Chapter 48 NURSING MANAGEMENT: diabetes mellitus
1. Describe the pathophysiology and clinical manifestations of diabetes mellitus.
2. Differentiate between type 1 and type 2 diabetes mellitus.
3. Describe the multidisciplinary care of the patient with diabetes mellitus.
4. Analyse the role of nutrition and exercise in the management of diabetes mellitus.
5. Explain the nursing management of a patient with newly diagnosed diabetes mellitus.
6. Apply knowledge of nursing management to the care of the patient with diabetes mellitus in the ambulatory and home care settings.
7. Recognise the pathophysiological and clinical manifestations of acute and chronic complications of diabetes mellitus.
8. Explain the multidisciplinary care and nursing management of the patient with acute and chronic complications of diabetes mellitus.
hyperosmolar hyperglycaemic non-ketotic syndrome (HHNS)
impaired fasting glucose (IFG)
impaired glucose tolerance (IGT)
DIABETES MELLITUS
Diabetes mellitus is a multisystem disease with metabolic, vascular and neuropathic components. Diabetes is characterised by chronic hyperglycaemia and alterations in carbohydrate, fat and protein metabolism related to absent or diminished insulin secretion and/or ineffective insulin action. The vascular component consists of abnormalities in both large and small vessels, and although vessel disease can occur in people without diabetes, it appears earlier and is more severe in people with diabetes. The neuropathic component consists of abnormalities in the peripheral and autonomic nervous systems caused by metabolic alterations.
Diabetes comes from the Greek word for siphon, suggesting a lot of urine is passed, and mellitus comes from the Latin word mel, meaning honey sweet. Diabetes has been documented since ancient times by Greeks, Chinese, Egyptians and Indians—for example, Indians tested for diabetes by observing whether ants were attracted to the person’s urine. Despite much more being known about diabetes today and the availability of sophisticated treatments, there is still no cure. Furthermore, diabetes is becoming much more common.
In 2010 the World Health Organization estimated a world prevalence rate for diabetes of 285,000,000 people and this number is predicted to rise to 439,000,000 people aged 20–79 years by 2030.1 Much of the increase will occur in developing countries due to population growth, ageing, unhealthy diets, obesity and sedentary lifestyles.1,2 In Australia, it is estimated that approximately 7.2% of the population have diabetes and another 9% have impaired glucose tolerance (prediabetes).2
The International Diabetes Federation estimated that in 2010 close to 4 million people in the 20–79 age group died from complications associated with diabetes, accounting for 6.8% of global all-cause mortality in this age group. In countries with a high prevalence of diabetes, such as the Pacific and the Middle East, as many as one in four deaths in adults aged between 35 and 64 years is due to diabetes.3 Although diabetes is sometimes considered a condition of developed nations, loss of life from premature death among people with diabetes is greatest in developing countries. The burden of premature death from diabetes is similar to that of HIV/AIDS, yet the problem goes largely unrecognised.
Diabetes has become one of the major causes of premature illness and death in many countries, mainly through the increased risk of cardiovascular disease (CVD). CVD is responsible for between 50% and 80% of deaths in people with diabetes. Diabetes is a leading cause of blindness, amputation and kidney failure. These complications account for much of the social and financial burden of diabetes.3 New Zealand’s annual health costs related to type 2 diabetes have been estimated at NZ$400 million and are predicted to increase to more than NZ$1 billion by 2021.4 In Australia, it has been estimated that the annual healthcare costs of type 2 diabetes could be in the vicinity of A$3 billion per year.2 The estimated global healthcare expenditure on the treatment and prevention of diabetes and its complications is expected to total $US376 billion in 2010 and climb to $US490 billion by 2030.5
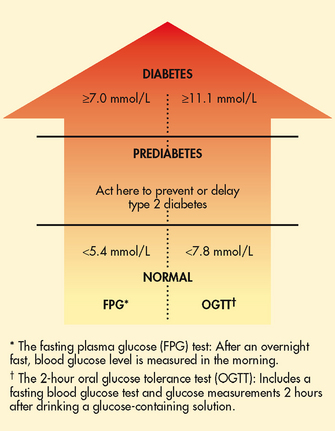
Prediabetes is a condition where individuals are at an increased risk for developing type 2 diabetes. In this condition the blood glucose levels are high but not high enough to meet the criteria for diabetes.6 People with prediabetes are defined as having impaired glucose tolerance (IGT) or impaired fasting glucose (IFG) with serum glucose levels of 5.6 mmol/L to 6.9 mmol/L. Individuals with IFG and/or IGT have a relatively high risk of developing diabetes in the future and, if no preventative measures are taken, usually develop diabetes within 10 years. Another condition that predisposes people to type 2 diabetes is metabolic syndrome (also known as syndrome X).7 This condition is characterised by central obesity and insulin resistance.8,9 (Metabolic syndrome is discussed in detail in Ch 40.)
Current theories link the causes of diabetes, either singly or in combination, to the following factors: genetics, autoimmune factors, viral factors and/or environmental factors (e.g. obesity, stress). Regardless of the cause, diabetes is primarily a disorder of glucose metabolism related to absent or insufficient insulin production and/or poor tissue uptake of available insulin.
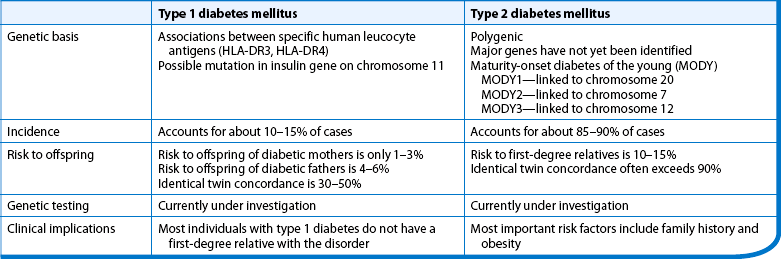
The two most common types of diabetes are classified as type 1 and type 2 diabetes mellitus. Table 48-1 outlines the different characteristics associated with both type 1 and type 2 diabetes. Gestational diabetes and secondary diabetes are other classifications of diabetes commonly seen in clinical practice.
TABLE 48-1 Characteristics of type 1 and type 2 diabetes mellitus
| Factor | Type 1 diabetes mellitus | Type 2 diabetes mellitus |
|---|---|---|
| Age at onset | More common in young people but can occur at any age | Usually age 30 years or older but can occur at any age; incidence is increasing in children |
| Type of onset | Signs and symptoms acute but disease process may be present for several months/years | Insidious |
| Prevalence | Accounts for 10–15% of all types of diabetes | Accounts for 85–90% of all types of diabetes |
| Environmental factors | Virus, toxins | Obesity, lack of exercise |
| Islet cell antibodies | Often present at onset | Absent |
| Endogenous insulin | Minimal or absent | Initially excessive; but reduces as β-cells exhaust with progression of the disease |
| Weight | Usually thin, with a history of weight loss prior to diagnosis | Commonly obese |
| Symptoms | Thirst, polyuria, polyphagia, fatigue, blurred vision | Frequently none or mild |
| Ketosis | Prone at onset or during insulin deficiency | Resistant, except during infection or stress |
| Nutritional therapy | Essential | Essential, may be sufficient for glycaemic control early in the disease process |
| Insulin | Required | Required as disease progresses and blood glucose levels can no longer be controlled by oral hypoglycaemic agents |
| Oral hypoglycaemic agents | Not beneficial | Beneficial in the early stages of the disease |
| Vascular and neurological complications | Associated with poor diabetes control | Associated with poor diabetes control |
Normal glucose metabolism
Insulin is a hormone produced by the beta- (β) cells in the islets of Langerhans in the pancreas. In healthy individuals, there is a continuous release of small amounts of insulin into the bloodstream (a basal rate). The pancreas releases a bolus of insulin in response to food and increasing blood glucose levels (see Fig 48-1).10
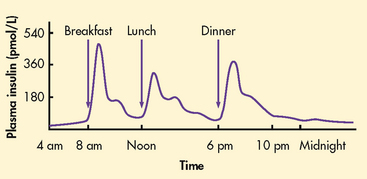
Figure 48-1 Normal endogenous insulin secretion. In the first hour or two after meals, insulin concentrations rise rapidly in blood and peak at about 1 hour. After meals, insulin concentrations promptly decline towards preprandial values as carbohydrate absorption from the gastrointestinal tract declines. After carbohydrate absorption from the gastrointestinal tract is complete and during the night, insulin concentrations are low and fairly constant, with a slight increase at dawn.
Insulin works by facilitating the movement of glucose out of the bloodstream into the muscle, fat and liver cells to be used for energy or stored. The activity of releasing insulin lowers blood glucose levels and facilitates a stable, normal glucose range of approximately 3.5–8 mmol/L. The average amount of insulin secreted daily by an adult is approximately 43–186 mmol/L, or 0.6 U/kg of body weight. Insulin is the only hormone that lowers the blood glucose.10
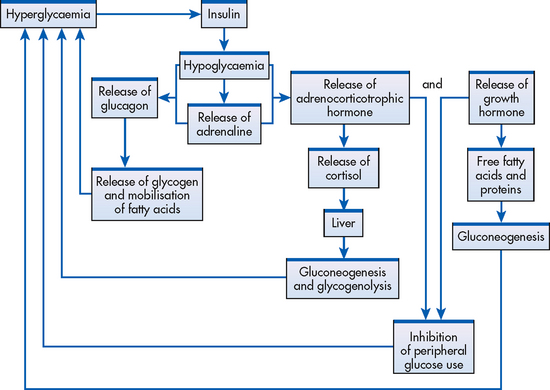
Insulin and glucagon are the two hormones produced by the pancreas that have a major effect on glucose metabolism. Insulin facilitates the uptake of glucose by skeletal muscle, liver and fat cells where it can be utilised for energy or stored as glycogen. Glucagon stimulates hepatic glucose production, facilitates the release of stored glucose from the liver and acts to raise blood glucose levels in conjunction with adrenaline, growth hormone and cortisol. This group of hormones is referred to as the counter-regulatory hormones. Insulin and glucagon provide a regulated release of glucose for energy in the both the fed and fasting state to maintain blood glucose levels within the normal range. Disorders of insulin secretion or insulin action, or both, occur in patients with diabetes.11 Disturbances in hepatic release of glucose also suggest disorders of glucagon regulation in patients with diabetes.
Proinsulin is released from the pancreatic β-cells as a precursor to insulin. Proinsulin is composed of two polypeptide chains—chain A and chain B—which are linked by a C-peptide chain. Insulin is formed when enzymes cleave off chain C, separating the A and B chains. The presence of C peptide in serum and urine is a useful indicator of β-cell function.10
Insulin promotes glucose transport from the bloodstream across the cell membrane to the cytoplasm of the cell. The rise in plasma insulin level after a meal also stimulates storage of glucose as glycogen in liver and muscle, enhances fat deposition in adipose tissue, increases protein synthesis and inhibits gluconeogenesis. This is why insulin is known as the anabolic or storage hormone. The fall in insulin level during normal overnight fasting stimulates the release of glucagon, which facilitates the release of stored glucose from the liver, protein from muscle and fat from adipose tissue. Skeletal muscle and adipose tissue have specific receptors for insulin and are considered insulin-dependent tissues. Other tissues (e.g. brain, liver, blood cells) do not directly depend on insulin for glucose transport but require adequate glucose supply from the bloodstream for normal function. Although liver cells are not considered insulin-dependent tissue, insulin receptor sites on the liver facilitate the hepatic uptake of glucose for conversion to glycogen.
Type 1 diabetes mellitus
Formerly known as juvenile onset or insulin-dependent diabetes mellitus (IDDM), type 1 diabetes mellitus is the most serious and common chronic disease of childhood. About half of all people with type 1 diabetes develop the disease before the age of 15 years.1 The incidence of type 1 diabetes appears to be increasing in many populations worldwide—evidence is mounting regarding the increase in type 1 diabetes in central and eastern Europe, but the data are sparse on sub-Saharan African and other underdeveloped countries.12
Typically, type 1 diabetes is seen in people with a lean body type, although it can occur in people who are overweight. Approximately 10–15% of all people with diabetes have type 1 diabetes and it is one of the most common childhood diseases in developed countries.13
AETIOLOGY AND PATHOPHYSIOLOGY
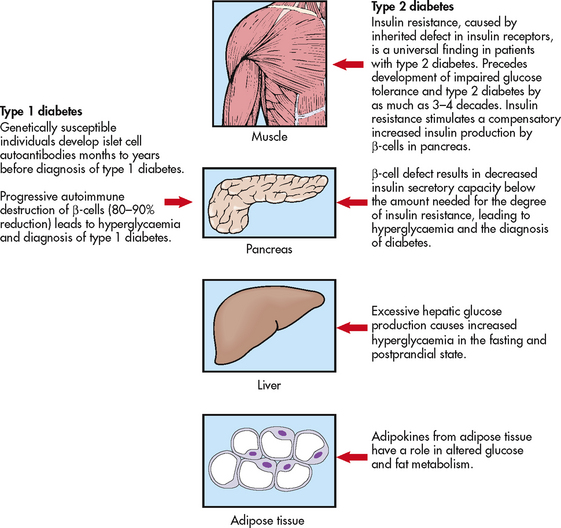
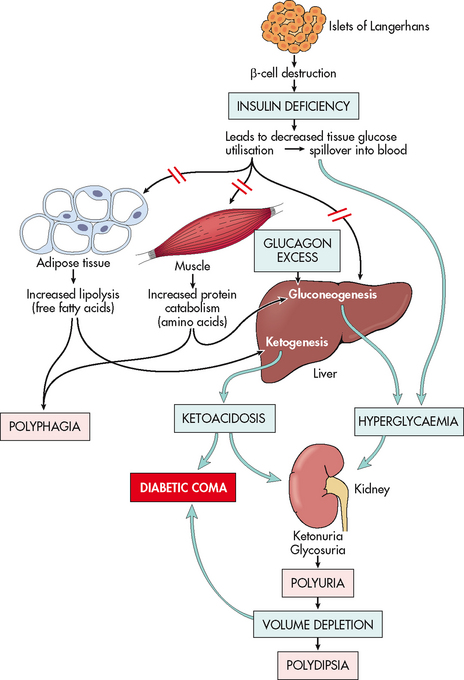
Type 1 diabetes results from progressive destruction of pancreatic β-cells due to an autoimmune process in susceptible individuals. Autoantibodies to the islet cells cause a reduction of 80–90% of normal β-cell function before hyperglycaemia and other manifestations occur (see Fig 48-2).
Predisposition to type 1 diabetes is related to human leucocyte antigens (HLAs). (See Ch 13 for a discussion of HLAs and disease associations.) Theoretically, when an individual with certain HLA types is exposed to an environmental trigger (e.g. viral infections such as coxsackievirus, enterovirus, measles, mumps), an autoimmune response follows whereby the β-cells of the pancreas are destroyed. The HLA types associated with an increased risk of type 1 diabetes include HLA-DR3 and HLA-DR4 (see the Health disparities box).
Genetic predisposition to type 1 diabetes and environmental factors may contribute to the pathogenesis of type 1 diabetes, but in many cases the cause is unknown. The presence of glutamic acid decarboxylase (GAD) antibodies, islet cell autoantibodies (ICA) or insulin autoantibodies (IAA) provides a differential diagnosis for type 1 diabetes, as these antibodies are not present in patients with type 2 diabetes.
CLINICAL MANIFESTATIONS
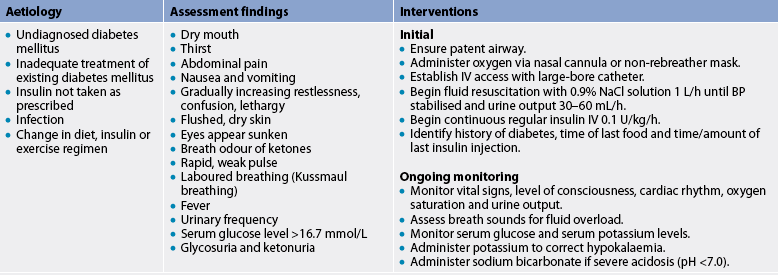
Type 1 diabetes is associated with a long preclinical period. The islet cell autoantibodies responsible for β-cell destruction may be present for months to years before the onset of symptoms. Type 1 diabetes is diagnosed when the person’s pancreas can no longer produce insulin. Islet cell function drops to around 10% before symptoms occur. When symptoms do occur they are very acute and the patient presents to the emergency department with impending or actual diabetic ketoacidosis (DKA), a life-threatening condition resulting in metabolic acidosis. DKA is a severe acute complication associated with type 1 diabetes and is discussed later in the chapter.
People with type 1 diabetes require exogenous insulin in the form of injections to sustain life. Without insulin, the patient will develop DKA. Newly diagnosed patients with type 1 diabetes often experience a remission soon after insulin initiation. During this time, the patient requires very little injected insulin as the β-cells have some relief from glucose toxicity and the β-cells can produce sufficient insulin for glucose control; however, progressive destruction continues to occur. Eventually, as β-cells are totally destroyed and the remission period ends, blood glucose levels rise and more insulin is required. This phenomenon is referred to as the honeymoon period and usually lasts for 3–12 months, after which time the person will require insulin injections on a permanent basis.
The classic symptoms of type 1 diabetes are polyuria (frequent urination), polydipsia (excessive thirst), polyphagia (excessive hunger) and visual disturbances. When the blood glucose levels rise above the renal threshold (10–12 mmol/L), glucose is excreted in the urine. The osmotic effect of glucose causes polyuria (where glucose goes water will follow), which results in polydipsia. Polyphagia is a consequence of cellular malnourishment when insulin deficiency prevents movement of glucose into the cells. Weight loss occurs as the body turns to alternative energy sources, such as breakdown of fat and muscle. Weakness and fatigue are common, as body cells cannot utilise glucose. Visual disturbances occur in relation to glucose in the vitreous and lens of the eye.
Type 2 diabetes mellitus
Some 85–90% of people with diabetes have type 2 diabetes mellitus, making type 2 diabetes by far the most prevalent type of diabetes.1 It is primarily a lifestyle disorder and is associated with obesity, overweight, low levels of physical activity and unhealthy eating practices. It usually occurs in people over 40 years of age, and 80–90% of patients are overweight at the time of diagnosis. It has a tendency to run in families and probably has a genetic basis (see the Health disparities box). In the past, the prevalence of type 2 diabetes was predominately from middle age onwards, although it is now seen in much younger age groups due to the obesity epidemic: with the increasing rates of obesity in our society, a growing number of children, adolescents and younger adults are being diagnosed with the disease.3,5 It is estimated that more than 7% of Australians aged 25 years or over have type 2 diabetes.2
Indigenous Australians, Māori, Pacific Islanders, Asians, Chinese and those from the Indian subcontinent are at a higher risk of developing type 2 diabetes.2,4 Aboriginal and Torres Strait Islander peoples have one of the highest prevalence rates of type 2 diabetes in the world, with an overall prevalence of 3.4 times that of non-Indigenous Australians.2 In New Zealand, while the overall incidence is much the same as in Australia, the prevalence rate for Māori and Pacific Islander peoples is more than three times higher than for Europeans, with Māori and Pacific Islander peoples five times more likely to die from diabetes.4 Both the New Zealand and Australian governments are developing national approaches to the prevention, early diagnosis and management of diabetes.2,4
The prevalence of type 2 diabetes increases with age, with about half of those diagnosed being over 55 years of age. In the past, type 2 diabetes was known as adult-onset diabetes or non-insulin dependent diabetes (NIDDM). The latter term is no longer considered appropriate because type 2 diabetes is now commonly treated with insulin. Insulin injections are usually required earlier in the disease course for management in children, adolescents and younger adults.1,3,11
AETIOLOGY AND PATHOPHYSIOLOGY
Type 2 diabetes is primarily a vascular disease that is characterised by a group of disorders consisting of hyperglycaemia, hyperlipidaemia and hypertension. The onset of type 2 is insidious and often has one or more microvascular (i.e. retinal, renal, possibly neuropathic), macrovascular (i.e. coronary, peripheral vascular) or neuropathic (i.e. autonomic, peripheral) complications present at diagnosis. Unlike patients with type 1 diabetes, patients with type 2 are not absolutely dependent on insulin for life, even though many of them are ultimately treated with insulin.10
Hyperinsulinaemia is present early in the disease course as the body attempts to overcome hyperglycaemia; as the disease progresses β-cell function deteriorates and insufficient insulin is produced, resulting in hyperglycaemia and diabetes. The presence or absence of endogenous insulin is the major pathophysiological distinction between types 1 and 2 and can be confirmed with the presence or absence of antibodies (GAD, ICA IAA).
The genetic determinants of type 2 diabetes remain poorly defined, except in the few people with an early-onset, dominantly-inherited form of the disorder (maturity-onset diabetes of the young, MODY), in whom specific genetic mutations have been identified (e.g. mutations of the glucokinase gene).13,14 Other genetic mutations that lead to insulin resistance and obesity have been found in some people with type 2 diabetes. It is likely that multiple genes are involved in this complex, multifactorial disorder (see the Health disparities box).
Three major metabolic abnormalities have a role in the development of type 2 diabetes. The first is insulin resistance, a condition in which the body tissues have an impaired response to insulin. Insulin normally binds to receptor sites located in skeletal muscle, fat and liver cells. In the person with diabetes, insulin receptor sites are unresponsive to insulin and deficient in number. When insulin cannot bind to the cell receptor sites, glucose cannot enter the cells, resulting in hyperglycaemia. In the early stages of insulin resistance, the pancreas responds to high blood glucose levels by producing more insulin (if β-cell function is normal). This creates a temporary state of hyperinsulinaemia, which coexists with the hyperglycaemia. As the disease progresses the β-cells become exhausted, resulting in deficient insulin production. Acanthosis nigricans is a physical marker of insulin resistance that is often present, particularly in children, and is easily identifiable in specific ethnic populations (e.g. Indigenous Australians). Acanthosis nigricans is characterised by a change in the texture and colour of the skin around the neck, in the skin folds under the arms, in the groin and on the knuckles of the hands.14 The second abnormality is a marked decrease in the ability of the pancreas to produce insulin as the β-cells become fatigued from the compensatory overproduction of insulin. The third abnormality is inappropriate glucose production by the liver. The liver’s ability to regulate glucose release is impaired, resulting in high fasting blood glucose levels. An increase in fasting blood glucose levels is often an indication of this factor. Figure 48-2 depicts the altered mechanisms in type 1 and type 2 diabetes.
CLINICAL MANIFESTATIONS
Disease onset in type 2 diabetes is gradual. The person may be asymptomatic for many years with undetected hyperglycaemia that produces few, if any, symptoms (e.g. lethargy), which may be attributed to other aspects of lifestyle. Some common manifestations associated with type 2 diabetes include recurrent infections, prolonged wound healing and visual changes. Some people may experience some of the classic symptoms associated with hyperglycaemia, such as polyuria, polydipsia and fatigue.
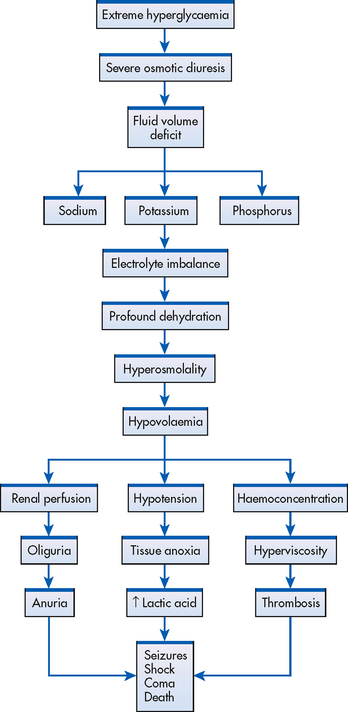
If the patient with type 2 diabetes has marked hyperglycaemia (27.6–55.1 mmol/L) but is still producing some endogenous insulin, DKA rarely occurs. However, osmotic fluid and electrolyte loss related to hyperglycaemia may become severe and lead to a hyperosmolar hyperglycaemic syndrome (HHS) coma (previously referred to as hyperosmolar non-ketotic [HONK] coma), particularly in the elderly.
Gestational diabetes
Gestational diabetes mellitus (GDM) is defined as carbohydrate intolerance of variable severity with onset first recognised in pregnancy. It goes away after the baby is born.15 It occurs in about 5% of pregnancies but could be as high as 20% in Indigenous women and women from high-risk populations, such as Māori and people from India, Asia and the Pacific Islands.4 During pregnancy women make 2–3 times more insulin to counteract the effects of placental hormones, which cause insulin resistance in the mother. GDM occurs when the woman cannot make enough extra insulin to keep her blood glucose levels within the normal range.
The Australasian Diabetes in Pregnancy Society (ADIPS)15 recommends that all pregnant women be screened for GDM with a 75 g oral glucose tolerance test (OGTT) at 24–28 weeks gestation. However, if resources are limited, screening may be reserved for those at highest risk. Risk factors include:
The International Association of Diabetes and Pregnancy Study Group Consensus Panel published recommendations on the diagnosis and classification of hyperglycaemia in pregnancy in March 2010.16 These recommendations are a result of the Hyperglycaemia and Pregnancy Outcome (HAPO) study, in which 25,000 women from nine countries were screened in the third trimester of pregnancy. The study results showed poor pregnancy outcomes at levels previously considered normal.17 As a result of these recommendations it is likely that the level for diagnosis in Australia and New Zealand will change in the near future, and it is estimated there will be a 30% increase in the number of women diagnosed with GDM once this occurs.
Self-blood glucose monitoring, nutritional therapy and physical activity are considered the cornerstones of management for GDM. If nutritional therapy and physical activity alone do not achieve the desired blood glucose levels, insulin therapy is indicated. Patients should be managed intensively to protect the developing fetus. Studies have shown that uncontrolled diabetes in pregnancy can lead to metabolic programming of the fetus in utero, which leads to the development of type 2 diabetes in childhood and early adulthood.18
Women with GDM have a higher risk of caesarean delivery, stillbirth or perinatal death and neonatal complications. Patients should be re-screened at 6 weeks postpartum with a 75 g OGTT to ensure that the diabetes has gone away. Although most women with gestational diabetes have normal glucose levels postpartum, studies show an increased risk of developing type 2 diabetes within 3–16 years.19 ADIPS suggests that patients should be re-screened with an OGTT every 2 years (or every year if insulin was required during pregnancy), as the risk of developing type 2 diabetes in the future is high. Women who have had GDM are encouraged to make healthy lifestyle choices with regard to physical activity and healthy eating for the rest of their lives.20
Management of GDM and the pregnant patient with type 1 or type 2 diabetes is a specialised area that requires a specialised multidisciplinary team and for this reason it is not covered in detail in this textbook.
Secondary diabetes
Diabetes occurs in some people because of another medical condition or related to the treatment of a medical condition that causes abnormal blood glucose levels. Conditions that may cause secondary diabetes include Cushing’s syndrome and the use of parenteral nutrition. Commonly used medications that can induce diabetes in some people include corticosteroids (e.g. prednisone), phenytoin and atypical antipsychotics (e.g. clozapine). Secondary diabetes usually resolves when the underlying condition is treated. Medication-induced diabetes should be treated. (Drugs that can alter blood glucose levels are listed in Box 48-4.)
Diagnostic studies
The diagnosis of diabetes can be made using the following tests. Whichever method is used, the diagnosis must be confirmed on a subsequent day by the first two methods.6 These methods and their criteria for diagnosis are as follows:
• fasting plasma glucose (FPG) level exceeding 7.0 mmol/L
• random, or casual, plasma glucose measurement exceeding 11.1 mmol/L, plus manifestations of diabetes, such as polyuria, polydipsia and unexplained weight loss—casual is defined as any time of day without regard to the time of the last meal
• 2-hour OGTT level exceeding 11.1 mmol/L, using a 75 g glucose load.
The FPG test, confirmed by repeat testing on another day, is the preferred method of diagnosis. When overt symptoms of hyperglycaemia (polyuria, polydipsia and polyphagia) coexist with FPG levels of 7.0 mmol/L or greater, further testing using the OGTT may not be necessary to make a diagnosis.6
When the OGTT is used, the accuracy of the test results depends on adequate patient preparation and attention to the many factors that may influence the outcome of such a test. For example, factors that can cause falsely elevated values include recent severe restrictions of dietary carbohydrate, acute illness, medications (e.g. contraceptives, glucocorticosteroids) and restricted activity, such as bed rest. A patient with impaired gastrointestinal absorption may also have false-negative test results.
HbA1c is a test used to measure the average blood glucose level over a 2–3 month period. Also known as glycosylated haemoglobin, this test measures the amount of glucose attached to red blood cells and is dependent on how much glucose was in the bloodstream at the time the cell became glycosylated. If a person’s blood glucose levels have been maintained in the recommended range, then HbA1c will be closer to normal. All patients with diabetes should have regular assessments of HbA1c levels. Major studies have shown that people with diabetes who maintain their HbA1c under 7% have a greatly reduced risk of developing retinopathy, nephropathy and neuropathy.21,22 Patients with diseases affecting red blood cells (e.g. sickle cell anaemia) and patients undergoing renal dialysis may have their HbA1c results affected and this should be taken into consideration in interpreting the test results.
MULTIDISCIPLINARY CARE
Diabetes management should be undertaken by a multidisciplinary team specially trained in diabetes care. The team should consist of an endocrinologist or diabetic specialist, a general practitioner, a diabetes nurse educator, a dietician, a podiatrist, a psychologist and an exercise physiologist. Patients with newly diagnosed diabetes should be referred to a diabetes educator and dietician (see Box 48-1, which outlines the roles of the different members of the healthcare team). The goals of diabetes management are to reduce symptoms, promote wellbeing, prevent acute complications of hyperglycaemia, and delay the onset and progression of long-term complications. Studies have shown that these goals are best achieved by a HbA1c under 7%.21,23 Patient teaching, which enables the patient to become the most active participant in their own care, is essential for a successful treatment plan. It is very important that the patient take responsibility for the day-to-day management of the disease. Since diabetes is a chronic progressive disease, patients may become depressed and refuse to accept that they have diabetes and do nothing towards self-management. Such patients are at risk of complications until they are prepared to take responsibility for managing their condition. Nutritional therapy, exercise, drug therapy and self-monitoring of blood glucose levels are the tools used in the management of diabetes (see Box 48-2).
BOX 48-1 Referral criteria: diabetes health professionals
Diabetes educator
Ophthalmologist/optometrist
• Type 2 diabetes within 1 year of diagnosis
• Type 1 diabetes within 5 years of diagnosis
• Comprehensive eye examination within first trimester if pregnant
• If no diabetic retinopathy, the patient should see the ophthalmologist/optometrist every 2 years
• Once diabetic retinopathy is identified, the patient should see the ophthalmologist/optometrist every year
Urgent referral to an ophthalmologist is essential if the patient has:
Psychologist
Source: Adapted from General Practice Queensland and Queensland Government. Standard care pathway for type 2 diabetes, 2008. Available at www.t2d.com.au.
MULTIDISCIPLINARY CARE
Diagnostic studies
History and physical examination
Blood tests, including fasting blood glucose, postprandial blood glucose, glycosylated haemoglobin (HbA1c), lipid profile, serum urea and serum creatinine, electrolytes
Urine for complete urinalysis, microalbuminuria, glucose and acetone (if indicated)
Funduscopic examination—dilated eye examination
Neurological examination, including monofilament test for sensation in lower extremities
Collaborative therapy
Nutritional therapy (see Table 48-6)
Exercise therapy (see Table 48-7 and Box 48-5)
Insulin and oral hypoglycaemic agents (OHAs) are the most common glucose-lowering agents (GLAs) used in the treatment of diabetes. All individuals with type 1 diabetes require insulin. For a select few people with type 2 diabetes, a regimen of good nutrition, regular physical activity and maintaining a desirable body weight will be sufficient to attain an optimal level of blood glucose control. For the majority, however, drug therapy, which may include insulin, will be necessary.
Drug therapy: insulin
Patients with type 1 diabetes need exogenous (injected) insulin for the duration of their life. Patients with type 2 diabetes may produce some insulin but it may be insufficient to meet their specific metabolic needs. Exogenous insulin may be required in combination with nutritional therapy, exercise and OHAs to maintain a satisfactory blood glucose level. Insulin may also be prescribed for patients with type 2 diabetes during periods of severe stress, such as illness or surgery.24
TYPES OF INSULIN
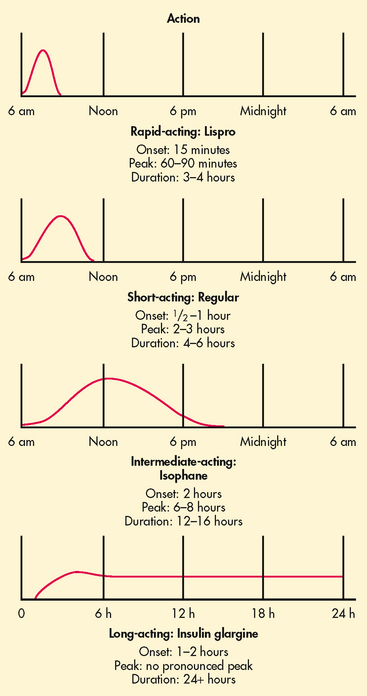
In the past, purified preparations of insulin made from beef (bovine) and pork (porcine) pancreases were used. However, human insulin is now the most widely used type of insulin. Human insulin is not directly harvested from human organs but is derived from common bacteria (e.g. Escherichia coli) or yeast cells using recombinant DNA technology (see Fig 13-19). Insulin differs in rate of onset, peak action and duration (see Fig 48-3).
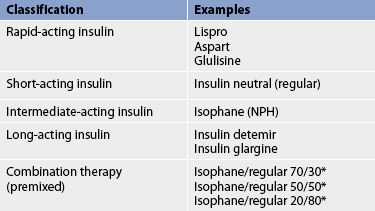
The specific properties of each type of insulin are matched with the patient’s diet and activity. Different combinations of insulin can be used to tailor treatment to the patient’s specific pattern of blood glucose levels, lifestyle, eating habits and activity patterns. Different types of insulin are listed in Table 48-2. All insulin preparations start with regular insulin as a base. By adding zinc, acetate buffers and protamine to insulin in various ways, the onset of activity, peak and duration times can be manipulated. Protamine is added to isophane insulin to slow absorption. These additives can cause an allergic reaction at the injection site.
The timing of insulin administration in relation to meals is important. Regular insulin should be taken 30–45 minutes before meals to ensure the onset of action in conjunction with meal absorption. Examples of insulin combination regimens, including the onset, peak and descriptions of the advantages and disadvantages of each regimen, are presented in Table 48-3. Ideally, the regimen should be mutually selected by the patient and the healthcare provider.25 The criteria for selection are based on the type of diabetes and the required, desired and feasible levels of glycaemic control.
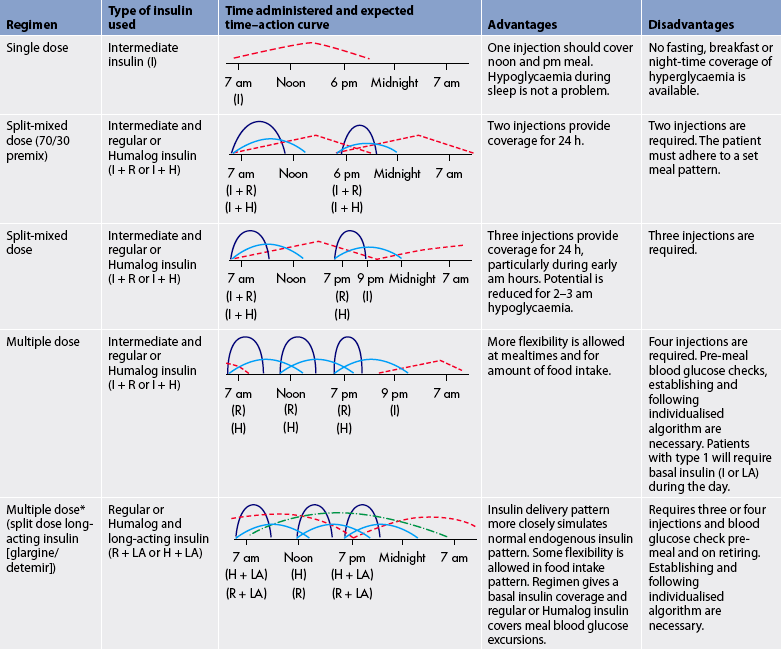
H, lispro (Humalog) or rapid-acting insulin =  ; I, intermediate insulin =
; I, intermediate insulin =  ; LA, long-acting insulin =
; LA, long-acting insulin =  ; R, regular insulin =
; R, regular insulin =  .
.
* Insulin delivery through a pump is similar to this regimen.
Synthetic rapid-acting insulins include lispro, aspart and glulisine insulin. They have an onset of action of approximately 5–15 minutes (compared to 30–60 minutes for regular insulin). Rapid-acting insulin is considered to be the type that best mimics natural insulin secretion in response to a meal. It is injected at the time of the meal or within 15 minutes of the meal. When this type of insulin is used as mealtime cover in people with type 1 diabetes, long-acting or basal insulin must also be used, as the action of rapid-acting insulin covers only the meal and is short term.
Insulin glargine and detemir are long-acting insulins that are released slowly over a 24-hour period. These insulins have minimal peaks of action (see Fig 48-3). The insulin is usually administered at bedtime but can be manipulated to suit the personal preference of the patient. As these insulins are peak-less, the risk of hypoglycaemia is reduced. Both glargine and detemir insulin should not be diluted or mixed with any other insulin or solution.26
Two different insulin types are commonly used in combination to mimic normal endogenous insulin secretion (see Table 48-3). Short- or rapid-acting insulin is often mixed with longer-acting insulin to provide both mealtime and basal coverage without having to administer two separate injections. Patients may mix the two types of insulin themselves in a syringe or may use commercially premixed insulin (see Table 48-2). These insulins offer convenience to patients and are especially helpful for those who lack the visual, manual or cognitive skills to mix insulin themselves. However, the convenience of these formulas sacrifices the potential for optimal blood glucose control because there is less opportunity for flexible dosing based on need.
Insulin requires special storage considerations. Heating or freezing insulin can alter the insulin molecule. Insulin vials in current use may be left at room temperature for up to 4 weeks unless the ambient temperature is greater than 25°C or is near freezing (<2°C), in which case the insulin should be stored in the refrigerator. Patients need to be aware that once insulin is open and in use it must be used within 28 days. The vial/pen should be dated when opened to avoid using insulin beyond this limit. Prolonged exposure to direct sunlight should be avoided. When prescribed, insulin vials/pens come in a box, so extra vials/pens will need to be stored in the refrigerator. The same principles apply for a patient who is travelling in hot climates. Insulin can be stored in a thermos or cooler to keep it cool (not frozen). Care should be taken not to leave insulin in the glove box of cars or in handbags.
Pre-filled syringes are stable for up to 30 days when stored in the refrigerator. This may be beneficial to patients who are sight impaired or who lack the manual dexterity to fill their own syringes at home. In these cases family members, friends and carers may pre-fill syringes on a periodic basis. Syringes pre-filled with a cloudy solution should be stored in a vertical position with the needle pointed up to avoid clumping of suspended insulin binders in the needle.27 Likewise, commercially prepared mixtures may be pre-filled and stored for later use. Some insulin combinations are not appropriate for pre-filling and storage because the mixture can alter the onset, action and/or peak times of either of the types of insulin. Advice should be sought from a pharmacist as needed when mixing and pre-filling different types of insulin. Pre-filled syringes should be gently rolled between the palms before injection to warm the refrigerated insulin and to re-suspend the particles.
ADMINISTRATION OF INSULIN
If insulin is ingested, it is broken down by gastric juices; therefore, it cannot be taken orally. Injection is the only route of administration currently approved for self-administration. Routine administration of insulin is generally carried out by subcutaneous (SC) injection, although regular insulin can be injected intravenously (IV) when immediate onset of action is desired.
Injection
The steps for SC insulin injection technique are outlined in Box 48-3. The technique should be taught to new insulin users and reassessed periodically with long-term users. It should never be assumed that because insulin is being used, the patient knows and practises the correct insulin injection technique. Poor eyesight may cause inaccurate drawing up of insulin as air bubbles in the syringe may be missed or the scale on the syringe read improperly.28,29 Nowadays insulin comes in insulin pens, which makes it much easier for the patient to dial up the required amount of insulin.
BOX 48-3 Insulin injection technique
PATIENT & FAMILY TEACHING GUIDE
2. Roll intermediate- or long-acting insulin bottle between palms of hands to mix insulin. Note: Always inspect insulin bottle before using it. Always check the insulin name, concentration and expiry date and that top of bottle is in perfect condition.
3. Prepare insulin injection in the same manner as for any injection, ensuring there are no air bubbles present in the syringe. If mixing short- and intermediate-acting insulin, always draw up the clear insulin first and expel any air bubbles before drawing up the intermediate-acting insulin.
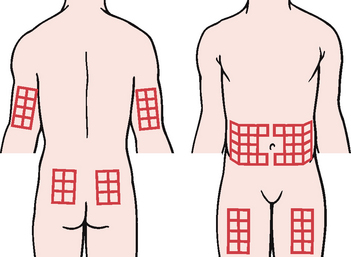
4. Select the injection site (see Fig 48-5) and inject, following the procedure for any SC injection. In sites where SC tissue is adequate, inject commercial insulin needles at 90° angle. For sites with minimal SC tissue, pinch up skin and insert needle at 45° angle.
5. If blood appears in the syringe after the needle is inserted, select a new site for injection. Aspiration of the syringe is not necessary.
6. After injecting the insulin, apply some pressure with a dry cottonwool ball or gauze pad at the site when withdrawing the needle.
7. Hold the cottonwool ball in place for a few seconds but do not massage.
Note: When instructing the patient to self-inject insulin, use the following guidelines (if appropriate):
The patient who mixes regular and intermediate-acting insulin in the same syringe needs to learn the technique for combining both in the same syringe. Commercially prepared premixed insulins are available, which reduces the need to mix insulins (see Fig 48-4). Insulins should not be mixed if they differ in purity or species origin.
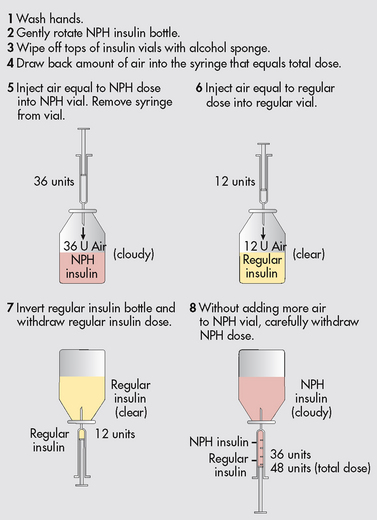
Figure 48-4 Mixing insulins. This step-order process avoids the problem of contaminating regular insulin with intermediate-acting (NPH [isophane]) insulin.
Insulin injection sites must be rotated (see Fig 48-5). Injecting in the same place regularly can cause development of hard fatty lumps called lipodystrophy. These lumps are not only unsightly, they also change the way insulin is absorbed, causing poor glycaemic control. In addition, insulin is absorbed at different rates depending on where it is injected in the body, so it is best to consistently use the same part of the body for the same daily injection—that is, the evening bolus dose should not be injected into the abdomen on Monday and the thigh on Tuesday. If the patient has chosen the thigh for the evening injection, then they should continue to use the thigh for all evening injections, but rotate sites within the thigh area over a period of time. Most insulin enters the blood fastest from the abdomen (stomach), a little more slowly from the arms, even more slowly from the legs and slowest from the buttocks. However, exercising an arm or leg after an injection can increase blood flow and speed insulin absorption from those areas, placing the patient at risk of hypoglycaemia. It is often better to inject the breakfast and lunch bolus doses into the abdomen. Insulin is absorbed fastest when injected into this area and fast absorption will help cover the carbohydrates at mealtimes. The supper or bedtime dose of long-acting insulin could be injected into the thigh, buttocks or upper arm, as long-acting insulin takes effect gradually and covers the patient’s needs throughout the night.
Most commercial insulin is available as U100 (i.e. 1 mL contains 100 units of insulin). U100 insulin must be used with a U100-marked syringe. Disposable plastic insulin syringes are available in a variety of sizes, including 1.0 mL, 0.5 mL and 0.3 mL. The 0.5 mL size may be used for doses of 50 units or less and the 0.3 mL syringe can be used for doses of 30 units or less. Smaller syringes offer a number of advantages. The major benefit is increased accuracy and reliability when delivering smaller doses because wider line markings are easier to see. Patients must be advised to check dosage lines carefully when changing syringe types because some use a scale of 0.5- or 1-unit increments and others use a 2-unit increment.
Needles are also available in various lengths: 4 mm, 5 mm, 6 mm, 8 mm and 12.7 mm. The longer needles are preferred for obese and larger patients. All patients need to allow at least 10 seconds after delivery of insulin before removing the needle, to prevent leaking from the site. Recapping the needle should only be done by the person using the syringe: the nurse must never recap a needle that has been used by a patient. It is no longer recommended to use an alcohol swab on the site before self-injection. Routine hygiene, such as washing the area with soap and rinsing with water twice a day (e.g. showering twice daily), is adequate hygiene for the patient giving their own injections at home. Nurses administering insulin in a hospital or another healthcare facility should check the facility’s policy regarding preparation of the injection site.
An insulin pen is a compact, portable device that serves the same function as a needle and syringe but is easier to use (see Fig 48-6). Pens can be disposable or non-disposable and are much more appealing to patients. Disposable pens are pre-filled with insulin and non-disposable pens require cartridges to be loaded. As the pens come in different shapes and sizes, patient capability in using the pen needs to be considered when prescribing the insulin.
Alternative delivery methods
Continuous SC insulin infusion can be administered using an insulin pump, which is a small battery-operated device that resembles a standard paging device in size and appearance (see Fig 48-7). Usually worn on the belt, the pump is connected via a small plastic tube to a catheter inserted into the SC tissue. Every 2–3 days the insertion site is changed and the pump is refilled with insulin and reprogrammed. The device is programmed to deliver a continuous infusion of rapid-acting insulin 24 hours a day, which is known as the ‘basal rate’. At mealtimes, the user programs the pump to deliver a bolus infusion of insulin appropriate to the amount of carbohydrate ingested and/or to bring down high pre-meal blood glucose levels, if necessary. A major advantage of the insulin pump therapy is the potential for tighter glucose control and a reduction in hypoglycaemic episodes.30 Tighter control is possible because insulin delivery mimics the normal physiological pattern. Pumps also offer the benefit of a more normal lifestyle, allowing users more flexibility with meal and activity patterns. The insertion site should be checked daily for redness and swelling.31 Drawbacks to using a pump include the cost of the pump and the consumables. The National Diabetes and Supply Scheme (NDSS) in Australia will only subsidise pump consumables for people with type 1 diabetes. In New Zealand, disposable and non-disposable insulin syringes and pen needles are subsidised if they are prescribed on the same form as that used for the supply of insulin.
An alternative to the insulin pump is intensive insulin therapy, which consists of multiple daily insulin injections together with frequent self-monitoring of blood glucose levels. The goal is to achieve a near-normal glucose level of 4–6 mmol/L before meals. The Diabetes Control and Complications Trial (DCCT)21 demonstrated that people with type 1 diabetes who have tight glucose control through intensive management develop fewer and less severe complications.32 Studies have shown comparable outcomes in patients receiving intensive insulin therapy and patients on insulin pumps. The disadvantages of multiple daily insulin injections are that three or more injections are needed daily. In addition, intermediate- and long-acting insulins such as isophane, detemir and glargine must be used as the basal component.33
PROBLEMS WITH INSULIN THERAPY
Allergic reactions, lipodystrophy, the Somogyi effect and the dawn phenomenon are the problems associated with insulin therapy. (Guidelines for assessing patients treated with insulin are presented in Table 48-4.)
TABLE 48-4 Assessing the patient treated with glucose-lowering agents
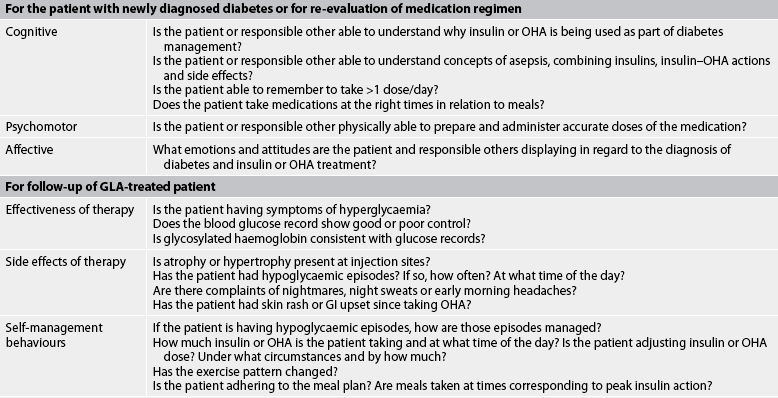
GI, gastrointestinal; GlA, glucose-lowering agent; OHA, oral hypoglycaemic agent.
Allergic reactions
Local inflammatory reactions to insulin may occur, such as itching, erythema and burning around the injection site. Local reactions may be self-limiting within 1–3 months or they may improve with a low dose of antihistamine. A true insulin allergy is a systemic urticarial response and possibly anaphylactic shock generally resulting from the use of bovine or porcine (animal) insulin. Fortunately, this type of allergy is rare, particularly since human insulin has become available.
Lipodystrophy
Lipodystrophy (hypertrophy or atrophy of SC tissue) may occur if the same injection sites are used frequently. Hypertrophy is thickening of the SC tissue, which eventually regresses if the patient does not use the site for at least 6 months. The use of hypertrophied sites may result in erratic insulin absorption.
The Somogyi effect and the dawn phenomenon
The Somogyi effect is associated with the occurrence of undetected hypoglycaemia during sleep (see Fig 48-8). The effect is thought to be induced by too much insulin. Nocturnal hypoglycaemia leads to rebound morning hyperglycaemia induced by counter-regulatory hormones (adrenaline, cortisol, growth hormone and glucagon).34,35 Counter-regulatory hormones stimulate lipolysis, gluconeogenesis and glycogenolysis, which in turn produce rebound hyperglycaemia and ketosis. The danger of this effect is that when blood glucose levels are measured in the morning, hyperglycaemia is apparent and the patient (or healthcare professional) may increase the evening insulin dose, further complicating the problem.36,37
The patient may report headaches on awakening and may recall night sweats or nightmares. If the Somogyi effect is suspected as a cause of early morning high blood glucose levels, the patient should be advised to check blood glucose levels between 2 am and 4 am to determine whether hypoglycaemia is present at that time. If this is the case, the long-acting insulin affecting the early morning blood glucose level may need to be reduced.
Named after the time of day it occurs, the dawn phenomenon is caused by insensitivity to insulin between approximately 4 am and 8 am due to a sleep-induced surge of growth hormone, which causes insulin resistance and hyperglycaemia. The dawn phenomenon is characterised by hyperglycaemia that is present on awakening in the morning. It affects the majority of people with diabetes and tends to be most severe when growth hormone is at its peak, in adolescence and young adulthood.37
Whether morning hyperglycaemia is caused by the Somogyi effect or the dawn phenomenon, the cause must be ascertained as the treatment for each is different. The treatment for the Somogyi effect is less insulin, whereas the treatment for the dawn phenomenon is an adjustment in the timing of insulin administration or an increase in insulin. The assessment must include insulin dose, injection sites and variability in the time of meals or insulin administration. In addition, the patient is asked to measure and document bedtime, night-time (2 am–4 am) and morning fasting blood glucose levels on several occasions. If the predawn levels are below 3.5 mmol/L and signs and symptoms of hypoglycaemia are present, the insulin dosage should be assessed and reduced. If the 2 am–4 am blood glucose level is high, the insulin dosage should be increased. The patient needs also to be counselled on appropriate bedtime snacks.
Drug therapy: oral agents
OHAs come in tablet form and work to improve the mechanisms by which insulin and glucose are produced and used by the body. For any of the OHAs to be effective, the patient must have some circulating endogenous insulin. OHAs may be used in combination with agents from other classes or with insulin to achieve blood glucose targets. OHAs are not used in the treatment of type 1 diabetes. Guidelines for assessing patients receiving OHAs are shown in Table 48-4.
A number of oral medications are available to improve diabetes control for patients with type 2 diabetes38 and these are listed in Table 48-5.
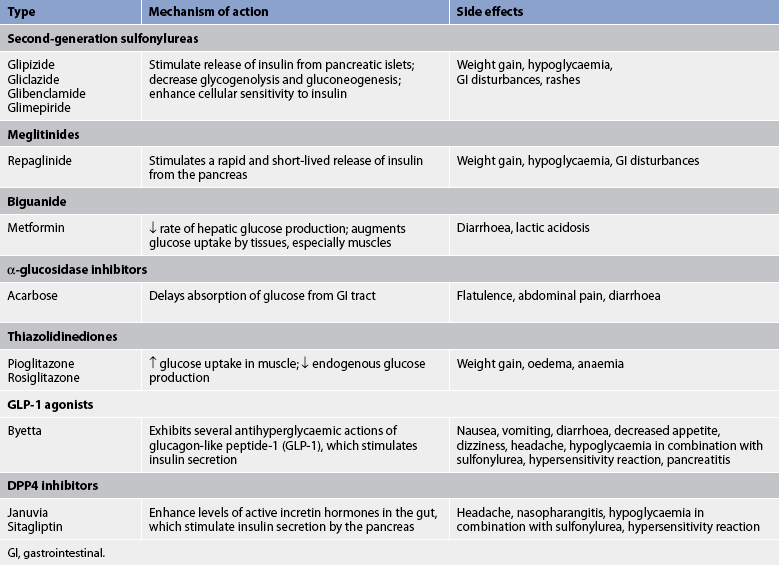
SULFONYLUREAS
Sulfonylureas have been widely used to treat type 2 diabetes since the 1950s. They are classified as either first-generation drugs (i.e. those that were produced first) or second-generation drugs (i.e. those that came later). First-generation drugs, including tolbutamide and chlorpropamide, which were used in the treatment of diabetes mellitus, are no longer available for use. The second generation of sulfonylureas includes glipizide, gliclazide, glimepiride and glibenclamide. Second-generation drugs have fewer adverse effects and are more potent by weight.
The primary action of the sulfonylureas is to increase insulin production by the pancreas, and patients taking these drugs need to be aware of the associated risks of hypoglycaemia. Therapy with sulfonylureas is generally more effective early in the course of type 2 diabetes when the pancreas is still producing insulin. The effectiveness of these medications will decrease as the disease progresses.38
MEGLITINIDES
Like the sulfonylureas, repaglinide increases insulin production by the pancreas but at a different binding site. However, because it is more rapidly absorbed and eliminated, it offers a reduced potential for hypoglycaemia. When taken just before meals, it stimulates insulin production during and after the meal, mimicking the normal blood glucose response to food intake. Patients should be instructed to take repaglinide 1–30 minutes before each meal. The side effects of this drug are weight gain and potential hypoglycaemia (much less common than with sulfonylureas).
BIGUANIDES
Biguanides (metformin) lower the blood glucose level by reducing hepatic glucose output and increasing tissue response to insulin. They can be used alone or with sulfonylureas, or in combination with other OHAs or insulin to treat type 2 diabetes. Besides being an effective blood glucose-lowering agent, metformin has other advantages. Unlike sulfonylureas and insulin, metformin does not promote weight gain and the risks of hypoglycaemia are minimal. It also has beneficial effects on plasma lipid levels. In addition, metformin can be used to treat prediabetes, especially in individuals who are obese or have other risk factors for diabetes. Care should be taken when administering metformin to patients who have renal impairment (creatinine >150 μmol/L) or who are undergoing procedures with contrast medium, as the drug is excreted by the kidneys. Metformin is contraindicated in patients who are heavy alcohol users. Side effects include nausea, gastrointestinal discomfort and diarrhoea. The drug should be started on a low dose and titrated up over a period of weeks. The patient is encouraged to take the medication with food to lessen gastrointestinal side effects.39
α-GLUCOSIDASE INHIBITORS
Also known as ‘starch blockers’, these drugs work by slowing down the absorption of carbohydrate in the small intestine. Acarbose is the available drug in this class. It is most effective in lowering postprandial blood glucose levels when taken at the beginning of each main meal. Effectiveness is measured by checking 2-hour postprandial glucose levels. However, this drug is not often used due to the gastrointestinal side effects. Medications from this class are not effective against fasting hyperglycaemia.38
THIAZOLIDINEDIONES
Sometimes referred to as ‘insulin sensitisers’, these agents include pioglitazone and rosiglitazone. They are most effective in people who have insulin resistance. They improve insulin sensitivity, transport and utilisation at target tissues. Because they do not increase insulin production, thiazolidinediones will not cause hypoglycaemia when used alone but the risk is still present when a thiazolidinedione is used in combination with a sulfonylurea or insulin. Patients should be aware it may take 6–8 weeks before improvement is seen in blood glucose levels. Patients taking these medications gain a secondary benefit of improved lipid profiles and blood pressure. Thiazolidinediones should not be used if the patient has stage III or IV cardiac disease as they cause fluid retention and have been implicated in heart failure in some patients.40
GLP-1 AGONISTS
Glucagon-like peptide 1 (GLP-1) agonists are rather unusual in that they are derived from a compound found in the saliva of the Gila monster, a large lizard native to the southwestern US. GLP-1 agonists are released into the blood by the intestine in response to food intake. GLP-1 impacts several organs including the intestines (where it slows food absorption), stimulates insulin production and restores first-phase insulin secretion. The overall effect is to decrease post-meal blood glucose levels and improve control without the risk of hypoglycaemia. Both an increase in β-cell mass and an increase in first-phase insulin release towards normal have been seen in people with type 2 diabetics who take GLP-1 agonists such as byetta.41 Rather than being glucagon-like and raising glucose levels, GLP-1 agonists lessen the excessive release of glucagon seen in type 2 (and type 1) diabetes. One of the positive side effects of this drug is weight loss. Other side effects include nausea, diarrhoea and a risk of hypoglycaemia when used in combination with a sulfonylurea.41
DPP-4 INHIBITORS
DPP-4 inhibitors work by blocking dipeptidyl peptidase, an enzyme that breaks down incretin hormones in the gut.42 These incretin hormones help increase insulin production after a meal and decrease the amount of glucose made by the liver. By blocking the enzyme that destroys incretin hormones, DPP-4 inhibitors allow these hormones more time to work, which lowers blood glucose levels.
Patients with kidney problems will need lower doses of DPP-4 to manage their blood glucose. Hypoglycaemia is not typically a problem with DPP-4 inhibitors when they are used alone, but can occur when they are used in combination with other diabetes medications that lower the blood sugar. DPP-4s are body weight neutral and may even induce slight weight loss in some patients. There is an increased risk of pancreatitis with use of these drugs. Long-term outcome studies are required to fully assess their morbidity and mortality risk.42
OTHER DRUGS AFFECTING BLOOD GLUCOSE LEVELS
Both the patient and the healthcare provider must be aware of drug interactions that can potentiate hypoglycaemic and hyperglycaemic effects. For example, β-adrenergic blockers can mask symptoms of hypoglycaemia and prolong the hypoglycaemic effects of insulin. Thiazide and loop diuretics can potentiate hyperglycaemia by inducing potassium loss, although low-dose therapy with a thiazide is usually considered safe. Cortisones cause hyperglycaemia and the patient can end up with drug-induced diabetes or, if they already have diabetes, a worsening of blood glucose control. A list of medications that may influence glycaemic control is presented in Box 48-4.
Nutritional therapy
Although nutritional therapy is one of the cornerstones of care for people with diabetes, it is also the most challenging for many people. Achieving nutritional goals requires a coordinated team effort that takes into account the patient’s behavioural, cognitive, socioeconomic, cultural and religious aspects. Because of these complexities it is recommended that a diabetes nurse educator and a dietician with expertise in diabetes management be members of the team.
Many people, both lay and professional, are misinformed about the nutritional management of diabetes. Over the past 50 years the eating plan has changed considerably; therefore, all people with diabetes should be referred to an accredited practising dietician for accurate information.43 When referring a patient to the dietician, the healthcare provider must ensure that any relevant cultural or religious requirements are acknowledged.
Within the context of an overall healthy eating plan, a person with diabetes can eat the same foods as a person who does not have diabetes. This means that the same principles of good nutrition that apply to the general population also apply to the person with diabetes. The healthy eating pyramid (see Fig 39-1) summarises and illustrates nutritional guidelines and nutrient needs. These are also appropriate in guiding the food choices of people with diabetes. Tools used to measure the effectiveness of nutritional therapy include blood glucose level, HbA1c and lipid values, tests of renal status and clinical measurements, such as body weight, body mass index, waist measurement and blood pressure.44 Table 48-6 describes nutritional therapy for type 1 and type 2 diabetes.
| Factor | Type 1 diabetes mellitus | Type 2 diabetes mellitus |
|---|---|---|
| Total kilojoules | Increased intake possibly necessary to achieve desirable body weight and restore body tissues | Reduction in intake desirable for overweight or obese patient |
| Effect of diet | Diet and insulin necessary for glucose control | Diet alone possibly sufficient for glucose control |
| Distribution of kilojoules | Equal distribution of carbohydrates through meals or adjustment of carbohydrates for insulin activity | Equal distribution recommended; low-fat diet desirable; consistency of carbohydrate at meals desirable |
| Consistency in daily intake | Necessary for glucose control | Desirable for weight reduction and moderation of blood glucose levels |
| Uniform timing of meals | Crucial for NPH insulin programs; flexibility with multidose rapid-acting insulin | Desirable but not essential |
| Between-meal and bedtime snacks | Frequently necessary | Not usually recommended |
| Nutritional supplement for exercise programs | Carbohydrates 20 g/h for moderate physical activities | May be necessary if patient controlled on sulfonylurea or insulin |
NPH, neutral protamine hagedorn.
The goal of nutritional therapy is to assist people with diabetes in making changes in nutrition and exercise habits that will lead to improved metabolic control. The overall dietary principles for diabetes are:45
1. Include high-fibre carbohydrate foods, which slow digestion, resulting in a gradual rise in blood glucose levels (low glycaemic index).
2. Limit saturated fat and have a moderate fat intake.
3. Be aware that moderate amounts of sugar can be included in a healthy eating plan.
4. Eat regular meals and snacks throughout the day, especially if taking insulin or medication.
5. Limit alcohol to two standard drinks for men and one standard drink for women per day and have at least two alcohol-free days per week.
6. Choose a variety of foods from the healthy eating pyramid.
TYPE 1 DIABETES MELLITUS
Meal planning should be based on the individual’s usual food intake and balanced with insulin and exercise patterns. The insulin regimen should be developed with the patient’s eating habits and activity pattern in mind. Patients using rapid-acting insulin can make adjustments in dosage before the meal based on the current blood glucose level and the carbohydrate content of the meal. Intensive insulin therapy, such as multiple daily insulin injections or the use of an insulin pump, allows considerable flexibility in food selection and can be adjusted for deviations from usual eating and exercise habits.
TYPE 2 DIABETES MELLITUS
The emphasis for nutritional therapy in type 2 diabetes should be placed on achieving glucose, lipid and blood pressure goals. Because 80–90% of people with type 2 diabetes are overweight, kilojoule reduction is an added goal.45
There is no one proven strategy or method that can be uniformly recommended. A nutritionally adequate meal plan with a reduction of total fat, especially saturated fats, and simple sugars can bring about decreased kilojoule and carbohydrate consumption. Spacing meals evenly throughout the day is another strategy that can be adopted to spread nutrient intake. A weight loss of 5–7% of body weight often improves glycaemic control, even if desirable body weight is not achieved. Weight loss is best attempted by a moderate decrease in kilojoules and an increase in energy expenditure. Regular exercise, at least 30 minutes per day, and learning new behaviours and attitudes can help facilitate long-term lifestyle changes. Monitoring blood glucose levels, HbA1c, lipids and blood pressure provides feedback on how well the goals of nutritional therapy are being met.
FOOD COMPOSITION
The meal plan for people with diabetes does not prohibit the consumption of any one type of food. All food groups should be represented in a daily meal plan that is nutritionally balanced. Although an individualised meal plan should be developed with a dietician, general guidelines and recommendations for people with diabetes include the following:43,45
• Protein—should constitute 15–20% of total daily kilojoules. Those with nephropathy should limit protein intake to 10%.
• Fat—should constitute less than 10% of daily kilojoules. Cholesterol intake should be less than 300 mg/day.
• Carbohydrate—should constitute the remaining percentage of daily kilojoules after determining protein and fat needs. Carbohydrates should include wholegrains, fresh vegetables and fresh fruits, which are considered to have a low glycaemic index. Although overall intake of simple sugar should be limited as much as possible, its consumption is acceptable in moderate amounts when counted as part of total carbohydrate intake.
• Sodium—intake should be less than 2400 mg/day.
• Fibre—intake should be approximately 25–30 g/day from a variety of food sources.
Alcohol
Alcohol is high in kilojoules, has no nutritive value and promotes hypertriglyceridaemia. In addition, it has detrimental effects on the liver (Ch 43). The inhibitory effect of alcohol on glucose production by the liver can cause severe hypoglycaemia in patients on insulin or OHAs that cause increased insulin secretion. Patients should be encouraged to discuss their alcohol use honestly with their healthcare provider in relation to its effect on glycaemic control.
Alcohol can also cause other serious adverse effects when used in conjunction with certain OHAs. For example, it may produce a disulfiram effect (nausea and vomiting, flushing, respiratory distress, chest pain) when ingested with sulfonylurea medications, such as glibenclamide. It can also increase the risk of lactic acidosis in patients on metformin.
Light to moderate alcohol consumption can be incorporated into the meal plan occasionally if blood glucose levels are well controlled and if the patient is not on medications that will cause adverse effects. Patients can reduce the risk of alcohol-induced hypoglycaemia by eating carbohydrates when drinking alcohol. One standard drink contains approximately 565 kJ. Patients with diabetes should have food when drinking alcohol, use sugar-free mixers and, if drinking wine, drink dry, light wines.
DIET EDUCATION
In the management of diabetes, diet is based on the national guidelines for healthy eating with additional information on managing carbohydrates. Whenever possible, a dietician should be part of the interdisciplinary diabetes care team. The patient should have a consultation with the dietician to learn the principles of nutrition therapy in the care of diabetes. However, in some instances, access to a dietician is not possible, especially for patients who live in remote areas. In these cases, nurses often assume responsibility for teaching basic dietary management.
The Healthy Eating Pyramid (see Ch 39) is an appropriate teaching tool for people with diabetes. The pyramid helps patients to visualise the recommended amounts of foods that should be eaten from each group on a daily basis. For many people, the graphic representation of the pyramid makes this method of meal planning more approachable and easier to understand than exchange/serve/portion lists.
An alternative method of presenting the basics of meal planning is to use the plate method. This simple method helps patients to visualise the amount and type of food that should fill a 22.5 cm plate. For lunch and dinner half of the plate is filled with non-starchy vegetables, one-quarter is filled with a carbohydrate and one-quarter is filled with 100–150 g of lean meat. A glass of low-fat milk and a small piece of fresh fruit complete the meal. The breakfast plate is filled halfway with carbohydrate and one-quarter of the plate contains an optional protein. Low-fat milk and fresh fruit complete the breakfast. Assuming low-fat and non-fat foods are selected, following the plate method will provide 5000–5900 kJ per day and a properly balanced meal plan.46
Diet teaching should include the patient’s family and significant others whenever possible. It is most effective to direct teaching efforts to the person who will be cooking. It is important, however, that the responsibility for maintaining the diabetic diet does not fall to someone other than the patient with diabetes. Reliance on another person to make health decisions fosters dependence and should be avoided except in special situations.
Exercise
Regular, consistent exercise is considered an essential part of diabetes management. Exercise increases insulin sensitivity and can have a direct effect on lowering blood glucose levels. In addition it contributes to weight loss, which also decreases insulin resistance. The therapeutic benefits of regular physical activity may result in a decreased need for diabetes medication in order to reach target blood glucose goals. Regular exercise may also help reduce triglyceride and LDL cholesterol levels, reduce blood pressure and improve circulation.45
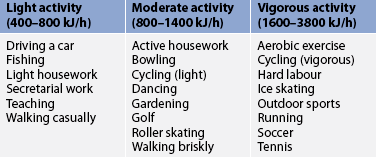
Patients who use insulin, sulfonylureas or meglitinides are at increased risk of hypoglycaemia when there is an increase in their physical activity, especially if they exercise at the time of peak drug action or if their food intake has not been sufficient to maintain adequate blood glucose levels. This can also occur if a normally sedentary patient with diabetes has an unusually active day. The glucose-lowering effects of exercise can last up to 48 hours after the activity, so it is possible for hypoglycaemia to occur for that long after the activity. It is recommended that patients who use medications that can cause hypoglycaemia schedule exercise about 1 hour after a meal or that they have a 10–15 g carbohydrate snack before exercising. Several small carbohydrate snacks can be taken every 30 minutes during exercise to prevent hypoglycaemia.45 Patients using medications that place them at risk of hypoglycaemia should always carry a fast-acting source of carbohydrate, such as glucose tablets or drinks, when exercising. Table 48-7 gives guidelines on the number of kilojoules burned per hour for different activities.
Although exercise generally has positive effects on blood glucose levels, the body can perceive strenuous activity as a stress, causing a release of counter-regulatory hormones that result in a temporary elevation of the blood glucose level. As a result, hyperglycaemia may occur in patients with poorly controlled type 2 diabetes or in patients with type 1 diabetes who exercise at a time of day when insulin action is waning. Some patients may have to inject a small bolus of rapid-acting or regular insulin if their blood glucose level is elevated before exercising, to prevent progressive hyperglycaemia. Additional information about exercise and diabetes should be provided in patient education (see the patient and family teaching guide in Box 48-5).
BOX 48-5 Exercise for patients with diabetes mellitus
PATIENT & FAMILY TEACHING GUIDE
1. Exercise does not have to be vigorous to be effective. The blood glucose-reducing effects of exercise can be attained with mild exercise, such as brisk walking. The exercises selected should be enjoyable to foster regularity.
2. Exercise is best done after meals, when the blood glucose level is rising.
3. Exercise plans should be individualised for each patient and monitored by the healthcare provider.
4. It is important to self-monitor blood glucose levels before, during and after exercise to determine the effect exercise has on the blood glucose level at particular times of the day.
5. Be alert to the possibility of delayed exercise-induced hypoglycaemia, which may occur several hours after the completion of exercise.
6. Taking a glucose-lowering medication does not mean that planned or spontaneous exercise cannot occur.
7. It is important to compensate for extensive planned and spontaneous activity by monitoring the blood glucose level to make adjustments in the insulin dose (if taken) and food intake.
Monitoring blood glucose
Self-monitoring of blood glucose (SMBG) is one of the cornerstones of diabetes management. It enables the patient to make self-management decisions regarding diet, exercise and medication. SMBG is also important for detecting hyperglycaemia and hypoglycaemia. In the past, monitoring was accomplished by checking for the presence and degree of glucose in the urine. This method of assessing glucose is rarely used now as urine testing does not provide an accurate measure of blood glucose. Instead, there are reliable point-of-care monitors available for monitoring blood glucose levels that provide an accurate measurement for early detection and treatment of hypoglycaemia and other management issues.
Portable blood glucose monitors are point-of-care devices used at the hospital bedside for patients with diabetes. Patients should be encouraged to continue self-management in hospital if they are willing and able. A wide variety of blood glucose monitors are available. Disposable lancets are used to obtain a small drop of capillary blood (usually from a finger), which is placed onto a reagent strip. After a specified time, the monitor displays a digital reading of the blood glucose level. SMBG technology is rapidly changing with newer, more convenient systems being introduced every year. Newer systems allow the user to collect blood from alternative sites, such as the forearm. Non-invasive approaches to blood glucose monitoring have also been developed and are currently in use in Europe, but they are not yet reliable.
Blood glucose levels reported by a laboratory are sometimes higher than those obtained by the patient’s home glucose monitor. The reason for this is that some monitors give capillary blood glucose values from whole blood (via the finger), whereas venous samples taken in the laboratory provide plasma readings. Plasma samples, or venous samples, are approximately 10–12% higher. Some monitors are automatically calibrated to give a ‘plasma’ test result (despite whole blood used for the sample), so that the home readings are more comparable with laboratory values. The literature accompanying a monitor will explain whether that particular monitor is calibrated to give plasma or whole blood readings.
Each blood glucose monitor comes with instructions for use. Since problems with monitoring technique can cause errors in management, thorough patient education in the use of the monitor is crucial. Initial training should be followed up at regular intervals with reassessment of patient technique. In addition, patients must be taught to calibrate and interpret calibration results for their blood glucose monitor.47 As control solutions are expensive and expire within a short period of time, patients may be able to ask their local pharmacist to calibrate their monitor with control solutions every 3–6 months. Box 48-6 lists the steps for patient education on SMBG.
BOX 48-6 Self-monitoring of blood glucose
PATIENT & FAMILY TEACHING GUIDE
1. Wash hands in warm water. It is not necessary to clean the site with alcohol and it may interfere with test results.
2. If it is difficult to obtain an adequate drop of blood for testing, warm the hands in warm water or let the arms hang dependently for a few minutes before the finger puncture is made.
3. If the puncture is made on the finger, use the side of the finger pad rather than near the centre. There are fewer nerve endings along the side of the finger pad. If an alternative site is used (e.g. forearm), special equipment may be needed. Refer to the manufacturer’s instructions for alternative site use.
4. The puncture should be only deep enough to obtain a sufficiently large drop of blood. Unnecessarily deep punctures may cause pain and bruising.
SMBG is recommended for all insulin-treated patients with diabetes. The frequency of monitoring depends on several factors, including the patient’s glycaemic goals, the type of diabetes, the patient’s ability to perform the test independently and the patient’s willingness to test. Patients with type 1 diabetes typically test four times per day (before meals and at bedtime); those using an insulin pump may test more frequently; and patients with type 2 diabetes will have more variable and individualised testing regimens of fasting and post-prandial levels.
Patients should be instructed to test blood glucose levels before and after exercise to determine its effects on metabolic control. This is especially important for patients with type 1 diabetes. Blood glucose testing should also be performed whenever hypoglycaemia is suspected so that immediate action can be taken if necessary. When the person with diabetes is ill, the blood glucose level may need to be tested at 2-hourly intervals to determine the effects of the illness on the blood glucose level.48
SMBG allows patients to be active partners in the treatment of diabetes. Achieving the desired level of patient participation does require time and effort from the healthcare professional. The diabetes nurse involved in this aspect of management should anticipate a close working relationship with patients as they refine their techniques and learn appropriate decision making about managing their diabetes. A patient who is visually impaired, cognitively impaired or limited in manual dexterity needs careful evaluation of the degree to which SMBG can be performed independently and whether it is indicated in that situation. Nurses working in home care and outpatient settings may need to identify carers who can assume this responsibility. Adaptive devices are available to help patients with certain limitations. These include talking meters and other equipment for the visually impaired, as well as devices to stabilise insulin vials and syringes for those with limitations affecting dexterity. Several blood glucose monitors also have an additional function that allows the readings to be downloaded onto a computer and displayed in various formats, such as graphs and electronic log books.
The limited literature available in this area has not shown support for SMBG in patients with type 2 diabetes: studies have shown no improvement in glycaemic control and demonstrated an increase in the number of patients who had higher scores on the depression subscales.49–51 While self-monitoring should clearly be part of any self-management program, more research is needed on the efficacy of SMBG in patients with diabetes.52 (See Ch 69 for further discussion on self-management.) Nevertheless, with good education, SMBG can be a useful tool to guide adjustments in food intake, activity patterns and medication dosages. It not only produces accurate records of daily glucose fluctuations and trends, but also alerts the patient to hyperglycaemic and hypoglycaemic episodes. Furthermore, it provides patients with a tool for achieving and maintaining specific glycaemic goals.
CONTINUOUS GLUCOSE MONITORING SYSTEMS
Continuous glucose monitoring systems (CGMSs) have been available in Australia and New Zealand for the past 10–15 years and provide non-invasive, real-time, continuous monitoring of blood glucose levels. The system consists of a cannula containing electrodes inserted into the subcutaneous tissue (usually the abdomen), which is attached to a monitoring device about the size of a pager worn on the patient’s belt. The system uses electrical current to measure the interstitial blood glucose level at 5-minute intervals. This information is then transmitted to the monitoring device and converted to a glucose measurement. The cannula can be left in situ for 3–6 days. Once the cannula is removed, the device is downloaded to a computer program, which provides a retrospective graphical display of blood glucose levels over the previous 3–6 days. Some insulin pumps display CGMS transmissions, enabling real-time monitoring of blood glucose levels. CGMSs are particularly useful for insulin/medication adjustment and detecting periods of hypoglycaemia.53
PANCREAS TRANSPLANTATION
Pancreas transplantation is used as a treatment option for patients with type 1 diabetes mellitus who have end-stage renal disease and who have had or plan to have a kidney transplant. Kidney and pancreas transplants are often done together. More than 75% of pancreas transplants are working 1 year postsurgery and they last an average of 8 years.
Successful pancreas transplantation can improve the quality of life of people with diabetes, primarily by eliminating the need for exogenous insulin, frequent daily blood glucose measurements and many of the dietary restrictions imposed by the disorder. Transplantation can also eliminate the acute complications commonly experienced by patients with type 1 diabetes (e.g. hypoglycaemia, hyperglycaemia). Patients who undergo pancreas transplantation require immunosuppressive drugs to prevent rejection of the graft and potential recurrence of the autoimmune process that might again destroy pancreatic islet cells. (Immunosuppressive therapy is discussed in Ch 13.)
Pancreatic islet cell transplantation is another potential treatment measure. The first islet transplant in the Australasian region was performed in Australia in 2002, and there is currently a national program for islet cell transplantation for patients meeting specific criteria, such as life-threatening hypoglycaemic events.54 Research is ongoing, but to date approximately 10% of transplant patients remain free of the need for insulin injections at 5 years. However, most recipients return to using insulin as transplanted islets lose their ability to function over time. Stem cell research may provide the answer in the future.
New developments in diabetic therapy
Many new insulin delivery systems are being researched, although they are not yet approved for use in Australia or New Zealand. These include: (1) inhaled insulin, which delivers a dose of liquid or dry powder insulin through the mouth and directly into the lungs, where it enters the blood as rapid-acting insulin; (2) a skin patch containing a reservoir of insulin, changed after every 12–24 hours; and (3) an oral spray, which is a liquid aerosol version of insulin that is absorbed by mucous membranes in the cheeks, tongue and throat. There is difficulty ascertaining how much of the insulin dose is absorbed via these methods.
Culturally competent care: diabetes mellitus
Because culture can have a strong influence on dietary preferences and meal preparation practices, cultural competence has special relevance for the care of patients with diabetes. This is especially pertinent when considering the prevalence of diabetes in such diverse cultural groups as Indigenous Australians, Māori and Pacific Islanders, and the populations of the Chinese and Indian subcontinents. The influences of culture on food choices and meal planning should be explored with the patient as part of the health history. When giving diet instructions, efforts should be made to consider the food preferences of the cultural group. Language barriers make cross-cultural education challenging and interpreters should be engaged at every possible opportunity.
 NURSING MANAGEMENT: DIABETES MELLITUS
NURSING MANAGEMENT: DIABETES MELLITUS
 Nursing assessment
Nursing assessment
Table 48-8 provides initial subjective and objective data that might be obtained from a person with diabetes mellitus. After the initial assessment, periodic patient assessments should be carried out on a regular basis.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses related to diabetes mellitus may include, but are not limited to, those found in NCP 48-1.
 Planning
Planning
The overall goals for the patient with diabetes mellitus include the following: (1) to be an active participant in the management of the diabetes regimen;55 (2) to experience few or no episodes of acute hyperglycaemic emergencies or hypoglycaemia; (3) to maintain blood glucose levels at normal or near-normal levels; (4) to prevent, minimise or delay the occurrence of chronic complications of diabetes; and (5) to adjust lifestyle to accommodate the diabetes regimen with a minimum of stress.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The role of the nurse in health promotion and maintenance relates to the identification, monitoring and education of the patient at risk of developing diabetes mellitus. Obesity is the number one predictor of type 2 diabetes mellitus. A modest weight loss of 5–7% of body weight and regular exercise of 30 minutes five times a week lowers the risk of developing type 2 diabetes significantly.10
Currently, routine screening for diabetes for all adults over age 55 is recommended. If normal, it should be repeated at 3-year intervals. The FPG is the preferred method for screening in clinical settings, although the OGTT is also suitable. Testing should be considered at a younger age or be carried out more frequently in individuals who meet the criteria listed in Box 48-7.5 It is important to know where an individual is on the glucose continuum (see Fig 48-9).
BOX 48-7 Criteria for testing in asymptomatic, undiagnosed individuals
TYPE 1 DIABETES MELLITUS
Testing presumably healthy individuals for the presence of any immune markers, outside a clinical trial setting, is not recommended.
TYPE 2 DIABETES MELLITUS
Testing for undiagnosed type 2 diabetes is recommended for the following high-risk individuals:
• people with impaired glucose tolerance or impaired fasting glucose
• Indigenous Australians aged 35 and over; Māori, Pacific Islander peoples aged 36 and over;
• certain high-risk non-English speaking background groups aged 35 and over (specifically people from the Indian subcontinent or of Chinese origin)
• people aged 45 and over who have either or both of the following risk factors:
• all people with clinical cardiovascular disease (myocardial infarction, angina or stroke)
Individuals presenting the following risk factors are also considered to be at high risk of having undiagnosed type 2 diabetes, but further studies are required in order to evaluate any net clinical or economic benefit of testing these groups:
• women with previous gestational diabetes
• people aged 45 and over who have a first-degree relative with type 2 diabetes.
National Health and Medical Research Council. National evidence-based guidelines for the management of type 2 diabetes mellitus. Available at www.nhmrc.gov.au/publications/synopses/di7todi13syn.htm.
The Australian type 2 diabetes risk assessment tool (AUSDRISK) is a new risk assessment tool based on similar models used elsewhere that the general population can use to assess their risk. It is a useful risk assessment tool for health promotion staff, GPs, healthcare workers and nurses (see Fig 48-10).
 Acute intervention
Acute intervention
Acute situations involving the patient with diabetes include hypoglycaemia, DKA and hyperosmolar hyperglycaemic non-ketotic syndrome. Nursing management for these situations is discussed in more detail later in this chapter. Other areas of acute intervention relate to management during stress, such as during acute illness and surgery.
 Stress of acute illness and surgery
Stress of acute illness and surgery
Both emotional and physical stress can increase blood glucose levels and result in hyperglycaemia. Because it is impossible to avoid stress totally in life, certain situations may require more intense management, such as extra insulin, to maintain glycaemic goals and avoid hyperglycaemia.
Acute illness, injury and surgery are situations that may evoke a counter-regulatory hormone response, resulting in hyperglycaemia.48 Even minor illnesses, such as a viral upper respiratory tract infection or the flu, can cause this. When patients with diabetes are ill, they should continue with the regular meal plan while increasing the intake of fluids with no kilojoules, such as broth, water and decaffeinated beverages. They should also continue taking oral agents and insulin as prescribed and check their blood glucose levels every 2 hours. If the blood glucose level is greater than 15.0 mmol/L on two consecutive occasions, urine or blood should be checked for ketones every 2–4 hours. Patients should report moderate-to-high ketone levels to their healthcare provider.
When the illness depresses the patient’s appetite, they should continue to take oral hypoglycaemic medications and/or insulin as prescribed while supplementing food intake with carbohydrate-containing fluids. Examples include soups, juices and regular decaffeinated soft drinks.48 The healthcare provider should be notified promptly if the patient is unable to keep anything down. The patient should understand that medication for diabetes, including insulin, should not be withheld during times of illness because counter-regulatory mechanisms often increase the blood glucose level dramatically. Food intake is also important because the body requires extra energy to deal with the stress of the illness. Extra insulin may be necessary to meet this demand and to prevent the onset of DKA in the patient with type 1 diabetes.48
During the intraoperative period, adjustments in the diabetes regimen should be planned to ensure glycaemic control. The patient may require dextrose/insulin infusions immediately before, during and after surgery when nil by mouth. OHAs should be discontinued 48 hours prior to surgery, and the patient may require insulin during the surgical period. Patients should be aware that this is a temporary measure and not a sign of worsening diabetes.
The nurse caring for an unconscious surgical patient receiving insulin must look for signs of hypoglycaemia, such as sweating, tachycardia and tremors. Frequent monitoring of blood glucose levels is required in unconscious patients.
 Ambulatory and home care
Ambulatory and home care
Successful management of diabetes requires ongoing interaction among the patient, the family and the healthcare team (see Ch 69). It is important that a diabetes nurse educator be involved in the care of the patient and the family. This nurse provides expertise in many areas of specialised care needs.
Because diabetes is a complex, chronic condition, a great deal of patient contact takes place in the outpatient and home environments. The goal of patient care in these settings is to enable the patient or carer to reach an optimal level of independence in self-care activities. Unfortunately, many patients with diabetes face challenges in reaching these goals. Diabetes increases the risk of other chronic conditions that can affect self-care activities. These include visual impairment, lower extremity problems that affect mobility and other functional limitations related to vascular disease. Therefore, important nursing functions are to assess the ability of patients and carers in such activities as SMBG and insulin injection techniques. Assistive devices for self-administration of insulin include syringe magnifiers, vial stabilisers, insulin pens and dosing aids for the visually impaired. In some cases, the nurse will make referrals to others who can help the patient achieve the self-care goal. These may include a diabetes educator, a dietician, a community nurse, an occupational therapist or a social worker.
A diagnosis of diabetes can affect the patient in a profound way. Patients with diabetes must continually contend with lifestyle choices that affect the food they eat, the activity they engage in, and the demands on their time and energy. In addition, they face the potential of experiencing the numerous and serious complications of this disease. Careful assessment of what it means to the patient to have diabetes should be the starting point of patient education. The nurse can help patients make adjustments by displaying an attitude that is supportive and non-judgemental. The goals of education should be mutually determined by the patient and the nurse, based on individual patient needs and therapeutic requirements.
The patient’s support system must be identified. If this is the family, they need to be involved in teaching so that they can care for the patient when self-care is no longer possible. The family and significant others need to be encouraged to provide emotional support and encouragement as the patient deals with the reality of living with a chronic disease.
 Insulin therapy
Insulin therapy
Nursing responsibilities for the patient receiving insulin include injection technique, assessment of the patient’s response to insulin therapy and education of the patient regarding administration, insulin adjustment and side effects of insulin (see Box 48-6). Table 48-4 lists guidelines for the nurse assessing a patient using GLAs, including insulin and OHAs.27
Assessment of the patient who is commencing insulin must include an evaluation of the patient’s ability to manage insulin therapy safely. This includes the patient’s ability to understand how insulin interacts with diet and activity and to be able to recognise and treat the symptoms of hypoglycaemia. If the patient does not have the cognitive skills to manage these tasks, a carer must be identified and trained. The patient or carer must have the cognitive and manual skills necessary to prepare and inject the insulin. If the patient or family lacks ability, additional resources will be needed to assist the patient.
Many patients are fearful when they begin using insulin. Some patients find it difficult to self-inject because they are afraid of needles or the pain associated with an injection. Some are afraid they will hurt themselves by giving too much or too little insulin. And in some cases, patients believe that using insulin is a ‘last ditch’ effort and that they are now in the final stages of the disease process. Therefore, it is important to explore the patient’s underlying apprehensions and fears before beginning the teaching session.
Follow-up assessment includes an inspection of injection sites for signs of lipodystrophy and other reactions, and a review of insulin preparation and injection techniques. The nurse should take a history of any hypoglycaemic episodes and how the patient manages hypoglycaemia. A review of the patient’s blood glucose record book is also important in assessing overall glycaemic control.
 Oral hypoglycaemic agents
Oral hypoglycaemic agents
Nursing responsibilities for the patient taking OHAs are similar to those for the patient taking insulin. Proper administration, assessment of the patient’s use of and response to the OHA, and education of the patient and the family about OHAs are all part of the nurse’s function.
The nurse’s assessment can be extremely valuable in determining the most appropriate OHA for a patient. Factors such as the patient’s mental status, eating habits, home environment, attitude towards diabetes and medication history all play a significant role in determining the most appropriate OHA for the individual patient. For example, frail older adults who live alone are at high risk of severe hypoglycaemia because a low blood glucose level is frequently undetected and/or untreated in this population. This is especially true if the patient has a short-term memory deficit. In these cases, an OHA that does not cause hypoglycaemia, or a shorter-acting OHA, would be most appropriate.
Patient education is an essential nursing function when caring for the patient on OHAs. Some patients may assume that their diabetes is not a serious condition if they ‘only’ need tablets for glycaemic control. Patients should be instructed that these agents will help keep blood glucose levels controlled and will help prevent serious long- and short-term complications of diabetes. They should also be advised that OHAs are used in addition to diet and activity as therapy for diabetes and that they should continue with their meal and activity plans. Patients should not take extra tablets if overeating has occurred, unless specifically instructed to do so by their healthcare provider. If the patient uses sulfonylureas, instructions should be given on prevention, symptom recognition and management of hypoglycaemia.
Patients should also be instructed to contact their healthcare provider if periods of illness or extreme stress occur. During such a period, insulin therapy may be required to prevent or treat hyperglycaemic symptoms and avoid an acute hyperglycaemia emergency.
 Personal hygiene
Personal hygiene
The potential for microvascular complications and infections requires diligent skin and dental hygiene practices on the part of the patient. Because of the susceptibility to periodontal disease, daily brushing and flossing should be encouraged in addition to regular visits to the dentist. When dental work must be done, the dentist should be informed that the patient has diabetes.
Routine care should include regular bathing, with particular emphasis given to foot care. Problems associated with the feet and lower extremities are presented later in the chapter. If cuts, abrasions or burns occur, they should be treated promptly and monitored carefully. The area should be washed and a non-abrasive or non-irritating antiseptic ointment may be applied. The area should be covered with a dry, sterile pad. If the injury does not begin to heal within 24 hours or if signs of infection develop, the healthcare provider should be notified immediately.
 Medical identification and travel
Medical identification and travel
All people with diabetes should wear a medic alert bracelet or necklace (see Fig 48-11). Patients should be instructed to carry medical identification at all times, indicating they have diabetes. Police, paramedics and many private citizens are aware of the need to look for identification when working with sick or unconscious persons. An identification card can supply valuable information, such as the name of the healthcare provider and the type and dose of insulin or OHA.

Figure 48-11 Medic Alert tags. Patients with diabetes should carry a card and wear a bracelet or necklace that indicates they have diabetes. If a patient with diabetes is unconscious, these measures will ensure prompt and appropriate attention.
Travel for patients with diabetes requires advanced planning. They should have diabetes supplies in their carry-on luggage when travelling by aeroplane, train or bus. This includes blood glucose monitoring equipment, insulin, syringes and needles. When syringes and lancet devices are carried onto a commercial airline, a letter from the prescribing doctor will be necessary to prevent delays at security checkpoints. Patients who use insulin or an OHA that can cause hypoglycaemia should include snack items and a quick-acting carbohydrate for treating hypoglycaemia in their carry-on luggage. Extra insulin should be available in case a bottle breaks or gets lost. In addition, they should carry a full day’s supply of food in the event of cancelled flights, delayed meals or closed restaurants. Some travel involves travelling through time zones, which can be confusing for the patient on insulin. The patient should contact the healthcare provider to plan an appropriate insulin schedule. Many patients find it easier and more predictable to take only regular insulin every 4–6 hours to cover insulin needs while on long aeroplane trips instead of trying to anticipate the peak of intermediate insulin and the availability of meals. During travel, most patients find it helpful to keep their watch set to the time of the city of origin until they reach their destination. The key to travelling well is to know the insulin type, its onset of action, its duration of action, the peak time and meal times.
 Patient and family teaching
Patient and family teaching
The goals of diabetes self-management education are to enable patients to become the most active participant in their care, while matching the level of self-management to the ability of the individual patient. Patients who manage their diabetes care actively have better outcomes than those who do not. For this reason, an educational approach that facilitates informed decision making on the part of the patient is widely advocated.55 Patients need to be aware that ‘natural’ therapies may affect their glucose levels (see the Complementary & alternative therapies box).
Unfortunately, patients can encounter a variety of physical, psychological and emotional barriers when it comes to managing their diabetes effectively. These barriers may include feelings of inadequacy about their own abilities, unwillingness to make the necessary behavioural changes, ineffective coping strategies, and cognitive deficits, anxiety and depression. Consultation with a psychologist may assist patients to overcome feelings of anxiety or those who are having emotional problems compounded or complicated by diabetes. If the patient is not able to manage the disease, a family member may be able to assume part of this role. If the patient or the family cannot make decisions related to diabetes management, the nurse can refer the patient to a social worker or other resources within the community. These resources can assist the patient and the family in outlining a feasible treatment program that meets their capabilities. Patient and healthcare provider resources are listed at the end of this chapter (see p 1395).
Herbs that affect glucose levels
COMPLEMENTARY & ALTERNATIVE THERAPIES
Effects
Herbs can affect glycaemic control. Herbs that can increase the antidiabetic effect of medications (i.e. lower blood glucose levels) include bay, basil, bee pollen, garlic, ginger, ginseng, milk thistle, wormwood, fenugreek, bitter melon and sage. Herbs that can decrease the antidiabetic effect of medications include St John’s wort.
An assessment of the patient’s knowledge of diabetes and lifestyle preferences is useful in planning a teaching program. Table 48-9 presents guidelines to use for patient teaching. The nurse should assess the patient’s knowledge base frequently so that gaps in knowledge or incorrect or inaccurate ideas can be quickly corrected.
TABLE 48-9 General guidelines for management of diabetes mellitus
PATIENT & FAMILY TEACHING GUIDE

HbA1c, glycosylated haemoglobin; OHA, oral hypoglycaemic agent.
Diabetes Australia and Diabetes New Zealand have informative online resources, facts sheets, recipes and advice for both patients and healthcare providers (see Resources on p 1395). They have offices across all states and territories. Both organisations publish resources for patients and provide information for healthcare professionals. They also provide diabetes education and run clinical research projects.
Acute complications of diabetes mellitus
The acute complications of diabetes mellitus arise from events associated with hyperglycaemia and insufficient insulin. A problem that may arise from too much insulin or an excessive dose of an OHA is hypoglycaemia (also referred to as insulin reaction or low blood glucose level). It is important for healthcare providers to be able to distinguish between hyperglycaemia and hypoglycaemia because hypoglycaemia worsens rapidly and constitutes a serious threat if action is not immediately taken. Table 48-10 compares the manifestations, causes, management and prevention of hyperglycaemia and hypoglycaemia.
TABLE 48-10 Comparison of hyperglycaemia and hypoglycaemia
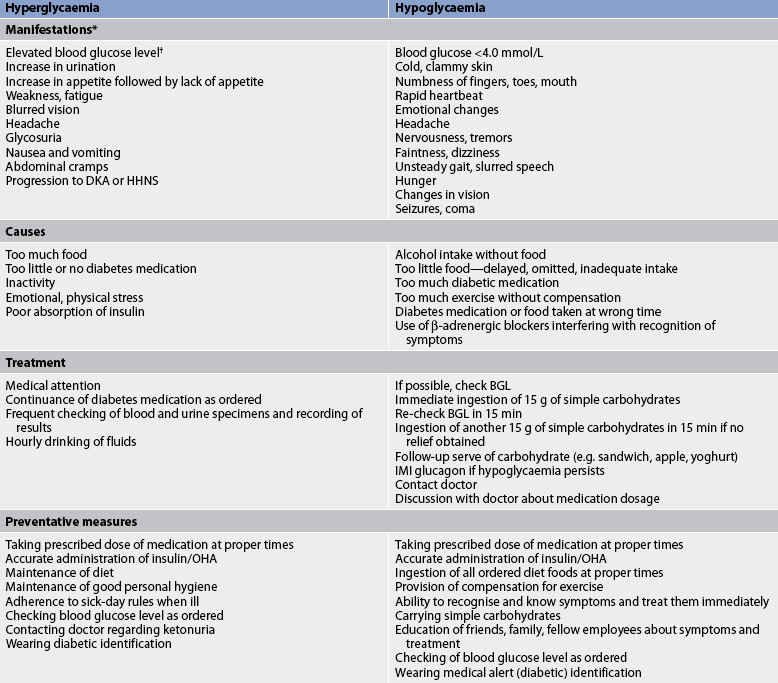
DKA, diabetic ketoacidosis; HHNS, hyperosmolar hyperglycaemic non-ketotic syndrome; IMI, intramuscular injection; OHA, oral hypoglycaemic agent.
* There is usually a gradual onset of symptoms in hyperglycaemia and a rapid onset in hypoglycaemia.
† Specific clinical manifestations related to elevated levels of blood glucose vary according to the patient.
Diabetic ketoacidosis
AETIOLOGY AND PATHOPHYSIOLOGY
Diabetic ketoacidosis (DKA), also referred to as diabetic acidosis and diabetic coma, is caused by a profound deficiency of insulin and is characterised by hyperglycaemia, ketosis, acidosis and dehydration. It is most likely to occur in people with type 1 diabetes but may be seen in type 2 diabetes in conditions of severe illness or stress when the pancreas cannot meet the extra demand for insulin. Precipitating factors include illness and infection, inadequate insulin dosage, undiagnosed type 1 diabetes, poor self-management and neglect.
When the circulating supply of insulin is insufficient, glucose cannot be used properly for energy, so the body breaks down fat stores as a secondary source of fuel (see Fig 48-12). Ketones are acidic by-products of fat metabolism that can cause serious problems when they become excessive in the blood. Ketosis alters the pH balance, causing metabolic acidosis to develop. Ketone bodies become problematic when they begin to be excreted in the urine. During this process, electrolytes become depleted as cations are eliminated along with the anionic ketones in an attempt to maintain electrolyte balance.
Insulin deficiency impairs protein synthesis and causes excessive protein degradation. This results in nitrogen losses from the tissues. Insulin deficiency also stimulates the production of glucose from amino acids (from proteins) in the liver and leads to further hyperglycaemia. But because there is a deficiency of insulin, the additional glucose cannot be used and the blood glucose level rises further, adding to the osmotic diuresis. Untreated, this leads to severe depletion of sodium, potassium, chloride, magnesium and phosphate. Vomiting caused by the acidosis results in more fluid and electrolyte losses. Eventually, hypovolaemia followed by shock will occur.
Renal failure may eventually occur from hypovolaemic shock. This causes the retention of ketones and glucose, and the acidosis progresses. Untreated, the patient becomes comatose as a result of dehydration, electrolyte imbalance and acidosis. If the condition is not treated, death is inevitable.
CLINICAL MANIFESTATIONS
Signs and symptoms of DKA include manifestations of dehydration, such as poor skin turgor, dry mucous membranes, tachycardia and orthostatic hypotension. Early symptoms may include lethargy and weakness. As the patient becomes severely dehydrated, the skin becomes dry and loose, and the eyeballs become soft and sunken. Abdominal pain is another symptom of DKA that may be accompanied by nausea and vomiting. Finally, Kussmaul respirations (rapid, deep breathing associated with dyspnoea) are the body’s attempt to reverse metabolic acidosis through the exhalation of excess carbon dioxide. Acetone is noted on the breath as a sweet, fruity odour. (See Ch 16 for a discussion of respiratory compensation of metabolic acidosis.) Laboratory findings include a blood glucose level above 14 mmol/L, arterial blood pH below 7.30, serum bicarbonate level less than 15 mmol/L, and ketones in the blood and urine.56
MULTIDISCIPLINARY CARE
Before the advent of self-monitoring of blood glucose levels, patients with DKA required hospitalisation for treatment. Today, hospitalisation may not be required if treated early. In instances where fluid and electrolyte imbalances are not severe and blood glucose levels can be safely monitored at home, less severe forms of DKA may be managed on an outpatient basis (see Box 48-8). However, other factors must be considered as to the location of where the patient is managed. These include the presence of fever, nausea, vomiting and diarrhoea; altered mental status; the nature of the cause of the ketoacidosis; and the availability of frequent communication with the healthcare provider (every few hours).
BOX 48-8 Diabetic ketoacidosis and hyperosmolar hyperglycaemic non-ketotic syndrome
MULTIDISCIPLINARY CARE
Regardless of the setting in which it occurs, DKA is a serious condition that proceeds rapidly and must be treated promptly. (See Table 48-11 for the emergency management of a patient with DKA.) Because fluid imbalance is potentially life-threatening, the initial goal of therapy is to establish intravenous access and begin fluid and electrolyte replacement. Typically, an infusion of 0.9% NaCl at a rate to restore urine output to 30–60 mL/h and to raise blood pressure constitutes the initial fluid therapy regimen. When blood glucose levels approach 12–14 mmol/L, 5% dextrose is added to each litre of fluid to prevent hypoglycaemia.56
The aim of fluid and electrolyte therapy is to replace extracellular and intracellular water and to correct deficits of sodium, chloride, bicarbonate, potassium, phosphate, magnesium and nitrogen. Early potassium replacement is essential because hypokalaemia is a significant cause of unnecessary and avoidable death during treatment of DKA. Although initial serum potassium levels may be normal or high, levels can rapidly decrease once therapy starts as insulin drives potassium into the cells, thus leading to life-threatening hypokalaemia.
IV insulin administration is therapy directed towards correcting hyperglycaemia and hyperketosis. Insulin therapy is withheld until fluid resuscitation is under way because insulin allows water to enter the cell along with glucose and can lead to a depletion of vascular volume. Initially a bolus of insulin is delivered, followed by a continuous infusion. Patients with DKA should be managed in the ICU.
Hyperosmolar hyperglycaemic non-ketotic syndrome
Hyperosmolar hyperglycaemic non-ketotic syndrome (HHNS) is a life-threatening syndrome that can occur in the patient with diabetes who is able to produce enough insulin to prevent DKA but not enough to prevent severe hyperglycaemia, osmotic diuresis and extracellular fluid depletion (see Fig 48-13). The main difference between HHNS and DKA is that the patient with HHNS usually has enough circulating insulin so that ketoacidosis does not occur. Because HHNS produces fewer symptoms in the earlier stages, blood glucose levels can climb quite high before the problem is recognised. The higher blood glucose levels increase serum osmolality and produce more severe neurological manifestations, such as somnolence, coma, seizures, hemiparesis and aphasia. HHNS often occurs in older patients with type 2 diabetes and is often related to impaired thirst sensation and/or a functional inability to replace fluids. There is usually a history of inadequate fluid intake, increasing mental depression and polyuria. Laboratory values in HHNS include a blood glucose level greater than 34 mmol/L and a marked increase in serum osmolality. Ketone bodies are absent or minimal in both blood and urine.
MULTIDISCIPLINARY CARE
HHNS constitutes a medical emergency and has a high mortality rate. Therapy is similar to that for the treatment of DKA and includes immediate IV administration of 0.9% NaCl at a rate that is dependent on cardiac status and the degree of fluid volume deficit.38 Regular insulin is given by IV bolus, followed by an infusion after fluid replacement therapy is instituted to aid in reducing the hyperglycaemia. When blood glucose levels fall to approximately 12–14 mmol/L, IV fluids containing 5% dextrose are administered to prevent hypoglycaemia. Electrolytes are monitored and replaced as needed.
Hypokalaemia is not as significant in HHNS as it is in DKA, although fluid losses may result in milder potassium deficits that require replacement. Vital signs, intake and output, tissue turgor, laboratory values and cardiac status are assessed to monitor the efficacy of fluid and electrolyte replacement. Patients with renal or cardiac compromise require special monitoring to avoid fluid overload during fluid replacement. This includes monitoring of serum osmolality and frequent assessment of cardiac, renal and mental status.
The management for both DKA and HHNS is similar, except that HHNS requires greater fluid replacement (see Table 48-11). Once the patient is stabilised, attempts to detect and correct the underlying precipitating cause should be initiated.
 NURSING MANAGEMENT: DIABETIC KETOACIDOSIS AND HYPEROSMOLAR HYPERGLYCAEMIC NON-KETOTIC SYNDROME
NURSING MANAGEMENT: DIABETIC KETOACIDOSIS AND HYPEROSMOLAR HYPERGLYCAEMIC NON-KETOTIC SYNDROME
When hospitalised, the patient is closely monitored with appropriate blood and urine tests. The nurse is responsible for monitoring blood glucose levels and urine for output and ketones, as well as using laboratory data to direct care.
Management requires careful monitoring of IV fluids to correct dehydration, administration of insulin therapy to reduce blood glucose and serum hydroxybutyrate levels, administration of electrolytes to correct electrolyte imbalance, assessment of renal status, assessment of cardiopulmonary status related to hydration and electrolyte levels, and monitoring of the level of consciousness.
The nurse must also monitor the signs of potassium imbalance resulting from low insulin levels and osmotic diuresis (see Ch 16). Treatment for hyperglycaemia is commenced with insulin, but there is a risk that serum potassium levels may decrease rapidly as potassium moves into the cells once insulin becomes available. Cardiac monitoring is necessary to detect hyperkalaemia and hypokalaemia as potassium excess or deficit is observable on electrocardiograph (ECG) tracings (see Ch 35). Vital signs should be assessed often to determine the presence of fever, hypovolaemic shock, tachycardia and Kussmaul respirations.
Hypoglycaemia
Hypoglycaemia, or low blood glucose levels, occurs when there is too much insulin in proportion to available glucose in the blood.57,58 This causes the blood glucose level to drop to less than 3.5 mmol/L. Because the brain requires a constant supply of glucose in sufficient quantities to function properly, hypoglycaemia can affect mental functioning. Common manifestations of hypoglycaemia include confusion, irritability, diaphoresis, tremors, hunger, weakness, headaches and visual disturbances. Manifestations of hypoglycaemia can mimic alcohol intoxication. Untreated hypoglycaemia can progress to loss of consciousness, seizures, coma and death.
Hypoglycaemic unawareness is a condition in which a person does not experience the warning signs and symptoms of hypoglycaemia, increasing their risk of dangerously low blood glucose levels. This is often related to autonomic neuropathy of diabetes, which interferes with the secretion of counter-regulatory hormones. Elderly patients and patients who use β-adrenergic blockers are also at risk of hypoglycaemic unawareness. It is usually not safe for patients with risk factors for hypoglycaemic unawareness to aim for tight blood glucose control because a major drawback of intensive treatment is hypoglycaemia. These patients are usually managed with blood glucose goals that are somewhat higher than those of patients who are able to detect and manage the onset of hypoglycaemia.
Hypoglycaemic symptoms may occur when a very high blood glucose level is brought down too rapidly (e.g. a blood glucose level of 16.7 mmol/L brought down quickly to 10 mmol/L). Although the blood glucose level is above normal by definition and measurement, the sudden metabolic shift can evoke hypoglycaemic symptoms. Too vigorous management of hyperglycaemia with insulin can induce this situation.
Causes of hypoglycaemia are often related to a mismatch in the timing of food intake and the peak action of insulin or OHAs that increase endogenous insulin secretion. The balance between blood glucose level and insulin can be disrupted by the administration of too much insulin or medication, the ingestion of too little food, delaying the time of eating and performing unusual amounts of exercise. Insulin reactions can occur at any time but most reactions occur when the OHA or insulin is at its peak of action or when the patient’s daily routine is disrupted without adequate adjustments in diet, medications and activity. Although hypoglycaemia is more common with insulin therapy, it can occur with OHAs and may be severe and persist for an extended time because of the longer duration of action.
 NURSING AND COLLABORATIVE MANAGEMENT: HYPOGLYCAEMIA
NURSING AND COLLABORATIVE MANAGEMENT: HYPOGLYCAEMIA
With prompt, effective treatment, hypoglycaemia can usually be reversed quickly. At the first sign of hypoglycaemia, the blood glucose level should be checked if possible (see Box 48-9). If it is below 3.5 mmol/L, the patient should immediately begin treatment for hypoglycaemia. If the blood glucose level is above 3.5 mmol/L, other causes of the signs and symptoms should be investigated. If the patient has manifestations of hypoglycaemia and monitoring equipment is not available, hypoglycaemia should be assumed and treatment should be initiated.
MULTIDISCIPLINARY CARE
Collaborative therapy
Conscious patient
Administer 15 g of quick-acting carbohydrate (e.g. 150 mL of regular soft drink, 8–10 jelly beans, 1 tbspn syrup or honey, 4 tspn jam, 120 mL juice, commercial glucose tablets [per label instructions])
Check blood glucose levels after 15 min
Repeat quick-acting carbohydrate after 15 min if no improvement in blood glucose levels
Administer additional food comprising longer-acting carbohydrate (e.g. slice of bread, dry biscuits) after symptoms subside
Immediately notify healthcare provider or emergency service (if patient outside hospital) if symptoms do not subside after two to three administrations of quick-acting carbohydrate
Hypoglycaemia is treated by ingesting 10–15 g of a simple (fast-acting) carbohydrate—for example, 150 mL of regular (non-diet) soft drink, or lollies such as 6–8 small jelly babies or jelly beans. Commercial products such as gels or tablets containing 15 g of glucose are convenient for carrying in a purse or pocket to be used in such situations.
Treatment with sweet foods that also contain fat, such as chocolate bars, biscuits and ice-cream, should be avoided because the high fat content will slow down the absorption of the sugar and delay the response to treatment. Over-treatment with large quantities of quick-acting carbohydrates should also be avoided so that a rapid fluctuation to hyperglycaemia does not occur. A prompt but moderate approach is best.
The blood glucose level should be checked about 15 minutes following the initial treatment for hypoglycaemia, and treatment should be repeated if the blood glucose level remains below 3.5 mmol/L. Once the blood glucose level is greater than 3.5 mmol/L, the patient should eat the regularly scheduled meal or a snack to prevent hypoglycaemia from recurring. Good snacks include a sandwich, a glass of milk, a piece of fruit, a tub of yoghurt or cheese and dry biscuits. The blood glucose level should also be checked again about 45 minutes after treatment to ensure that hypoglycaemia is not recurring.
If there is no significant improvement in the patient’s condition after two to three doses of 15 g of fast acting carbohydrate, or if the patient is not alert enough to swallow, 1 mg of glucagon may be administered by intramuscular (IM) or SC injection. An IM injection in a site such as the deltoid muscle will result in a quicker response. Glucagon stimulates the liver to convert glycogen to glucose, making glucose rapidly available. Rebound hypoglycaemia is a potential adverse effect of glucagon. Encouraging the patient to ingest a complex carbohydrate after recovery may prevent this from happening.
Once the acute hypoglycaemia has been reversed, the nurse should explore with the patient the reasons why the situation developed. This assessment may indicate the need for additional education to avoid future episodes of hypoglycaemia. The danger of hypoglycaemic reactions must be stressed, as memory and learning impairment can result from repeated episodes of severe hypoglycaemia.
Chronic complications of diabetes mellitus
Chronic complications of diabetes are primarily those of end-organ from damage to the large and small blood vessels secondary to chronic hyperglycaemia (see Fig 48-14). Several theories exist as to how and why chronic hyperglycaemia damages cells and tissues. Possible causes include: (1) the accumulation of damaging by-products of glucose metabolism, such as sorbitol, which is associated with damage to nerve cells; (2) the formation of abnormal glucose molecules in the basement membrane of small blood vessels, such as those that circulate to the eyes and kidneys; and (3) a derangement in RBC function that leads to a decrease in oxygenation to the tissues.
Two landmark studies in diabetes—the Diabetes Control and Complications Trial (DCCT) in type 1 diabetes and the United Kingdom Prospective Diabetes Study (UKPDS) in type 2 diabetes—have greatly influenced diabetes management since the 1990s.21,22,32 Both studies showed a reduced risk of microvascular complications by maintaining tight blood glucose control. The studies were randomised controlled trials in which patients were randomly assigned to one of two groups: intensive or standard treatment.
In the UKPDS, the average HbA1c in the intensive treatment group was around 7% and in the standard treatment group it was 9%. (The normal range for HbA1c in a person without diabetes is 3.5–6%). The intensive treatment group achieved a lower HbA1c than the standard treatment group and this finding has persisted over time despite a lapse back to suboptimal glycaemic control, suggesting that early intensive treatment may trigger the body’s metabolic memory, which is maintained over time. In addition, the results showed that patients in the intensive treatment group reduced their risk of developing retinopathy and nephropathy. The main adverse effect associated with intensive therapy was an increase in cases of severe hypoglycaemia. When determining specific targets for individual patients, the risk of severe or undetected hypoglycaemia as a side effect of tight glucose control must be taken into account.
The UKPDS showed that people newly diagnosed with diabetes who maintained tight blood glucose control (HbA1c 7.1% ±1.5%) significantly reduced the risk of complications. Each 1% reduction in mean HbA1c was associated with a risk reduction of 21% for any end point related to diabetes. In patients with type 2 diabetes, the risk of diabetic complications was strongly associated with previous hyperglycaemia. Any reduction in HbA1c is likely to reduce the risk of complications, with the lowest risks being found in those with HbA1c values closest to or in the normal range (<6%).23
The DCCT had similar findings in people with type 1 diabetes but the closer the HbA1c was to normal, the higher the risk of hypoglycaemia. Retinopathy worsened at near-normal levels (5.5–6.0%)
The ADVANCE, ACCORD and VADT studies were undertaken to investigate the effects of tight blood glucose control on macrovascular (in particular, cardiovascular) outcomes.59–62 The ACCORD study was halted before completion because of increased cardiovascular mortality when blood glucose was very tightly controlled (HbA1c <6.5%).63 The results from these three studies suggest that tight glycaemic control late in the course of type 2 diabetes offers little benefit for protection against cardiovascular disease.
The standards of diabetes care have improved considerably during the past decade due to the publication of these five studies. Reports from the UKPDS have helped establish that optimal treatment of diabetes requires not only attention to glucose control but also appropriate evaluation and management of cardiovascular risk.23 These studies have clearly shown that it is not only control of blood glucose, but also control of blood pressure and blood lipid levels, which helps prevent complications in people with type 1 or type 2 diabetes. The National Health and Medical Research Council in Australia has developed guidelines for the management of type 1 and type 2 diabetes based on the best available literature (see Resources on p 1395).
When setting targets for HbA1c, the following factors need to be considered:
• Asses the threshold for risk of cardiovascular disease if aiming for a HbA1c below 6%.
• Tight control for early stages of the disease in early treatment of diabetes reduces complications and mortality.
• Patients with cardiovascular disease or long duration of diabetes are at increased risk of cardiovascular disease mortality if aggressively treated.
• HbA1c ≤7% reduces microvascular complications, irrespective of the duration of the disease.
• Aggressive treatment needs to be balanced against the risk of hypoglycaemia.
What level of HbA1c is associated with fewer diabetes mellitus complications?
EVIDENCE-BASED PRACTICE
Clinical question
For patients with diabetes mellitus (P), does maintaining a HbA1c level <7% (I) compared to maintaining a level >7% (C) decrease microvascular and neuropathic complications (O)?
Critical appraisal and synthesis of evidence
• Studies included adults, older adults and children with type 1 and type 2 diabetes
• Assessed were blood glucose, HbA1c, blood lipids, blood pressure, vascular disease, obesity, dyslipidaemia, hypertension, nephropathy and quality of life
• Lowering HbA1c (to average of 7%) reduced microvascular and neuropathic complications
• Tighter glycaemic control (a normal HbA1c <6%) may further reduce complications but increases hypoglycaemia risk
Implications for nursing practice
• Counsel patients that recommendations are to maintain HbA1c at <7%.
• Individualise goals based on the patient’s health status.
• Urge patients to actively manage their blood glucose and HbA1c levels.
P, patient population of interest; I, intervention or area of interest; C, comparison of interest or comparison group; O, outcome(s) of interest.
Long-term complications can have devastating effects on the patient’s quality of life. People with diabetes require scheduled, ongoing monitoring for the detection and prevention of chronic complications. The recommendations for ongoing evaluation are listed in Table 48-12.45 It is imperative that patients understand the importance of participating in regular follow-up examinations, also known as the annual cycle of care.
TABLE 48-12 Prevention, detection and monitoring of long-term complications of diabetes mellitus*

* Based on the recommendations of the American Diabetes Association. ECG, electrocardiogram.
Angiopathy
Angiopathy, or blood vessel disease, is estimated to account for the majority of deaths among patients with diabetes. These chronic blood vessel dysfunctions are divided into two categories: macrovascular complications and microvascular complications.
MACROVASCULAR COMPLICATIONS
Macrovascular complications are diseases of the large and medium-sized blood vessels that occur with greater frequency and with an earlier onset in people with diabetes. Although atherosclerotic plaque formation is believed to have a genetic origin, its development seems to be promoted by the altered lipid metabolism common to diabetes. Tight glucose control may help delay the atherosclerotic process.21 Macrovascular diseases include cerebrovascular disease (stroke), cardiovascular disease (heart attack) and peripheral vascular disease. Although genetic make-up cannot be altered, a patient with diabetes can diminish other risk factors such as obesity, smoking, hypertension, high fat intake and sedentary lifestyle associated with macroangiopathy. Smoking, which is detrimental to health in general, is especially injurious to people with diabetes. Smoking significantly increases the risk of blood vessel disease in people with diabetes and increases the risk of cardiovascular disease, stroke and lower extremity amputation.
Insulin resistance seems to play an important role in the development of cardiovascular disease and is implicated in the pathogenesis of essential hypertension and alterations to serum lipid levels (dyslipidaemia). The term insulin resistance syndrome is applied to the clinical association of insulin resistance, hypertension, increased very-low-density lipoprotein (VLDL) concentrations and decreased HDL cholesterol concentrations. The role of insulin resistance in the pathogenesis of cardiovascular disease is not well understood but it seems to combine with dyslipidaemia in contributing to a greater risk of cardiovascular disease in patients with diabetes mellitus.22 All patients with diabetes should be screened for dyslipidaemia at the time that diabetes is diagnosed. These abnormalities typically include an elevated triglyceride level and reduced HDL cholesterol level.
MICROVASCULAR COMPLICATIONS
Microvascular complications result from thickening of the vessel membranes in the capillaries and arterioles in response to chronic hyperglycaemia. They differ from the macrovascular complications in that they are specific to diabetes. Although microangiopathy can be found throughout the body, the areas most noticeably affected are the eyes (retinopathy), the kidneys (nephropathy) and the skin (dermopathy). Thickening of the basement membrane has been found in some people with diabetes before or at the time of diagnosis or before the onset of symptoms of diabetes mellitus. However, clinical manifestations usually do not appear until 10–20 years after the onset of diabetes.
Diabetic retinopathy
AETIOLOGY AND PATHOPHYSIOLOGY
Diabetic retinopathy refers to the process of microvascular damage to the retina as a result of chronic hyperglycaemia in patients with diabetes. After 15 years with diabetes mellitus, nearly all patients with type 1 diabetes and 80% with type 2 diabetes will have some degree of retinal disease. Diabetic retinopathy is estimated to be the most common cause of new cases of blindness in people aged 20–74 years.64
Retinopathy can be classified as non-proliferative or proliferative. In non-proliferative retinopathy, the most common form, partial occlusion of the small blood vessels in the retina causes the development of microaneurysms in the capillary walls. The walls of these microaneurysms are so weak that capillary fluid leaks out, causing retinal oedema and eventually hard exudates or intraretinal haemorrhages. Vision may be affected if the macula is involved.
Proliferative retinopathy, the most severe form, involves the retina and the vitreous humour. When retinal capillaries become occluded, the body compensates by forming new blood vessels to supply the retina with blood, a pathological process known as neovascularisation. These new vessels are extremely fragile and haemorrhage easily, producing vitreous contraction. Eventually, light is prevented from reaching the retina as the vessels become torn and bleed into the vitreous cavity. The patient sees black or red spots or lines. If these new blood vessels pull the retina while the vitreous humour contracts, causing a tear, partial or complete retinal detachment will occur. If the macula is involved, vision is lost. Without treatment, more than half the patients with proliferative diabetic retinopathy will become blind.
MULTIDISCIPLINARY CARE
The earliest and most treatable stages of diabetic retinopathy often produce no changes in vision. Because of this, patients with diabetes must have regular dilated eye examinations by an ophthalmologist or a specially trained optometrist for early detection and treatment.
The most common forms of treatment for diabetic retinopathy are early photocoagulation of the retina, cryotherapy (cryopexy) and vitrectomy.
• Photocoagulation by laser destroys the ischaemic areas of the retina that produce growth factors that encourage neovascularisation.64 (Photocoagulation is discussed in Ch 21.)
• Cryotherapy (cryopexy) is sometimes used to treat peripheral areas of the retina that cannot be reached with lasers or when retinal haemorrhage prevents complete photocoagulation. In this procedure, topical anaesthesia is used so that a cryoprobe can be placed directly on the surface of the eye. When the probe is properly located, its tip creates a frozen area that extends through the external tissue and through the eyeball until it reaches a specific point on the retina. Multiple points on the retina can be treated in this way. (Cryotherapy is discussed in Ch 21.)
• Vitrectomy is the aspiration of blood, membrane and fibres from the inside of the eye through a small incision just behind the cornea. Vitrectomy is indicated when there is vitreal haemorrhage that does not clear in 6 months or when there is threatened or actual retinal detachment. (Vitrectomy is discussed in Ch 21.)
People with diabetes are also prone to other visual problems. Glaucoma occurs as a result of the occlusion of the outflow channels secondary to neovascularisation. This type of glaucoma is difficult to treat and often results in blindness. Cataracts develop at an earlier age and progress more rapidly in people with diabetes.
Nephropathy
Diabetic nephropathy is a microvascular complication associated with damage to the small blood vessels that supply the glomeruli of the kidneys. In Australia and New Zealand, diabetes is one of the most common causes of primary kidney disease.4,5 Risk factors for the development of diabetic nephropathy include hypertension, genetic predisposition, smoking and chronic hyperglycaemia. Results from the UKPDS have demonstrated that kidney disease can be significantly reduced when near-normal blood glucose control is achieved and maintained.21
Hypertension significantly accelerates the progression of diabetic nephropathy. Therefore, aggressive blood pressure management is indicated for all patients with diabetes. Angiotensin-converting enzyme (ACE) inhibitor drugs (e.g. lisinopril) are commonly prescribed for patients with diabetes because they are effective blood pressure-lowering agents with few side effects. In fact, ACE inhibitors are often prescribed for patients with diabetes even when they are not hypertensive, because drugs in this class have a protective effect on the kidneys that prevents the progression of diabetic nephropathy independent of hypertension control.65 Angiotensin II receptor antagonists (e.g. losartan) may also be used for their kidney-protective benefits. (See Ch 32 for a discussion of hypertension and Ch 46 for a discussion of renal failure.)
Standards for the prevention and detection of nephropathy in patients with diabetes include yearly screening for the presence of microalbuminuria (MAU) in the urine. This test detects kidney damage at an earlier stage than the standard dipstick test for urine protein. The presence of protein in the urine as detected by MAU urinalysis should be followed-up with an albumin/creatinine ratio or 24-hour urine collection for determination of the creatinine clearance and serum creatinine level.
Neuropathy
Diabetic neuropathy is nerve damage that occurs because of the metabolic derangements associated with diabetes mellitus. About 60–70% of patients with diabetes have some degree of neuropathy, with neurological complications occurring equally in type 1 and type 2 diabetes.66 The most common type of neuropathy affecting people with diabetes is sensory neuropathy. This can lead to the loss of protective sensation in the lower extremities and, coupled with other factors, this significantly increases the risk of complications that result in a lower limb amputation.
AETIOLOGY AND PATHOPHYSIOLOGY
The pathophysiological processes of diabetic neuropathy are not well understood. Several theories exist, including metabolic, vascular and autoimmune elements. The prevailing theory suggests that persistent hyperglycaemia leads to an accumulation of sorbitol and fructose in the nerves, which causes damage by an unknown mechanism destroying the myelin sheath that protects the nerve. The result is reduced nerve conduction and demyelination. Ischaemia in blood vessels damaged by chronic hyperglycaemia that supply the peripheral nerves has also been implicated in the development of diabetic neuropathy.
CLASSIFICATION
The two major categories of diabetic neuropathy are sensory neuropathy, which affects the peripheral nervous system, and autonomic neuropathy. Each of these types can take on several forms.
Sensory neuropathy
The most common form of sensory neuropathy is distal symmetrical neuropathy, which affects the hands and/or feet bilaterally. This is sometimes referred to as ‘stocking–glove neuropathy’. Characteristics of distal symmetrical neuropathy include loss of sensation, abnormal sensations, pain and paraesthesias. The pain, which is often described as burning, cramping, crushing or tearing, is usually worse at night and may occur only at that time. The paraesthesias may be associated with tingling, burning and itching sensations. The patient may report a feeling of walking on pillows or numb feet. At times the skin becomes so sensitive (hyperaesthesia) that even light pressure from bed sheets cannot be tolerated. Complete or partial loss of sensitivity to touch and temperature is common. Foot injury and ulcerations can occur without the patient ever having pain (see Figs 48-15 and 48-16). Neuropathy can also cause atrophy of the small muscles of the hands and feet, causing deformity and limiting fine movement.
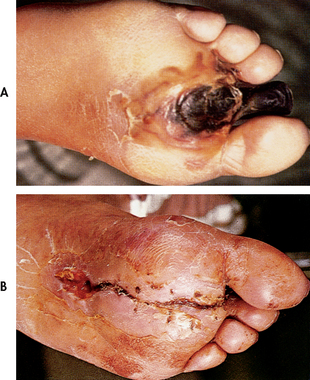
Figure 48-16 The necrotic toe developed as a complication of diabetes. A, Before amputation. B, After amputation.
Control of blood glucose levels is the only treatment for diabetic neuropathy. It is effective in many, but not all, cases. Drug therapy may be used to treat neuropathic symptoms, particularly pain. Medications commonly used include topical creams (e.g. capsaicin), tricyclic antidepressants (e.g. amitriptyline) and antiseizure medications (e.g. gabapentin). Capsaicin is a moderately effective topical cream made from chilli peppers. It depletes the accumulation of pain-mediating chemicals in the peripheral sensory neurons. The cream is applied three to four times a day. There is usually an increase in symptoms at the start of therapy, which is followed by relief of pain in 2–3 weeks. Tricyclic antidepressants are also moderately effective in treating the symptoms of diabetic neuropathy. They work by inhibiting the reuptake of noradrenaline or serotonin, neurotransmitters that are believed to play a role in the transmission of pain through the spinal cord. Although gabapentin has been found to be effective in treating the pain of diabetic neuropathy, its mechanism of action is not well understood.67
Autonomic neuropathy
Autonomic neuropathy can affect nearly all body systems and lead to hypoglycaemic unawareness, faecal incontinence and diarrhoea, and urinary retention. Delayed gastric emptying (gastroparesis) is a complication of autonomic neuropathy that can produce anorexia, nausea, vomiting, gastro-oesophageal reflux and persistent feelings of fullness. Gastroparesis can trigger hypoglycaemia by delaying food absorption. Cardiovascular abnormalities associated with autonomic neuropathy are postural hypotension, resting tachycardia and painless myocardial infarction. A patient with postural hypotension should be instructed to change slowly from a lying or sitting position.
Diabetes can affect sexual function in men and women. Erectile dysfunction associated with diabetes mellitus is believed to result from damage to the sacral parasympathetic nerves. Determining whether this problem is of organic or psychological origin is an important part of the assessment. Decreased libido is a problem in some women with diabetes. Candida and non-specific vaginitis are also common. Organic impotence or sexual dysfunction in either the male or the female patient requires sensitive therapeutic counselling for both the patient and their partner. (See Ch 54 for a further discussion of erectile dysfunction.)
A neurogenic bladder may develop as sensation in the inner bladder wall decreases, causing urinary retention. A patient with retention has infrequent voiding, difficulty in voiding and a weak stream of urine. Emptying the bladder every 3 hours in a sitting position helps prevent stasis and subsequent infection. Tightening the abdominal muscles during voiding and using the Credé manoeuvre (mild massage downwards over the lower abdomen and bladder) may also help with complete bladder emptying. Cholinergic agonist drugs, such as bethanechol, may be used. The patient may also have to learn self-catheterisation.
Complications of the feet and lower extremities
Among people with known and newly diagnosed diabetes, 19.6% are at risk of foot ulcer; those with the greatest risk are those who have been diagnosed for 10 years or more.11 The development of diabetic foot complications is a multifactorial process. They result from a combination of microvascular and macrovascular diseases that place the patient at risk of injury and serious infection that may lead to amputation. Sensory neuropathy and peripheral vascular disease (PVD) are risk factors, and clotting abnormalities, impaired immune function and autonomic neuropathy also play important roles. Smoking is deleterious to the health of lower extremity blood vessels and increases the risk of amputation.
PVD increases the risk of amputation by causing a reduction in blood flow to the lower extremities. When blood flow is decreased, oxygen, white blood cells and vital nutrients are not available to the tissues. Therefore, wounds take longer to heal and the risk of infection increases. Signs of PVD include intermittent claudication, pain at rest, cold feet, loss of hair, delayed capillary filling and dependent rubor (redness of the skin that occurs when the extremity is in a dependent position). The disease is diagnosed by history, Doppler ultrasound findings and angiography. Management includes control or reduction of risk factors, particularly smoking, high cholesterol intake and hypertension. Bypass or graft surgery is indicated in some patients. Proper care of the feet is essential for patients with PVD: guidelines for patient teaching are listed in Box 48-10.
PATIENT & FAMILY TEACHING GUIDE
1. Wash feet daily with a mild soap and warm water. Test water temperature with hands first.
2. Pat feet dry gently, especially between the toes.
3. Examine feet daily for cuts, blisters, swelling and red, tender areas. Do not depend on feeling sores. If eyesight is poor, have others inspect feet.
4. Use lanolin on feet to prevent skin from drying and cracking. Do not apply between toes.
5. Use mild foot powder on sweaty feet.
6. Do not use commercial remedies to remove calluses or corns.
7. Clean cuts with warm water and mild soap, covering with clean dressing. Do not use iodine, rubbing alcohol or strong adhesives.
8. Report skin infections or non-healing sores to healthcare provider immediately.
9. Cut toenails evenly with rounded contour of toes. Do not cut down corners. The best time to trim nails is after a shower or bath.
10. Separate overlapping toes with cottonwool or lamb’s wool.
11. Avoid open-toe, open-heel and high-heel shoes. Leather shoes are preferred to plastic ones. Wear slippers with soles. Do not go barefoot. Shake out shoes before putting on.
12. Wear clean, absorbent (cotton or wool) socks or stockings that have not been mended. Coloured socks must be colourfast.
13. Do not wear clothing that leaves impressions, hindering circulation.
14. Do not use hot-water bottles or heating pads to warm feet. Wear socks for warmth.
16. Exercise feet daily either by walking or by flexing and extending feet in suspended position. Avoid prolonged sitting, standing and crossing of legs.
Sensory neuropathy is a major risk factor for lower extremity amputation in patients with diabetes. Loss of protective sensation (LOPS) often prevents the patient from becoming aware that a foot injury has occurred. Improper footwear and injury from stepping on foreign objects while barefoot are common causes of undetected foot injury in people with LOPS. Because the primary risk factor for lower extremity amputation is LOPS, annual screening using a monofilament is an extremely important preventative measure. This is done by applying a thin, flexible filament to several spots on the plantar surface of the foot, with the patient looking away, and asking the patient to report whether or not they can feel it. Insensitivity to a 5.07 Semmes-Weinstein monofilament has been shown to greatly increase the risk of diabetic foot ulcers that can lead to amputation. If the patient has LOPS, aggressive measures must be taken to teach the patient how to prevent foot ulceration. These measures include selecting proper footwear (including prescription shoes), avoiding injury to the feet, practising diligent skin and nail care, inspecting the feet thoroughly each day and treating small problems promptly.
The Doppler ultrasound instrument is used to diagnose the presence or degree of PVD. Similar to an electronic stethoscope, this device amplifies sound. The procedure is non-invasive and can measure blood pressure in the lower extremities and blood flow velocity. It can indicate areas of stenosis or occlusion and is useful as an indicator of the need for additional vascular tests.
Neuropathic arthropathy, or Charcot’s foot, results in ankle and foot changes that ultimately lead to joint dysfunction and footdrop. These changes occur gradually and promote an abnormal distribution of weight over the foot, further increasing the chances of developing a foot ulcer as new pressure points emerge. Neuropathic ulcers resemble a ‘punched out’ wound and are usually painless. Infection is a danger and necessitates the long-term use of antibiotics and weeks of avoidance of weight bearing on the affected limb.
Integumentary complications
Diabetic dermopathy is a skin disorder characterised by brown shin spots located on the anterior surfaces of the lower extremities. It is thought to develop from leakage of small blood vessels into the skin, attributed to microangiopathy. The spots are harmless and painless and initially measure less than 1 cm in diameter.
Necrobiosis lipoidica diabeticorum is another skin disorder believed to be the result of the breakdown of collagen in the skin. It usually appears as reddish–yellow lesions, with atrophic skin that becomes shiny and transparent, revealing tiny blood vessels under the surface. The lesions may develop into sores, so special care must be taken to protect affected areas from injury and ulceration. This condition is not common but it may appear before other clinical signs or symptoms of diabetes and is more frequently seen in young women.
Xanthoma is characterised by small, raised yellowish lipid-laden lesions on the surface of the skin. It is common in patients who have hyperlipidaemia.68–70
Infection
Patients with diabetes are more susceptible to infections than other patients. The mechanisms for this phenomenon include a defect in the mobilisation of inflammatory cells and an impairment of phagocytosis by neutrophils and monocytes. Recurring or persistent infections such as Candida albicans, as well as boils and furuncles, in the undiagnosed patient often lead the healthcare provider to suspect diabetes. Loss of sensation (neuropathy) may delay the detection of an infection.
Persistent glycosuria may predispose to bladder infections, especially in patients with a neurogenic bladder. Decreased circulation resulting from angiopathy can prevent or delay the immune response. Antibiotic therapy has reduced infection from being a major cause of death in diabetes patients. Treatment of infections must be prompt and vigorous.
Gerontological considerations: diabetes mellitus
Diabetes is more prevalent in older populations. A major reason for this is that the process of ageing involves insulin resistance and glucose intolerance, which are believed to be precursors to type 2 diabetes.71 Ageing is also associated with a number of conditions that are more likely to be treated with medications that impair insulin action (e.g. corticosteroids, antihypertensives, phenothiazines). Undiagnosed and untreated diabetes is more common in the elderly, partly because many of the normal physiological changes of ageing resemble those of diabetes, such as visual changes and decreased glomerular filtration.
Although good glycaemic control is important to people of all ages with diabetes, several factors are taken into account when determining glycaemic goals for older adults. One is that hypoglycaemic unawareness is more common in this age group, making these patients more likely to suffer adverse consequences from blood glucose-lowering therapy. They may also have delayed psychomotor function that could interfere with the ability to treat hypoglycaemia. Other factors to consider in establishing glycaemic goals for the older patient include the patient’s own desire for treatment and whether the patient has other coexisting medical problems, such as cognitive impairment. Although it is generally agreed that treatment is indicated for older adults with diabetes to prevent acute complications and avoid unpleasant symptoms, strict glycaemic control may be difficult to achieve.62
As with any group, diet and exercise are recommended as therapy for older adults with diabetes. This should take into account functional limitations that may interfere with physical activity and the ability to prepare meals. Because of the physiological changes that occur with ageing, the therapeutic outcome for the older adult with diabetes who receives OHAs may be altered. The second-generation sulfonylurea drugs (e.g. glipizide) are usually well tolerated and have increased potency and appear to have fewer side effects and fewer drug interaction problems when compared with the first-generation agents. Other OHAs described earlier in this chapter may also be used in older patients with diabetes. Insulin therapy may be instituted if OHAs fail. However, it is important to recognise that elderly patients are more likely to have limitations in manual dexterity and visual acuity, which will affect accurate insulin administration.
Patient teaching should be based on the individual’s needs, using a slower pace with simple printed or audio materials. It is important to include family or a support person in the teaching. Patient education issues for the older patient include those related to vision, mobility, mental status, functional ability, financial and social situation, the effect of multiple medications, eating habits, the potential for undetected hypoglycaemia and quality-of-life issues.62
The patient with diabetic ketoacidosis
CASE STUDY
Patient profile
John Adams, a 34-year-old diabetic, was admitted to the emergency department after he was found comatose at home by his wife.
Subjective data (provided by wife)
• Was diagnosed with diabetes mellitus 12 months ago
• Was taking 48 U of insulin daily: 12 U of regular insulin plus 20 U of isophane (NPH) before breakfast, 8 U of regular insulin before dinner and 8 U of NPH at bedtime
• Has a history of flu for 1 week with vomiting and anorexia
• Stopped taking insulin 2 days ago when he was unable to eat
CRITICAL THINKING QUESTIONS
1. Briefly explain the pathophysiology of the development of diabetic ketoacidosis (DKA) in this patient.
2. What clinical manifestations of DKA does this patient exhibit?
3. What factors precipitated this patient’s DKA?
4. What distinguishes this case history from one of hyperosmolar hyperglycaemic non-ketotic syndrome or hypoglycaemia?
5. What teaching should be done with this patient and his family?
6. What role could the patient’s wife have in the management of his diabetes?
7. Based on the assessment data presented, write one or more appropriate nursing diagnoses. Are there any collaborative problems?
1. The polydipsia and polyuria related to diabetes mellitus are primarily caused by:
2. When a patient with type 2 diabetes mellitus is admitted to the hospital with pneumonia, the nurse recognises that the patient:
3. Effective collaborative management of diabetes includes:
4. The nurse assists the patient with nutritional therapy of diabetes with the knowledge that a healthy eating plan is designed:
5. In teaching ‘survival skills’ to a newly diagnosed type 1 diabetic, the nurse includes information about:
6. An appropriate teaching measure related to care of the feet for the patient with diabetes mellitus is to:
7. A diabetic patient has a serum glucose level of 45.7 mmol/L and is unresponsive. Following assessment of the patient, the nurse suspects diabetic ketoacidosis rather than hyperosmolar hyperglycaemic non-ketotic syndrome based on the finding of:
8. Which of the following is not an appropriate therapy for patients with diabetes mellitus?
1 World Health Organization (WHO). Diabetes facts & figures. Available at www.who.int/diabetes/facts/en/index.html. accessed 15 September 2010.
2 Magliano DJ, Peeters A, Vos T, Sicree R, Shaw J, Sindall C, Haby M, Begg SJ, Zimmet PZ. Projecting the burden of diabetes in Australia: what is the size of the matter? Aust New Zeal J Publ Health. 2009;33(6):540–543.
3 van Dieren S, Beulens J, van der Schouw YT, Grobbee DE, Neal B. The global burden of diabetes and its complications: an emerging pandemic, Eur J Cardiovasc Prev Rehabil. 2010;17:S3–S8. Available at http://journals.lww.com/ejcpr/Fulltext/2010/05001/The_global_burden_of_diabetes_and_its.2.aspx accessed 3 January 2011.
4 New Zealand Ministry of Health (NZMOH). Diabetes surveillance population-based estimates and projections for New Zealand, 2001–2011. Public Health Intelligence Occasional Bulletin no. 46. Available at www.moh.govt.nz/moh.nsf/indexmh/diabetes-suveillance-population-estimates-projections-2001-2011?Open, September 2007. accessed 4 January 2011.
5 American Diabetes Association. Total prevalence of diabetes and prediabetes. Available at www.diabetes.org, 2009. accessed 3 January 2011.
6 American Diabetes Association. Position statement: the diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(1):S62–S69.
7 Aronoff S, Bekowitz K, Shreiner B. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectrum. 2004;17(3):183–190.
8 Wassink AM, Van Der Graaf Y, Soedamah-Muthu SS, et al. Metabolic syndrome and incidence of type 2 diabetes in patients with manifest vascular disease. Diab Vasc Dis Res. 2008 Jun;5(2):114–122.
9 Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428.
10 Craft J, Gordon C. Diabetes mellitus. In: Craft J, Gordon C, Tiziani A, eds. Understanding pathophysiology. Sydney: Elsevier, 2011.
11 National Institute for Clinical Excellence. Type 2 diabetes: prevention and management of foot problems. Available at www.nice.org.uk/nicemedia/pdf/CG010 NICEguideline.pdf, Updated 2010. accessed 19 December 2010.
12 International Diabetes Federation. Diabetes in the young: a global perspective. In: Diabetes atlas. Available from www.diabetesatlas.org/content/diabetes-young-global-perspective. accessed 13 April 2011.
13 Australian Bureau of Statistics (ABS). Health of children in Australia: a snapshot, 2004–05. Canberra: ABS; 2008. Available at www.abs.gov.au/AUSSTATS/abs@.nsf/mf/4829.0.55.001/ accessed 4 January 2011.
14 Eppens MC, Craig ME, Cusumano J, Hing S, Chan A, Howard N, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes, Diabetes Care. 2006;29(6):1300–1306. Available at http://care.diabetesjournals.org/content/29/6/1300.full.pdf+html accessed 4 January 2011.
15 Hoffman L, Nolan C, Wilson D, Oats J, Simmons D. Australasian Diabetes in Pregnancy Society: gestational diabetes mellitus management guidelines. Med J Aust. 2004;181(6):342.
16 The International Association of Diabetes and Pregnancy Study Group Consensus Panel. Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682.
17 The HAPO Study Cooperative Research Group. Hyperglycaemia and adverse pregnancy outcome. N Engl J Med. 2008;358(19):1991–2002.
18 McLean M, Chipps D, Wah Cheung N. Mother to child transmission of diabetes mellitus: does gestational diabetes program type 2 diabetes in the next generation? Diabetic Medicine. 2006;23:1213–1215.
19 Ben-Haroush A, Yogev Y, Hod M. Epidemiology of gestational diabetes mellitus and its association with type 2 diabetes. Diabetic Medicine. 2003;21:103–113.
20 McIntyre HD, Cheung NW, Oats JJ, Simmons D. Gestational diabetes mellitus: from consensus to action on screening and treatment. Med J Aust. 2005;183(6):288–289.
21 Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. Diabetes Control and Complications Trial, N Engl J Med. 1993;329(14):977–986. Available at http://diabetes.niddk.nih.gov/dm/pubs/control/ accessed 19 December 2010. (Seminal text)
22 Bloomgarden ZT. Third Annual World Congress on the Insulin Resistance Syndrome: atherothrombotic disease. Diabetes Care. 2006;29(8):1973–1980.
23 UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352:837–853. [Erratum, Lancet 1999;354.] Available at www.dtu.ox.ac.uk/ukpds/index.php accessed 19 December 2010.
24 Neumiller J, Oedegard PS, Wysham CH. Update on insulin management in type 2 diabetes, Diabetes Spectrum. 2009;22(2):85–91. Available at http://spectrum.diabetesjournals.org/content/22/2/85.full.pdf+html accessed 4 January 2011.
25 National Health and Medical Research Council (NHMRC). The Diabetes Unit, Menzies Centre for Health Policy national evidence based guidelines for patient education in type 2 diabetes. Sydney: NHMRC, Diabetes Australia, University of Sydney; 2009. Available at www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/di16-diabetes-patient-education.pdf accessed 4 January 2011.
26 Aventis Pharmaceuticals. Lantus [insulin glargine] prescribing information. Available at http://products.sanofi-aventis.us/lantus/lantus.html. accessed 20 December 2010.
27 Institute for Clinical Systems Improvement. Health care order set: subcutaneous insulin management. 5th edn. Available at www.icsi.org/insulin_management__order_set_/insulin_management__subcutaneous_order_set__pdf_.html, July 2010. accessed 4 January 2011.
28 Australian Diabetes Educators Association. National standards for the development and quality assessment of services initiating insulin in the ambulatory setting. Available at www.chpcp.org/resources/Prioritizing%20clients%20with%20diabetes%2031-8-08%20(1).pdf, 2008. accessed 4 January 2011.
29 Australian Diabetes Educators Association. Position statement: use of subcutaneous (S/C) insulin delivery devices. Available at www.adea.com.au/main/forhealthprofessionals/positionstatements/ourpositionstatements, 2006. accessed 19 December 2010.
30 Battelino T. Risk and benefits of continuous subcutaneous insulin infusion (CSII) treatment in school children and adolescents. Pediatr Diabetes. 2006;7(suppl 4):20–24.
31 White R. Insulin pump therapy (continuous subcutaneous insulin infusion). Prim Care. 2007;34:4.
32 The Boden Institute of Obesity, Nutrition and Exercise. University of Sydney national evidence based guideline for blood glucose control in type 2 diabetes. Sydney: NHMRC, Diabetes Australia, University of Sydney; 2009. Available at www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/di19-diabetes-blood-glucose-control.pdf accessed 4 January 2011.
33 Lepore G, Dodesini AR, Nosari I, Trevisan R. Both continuous subcutaneous insulin infusion and a multiple daily injection regimen with glargine as basal insulin are equally better than traditional multiple daily injection treatment. Diabetes Care. 2003;26(4):1321–1322.
34 Shanik MH, Xu Y, Skrha J, et al. Insulin resistance and hyperinsulinemia: is hyperinsulinemia the cart or the horse? Diabetes Care. 2008;31(suppl 2):S262–S268.
35 Bolli GB, Gerich JE. The ‘dawn phenomenon’—a common occurrence in both non-insulin-dependent and insulin-dependent diabetes mellitus. New Engl J Med. 1984;310(12):746–750. Seminal text
36 Campbell PJ, Bolli GB, Cryer PE. Pathogenesis of the dawn phenomenon in patients with insulin-dependent diabetes mellitus. Accelerated glucose production and impaired glucose utilization due to nocturnal surges in growth hormone secretion. N Engl J Med. 1985;312(23):1473–1479. Seminal text
37 Harmel A, Mathur R, Davidson M. Fasting hyperglycaemia. In: Diabetes mellitus: diagnosis and treatment. Philadelphia: Saunders; 2004:270.
38 Lehne RA. Pharmacology for nursing care, 7th edn. St Louis: Mosby, 2007.
39 Nisbet JC, Sturtevant JM, Prins JB. Metformin and serious adverse effects. Med J Aust. 2004;180(2):53–54.
40 Kormajda M, McMurray J, Beck-Neilson H, et al. Heart failure events with rosiglitazone in type 2 diabetes: data from the RECORD clinical trial. Eur Heart J. 2010;31:824–831.
41 MIMS Online. Byetta. Available at https://www-mimsonline-com-au.cknservices.dotsec.com/Search/AbbrPI.aspx?ModuleName=Product%20Info&searchKeyword=byetta&PreviousPage=∼/Search/QuickSearch.aspx&SearchType=&ID=78450001_2. accessed 30 September 2010.
42 MIMS Online. Januvia. Available at https://www-mimsonline-com-au.cknservices.dotsec.com/Search/QuickSearch.aspx?ModuleName=Product%20Info&searchKeyword=Januvia. accessed 30 September 2010.
43 Australian Diabetes Council. Living with diabetes. Available at www.australiandiabetescouncil.com/Footer/Sitemap.aspx, 2010. accessed 19 December 2010.
44 www.health.qld.gov.au/chronicdisease/documents/pub_bpg_t2d_diet.pdf accessed April 2011.
45 Australian Diabetes Educators Association. Joint statement on the role of accredited practicing dieticians and diabetes educators in the delivery of nutrition and diabetes self-management education services for people with diabetes. Available at www.docstoc.com/docs/34640395/joint-statement-on-the-role-of-accredited-practising-dietitians, 2005. accessed 19 December 2010.
46 US National Library of Medicine, National Institutes of Health. Diabetes meal planning. Available at www.nlm.nih.gov/medlineplus/tutorials/diabetesmealplanning/htm/index.htm. accessed 4 January 2011.
47 Australian Diabetes Educators Association. Position statement: use of blood glucose meters. Available at www.adea.com.au/asset/view_document/979316083, 2008. accessed 19 December 2010.
48 Australian Diabetes Council. Guidelines for sick day management for the person with type 1 diabetes. Available at www.australiandiabetescouncil.com/About-Diabetes/Types-of-diabetes/Type-1/Sick-day-management.aspx, 2010. accessed 4 January 2011.
49 O’Kane M, Bunting B, Copland M. Efficacy of self-monitoring of blood glucose in patients with newly diagnosed type 2 diabetes (ESMON study): randomised controlled trial. BMJ. 2008;336:1174.
50 Farmer A, Wade A, Goyder E. Impact of self-monitoring of blood glucose in the management of patients with non-insulin treated diabetes: open parallel group randomised trial. BMJ. 2007;335:132.
51 Davis W, Bruce D, Davis T. Is self blood glucose monitoring appropriate for all type 2 diabetic patients? The Fremantle Diabetes Study. Diabetes Care. 2006;28(8):1764–1770.
52 Lowe J. Self monitoring of blood glucose: a systematic review. Australian Prescriber. 2010;33:138–140.
53 Burge M, Mitchell S, Sawyer A, et al. Continuous glucose monitoring: the future of diabetes management. Diabetes Spectum. 2008;21(2):112–119.
54 O’Connell PJ, Hawthorne WJ, Holmes-Walker DJ, et al. Clinical islet transplantation in type 1 diabetes mellitus: results of Australia’s first trial. Med J Aust. 2006;184(5):221–225.
55 Victorian Government Health Information, Community Health. Diabetes self-management-guidelines for providing services to people newly diagnosed with type 2 diabetes. Available at www.health.vic.gov.au/communityhealth/publications/diabetes.htm, March 2007. accessed 4 January 2011.
56 Rucker DW. Diabetic ketoacidosis. eMedicine. Available at http://emedicine.medscape.com/article/766275-overview, Updated 4 June 2010. accessed 4 January 2011.
57 Brisco V, Davis S. Hypoglycaemia in type 1 & type 2 diabetes: physiology, pathophysiology and management. Clin Diabetes. 2006;24(3):115–121.
58 Clarke W, Jones T, Rewers A, et al. Assessment and management of hypoglycemia in children and adolescents with diabetes. Paed Diabetes. 2009;10(12):134–145.
59 Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC, Jr., et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–2559.
60 ACCORD Study Group. Effects of intensive blood pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1575–1585.
61 ADVANCE Study Group. A summary of the ADVANCE Trial. Diabetes Care. 2009;32:S357–S361.
62 Duckworth W, et alVADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Eng J Med. 2009;360:129–139.
63 Nilsson P. ACCORD and risk factor control in type 2 diabetes. N Engl J Med. 2010;362:1628–1630.
64 Juvenile Diabetes Research Foundation. Clinical trial results are promising for diabetic eye disease. Available at www.jdrf.org.au/blog/2010/08/09/clinical-trial-results-are-promising-for-diabetic-eye-disease, August 2010. accessed 19 December 2010.
65 Strippoli GF, Craig M, Schena FP, Craig JC. Antihypertensive agents for primary prevention of diabetic nephropathy. J Am Soc Nephrol. 2005;16(10):3081–3091.
66 Juvenile Diabetes Research Foundation. Complications of diabetes. Available at www.jdrf.org.au/living-with-type-1-diabetes/type-1-diabetes-complications, 2010. accessed 19 December 2010.
67 Quann D. Diabetic neuropathy. eMedicine. Available at http://emedicine.medscape.com/article/1170337-overview, Updated 11 June 2010. accessed 4 January 2011.
68 Nakajima T, Tanemura A, Inui S, Katayama I. Venous insufficiency in patients with necrobiosis lipoidica. J Dermatol. 2009;36(3):166–169.
69 Oumeish OY. Skin disorders in patients with diabetes. Clin Dermatol. 2008;26(3):235–242.
70 Xanthoma. Nurs Times. 2006;102(26):25.
71 Australian Diabetes Educators Association. Guidelines for the management and care of diabetes in the elderly. Available at www.adea.com.au/asset/view_document/979316043, 2006. accessed 19 December 2010.
American Diabetes Association. www.diabetes.org
Australian Diabetes Society. www.diabetessociety.com.au
Australian Diabetes Educators Association. www.adea.com.au
Australasian Diabetes in Pregnancy Society. www.adips.org
Australasian Society for the Study of Obesity. www.asso.org.au
Diabetes Australia. www.diabetesaustralia.com.au
Diabetes New Zealand. www.diabetes.org.nz
Juvenile Diabetes Research Foundation Australia. www.jdrf.org.au
International Diabetes Federation. www.idf.org/home
National Health and Medical Research Council (Australia): diabetes guidelines. www.nhmrc.gov.au/_files_nhmrc/file/publications/synopses/cp102.pdf and www.nhmrc.gov.au/publications/synopses/di7todi13syn.htm
New Zealand Guidelines Group. www.nzgg.org.nz