow-directed catheter during progressive stages of insertion with corresponding pressure waveforms.
In addition to these relatively standard and common features of the PA flow-directed catheter, catheters with other features are available. One modification is the inclusion of an atrial electrode, which is useful in recording the atrial ECG or pacing the heart. Another common modification is inclusion of a fibreoptic sensor in the distal tip that detects mixed venous oxygen saturation. Another type of catheter provides continuous measurement of right ventricular volume and ejection fraction, whereas another catheter provides continuous CO monitoring.33 The PA catheter sheath/introducer usually has a side port that serves as another intravenous line. Most catheters also have a plastic ‘sleeve’ connected to the sheath/introducer, which permits manipulation of the catheter’s position while maintaining sterility.
Pulmonary artery catheter insertion
Before PA catheter insertion, the nurse notes the patient’s electrolyte, acid–base, oxygenation and coagulation status. Imbalances such as hypokalaemia, hypomagnesaemia, hypoxaemia or acidosis can make the heart more irritable and increase the risk of ventricular arrhythmia during catheter insertion. Coagulopathy increases the risk of haemorrhage. The nurse prepares for the procedure by arranging the monitor, cables, and flush and infusion solutions. The system is zero referenced to the phlebostatic axis. The procedure is explained to the patient and informed consent is obtained. The patient is positioned supine with the head of the bed flat.33 The PA catheter is inserted through a sheath percutaneously into the internal jugular, subclavian, antecubital or femoral vein using surgical asepsis. Venous cut-down is rarely required. The line is then advanced through the venous system to the right side of the heart.
Catheter insertion is guided by continuously observing the characteristic waveforms on the monitor as the catheter is advanced through the right side of the heart to the left until it reaches the PA (see Fig 65-7). When the tip reaches the right atrium, the balloon is inflated.33 Inflation of the balloon should not exceed the balloon’s capacity (usually 1–1.5 mL of air). The catheter is then floated through the tricuspid valve into the right ventricle and then through the pulmonary valve and into the PA. Once a typical PAWP tracing is observed, the balloon is deflated and the PA waveform should return on the monitor. Following catheter insertion, a chest X-ray is obtained to confirm the position. To maintain the catheter in its proper position, the catheter is secured at its point of entry into the skin. The measurement at the exit point should be noted and recorded. An occlusive dressing is applied and changed according to unit protocol.
Pulmonary artery pressure measurements
Systolic, diastolic and mean pressures are routinely monitored. PA systolic (PAS) pressure is the peak pressure and PAD pressure is the lowest pressure point on the PA waveform. Mean PA pressure is the time-weighted average. Because PA ports are in the chest, intrathoracic pressures alter PA pressure. To produce accurate data, PA measurements are usually obtained at the end of expiration.37,38
The measurement of PAWP is obtained by slowly inflating the balloon with air (not to exceed balloon capacity) until the PA waveform changes to a PAWP waveform (see Fig 65-8). This measurement should be performed only by staff who have been deemed competent with this technique. Before inflation the PA pressure tracing on the monitor looks like an arterial tracing, with a systolic peak, dicrotic notch and then the diastolic low point. As the waveform becomes ‘wedged’, the tracing changes shape and amplitude. Generally, the PAWP waveform is characterised by two, small positive waves, the a and v waves. The a wave indicates atrial contraction and is followed by the x descent, indicating atrial relaxation. At times, a c wave may be seen following the a wave and indicates closure of the mitral valve. The v wave is seen during the interval between the T and P waves of the ECG. The v wave indicates inflow into the left atrium when the mitral valve is closed and the ventricle is contracting. The v wave is followed by the y descent, indicating the emptying of the left atrium when the mitral valve opens and the ventricle fills.38
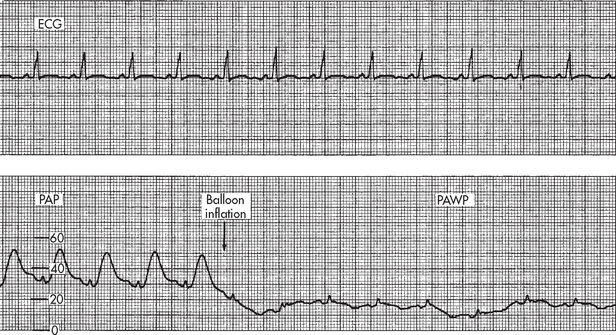
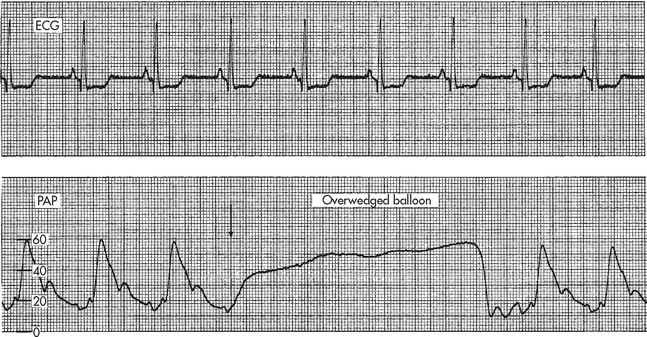
Figure 65-8 Change in pulmonary artery pressure (PAP) waveform to pulmonary artery wedge pressure (PAWP) waveform with balloon inflation. The balloon is inflated while observing the bedside monitor for change in the waveform. Balloon inflation (arrow) in patient with a normal PAWP.
When measuring the PAWP, the balloon should be inflated for no more than four respiratory cycles or 8–15 seconds.37,38 There is a danger of rupture of the PA if the catheter migrates distally into a smaller vessel or if the balloon is overinflated. This is suspected when less than 1 mL is needed to wedge the tracing or an ‘overwedge’ tracing is obtained (see Fig 65-9). Readings should be acquired from an analogue strip pressure recording and the strip should be placed into the patient’s record. If a printout of the tracing is not available, the readings can be taken from the monitor using the cursor and scale mode.
Central venous or right atrial pressure measurement
CVP is a measurement of right ventricular preload. It can be measured with a PA catheter using one of the proximal lumens or with a central venous catheter placed in the internal jugular or subclavian vein. CVP is measured as a mean pressure at the end of expiration. CVP waveforms (see Fig 65-10) are similar to PAWP waveforms. Although the PAD pressure and PAWP are more sensitive indicators of fluid volume status, CVP may also reflect fluid volume problems. An elevated CVP may indicate right ventricular failure or volume overload. A low CVP may be the result of intravascular hypovolaemia. However, CVP monitoring can produce erroneous results: a low CVP does not always mean low volume and it may reflect other pathology, including peripheral dilation due to sepsis. Additionally, hypovolaemic patients may have normal CVP due to sympathetic nervous system activity increasing vascular tone.
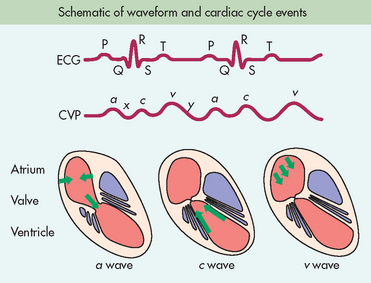
Figure 65-10 Cardiac events that produce the CVP waveform with a, c and v waves. a wave represents atrial contraction. x descent represents atrial relaxation. c wave represents the bulging of the closed tricuspid valve into the right atrium during ventricular systole. v wave represents atrial filling. y descent represents opening of the tricuspid valve and filling of the ventricle.
Invasive cardiac output measurement techniques
CO is frequently monitored in patients with haemodynamic instability. Normal resting CO is 4–8 L per minute and varies with body size. CI accounts for variations in body size as it is calculated taking the patient’s height and weight into consideration and is normally 2.2–4 L/min/m2. CO is decreased in conditions such as hypovolaemia, cardiogenic shock and heart failure. Under normal conditions, CO increases with exercise. Increases in CO at rest indicate a hyperdynamic state seen with fever or sepsis.
The PA catheter is commonly used to measure CO via the intermittent bolus thermodilution CO (TDCO) method or the continuous CO (CCO) method. With the TDCO method, a fixed volume (5–10 mL) of 5% dextrose solution (or saline, if contraindicated) at room temperature (or iced for patients with low or high CO) is injected rapidly (≤4 s) and smoothly into the proximal lumen port of the PA catheter.39 The thermistor lumen port located near the distal tip of the PA catheter detects the drop in blood temperature. CO is mathematically calculated from the area under the temperature curve by the computer. The larger the area under the curve, the smaller the CO; conversely, the smaller the area under the curve, the larger the CO (see Fig 65-11).39 This procedure is repeated three times, with each measurement 1–2 minutes apart. Any CO measurement that does not have a normal curve is discarded. An average of three acceptable measurements is calculated to determine the CO.
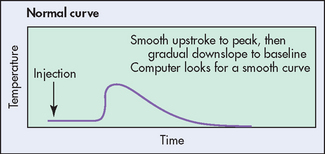
Figure 65-11 Normal cardiac output curve. Cardiac output is calculated from the temperature change in the pulmonary artery when a fixed volume of a solution at a known temperature is injected into the proximal port in the right atrium. The nurse should observe the curve during injection to make sure that it is smooth.
The CCO method uses a heat-exchange CO catheter. This PA catheter contains a thermal filament that is located in the right atrium. This filament emits a pulsed signal every 30–60 seconds that allows for the mixing of blood with heat as it passes through the right ventricle. The thermistor lumen port detects the change in temperature. A bedside computer displays digital measurements every 30–60 seconds that reflect the average CO for the past 3–6 minutes. The CCO method eliminates the need for fluid boluses, reduces the risk of contamination and permits ongoing evaluation (or trending) of the CO. Comparisons of the TDCO method with the CCO method have shown the CCO method to be reliable.39
The SVR, SVR index (SVRI), SV and SV index (SVI) can be calculated each time the CO is measured. The formulas for calculating these are shown in Table 65-2. Increased SVR (>1200 dynes/s/cm−5) indicates vasoconstriction from shock, increased release or administration of adrenaline or noradrenaline, or left ventricular failure. A low SVR (<800 dynes/s/cm−5) indicates vasodilation, which may occur during sepsis, septic shock or neurogenic shock or with drugs that reduce afterload. Changes in SV are rapidly becoming more important indicators of the pumping status of the heart. A high SV may be seen in bradycardia and exercise and with the use of positive inotropes (e.g. dobutamine). Low SV is seen with tachyarrhythmias, extreme vasodilation and cardiac tamponade.
Venous oxygen saturation
Both CVP and PA catheters can include sensors to measure the oxygen saturation of haemoglobin of PA blood. The oxygen saturation of blood from the PA catheter is termed mixed venous oxygen saturation (SvO2). Similarly, the oxygen saturation of venous blood from the CVP catheter is termed ScvO2. Either measurement is useful in determining the adequacy of tissue oxygenation. SvO2 reflects the dynamic balance between oxygenation of the arterial blood, tissue perfusion and tissue oxygen consumption (vO2). SvO2/ScvO2, when considered in conjunction with the arterial oxygen saturation, is useful in analysing haemodynamic status and response to treatments or activities (see Table 65-3). Normal SvO2 or ScvO2 at rest is 60–80%.
TABLE 65-3 Clinical interpretation of SvO2/ScvO2* measurements
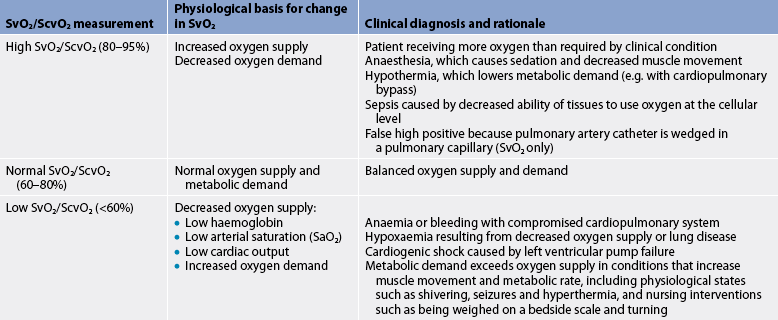
* ScvO2 values are generally slightly higher than SvO2 values.
Source: Urden lD, Stacy KM, lough Me. Thelan’s critical care nursing: diagnosis and management. 6th edn. St louis: Mosby; 2010
Sustained decreases and increases in SvO2/ScvO2 must be analysed carefully. Decreased SvO2/ScvO2 may indicate decreased arterial oxygenation, low CO, low haemoglobin or increased oxygen consumption. If the SvO2/ScvO2 falls, the nurse determines which of these factors has changed. The nurse observes for changes in arterial oxygenation by monitoring pulse oximetry or ABGs. By noting any changes in level of consciousness, strength and quality of peripheral pulses, urine output, and skin colour and temperature, the nurse can grossly assess CO and tissue perfusion. If arterial oxygenation, CO and haemoglobin are unchanged, a fall in SvO2 or ScvO2 indicates increased oxygen consumption or extraction, which could result from an increased metabolic rate, pain, movement or fever. If oxygen consumption increases without a comparable increase in oxygen delivery, more oxygen is extracted from the blood and the SvO2/ScvO2 will continue to fall.33
Increased SvO2/ScvO2 is also clinically significant and may indicate a clinical improvement (e.g. increased arterial oxygen saturation, improved perfusion, decreased metabolic rate) or problems (e.g. sepsis, ventricular septal defect). In sepsis, oxygen may not be extracted properly at the tissue level, resulting in increased mixed venous oxygen saturation.
Nursing interventions may be guided by changes in SvO2/ScvO2. The nurse may note that the patient’s heart rate increased moderately during repositioning but that the SvO2/ScvO2 remained stable. In this case the nurse may conclude that the position change was tolerated. If the SvO2/ScvO2 had dropped, this would be an indication to stop the activity until the SvO2/ScvO2 returns to the previous level.
In many cases as activity or metabolism increases, heart rate and CO increase, and SvO2/ScvO2 remains constant or varies slightly. However, it is not uncommon for critically ill patients to have conditions that prevent substantial increases in CO. For example, this could occur in the patient with heart failure, shock, arrhythmias or cardiac transplantation. In these cases, SvO2/ScvO2 can provide a useful indicator of the balance between oxygen delivery and consumption.
Complications with PA catheters
Infection and sepsis are serious problems associated with PA catheters. Careful surgical asepsis for insertion and maintenance of the catheter and tubing line is important to prevent infection. The skin is cleaned, often with an alcohol-based chlorhexidine solution. The insertion site is covered with a sterile occlusive dressing. The nurse should monitor the patient for local and systemic signs of infection (e.g. redness and exudate at the insertion site, fever and increased white blood cell count). The PA catheter must be removed if there are local or systemic signs of infection.40 To reduce the risk of infection, the flush bag, pressure tubing, transducer and stopcock are usually changed every 72 hours, and the PA catheter should be removed once haemodynamic monitoring is no longer needed.37
Air embolus is another risk associated with PA catheters. Air embolus can be caused by injection of air into the lumen of a ruptured balloon or by balloon rupture. The risk of air embolus may be decreased by first aspirating to check for the absence or presence of blood and by injecting only the prescribed volume of air into the balloon before obtaining the PAWP. Catheters are also checked for balloon leak before insertion; defective catheters are not used. If blood is aspirated from the balloon port or if it is observed that injected air does not passively flow back into the syringe, the catheter should be so labelled, the intensivist notified and the catheter should not be used again. Air can also be introduced into the system if connections are not tight; Luer-Lock connections should be used on all pressure lines. In addition, the low-pressure alarm is activated for all pressure lines to signal any substantial drop in pressure. Any time the line needs to be disconnected to change the apparatus, the line should be closed to the patient via clamping or stopcocks.
The patient with a PA catheter is at risk of pulmonary infarction or PA rupture from the following causes: (1) the balloon may rupture, releasing fragments that could embolise; (2) prolonged balloon inflation may obstruct blood flow; (3) the catheter may advance into a wedge position, obstructing blood flow; and (4) a thrombus could form and embolise. To reduce the risk of pulmonary infarction and rupture, the balloon must never be inflated beyond the balloon’s capacity (usually 1–1.5 mL of air). The balloon must not be left inflated for more than four breaths (except during insertion) or 15 seconds.37,38 PA pressure waveforms are monitored continuously for evidence of catheter occlusion, dislocation or spontaneous wedging. The pressure tracing will be blunted if the catheter starts to be occluded. The pressure tracing will appear wedged if the PA catheter advances and becomes spontaneously wedged. In each of these cases, the catheter must be repositioned immediately. To reduce the risk of thrombus and embolus formation, the PA catheter is continuously flushed with a slow infusion of non-heparinised saline solution (unless otherwise desired) to prevent thrombus formation.38
Ventricular arrhythmias can occur during PA catheter insertion or removal or if the tip migrates back from the PA to the right ventricle and irritates the ventricular wall. In addition, the nurse may observe that the PA catheter cannot be wedged. In these situations, the catheter may need to be repositioned by the doctor or an accredited nurse.
Pulse-induced contour cardiac output
Pulse-induced contour cardiac output (PICCO) is a more recently adopted method of invasive cardiac output measurement. This method allows continuous assessment of CO and, unlike the pulmonary artery catheter, requires only a central venous line and an arterial line with a thermistor.41 By injecting a bolus (usually room-temperature saline) into the central vein, the cardiac output can be calculated from thermal signals between the central catheter and the arterial catheter. In addition, beat-by-beat cardiac output, obtained from the shape of the arterial pressure wave, can be obtained from the arterial catheter. This is calculated by measuring the area under the systolic portion of the arterial pulse wave from the end of diastole to the end of the ejection phase.
In addition to measuring cardiac output, other parameters measured by PICCO include:
• global end-diastolic volume (GEDV)—the volume of blood contained in the four chambers of the heart at end diastole; the normal value for the GEDV index is 680–800 mL/m2
• intrathoracic blood volume (ITBV)—the volume of the four chambers of the heart plus the blood volume in the pulmonary vessels; the normal value for the ITBV index 850–1000 mL/m2
• extravascular lung water (EVLW)—the amount of water content in the lungs; this allows for quantification of the degree of pulmonary oedema; the normal value for the EVLW index is 3.0–7.0 mL/kg.
One of the main advantages of PICCO is that most critically ill patients require central venous and arterial monitoring catheters for haemodynamic management and the PICCO system does not require insertion of a more invasive PA catheter. Additionally, the information reflecting EVLW has the potential to guide patient management giving parameters reflecting pulmonary oedema that would otherwise require a chest X-ray. The system is relatively easy to set up and allows monitoring of real-time responses to treatment.
Non-invasive haemodynamic monitoring
Impedance cardiography
Impedance cardiography (ICG) is a continuous or intermittent, non-invasive method of obtaining the CO and assessing thoracic fluid status. Based on the concepts of impedance (the resistance to the flow of electrical current [Z]), ICG uses four sets of external electrodes to deliver a high-frequency, low-amplitude current that is similar to that used in apnoea monitors. Blood is an excellent conductor of electricity (lower impedance) and pulsatile blood flow generates electrical impedance changes. ICG measures the change in impedance (dZ) in the ascending aorta and left ventricle over time (dt) and is represented as dZ/dt. ZO is the measurement of the average impedance of the fluid in the thorax. Impedance-based haemodynamic parameters (CO, SV and SVR) can be calculated from ZO, dZ/dt, mean arterial pressure (MAP), CVP and the ECG. Major indications for ICG include early signs and symptoms of pulmonary or cardiac dysfunction, differentiation of cardiac or pulmonary cause of shortness of breath, evaluation of aetiology and management of hypotension, monitoring after discontinuing a PA catheter or justification for insertion of a PA catheter, evaluation of pharmacotherapy and diagnosis of rejection following cardiac transplantation.33 ICG is not recommended in patients who have generalised oedema or third spacing, as the excess volume interferes with accurate signals.
Non-invasive arterial oxygenation monitoring
Pulse oximetry is a non-invasive and continuous method of determining arterial oxygenation (SpO2) and monitoring SpO2 may reduce the frequency of ABG sampling (see Ch 25). SpO2 is normally 95–100%. A common use for pulse oximetry is to evaluate the effectiveness of oxygen therapy. Decreased SpO2 indicates inadequate oxygenation of the blood in the pulmonary capillaries. This may be corrected by increasing the fraction of inspired oxygen (FiO2) and evaluating the patient’s response. Similarly, the nurse uses SpO2 to monitor how the patient tolerates decreases in FiO2 and responds to changes in position and treatments. For example, the nurse may note that the SpO2 falls when the patient is positioned in a left lateral recumbent position. The nurse can then plan position changes that pose less risk for the patient.
Accurate SpO2 measurements may be difficult to obtain for patients who are hypothermic, receiving IV vasopressor therapy (e.g. noradrenaline) or experiencing hypoperfusion (e.g. shock). Alternative locations for placement of the pulse oximetry probe may need to be considered (e.g. forehead, earlobe).
 NURSING MANAGEMENT: HAEMODYNAMIC MONITORING
NURSING MANAGEMENT: HAEMODYNAMIC MONITORING
Assessment of haemodynamic status requires integration of data from many sources and comparison of the data over time. Thorough nursing observations provide important clues about the patient’s haemodynamic status. The nurse should begin by obtaining baseline data about the patient’s general appearance, level of consciousness, skin colour and temperature, vital signs, peripheral pulses and urine output. (Does the patient appear tired, weak, exhausted?) There may be too little cardiac reserve to sustain even minimum activity. Pallor, cool skin and diminished pulses may indicate decreased CO. Changes in mental clarity may reflect problems with cerebral perfusion or oxygenation. Monitoring urine output reflects the adequacy of perfusion to the kidneys. The patient with diminished perfusion to the GI tract may develop hypoactive or absent bowel sounds. If the patient is bleeding and developing shock, blood pressure may initially be relatively stable, yet the patient may become increasingly pale and cool from peripheral vasoconstriction. Conversely, the patient experiencing septic shock may remain warm and pink yet develop tachycardia and blood pressure instability. Although heart rates of 100 beats per minute are common among stressed, compromised, critically ill patients, sustained tachycardia greatly increases myocardial oxygen demand and may result in diminished CO.
The astute critical care nurse correlates observational data with data obtained from technology devices (e.g. ECG; arterial, PA, PAWP pressures; SvO2/ScvO2). Single haemodynamic values are rarely significant—generally a trend in values offers greater insight. The nurse must evaluate the whole clinical picture with the goals of recognising early clues and intervening before problems escalate.
Circulatory assist devices
Mechanical circulatory assist devices (CADs), such as the intraaortic balloon pump (IABP) and left ventricular assist device (VAD), are used to decrease cardiac work and improve organ perfusion in patients with heart failure when conventional drug therapy is no longer adequate. The type of device used depends on the extent and nature of the myocardial problem and the capabilities of the institution and staff. CADs provide interim support in three types of situations: (1) the left ventricle requires support while recovering from acute injury; (2) the heart requires surgical repair (e.g. a ruptured septum) but the patient must be stabilised; and (3) the heart has failed and the patient is awaiting cardiac transplantation. All CADs decrease left ventricular workload, increase myocardial perfusion and augment circulation. The most commonly used CAD is the IABP. Several types of VADs are available, and additional devices are under development.
INTRAAORTIC BALLOON PUMP
The intraaortic balloon pump (IABP) provides temporary circulatory assistance to the compromised heart by reducing afterload (via reduction in systolic pressure) and augmenting the aortic diastolic pressure. Box 65-3 lists the clinical conditions for which the IABP is used.
BOX 65-3 Indications and contraindications for the intraaortic balloon pump
Indications
Refractory unstable angina (when drugs have failed)
Short-term bridge to cardiac transplantation
Acute myocardial infarction with any of the following:*
Preoperative, intraoperative and postoperative cardiac surgery (e.g. prophylaxis before surgery, failure to wean from cardiopulmonary bypass, left ventricular failure after cardiopulmonary bypass)
Contraindications
Terminal or untreatable diseases of any major organ system
Abdominal aortic and thoracic aneurysms
Moderate-to-severe aortic insufficiency
Generalised peripheral vascular disease†
The IABP consists of a ‘sausage-shaped’ balloon, a pump that inflates and deflates the balloon, control devices for synchronising the balloon inflation to the cardiac cycle, and fail-safe devices (see Fig 65-12). The balloon is inserted percutaneously or surgically, under strict aseptic technique, into the femoral artery, advanced towards the heart and positioned in the descending thoracic aorta just below the left subclavian artery (see Fig 65-13). Following placement, the position is confirmed by X-ray. A pneumatic device cyclically fills the balloon with helium at the start of diastole (immediately after aortic valve closure) and deflates it just before systole. In a patient with a regular rhythm, the ECG is the primary trigger used to initiate deflation on the R wave (of the QRS) and inflation on the T wave (diastolic period), and the dicrotic notch of the arterial pressure tracing is used to refine timing (see Fig 65-14, A). IABP support is referred to as counterpulsation because the timing of balloon inflation is during diastole, the opposite to ventricular contraction, with deflation just prior to the patient’s ventricular contraction. The IAPB assist ratio is 1:1 in the acute phase of treatment—that is, one IABP cycle of inflation and deflation for every heartbeat.42
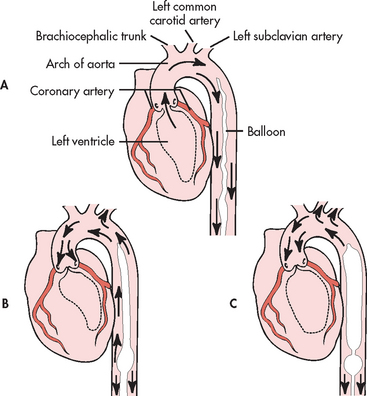
Figure 65-13 Intraaortic balloon pump. A, During systole the balloon is deflated, which facilitates ejection of the blood into the periphery. B, In early diastole, the balloon begins to inflate. C, In late diastole, the balloon is totally inflated, which augments aortic pressure and increases the coronary perfusion pressure, with the end result of increased coronary and cerebral blood flow.
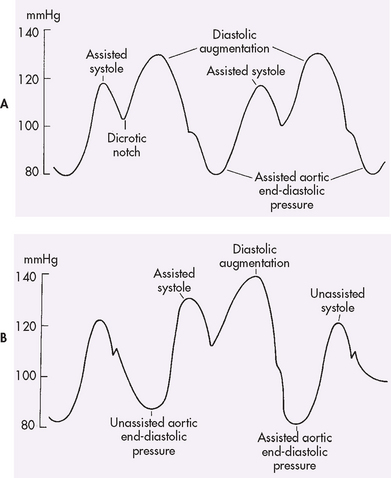
Figure 65-14 A, Correct 1:1 intraaortic balloon pump frequency. B, Correct 1:2 intraaortic balloon pump frequency.
Effects of counterpulsation
In late diastole when the balloon is totally inflated, blood is forcibly displaced distally to the extremities and proximally to the coronary arteries and main branches of the aortic arch. Diastolic arterial pressure rises (diastolic augmentation), increasing coronary artery perfusion pressure and perfusion of vital organs. The rise in coronary artery perfusion pressure causes an increase in blood flow to the myocardium. The balloon is rapidly deflated just before systole. The suddenly created vacuum causes aortic pressure to drop. With aortic resistance to left ventricular ejection reduced (reduced afterload), the left ventricle empties more easily and completely. As with other types of afterload reduction, the SV increases, yet the myocardial oxygen consumption decreases.42 Haemodynamic effects of the IABP are summarised in Box 65-4.
BOX 65-4 Haemodynamic effects of intraaortic balloon pumps
Effects of inflation during diastole
Complications with intraaortic balloon pumps
Complications that occur with the IABP are listed in Table 65-4. Vascular injuries, such as dislodging of plaque, aortic dissection and compromised distal circulation, are common.43 Major complications may include significant limb ischaemia, septicaemia, bleeding, balloon rupture and thrombus formation. To reduce these risks, cardiovascular, neurovascular and haemodynamic assessments are necessary every 15–60 minutes depending on the patient’s status. The action of the balloon pump can commonly cause physical destruction of platelets and thrombocytopenia. Coagulation profiles must be monitored and the patient must be assessed for evidence of systemic bleeding. Displacement of the balloon can occlude the left subclavian, renal or mesenteric arteries and can result in diminished or absent radial pulse, decreased urine output and diminished or absent bowels sounds. Patients receiving IABP therapy are prone to infection, and local or systemic signs of infection necessitate catheter removal.43
TABLE 65-4 Nursing management: potential complications of intraaortic balloon pump therapy
| Potential complication | Nursing management |
|---|---|
| Site infection from invasive lines | Use strict aseptic technique for insertion and dressing changes for all lines. Cover all insertion sites with occlusive dressings. Administer prescribed prophylactic antibiotic for entire course of therapy. |
| Pneumonia associated with immobilisation | Reposition patient every 2 h, being careful not to displace balloon. If patient requires chest physiotherapy, avoid introducing an ECG artefact. |
| Arterial trauma caused by insertion or displacement of balloon | Evaluate and mark peripheral pulses before insertion of balloon to use as baseline for assessing pulses after insertion. After insertion of balloon, evaluate perfusion to both upper and lower extremities at least every hour. Measure urine output at least every hour (occlusion of renal arteries causes severe decrease in urine output). Observe arterial waveforms for sudden changes. Keep head of bed <45°. Do not flex cannulated leg at the hip. Immobilise cannulated leg to prevent flexion using a draw sheet tucked under the mattress, soft ankle restraint or knee immobiliser. |
| Thromboembolism caused by trauma, balloon obstruction of blood flow distal to catheter | Administer prophylactic heparin if ordered. Evaluate pulses, urine output and level of consciousness at least every hour. Check circulation, sensation and movement in both legs at least every hour. |
| Haematological complications caused by platelet aggregation along the balloon (decrease in platelets possible) | Administer low-molecular-weight dextran (Rheomacrodex) if ordered. Monitor for allergic reaction to dextran. Monitor coagulation profiles, haematocrit and platelet count. |
| Haemorrhage from insertion site | Check site for bleeding at least every hour. Observe vital signs for hypovolaemia with each vital sign check. |
| Balloon leak or rupture | Prepare for emergency removal and possible reinsertion. |
ECG, electrocardiogram.
Mechanical complications are rare but may occur. Incorrect timing of balloon inflation may cause increased afterload, decreased CO, myocardial ischaemia and increased myocardial oxygen use, and must be recognised immediately by the nurse. If the balloon develops a leak, the catheter must be changed immediately to avoid a helium gas embolus. Signs of a leak include less effective augmentation, repeated alarms for gas loss and blood backing up into the catheter. A malfunction of the balloon or console triggers fail-safe alarms and automatic shutdown of the unit.
The patient with an IABP is relatively immobile, limited to side-lying or supine positions with the head of the bed elevated less than 45°.42 The leg in which the catheter is inserted must not be flexed at the hip to avoid kinking or dislodgement of the catheter. The patient may be receiving ventilator support and is likely to have multiple invasive lines that increase the challenge of comfortable positioning. The patient may experience sleeplessness and anxiety. Adequate sedation, pain relief, skin care and comfort measures are required.
IABP therapy is weaned as the patient improves; that is, circulatory support provided by the IABP is gradually reduced. Weaning involves reducing the IABP assist ratio from 1:1 to 1:2 and assessing the patient’s response (see Fig 65-14, B). If haemodynamic parameters remain stable, the ratio can be changed from 1:3 to 1:8 until the IABP catheter is removed. Even if the patient is stable without the IABP, pumping is continued until the line is removed.42 This reduces the risk of thrombus formation around the catheter. Frequent haemodynamic assessment continues to be required during the weaning phase.
VENTRICULAR ASSIST DEVICES
The ventricular assist device (VAD) provides longer-term support for the failing heart (usually months) and allows more mobility than the IABP. VADs are inserted into the path of flowing blood to augment or replace the action of the ventricle. Some VADs are implanted (e.g. peritoneum) and others are positioned externally. A typical VAD would shunt the blood from the left atrium or ventricle to the device and then to the aorta (see Fig 65-15). Some VADs provide biventricular support.
Failure to wean from cardiopulmonary bypass (CPB) after surgery has been the primary indicator for VAD support. Increasingly the VAD is used to support patients with ventricular failure caused by myocardial infarction and patients awaiting cardiac transplantation. A VAD is a temporary device with the capability to partially or totally support circulation until the heart recovers or a donor heart can be obtained. Cannula sites depend on the type of device used. For support of the right side of the heart, the right atrium and PA are cannulated. The left ventricular apex can be cannulated for left VADs. Direct cannulation of the atria and great vessels occurs in the operating room through a sternotomy.
Appropriate patient selection for VAD therapy is critical. Indications for VAD therapy include: (1) extension of CPB for failure to wean or postcardiotomy cardiogenic shock; (2) bridge to recovery or cardiac transplantation; and (3) patients with New York Heart Association classification IV (see Box 34-1) who have failed medical therapy. Relative contraindications for VAD therapy include: (1) body surface area <1.5 m2; (2) renal or liver failure unrelated to cardiac incident; and (3) comorbidities that would limit life expectancy to <3 years.44
 NURSING MANAGEMENT: CIRCULATORY ASSIST DEVICES
NURSING MANAGEMENT: CIRCULATORY ASSIST DEVICES
The patient with an IABP requires highly skilled nursing care. Detailed cardiovascular assessment, including measurement of haemodynamic parameters (e.g. PA and arterial pressures, CO, CI, SVR, SV), cardiac and thoracic auscultation and evaluation of the ECG (e.g. rate, rhythm), is performed frequently. Assessment of adequate tissue perfusion (e.g. skin colour and temperature, mental status, peripheral pulses, urine output, bowel sounds) is also performed regularly.42 It is expected that with IABP therapy these parameters should improve.
Nursing care of the patient with a VAD is similar to that of the patient with an IABP. The patient is observed for bleeding, cardiac tamponade, ventricular failure, infection, arrhythmias, renal failure, haemolysis and thromboembolism. Unlike the patient with an IABP, who must remain in bed with limited position changes, the patient with VAD may be mobile and require an activity plan.44 In some cases, patients with VADs may go home. Preparation for discharge is complex and requires in-depth teaching about the device. Patients must have a competent carer present at all times.
Ideally, patients with VADs will recover with ventricular improvement, heart transplantation or artificial heart implantation. However, many patients die or the decision to terminate the device is made and death follows. Both the patient and the family require psychological support. Nursing care should include the family as much as possible. Other members of the healthcare team, such as social workers or clergy, should be consulted and involved in care as needed.
Artificial airways
The patient in the ICU often requires mechanical assistance to maintain airway patency. Inserting a tube into the trachea, thus bypassing upper airway and laryngeal structures, creates an artificial airway. The tube is placed into the trachea via the mouth or nose past the larynx (endotracheal [ET] intubation) or through a stoma in the neck (tracheostomy). ET intubation is more common in ICU patients. It can be performed quickly and safely at the bedside. Indications for ET intubation include: (1) upper airway obstruction (e.g. secondary to trauma, tumour, bleeding); (2) apnoea; (3) high risk of aspiration; (4) ineffective clearance of secretions; and (5) respiratory distress.45 ET tubes are illustrated in Figure 65-16. A tracheostomy is a surgical procedure that is performed when the need for an artificial airway is long term. Tracheostomy tubes and related nursing management are discussed in Chapter 26.
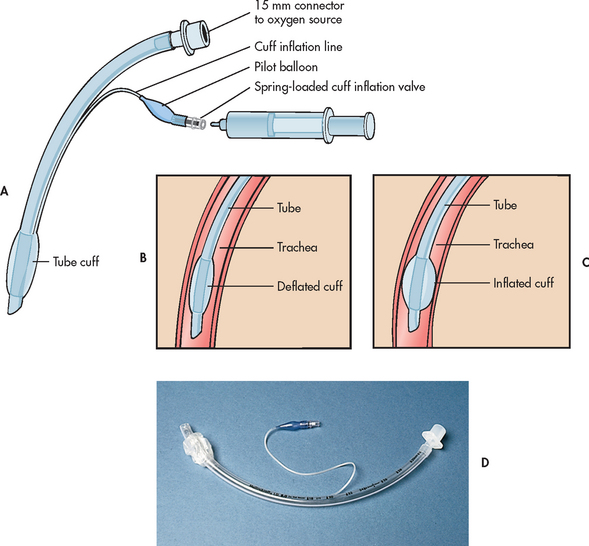
Figure 65-16 Endotracheal tube. A, Parts of an endotracheal tube. B, Tube in place with the cuff deflated. C, Tube in place with the cuff inflated. D, Actual tube before placement.
ENDOTRACHEAL TUBES
In oral intubation the ET tube is passed through the mouth and vocal cords into the trachea with the aid of a laryngoscope or bronchoscope. In nasal intubation, the ET tube is manipulated through the nose, nasopharynx and vocal cords. Oral ET intubation is the procedure of choice for most emergencies because the airway can be secured rapidly. Compared with the nasal route, a larger-diameter tube can be used for oral intubation. With a larger-bore ET tube, the work of breathing is reduced because there is less airway resistance. It is easier to remove secretions and perform fibreoptic bronchoscopy if needed. Nasal ET intubation is indicated when head and neck manipulation is risky.
Risks are associated with oral ET intubation. It is difficult to place an oral tube if head and neck mobility are limited (e.g. suspected spinal cord injury). Teeth can be chipped or inadvertently dislodged during the procedure. Salivation is increased and swallowing is difficult. Often a patient will obstruct the ET tube by biting down on it. A bite block or oropharyngeal airway can be used to avoid this, along with use of sedative mediations. The ET tube and bite block (if used) should be secured (separately) to the face. Mouth care is a challenge due to the limitations of space in the oral cavity but can be achieved with smaller or paediatric-sized oral products for teeth brushing, cleaning and suctioning.
Nasal intubation is contraindicated in patients with facial fractures, suspected fractures of the base of the skull and postoperatively after cranial surgeries.46 The nasal tube may be uncomfortable for some patients because it presses on the septum, whereas others may prefer it because there is no need for a bite block and mouth care is more easily accomplished. However, nasal ET tubes are more subject to kinking than oral tubes; the work of breathing is greater because the longer, narrower tube offers more airflow resistance; and suctioning and secretion removal are more difficult.
ENDOTRACHEAL INTUBATION PROCEDURE
Unless endotracheal intubation is an emergency, consent for the procedure should be obtained. The patient and family should be told the reason for ET intubation, the steps that will occur in the procedure, and the patient’s role in the procedure (if indicated). It is also important to explain that while intubated, the patient will not be able to speak but that other means of communication will be provided.
All patients undergoing intubation and receiving mechanical ventilation need to have a self-inflating bag-valve-mask (BVM) device (e.g. Ambu bag) attached to oxygen and suctioning equipment ready and available at the bedside at all times. The BVM device should contain a reservoir to sequester oxygen so that oxygen concentrations of 90–95% can be delivered. The slower the bag is deflated and inflated, the higher the oxygen concentration that will be delivered. The nurse assembles and checks the equipment to be used, removes the patient’s dentures and/or partial plates (for oral intubation) and administers drugs as ordered. Premedication varies, depending on the patient’s level of consciousness and the nature of the procedure (e.g. urgent or elective). Rapid-sequence intubation (RSI) is the rapid, concurrent administration of a combination of both a paralytic agent and a sedative agent during emergency airway management to decrease the risks of aspiration, combativeness and injury to the patient. RSI is not indicated in patients who are comatose or during cardiac arrest.46,47 A sedative–hypnotic–amnesic (e.g. midazolam) is used if the patient is agitated, disoriented or combative. A rapid-onset opioid, such as fentanyl, may be used to blunt the pain of laryngoscopy and intubation. A paralytic drug, such as suxamethonium, may be used to produce skeletal muscle paralysis following adequate sedation. Atropine may be used to limit secretions. Pulse oximetry is used during the procedure to assess oxygenation.
For oral intubation, the patient is placed supine with the head extended and the neck flexed (‘sniffing position’). This position allows for visualisation of the vocal cords by aligning the axes of the mouth, pharynx and trachea.47 For nasal intubation it may be helpful to ask the patient to extrude the tongue if they can. Before intubation is attempted, the patient is pre-oxygenated using a self-inflating BVM device with 100% oxygen for 3–5 minutes. Each intubation attempt is limited to 30 seconds. If unsuccessful, the patient is ventilated between successive attempts using the BVM device with 100% oxygen.45,47
Following intubation, the cuff is inflated and the placement of the ET tube is confirmed while manually ventilating the patient with 100% oxygen.48 An end-tidal CO2 detector is used to confirm proper tube placement by measuring the amount of exhaled CO2 from the lungs. The detector is placed between the BVM and ET tube and either observed for a colour change (indicating the presence of CO2) or a number. If no CO2 is detected, then the tube is likely to be in the oesophagus.49 The lung bases and apices are auscultated for bilateral breath sounds and the chest is observed for symmetrical chest wall movement. In addition, the SpO2 should be stable or improved.45 If the evidence supports proper ET tube placement, the tube is connected to an oxygen source and secured in place as per institutional policy (see Fig 65-17).
Figure 65-17 Method for securing an endotracheal tube using adhesive tape. An example protocol:
1. Ensure the patient’s skin is clean and dry.
2. Cut two lengths of tape—one long enough to be positioned around the head and extend around and slightly beyond the tube.
3. Double a shorter length of tape on the first piece, over the area that would contact the patient’s skin or hair.
4. Split both ends of the longer length of tape in half, to adhere around the tube.
5. Position the doubled tape, then wrap each adhesive end around the tube, then across the face, above the top lip.
The ET tube and the pharynx should be suctioned as needed. A chest X-ray is obtained immediately to confirm tube placement (3–5 cm above the carina in the adult). This position allows the patient to move the neck without dislodging the tube or causing it to enter the right main bronchus. Once proper positioning is confirmed with X-ray, the position of the tube at the teeth (usually 21 cm for women and 23 cm for men) or nose (‘exit mark’) is recorded and marked.50 Excess tubing is cut to reduce dead space.
The ET tube is connected to either humidified air or oxygen or a mechanical ventilator. ABGs should be obtained 25 minutes after intubation to determine oxygenation and ventilation status. ABG values are reviewed and used to guide oxygenation and ventilation changes. Pulse oximetry provides useful continuous monitoring of arterial oxygenation.
 NURSING MANAGEMENT: ARTIFICIAL AIRWAY
NURSING MANAGEMENT: ARTIFICIAL AIRWAY
Nursing responsibilities for the patient with an artificial airway include: (1) maintaining correct tube placement; (2) maintaining proper cuff inflation; (3) monitoring oxygenation and ventilation; (4) maintaining tube patency; (5) assessing for complications; (6) providing oral care and maintaining skin integrity; and (7) fostering comfort and communication (see NCP 65-1).
 Maintaining correct tube placement
Maintaining correct tube placement
When a patient is intubated (± mechanical ventilation) the nurse’s presence close to the patient’s bedside is paramount. Ventilation and oxygenation can be severely compromised if the patient is inadvertently extubated or disconnected from the ventilator. The nurse must monitor the ET tube for proper placement continuously and record the position at least every 2–4 hours.46 If the tube is dislodged, it could terminate in the pharynx or enter the oesophagus or the right main bronchus (thus ventilating only the right lung). The nurse maintains proper tube position by confirming that the exit mark on the tube remains constant while at rest and during patient care, repositioning and patient transport. The nurse observes for symmetrical chest wall movement and auscultates to confirm bilateral breath sounds. It is an emergency if the ET tube is not positioned properly. The nurse stays with the patient, maintains the airway, supports ventilation and secures the appropriate assistance to reposition the tube immediately. It may be necessary to ventilate the patient with a BVM device. If a malpositioned tube is not repositioned, no oxygen will be delivered to the lungs or the entire tidal volume will be delivered to one lung, placing the patient at risk of barotrauma and pneumothorax.
 Maintaining proper cuff inflation
Maintaining proper cuff inflation
The cuff is an inflatable, pliable sleeve encircling the outer wall of the ET tube (see Fig 65-16). The high-volume, low-pressure cuff stabilises and seals the ET tube within the trachea and prevents the escape of ventilating gases. However, it can cause tracheal damage. To avoid damage, the cuff is inflated with air and the pressure in the cuff is measured and monitored. Normal tracheal arterial perfusion is estimated at 30 mmHg. To ensure adequate tracheal perfusion, cuff pressure should be maintained at 20–25 mmHg.46 The nurse measures and records cuff pressure after intubation and on a routine basis (e.g. every 8 hours) using a pressure manometer, the minimal occluding volume (MOV) technique or the minimal leak technique (MLT).
The steps in the MOV technique for cuff inflation are: (1) for the mechanically ventilated patient, place a stethoscope over the trachea and inflate the cuff to the MOV by adding air until no air leak is heard at peak inspiratory pressure (end of ventilator inspiration); (2) for the spontaneously breathing patient, inflate until no sound is heard after a deep breath or after inhalation with a BVM; (3) use a manometer to verify that cuff pressure is between 20 and 25 mmHg; and (4) record cuff pressure in the chart. If adequate cuff pressure cannot be maintained or larger volumes of air are needed to keep the cuff inflated, the cuff could be leaking or there could be tracheal dilation at the cuff site. In these situations the ET tube should be repositioned or the intensivist notified and the tube changed. The procedure for MLT is similar with one exception. A small amount of air is removed from the cuff until a slight leak is auscultated at peak inflation. Both techniques are intended to prevent the risks of tracheal trauma due to high cuff pressures.
 Monitoring oxygenation and ventilation
Monitoring oxygenation and ventilation
The patient with an ET tube is monitored vigilantly for adequate oxygenation by assessing clinical findings, ABGs and SpO2. The nurse assesses for clinical signs of hypoxaemia such as changes in mentation (e.g. confusion), anxiety, dusky skin and cardiac arrhythmias. Periodic ABGs (specifically PaO2) and continuous SpO2 provide objective data regarding oxygenation. Lower values are expected in patients with obstructive pulmonary disease. PA or CVP catheters with SvO2/ScvO2 capability can give an indirect indication about the patient’s oxygenation status (see Box 65-1).
Indicators of ventilation include assessment of clinical findings, PaCO2 and partial pressure of end-tidal CO2 (PetCO2). The patient’s respirations should be assessed for rate and rhythm and use of accessory muscles. The patient who is hyperventilating will be breathing rapidly and deeply and may experience dizziness and peripheral numbness and tingling. The patient who is hypoventilating will be breathing shallowly or slowly and may appear dusky. PaCO2 is the best indicator of alveolar hyperventilation (decreased PaCO2, increased pH indicates respiratory alkalosis) or hypoventilation (increased PaCO2, decreased pH indicates respiratory acidosis).
PetCO2 monitoring is done by analysing exhaled gas directly at the patient–ventilator circuit (mainstream sampling) or by transporting a sample of gas via a small-bore tubing to a bedside monitor (sidestream sampling).48 Continuous PetCO2 monitoring can be used to assess the patency of the airway and the presence of breathing. In addition, gradual changes in PetCO2 values may accompany an increase in CO2 production (e.g. sepsis, hypoventilation, neuromuscular blockade) or a decrease in CO2 production (e.g. hypothermia, decreased CO, metabolic acidosis). In patients with normal ventilation-to-perfusion ratios, PetCO2 can be used as an estimate of PaCO2, with PetCO2 generally 1–5 mmHg (0.135–0.675 kPa) lower than PaCO2. However, in patients with an unusually large dead space or serious mismatch between ventilation and perfusion, PetCO2 is not a reliable estimate of PaCO2.
 Maintaining tube patency
Maintaining tube patency
The patient should be assessed routinely to determine the need for suctioning but should not receive suctioning routinely. Indications for suctioning include: (1) visible secretions in the ET tube; (2) sudden onset of respiratory distress; (3) suspected aspiration of secretions; (4) increase in peak airway pressures; (5) auscultation of adventitious breath sounds over the trachea and/or bronchi; (6) increase in respiratory rate and/or sustained coughing; and (7) sudden or gradual decrease in PaO2 and/or SpO2.48
Two recommended suctioning methods, the closed-suction technique (CST) and the open-suction technique (OST), are described in Box 65-5. The CST uses a suction catheter that is enclosed in a plastic sleeve connected directly to the patient–ventilator circuit (see Fig 65-17). With the CST, oxygenation and ventilation are maintained during suctioning and exposure to the patient’s secretions is reduced. The CST should be considered for patients who require high levels of positive end-expiratory pressure (PEEP) (>7.5 cm H2O) and/or FiO2 (>0.50), who have bloody or infected pulmonary secretions, who require frequent suctioning and who experience haemodynamic instability with the OST.50
BOX 65-5 Suctioning procedures for a patient on a mechanical ventilator
General measures
2. Wash hands and don personal protective equipment.
3. Explain the procedure and the patient’s role in assisting with secretion removal by coughing.
4. Monitor the patient’s cardiopulmonary status (e.g. vital signs, SpO2, SvO2, ScvO2, ECG, level of consciousness) before, during and after the procedure.
Open-suction technique
1. Open sterile catheter package using the inside of the package as a sterile field. Note: Suction catheter should be no wider than half the diameter of the ET tube (e.g. for a 7-mm ET tube, select a 10-French suction catheter).
2. Fill the sterile solution container with sterile normal saline or water.
4. Pick up sterile suction catheter with dominant hand. Using non-dominant hand, secure the suction tubing to the suction catheter.
5. Check equipment for proper functioning by suctioning a small volume of sterile saline solution from the container. (Go to step 7.)
Closed-suction technique
6. Connect the suction tubing to the closed suction port.
7. Hyperoxygenate the patient for 30 seconds using one of the following methods:
8. With suction off, gently and quickly insert the catheter using the dominant hand. When resistance is met, pull back 1–2 cm.
9. Apply continuous or intermittent suction using the non-dominant thumb. Rotate the catheter between the dominant thumb and forefinger and withdraw the catheter from the ET/tracheostomy tube over 10 seconds or less.
10. Hyperoxygenate for 30 seconds, as described in step 7.
11. If secretions remain and the patient has tolerated the procedure, two to three suction passes may be performed, as described in steps 8 and 9. Note: Rinse the suction catheter with sterile saline solution between suctioning passes as needed.
12. Reconnect the patient to the ventilator (open-suction technique).
13. At the completion of ET tube suctioning, rinse the catheter and connecting tubing with the sterile saline solution.
14. Suction nasal and/or oral pharynx. Note: A separate catheter must be used for this step when using the closed-suction technique.
15. Discard the suction catheter and rinse the connecting tubing with the sterile saline solution (open-suction technique).
BVM, bag-valve-mask; ECG, electrocardiogram; ET, endotracheal; PEEP, positive end-expiratory pressure.
Source: Suctioning: endotracheal or tracheostomy tube. In: Wiegand DL, Carlson KK, eds. AACN procedure manual for critical care. 6th edn. St Louis: Mosby; 2010.
*Attach a PEEP valve to the BVM for patients on >5 cm H2O PEEP.
Potential complications associated with suctioning include hypoxaemia, bronchospasm, increased intracranial pressure, arrhythmias, hyper-/hypotension, mucosal damage, pulmonary bleeding and infection.50 The nurse must closely assess the patient before, during and after the suctioning procedure. If the patient does not tolerate suctioning (e.g. decreased SpO2, increased blood pressure, sustained coughing or development of arrhythmias), the procedure is halted and the patient is manually ventilated with 100% oxygen if performing CST, hyperoxygenated until equilibrium occurs and before another suction pass is attempted. Hypoxia is prevented by hyperoxygenating the patient before and after each suctioning pass and limiting each suctioning pass to 10 seconds or less (see Box 65-5). Research has shown that there are no differences in outcomes between the use of hyperventilation and hyperoxygenation in preventing suction-induced hypoxia.50 If the SvO2/ScvO2 and/or SpO2 are used, trends should be assessed throughout the suctioning procedure.
Causes of cardiac arrhythmias during suctioning include hypoxaemia resulting in myocardial hypoxia, vagal stimulation caused by tracheal irritation, and sympathetic nervous system stimulation caused by anxiety, discomfort or pain. Arrhythmias include tachy- and bradyarrhythmias, premature beats and asystole. Suctioning should be halted if any new arrhythmias develop. Excessive suctioning should be avoided in patients with severe hypoxaemia or bradycardia.
Tracheal mucosal damage may occur because of excessive suction pressures (>120 mmHg), overly vigorous catheter insertion and the characteristics of the suction catheter itself. The presence of blood streaks or tissue shreds in aspirated secretions may indicate that mucosal damage has occurred. Mucosal damage increases the risk of infection and bleeding, particularly if the patient is receiving anticoagulants.49 Trauma to the mucosa can be prevented by the steps described in Box 65-5.
Secretions may be thick and difficult to suction because of inadequate hydration, inadequate humidification, infection or inaccessibility of the left main stem bronchus or lower airways. Adequately hydrating the patient (e.g. oral or IV fluids) and providing supplemental nebulised saline or humidification of inspired gases may assist in thinning secretions. Instillation of normal saline into the ET tube is not routinely encouraged and its efficacy is controversial.51,52 The SpO2 has been shown to decrease during suctioning with instillation of normal saline. Normal saline is unable to liquefy or thin secretions due to its lack of mucolytic properties.51,52 If infection is the cause of thick secretions, the patient should be assessed for the need of appropriate antibiotics. Physiotherapy, including postural drainage, percussion and turning the patient every 2 hours, may help move secretions into larger airways.
 Providing oral care and maintaining skin integrity
Providing oral care and maintaining skin integrity
When an oral ET tube is in place, the patient’s mouth can never be fully closed so the lips and mouth should be moistened with saline or water swabs to prevent mucosal drying. Oral care should include gentle brushing of the teeth, tongue and gums twice a day, along with using moistened mouth swabs and oral/pharyngeal suctioning every 2–4 hours and as needed to provide comfort and to prevent injury to the gums and plaque accumulation (see Box 65-6).
BOX 65-6 Oral care procedures for a patient on a mechanical ventilator
General measures
2. Wash hands and don personal protective equipment.
3. Explain the procedure to the patient and family, if present.
4. Perform oral care using paediatric or adult soft toothbrushes at least twice a day by gently brushing teeth and tongue to clean/remove plaque.
5. Use oral swabs with a normal saline or 1.5% hydrogen peroxide solution every 2–4 hours. Note: Postoperative cardiac surgery patients are the only population in whiCh 2% chlorhexidine gluconate is recommended twice a day.
6. Apply a water-based mouth moisturiser to oral mucosa and lips with each cleaning.
7. Suction oral cavity/pharynx frequently. See Figure 65-19 for an example of an endotracheal tube that can provide continuous subglottic suctioning.
Source: Endotracheal tube and oral care. In: Wiegand DL, Carlson KK, eds. AACN procedure manual for critical care. 6th edn. St Louis: Mosby; 2010.
Meticulous care is required to prevent skin breakdown on the face, lips, tongue and/or nares as a result of pressure from the ET tube or from the method used to secure the ET tube to the patient’s face. The ET tube should be repositioned and retaped every 24 hours and as needed.47 If the patient is nasally intubated, the nurse should remove the old tape or ties and clean the skin around the ET tube with saline-soaked gauze or cotton swabs. If the patient is orally intubated, the nurse should remove the old tape or ties. Oral hygiene should be provided and the ET tube should be repositioned to the opposite side of the mouth. The nurse reconfirms proper cuff inflation and tube placement. The ET tube is resecured according to institutional policy (see Fig 65-18).
If a manufactured tube holder is used, the straps can be loosened, the area under the straps massaged and the straps reapplied. If the patient is anxious or uncooperative, it is recommended that two caregivers perform the repositioning procedure to prevent accidental dislodgement. The patient should be monitored for any signs of respiratory distress throughout the procedure.
 Fostering comfort and communication
Fostering comfort and communication
Patients have reported that intubation is a major stressor in the ICU.53 The intubated patient may experience anxiety because of the inability to communicate and not knowing what to expect. Communicating with the intubated patient can be a frustrating experience for the patient, the family and the nurse. To communicate more effectively, the nurse should employ a variety of methods.
The physical discomfort associated with ET intubation and mechanical ventilation often necessitates sedating the patient or administering an analgesic until the ET tube is no longer required. The patient may require morphine, midazolam or other sedatives to blunt the anxiety and discomfort related to intubation. The nurse should evaluate the effectiveness of the drugs used to achieve an acceptable level of patient comfort. In addition, the nurse should consider initiating alternative therapies (e.g. music therapy or massage) to complement drug therapy.
COMPLICATIONS OF ENDOTRACHEAL INTUBATION
Two major complications of ET intubation are inadvertent extubation and aspiration. Inadvertent (unplanned) extubation (i.e. removal of the ET tube from the trachea) can be a catastrophic event and usually complicates the patient’s recovery. Unplanned extubations can be due to patient removal of the ET tube or accidental (i.e. result of movement or procedure-related) removal. Usually the unplanned extubation is obvious (the patient is holding the ET tube). At other times, the tip of the ET tube is in the hypopharynx or oesophagus and the extubation is not so obvious. Signs of unplanned extubation may include patient vocalisation, activation of the low-pressure ventilator alarm, diminished or absent breath sounds, respiratory distress and gastric distension. The nurse is responsible for preventing unplanned extubation by continued vigilance by the bedside, ensuring the ET tube is adequately secured, and observation and support of the ET tube during repositioning, procedures and patient transfer. Additionally, immobilising the patient’s hands through the use of soft wrist restraints (using consent and institutional policy) and providing sedation and analgesia as ordered may be needed. Reassessment for continued need of restraints is done according to institutional policy.
Should an unplanned extubation occur, the nurse should stay with the patient and call for help. Interventions are directed at maintaining the patient’s airway, supporting ventilation (usually by manually ventilating the patient with 100% oxygen) and securing the appropriate assistance to immediately reintubate the patient (if necessary) and provide psychological support to the patient.
Aspiration is a potential hazard for the patient with an ET tube. The ET tube passes through the epiglottis, splinting it in an open position. Thus, the intubated patient cannot protect the airway from aspiration. The cuff cannot totally prevent the trickle of oral or gastric secretions into the trachea. Also, secretions accumulate above the cuff. When the cuff is deflated, those secretions move into the lungs. Some ET tubes provide the ability to perform continuous or intermittent suctioning of secretions above the cuff (see Fig 65-19).
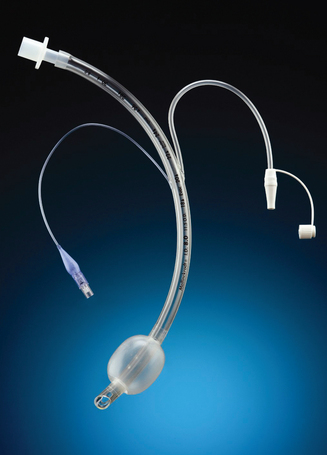
Figure 65-19 Continuous subglottal suctioning can be provided by the Hi Lo Evac Tube. A dorsal lumen above the cuff allows for suctioning of secretions from the subglottic area.
Source: Reprinted by permission of Nellcor Puritan Bennett Inc., Pleasanton, CA.
Oral intubation increases salivation, yet swallowing is difficult, so the mouth must be suctioned frequently. The posterior pharynx should always be suctioned before cuff deflation. This may be performed with a Yankauer (tonsil-tip) suction catheter or a sterile single-use catheter. Other contributing factors to aspiration include improper cuff inflation and tracheo-oesophageal fistula. The patient with an ET tube is at risk of aspiration of gastric contents. Even when the cuff is properly inflated, the nurse must take precautions to avoid emesis, which can lead to aspiration. Most often, a nasogastric (NG) tube is inserted and connected to low, intermittent suction or free drainage when a patient is intubated. All intubated patients and patients receiving enteral feedings should have the head of the bed elevated to a minimum of 30–45° unless contraindicated.
Mechanical ventilation
Mechanical ventilation is the process by which room air or oxygen-enriched air is moved into and out of the lungs by a mechanical ventilator. Mechanical ventilation is not curative. It is a means of supporting patients until they recover the ability to breathe independently, as a bridge to long-term mechanical ventilation or until a decision is made to withdraw ventilator support. Indications for mechanical ventilation include: (1) apnoea or impending inability to breath; (2) acute respiratory failure generally defined as pH ≤ 7.25 with a PaCO2 ≥50 mmHg; (3) severe hypoxia; and (4) respiratory muscle fatique.54
Patients with chronic pulmonary disease and their families should be given the opportunity to decide the issue of mechanical ventilation before terminal respiratory disease develops. All patients, particularly those with grave or chronic illnesses, should be encouraged to discuss the subject with their families and healthcare providers along with formalising the results of that discussion perhaps in an advance directive. The decision to use, withhold or withdraw mechanical ventilation must be made carefully, respecting the informed wishes of the patient and family.
TYPES OF MECHANICAL VENTILATION
The two major types of mechanical ventilation are negative pressure ventilation and positive pressure ventilation.
Negative pressure ventilation
Negative pressure ventilation involves the use of chambers that encase the chest or body and surround it with intermittent subatmospheric or negative pressure. The ‘iron lung’ was the first form of negative pressure ventilation that evolved during the polio epidemics of the 1950s. Intermittent negative pressure around the chest wall causes the chest to be pulled outwards. This reduces intrathoracic pressure. Air rushes in via the upper airway, which is outside the sealed chamber. Expiration is passive; the machine cycles off, allowing chest retraction. This type of ventilation is similar to normal ventilation in that decreased intrathoracic pressures produce inspiration and expiration is passive. Negative pressure ventilation is delivered as non-invasive ventilation and an artificial airway is not required.
There are several portable negative pressure ventilators that are used in the home for patients with neuromuscular diseases, central nervous system disorders, diseases and injuries of the spinal cord, and severe chronic obstructive pulmonary disease (COPD) (see Fig 65-20). Negative pressure ventilators are not used extensively for acutely ill patients. However, some research has demonstrated positive outcomes with the use of negative pressure ventilation in acute exacerbations of chronic respiratory failure.54
Positive pressure ventilation
Positive pressure ventilation (PPV) is the primary method used with acutely ill patients (see Fig 65-21). During inspiration the ventilator pushes air into the lungs under positive pressure. Unlike spontaneous ventilation, intrathoracic pressure is raised during lung inflation rather than lowered. Expiration occurs passively as in normal expiration. Positive pressure ventilators are categorised into two groups: volume and pressure ventilation.
Volume ventilation
With volume ventilation, a predetermined tidal volume (VT) is delivered with each inspiration and the amount of pressure needed to deliver the breath varies based on the compliance and resistance factors of the patient–ventilator system. Consequently, the VT is consistent from breath to breath but airway pressures will vary.55
Pressure ventilation
With pressure ventilation, the peak inspiratory pressure is predetermined and the VT delivered to the patient varies based on the selected pressure and the compliance and resistance factors of the patient–ventilator system.53 With this understanding, careful attention must be given to the VT to prevent unplanned hyperventilation or hypoventilation. For example, when the patient breathes out of synchrony with the ventilator, the pressure limit may be reached quickly and the volume of gas delivered may be small. Initially, pressure ventilation was used only in stable patients being weaned from the ventilator. Today, pressure ventilation is frequently selected to treat critically ill patients.55
SETTINGS OF MECHANICAL VENTILATORS
Mechanical ventilator settings regulate the rate, depth and other characteristics of ventilation (see Table 65-5). Settings are based on the patient’s requirements and characteristics (ABGs, body weight, level of consciousness, muscle strength). The ventilator is adjusted to be synchronised with the patient’s ventilation pattern. Settings are evaluated and adjusted frequently until optimal ventilation and oxygenation is achieved. Some settings serve as a fail-safe mechanism, alerting staff to problems with ventilation. It is important that the nurse ensures and documents that all ventilator alarms are on at all times. Alarms alert the staff of potentially dangerous situations, such as mechanical malfunction, apnoea or patient asynchrony with the ventilator. On many ventilators the alarms can be temporarily suspended or silenced for up to 2 minutes for suctioning or testing. After that period of time, the alarm system automatically becomes functional again.
TABLE 65-5 Settings of mechanical ventilation
| Parameter | Description |
|---|---|
| Respiratory rate (f) | Number of breaths the ventilator delivers per minute; usual setting is 6–20 breaths/min |
| Tidal volume (VT) | Volume of gas delivered to patient during each ventilator breath; usual volume is 10–12 mL/kg; 6–8 mL/kg in acute lung injury |
| Oxygen concentration (FiO2) | Fraction of inspired oxygen delivered to the patient; may be set between 21% (essentially room air) and 100%; usually adjusted to maintain PaO2 level >60 mmHg or SpO2 level >90% |
| Positive end-expiratory pressure (PEEP) | Positive pressure applied at the end of expiration of ventilator breaths; usual setting is 5 cm H2O |
| Pressure support | Positive pressure used to augment patient’s inspiratory pressure; usual setting is 5–10 cm H2O |
| I:E ratio | Duration of inspiration (I) to duration of expiration (E); usual setting is 1:2 to 1:1.5 unless IRV is desired |
| Inspiratory flow rate and time | Speed with which the VT is delivered; usual setting is 40–80 L/min and time is 0.8–1.2 seconds |
| Sensitivity | Determines the amount of effort the patient must generate to initiate a ventilator breath; it may be set for pressure triggering or flow triggering; usual setting for a pressure trigger is 0.5–1.5 cm H2O below baseline pressure and for a flow trigger is 1–3 L/min below baseline flow |
| High pressure limit | Regulates the maximal pressure the ventilator can generate to deliver the VT; when the pressure limit is reached, the ventilator terminates the breath and spills the undelivered volume into the atmosphere; usual setting is 10–20 cm H2O above peak inspiratory pressure (up to 40 cm H2O) |
IRV, inverse ratio ventilation.
Source: Urden LD, Stacy KM, Lough ME. Thelan’s critical care nursing: diagnosis and management. 6th edn. St Louis: Mosby; 2010.
MODES OF VOLUME VENTILATION
The variable methods by which the patient and the ventilator interact to deliver effective ventilation are called modes. The ventilator mode selected is based on how much work of breathing the patient ought to, or can, perform and is determined by the patient’s ventilation status, respiratory drive and ABGs. Work of breathing (WOB) refers to the inspiratory effort needed to overcome the elasticity and viscosity of the lungs along with the airway resistance. Generally, ventilator modes are controlled or assisted. With controlled ventilation support, the ventilator does all of the WOB, and with assisted ventilation support, the ventilator and the patient share the WOB.53 For the past 25 years, volume modes, such as controlled mechanical ventilation, assist-control ventilation and synchronised intermittent mandatory ventilation, have been used to treat critically ill patients. Over the last decade, pressure modes such as pressure support ventilation and pressure-controlled inverse ratio ventilation have become more widespread.56 These modes are described in Box 65-7.
BOX 65-7 Modes of mechanical ventilation
Volume modes
Control ventilation (CV) or controlled mandatory ventilation (CMV)
With this mode, the ventilator provides all of the patient’s minute ventilation. The clinician sets the rate, tidal volume (VT), inspiratory time and positive end-expiratory pressure (PEEP). Generally, this term is used to describe those situations in which the patient is chemically relaxed or is paralysed from a spinal cord or neuromuscular disease and is therefore unable to initiate spontaneous breaths. The ventilator mode setting may be set on CMV, assist-control (AC) or synchronised intermittent mandatory ventilation (SIMV) because all these options provide breaths at the clinician-selected rate.
Assist-control (AC) or assisted mandatory ventilation (AMV)
This option requires that a rate, VT, inspiratory time and PEEP be set for the patient. The ventilator sensitivity is also set, and when the patient initiates a spontaneous breath, a full machine breath is delivered.
Intermittent mandatory ventilation (IMV) and synchronised intermittent mandatory ventilation (SIMV)
This mode requires that rate, VT, inspiratory time, sensitivity and PEEP are set by the clinician. In between ‘mandatory breaths’, the patient can spontaneously breathe at their own rate and VT. With SIMV, the ventilator synchronises the mandatory breaths with the patient’s own inspirations.
Pressure modes
Pressure support ventilation (PSV)
This mode provides an augmented inspiration to a spontaneously breathing patient. With PSV the clinician selects an inspiratory pressure level, PEEP and sensitivity. When the patient initiates a breath, a high flow of gas is delivered to the preselected pressure level and pressure is maintained throughout inspiration. The patient determines the parameters of VT, rate and inspiratory time.
Pressure-controlled inverse ratio ventilation (PC-IRV)
This mode combines pressure-limited ventilation with an inverse ratio of inspiration to expiration. The clinician selects the pressure level, rate, inspiratory time (1:1, 2:1, 3:1, 4:1) and the PEEP level. With the prolonged inspiratory times, auto-PEEP may result. The auto-PEEP may be a desirable outcome of the inverse ratios. Some clinicians use PC without IRV. Conventional inspiratory times are used and rate, pressure level and PEEP are selected.
Positive end-expiratory pressure (PEEP) and continuous positive airway pressure (CPAP)
Source: Ventilatory management: volume and pressure modes. In: Wiegand DL, Carlson KK, eds. AACN procedure manual for critical care. 6th edn. St Louis: Mosby; 2010.
Controlled mandatory ventilation
In controlled mandatory ventilation (CMV), breaths are delivered at a set rate per minute and a set VT, which are independent of the patient’s respiratory efforts. Although CMV is used infrequently, it can be used when the patient has no drive to breathe (e.g. the anaesthetised patient) or is unable to breathe spontaneously (e.g. the paralysed patient). In the CMV mode, the patient performs no WOB and cannot adjust respirations to changing demands.
Assist-control mechanical ventilation
In assist-control ventilation (ACV), the ventilator delivers a preset VT at a preset frequency and when the patient initiates a spontaneous breath, a full VT is delivered. The ventilator senses a decrease in intrathoracic pressure and then delivers the preset VT. The patient can breathe faster than the preset rate but not slower. This mode has the advantage of allowing the patient some control over ventilation while providing some assistance. ACV is used in patients with a variety of conditions, including neuromuscular disorders (e.g. Guillain-Barré syndrome), pulmonary oedema and acute respiratory failure in the ACV mode. The patient has the potential for hypoventilation and hyperventilation. The spontaneously breathing patient can easily be overventilated, resulting in hyperventilation. If the volume or minimum rate is set low and the patient is apnoeic or weak, the patient will be hypoventilated. Thus, these patients require vigilant assessment and monitoring of ventilation status, including respiratory rate, ABGs, SpO2 and SvO2/ScvO2. It is also important that the amount of negative pressure required to initiate a breath (trigger sensitivity) is appropriate to the patient’s condition. For example, if it is too difficult to initiate a breath, the WOB is increased and the patient may tire and/or develop ventilator asynchrony (i.e. the patient ‘fights’ the ventilator).
Synchronised intermittent mandatory ventilation
During synchronised intermittent mandatory ventilation (SIMV), the ventilator delivers a preset VT at a preset frequency in synchrony with the patient’s spontaneous breathing. Between ventilator-delivered breaths, the patient is able to breathe spontaneously through the ventilator circuit. Thus, the patient receives the preset FiO2 concentration during the spontaneous breaths but self-regulates the rate and depth of those breaths. This mode of ventilation differs from ACV, in which all breaths are of the same preset volume. It is used during continuous ventilation and during weaning from the ventilator. SIMV may also be combined with pressure support ventilation (described below). Potential benefits of SIMV include improved patient–ventilator synchrony (the patient ‘fights’ the ventilator less), lower mean airway pressure and prevention of muscle atrophy as the patient takes on more of the WOB.54
There are disadvantages with SIMV. If spontaneous breathing decreases when the preset rate is low, ventilation may not be adequately supported. Low-rate SIMV should be used only in patients with regular, spontaneous breathing. Weaning with SIMV demands close monitoring and may take longer because the rate of breathing is gradually reduced. Patients being weaned with SIMV may also have increased muscle fatigue, associated with spontaneous breathing efforts.
MODES OF PRESSURE VENTILATION
Pressure support ventilation
With pressure support ventilation (PSV), positive pressure is applied to the airway only during inspiration and is used in conjunction with the patient’s spontaneous respirations. The patient must be able to initiate a breath in this modality. A preset level of positive airway pressure is selected so that the gas flow rate is greater than the patient’s inspiratory flow rate. As the patient initiates a breath, the machine senses the spontaneous effort and supplies a rapid flow of gas at the initiation of the breath and variable flow throughout the breath. With PSV the patient determines inspiratory length, VT and respiratory rate. VT depends on the pressure level and airway compliance. PSV is used with continuous ventilation and is especially helpful in combination with SIMV during weaning. PSV is not used as a sole ventilation support during acute respiratory failure because of the risk of hypoventilation. Advantages to PSV include increased patient comfort, decreased WOB (because inspiratory efforts are augmented), decreased oxygen consumption (because inspiratory work is reduced) and increased endurance conditioning (because the patient is exercising respiratory muscles).57
Pressure-controlled inverse ratio ventilation
Pressure-controlled inverse ratio ventilation (PC-IRV) combines pressure-limited ventilation with an inverse ratio of inspiration (I) to expiration (E). The I:E ratio is the ratio of duration of inspiration (I) to the duration of expiration (E). This value is normally 1:2. With IRV the I:E ratio begins at 1:1 and may progress to 4:1. A prolonged positive inspiratory pressure is applied, which increases inspiratory time. IRV progressively expands collapsed alveoli. The short expiratory time has a PEEP-like effect, preventing alveolar collapse. Because IRV imposes a non-physiological breathing pattern, the patient requires sedation or paralysis. IRV is indicated for patients with acute respiratory distress syndrome (ARDS) who continue to have refractory hypoxaemia despite high levels of PEEP. (ARDS is discussed in Ch 67.) Not all patients with poor oxygenation respond to PC-IRV.
Other modes
Improved technology and the sophistication of ventilator manufacturers have led to the development of additional pressure modes. However, due to the non-standardisation of these options, the names and features are manufacturer specific. The superiority of these modes has not been established. Some examples include volume-assured pressure ventilation (VAPS) and pressure release ventilation (PRV).
OTHER VENTILATORY MANOEUVRES
Positive end-expiratory pressure
Positive end-expiratory pressure (PEEP) is a ventilation manoeuvre in which positive pressure is applied to the airway during exhalation. During exhalation, with an ET tube inserted, airway pressure drops to zero and exhalation occurs passively. With PEEP, exhalation remains passive but pressure falls to a preset level greater than zero, often 3–10 cm H2O. With PEEP, lung volume during expiration and between breaths is greater than normal. Thus, PEEP increases functional residual capacity (FRC) and this often improves oxygenation. The mechanisms by which PEEP increases FRC and oxygenation include increased aeration of patent alveoli, aeration of previously collapsed alveoli and prevention of alveolar collapse throughout the respiratory cycle.57
PEEP is titrated to the point that oxygenation improves without compromising haemodynamic status.58 This is termed ‘best’ or ‘optimal’ PEEP. Often 5 cm H2O PEEP (referred to as physiological PEEP) is used prophylactically to replace the glottic mechanism, help maintain a normal FRC and prevent alveolar collapse. A PEEP of 5 cm H2O is also used for patients with a history of alveolar collapse during weaning. PEEP has demonstrated improvements in gas exchange, vital capacity and inspiratory force when used during weaning.
In contrast, auto-PEEP is not purposely set on the ventilator but is a result of inadequate exhalation time during ventilation.59 Auto-PEEP is additional PEEP over and above that set by the clinician and can be measured at end-expiration by pressing the hold button located on most ventilators. This additional PEEP may not be desirable and may result in increased work of breathing, barotrauma and haemodynamic instability. However, during some ventilator modes (PC-IRV), auto-PEEP may be desirable. Interventions to limit auto-PEEP include sedation and analgesia, large diameter ET tube, bronchodilators, shorter inspiratory times, decreased respiratory rates and reducing water accumulation in the ventilator circuit by frequent emptying or use of dual heated circuits. In patients with short exhalation times and early airway closure (e.g. COPD, asthma), setting PEEP can offset auto-PEEP by splinting the airway open during exhalation and preventing ‘air trapping’.
In general, the major purpose of PEEP is to maintain or improve oxygenation while limiting the risk of oxygen toxicity. FiO2 can often be reduced when PEEP is used. PEEP is thought to be useful in pulmonary oedema, providing a counterpressure opposing fluid extravasation. PEEP is indicated in lungs with diffuse disease, severe hypoxaemia unresponsive to an FiO2 >0.5 (50% oxygen), and loss of compliance or stiffness. The classic indication for PEEP therapy is ARDS (see Ch 67). PEEP is generally contraindicated or used with extreme caution in patients with highly compliant lungs (e.g. COPD), unilateral or non-uniform disease, hypovolaemia and low CO. In these situations the adverse effects of PEEP may outweigh any benefits.
Continuous positive airway pressure
Continuous positive airway pressure (CPAP) restores FRC and is similar to PEEP. However, the pressure in CPAP is delivered continuously during spontaneous breathing, thus preventing the patient’s airway pressure from falling to zero. For example, if the CPAP is 5 cm H2O, airway pressure during expiration is 5 cm H2O. During inspiration, 1–2 cm H2O of negative pressure is generated, thus reducing airway pressure from 5 to 3 or 4 cm H2O. The patient receiving SIMV with PEEP receives CPAP when breathing spontaneously. CPAP is commonly used in the treatment of obstructive sleep apnoea. CPAP can be administered non-invasively by a tight-fitting mask or an ET or a tracheal tube. CPAP increases WOB because the patient must forcibly exhale against the CPAP and so must be used with caution in patients with clinically significant myocardial compromise.
Bilevel positive airway pressure
Bilevel positive airway pressure (BiPAP) provides two levels of positive pressure support—higher inspiratory positive airway pressure (IPAP) and a lower expiratory positive airway pressure (EPAP)—along with oxygen. It is a non-invasive modality and is delivered through a tight-fitting face mask, nasal mask or nasal pillows. Similar to PSV, which is delivered through an artificial airway, the patient must be able to spontaneously breathe and cooperate with this treatment. Indications include acute respiratory failure in patients with COPD and heart failure, and sleep apnoea. BiPAP may also be used after extubation to prevent reintubation.60 Patients with shock, altered mental status or increased airway secretions are not good candidates for BiPAP.
High-frequency ventilation
High-frequency ventilation (HFV) involves delivery of a small tidal volume (usually 1–5 mL per kg of body weight) at rapid respiratory rates (100–300 breaths per minute) in an effort to recruit and maintain lung volume and reduce intrapulmonary shunting (see Ch 66). One benefit of HFV may be the ability to support gas exchange while minimising the risk of barotrauma. HFV has been widely accepted in neonatal and paediatric ICUs but its use in adults is considered investigational and limited to patients with ARDS.61
There are three types of HFV. High-frequency jet ventilation (HFJV) delivers humidified gas from a high-pressure source through a small-bore cannula positioned in the airway. With HFJV, precise VT is difficult to predict and is a function of numerous variables. High-frequency percussive ventilation (HFPV) attempts to combine the positive effects of both HFV and conventional mechanical ventilation. A piston mechanism positioned at the end of the ET tube is driven by a high-pressure gas supply at a rate of 200–900 beats per minute. These high-frequency beats are superimposed on a conventional pressure-controlled ventilator mode. High-frequency oscillatory ventilation (HFOV) uses a diaphragm or a piston in the ventilator to generate vibrations (or oscillations) of subphysiological volumes of gas. HFOV can produce respiratory frequencies in excess of 3000 breaths per minute. Patients receiving HFV must be paralysed to suppress spontaneous respiration. In addition, patients must receive concurrent sedation and analgesia as necessary adjuncts when inducing paralysis.
Nitric oxide
Nitric oxide (NO) is a gaseous molecule that is synthesised intravascularly and participates in the regulation of pulmonary vascular tone. Inhibition of NO production results in pulmonary vasoconstriction, and administration of continuous inhaled NO results in pulmonary vasodilation. Current indications include a diagnosis of ARDS, use as a diagnostic screening tool for pulmonary hypertension during a cardiac catheterisation, and during or after cardiac surgery. Administration may be given invasively through an ET/tracheostomy or via a face mask. NO therapy does not appear to pose a risk to healthcare providers during routine delivery of up to 20 ppm.62
Prone positioning
Prone positioning is the repositioning of a patient from a supine or lateral position to a prone (on the stomach with face down) position. This repositioning is used to improve lung recruitment through various mechanisms. The effects of fluid in the dependent parts of the lungs are reversed via gravity as the patient is changed from supine to prone. In this position, the heart rests on the sternum, away from the lungs, contributing to an overall uniformity of pleural pressures. This relatively safe, although nurse-intensive therapy, is used as supportive therapy in critically ill patients with acute lung injury or ARDS to improve oxygenation.63
Extracorporeal membrane oxygenation
Extracorporeal membrane oxygenation (ECMO) is a form of pulmonary support for the patient with severe respiratory failure. It is more commonly used in the paediatric and neonatal populations with increasing use in the adult patient.64 ECMO is a modification of cardiac bypass and involves partially removing blood from a patient through the use of large-bore catheters, infusing oxygen, removing CO2 and returning the blood back to the patient. This intensive therapy requires systemic anticoagulation and is a time-limited intervention. A skilled team of specialist healthcare staff, including a cardiac perfusionist, is usually required continuously at the bedside.
COMPLICATIONS OF POSITIVE PRESSURE VENTILATION
Although mechanical ventilation may be essential to maintain ventilation and oxygenation, it can cause adverse effects. It is often difficult to distinguish complications of mechanical ventilation from the underlying disease.
Cardiovascular system
PPV can affect circulation because of the transmission of increased mean airway pressure to the thoracic cavity. With increased intrathoracic pressure, thoracic vessels are compressed. This results in decreased venous return to the heart, decreased left ventricular end-diastolic volume (preload), decreased CO and hypotension. Mean airway pressure is further increased if titrating PEEP (>5 cm H2O) to improve oxygenation.
If the lungs are non-compliant (as in ARDS), airway pressures are not as easily transmitted to the heart and blood vessels. Thus, the effects of PPV on CO are reduced. Conversely, with compliant lungs (e.g. emphysema), there is an increased danger of transmission of high airway pressures and a negative effect on haemodynamic status.
Compromise of venous return by PPV is exaggerated by hypovolaemia (e.g. haemorrhage, multiple trauma) and decreased venous tone (e.g. sepsis, spinal shock). Restoration and maintenance of the circulating blood volume are important in minimising cardiovascular complications.
Pulmonary system
Barotrauma
As lung inflation pressures increase, risk of barotrauma increases. Patients with compliant lungs (e.g. COPD) are at greater risk of barotrauma because the increased airway pressure readily distends the lungs and may rupture alveoli or emphysematous blebs. Patients with stiff lungs (e.g. ARDS) who are given high inspiratory pressures and high levels of PEEP (>5 cm H2O), as well as patients with suppurative lung abscesses resulting from necrotising organisms (e.g. staphylococci), are also susceptible to barotrauma.
Air can escape into the pleural space from the alveoli or the interstitium, accumulate and become trapped. Pleural pressure increases and collapses the lung, causing pneumothorax. (Clinical manifestations of pneumothorax are discussed in Ch 27.) The lung receives air during inspiration but cannot expel it during expiration. Respiratory bronchioles are larger on inspiration than expiration. They may close on expiration and air becomes trapped. With PPV, a simple pneumothorax can become a life-threatening tension pneumothorax. With tension pneumothorax, the mediastinum and contralateral lung are compressed, compromising CO. Immediate treatment of the pneumothorax is required. For some patients, chest tubes may need to be inserted prophylactically.
Pneumomediastinum usually begins with rupture of the alveoli into the lung interstitium; progressive air movement then occurs into the mediastinum and subcutaneous neck tissue. This is commonly followed by pneumothorax. Occurrence of new, unexplained subcutaneous emphysema is an indication for immediate chest X-ray. Pneumomediastinum and subcutaneous emphysema in the neck may be too small to be detected radiographically or clinically before the development of a pneumothorax.
Volu-pressure trauma
The concept of volu-pressure trauma in PPV relates to the lung injury that occurs when large tidal volumes are used to ventilate non-compliant lungs (e.g. ARDS). Volu-pressure trauma results in alveolar ruptures and movement of fluids and proteins into the alveolar spaces. The ARDS Network Study demonstrated a change in mortality of patients with ARDS by using a smaller VT of 6 mL/kg. The use of low-volume ventilation rather than pressure ventilation is the suggested strategy for lung protection in ARDS patients.65
Alveolar hypoventilation
Hypoventilation can be caused by inappropriate ventilator settings, leakage of air from the ventilator tubing or around the ET tube or tracheostomy cuff, lung secretions or obstruction, and low ventilation–perfusion ratio. Low VT or respiratory rate decreases minute ventilation, causing hypoventilation. A leaking cuff or tubing that is not secured may cause air leakage, lowering the delivered VT. Too low a preset mandatory rate in a patient who is unable to produce adequate spontaneous respirations causes hypoventilation, respiratory acidosis and additional problems related to acidosis, such as cardiac arrhythmias. Excess lung secretions can cause hypoventilation. Turning the patient every 1–2 hours, providing chest physiotherapy to lung areas with increased secretions, encouraging deep breathing, coughing and tracheal suctioning as indicated can all help alleviate this. Atelectasis, defined as the lack of gas exchange within alveoli due to alveolar collapse or fluid consolidation, may develop. Increasing the VT and adding small increments of PEEP decreases the likelihood of atelectasis.
Alveolar hyperventilation
Respiratory alkalosis can occur if the respiratory rate or VT is set too high (mechanical overventilation) or if the patient is hyperventilating spontaneously. It is easy to overventilate a patient on PPV. Particularly at risk are patients with chronic alveolar hypoventilation and CO2 retention (e.g. patients with COPD). The patient with COPD may have a chronic PaCO2 elevation (acidosis) and compensatory bicarbonate retention by the kidneys. When the patient is ventilated, the patient’s ‘normal baseline’, rather than the standard normal values, should be the therapeutic goal. If the patient with COPD is returned to a standard normal PaCO2, they will develop alkalosis because of the retained bicarbonate. Such a patient could move from compensated respiratory acidosis to serious metabolic alkalosis. The presence of alkalosis makes weaning from the ventilator difficult. Alkalosis, especially if the onset is abrupt, can have additional serious consequences, including hypokalaemia, hypocalcaemia and arrhythmias. Neuromuscular irritability, seizures, coma and death can occur if alkalosis progresses. Usually the patient with COPD who is supported by a ventilator does better with a short inspiratory and longer expiratory time.
If hyperventilation is spontaneous, it is important to determine the cause and treat it. Causes may include hypoxaemia, pain, fear, anxiety or compensation for metabolic acidosis. Patients who ‘fight’ the ventilator or breathe out of synchrony may be anxious or in pain. If the patient is anxious and fearful, sitting with the patient and verbally coaching the patient to breathe with the ventilator may help. If these measures fail, manually hand ventilating (‘bagging’) the patient slowly with a 100% oxygen source may slow breathing enough to bring it into synchrony with the ventilator.
Ventilator-associated pneumonia
The risk of iatrogenic pneumonia is highest in patients requiring PPV because the ET or tracheostomy tube bypasses normal upper airway defences. In addition, poor nutritional state, immobility and the underlying disease process (e.g. immunosuppression, organ failure) make the patient more prone to infection. Ventilator-associated pneumonia (VAP) is defined as a pneumonia that occurs 48 hours or more after endotracheal intubation.66 Staff in ICUs work hard to prevent VAP, and research in Australia in one ICU found that VAP was lower than in European ICUs (6.2 per 1000 ventilator days compared with 9.5).67 Patients who develop VAP have significantly longer hospital stays and higher mortality rates than those who do not develop VAP.
In patients with early VAP (within 96 hours of mechanical ventilation), sputum cultures often grow Gram-negative bacteria, such as Escherichia coli, Klebsiella, Proteus, Streptococcus pneumoniae, Haemophilus influenzae and oxacillin-sensitive S. aureus. Organisms associated with late VAP include antibiotic-resistant microorganisms such as Pseudomonas aeruginosa and oxacillin-resistant Staphylococcus aureus. These are abundant in the hospital environment and are also naturally present in the patient’s GI tract. Organisms can spread in a number of ways, including contaminated respiratory equipment, inadequate hand-washing, adverse environmental factors such as poor room ventilation and high traffic flow, and decreased patient ability to cough and clear secretions. Colonisation of the oropharynx by Gram-negative organisms is a predisposing factor in the development of Gram-negative pneumonia.
Clinical evidence suggesting VAP includes fever, elevated white blood cell count, purulent sputum, malodorous sputum or crackles on auscultation, and pulmonary infiltrates noted on chest X-ray. The patient is treated with antibiotics after appropriate cultures are taken by tracheal suctioning or bronchoscopy, and when infection is evident.
Guidelines on VAP prevention include: (1) head of bed elevation at a minimum of 30–45° unless medically contraindicated; (2) no routine changes of the patient’s ventilator circuit tubing; and (3) the use of an ET with a dorsal lumen above the cuff to allow continuous suctioning of secretions in the subglottic area (see Fig 65-19).68 Continuous subglottic suctioning appears effective in preventing early-onset VAP.69 Prevention also includes effective and frequent hand-washing before and after suctioning, whenever ventilator equipment is touched and after contact with any respiratory secretions. The nurse should wear gloves when in contact with the patient and change gloves between activities (e.g. bathing the patient, administering an intravenous drug). Compliance with the simplest preventative measures (e.g. washing hands and changing gloves) continues to be inconsistent among healthcare workers and yet is one of the best ways of preventing hospital-acquired infection. Finally, condensation that collects in the ventilator tubing should be drained away from the patient as it collects.
Sodium and water imbalance
Progressive fluid retention often occurs after 48–72 hours of PPV. PPV, especially with PEEP, is associated with decreased urinary output and increased sodium retention. Fluid balance changes may be due to decreased CO, which in turn results in diminished renal perfusion. Consequently, renin release is stimulated with the subsequent production of angiotensin and aldosterone. This results in sodium and water retention. It is also possible that pressure changes within the thorax are associated with decreased release of atrial natriuretic peptide, also causing sodium retention. Mild water retention is also associated with PPV. There is less insensible water loss via the airway because ventilated delivered gases are humidified. In addition, as a part of the stress response, release of antidiuretic hormone and aldosterone may be increased, contributing to sodium and water retention.
Neurological system
In patients with a head injury, PPV, especially with high levels of PEEP, can impair cerebral blood flow, particularly in patients prone to rises in intracerebral pressure. This is related to increased intrathoracic positive pressure impeding venous drainage from the head, as evidenced by jugular venous distension. As a result of the impaired venous return and increase in cerebral volume, the patient may exhibit increases in intracranial pressure. Elevating the head of the bed, keeping the patient’s head in alignment and avoiding high levels of PEEP may decrease the deleterious effects of PPV on intracranial pressure.
Gastrointestinal system
Patients receiving PPV are often stressed because of serious illness, immobility and discomfort associated with the ventilator. Thus, the ventilated patient is at risk of developing stress ulcers and GI bleeding. Patients with a pre-existing ulcer or those receiving corticosteroid therapy are at an especially increased risk. Any kind of circulatory compromise, including reduction of CO caused by PPV, may contribute to ischaemia of the gastric and intestinal mucosa and possibly increase the risk of translocation of GI bacteria.70
Clinical practice for peptic ulcer prophylaxis frequently includes the administration of histamine H2-receptor antagonists (e.g. ranitidine) or proton pump inhibitors (e.g. omeprazole) and tube feedings in an effort to decrease gastric acidity and diminish the risk of stress ulcer and haemorrhage. Although research findings about the use of H2-receptor blockers or proton pump inhibitors are conflicting, peptic ulcer prophylaxis in patients who are mechanically ventilated is frequently prescribed to decrease the risk of VAP.
Gastric and bowel dilation may occur as a result of gas accumulation in the GI tract from swallowed air. The irritation of an artificial airway may cause excessive air swallowing and subsequent gastric dilation. Gastric or bowel dilation may put pressure on the vena cava, decrease CO and prohibit adequate diaphragmatic excursion during spontaneous breathing. Elevation of the diaphragm as a result of paralytic ileus or bowel dilation leads to compression of the lower lobes of the lungs, which may cause atelectasis and compromise respiratory function. Decompression of the stomach can be accomplished by the insertion of an NG tube.
Immobility, sedation, circulatory impairment, decreased oral intake, use of opioid pain medications and stress contribute to decreased peristalsis. The patient’s inability to exhale against a closed glottis may make defecation difficult. As a result, the ventilated patient could be predisposed to constipation.
Musculoskeletal system
Maintenance of muscle strength and prevention of the problems associated with immobility are important. Exercise tolerance is enhanced by adequate analgesia and nutrition. Progressive ambulation of patients receiving long-term PPV who are haemodynamically stable can be attained without interruption of mechanical ventilation. The ventilator can be pushed around the room or the patient can be manually ventilated with a BVM device while ambulating. Passive and active exercises, consisting of movements to maintain muscle tone in the upper and lower extremities, should be done in bed. Simple manoeuvres, such as leg lifts, knee bends, quadriceps setting or arm circles, are appropriate. Prevention of contractures, pressure ulcers, footdrop and external rotation of the hip and legs by proper positioning is important.
Psychosocial needs
The patient receiving mechanical ventilation may experience physical and emotional stress. In addition to the issues discussed at the beginning of this chapter, the patient supported by a mechanical ventilator is unable to speak, eat, move or breathe normally. Tubes and machines may cause pain and contribute to fear and anxiety.71 Ordinary activities of daily living, such as eating, elimination and even coughing, are extremely complicated. In a recent study of former ICU patients, ‘support’ dominated as one of the most important themes.71 The nurse should work to strengthen the various factors that affect supporting the patient and promote feeling safe. Communication must be creative in the case of the intubated patient and information forthright. Patients should be involved in decision making as much as possible. The nurse should encourage hope where appropriate and build trusting relationships with the patient and family.
Patients receiving PPV usually require some type of sedation (e.g. midazolam) and/or analgesia (e.g. fentanyl) to facilitate optimal ventilation. Before initiating sedation and/or analgesia in the mechanically ventilated patient who is agitated or anxious, it is important to assess for the cause of distress. Common problems that can result in patient agitation or anxiety include PPV, nutritional deficits, pain, hypoxaemia, hypercapnia, drugs and environmental stressors (e.g. sleep deprivation). It is important to note that delirium is an acute change in mental status and is a marker of cerebral insufficiency associated with longer hospital stays and a higher mortality rate. ICU patients are particularly vulnerable to delirium and every effort should be made to accurately assess and treat it.72,73
At times the decision is made to paralyse the patient with a neuromuscular blocking agent (e.g. pancuronium) to provide more effective synchrony with the ventilator, to increase oxygenation or to reduce metabolic oxygen needs. If the patient is paralysed, the nurse should remember that the patient can hear, see, smell, think and feel. IV sedation and analgesia must always be administered concurrently when the patient is paralysed, ensuring that the patient is first sedated, then paralysed. Assessment of the patient should include train-of-four (TOF) peripheral nerve stimulation, physiological signs of pain or anxiety (changes in heart rate and blood pressure) and monitoring for signs of ventilator asynchrony.74 The TOF assessment involves the use of a peripheral nerve stimulator to deliver four successive stimulating currents to elicit muscle twitches (see Fig 65-22). The number of twitches will vary with the percentage of neuromuscular blockade and the usual goal is one to two twitches out of four. Excessive administration of neuromuscular blocking agents may predispose the patient to prolonged paralysis and muscle weakness even after these agents are discontinued.
Figure 65-22 Placement of electrodes along the ulnar nerve.
Source: Wiegand DL, Carson KK, eds. AACN procedure manual for critical care. 5th edn. St Louis: Mosby; 2009.
Many patients have few memories of their time in the ICU, whereas others remember vivid details. Although appearing to be asleep, sedated or paralysed, patients may be aware of their surroundings and should always be addressed as though awake and alert.
Machine disconnection or malfunction
Mechanical ventilators may become disconnected or malfunction. When turned on and operative, alarms alert the nurse to problems. Most deaths from accidental ventilator disconnection occur while the alarm is turned off and most accidental disconnections in critical care settings are discovered by low-pressure alarm activation. The most frequent site for disconnection is between the tracheal tube and the adapter. Connections should be pushed together and then twisted to secure more tightly. The nurse should ascertain that alarms are set at all times and should chart that this is the case. Alarms can be paused (not inactivated) during suctioning or removal from the ventilator and should always be reactivated before leaving the patient’s bedside.
Ventilator malfunction may also occur and may be related to several factors. Although most institutions have emergency generators in the event of a power failure, the nurse should always consider the possibility that power may fail and have a plan for manually ventilating all the patients who are dependent on a ventilator. If, at any time, the nurse determines that the ventilator is malfunctioning (e.g. failure of oxygen supply), the patient should be disconnected from the machine and manually ventilated with 100% oxygen until the ventilator is fixed or replaced.
NUTRITIONAL THERAPY: PATIENT RECEIVING POSITIVE PRESSURE VENTILATION
PPV and the hypermetabolism associated with critical illness can contribute to inadequate nutrition. The presence of an ET tube eliminates the normal route for eating. Although patients who are nasotracheally intubated may be allowed liquid and semiliquid feedings orally, it is difficult to ingest sufficient kilojoules including protein and fat. A patient with a tracheostomy can eat normally once the stoma has healed. When a tracheostomy tube is present, the patient should tilt the head slightly forwards to facilitate swallowing and to prevent aspiration. Often, soft foods (e.g. puddings, ice-cream) are more easily swallowed than liquids.
Patients who are likely to be without food for 3–5 days should have a nutritional program initiated. Inadequate nutrition makes the patient receiving prolonged mechanical ventilation more prone to poor oxygen transport secondary to anaemia and to poor tolerance of minimal exercise. Poor nutrition and the disuse of respiratory muscles contribute to decreased respiratory muscle strength. In addition, the hypermetabolism associated with critical illness, trauma and surgery and the presence of anxiety, pain and increased WOB greatly increase energy expenditure. Serum protein levels (e.g. albumin, prealbumin, transferrin, total protein) are usually decreased. Inadequate nutrition can delay weaning, decrease resistance to infection and decrease the speed of recovery. Enteral feeding via a small-bore feeding tube is the preferred method to meet the kilojoule needs of ventilated patients. (See Ch 39 for a discussion of enteral feeding.)
Verification of feeding tube placement includes: (1) X-ray confirmation before initial use; (2) marking and ongoing assessment of the tube’s exit site; and (3) ongoing review of routine X-rays and aspirate. The traditionally used auscultatory method of assessment (i.e. listening for air after injection) is not a reliable method for verifying placement of feeding tubes.10
WEANING FROM POSITIVE PRESSURE VENTILATION AND EXTUBATION
The process of reducing ventilator support and resuming spontaneous ventilation is termed weaning. The weaning process differs for patients requiring short-term ventilation (<3 days) versus long-term ventilation (>3 days). Patients requiring short-term ventilation (e.g. after cardiac surgery) will experience a linear weaning process. Patients who are likely to require prolonged PPV (e.g. patients with COPD who develop respiratory failure) will most likely experience a weaning process that consists of peaks and troughs.76 Conceptually, preparation for weaning should begin when PPV is initiated and should involve a team approach (e.g. nurse, intensivist, physiotherapist, patient, family and dietician). The indicators for weaning are shown in Table 65-6.
TABLE 65-6 Indicators for weaning
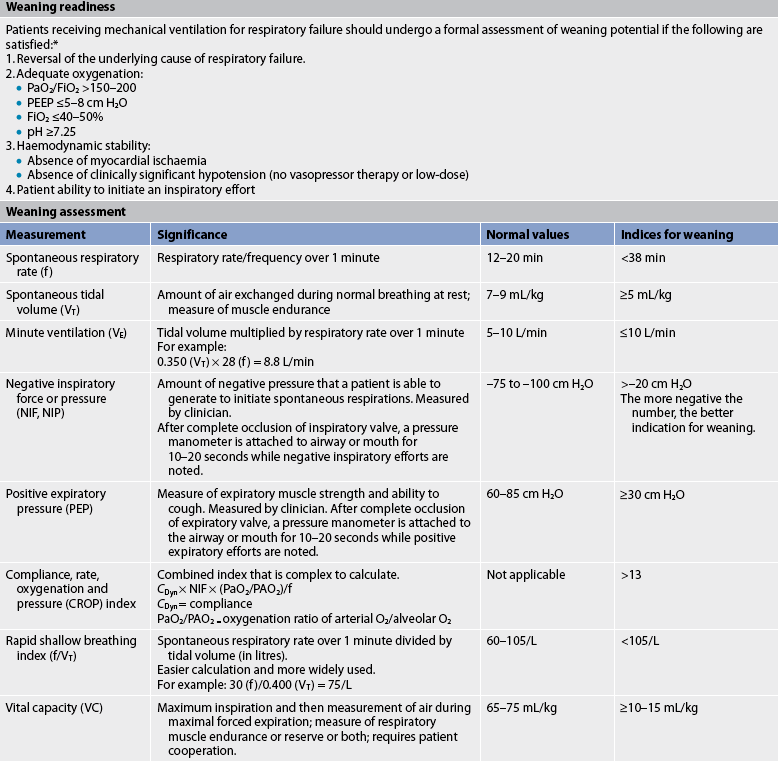
* The decision to use these criteria must be individualised to the patient.
Source: Macintyre NR, Cook DJ, Ely Ew Jr et al. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest 2001; 120(6 Suppl):375–95S. Burns SM. Weaning process. In: Wiegand DL, Carlson KK, eds. AACN procedure manual for critical care. 6th edn. St louis: Mosby; 2010.
Weaning can be viewed as consisting of three phases: the pre-weaning phase, the weaning process and the outcome phase. The pre-weaning or assessment phase determines the patient’s ability to breathe spontaneously. Assessment in this phase depends on a combination of respiratory and non-respiratory factors.
Weaning assessment parameters should include criteria to assess muscle strength (negative inspiratory pressure [NIP]) and endurance (spontaneous tidal volume [SVT] and vital capacity [VC]), minute ventilation (VE) and rapid shallow breathing index (RSBI).75,76 In addition, the patient’s lungs should be reasonably clear on auscultation and chest X-ray. Non-respiratory factors include the assessment of the patient’s neurological status, haemodynamic status, fluid and electrolytes/acid–base balance, nutrition and haemoglobin level.75 It is important to have an alert, well-rested and well-informed patient relatively free from pain who can cooperate with the weaning plan. This does not mean complete withdrawal from sedatives or analgesics. Instead, drugs should be titrated to achieve comfort without causing excessive drowsiness.
Additionally, the use of a standard approach for weaning or weaning protocols has shown to decrease ventilator days. The components of the protocol are not as important as the use of a protocol to prevent delays in weaning. All methods can be delivered with the patient remaining connected to the ventilator circuit. The patient receiving SIMV can have the ventilator breaths gradually reduced as the patient’s ventilation status permits. CPAP or PSV can be added to SIMV. Another method involves PSV, CPAP or both delivered without SIMV. PSV is thought to provide gentle, slow respiratory muscle conditioning and may be especially beneficial for patients who are deconditioned or have cardiac problems. Some patients, especially patients ventilated for elective procedures, may be weaned by simply providing humidified oxygen (T-piece or flow-by method).75,76
Weaning is usually carried out during the day, with the patient ventilated at night in a rest mode. The rest mode should be a stable, non-fatiguing and comfortable form of support for the patient. Regardless of the weaning mode selected, all team members should be familiar with the weaning plan. Additionally, regardless of the method used, it is important to permit the patient’s respiratory muscles to rest between weaning trials. Once the respiratory muscles become fatigued, they may require 12–24 hours to recover.
The patient being weaned and the family should be provided continuing psychological support. The weaning process should be explained and the patient and family informed of progress. The patient should be placed in a sitting or semirecumbent position and made comfortable. Baseline vital signs and respiratory parameters are measured. During the weaning trial, the patient must be monitored closely for non-invasive criteria that may signal intolerance and result in cessation of the trial (e.g. tachypnoea, dyspnoea, tachycardia, arrhythmias, sustained desaturation [SpO2 <91%], hypertension or hypotension, agitation, diaphoresis, anxiety, sustained VT <5 mL/kg, changes in level of consciousness). Documentation of the patient’s tolerance throughout the weaning process is important and should include statements regarding the patient’s and family’s perceptions.
The weaning outcome phase refers to the period when weaning stops and the patient is extubated or weaning is stopped because no further progress is being made. The patient who is ready for extubation should receive hyperoxygenation and suctioning (i.e. oropharynx, ET tube). The patient should be instructed to take a deep breath and, at the peak of inspiration, the cuff should be deflated and the tube removed in one motion. After removal, the patient should be encouraged to cough, and the pharynx should be suctioned as needed. Supplemental oxygen should be applied and naso-oral care provided. The nurse must carefully monitor the patient’s vital signs, respiratory status and oxygenation immediately following extubation, within 1 hour, and per institutional policy. If the patient cannot tolerate extubation, immediate reintubation or use of non-invasive ventilation may be required.
 NURSING MANAGEMENT: MECHANICAL VENTILATION
NURSING MANAGEMENT: MECHANICAL VENTILATION
Nursing management of the patient receiving mechanical ventilation is presented in NCP 65-1.
Critical care and mechanical ventilation
CASE STUDY
Patient profile
An elderly man was found lying on the street by the police. He was unresponsive on admission to the emergency department and remains unresponsive. He has an oral ET tube in place and is receiving mechanical ventilation. He weighed approximately 90 kg. A subclavian central line was inserted to monitor CVP and administer fluids.
Multidisciplinary care
• Positive pressure ventilation settings: assist-control mode at 16 breaths per minute; VT, 900 mL; FiO2, 0.6 (60%)
• Enteral nutrition at 25 mL/h via small-bore feeding tube
• Indwelling urinary catheter on free drainage
• Change position every 2 hours
• Perform chest physiotherapy every 2–4 hours
• Gentamycin 80 mg IV 8 hourly
CRITICAL THINKING QUESTIONS
1. Identify two reasons for intubating and providing mechanical ventilation for this patient.
2. What do this patient’s ABGs indicate and which ventilator setting(s) should be changed?
3. This patient’s systolic blood pressure drops to 80 mmHg and he remains in atrial fibrillation with a ventricular rate of 138 beats/min. A pulmonary artery (PA) catheter is inserted for haemodynamic monitoring. What would be the purpose of haemodynamic monitoring in this patient? Identify two major nursing considerations for a patient with a PA catheter.
4. This patient’s initial pulmonary artery wedge pressure is 14 mmHg and cardiac index is 2 L/min/m2. How would you interpret these values? What medical interventions might be considered?
5. This patient’s pulmonary condition deteriorates. The PaO2 drops to 70 mmHg (9.5 kPa) and the SpO2 is 89%. The peak end-expiratory pressure is added to the ventilator settings. What implications does this have for this patient given his haemodynamic status?
6. Based on the data presented, identify two priority nursing issues.
7. After 6 days, this patient remains unresponsive and is developing renal failure. The intensivist believes the situation is hopeless and wishes to discuss termination of supportive therapy. What approach should the nurse take in locating this patient’s next of kin?
1. An appropriate nursing intervention for the patient with delirium in the intensive care unit (ICU) is to:
2. The critical care nurse recognises that an ideal plan for family involvement includes:
3. To establish haemodynamic monitoring for a patient, the nurse zeroes the:
4. The nursing management of a patient with an artificial airway includes:
5. The purpose of adding positive end-expiratory pressure to positive pressure ventilation is to:
6. The nurse monitors the patient with positive pressure mechanical ventilation for:
1 Nightingale F. Notes on hospitals, 3rd edn. London: Longman, Roberts & Green, 1863.
2 Wiles V, Daffern K. There’s a bird in my hand and a bear by the bed—I must be in ICU. The pivotal years of Australian critical care nursing. Sydney: ACCCN, 2002.
3 Urden L, Stacy KL, Lough ME, eds. Critical care nursing: diagnosis and management, 6th edn., St Louis: Elsevier, 2010.
4 Williams TA, Leslie G, Finn J, et al. Clinical effectiveness of a critical care nursing outreach service in facilitating discharge from the intensive care unit. Am J Crit Care. 2010;19(5):e63–e72.
5 Australian College of Critical Care Nurses (ACCCN). ACCCN ICU staffing position statement (2003) on intensive care nursing staffing. Available at www.acccn.com.au/images/stories/downloads/staffing_intensive_care_nursing.pdf. accessed 9 January 2011.
6 The Joint Faculty of Intensive Care Medicine. Minimum standards for intensive care units. Available at www.cicm.org.au/cmsfiles/IC-1%20Minimum%20Standards%20for%20Intensive%20Care%20Units.pdf. accessed 12 December 2010.
7 Australian College of Critical Care Nurses (ACCCN). Competency standards for specialist critical care nurses. Sydney: ACCCN, 2002. Current to 2012.
8 Australian Health Workforce Advisory Committee. The critical care nurse workforce in Australia. Sydney: NSW Health Department; 2001–2011. Available at www.ahwo.gov.au/documents/Publications/2002/The%20critical%20care%20nurse%20workforce%20in%20Australia.pdf accessed 9 January 2011.
9 Higlett T, Anderson T, Hart G. Review of intensive care resources and activity, 2005/2006. Melbourne: Australian & New Zealand Intensive Care Society; 2006. Available at www.anzics.com.au/downloads/doc_download/40-arcccr-report-2006 accessed 9 January 2011.
10 Marshall A, Boyle M. Support of metabolic function. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
11 Heyland DK, Stephens KE, Day AG, McClave SA. The success of enteral nutrition and ICU-acquired infections: a multicenter observational study. Clin Nutr. 2011;30(2):148–155.
12 Doig GS, Heighes PT, Simpson F, Sweetman EA, Davies AR. Enteral nutrition within 24 h of ICU admission significantly reduces mortality: a meta-analysis of RCTs. Intensive Care Med. 2009;35(12):2018–2027.
13 Papathanassoglou ED. Psychological support and outcomes for ICU patients. Nurs Crit Care. 2010;15(3):118–128.
14 Rattray J, Crocker C, Jones M, Connaghan J. Patients’ perceptions of and emotional outcome after intensive care: results from a multicentre study. Nurs Crit Care. 2010;15(2):86–93.
15 Mitchell M, Wilson D, Wade V. Psychosocial and cultural care of the critically ill. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
16 Wang HL, Tsai YF. Nurses’ knowledge and barriers regarding pain management in intensive care units. J Clin Nurs. 2010;19(21–22):3188–3196.
17 Botha J, Le Blanc V. The state of sedation in the nation: results of an Australian survey. Crit Care Resusc. 2005;7:92–96.
18 O’Connor M, Bucknall T, Manias E. A critical review of daily sedation interruption in the intensive care unit. J Clin Nurs. 2009;18(9):1239–1249.
19 Sessler CN, Pedram S. Protocolized and target-based sedation and analgesia in the ICU. Crit Care Clin. 2009;25(3):489–513.
20 Elliott R, McKinley S, Aitken LM, Hendrikz J. The effect of an algorithm-based sedation guideline on the duration of mechanical ventilation in an Australian intensive care unit. Intensive Care Med. 2006;32(10):1506–1514.
21 Elliott R, McKinley S, Aitken L. Adoption of a sedation scoring system and sedation guideline in an intensive care unit. J Adv Nurs. 2006;54(2):208–216.
22 Elliott R, McKinley S, Eager D. A pilot study of sound levels in an Australian adult general intensive care unit. Noise Health. 2010;12(46):26–36.
23 Roberts B, Rickard CM, Rajbhandari D, et al. Multicentre study of delirium in ICU patients using a simple screening tool. Aust Crit Care. 2005;18(1):6–16.
24 Girard TD, Pandharipande PP, Ely EW. Delirium in the intensive care unit. Crit Care. 2008;12(3):S3.
25 Bosma KJ, Ranieri VM. Filtering out the noise: evaluating the impact of noise and sound reduction strategies on sleep quality for ICU patients. Crit Care. 2009;13(3):151.
26 Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest. 2009;136(1):284–294.
27 Weinhouse GL. Pharmacology I: effects on sleep of commonly used ICU medications. Crit Care Clin. 2008;24(3):477–491.
28 Swift MC, Scholten I. Not feeding, not coming home: parental experiences of infant feeding difficulties and family relationships in a neonatal unit. J Clin Nurs. 2009;19(1–2):249–258.
29 Bailey S, Rischbieth A. Ethical issues in critical care. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
30 Filan SL, Llewellyn-Jones RH. Animal-assisted therapy for dementia: a review of the literature. Int Psychogeriatr. 2006;18(4):597–611.
31 American Association of Critical-Care Nurses (AACN). AACN practice alert: family presence during CPR and invasive procedures. Available at www.aacn.org/AACN/practiceAlert.nsf/Files/FP/$file/Family%20Presence%20During%20CPR%2011-2004.pdf. accessed 10 December 2010.
32 Urden L, Stacy KL, Lough ME, eds. Critical care nursing: diagnosis and management, 6th edn., St Louis: Elsevier, 2010.
33 Kent B, Dowd B. Assessment, monitoring and diagnostics. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
34 Preuss T, Wiegand DL. Single- and multiple-pressure transducer system. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
35 Becker DE. Arterial catheter insertion (perform). In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
36 Shaffer RB. Arterial catheter insertion (assist), care, and removal. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
37 Fleck DA. Pulmonary artery catheter insertion (perform). In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
38 Preuss T, Wiegand DL. Pulmonary artery catheter and pressure lines: troubleshooting. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
39 Klein DG. Cardiac output measurement techniques (invasive). In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
40 McCann M, Einarsdottir H, Van Waeleghem JP, Murphy F, Sedgewick J. Vascular access management III: central venous catheters. J Ren Care. 2010;36(1):25–33.
41 Oren-Grinberg A. The PiCCO Monitor. Int Anesthesiol Clin. 2010;48(1):57–85.
42 Dennis M, Gallagher R. Specialty cardiac care. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
43 Christenson JT, Sierra J, Romand JA, Licker M, Kalangos A. Long intraaortic balloon treatment time leads to more vascular complications. Asian Cardiovasc Thorac Ann. 2007;15(5):408–412.
44 Currey J, Dimovski S, Treloggen J. Organ donation and transplantation. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
45 Cuthbertson S, Kelly M. Support of respiratory function. In: Elliott D, Aitken L, Chaboyer W, eds. ACCCN’s critical care nursing. Sydney: Elsevier, 2007.
46 Anwer HM, Zeitoun IM, Shehata EA. Submandibular approach for tracheal intubation in patients with panfacial fractures. Br J Anaesth. 2007;98(6):835–840.
47 Reynolds SF, Heffner J. Airway management of the critically ill patient: rapid-sequence intubation. Chest. 2005;127(4):1397–1412.
48 Goodrich CA. Endotracheal intubation (assist). In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
49 Good VS, Luehrs P. Continuous end-tidal carbon dioxide monitoring. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
50 Goodrich CA. Endotracheal intubation (perform). In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
51 Celik SA, Kanan N. A current conflict: use of isotonic sodium chloride solution on endotracheal suctioning in critically ill patients. Dimens Crit Care Nurs. 2006;25(1):11–14.
52 Paratz JD, Stockton KA. Efficacy and safety of normal saline instillation: a systematic review. Physiotherapy. 2009;95(4):241–250.
53 Lusk B, Lash AA. The stress response. Psychoneuroimmunology and stress among ICU patients. Dimens Crit Care Nurs. 2005;24(1):25–31. (Nursing research-based reference)
54 Chakrabarti B, Calverley PM. Management of acute ventilatory failure. Postgrad Med J. 2006;82:438–445.
55 Pertab D. Principles of mechanical ventilation: a critical review. Br J Nurs. 2009;18(15):915–918.
56 Burns SM. Invasive mechanical ventilation (through an artificial airway): volume and pressure modes. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
57 Uyar M, Demirag K, Olgun E, et al. Comparison of oxygen cost of breathing between pressure-support ventilation and airway pressure release ventilation. Anaesth Intensive Care. 2005;33(2):218–222.
58 Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13(1):R22.
59 Burns SM. Auto-positive end-expiratory pressure (auto-PEEP) calculation. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
60 Burns KE, Adhikari NK, Keenan SP, Meade MO. Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. (8):2010. CD004127.
61 Ten IS, Anderson MR. Is high-frequency ventilation more beneficial than low-tidal volume conventional ventilation? Respir Care Clin N Am. 2006;12(3):437–451.
62 Afshari A, Brok J, Moller AM, Wetterslev J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev. (7):2010. CD002787.
63 Gattinoni L, Caironi P. Prone positioning: beyond physiology. Anesthesiology. 2010;113(6):1262–1264.
64 Grist G, Whittaker C, Merrigan K, Fenton J, Pallotto E, Lofland G. Defining the late implementation of extracorporeal membrane oxygenation (ECMO) by identifying increased mortality risk using specific physiologic cut-points in neonatal and pediatric respiratory patients. J Extra Corpor Technol. 2009;41(4):213–219.
65 Erickson SE, Martin GS, Davis JL, Matthay M, Eisner A. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37(5):1574–1579.
66 American Thoracic Society, Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171(4):388–416.
67 Sogaard OS, Lemoh C, Spelman D, et al. A binational cohort study of ventilator-associated pneumonia in Denmark and Australia. Scand J Infect Dis. 2006;38(4):256–264.
68 Rotstein C, Evans G, Born A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol. 2008;19(1):19–53.
69 Dezfulian C, Shojania K, Collard HR, et al. Subglottic secretion drainage for preventing ventilator-associated pneumonia: a meta-analysis. Am J Med. 2005;118(1):11–18.
70 deFoneska A, Kaunitz JD. Gastroduodenal mucosal defense. Curr Opin Gastroenterol. 2010;26(6):604–610.
71 Hofhuis JG, Spronk PE, van Stel HF, Schrijvers AJ, Rommes JH, Bakker J. Experiences of critically ill patients in the ICU. Intensive Crit Care Nurs. 2008;24(5):300–313.
72 Weinhouse GL, Schwab RJ, Watson PL, et al. Bench-to-bedside review: delirium in ICU patients—importance of sleep deprivation. Crit Care. 2009;13(6):234.
73 Roberts BL, Rickard CM, Rajbhandari D, Reynolds P. Patients’ dreams in ICU: recall at two years post discharge and comparison to delirium status during ICU admission. A multicentre cohort study. Intensive Crit Care Nurs. 2006;22(5):264–273.
74 Whetstone Foster JG. Peripheral nerve stimulators. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
75 Burns SM. Weaning process. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
76 Burns SM. Standard weaning criteria: negative inspiratory forces or pressure, positive expiratory pressure, spontaneous tidal volume, vital capacity and rapid shallow breathing index. In Wiegand DL, Carlson KK, eds.: AACN procedure manual for critical care, 6th edn., St Louis: Mosby, 2010.
Australian College of Critical Care Nurses. www.acccn.com.au
Australian and New Zealand Intensive Care Society. www.anzics.com.au
British Association of Critical Care Nurses. www.baccn.org.uk
Critical Care Forum. www.ccforum.com/home
Intensive Care. www.intensivecare.com
World Federation of Critical Care Nurses. www.wfccn.org