Chapter 32 NURSING MANAGEMENT: hypertension
1. Describe the mechanisms involved in the regulation of blood pressure.
2. Identify the pathophysiological mechanisms associated with primary hypertension.
3. Evaluate the clinical manifestations and complications of hypertension.
4. Describe strategies for the prevention of primary hypertension.
5. Explore the multidisciplinary care for hypertension, including drug therapy and lifestyle modifications.
6. Explore the multidisciplinary care of the older adult with hypertension.
7. Describe the nursing management of the patient with hypertension, emphasising patient education.
8. Explain the clinical manifestations and multidisciplinary care of a hypertensive crisis.
Hypertension, or high blood pressure (BP), is defined as persistent systolic blood pressure (SBP) greater than or equal to 140 mmHg, diastolic blood pressure (DBP) greater than or equal to 90 mmHg, or current use of antihypertensive medication.1 Hypertension means that the heart is working harder than normal, putting both the heart and the blood vessels under strain. There is a direct relationship between hypertension and cardiovascular disease (CVD). There is a proportional increase in the risk of myocardial infarction, heart failure, stroke and renal disease with higher BP.1
Hypertension is an important medical and public health issue. It exists worldwide at epidemic rates, affecting an estimated 1.2 billion people. At least 3.7 million Australians over the age of 25 have hypertension or are on medication for that condition.2 The prevalence of hypertension among Australians over 25 is 32% of men and 27% of women, and among New Zealanders over 15 it is 22% of men and 18% of women.2,3
The prevalence of hypertension increases with age; people who do not have hypertension at age 55 have a 90% chance of developing it later in life.4 An SBP of >140 mmHg is a more important cardiovascular risk factor for developing hypertension than an elevated DBP in individuals older than 50 years.1 Hypertension is two to three times more prevalent among Indigenous Australians and Māori and Pacific Islander peoples, where it appears at a younger age and is more often undiagnosed and poorly controlled.5,6
CVD is the number one cause of death in women in Australia and New Zealand and in most of the developed countries of the world.2–4 Despite this, when surveyed, only one in four Australian women recognised that CVD is the leading cause of death in women.7 While the Heart Foundations of New Zealand and Australia have much useful information on their web pages,5,8 they do not specifically focus on women. However, the American Heart Association has published a set of evidence-based guidelines specifically for the prevention of CVD in women,9 which focus on lifestyle recommendations and major risk factor interventions that can be used as an educational tool.
The status of hypertension control has improved considerably over the past 20 years. Large-scale education programs provided by national organisations have increased awareness of hypertension. The Heart Foundations of Australia and New Zealand have released guidelines for the management of hypertension and cardiovascular risk.5,8 Numerical absolute cardiovascular risk assessment is recommended for all Australians and New Zealanders aged 45–74 (or 35 and over for Māori, Pacific Islanders, people from the Indian subcontinent and Indigenous Australians) who are not already known to be at high risk, whether or not they have hypertension (see Table 32-2). The management of hypertension should be based on a thorough clinical assessment that includes an estimate of the patient’s absolute risk for cardiovascular disease, as well as BP levels and other clinical investigations. However, considerable work remains for diagnosing, educating and treating hypertension. Many people (up to 30%) with hypertension do not know they have it because it is generally an asymptomatic condition. Two-thirds of hypertensive patients do not have their BP controlled.1 Individuals who remain undiagnosed and untreated for hypertension present the greatest challenge and opportunity for healthcare providers. Both the New Zealand and the Australian governments have set national health priorities that include the prevention and management of cardiovascular risk factors, with goals to reduce the proportion of adults with hypertension.8,10
TABLE 32-2 Definitions and classification of blood pressure levels (mmHg)
| Category | Systolic | Diastolic |
|---|---|---|
| Normal | <120 | <80 |
| High–normal | 120-139 | 80-89 |
| Grade 1 (mild) | 140-159 | 90-99 |
| Grade 2 (moderate) | 160-179 | 100-109 |
| Grade 3 (severe) | ≥180 | ≥110 |
| Isolated systolic hypertension | ≥140 | <90 |
| Isolated systolic hypertension with widened pulse pressure | ≥ 160 | ≤ 70 |
Source: national Heart foundation. Guide to management of hypertension, 2008. Canberra: national Heart foundation of australia; 2008.
Prevention and control of hypertension
HEALTH PROMOTION
Normal regulation of blood pressure
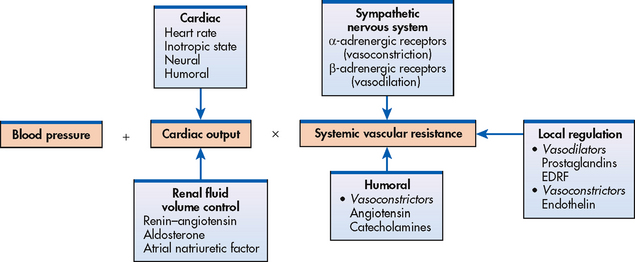
Blood pressure is the force exerted by the blood against the walls of the blood vessel. It must be adequate to maintain tissue perfusion during activity and rest. The maintenance of normal BP and tissue perfusion requires the integration of both systemic factors and local peripheral vascular effects. Arterial BP is primarily a function of cardiac output (CO) and systemic vascular resistance (SVR). The relationship is summarised by the following equation:
Arterial BP = CO × SVR
CO is the total blood flow through the systemic or pulmonary circulation per minute. CO is described as the stroke volume (amount of blood pumped out of the left ventricle per beat, approximately 70 mL) multiplied by the heart rate (HR) for 1 minute. SVR is the force opposing the movement of blood within the blood vessels. The radius of the small arteries and arterioles is the principal factor determining vascular resistance. A small change in the radius of the arterioles creates a major change in the SVR. If SVR is increased and CO remains constant or increases, arterial BP will increase.
The mechanisms that regulate BP can affect either CO or SVR, or both. Regulation of BP is a complex process involving nervous, cardiovascular, renal and endocrine functions (see Fig 32-1). BP is regulated by both short-term (seconds to hours) and long-term (days to weeks) mechanisms. Short-term mechanisms, including the sympathetic nervous system and vascular endothelium, are active within a few seconds. Long-term mechanisms include renal and hormonal processes that regulate arteriolar resistance and blood volume.
SYMPATHETIC NERVOUS SYSTEM
The nervous system, which reacts within seconds after a decrease in arterial pressure, increases BP primarily by activation of the sympathetic nervous system (SNS). Increased SNS activity increases the HR and cardiac contractility, produces widespread vasoconstriction in the peripheral arterioles and promotes the release of renin from the kidneys. The net effect of SNS activation is to increase arterial pressure by increasing both CO and SVR.
Changes in BP are sensed by specialised nerve cells called baroreceptors, located in the carotid artery and arch of the aorta and transmitted to the vasomotor centres in the brainstem. Information received in the brainstem is relayed throughout the brain by complex networks of interneurons exciting or inhibiting efferent nerves, thereby influencing cardiovascular function. SNS efferent nerves innervate cardiac and vascular smooth muscle cells. Under normal conditions, a low level of continuous SNS activity maintains tonic vasoconstriction. BP may be reduced by withdrawal of SNS activity or by stimulation of the parasympathetic nervous system, which decreases the HR (via the vagus nerve) and thereby decreases CO.
The neurotransmitter noradrenaline is released from SNS nerve endings. It activates receptors located in the sinoatrial node, myocardium and vascular smooth muscle. The response to noradrenaline depends on the type and density of receptors present. SNS receptors are classified as alpha-1 (α1), alpha-2 (α2), beta-1 (β1) and beta-2 (β2) (see Table 32-1). α1-adrenergic receptors located in the peripheral vasculature cause vasoconstriction when stimulated by noradrenaline. β1-adrenergic receptors in the heart respond to noradrenaline and adrenaline with increased HR (chronotropic), increased force of contraction (inotropic) and increased speed of conduction. The smooth muscle of the blood vessels has α1-adrenergic and β2-adrenergic receptors. β2-adrenergic receptors are activated primarily by adrenaline released from the adrenal medulla and cause vasodilation. Diminished responsiveness of cardiovascular cells to SNS stimulation is one of the most significant cardiovascular effects of ageing.
The sympathetic vasomotor centre, located in the medulla, interacts with many areas of the brain to maintain normal BP under various conditions. During exercise the motor area of the cortex is stimulated, activating the vasomotor centre and the SNS through neuronal connections. This causes an appropriate increase in CO and BP to accommodate the increased oxygen demand of the exercising muscles. During postural change from lying to standing, there is a transient decrease in BP. The vasomotor centre is stimulated and activates the SNS, causing peripheral vasoconstriction and increased venous return to the heart. If this response did not occur, there would be inadequate blood flow to the brain, resulting in dizziness or syncope. Cerebral cortical perceptions such as pain and stress activate the vasomotor centres through the neuronal connections.
Baroreceptors
Baroreceptors (pressoreceptors) are specialised nerve cells located in the carotid artery and arch of the aorta. They have an important role in the maintenance of BP stability during normal activities. They are sensitive to stretching and, when stimulated by an increase in BP, send inhibitory impulses to the sympathetic vasomotor centre in the brainstem. Inhibition of sympathetic activity results in decreased HR, decreased force of contraction and vasodilation in peripheral arterioles. Increased parasympathetic activity (vagus nerve) reduces HR.
A fall in BP, sensed by the baroreceptors, leads to activation of the SNS. The result is constriction of the peripheral arterioles, increased HR and increased contractility of the heart. In the presence of longstanding hypertension, the baroreceptors become adjusted to elevated levels of BP and recognise this level as ‘normal’. The baroreceptor reflex is less responsive in some older adults.
VASCULAR ENDOTHELIUM
The vascular endothelium is a single cell layer that lines the blood vessels. Previously considered inert, it is now known to produce vasoactive substances and growth factors. Nitric oxide, an endothelium-derived relaxing factor (EDRF), helps maintain low arterial tone at rest, inhibits growth of the smooth muscle layer and inhibits platelet aggregation. Other substances released by the vascular endothelium with local vasodilator effects include prostacyclin and endothelium-derived hyperpolarising factor.
Endothelin (ET), produced by the endothelial cells, is an extremely potent vasoconstrictor. There are three subclasses of endothelins (ET-1, ET-2 and ET-3). ET-1 is the most potent endothelin in producing vasoconstriction. ET-1 also causes adhesion and aggregation of neutrophils and stimulates smooth muscle growth. Endothelial cell function and dysfunction are areas of ongoing investigation. There is some evidence that vascular endothelial dysfunction may contribute to atherosclerosis and primary hypertension. The prevention or reversal of endothelial dysfunction may become important for therapeutic interventions in the future.
RENAL SYSTEM
The kidneys contribute to BP regulation by controlling sodium excretion and extracellular fluid (ECF) volume (see Ch 44). Sodium retention results in water retention, which causes an increased ECF volume. This increases the venous return to the heart, increasing the stroke volume, which elevates the BP through an increase in CO.
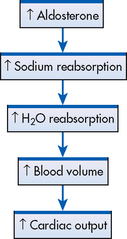
The renin–angiotensin–aldosterone system (RAAS) also plays an important role in BP regulation. In response to SNS stimulation, decreased blood flow through the kidneys or decreased serum sodium concentration, renin is secreted from the juxtaglomerular apparatus in the kidneys. Renin is an enzyme that converts angiotensinogen to angiotensin I. Angiotensin-converting enzyme (ACE) converts angiotensin I into angiotensin II, which can increase BP by two different mechanisms (see Ch 45). First, angiotensin II is a potent vasoconstrictor and increases vascular resistance, resulting in an immediate increase in BP. Second, over a period of hours or days, angiotensin II increases BP indirectly by stimulating the adrenal cortex to secrete aldosterone, which causes sodium and water retention by the kidneys, resulting in increased blood volume and increased CO (see Fig 32-2).
Angiotensin II also functions at a local level within the heart and blood vessels. The local vasoactive effects of angiotensin II include vasoconstriction and tissue growth, which results in remodelling of the vessel walls. These changes are associated with the development of primary hypertension and also the long-term effects of hypertension (e.g. atherosclerosis, renal disease, cardiac hypertrophy).
Prostaglandins (PGE2 and PGI2) secreted by the renal medulla have a vasodilator effect on the systemic circulation. This results in decreased systemic vascular resistance and lowering of BP. (Prostaglandins are discussed in Ch 12.) The natriuretic peptides (atrial natriuretic peptide [ANP] and type B natriuretic peptide [BNP]) secreted by cardiac cells antagonise the effects of antidiuretic hormone (ADH) and aldosterone by promoting natriuresis (excretion of sodium in urine) and diuresis, resulting in reduced blood volume and a decrease in BP.
ENDOCRINE SYSTEM
Stimulation of the SNS results in the release of adrenaline along with a small fraction of noradrenaline by the adrenal medulla. Adrenaline increases CO by increasing the HR and myocardial contractility. Adrenaline activates β2-adrenergic receptors in peripheral arterioles of skeletal muscle, causing vasodilatation. In peripheral arterioles with only α1-adrenergic receptors (skin and kidneys), adrenaline causes vasoconstriction.
The adrenal cortex is stimulated by angiotensin II to release aldosterone. (Release of aldosterone is also regulated by other factors, such as low sodium levels [see Chs 47 and 49].) Aldosterone stimulates the kidneys to retain sodium and water. This increases BP by increasing CO (see Fig 32-2).
An increased blood sodium and osmolarity level stimulates the release of ADH from the posterior pituitary gland. ADH increases the ECF volume by promoting the reabsorption of water in the distal and collecting tubules of the kidneys. The resulting increase in blood volume can cause an elevation in BP.
In the healthy person, these regulatory mechanisms function in response to the demands of the body. When hypertension develops, one or more of the BP-regulating mechanisms are defective.
Hypertension
CLASSIFICATION OF HYPERTENSION
Table 32-2 describes the BP classification for people 18 years of age and older. The classification of BP is based on the average of two or more properly measured, seated BP readings on each of two or more visits to a health practitioner.5
SUBTYPES OF HYPERTENSION
Isolated systolic hypertension
Isolated systolic hypertension (ISH) is defined as an average systolic BP ≥140 mmHg coupled with an average DBP <90 mmHg.7 Although ISH does occur in younger adults, it is much more common in older adults. Blood pressure patterns change as part of the ageing process. The SBP continues to rise with ageing; the DBP rises until approximately 55 years and then declines. Older adults often have ISH caused by loss of elasticity in large arteries from atherosclerosis. An SBP greater than 140 mmHg is a more important risk factor for cardiovascular disease than an elevated DBP in individuals older than 50 years. ISH is the most common form of hypertension for this age group. Control of ISH decreases the incidence of stroke, heart failure, cardiovascular mortality and total mortality.1
Pseudohypertension
Pseudohypertension, or false hypertension, can occur with advanced (often calcified) arteriosclerosis. Sclerotic arteries do not collapse when the cuff is fully inflated, presenting much higher cuff pressures than are actually present within the vessels. Pseudohypertension is suspected if arteries feel rigid or when few retinal or cardiac signs are found relative to the pressures obtained by cuff. Osler’s sign (a palpable radial or brachial artery after the blood pressure cuff is fully inflated) has a low sensitivity and specificity for pseudohypertension and is not recommended. The only way to accurately measure BP in pseudohypertension is through the use of an intra-arterial catheter.
AETIOLOGY
The aetiology of hypertension can be classified as either primary or secondary.
Primary hypertension
Primary (essential or idiopathic) hypertension is elevated BP without an identified cause; it accounts for 90–95% of all cases of hypertension. Although the exact cause of primary hypertension is unknown, several contributing factors have been identified, including increased SNS activity, overproduction of sodium-retaining hormones and vasoconstrictors, increased sodium intake, greater than ideal body weight, diabetes mellitus and excessive alcohol consumption. Primary hypertension is the focus of this chapter because of its prevalence in clinical practice and its impact on health.
Secondary hypertension
Secondary hypertension is elevated BP with a specific cause that can often be identified and corrected. This type of hypertension accounts for 5–10% of hypertension in adults and more than 80% of hypertension in children. If a person below age 20 or over age 50 suddenly develops hypertension, especially if it is severe, a secondary cause should be suspected. Clinical findings that suggest secondary hypertension include unprovoked hypokalaemia, abdominal bruit heard over the renal arteries, variable BP with a history of tachycardia, sweating and tremor or a family history of renal disease.
Causes of secondary hypertension include the following: (1) coarctation or congenital narrowing of the aorta; (2) renal disease such as renal artery stenosis and parenchymal disease (see Ch 46); (3) endocrine disorders such as phaeochromocytoma, Cushing’s syndrome and hyperaldosteronism (see Ch 49); (4) neurological disorders such as brain tumours, tetraplegia and head injury; (5) sleep apnoea; (6) medications such as sympathetic stimulants (including cocaine), monoamine oxidase inhibitors taken with tyramine-containing foods, oestrogen replacement therapy, oral contraceptive pills and non-steroidal anti-inflammatory drugs (NSAIDs); (7) cirrhosis; and (8) pregnancy-induced hypertension. Treatment of secondary hypertension is directed at eliminating the underlying cause. Secondary hypertension is a contributing factor to hypertensive crisis (see p 850).
PATHOPHYSIOLOGY OF PRIMARY HYPERTENSION
For arterial pressure to rise there must be an increase in either CO or SVR. Increased CO is sometimes found in the prehypertensive and borderline hypertensive person. Later in the course of hypertension, SVR rises and the CO returns to normal. The haemodynamic hallmark of hypertension is persistently increased SVR. This persistent elevation in SVR may come about in various ways. Factors that are known to be related to the development of primary hypertension or contribute to its consequences are presented in Box 32-1.
BOX 32-1 Risk factors for primary hypertension
When managing raised blood pressure, risk factors that should be considered include:
• Personal history of associated clinical conditions or presence of target organ disease
• Age (>55 years male, >65 years female)
• Male gender (increased risk at any age compared to females)
• Family history of hypertension or premature cardiovascular disease (in first-degree relative age <60 years)
• Dyslipidaemia (raised total or LDL cholesterol or reduced HDL cholesterol)
• Obesity (BMI ≥30 kg/m2); overweight (BMI ≥25 kg/m2); and/or increased waist circumference (‘increased risk’: >94 cm males, >80 cm females; ‘high risk’: >102 cm males; >88 cm females)†
BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Source: Compiled from National Heart Foundation. Guide to management of hypertension, 2008. Canberra: National Heart Foundation of Australia; 2008.
†These categories of risk may not be appropriate for all population groups.
Heredity
Genetic abnormalities associated with several rare forms of hypertension have been identified. However, the contribution of genetic factors to BP levels in the general population is very small.1 Environmental factors also have a role in contributing to the development of high BP. In practice, children and siblings of persons with hypertension should be more carefully screened and strongly advised to adopt healthy lifestyles to prevent hypertension.
Water and sodium retention
Excessive sodium intake is considered responsible for initiating hypertension in some people. Populations with a low sodium intake (usually hunter–gatherer societies) show little or no hypertension and no progressive increase in BP with age as is found in industrialised societies. In addition, when people from these societies adopt industrialised lifestyles, the prevalence of hypertension increases. When sodium is restricted in many hypertensive people, their BP falls. A high sodium intake may activate a number of pressor mechanisms and cause water retention. Although almost everyone in Western countries consumes a high-sodium diet, only about 20% develop hypertension. This indicates that some degree of sodium sensitivity must be present for high sodium intake to trigger the development of hypertension. In clinical practice, there is not an easy or simple test to identify individuals whose BP will rise with even a small increase in salt intake (salt sensitive) versus those who can ingest large amounts of sodium without much change in BP (salt resistant).11 Certain demographic factors, such as obesity, increasing age, ethnicity, diabetes mellitus and chronic kidney disease, are more commonly associated with BP salt sensitivity.11
HEALTH DISPARITIES
Women
• After age 55, hypertension is more common in women than in men.
• Part of the rise in BP in women is attributed to menopause-related factors, such as oestrogen withdrawal, overproduction of pituitary hormones and weight gain.
• Women with hypertension are more likely to suffer a stroke than an MI.
• ACE-inhibitor-induced cough and diuretic-induced hyponatraemia are more common in women.
• Oral contraceptives may cause small increases in BP in many women and frank hypertension in a small percentage of women.
• Effects usually seen in initial period of contraceptive use and during dosage changes.
ACE, angiotensin-converting enzyme; BP, blood pressure; MI, myocardial infarction.
Altered renin–angiotensin mechanism
High plasma renin activity (PRA) results in the increased conversion of angiotensinogen to angiotensin I (see Fig 44-4). Angiotensin II causes direct arteriolar constriction, promotes vascular hypertrophy and induces aldosterone secretion. Thus altered renin–angiotensin mechanisms may contribute to the development and maintenance of hypertension. Any rise in BP inhibits the release of renin from the renal juxtaglomerular cells. Based on this feedback loop, low levels of PRA would be expected in patients with primary hypertension. However, only about 30% have low PRA, 50% have normal levels and 20% have high PRA. These normal or high PRA levels may be related to excess renin secretion from ischaemic nephrons.
Stress and increased sympathetic nervous system activity
It has long been recognised that arterial pressure is influenced by factors such as anger, fear and pain. Physiological responses to stress, which are normally protective, may persist to a pathological degree, resulting in prolonged increase in SNS activity. Increased SNS stimulation produces increased vasoconstriction, increased HR and increased renin release. Increased renin activates the angiotensin mechanism and increases aldosterone secretion, both leading to elevated BP. People exposed to high levels of repeated psychological stress develop hypertension to a greater extent than those who do not experience as much stress.
Insulin resistance and hyperinsulinaemia
Abnormalities of glucose, insulin and lipoprotein metabolism are common in primary hypertension. They are not present in secondary hypertension and do not improve when hypertension is treated. Insulin resistance is a risk factor for the development of hypertension and cardiovascular disease. High insulin concentration in the blood stimulates SNS activity and impairs nitric oxide-mediated vasodilation. Additional pressor effects of insulin include vascular hypertrophy and increased renal sodium reabsorption.
Endothelial cell dysfunction
Vascular endothelial cells are known to be the source of multiple vasoactive substances. Some hypertensive people have a reduced vasodilator response to nitric oxide. Endothelin produces pronounced and prolonged vasoconstriction. The role of endothelial dysfunction in the pathogenesis and treatment of hypertension is an area of ongoing investigation.
CLINICAL MANIFESTATIONS
Hypertension is often called the ‘silent killer’ because it is frequently asymptomatic until it becomes severe and target organ disease has occurred. A patient with severe hypertension may experience a variety of symptoms secondary to effects on blood vessels in the various organs and tissues or to the increased workload of the heart. These secondary symptoms include fatigue, reduced activity tolerance, dizziness, palpitations, angina and dyspnoea. In the past, symptoms of hypertension were thought to include headache, nosebleeds and dizziness. However, unless BP is very high or low these symptoms are not more frequent in people with hypertension than in the general population.11
COMPLICATIONS
The most common complications of hypertension are target organ diseases (see Table 32-3) occurring in the heart (hypertensive heart disease), brain (cerebrovascular disease), peripheral vasculature (peripheral vascular disease), kidneys (nephrosclerosis) and eyes (retinal damage).
TABLE 32-3 Categories of associated clinical conditions and target organ disease*
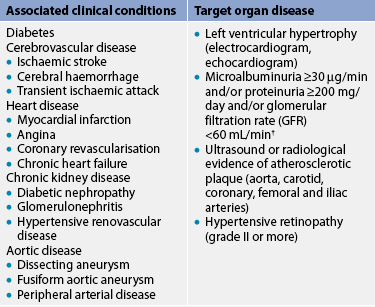
* As ‘markers’ for those at high or very high absolute risk of a primary or secondary cardiovascular event.
† GFR is estimated by the Cockcroft gault formula = (140 – age) × weight (kg)/(serum creatinine (μmol/L) × 0.814) for males. For females, multiply result by 0.85.
Source: National Heart foundation. Guide to management of hypertension, 2008. Canberra: national Heart foundation of australia; 2008. Available at www.heartfoundation.org.nz/index.asp?PageID=2145860168, accessed December 2010.
Hypertensive heart disease
Coronary artery disease
Hypertension is a major risk factor for coronary artery disease (CAD). In people older than 50 years, systolic hypertension is a much more important risk factor for cardiovascular disease than diastolic hypertension.1 The mechanisms by which hypertension contributes to the development of atherosclerosis are not fully known. The ‘response-to-injury’ hypothesis of atherogenesis suggests that hypertension disrupts the coronary artery endothelium, thus exposing the intimal layer to activated white blood cells and platelets. Growth factors released by the vascular endothelium and platelets may induce smooth muscle proliferation within the lesion. These arteriolar changes result in a stiffened arterial wall and a narrowed internal lumen and account for a high incidence of coronary artery disease and the resulting problems of angina and myocardial infarction (MI).
Left ventricular hypertrophy
Sustained high BP increases the cardiac workload and produces left ventricular hypertrophy (LVH) (see Fig 32-3). Initially, LVH is an adaptive or compensatory mechanism that strengthens cardiac contraction and increases CO. However, increased contractility increases myocardial work and oxygen consumption. When the heart can no longer meet the demands for myocardial oxygen, heart failure develops. Progressive LVH, especially in association with CAD, is associated with the development of heart failure.
Heart failure
Heart failure occurs when the heart’s compensatory adaptations are overwhelmed and the heart can no longer pump enough blood to meet the metabolic needs of the body (see Ch 34). Contractility is depressed and stroke volume and CO are decreased. The patient may complain of shortness of breath on exertion, paroxysmal nocturnal dyspnoea and fatigue. Signs of an enlarged heart may be present on X-ray and an electrocardiogram (ECG) may show electrical changes indicative of LVH.
Cerebrovascular disease
Atherosclerosis is the most common cause of cerebrovascular disease. Hypertension is a major risk factor for cerebral atherosclerosis and stroke. Even in mildly hypertensive people, the risk of stroke is four times higher than in normotensive people. Adequate control of BP diminishes the risk of stroke.
Atherosclerotic plaques are commonly distributed at the bifurcation of the common carotid artery into the internal and external carotid arteries. Portions of the atherosclerotic plaque or the blood clot that forms with disruption of the plaque may break off and travel to intracerebral vessels, producing a thromboembolism. The patient may experience transient ischaemic attacks or a stroke. (These conditions are discussed in Ch 57.)
Hypertensive encephalopathy may occur after a marked rise in BP if the cerebral blood flow is not decreased by autoregulation. Autoregulation is a physiological process that maintains constant cerebral blood flow despite fluctuations in arterial BP. Normally as pressure in the cerebral blood vessels rises, the vessels constrict to maintain constant flow. When arterial BP exceeds the body’s ability to autoregulate, the cerebral vessels suddenly dilate, capillary permeability increases and cerebral oedema develops, producing a rise in intracranial pressure. If left untreated, patients die quickly from brain damage. (Cerebral blood flow and autoregulation are discussed in Ch 56.)
Peripheral vascular disease
As it does with other vessels, hypertension speeds up the process of atherosclerosis in the peripheral blood vessels, leading to the development of peripheral vascular disease, aortic aneurysm and aortic dissection (see Ch 37). Intermittent claudication (ischaemic muscle pain precipitated by activity and relieved with rest) is a classic symptom of peripheral vascular disease involving the arteries.
Nephrosclerosis
Hypertension is one of the leading causes of end-stage renal disease. Some degree of renal dysfunction is usually present in the hypertensive patient, even one with a minimally elevated BP. Renal dysfunction is the direct result of ischaemia caused by the narrowed lumen of the intrarenal blood vessels. Gradual narrowing of the arteries and arterioles leads to atrophy of the tubules, destruction of the glomeruli and eventual death of nephrons. Initially intact nephrons can compensate, but these changes may eventually lead to renal failure. Common laboratory indications of renal dysfunction are microalbuminuria, proteinuria, microscopic haematuria and elevated serum urea and serum creatinine levels. The earliest manifestation of renal dysfunction is usually nocturia (see Ch 45).
Retinal damage
The appearance of the retina provides important information about the severity and duration of the hypertensive process. The retina is the only place in the body where the blood vessels can be directly visualised. Therefore damage to retinal vessels provides an indication of concurrent vessel damage in the heart, brain and kidneys. An ophthalmoscope is used to visualise the blood vessels of the eye. Manifestations of severe retinal damage include blurring of vision, retinal haemorrhage and loss of vision.
DIAGNOSTIC STUDIES
Measurement of BP is discussed in Chapter 31 and on page 846.
There is some controversy as to how extensive a diagnostic examination should be performed in the initial evaluation of a person with hypertension. Because most hypertension is classified as primary hypertension, testing for secondary causes is not routinely done. Basic laboratory studies are performed to: (1) identify or rule out causes of secondary hypertension; (2) evaluate target organ disease; (3) determine overall cardiovascular risk; or (4) establish baseline levels before initiating therapy.
Box 32-2 lists basic diagnostic studies that are performed in a person with hypertension. Routine urinalysis and serum urea and creatinine levels are used to screen for renal involvement and to provide baseline information about kidney function. Creatinine clearance, the rate at which creatinine is cleared from the circulation, reflects the glomerular filtration rate. Decreases in creatinine clearance indicate renal insufficiency. Creatinine clearance can be measured quantitatively in a timed urine collection. It can also be estimated from the serum creatinine level. (Serum creatinine and creatinine clearance are discussed in Chs 44 and 46.)
MULTIDISCIPLINARY CARE
Diagnostic studies
History and physical examination
Basic metabolic panel (serum glucose, sodium, potassium, chloride, carbon dioxide, calcium, nitrogen and creatinine)
Serum lipid profile (total lipids, triglycerides, HDL and LDL cholesterol, total-to-HDL cholesterol ratio)
Collaborative therapy
Ambulatory BP monitoring (if indicated)
Every 3–6 months by healthcare provider once BP is stabilised
Nutritional therapy (Table 32-4)
Restrict cholesterol and saturated fats
Maintain adequate intake of potassium
Maintain adequate intake of calcium and magnesium
Regular, moderate physical activity
Stop smoking (see Ch 10)
Antihypertensive drugs (see Table 32-5)
BP, blood pressure; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Measurement of serum electrolytes, especially potassium levels, is important to detect hyperaldosteronism, a cause of secondary hypertension. Blood glucose levels should be assessed to assist in the diagnosis of diabetes mellitus. A lipid profile provides information about additional risk factors that predispose to atherosclerosis and cardiovascular disease. Uric acid levels are determined to establish a baseline, because the levels often rise with diuretic therapy. An ECG provides baseline information about the cardiac status. It is helpful in identifying the presence of LVH, cardiac ischaemia or previous MI. Because of the prognostic importance of LVH, echocardiography may also be performed. If the patient’s age, history, physical examination findings or severity of hypertension points to a secondary cause, further diagnostic testing is indicated.
Ambulatory blood pressure monitoring
Some patients have elevated BP readings in a clinical setting and normal readings when BP is measured elsewhere. This phenomenon is referred to as ‘white coat’ hypertension. Self-monitoring of BP at home and at work is a practical, economical approach that may be considered prior to ambulatory blood pressure monitoring (ABPM). ABPM is a non-invasive, fully automated system that measures BP at preset intervals over a 24-hour period. The equipment includes a BP cuff and a small microprocessing unit that fits into a pouch worn on a shoulder strap or belt. Patients are instructed to hold their arm still by their side when the device is taking a reading and asked to maintain a diary of activities that may affect BP. Other potential applications for ABPM include apparent drug resistance, hypotensive symptoms with hypertensive medications, episodic hypertension or SNS dysfunction.
As with most physiological phenomena, BP demonstrates diurnal variability expressed as sleep–wakefulness difference. For day-active people, BP is highest in the early morning, decreases during the day and is lowest at night. BP at night (when asleep) usually drops by 10% or more from daytime (awake) BP.1 Some patients with hypertension do not show a normal, nocturnal dip in BP and are referred to as ‘non-dippers’. The absence of diurnal variability has been associated with more target organ damage and an increased risk of cardiovascular events. The presence or absence of diurnal variability can be determined by ABPM.
MULTIDISCIPLINARY CARE
There are published clinical guidelines for the management of hypertension.5,6,8–10 The goal in treating a patient with hypertension is to control BP and reduce overall cardiovascular risk. (Treatment recommendations are summarised in Box 32-2, Table 32-4 and Fig 32-4.) Treatment goals are to lower BP to less than 140 mmHg systolic and less than 90 mmHg diastolic for most patients with hypertension (<130 mmHg systolic and <80 mmHg diastolic for those with diabetes mellitus and chronic kidney disease). Lifestyle modifications are indicated for all patients with prehypertension and hypertension.
TABLE 32-4 DASH diet for hypertension
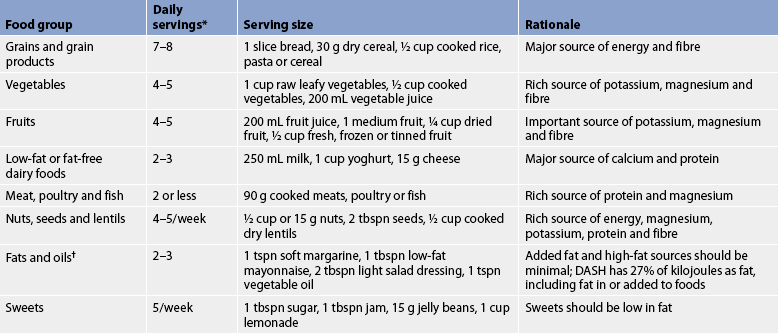
DASH, Dietary Approaches to Stop Hypertension. The DASH eating plan above is based on approximately 8.37 kJ per day. The number of daily servings in a food group may vary from those listed, depending on specific energy or health needs.
† fat content changes serving counts for fats and oils. For example, 1 tbspn of regular salad dressing equals 1 serving; 1 tbspn of a low-fat dressing equals ½ serving; 1 tbspn of a fat-free dressing equals 0 servings.
Source: National Heart Lung and Blood Institute. The DASH diet. Washington, DC: national Institutes of Health. Available at: www.nhlbi.nih.gov/hbp/prevent/h_eating/h_e_dash.htm, accessed December 2010.

Figure 32-4 When to initiate blood pressure-lowering drug treatment. BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
*For Indigenous Australian, Māori and Pacific Islander adults, consider managing as though at a higher risk level.
†For example, diabetes (strict glycaemic control lowers cardiovascular risk); lipid disorders (cholesterol-lowering therapy reduces the risk of primary and secondary coronary events).
‡Continue lifestyle modification, monitor BP and reassess absolute cardiovascular risk regularly. Note that patients with mild hypertension will require antihypertensive drug treatment if their absolute risk of cardiovascular disease is elevated due to changes in other risk factors.
Source: National Heart Foundation of Australia, 2008. Available at www.heartfoundation.org.au/SiteCollectionDocuments/A_Hypert_Guidelines2008_2009Update_Figure2_FINAL.pdf.
Lifestyle modifications
Lifestyle modifications should be used in all patients with prehypertension and hypertension.1,11,12 These modifications are directed towards reducing BP and overall cardiovascular risk. Modifications include: (1) weight reduction; (2) DASH (dietary approaches to stop hypertension) eating plan; (3) dietary sodium reduction; (4) moderation of alcohol consumption; (5) regular aerobic physical activity; and (6) avoidance of tobacco use (smoking and chewing).
Weight reduction
Overweight individuals have an increased incidence of hypertension and an increased risk of cardiovascular disease. Weight reduction has a significant effect on lowering BP in many people and the effect is seen even with moderate weight loss. A weight loss of 10 kg may decrease SBP by between 5 and 20 mmHg.1 When a person decreases kilojoule intake, sodium and fat intake are usually also reduced. Although reducing the fat content of the diet has not been shown to produce sustained benefits in BP control, it may slow the progress of atherosclerosis and reduce overall cardiovascular disease risk (see Ch 33). Weight reduction through a combination of dietary kilojoule restriction and moderate physical activity is recommended for overweight hypertensive patients (see Ch 40).
DASH eating plan
This diet involves eating several servings of fish each week, eating plenty of fruits and vegetables, increasing fibre intake and drinking a lot of water (see Table 32-4).12,13 The DASH diet significantly lowers BP. These decreases have been compared to those achieved with BP-lowering medication.1
Dietary sodium reduction
The average Australian and New Zealand adult’s intake of sodium is higher than recommended. The most common source of sodium in the diet is processed foods (e.g. tinned soups, frozen dinners). The Heart Foundations of Australia and New Zealand recommend restricting salt intake and the JNC 7 recommends restricting intake to less than 6 g of salt (sodium chloride [NaCl]) or less than 2.4 g of sodium per day for healthy adults.1,5,6 This involves avoiding foods known to be high in sodium and not adding salt in the preparation of foods or at meals (see Tables 34-4, 34-5 and 34-6).
The patient and family, especially the member who prepares the meals, should be taught about sodium-restricted diets. Instruction should include reading labels of over-the-counter medications, packaged foods and health products (e.g. toothpaste containing baking soda) to identify hidden sources of sodium. It is helpful to review the patient’s normal diet and to identify foods high in sodium. Analysis of a 3-day diet history will help identify foods high in sodium in the patient’s usual diet.
Sodium restriction may be enough to control BP in some patients with hypertension. If drug therapy is needed, a lower dose may be effective if the patient also restricts sodium intake. Furthermore, moderate sodium restriction lessens the risk of hypokalaemia associated with diuretic therapy. However, the response may differ between individuals who are salt-sensitive or salt-resistant.
The significance of other dietary elements for the control of hypertension is not certain. There is evidence that greater levels of dietary potassium, calcium, vitamin D and omega-3 fatty acids are associated with lower BP in the general population and in those with hypertension. Based on available data, it is recommended that people with hypertension maintain adequate potassium and calcium intake from food sources. Although it is important to maintain an adequate intake of calcium and vitamin D for general health, calcium supplements are not recommended to lower BP. There is evidence that omega-3 fatty acids found in certain fish oils can contribute to reductions in BP and triglycerides (see ‘Complementary & alternative therapies’ box). Caffeine may raise BP acutely, but there is no long-term relationship between caffeine intake and elevated BP.
Moderation of alcohol consumption
Excessive alcohol consumption is strongly associated with hypertension. Consumption of three or more alcoholic drinks daily is also a risk factor for heart disease and stroke. Men should limit their intake of alcohol to no more than two drinks per day and women and lighter weight men to no more than one drink per day (one standard drink equals 570 mL beer, 250 mL wine or 60 mL 100-proof spirits).
Regular physical activity
It is recommended that all adults undertake regular aerobic physical activity (e.g. brisk walking) at least 30 minutes per day most days of the week. Moderately intense activity, such as brisk walking, jogging and swimming, can lower BP, promote relaxation and decrease or control body weight. Regular activity of this type can reduce SBP in the hypertensive patient by approximately 4–9 mmHg. Sedentary people should be advised to increase activity levels gradually. People with heart disease or other serious health problems need a thorough examination, possibly including a stress test, before beginning an exercise program.
COMPLEMENTARY & ALTERNATIVE THERAPIES
Dietary sources of omega-3 fatty acids include fish oil, which contains both docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Fish oil may be obtained by eating oily fish or by taking a supplement. Oily fish include anchovies, bluefish, carp, catfish, halibut, herring, lake trout, mackerel, pompano, salmon, striped sea bass, tuna (albacore) and whitefish. Potentially harmful contaminants, such as dioxins, methylmercury and polychlorinated biphenyls (PCBs), are found in some species of fish.
Omega-3 fatty acids should not be confused with omega-6 fatty acids.
Effects
May include reduction of serum triglyceride levels and small reductions in blood pressure. Effects appear to be dose-dependent. May also reduce inflammation, reduce blood clotting and reduce build-up of atherosclerotic plaques in arteries of the heart. The American Heart Association recommends including fish in the diet of all individuals and fish oil supplements for those with a history of cardiovascular disease.
Nursing implications
Generally safe in recommended dosages for up to 2–3.5 years. Dosages greater than 3 g per day may result in increased risk of bleeding. Use with caution in diabetic patients or patients with bleeding disorders. Use with caution in patients taking medications, herbs or supplements that also increase the risk of bleeding. Advise patients to consult a healthcare provider before using fish oils at dosages greater than 3 g per day. Fish oil taken for many months may cause a deficiency of vitamin E. Vitamin E is added to many fish oil products. More research is needed to determine the safety of supplements during pregnancy or lactation. Advise women who are pregnant or lactating to limit intake of oily fish and to seek the recommendation of their healthcare provider.
Source: Agency for Healthcare Research and Quality. Health effects of omega-3 fatty acids on cardiovascular disease. Evidence Report/Technology Assessment no. 94. Available at: www.ahrq.gov/clinic/epcsums/o3cardsum.htm, accessed 27 December 2010.
Avoidance of tobacco products
Nicotine contained in tobacco causes vasoconstriction and increases BP in hypertensive people. In addition, smoking tobacco is a major risk factor for cardiovascular disease. The cardiovascular benefits of discontinuing tobacco use can be seen within a year in all age groups. Everyone, especially a hypertensive patient, should be strongly advised to avoid tobacco use. The lower amounts of nicotine contained in smoking cessation aids usually will not raise BP and may be used as indicated. People who continue to use tobacco products should be advised to monitor their BP during use. (Tobacco use and smoking cessation are discussed in Ch 10.)
Stress management
Stress can raise BP on a short-term basis and has been implicated in the development of hypertension. Relaxation therapy, guided imagery and biofeedback may be useful in helping patients manage stress, thus decreasing BP. Psychosocial factors such as time urgency and impatience and hostility have been associated with an increase in the long-term risk of hypertension in young adults.
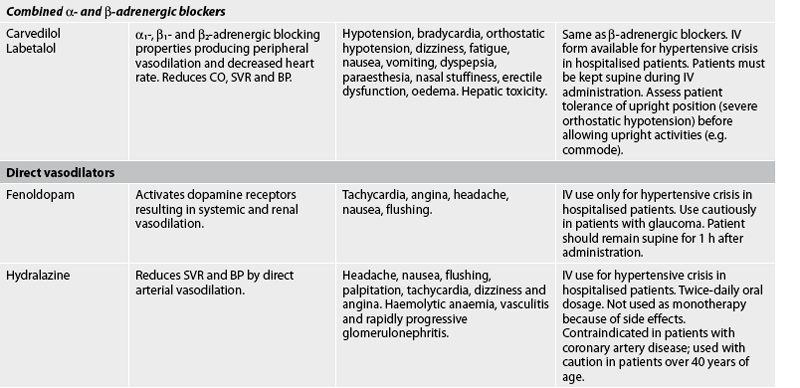
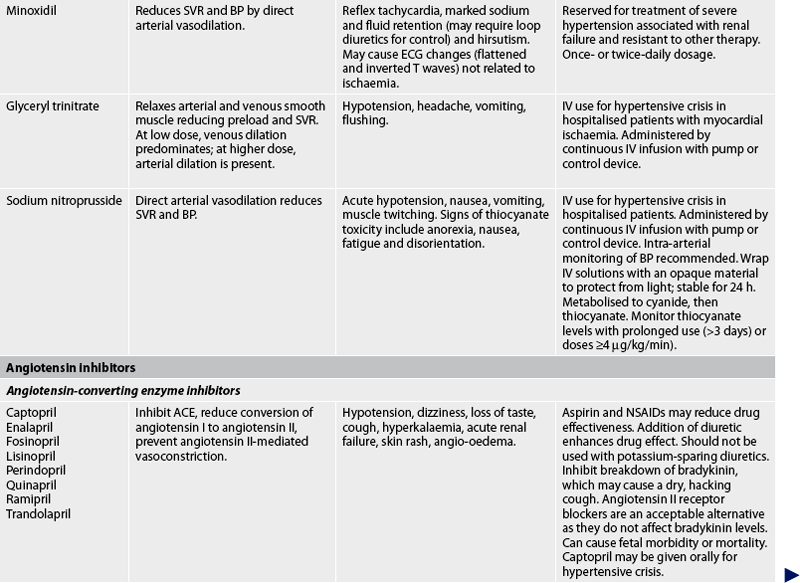
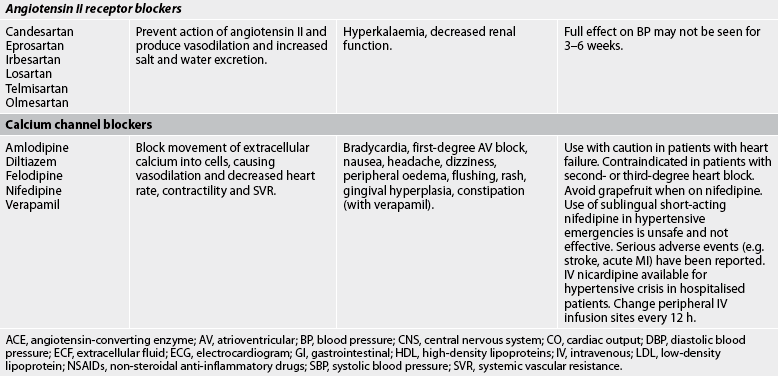
Drug therapy
The general goals of drug therapy are to achieve BP less than 140/90 mmHg (<130/80 mmHg for patients with diabetes mellitus or chronic kidney disease). The drugs currently available for treating hypertension have two main actions: (1) they decrease the volume of circulating blood; and (2) they reduce SVR (see Table 32-5). The drugs used in the treatment of hypertension include diuretics, adrenergic (SNS) inhibitors, direct vasodilators, angiotensin inhibitors and calcium channel blockers. The various sites and methods of action are presented in Figure 32-5.
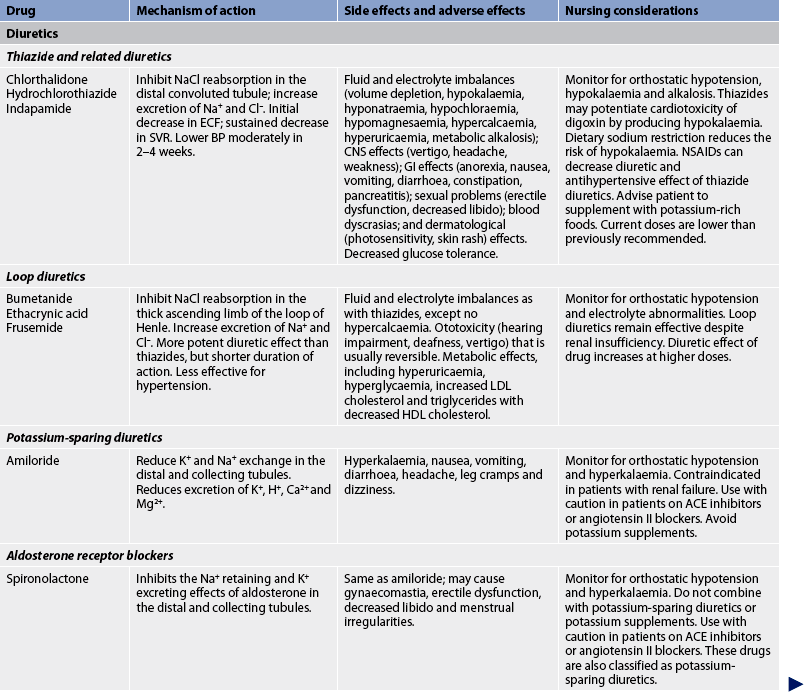
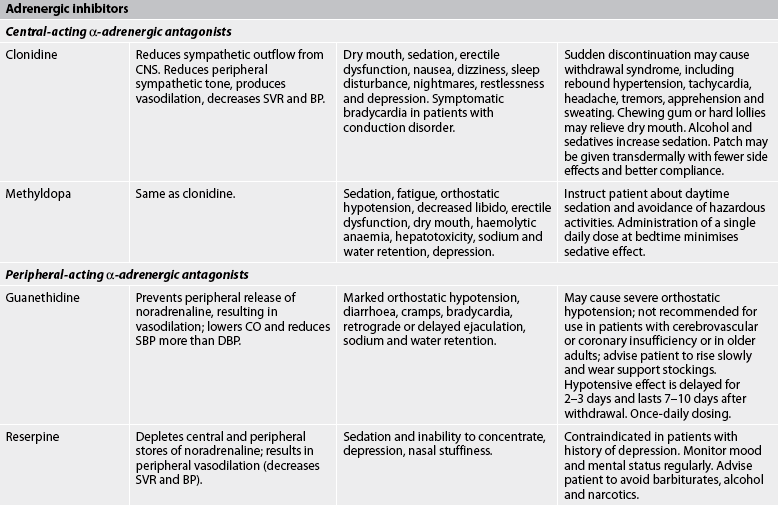

ACE, angiotensin-converting enzyme; AV, atrioventricular; BP, blood pressure; CNS, central nervous system; CO, cardiac output; DBP, diastolic blood pressure; ECF, extracellular fluid; ECG, electrocardiogram; GI, gastrointestinal; HDL, high-density lipoproteins; IV, intravenous; LDL, low-density lipoprotein; NSAIDS, non-steroidal anti-inflammatory drugs; SBP, systolic blood pressure; SVR, systemic vascular resistance.
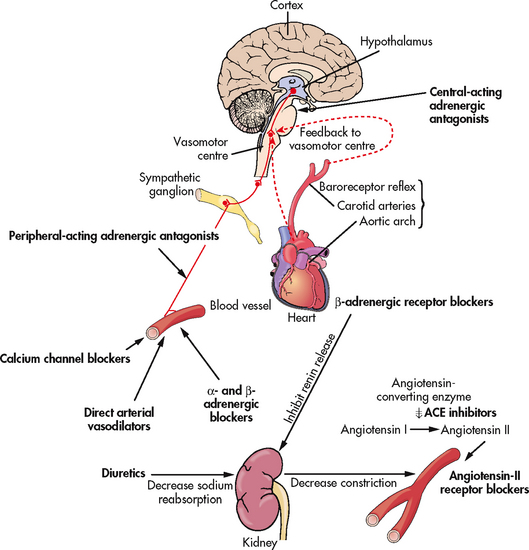
Figure 32-5 The site and method of action of various antihypertensive drugs (bold type). ACE, angiotensin-converting enzyme.
Although the precise action of diuretics in the reduction of BP is unclear, it is known that they promote sodium and water excretion, reduce plasma volume, decrease sodium in the arteriolar walls and reduce the vascular response to catecholamines. Adrenergic-inhibiting agents act by diminishing the SNS effects that increase BP. Adrenergic inhibitors include drugs that act centrally on the vasomotor centre and peripherally to inhibit noradrenaline release or to block the adrenergic receptors on blood vessels. Direct vasodilators decrease the BP by relaxing vascular smooth muscle and reducing SVR. Calcium channel blockers increase sodium excretion and cause arteriolar vasodilation by preventing the movement of extracellular calcium into cells.
There are two types of angiotensin inhibitors. The first type is ACE inhibitors, which prevent the conversion of angiotensin I to angiotensin II and thus reduce angiotensin II-mediated vasoconstriction and sodium and water retention. The second type is angiotensin II receptor blockers (ARBs), which prevent angiotensin II from binding to its receptors in the walls of the blood vessels.
Thiazide-type diuretics are used as initial therapy for most patients with hypertension, either alone or in combination with one of the other classes. Compelling indications requiring the use of other antihypertensive drugs as initial therapy include heart failure, post-MI, high risk of coronary artery disease, diabetes mellitus, chronic kidney disease and recurrent stroke prevention. If a drug is not tolerated or is contraindicated, then one of the other drug classes should be used (see Table 32-5).
Most patients who are hypertensive will require two or more antihypertensive medications to achieve their BP goals. Addition of this second drug from a different class should be initiated when use of a single drug in adequate doses fails to achieve the BP goal. When BP is more than 20/10 mmHg above SBP and DBP goals, consideration should be given to initiating therapy with two drugs, either as separate prescriptions or in combination therapy (see Table 32-6).
Once antihypertensive therapy is initiated, most patients should return for follow-up and adjustment of medications at approximately monthly intervals until the BP goal is reached. More frequent visits will be necessary for patients with stage 2 hypertension or with complicating coexisting conditions. After BP is at goal and stable, follow-up visits can usually be at 3–6-month intervals. Comorbidities, such as heart failure, associated diseases such as diabetes mellitus and the need for ongoing monitoring (e.g. laboratory testing), influence the frequency of visits.
Side effects and adverse effects of antihypertensive drugs may be so severe or undesirable that the patient does not comply with therapy. Table 32-5 describes the major side effects of antihypertensive drugs. Hyperuricaemia, hyperglycaemia and hypokalaemia are common side effects with both thiazide and loop diuretics. ACE inhibitors lead to high levels of bradykinin, which can cause coughing. An individual who develops a cough with the use of ACE inhibitors may be switched to an angiotensin II receptor blocker. Hyperkalaemia can be a serious side effect of the potassium-sparing diuretics and ACE inhibitors. Sexual dysfunction may occur with some of the diuretics. Orthostatic hypotension and sexual dysfunction are two undesirable effects of adrenergic-inhibiting agents. Tachycardia and orthostatic hypotension are potential adverse effects of both vasodilators and angiotensin inhibitors.
Patient teaching related to drug therapy
Patient and family teaching related to drug therapy is needed to identify and minimise side effects and to cope with therapeutic effects. Side effects of antihypertensive drug therapy are common. Side effects may be an initial response to a drug and may decrease with continued use of the drug. Informing the patient about side effects that lessen with time may enable the individual to continue taking the drug. The number or severity of side effects may be related to the dosage and it may be necessary to change the drug or decrease the dosage. In this case, the patient should be advised to report the side effects to the healthcare provider who prescribed the medication.
A common side effect of several of these drugs is orthostatic hypotension. This condition is caused by an alteration of the autonomic nervous system’s mechanisms for regulating pressure, which are required for position changes. Consequently, the patient may feel dizzy, weak and faint when assuming an upright position after sitting or lying down. (Specific measures to control or decrease orthostatic hypotension are presented in Box 32-6.)
PATIENT & FAMILY TEACHING GUIDE
When presenting information to the patient and/or family, the nurse should do the following:
General instructions
1. Provide the numerical value of the patient’s BP and explain what it means (e.g. high, low, normal, borderline). Encourage the patient to monitor BP at home.
2. Inform the patient that hypertension is usually asymptomatic and symptoms do not reliably indicate BP levels.
3. Explain that hypertension means high BP and does not relate to a ‘hyper’ personality.
4. Explain that long-term follow-up and therapy are necessary to treat hypertension. Therapy involves lifestyle changes (e.g. weight management, sodium reduction, smoking cessation) and, in most cases, medications.
5. Explain that therapy will not cure but should control hypertension.
6. Tell the patient that controlled hypertension is usually compatible with an excellent prognosis and a normal lifestyle.
7. Explain the potential dangers of uncontrolled hypertension.
Instructions related to medications
1. Be specific about the names, actions, dosages and side effects of prescribed medications.
2. Tell the patient to plan regular and convenient times for taking medications and measuring BP.
3. Tell the patient not to discontinue medications abruptly because withdrawal may cause a severe hypertensive reaction.
4. Tell the patient not to double doses when a dose is missed.
5. Inform the patient that if BP increases, the patient should not take an increased medication dosage before consulting with the healthcare provider.
6. Tell the patient not to take a medication belonging to someone else.
7. Tell the patient to supplement diet with foods high in potassium (e.g. citrus fruits and green leafy vegetables) if taking potassium-losing diuretics.
8. Tell the patient to avoid hot baths, excessive amounts of alcohol and strenuous exercise within 3 h of taking medications that promote vasodilation.
9. Many medications cause orthostatic hypotension. Explain to the patient that the effects of orthostatic hypotension can be reduced by arising slowly from bed, sitting on the side of the bed for a few minutes, standing slowly, not standing still for prolonged periods of time, doing leg exercises to increase venous return, sleeping with the head of the bed raised or on pillows and lying or sitting down when dizziness occurs.
10. Many medications cause sexual problems (e.g. erectile dysfunction, decreased libido). Encourage the patient to consult with the healthcare provider about changing drugs or dosages if sexual problems develop.
11. Inform the patient that the side effects of medication(s) often diminish with time.
12. Caution the patient about potentially high-risk over-the-counter medications, such as high-sodium antacids, appetite suppressants and cold and sinus medications. Advise the patient to read warning labels and to consult with the pharmacist.
Sexual dysfunction may occur with many of the antihypertensive drugs (see Table 32-5) and can be a major reason that a patient does not adhere to the treatment plan. Problems can range from decreased libido to erectile dysfunction. Rather than discussing a sexual problem with a healthcare professional, the patient may decide to simply stop taking the drug. The nurse should approach the patient on this sensitive subject and encourage discussion of any sexual dysfunction that may be experienced. It may be easier for the patient to discuss and handle sexual problems once it has been explained that the drug may be the source of the problems and that the side effects can be decreased or eliminated by changing to another antihypertensive drug. The patient should be encouraged to discuss side effects with the healthcare provider who prescribed the medication. If the patient is reluctant to do so, the nurse may offer to alert the healthcare provider to the sexual side effect that the patient is experiencing. There are so many options now in treating hypertension that a plan that is acceptable to the patient should be achievable.
Some unpleasant effects of drugs result from their therapeutic effect, but the impact can be minimised. For example, dry mouth and frequent voiding are unpleasant effects of diuretics. Sugarless gum or lollies may relieve the dry mouth. The nurse can assist the patient to develop a medication schedule to minimise unpleasant effects. When frequent urination interrupts sleep, taking the diuretic earlier in the day may be beneficial. Side effects of vasodilators and adrenergic inhibitors decrease if the drugs are taken in the evening. It should be remembered that BP is lowest during the night and highest shortly after awakening. Therefore drugs with 24-hour duration of action should be taken as early in the morning as possible (e.g. 4 or 5 am if the patient awakens to void).
Resistant hypertension
Resistant hypertension is the failure to reach goal BP in patients who are adhering to full doses of an appropriate three-drug therapy regimen that includes a diuretic. Healthcare professionals need to carefully explore reasons why the patient is not at goal BP (see Box 32-3).
BOX 32-3 Causes of resistant hypertension
Medications that can interfere with blood pressure control
Non-steroidal anti-inflammatory agents, including aspirin
Sympathomimetic agents (decongestants, diet pills, cocaine)
Stimulants (methylphenidate, dexmethylphenidate, dextroamphetamine, amphetamine, methamphetamine, modafinil)
Source: Calhoun DA, Jones D, Textor S et al. Resistant hypertension: diagnosis, evaluation, and treatment: a scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 2008; 51: 1403–1419. Available at http://hyper.ahajournals.org/cgi/reprint/HYPERTENSIONAHA.108.189141, accessed December 2010.
 NURSING MANAGEMENT: PRIMARY HYPERTENSION
NURSING MANAGEMENT: PRIMARY HYPERTENSION
 Nursing assessment
Nursing assessment
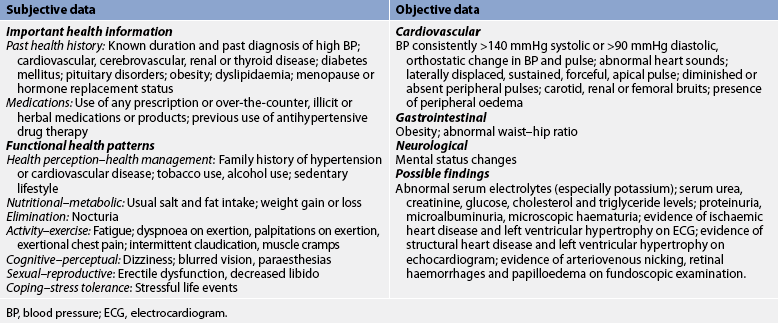
Subjective and objective data that should be obtained from a patient with hypertension are presented in Table 32-7.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses and collaborative problems for the patient with hypertension include, but are not limited to, those presented in Box 32-4.
NURSING DIAGNOSES & COLLABORATIVE PROBLEMS
Nursing diagnoses
• Ineffective health maintenance related to lack of knowledge of pathology, complications and management of hypertension
• Anxiety related to complexity of management regimen, possible complications and lifestyle changes associated with hypertension
• Sexual dysfunction related to side effects of antihypertensive medication
• Ineffective therapeutic regimen management related to:
• Ineffective tissue perfusion related to complications of hypertension (specify):
 Planning
Planning
The overall goals for the patient with hypertension are that the patient will: (1) achieve and maintain the individually determined goal BP; (2) understand, accept and implement the therapeutic plan; (3) experience minimal or no unpleasant side effects of therapy; and (4) be confident of their ability to manage and cope with this condition.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
Primary prevention of hypertension provides an attractive alternative to the costly cycle of managing hypertension and its complications. Current recommendations for primary prevention are based on lifestyle modifications that have been shown to prevent or delay the expected rise in BP in susceptible people. A diet rich in fruits, vegetables and low-fat dairy foods, with reduced saturated and total fats, significantly lowers BP (see Table 32-4). This diet has been recommended for primary prevention in the general population. Dietary modifications that do not require active participation of the individual, such as industry reducing the amount of salt added to processed foods, may be even more effective.
To raise awareness about the dangers of high BP, the National Heart Foundations of Australia and New Zealand have developed special internet pages and educational materials for healthcare professionals, patients and the public, including updated guides to assist with lowering high BP (see Resources on p 853).
 Individual patient evaluation
Individual patient evaluation
The majority of cases of hypertension are identified through routine screening procedures such as insurance, pre-employment and defence forces physical examinations. The nurse in these settings, as well as in most other practice settings, is in an ideal position to assess for the presence of hypertension, identify the risk factors for hypertension and coronary artery disease and teach the patient about these conditions. In addition to BP determination, a complete health assessment should include such factors as age, sex and race; diet history (including sodium and alcohol intake); weight patterns; and family history of heart disease, stroke, renal disease and diabetes mellitus. Medications taken, both prescribed and over-the-counter, should be noted. The patient should be asked about a previous history of high BP and the results of treatment (if any) (see Box 32-4).
 Blood pressure measurement
Blood pressure measurement
The auscultatory method of BP measurement is recommended (see Box 32-5). Initially, the BP is taken at least two times, at least 1 minute apart, with the average pressure recorded as the value for that visit. Waiting for at least 1 minute between readings allows the venous blood to drain from the arm and prevents inaccurate readings. The size and placement of the BP cuff are important considerations for accurate measurement. Using a cuff that is too small or too large will result in readings that are falsely high or falsely low, respectively. The cuff should be placed snugly around the patient’s bare upper arm with the midline of the bladder of the cuff (usually marked on the cuff by the manufacturer) placed above the brachial artery. Using the forearm is acceptable in patients who have an upper arm circumference over 51 cm and a thigh or extra-large cuff does not fit the upper arm. When this procedure is used, the forearm is supported at heart level and the clinician auscultates Korotkoff sounds over the radial artery, detects SBP with a Doppler or uses an oscillometric device. This method, which provides only a general estimate of SBP and DBP, is largely overestimated. The accuracy of these methods has not been validated.8
BOX 32-5 How to measure blood pressure accurately
• It is still recommended to use a mercury sphygmomanometer. If another form of sphygmomanometer is used, it should be calibrated regularly against a mercury sphygmomanometer to ensure accuracy. Because of environmental concerns about the toxicity of mercury, there is a move to phase out mercury sphygmomanometers. At present there has been no agreement relating to an optimal replacement, as the accuracy of other available devices is very dependent on the particular product chosen.
• The patient should be seated and relaxed, preferably for several minutes prior to measurement, and in a quiet room.
• The arm being used for measurement should be free of constricting clothing so that the cuff can be wrapped around the upper arm without impediment.
• Select an appropriate cuff size. The bladder length should be at least 80% and the width at least 40% of the circumference of the mid-upper arm. Use of a ‘standard size’ cuff in people with large arms can result in artificially high blood pressure readings. If an oversized cuff cannot be satisfactorily fitted on a large arm, then the utilisation of an appropriately sized cuff on the forearm with radial artery auscultation should be considered.
• Wrap the cuff snugly around the upper arm, with the centre of the bladder of the cuff positioned over the brachial artery and the lower border of the cuff about 2 cm above the bend of the elbow.
• Ensure the cuff is at heart level by appropriately supporting the arm whatever the position of the patient.
• Inflate the cuff to the pressure at which the radial pulse disappears.
• Deflate the cuff at a rate of 2–3 mmHg/beat (2–3 mmHg/s) or less and note the pressure at which the radial pulse reappears.
• Fully deflate the cuff, wait approximately 30 s and then inflate the cuff to at least 30 mmHg above that at which the radial pulse reappeared.
• While deflating the cuff at a rate of 2–3 mmHg/beat (2–3 mmHg/s) or less, auscultate over the brachial artery at the elbow.
• Record the result for systolic and diastolic pressures to the nearest 2 mmHg. For the systolic reading, record the level at which the first (at least two consecutive) sounds are heard, even if they subsequently transiently disappear with progressive deflation (the ‘auscultatory gap’). For the diastolic reading, use phase V Korotkoff (disappearance of sound). Only use phase IV Korotkoff (muffling of sound) if sound continues towards zero.
• Wait 30 s before repeating the procedure in the same arm.
• Average the readings. If the first two readings differ by more than 10 mmHg systolic or 6 mmHg diastolic or if the initial readings are high, take several readings after 5 min of quiet rest, until consecutive readings do not vary by greater than these amounts.
• Ideally, patients should not take caffeine-containing beverages or smoke for at least 2 h before blood pressure is measured, as both tend to produce acute increases in blood pressure (and particularly in combination).
Source: National Heart Foundation. Guide to management of hypertension, 2008. Canberra: National Heart Foundation of Australia; 2008.
Does a modest reduction in dietary salt decrease blood pressure in different ethnic groups?
EVIDENCE-BASED PRACTICE
Clinical question
In persons with normal or elevated blood pressure of different ethnicities (P), what is the effect of a modest reduction in salt intake (I) compared to no change in salt intake (C) on blood pressure (BP) reduction (O)?
Best available evidence
A reduction in salt intake lowers blood pressure and this randomised double-blind crossover trial examined the effects in three different ethnic groups: Caucasian (n = 71), African (n = 69) and Asian (n = 29) individuals who had untreated mildly raised blood pressure. With a reduction in salt intake, there was a significant decrease in blood pressure from 146+/–13/91+/–8 to 141+/–12/88+/–9 mmHg (P <0.001), in urinary albumin from 10.2 (IQR: 6.8 to 18.9) to 9.1 (6.6 to 14.0) mg/24 hours (P <0.001), in the albumin/creatinine ratio from 0.81 (0.47 to 1.43) to 0.66 (0.44 to 1.22) mg/mmol (P <0.001) and in carotid–femoral pulse wave velocity from 11.5+/–2.3 to 11.1+/–1.9 m/s (P <0.01). All reductions were significant in all groups except for pulse wave velocity, with reductions for this outcome significant only in Africans.
Conclusion
A modest reduction in salt intake, which is more likely to be achieved, causes significant and universal effects on blood pressure. Furthermore, it reduces urinary albumin and improves large artery compliance.
Implications for nursing practice
• Include assessment of usual salt intake during nutritional assessment.
• Include in patient education that modestly reducing salt intake by 5–6 g/day will decrease BP.
• Provide appropriate educational materials on how to achieve this reduction in salt intake (e.g. DASH eating plan [see Table 32-4])
• Have patient consult with dietician to assist in teaching about salt reduction in diet.
BP measurements of both arms should be performed initially to detect any differences between arms. Atherosclerotic narrowing of the subclavian artery can cause a falsely low reading on the side where the narrowing occurs. Therefore the arm with the higher reading should be used for all subsequent BP measurements.
The patient’s arm is uncovered and placed at the level of the heart for BP measurement. For BP measurements taken in the sitting position, the arm is raised to the level of the heart and supported. For measurements taken in a supine position, the arm should also be raised to heart level and supported (e.g. small pillow). If the arm is resting on the bed, it will be below heart level.
Orthostatic (or postural) changes in BP and pulse should be measured in older adults, people taking antihypertensive drugs and patients who report symptoms consistent with reduced BP upon standing (e.g. light-headedness, dizziness, syncope). Serial BP and pulse are measured with the patient in the supine, sitting and standing positions. BP and pulse are initially measured with the patient in the supine position after at least 2–3 minutes of rest, measured again within 1–2 minutes of repositioning the patient in the sitting position with legs dangling and measured within 1–2 minutes of repositioning the patient to the standing position. Usually the SBP decreases slightly (<10 mmHg) on standing, whereas the DBP and pulse increase slightly. A decrease of 20 mmHg or more in SBP, a decrease of 10 mmHg or more in DBP, and/or an increase of 20 beats per minute or more in pulse from supine to standing is defined as orthostatic hypotension. Common causes of orthostatic hypotension include intravascular volume loss and inadequate vasoconstrictor mechanisms related to disease or medications.
In the acute care setting, BP measurement is typically performed to evaluate vital signs, volume status and effects of medications as opposed to the diagnosis of hypertension. Medications, acute illness, bed rest and alterations in usual diet all have an impact on BP values. The healthcare provider should be informed of any patient with a persistent elevation in BP. These patients should also be evaluated for hypertension after discharge.5
 Screening programs
Screening programs
Screening programs in the community are widely used to assess people’s BP. At the time of the BP measurement, each person should be informed in writing of the numeric value of the reading and, if necessary, why further evaluation is important. Effort and resources should be focused on controlling BP in the person already identified as having hypertension; identifying and controlling BP in at-risk groups such as Indigenous Australians, Māori and Pacific Islander peoples, obese people and blood relatives of people with hypertension; and screening those with limited access to the healthcare system.
 Cardiovascular risk factor modification
Cardiovascular risk factor modification
Education regarding cardiovascular risk factors is appropriate for individual and targeted screening programs. Modifiable cardiovascular risk factors include hypertension, obesity, diabetes mellitus, tobacco use and physical inactivity. Risk factors can easily be identified and modification discussed with the patient. (Health-promoting behaviours for cardiovascular risk factors are discussed in Table 33-3.)
 Ambulatory and home care
Ambulatory and home care
The primary nursing responsibilities for long-term management of hypertension are to assist the patient in reducing BP and complying with the treatment plan. Nursing actions include patient and family teaching (see Box 32-6), detection and reporting of adverse treatment effects, compliance assessment and enhancement, and evaluation of therapeutic effectiveness. Patient and family teaching includes the following: (1) nutritional therapy; (2) drug therapy; (3) physical activity; (4) home monitoring of BP (if appropriate); and (5) tobacco cessation (if applicable).
 Physical activity
Physical activity
Physical activity is bodily movement produced by skeletal muscles that requires energy expenditure. Health benefits from physical activity can be achieved with moderate-intensity aerobic activities (e.g. brisk walking). The goal for all adults is at least 30 minutes per day, most days of the week.1 Generally, physical activity is more likely to be sustained if it is safe and enjoyable, fits easily into the daily schedule and does not result in excessive financial or social costs. In some communities, health clubs offer special ‘off-peak’ rates to encourage physical activity among older adults. Cardiac rehabilitation programs offer supervised exercise with education about reduction of cardiovascular risk factors. Nurses can assist people with hypertension to increase their physical activity by identifying and communicating the need for increased activity, explaining the difference between physical activity and moderate-intensity aerobic exercise, assisting in initiating an exercise plan and following up appropriately.
 Home BP monitoring
Home BP monitoring
Some patients benefit from regularly monitoring their BP at home. Home BP measurement may give a more valid indication of BP because the patient is more relaxed. The patient should be instructed to take BP readings after resting quietly for 3–5 minutes in a comfortable chair with the upper arm at heart level. The average of three readings, separated by at least 1 minute, should be used as the BP reading.3 The patient should be advised not to smoke, exercise or ingest caffeine for 30 minutes prior to taking BP measurements. It is important to emphasise to the patient that a single reading is not as important as a series of readings over a period of time. The patient should be instructed to take BP readings daily when treatment is initiated or medications are adjusted and weekly once the BP has stabilised. A log of the BP measurements should be maintained by the patient and brought to surgery visits. Devices that have memory or printouts of the readings are recommended to facilitate accurate reporting.8
Home BP readings may help achieve patient compliance by reinforcing the need to remain on therapy. A patient may become excessively concerned with the BP readings when using home monitoring. Generally, however, this practice should reassure the patient that the treatment is effective.
 Patient compliance
Patient compliance
A major problem in the long-term management of the patient with hypertension is poor compliance with the prescribed treatment plan. The reasons are many and include inadequate patient teaching, unpleasant side effects of drugs, return of BP to normal range while on medication, lack of motivation, high cost of drugs, having to regularly revisit the doctor for prescriptions and lack of a trusting relationship between the patient and the healthcare provider. In addition to using BP determinations as an indicator of compliance, the nurse should also assess the patient’s diet, activity level and lifestyle.
Individual assessment to determine the reasons why the patient is not complying with the treatment plan and development of an individualised plan with the patient’s assistance are essential. The plan should be compatible with the patient’s personality, habits and lifestyle. Active patient participation increases the likelihood of adherence to the treatment plan. Measures such as involving the patient in scheduling medication convenient to a daily routine, helping the patient to link pill taking with another daily activity, selecting medications that are affordable and involving family members (if necessary) help increase patient compliance. Substituting combination tablets for multiple drugs once the BP is stabilised may also facilitate compliance, because the patient has to take fewer medications each day and the cost may be less. It is important to help the patient and family understand that hypertension is a chronic condition that cannot be cured but can be controlled with drug therapy, diet therapy, physical activity, periodic evaluation and other relevant lifestyle changes.
 Evaluation
Evaluation
The overall expected outcomes are that the patient with hypertension will:
• achieve and maintain goal BP as defined for the individual
• understand, accept and implement the therapeutic plan
• experience minimal or no unpleasant side effects of therapy.
Gerontological considerations: hypertension
The number of Australians and New Zealanders aged 65 and older continues to rise and this age group will comprise 26% of the Australian population by the year 2051.14 The prevalence of hypertension increases with age. The lifetime risk of developing hypertension is approximately 90% for middle-aged (age 55–65 years) and older (age >65 years) normotensive men and women.14 In industrialised countries, SBP rises throughout the life span, whereas DBP rises until approximately age 55 and then declines due to an increase in central arterial stiffness.15 ISH is the most common form of hypertension in individuals over 50 years of age.16,17 Additionally, older adults are more likely to have ‘white coat’ hypertension.8
The following age-related physical changes play a role in the pathophysiology of hypertension in the older adult: (1) loss of tissue elasticity; (2) increased collagen content and stiffness of the myocardium; (3) increased peripheral vascular resistance; (4) decreased adrenergic receptor sensitivity; (5) blunting of baroreceptor reflexes; (6) decreased renal function; and (7) decreased renin response to sodium and water depletion. In the older adult taking antihypertensive medication, absorption of some drugs may be altered as a result of decreased splanchnic blood flow. Metabolism and excretion of drugs may also be prolonged.
Careful technique is important in assessing BP in older adults. In some older people, there is a wide gap between the first Korotkoff sound and subsequent beats. This is called the auscultatory gap. Failing to inflate the cuff high enough may result in seriously underestimating the SBP. This problem can be avoided by palpating the brachial or radial artery while inflating the cuff to a level above the disappearance of the pulse.
Older adults are sensitive to BP changes. Therefore reducing SBP to less than 120 mmHg in a person with longstanding hypertension could lead to inadequate cerebral blood flow. Older adults also produce less renin and are more resistant to the effects of ACE inhibitors and angiotensin II receptor blockers.
Because of varying degrees of impaired baroreceptor reflex mechanisms, orthostatic hypotension occurs often in older adults, especially in those with ISH. Orthostatic hypotension in this age group is often associated with volume depletion or chronic disease states, such as decreased renal and hepatic function or electrolyte imbalance. To reduce the likelihood of orthostatic hypotension, antihypertensive drugs should be started at low doses and increased cautiously. BP and pulse should be measured in the supine, sitting and standing positions at every visit.
Hypertensive crisis
Hypertensive crisis is a severe and abrupt elevation in BP, arbitrarily defined as a DBP greater than 140 mmHg.18 The rate of rise of BP is more important than the absolute value in determining the need for emergency treatment. Patients with chronic hypertension can tolerate much higher BP than previously normotensive people. Prompt recognition and management of hypertensive crisis is essential to decrease the threat to organ function and life.
Hypertensive crisis occurs most commonly in patients with a history of hypertension who have failed to comply with their prescribed medications or who have been under-medicated. In this setting, rising BP is thought to trigger endothelial damage and the release of vasoconstrictor substances. A vicious cycle of BP elevation ensues leading to life-threatening damage to target organs. Hypertensive crisis related to cocaine or crack use is becoming a more frequent problem. Other drugs, such as amphetamines, phencyclidine (PCP) and lysergic acid diethylamide (LSD), may also precipitate hypertensive crisis that may be complicated by drug-induced seizures, stroke, myocardial infarction or encephalopathy. Box 32-7 lists causes of hypertensive crisis.
Hypertensive crisis is classified by the degree of organ damage and the rapidity with which the BP must be lowered. Hypertensive emergency, which develops over hours to days, is a situation in which a patient’s BP is severely elevated (>180/120 mmHg) with evidence of acute target organ damage, especially damage to the central nervous system.1 Hypertensive emergencies can precipitate encephalopathy, intracranial or subarachnoid haemorrhage, acute left ventricular failure with pulmonary oedema, myocardial infarction, renal failure, dissecting aortic aneurysm and retinopathy.18 Hypertensive urgency, which develops over days to weeks, is a situation in which a patient’s BP is severely elevated but there is no clinical evidence of target organ damage.
CLINICAL MANIFESTATIONS
A hypertensive emergency is often manifested as hypertensive encephalopathy, a syndrome in which a sudden rise in BP is associated with headache, nausea, vomiting, seizures, confusion, stupor and coma. The manifestations of encephalopathy are the results of increased cerebral capillary permeability leading to cerebral oedema and a disruption in cerebral function.18 Other common manifestations are blurred vision and transient blindness from papilloedema and retinopathy.
Renal insufficiency ranging from minor impairment to complete renal shutdown may occur. Rapid cardiac decompensation ranging from unstable angina to infarction and pulmonary oedema is also possible with patients presenting with chest pain and dyspnoea. Aortic dissection causes excruciating chest and back pain often accompanied by diaphoresis and the loss of pulses in an extremity.
Patient assessment is extremely important, especially monitoring for signs of neurological dysfunction, retinal damage, heart failure, pulmonary oedema and renal failure. The neurological manifestations are often similar to the presentation of a stroke. However, a hypertensive crisis does not show focal or lateralising signs often seen with a stroke.
 NURSING AND COLLABORATIVE MANAGEMENT: HYPERTENSIVE CRISIS
NURSING AND COLLABORATIVE MANAGEMENT: HYPERTENSIVE CRISIS
BP level alone is a poor indicator of the seriousness of the patient’s condition and is not the major factor in deciding the treatment for a hypertensive crisis. The association between elevated BP and signs of new or progressive target organ damage (e.g. cerebrovascular, cardiac, retinal or renal involvement) determines the seriousness of the situation.
Hypertensive emergencies require hospitalisation, parenteral (intravenous [IV]) administration of antihypertensive drugs and intensive care monitoring. When treating hypertensive emergencies, the mean arterial pressure (MAP) is often used instead of systolic and diastolic readings to guide and evaluate therapy. MAP is calculated as follows:
MAP = (SBP + 2 DBP) ÷ 3
The initial treatment goal is to decrease MAP by no more than 25% within minutes to 1 hour. If the patient is stable, the target goal for BP is 160/100–110 mmHg over the next 2–6 hours. Lowering the BP excessively may decrease cerebral, coronary or renal perfusion and could precipitate a stroke, acute MI or renal failure. Additional gradual reductions towards a normal BP should be implemented over the next 24–48 hours if the patient is clinically stable. Special circumstances include patients with aortic dissection who should have their SBP lowered to below 100 mmHg if tolerated. Another exception is patients with acute ischaemic stroke, where BP may be lowered to enable the use of thrombolytic agents. Finally, an elevated BP in the immediate post-stroke period may be a compensatory response to improve cerebral perfusion to ischaemic brain tissue. Currently, there is no clear evidence supporting the use of antihypertensive medications in these patients.1
The IV drugs used for hypertensive emergencies include vasodilators (e.g. sodium nitroprusside, glyceryl trinitrate, hydralazine), adrenergic inhibitors (e.g. phentolamine, esmolol) and the ACE inhibitor enalaprilat.1 Sodium nitroprusside is the most effective IV drug for the treatment of hypertensive emergencies. Oral agents may be administered in addition to IV drugs to help make an earlier transition to long-term therapy. The mechanisms of action and the adverse effects of these drugs are presented in Table 32-5.
Administered intravenously, the drugs have a rapid (within seconds to minutes) onset of action. The patient’s BP and pulse should be taken every 2–3 minutes during the initial administration of these drugs. The use of an intra-arterial line (see Ch 65) or an automated, non-invasive BP machine to monitor the BP is required. The rate of drug administration is titrated according to the level of MAP or BP. It is important to prevent hypotension and its effects in a person whose body has adjusted to an elevated BP. An excessive reduction in BP may cause stroke, MI or visual changes. Continual ECG monitoring is frequently done to observe for cardiac arrhythmias. Extreme caution is needed in treating the patient with coronary artery disease or cerebrovascular insufficiency. Hourly urinary output should be measured to assess renal perfusion. Careful monitoring of vital signs and urinary output provides information regarding the effectiveness of these drugs and the patient’s response to therapy. Patients receiving IV antihypertensive drugs may be restricted to bed; getting up (e.g. to use the commode) may cause severe cerebral ischaemia and fainting.
Regular, ongoing assessment is essential to evaluate the patient with severe hypertension. Frequent neurological checks, including level of consciousness, pupillary size and reaction, movement of extremities and reactions to stimuli help detect any changes in the patient’s condition. Cardiac, pulmonary and renal systems should be monitored for decompensation caused by the severe elevation in BP (e.g. pulmonary oedema, heart failure, angina, renal failure).
Hypertensive urgencies usually do not require IV medications but can be managed with oral agents. The patient with a hypertensive urgency may not need hospitalisation, but requires frequent follow-up. The oral drugs most frequently used for hypertensive urgencies are captopril, labetolol and clonidine (see Table 32-5). The disadvantage of oral medications is the inability to regulate the dosage moment to moment, as can be done with IV medications. If a patient with a hypertensive urgency is not hospitalised, outpatient follow-up should be arranged within 24 hours.
A patient with severe elevation of BP but without target organ damage may not require urgent drug therapy or hospitalisation.19 Allowing the patient to sit for 20–30 minutes in a quiet environment may significantly reduce BP. Oral drugs may then be instituted or adjusted. Additional nursing interventions include encouraging the patient to verbalise any concerns or fears, answering questions regarding hypertension and eliminating any adverse stimuli (e.g. excess noise) in the patient’s environment.
Once the hypertensive crisis is resolved, it is important to determine the cause. The patient will need appropriate management and extensive education to avoid future crises.
The patient with primary hypertension
CASE STUDY
Patient profile
Taku is a 45-year-old Pacific Islander man with no previous history of hypertension. At a screening clinic 1 month ago his blood pressure was found to be 180/120 mmHg. He has been followed up by his nurse practitioner for the past month. During this time he has been taking hydrochlorothiazide 12.5 mg/day.
Subjective data
• Father died of stroke at age 60 years
• Mother is alive but has hypertension
• States that he feels fine and is not a ‘hyper’ person
• Smokes one pack of cigarettes daily
• Drinks a six-pack of beer on Friday and Saturday nights
• Has been told that blood pressure medication interferes with sexual relationships
CRITICAL THINKING QUESTIONS
1. What risk factors for hypertension does this patient have?
2. What evidence of target organ damage is present?
3. What misconceptions about hypertension should be corrected?
4. Based on the assessment data presented, what are the nursing priorities for this patient? Include one or more priority nursing diagnoses. What are the collaborative problems?
5. What areas would you focus on in teaching this patient about his illness?
1. If a patient has decreased cardiac output caused by fluid volume deficit and marked vasodilation, the regulatory mechanism that will increase the blood pressure by improving both of these is:
2. While obtaining subjective assessment data from a patient with hypertension, the nurse recognises that a modifiable risk factor for the development of hypertension is:
3. Target organ damage that can occur from hypertension includes:
4. A high-risk population that should be targeted in the primary prevention of hypertension is:
5. In teaching a patient with hypertension about controlling the condition, the nurse recognises that:
6. A major consideration in the management of the older adult with hypertension is to:
7. A man with newly diagnosed hypertension has a BP of 158/98 mmHg after 12 months of exercise and diet modifications. The nurse advises the patient that:
8. A patient is admitted to the hospital in hypertensive crisis. The nurse recognises that a hypertensive urgency differs from a hypertensive emergency in that:
1 . The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC-7), JAMA. 2003;289:2560. Available at www.nhlbi.nih.gov/guidelines/hypertension/index.htm accessed 20 November 2010.
2 Australian Institute of Health & Welfare (AIHW). Chronic disease and associated risk factors in Australia. Canberra: AIHW, 2006.
3 New Zealand Ministry of Health (NZMOH). DHB toolkit. Cardiovascular disease. 2nd edn. Wellington: NZMOH; 2003. Available at www.moh.govt.nz/moh.nsf/0/31E369C74DB3774DCC257234000461F0 accessed 20 November 2010.
4 American Heart Association. Heart disease and stroke statistics: 2010 update. Available at www.americanheart.org/presenter.jhtml?identifier=1200026. accessed 20 November 2010.
5 Heart Foundation. Guide to management of hypertension, 2008. Canberra: National Heart Foundation of Australia; 2008. Available at www.heartfoundation.org.au/SiteCollectionDocuments/A_Hypert_Guidelines2008_2009Update_FINAL.pdf accessed 20 November 2010.
6 Heart Foundation New Zealand. Assessment and management of cardiovascular risk: best practice evidence-based guidelines. Available at www.nzgg.org.nz/guidelines/0035/CVD_Risk_Summary.pdf, 2003. accessed 20 November 2010.
7 Australian Bureau of Statistics (ABS). Causes of death, Australia. Canberra: ABS; 2008. Available at www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3303.0Main+Features12008?OpenDocument accessed 20 November 2010.
8 Crooke M. New Zealand cardiovascular guidelines: best practice evidence-based guideline: the assessment and management of cardiovascular risk, December 2003, Clin Biochem Rev. 2007;28(1):19–29. Available at www.ncbi.nlm.nih.gov/pmc/articles/PMC1904419/ accessed 20 November 2010.
9 Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women, Circulation. 2007;115(11):1481–1501. Available at http://circ.ahajournals.org/cgi/reprint/115/11/1481 accessed 20 November 2010.
10 Department of Health and Ageing. National chronic disease strategy. Available at www.health.gov.au/internet/main/publishing.nsf/Content/7E7E9140A3D3A3BCCA257140007AB32B/$File/stratal3.pdf, 2006. accessed 20 November 2010.
11 Appel LJ. DASH position paper: dietary approaches to lower blood pressure. J Clin Hypertension. 2009;11:358.
12 Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella E. Trends in hypertension prevalence, awareness, treatment and control rates in United States adults between 1988–1994 and 1994–2004. Hypertension. 2008;52:818–827.
13 National Heart, Lung, and Blood Institute. Your guide to lowering your blood pressure with DASH. NIH pub. no. 06-4082. Bethesda, MD: National Heart, Lung, and Blood Institute; 2006. Available at www.nhlbi.nih.gov/health/public/heart/hbp/dash/new_dash.pdf accessed 20 November 2010.
14 Australian Bureau of Statistics (ABS). Population projections for Australia. Canberra: ABS; 2004–2101. Available at: www.abs.gov.au/AUSSTATS/abs@.nsf/Lookup/3222.0Explanatory%20Notes12004%20to%202101?OpenDocument accessed 1 September 2010.
15 Besdine RW, Wetle TF. Improving health for elderly people: an international health promotion and disease prevention agenda. Aging Clin Exp Res. 2010;22(3):219–230.
16 Vasan RS, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men: the Framingham Heart Study. JAMA. 2002;287(8):1003–1010. Seminal study
17 Redberg RF, Benjamin EJ, Bittner V. ACCF/AHA 2009 performance measures for primary prevention of cardiovascular disease in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on performance measures (Writing Committee to Develop Performance Measures for Primary Prevention of Cardiovascular Disease). J Am Coll Cardiol. 2009;54:1364.
18 Pergolini MS. The management of hypertensive crises: a clinical review. Clin Ter. 2009;160(2):151–157.
19 Libby P, Bonow R, Zipes D, Mann D. Braunwald’s heart disease: a textbook of cardiovascular medicine, 8th edn. Philadelphia: Saunders, 2008.
Department of Health and Ageing (Australia): cardiovascular disease. www.health.gov.au/internet/main/publishing.nsf/Content/chronic-cardio
National Heart Foundation of Australia. www.heartfoundation.com.au
National Heart Foundation New Zealand. www.heartfoundation.org.nz
New Zealand Ministry of Health: cardiovascular toolkit. www.moh.govt.nz/moh.nsf