Chapter 37 NURSING MANAGEMENT: vascular disorders
1. Describe the aetiology and pathophysiology of peripheral arterial disease.
2. Identify the major risk factors associated with peripheral arterial disease.
3. Describe the pathophysiology, clinical manifestations and multidisciplinary care of aortic aneurysms.
4. Apply knowledge of perioperative nursing care to a patient having an aortic aneurysm repair.
5. Describe the pathophysiology, clinical manifestations and multidisciplinary care of aortic dissection.
6. Discuss the clinical manifestations, multidisciplinary care and surgical management of peripheral arterial disease of the lower extremities.
7. Discuss the nursing management of the patient with acute arterial insufficiency affecting the lower extremities.
8. Differentiate between the pathophysiology, clinical manifestations and multidisciplinary care of thromboangiitis obliterans (Buerger’s disease) and Raynaud’s phenomenon.
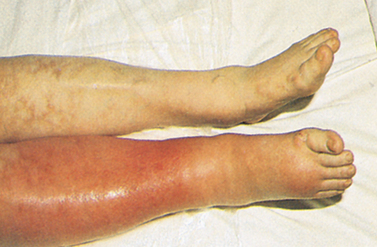
9. Identify the risk factors predisposing to the development of superficial thrombophlebitis and deep vein thrombosis.
10. Differentiate between the clinical characteristics of superficial thrombophlebitis and deep vein thrombosis.
11. Describe the nursing management of the patient with deep vein thrombosis.
12. Explain the purpose and actions of commonly used anticoagulants and the nursing management of the patients receiving them.
13. Discuss the pathophysiology, clinical manifestations, and collaborative and nursing management of venous leg ulcers.
chronic venous insufficiency (CVI)
peripheral arterial disease (PAD)
PERIPHERAL ARTERIAL DISEASE
Problems of the vascular system include disorders of the arteries and veins. Peripheral vascular disease is a term commonly used in New Zealand and Australia and is a general term used to describe reduced arterial blood supply. Peripheral arterial disease (PAD) is a term used to describe a wide variety of conditions affecting arteries in the neck, abdomen and extremities. PAD can be subdivided into occlusive disease, aneurysmal disease and vasospastic phenomenon. In contrast, venous diseases primarily affect the lower extremities and can be categorised into venous thrombosis and chronic venous insufficiency.
Peripheral arterial disease involves progressive narrowing and degeneration of the arteries of the neck, abdomen and extremities. Regardless of the anatomical location, atherosclerosis is responsible for the majority of PAD, both occlusive and aneurysmal.1 Although PAD typically appears in the sixth to eighth decades of life, it occurs at an earlier age in people with diabetes mellitus. Men in their sixties are almost twice as likely to have PAD as are women. However, as women age, the incidence of PAD is similar to or greater than that in men. After age 85, 30–50% of both men and women have PAD.2,3 Thus, as the population ages, PAD will become a major healthcare problem in Australia and New Zealand. Recent Australian data show PAD claims more than 2500 lives annually (1.9% of all deaths) and is responsible for 24,288 hospitalisations per year.4
PAD is strongly related to other manifestations of cardiovascular disease and its risk factors. Specifically, people with PAD have a twofold to threefold risk of cardiovascular morbidity and mortality.2 Therefore, PAD must be thought of as a marker of advanced systemic atherosclerosis. If an individual has PAD, it is likely that they will also have coronary artery disease and/or carotid artery disease.
AETIOLOGY AND PATHOPHYSIOLOGY
The leading cause of PAD is atherosclerosis, a gradual thickening of the intima and media of the arteries, which leads to progressive narrowing of the vessel lumen. Although the exact cause (or causes) of atherosclerosis remains unknown, several theories exist (see Ch 33). The pathological changes that occur with atherosclerosis consist of migration and replication of smooth muscle cells, deposition of connective tissue, lymphocyte and macrophage infiltration, and accumulation of lipids.
The four most significant risk factors for PAD are cigarette smoking, hyperlipidaemia, hypertension and diabetes mellitus, with the most important being cigarette smoking. If smoking starts at or before the age of 16 there is evidence that the risk of developing PAD is more than doubled, regardless of the amount smoked. Other risk factors include being overweight or obese, hypertriglyceridaemia, hyperuricaemia, family history, sedentary lifestyle and stress. Additional risk factors under investigation include elevated homocysteine and ferritin levels.5,6
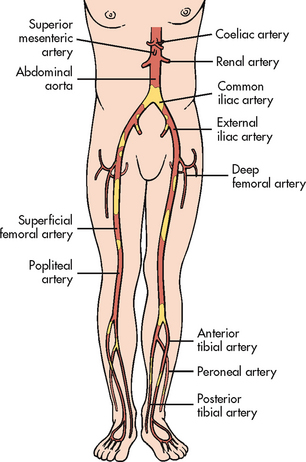
Although atherosclerosis is a diffuse process, certain segments of the arterial tree are more commonly involved, including the coronary arteries (see Ch 33), carotid arteries (see Ch 57), aortic bifurcation, iliac and common femoral arteries, femoral profunda, superficial femoral artery and distal popliteal artery (especially in diabetics) (see Fig 37-1).1 The involvement is generally segmental, with normal segments interspersed between involved ones. Clinical manifestations occur when the vessel is between 60% and 75% occluded.
Carotid artery disease
Atherosclerosis is the most common cause of carotid artery disease (cerebrovascular disease) in New Zealand and Australia. Over 80% of all strokes are ischaemic in nature and result from an atherothrombotic event.4 If carotid artery disease is identified early and treated, the risk of stroke decreases. Cerebrovascular disease and stroke are discussed in Chapter 57.
DISORDERS OF THE AORTA
The aorta is the largest artery and is responsible for supplying oxygenated blood to essentially all vital organs in the body. The most common vascular problems that affect the aorta are aortic aneurysms and aortic dissection. Although the underlying aetiology, pathophysiology and clinical manifestations of these three aortic problems are slightly different, the diagnostic studies, surgical therapy and nursing management are similar.
Aortic aneurysms
Aneurysms are outpouchings or dilations of the arterial wall and are common problems involving the aorta. Aneurysms of peripheral arteries can also occur but are far less common. Aneurysms occur in men more often than in women and their incidence increases with age.7 Abdominal aortic aneurysms occur in 5–7% of people over 60 years of age, and the incidence is almost five times higher in men than in women.4
AETIOLOGY AND PATHOPHYSIOLOGY
Aortic aneurysms may involve the aortic arch, thoracic aorta and/or abdominal aorta. Most aneurysms, however, are found in the abdominal aorta below the level of the renal arteries. The growth rate of aneurysms is unpredictable, but the larger the aneurysm, the greater the risk of rupture. The dilated aortic wall becomes lined with thrombi that can embolise, leading to acute ischaemic symptoms to distal (downstream) branches. Three-quarters of true aortic aneurysms occur in the abdomen (see Fig 37-2) and one-quarter in the thoracic aorta. Popliteal artery aneurysms rank third in frequency. Patients may have an aneurysm in more than one location.
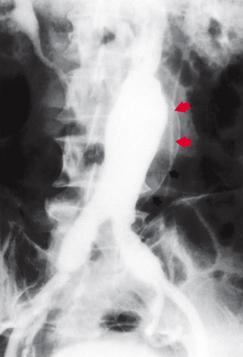
Figure 37-2 Angiography demonstrating fusiform abdominal aortic aneurysm. Note calcification of the aortic wall (arrows) and extension of the aneurysm into the common iliac arteries.
Although the exact cause of aneurysms remains unknown, several theories of pathogenesis exist. The most commonly accepted aetiology of aneurysms is atherosclerosis.7 It is known that atherosclerotic plaques deposit beneath the intima (the innermost layer of the arterial wall). This plaque formation is thought to cause degenerative changes in the media (middle layer of the arterial wall), leading to loss of elasticity, weakening and eventual dilation of the aorta.
Several studies have shown a strong genetic predisposition in the development of abdominal aortic aneurysms. The familial tendency to develop aneurysms is related to either a specific defect in collagen (Ehlers-Danlos syndrome) or a premature degeneration of vascular elastic tissue (Marfan’s syndrome).1 Less common causes of aneurysm formation include penetrating or blunt trauma, acute or chronic infections (e.g. Salmonella) and disruptions of anastomoses.7
CLASSIFICATION
Aneurysms are generally divided into two basic classifications: true and false aneurysms (see Fig 37-3). A true aneurysm is one in which the wall of the artery forms the aneurysm, with at least one vessel layer still intact. True aneurysms can be further subdivided into fusiform and saccular dilations. A fusiform aneurysm is circumferential and relatively uniform in shape. A saccular aneurysm is pouch-like with a narrow neck connecting the bulge to one side of the arterial wall.
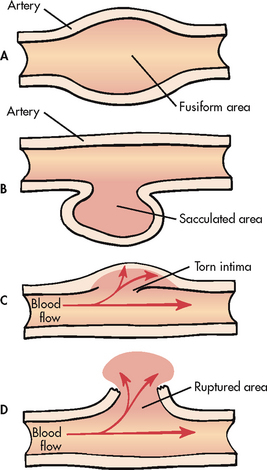
Figure 37-3 A, True fusiform abdominal aortic aneurysm. B, True saccular aortic aneurysm. C, Aortic dissection. D, False aneurysm or pseudoaneurysm.
A false aneurysm, or pseudoaneurysm, is not an aneurysm but a disruption of all layers of the arterial wall resulting in bleeding that is contained by surrounding structures. False aneurysms may result from trauma or infection or after peripheral artery bypass graft surgery at the site of the graft-to-artery anastomosis. They may also result from arterial leakage after removal of cannulae, such as upper or lower extremity arterial catheters and intraaortic balloon pump devices.7
CLINICAL MANIFESTATIONS
Thoracic aortic aneurysms are usually asymptomatic. When manifestations are present, they are varied. The most common manifestation is deep, diffuse chest pain. Aneurysms located in the ascending aorta and the aortic arch can produce hoarseness in the patient as a result of pressure on the recurrent laryngeal nerve. Pressure on the oesophagus can cause dysphagia. If the aneurysm presses on the superior vena cava, it can cause decreased venous drainage resulting in distended neck veins and oedema of the head and arms.
Abdominal aortic aneurysms are most often asymptomatic. They are detected on routine physical examination or coincidentally when the patient is examined for an unrelated problem (e.g. abdominal X-ray, ultrasound, computed tomography [CT] scan, intravenous pyelogram or abdominal surgery). On physical examination, a pulsatile mass in the periumbilical area slightly to the left of the midline may be detected. Bruits may be audible with a stethoscope placed over the aneurysm. These physical findings may be more difficult to detect in obese individuals.
Symptoms of an abdominal aortic aneurysm may mimic pain associated with any abdominal or back disorder. Symptoms may result from compression of nearby anatomical structures. These include back pain caused by lumbar nerve compression and epigastric discomfort with or without alteration in bowel elimination resulting from compression on the bowel. Occasionally aneurysms, even small ones, spontaneously embolise plaque. This can cause the ‘blue toe syndrome’, in which patchy mottling of the feet and toes occurs in the presence of palpable pedal pulse.
COMPLICATIONS
The most serious complication related to an untreated aneurysm is rupture. If rupture occurs posteriorly into the retroperitoneal space, bleeding may be tamponaded by surrounding structures, preventing exsanguination and death. In this case the patient often has severe back pain and may or may not have back or flank ecchymosis (Grey Turner’s sign).
If rupture occurs anteriorly into the abdominal cavity, most patients do not survive long enough to get to the hospital; they die from massive haemorrhage. If the patient does reach the hospital, they are in hypovolaemic shock with tachycardia, hypotension, pale clammy skin, decreased urine output, altered sensorium and abdominal tenderness on palpation. (Shock is discussed in Ch 66.) In this situation, simultaneous resuscitation and immediate surgical repair are necessary.
DIAGNOSTIC STUDIES
Most aneurysms are found on routine physical or X-ray examination. Chest X-rays are useful in demonstrating the mediastinal silhouette and any abnormal widening of the thoracic aorta. A plain X-ray of the abdomen may show calcification within the wall of an abdominal aortic aneurysm.
An electrocardiogram (ECG) may be performed to rule out evidence of myocardial infarction (MI) because some people with thoracic aneurysms may have symptoms suggestive of angina. Echocardiography assists in the diagnosis of aortic insufficiency related to ascending aortic dilation. Ultrasonography is useful in screening for aneurysms and, in the case of a non-surgical candidate, is used to monitor the aneurysm’s size serially. A CT scan is the most accurate test to determine the anterior-to-posterior length and cross-sectional diameter of the aneurysm and to identify the presence of thrombus in the aneurysm. Magnetic resonance imaging (MRI) may also be used to diagnose and assess the location and severity of aneurysms.
Angiography (anatomical mapping of the aortic system by contrast imaging) is not a reliable method of determining the diameter or length of an aneurysm. It may, however, be helpful in providing the surgeon with accurate information about the involvement of intestinal, renal or distal vessels. It is also useful if a suprarenal or thoracoabdominal aneurysm is suspected.
MULTIDISCIPLINARY CARE
The goal of management is to prevent rupture of the aneurysm. Therefore, early detection and prompt treatment are imperative. Once an aneurysm is suspected, studies are performed to determine its exact size and location. A careful review of all body systems is necessary to identify any coexisting disorders, especially of the lungs, heart or kidneys, because they may influence the patient’s risk of surgery. The carotid and coronary arteries should be assessed for atherosclerotic disease. If obstructions in these vessels are present, they may need to be corrected before the aneurysm is repaired. For individuals with small aneurysms (<4 cm), conservative therapy may be initiated, which consists of risk factor modification, decreasing blood pressure (BP) and monitoring the size of the aneurysm every 6 months using ultrasound, MRI or CT scan.8 Generally, if coexisting medical problems are stable, surgical repair is the treatment of choice for aneurysms larger than 5–6 cm or if the aneurysm is expanding rapidly (≥0.5 cm increase in diameter over a 6-month period) in a patient who is symptomatic. In individuals with stable, chronic coexisting conditions (e.g. chronic obstructive pulmonary disease, coronary artery disease, cerebrovascular disease) that meet specific anatomical criteria, endovascular repair of the aneurysm may be the treatment of choice.8
Surgical therapy
Before surgery, the patient is hydrated and any abnormalities in electrolytes, coagulation and haematocrit are corrected. The patient may receive preoperative antibiotics and showers with antiseptics before surgery to decrease the risk of a postoperative infection. However, if the aneurysm has ruptured, immediate surgical intervention is required. Even with prompt care, the mortality rate after rupture is very high (50–70%) and increases with the age of the patient.9 Aneurysms repaired electively have a surgical risk of 1–5%.10
The surgical technique involves: (1) incising the diseased segment of the aorta; (2) removing intraluminal thrombus or plaque; (3) inserting a synthetic graft (Dacron or polytetrafluoroethylene), which is sutured to the normal aorta proximal and distal to the aneurysm; and (4) suturing the native aortic wall around the graft so that it will act as a protective cover (see Fig 37-4). If the iliac arteries are also aneurysmal, the entire diseased segment is replaced with a bifurcation graft. With saccular aneurysms, it may be possible to excise only the bulbous lesion, repairing the artery by primary closure (suturing the artery together) or by application of an autogenous or synthetic patch graft over the arterial defect. Use of autotransfusion, which recycles the patient’s own blood, has markedly reduced the need for blood transfusions in surgery. (Autotransfusion is discussed in Ch 30.)
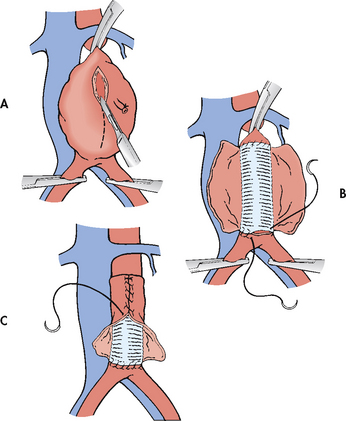
Figure 37-4 Surgical repair of an abdominal aortic aneurysm. A, Incising the aneurysmal sac. B, Insertion of synthetic graft. C, Suturing native aortic wall over synthetic graft.
All aneurysm resections require cross-clamping of the aorta proximal and distal to the aneurysm. Most resections can be completed in 30–45 minutes, after which time the clamps are removed and blood flow to the lower extremities is restored. Fortunately, most abdominal aortic aneurysms originate below the origin of the renal arteries. However, if the aneurysm extends above the renal arteries or if the clamp must be applied above the renal arteries, adequate renal perfusion after removal of the clamp should be ascertained before closure of the abdominal incision. The risk of postoperative renal complications is significantly increased in patients who have surgical repair of aneurysms above the renal arteries.
Endovascular graft procedure
The newest alternative to conventional surgical repair of an abdominal aortic aneurysm is the minimally invasive endovascular grafting technique.10 This technique involves the placement of a sutureless aortic graft into the abdominal aorta inside the aneurysm through the femoral artery. The graft is constructed from a Dacron cylinder and the surface of the graft is supported with multiple rings of extra-flexible wire. After the compactly folded graft is delivered through the sheath to the predetermined point, the graft is deployed, pressed/implanted against the vessel wall by balloon inflation (creating a circumferential seal) and anchored to the vessel by a series of small hooks. The blood then flows through the endovascular graft, thus preventing further expansion of the aneurysm due to pressure. The aneurysmal wall will begin to shrink over time because the blood is now being diverted through the endograft. After the endovascular graft is in place, intravascular ultrasound (IVUS) may be used to assess how well the graft is seated in the aorta and the graft’s proximity to the renal and/or hypogastric arteries.11
Patients must meet strict eligibility criteria to be a potential candidate for use of the endovascular devices. For example, patients are not considered suitable candidates if the aneurysm involves the renal arteries. Some of the devices are custom-made for each patient using data from CT scans, angiography and ultrasound. In some institutions, surgeons use knitted Dacron grafts combined with balloon-expandable stents.
The benefits of endovascular repair include decreased anaesthesia and operative time, smaller operative blood loss, decreased morbidity and mortality rates, small bilateral groin incisions (as opposed to a large abdominal incision), more rapid resumption of physical activity, shortened length of hospital stay, quicker recovery, higher patient satisfaction and reduction in overall costs.8,12,13 Potential complications include perigraft leaks (endoleaks), aortic dissection, bleeding, graft dislocation and embolisation, renal artery occlusion due to graft migration, graft thrombosis, incisional site haematoma and incisional infection. The most commonly reported complication is perigraft leak (8–44%), which may require the insertion of coils (beads) for haemostasis.12 Graft dysfunction may require conversion to a traditional surgical repair. The long-term complications associated with this technique are not known. With endovascular repair there is a higher reintervention rate and a need for long-term follow-up.13 Although the long-term complications associated with this technique are not known, 2- and 5-year follow-up studies indicate that aneurysm rupture is rare and long-term graft patency is similar between endovascular repair and the standard open repair.14,15
A new approach to endovascular repair is percutaneous femoral access as opposed to the more traditional femoral approach via an incision (cutdown).16 Advantages to percutaneous endograft placement include shorter operative time, shorter anaesthesia time, a reduction in the use of general anaesthesia and reduced groin complications within the first 6 months after surgery.
 NURSING MANAGEMENT: AORTIC ANEURYSMS
NURSING MANAGEMENT: AORTIC ANEURYSMS
 Nursing assessment
Nursing assessment
A thorough nursing history and physical assessment should be performed. Because atherosclerosis is a systemic disease process, it is likely that the disease process is present throughout the body. Therefore, it is important for the nurse to watch for signs of cardiac, pulmonary, cerebral and lower extremity vascular problems. The patient should be monitored for indications of rupture of the aneurysm, such as diaphoresis; paleness; weakness; tachycardia; hypotension; abdominal, back, groin or periumbilical pain; changes in sensorium; or a pulsating abdominal mass.
Establishing baseline data is important for later postoperative assessment and intervention. In addition to gathering data, the nurse should observe the patient closely for any abnormalities. Special attention should be paid to the character and quality of the peripheral pulses and the neurological status. Pedal pulse sites (dorsalis pedis and posterial tibial) and skin lesions on the lower extremities should be marked and documented before surgery.
 Planning
Planning
The overall goals for a patient undergoing aortic surgery include: (1) normal tissue perfusion; (2) intact motor and sensory function; and (3) no complications related to surgical repair, such as thrombosis or infection.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The nurse must be aware of cardiovascular disease risk factors and be alert for opportunities to teach health promotion measures to patients in the hospital and the community (see Ch 33). Special attention should be given to the patient with a strong family history of aneurysm or any evidence of other cardiovascular disease.
The patient should be encouraged to reduce risk factors known to be associated with atherosclerosis (see Ch 33). These include controlling hypertension, smoking cessation and following a diet low in fats and cholesterol. These measures are also undertaken to ensure continued graft patency following surgical repair.
 Acute intervention
Acute intervention
The nursing role during the preoperative period includes patient and family teaching, providing support for the patient and family, and careful assessment of all body systems. Preoperative teaching should include a brief explanation of the disease process, the planned surgical procedure(s), preoperative routines, what to expect immediately after surgery (e.g. recovery room, tubes/drains) and usual postoperative timelines. Although specific preoperative routines often vary by institution and/or surgeon, in general patients undergoing aortic surgery typically undergo some sort of bowel preparation (i.e. laxatives, enemas) and have a preoperative shower with an antimicrobial soap the day before surgery, receive nil by mouth (NBM) after midnight the day before surgery and often take preoperative intravenous (IV) antibiotics immediately before surgery. If the patient will be going to the intensive care unit (ICU) after surgery, a tour of the ICU before surgery may be of interest to the patient and family (see Ch 65).
Postoperatively, patients undergoing aortic surgery typically go to an ICU where there are appropriate support services and equipment. When the patient arrives in the ICU, an endotracheal tube, an arterial line, a central venous pressure or pulmonary artery catheter, peripheral IV lines, an indwelling urinary catheter and a nasogastric tube will likely be in place with continuous ECG and pulse oximetry monitoring. If the thorax is entered during surgery, chest tubes will also be in place. Pain medication may be administered via epidural catheter or patient-controlled analgesia.
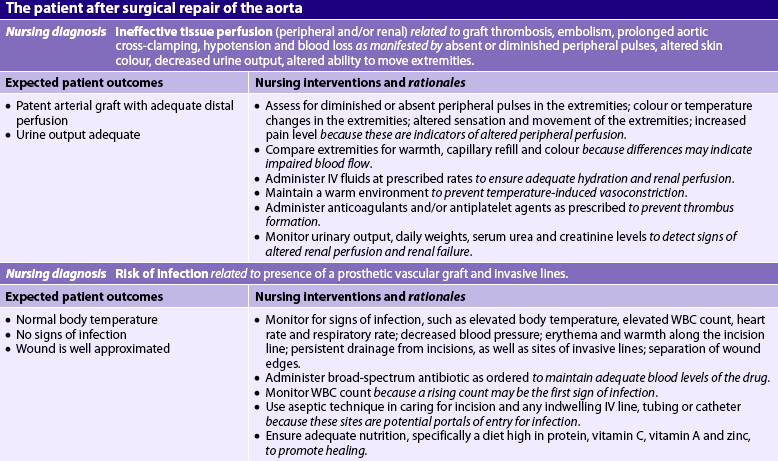
In addition to the usual goals associated with the care of a postoperative patient (e.g. maintaining adequate respiratory function, fluid and electrolyte balance, and pain control; see Ch 19), the nurse must monitor graft patency and renal perfusion. The nurse can also assist in preventing arrhythmias, infections and neurological complications. Care of the patient with an aneurysm repair or other aortic surgery is described in NCP 37-1.
 Graft patency
Graft patency
It is important to maintain adequate blood pressure to promote graft patency. Prolonged hypotension may result in graft thrombosis due to decreased blood flow. Administration of IV fluids and blood components (as indicated) is essential to maintaining adequate blood flow to the graft. Central venous pressure (CVP) readings or pulmonary artery (PA) pressures and urinary output should be monitored hourly in the immediate postoperative period to help assess the patient’s state of hydration.
Severe hypertension may cause undue stress on the arterial anastomoses, resulting in leakage of blood or rupture at the suture lines. Drug therapy with diuretics or IV antihypertensive agents may be indicated if severe hypertension persists.
 Cardiovascular status
Cardiovascular status
In patients with pre-existing coronary artery disease, myocardial ischaemia or infarction may occur in the perioperative period due to decreased oxygen supply to the heart or increased oxygen demands on the heart. Cardiac arrhythmias also may occur due to electrolyte imbalances, hypoxaemia, hypothermia or myocardial ischaemia. Nursing interventions include continuous ECG monitoring, frequent electrolyte and arterial blood gas (ABG) determinations, administration of oxygen and IV antiarrhythmic medications as needed, replacement of electrolytes as indicated, adequate pain control and resumption of preoperative cardiac medications.
 Infection
Infection
The development of a prosthetic vascular graft infection is a relatively rare but possibly life-threatening complication. Nursing intervention to prevent infection should include ensuring that the patient receives a broad-spectrum antibiotic as prescribed. It is important to assess body temperature regularly and to report any elevations. Laboratory data should be monitored for elevated white blood cell (WBC) count, which may be the first indication of an infection. In addition, the nurse should ensure adequate nutrition and observe the surgical incision for any evidence of delayed healing, signs of infection or prolonged drainage.
All IV, arterial and central venous catheter insertion sites should be cared for carefully with the use of sterile technique because they are frequently a portal of entry for bacteria. Meticulous perineal care for the patient with an indwelling urinary catheter is also essential to minimise the risk of urinary tract infection. Surgical incisions should be kept clean and dry.
 Gastrointestinal status
Gastrointestinal status
After abdominal aortic surgery, paralytic ileus may develop as a result of anaesthesia and the manual manipulation and displacement of the bowel for long periods during surgery. The intestines may become swollen and bruised, and peristalsis ceases for variable intervals. A retroperitoneal surgical approach can be used to decrease the risk of bowel complications.
A nasogastric tube is inserted during surgery and connected to low, intermittent suction. This decompresses the stomach and duodenum, prevents aspiration of stomach contents and decreases pressure on suture lines. The nasogastric tube should be irrigated with normal saline solution as needed, and the amount and character of the drainage recorded. The nurse should auscultate for the return of bowel sounds. The passing of flatus is a key sign of returning bowel function and should be noted. Early ambulation will assist with the resumption of bowel functioning. It is unusual for paralytic ileus to persist beyond the fourth postoperative day.
If the blood supply to the bowel is disrupted during surgery, temporary ischaemia or infarction (death) of intestinal tissue may result. This is evidenced by lack of bowel sounds, fever, abdominal distension, diarrhoea and bloody stools. When bowel infarction does occur as a result of mesenteric ischaemia, reoperation is necessary as soon as possible to restore blood flow, with likely resection of the infarcted bowel. Fortunately, this serious complication is uncommon.
While the patient is NBM, meticulous mouth care should be given every few hours. In some situations ice chips or lozenges may be given to the patient to soothe an irritated throat.
 Neurological status
Neurological status
Neurological complications can occur after surgical procedures on the aorta. When the ascending aorta and aortic arch are involved, nursing interventions should include assessment of the level of consciousness, pupil size and response to light, facial symmetry, tongue deviation, speech, ability to move upper extremities and quality of hand grasps (see Ch 55). When the descending aorta is involved, nursing assessment of the ability to move the lower extremities is also important. These assessments should be recorded in detail with a careful description of the patient’s response. Any alteration from the baseline assessment should be reported to the doctor immediately.
 Peripheral perfusion status
Peripheral perfusion status
The anatomical location of the aneurysm indicates the areas of major concern related to peripheral perfusion. All peripheral pulses should be checked regularly and recorded. This should be done every hour for several hours, depending on the nursing policy, and routinely thereafter at frequent intervals. When the ascending aorta and aortic arch are involved, the carotid, radial and temporal artery pulses should be assessed. After surgery involving the descending aorta, pulses to be assessed may include the femoral, popliteal, posterior tibial and dorsalis pedis (see Ch 31).
When checking the pulses, the nurse should mark the locations lightly with a felt-tip pen so that others can locate them easily. Doppler ultrasonography is useful in assessment of peripheral pulses. It is also important to note the skin temperature and colour, capillary refill time, and sensation and movement of the extremities.
Occasionally, pulses in the lower extremities may be absent for a short time following surgery. This is usually due to vasospasm and hypothermia. A decreased or absent pulse in conjunction with a cool, pale, mottled or painful extremity may indicate embolisation of aneurysmal thrombus or plaque or occlusion of the graft. Graft occlusion is treated with reoperation if identified early. In rare instances, thrombolytic therapy may also be considered. Therefore, these findings should be reported to the surgeon immediately. In some patients the pulses may have been absent preoperatively because of coexistent peripheral arterial occlusive disease. Comparison with the preoperative status is essential to determine the aetiology of a decreased or absent pulse and the proper treatment required.
 Renal perfusion status
Renal perfusion status
The patient returns from surgery with an indwelling urinary catheter in place. In the immediate postoperative period, hourly urine output is recorded. An accurate record of fluid intake and urinary output should be kept until the patient resumes the preoperative diet. Daily weights should also be obtained. CVP readings and PA pressures provide important information regarding hydration status. Daily serum urea and electrolyte levels and serum creatinine studies are performed to evaluate renal function. (For signs and symptoms of acute renal injury, see Ch 46.) Irreversible renal failure may occur after aortic surgery, particularly in high-risk individuals (e.g. patients with diabetes mellitus).
One cause of decreased renal perfusion is embolisation of a fragment of thrombus or plaque from the aorta that subsequently lodges in one or both of the renal arteries. This can cause ischaemia of one or both kidneys. Hypotension, dehydration, prolonged aortic clamping during surgery or blood loss can also lead to decreased renal perfusion.
 Ambulatory and home care
Ambulatory and home care
The patient may be apprehensive about returning home after major surgery involving the aorta. The nurse should encourage the patient to express any concerns and reassure the patient that normal activities of daily living can be resumed. The patient should be instructed to increase activities gradually. Fatigue, poor appetite and irregular bowel habits are to be expected. Heavy lifting should be avoided for at least 4–6 weeks following the traditional surgery. Observation of incisions for signs and symptoms of infection is encouraged. Any redness, swelling, increased pain, drainage from incisions or fever greater than 37.8°C should be reported to the doctor.
The patient should be taught to observe for changes in colour or warmth of the extremities. Patients may be taught to palpate peripheral pulses and to assess changes in their quality. The patient who has received a synthetic graft should be aware that prophylactic antibiotics may be required before future invasive procedures, including any dental procedures.
Sexual dysfunction in male patients is not uncommon after aortic surgery; it may occur because the internal hypogastric artery is interrupted, leading to decreased arterial blood flow to the penis. In addition, the periaortic sympathetic plexus may be disrupted by the surgical procedure. Preoperatively, baseline sexual function should be documented and patient counselling is recommended. Postoperatively, referral to a urologist may be considered if impotence is a problem.
There are situations in which operative repair is not performed. Examples of this are the presence of a very small aneurysm (<4 cm), a patient who is not a surgical candidate (e.g. because of severe lung or cardiac disease) or a patient or family’s refusal to undergo repair. The patient who does not undergo surgical repair should be urged to receive regular routine physical examinations and should be reminded that any symptom, no matter how minor, must be investigated if it persists.
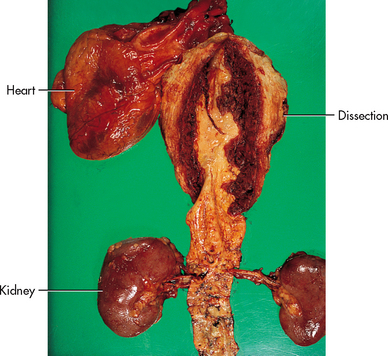
Aortic dissection
Aortic dissection, often misnamed ‘dissecting aneurysm’, is not a type of aneurysm. It occurs most commonly in the thoracic aorta and is the result of a tear in the intimal (innermost) lining of the arterial wall that allows blood to enter between the intima and media, thus creating a false lumen (Figs 37-3 and 37-5). Aortic dissection affects men more often than women and occurs most frequently between the fourth and seventh decades of life. This process is usually acute and life-threatening. However, it also may be self-limiting and result in a chronic and stable process for a period of time. If patients have an acute ascending aortic dissection and are not surgically treated, the mortality rate is 90%.14
AETIOLOGY AND PATHOPHYSIOLOGY
Aortic dissection results from a small tear in the intimal lining of the artery, allowing blood to ‘track’ between the intima and the media, thus creating a false lumen of blood flow. As the heart contracts, each systolic pulsation causes increased pressure on the damaged area, which further increases the dissection. As it extends proximally or distally, it may occlude major branches of the aorta, cutting off blood supply to areas such as the brain, abdominal organs, kidneys, spinal cord and extremities. Occasionally a small tear develops distally and the blood flow re-enters the true vessel lumen.
The exact cause of dissection is uncertain, although many authorities attribute the cause to the destruction of the medial layer’s elastic fibres. Most people with dissection problems are older and have chronic hypertension. Patients with Marfan’s syndrome (a premature degeneration of vascular elastic tissue) have a high incidence of aortic dissection. Pregnancy promotes increased vascular stress because of increased total blood volume, decreased peripheral vascular resistance and increased aortic compliance.14 Blunt trauma is also a precipitating factor associated with aortic dissection. Areas that undergo the greatest amount of stress and are thus most prone to dissection are the ascending aorta, the aortic arch and the descending aorta beyond the origin of the left subclavian artery.
CLINICAL MANIFESTATIONS
Clinical manifestations depend on the location of the intimal tear and the extent of the dissection. The typical patient with acute aortic dissection usually has sudden, severe pain in the anterior part of the chest or intrascapular pain radiating down the spine into the abdomen or legs.15 The pain is described as ‘tearing’ or ‘ripping’. The severe pain may mimic that of an MI. As the dissection progresses, pain may be located both above and below the diaphragm. Cardiovascular, neurological and respiratory signs may also be present.
If the arch of the aorta is involved, the patient may exhibit neurological deficiencies, including an altered level of consciousness, dizziness, and weakened or absent carotid and temporal pulses. An ascending aortic dissection usually produces some degree of aortic valvular insufficiency and a new high-pitched, diastolic cardiac murmur is audible on auscultation. Severe aortic valve insufficiency may produce left ventricular failure with the development of dyspnoea and orthopnoea caused by pulmonary oedema. When either subclavian artery is involved, radial, ulnar and brachial pulse quality and BP readings may be significantly different between the left and right arms. As the dissection progresses down the aorta, the abdominal organs and lower extremities demonstrate evidence of altered tissue perfusion.
COMPLICATIONS
A severe and life-threatening complication of aortic dissection of the ascending aortic arch is cardiac tamponade, which occurs when blood escapes from the dissection into the pericardial sac. Clinical manifestations of cardiac tamponade include hypotension, narrowed pulse pressure, distended neck veins, muffled heart sounds and pulsus paradoxus (see Ch 36.)
Because the aorta is weakened by the medial dissection, it may rupture. Haemorrhage may occur into the mediastinal, pleural or abdominal cavities. Rupture of a dissected aorta typically results in exsanguination and death. Research indicates that 86% of deaths associated with aortic dissection are due to aortic rupture.15
Dissection can lead to occlusion of the arterial supply to many vital organs, such as the spinal cord, kidneys and abdominal organs. Ischaemia of the spinal cord produces symptoms varying from weakness and decreased pain sensation to complete paralysis of the lower extremities. Renal ischaemia can lead to renal failure. Manifestations of abdominal ischaemia include abdominal pain, decreased bowel sounds and altered bowel elimination.
DIAGNOSTIC STUDIES
The diagnostic studies used to assess aortic dissection are similar to those performed for aortic aneurysms (see Box 37-1). Left ventricular hypertrophy is a common finding on an echocardiogram and is possibly related to changes caused by systemic hypertension. A chest X-ray may show a widening of the mediastinal silhouette and left pleural effusion. A transoesophageal echocardiogram (TOE) can identify dissections that are close to the aortic root. A CT scan or MRI provides valuable information on the presence and severity of the dissection. After the patient’s condition has stabilised, angiography may be necessary to assess the extent of the dissection.
MULTIDISCIPLINARY CARE
The initial goal of therapy for aortic dissection without complications is to lower the BP and myocardial contractility to diminish the pulsatile forces within the aorta (see Box 37-1). IV sodium nitroprusside rapidly reduces the systolic BP. In addition, IV β-adrenergic blockers, such as propranolol, decrease the force of myocardial contractility.
Conservative therapy
The patient with aortic dissection without complications can be treated conservatively for a period. Supportive treatment is directed towards pain relief, blood transfusion (if required) and management of heart failure (if indicated). If the dissection is limited to the descending aorta, conservative therapy may be adequate to treat the problem. Success of the treatment is judged by the relief of pain, which is an indication of stabilisation of the aortic dissection. However, if the dissection involves the ascending aorta, immediate surgery is usually indicated.
Surgical therapy
Surgery is indicated when drug therapy is ineffective or when complications of aortic dissection (e.g. heart failure, leaking dissection, occlusion of an artery) are present. The aorta is fragile following the dissection. Therefore, surgery is delayed for as long as possible to allow time for oedema in the area of the dissection to decrease, to permit clotting of the blood in the false lumen and to allow the healing process to begin. Surgery for aortic dissection involves resection of the aortic segment containing the intimal tear and replacement with synthetic graft material. The extent of aortic replacement depends on the extent of the dissection. Even with prompt surgical intervention, 30-day mortality of acute aortic dissections remains high (10–28%), with causes of death related to MI, cerebral ischaemia, uncontrolled bleeding, abdominal ischaemia, sepsis and multi-organ failure.
 NURSING MANAGEMENT: AORTIC DISSECTION
NURSING MANAGEMENT: AORTIC DISSECTION
Preoperatively, nursing management related to aortic dissection includes keeping the patient in bed in a semi-Fowler position and maintaining a quiet environment. These measures assist in keeping the systolic BP at the lowest possible level that maintains vital organ perfusion (typically maintaining systolic BP between 110 and 120 mmHg or mean arterial pressure between 70 and 80 mmHg).13 Narcotics and tranquillisers should be administered as ordered. Pain and anxiety must be managed for patient comfort, especially since they may cause elevations in systolic BP.
Continuous IV administration of antihypertensive agents requires close nursing supervision. An ECG monitoring device is used and an intraarterial pressure line is usually inserted (see Ch 65). The nurse should observe for changes in the quality of peripheral pulses and for signs of increasing pain, restlessness and anxiety. Vital signs are taken frequently, sometimes as often as every 2–3 minutes, while obtaining control of the BP. If the blood vessels branching off the aortic arch are involved, decreased cerebral blood flow may alter the sensorium and level of consciousness. Postoperative care after surgery to correct the dissection is similar to that after aneurysmectomy (see NCP 37-1).
In preparation for discharge, the nurse should focus on patient and family teaching. The therapeutic regimen includes antihypertensive drugs, which are usually taken orally. The patient needs to understand that these drugs must be taken to control BP. The nurse should instruct the patient that if the pain returns or other symptoms progress, the patient must seek immediate help at the nearest healthcare facility. β-adrenergic blockers (e.g. propranolol) can be taken orally to continue to decrease myocardial contractility. It is important that the patient understands the drug regimen and potential side effects. The patient should be told to discuss any side effects with the healthcare provider before discontinuing the drug.
Peripheral arterial disease of the lower extremities
PAD of the lower extremities may affect the aortoiliac, femoral, popliteal, tibial or peroneal arteries or any combination of these areas (see Fig 37-1). The femoral–popliteal area is the site most commonly affected in non-diabetic patients. Patients with diabetes mellitus tend to develop disease in the arteries below the knee, especially the anterior tibial, posterior tibial and peroneal arteries. In advanced stages, multiple levels of occlusions are found.
CLINICAL MANIFESTATIONS
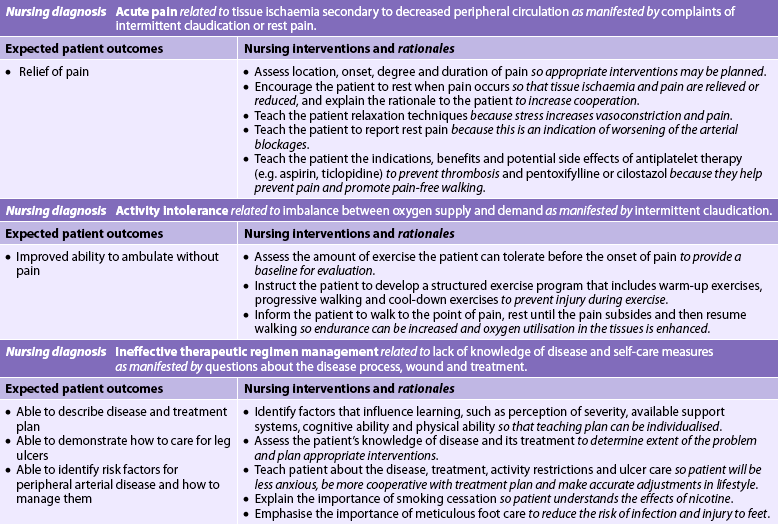
The severity of the clinical manifestations depends on the site and extent of the obstruction, and the extent and amount of collateral circulation. The classic symptom of PAD of the lower extremities is intermittent claudication, which is ischaemic muscle ache or pain that is precipitated by a consistent level of exercise, resolves within 10 minutes or less with rest and is reproducible.2 The ischaemic pain is attributable to end products of anaerobic cellular metabolism, such as lactic acid accumulation. Once the patient stops exercising, the metabolites are cleared and the pain subsides. Disease involving the femoral or popliteal arteries causes claudication in the calf. PAD of the aortoiliac arteries produces claudication in the buttocks and thighs. It should be noted, however, that sedentary patients with PAD of the lower extremities may never exert themselves sufficiently to experience claudication. If disease extends into the internal iliac (hypogastric) arteries, impotence often results. Sexual dysfunction occurs in as many as 30–50% of patients with aortoiliac occlusion.16
Paraesthesia, manifested as numbness or tingling occurring in the toes or feet, may result from nerve tissue ischaemia. True peripheral neuropathy occurs more commonly in patients with diabetes (see Ch 48) and in those with progressive longstanding ischaemia. The neuropathy produces excruciating shooting or burning pain in the extremity. It does not follow any particular nerve roots but may be present near ulcerated areas. Gradually diminishing perfusion to neurons produces loss of both pressure and deep pain sensations. Therefore, injuries to the extremity often go unnoticed by the patient.
The physical appearance of the limb provides important information about the adequacy of blood flow. Trophic changes occur to the skin. The skin becomes thin, shiny and taut, and there is a loss of hair on the lower legs. Diminished or absent pedal, popliteal or femoral pulses may be noted. Pallor or blanching of the foot is noted in response to leg elevation (elevation pallor). Conversely, reactive hyperaemia (redness of the foot) is observed when the limb is hung in a dependent position (dependent rubor).
However, as the disease process advances and involves multiple arterial segments, continuous pain develops at rest. Rest pain most often occurs in the forefoot or toes and is aggravated by limb elevation. Rest pain occurs when there is insufficient blood flow to maintain basic metabolic requirements of the tissues and nerves of the distal extremity. Rest pain occurs more often at night because cardiac output tends to drop during sleep and the limbs are at the level of the heart. At this severity of disease, patients will try to achieve partial pain relief by dangling the leg over the side of the bed to allow gravity to maximise arterial blood flow. Without revascularisation, the limb may progress to ulceration and gangrene. Every attempt is made to save the limb and surgery is usually indicated unless the patient is at high surgical risk and/or has numerous coexisting medical conditions.
COMPLICATIONS
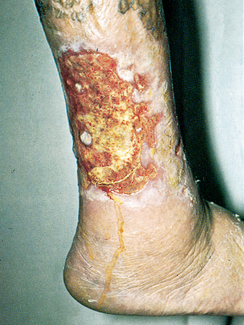
PAD of the lower extremities progresses slowly. Prolonged ischaemia leads to atrophy of the skin and underlying muscles. Because of the decreased arterial blood flow to the lower extremities, even minor trauma to the feet (e.g. stubbing one’s toe, a blister from ill-fitting shoes) may result in delayed healing, wound infection and tissue necrosis, especially in diabetic patients. Arterial (ischaemic) ulcers most commonly occur over bony prominences on the toes, feet and lower leg (see Table 37-1). Non-healing arterial ulcers and gangrene are the most serious complications of end-stage PAD and may result in lower extremity amputation if blood flow is not adequately restored or if severe infection occurs. If atherosclerosis has been present for an extended period, collateral circulation may prevent gangrene of the extremity.
TABLE 37-1 Comparison of arterial and venous leg ulcers
| Characteristic | Arterial | Venous |
|---|---|---|
| Peripheral pulses | Decreased or absent | Present; may be difficult to palpate with oedema |
| Capillary refill | >3 s | <3 s |
| Ankle–brachial index | <0.75 | >0.90 |
| Oedema | No oedema | Lower leg oedema |
| Hair | Loss of hair on legs, feet, toes | Hair may be present or absent |
| Ulcer location | Tips of toes, foot or lateral malleolus | Near medial malleolus |
| Ulcer margin | Rounded, smooth, looks ‘punched out’ | Irregularly shaped |
| Ulcer drainage | Minimal | Moderate-to-large amount |
| Pain | Intermittent claudication or rest pain in foot; ulcer may or may not be painful | Dull ache or heaviness in calf or thigh; ulcer often painful |
| Nails | Thickened; brittle | Normal or thickened |
| Skin colour | Dependent rubor; elevation pallor | Bronze-brown pigmentation; varicose veins may be visible |
| Skin texture | Thin, shiny, friable, dry | Skin thick, hardened and indurated |
| Skin temperature | Cool; temperature gradient down the leg | Warm, no temperature gradient |
| Dermatitis | Rarely occurs | Frequently occurs |
| Pruritus | Rarely occurs | Frequently occurs |
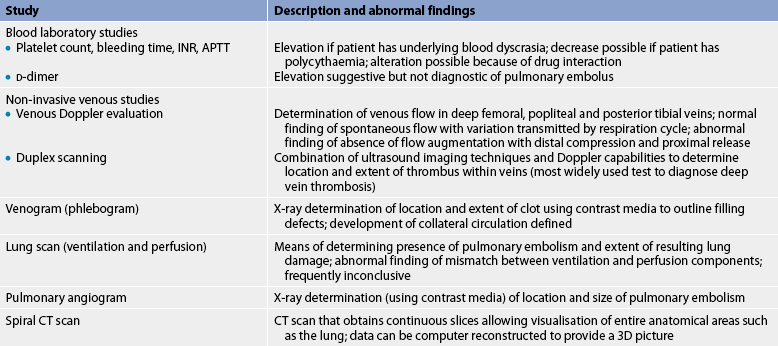
DIAGNOSTIC STUDIES
Various tests have been developed to assess blood flow and to outline the vascular system (see Box 37-2). Doppler ultrasound consists of a probe transducer containing a crystal that directs high-frequency sound waves towards the artery or vein being examined. The sound waves bounce off the blood cells at a rate that corresponds with the velocity (or speed) of blood flow through the vessel. This emits an audible signal. When palpation of a peripheral pulse is difficult because of severe PAD, Doppler ultrasound can be useful in determining the presence of blood flow. A palpable pulse and a pulse heard via Doppler ultrasound are not equivalent, and these terms should not be used interchangeably. In addition, segmental blood pressures are obtained (using Doppler ultrasound and sphygmomanometer) at the thigh, below the knee and at ankle level while the patient is supine. A fall-off in segmental BP of more than 30 mmHg indicates PAD.
BOX 37-2 Peripheral arterial disease
MULTIDISCIPLINARY CARE
Diagnostic studies
Health history and physical examination, including palpation of peripheral pulses
Collaborative therapy
Proper foot care (see Box 48-10)
Percutaneous transluminal angioplasty with or without stent
Peripheral arterial bypass surgery
Patch graft angioplasty, often in conjunction with bypass surgery
The ankle–brachial index (ABI) is undertaken using hand-held Doppler ultrasound. The ABI is calculated by dividing the ankle systolic BP by the highest brachial systolic BP.15 A normal ABI is 0.90–1.30. An ABI between 0.41 and 0.89 indicates mild-to-moderate PAD, and an index of 0–0.40 indicates severe PAD. The ABI technique is also used to follow patients postoperatively after revascularisation to monitor patency of bypass grafts. This procedure has limited usefulness when arteries are calcified and non-compressive, as occurs in patients with diabetes mellitus. In these patients, the ABI is frequently falsely elevated.
Duplex imaging, another non-invasive test, uses a bidirectional, colour Doppler system to map blood flow systematically throughout the entire region of an artery. It provides anatomical and physiological information about the blood vessels.
Angiography is used to further delineate the location and extent of the disease process. In addition, it provides information on inflow and outflow vessels to plan for surgery. Angiography is useful when an intervention (i.e. surgery, angioplasty) is indicated. Magnetic resonance angiography (MRA) is sometimes used alternatively. (MRA is described in Ch 31.)
MULTIDISCIPLINARY CARE
Risk factor modification
Regardless of the severity of symptoms, it is paramount that all patients with PAD undergo risk factor modification.17,18 Smoking cessation is essential for slowing the progression of PAD to critical limb ischaemia and reducing the risk of MI and death.17 Smoking cessation is a complex and difficult process with a high incidence of smoking relapse.19 (Smoking cessation is discussed in Ch 10.)
Aggressive treatment of hyperlipidaemia (low-density lipoproteins [LDLs] <3.3 mmol/L and triglycerides <1.60 mmol/L) is another goal of therapy in PAD patients. Research has shown that treatment of PAD patients with a lipid-lowering agent such as a statin (e.g. simvastatin) lowers serum cholesterol levels, improves endothelial function and stabilises or reduces femoral atherosclerosis.19 (Therapy to lower cholesterol is discussed in Ch 33.)
Hypertension and diabetes mellitus are risk factors for PAD. However, data are not conclusive as to whether aggressive treatment will alter the progression of PAD.17,19 Nonetheless, tight control of these two risk factors is likely to decrease the risk of other cardiovascular-related morbidity and mortality (i.e. stroke, MI). An aglycosylated haemoglobin (HbA1C) level less than 0.07 is recommended for diabetics. BP should be maintained at less than 130/80 mmHg.
Drug therapy
Antiplatelet agents such as aspirin, ticlopidine and clopidogrel are considered important for reducing the risks of MI, ischaemic stroke and cardiovascular-related death in patients with PAD.17 However, aspirin is not tolerated by some patients because of gastrointestinal distress. Ticlopidine and clopidogrel, both drugs that inhibit platelet activation, are also effective in reducing the risk of MI and stroke. Patients taking ticlopidine must be monitored carefully for thrombocytopenia, neutropenia and thrombotic thrombocytopenic purpura, all of which necessitate stopping the drug. Clopidogrel is more effective than aspirin at reducing the risk of MI, stroke and cardiovascular-related death in patients with PAD.20
Various drugs are prescribed to treat intermittent claudication. The most common drug therapy has been oxpentifylline, which increases erythrocyte flexibility and reduces blood viscosity, thus improving the supply of oxygenated blood to ischaemic muscle. The use of heparin, low-molecular-weight heparin and oral anticoagulants is not recommended for treating intermittent claudication.21,22
Exercise
The primary non-pharmacological treatment for claudication is a formal exercise training program.23 Although exercise training does not cause increased collateral blood flow to the legs, it does improve oxygen extraction in the legs, skeletal muscle metabolism and vascular endothelial function.22 Unfortunately, recent nursing research indicates that only about 50% of PAD patients participate in regular exercise.
Walking is the most effective exercise for individuals with claudication. A supervised, hospital-based PAD rehabilitation program is an effective means for improving exercise performance.23 Such programs typically include exercise for 30–60 minutes/day, 3–5 times/week, for 3–6 months. One advantage of a formal rehabilitation program is that patients can exercise on other equipment (e.g. exercise bicycles, rowing machines) to improve whole body fitness and minimise boredom.
A home exercise program is an alternative to a formal program. Slow, progressive physical activity should be encouraged after a warm-up period. The patient should be instructed to walk to the point of discomfort, stop and rest, and then resume walking until the discomfort recurs. Walking should be done for a prescribed time, usually 30–40 minutes/day, 3–5 times/week. An exercise therapy program also should be implemented in PAD patients after balloon angioplasty and/or peripheral arterial bypass surgery.
Nutritional therapy
Patients with PAD should be taught how to alter their dietary intake. Overall kilojoule intake should be adjusted so that ideal body weight can be achieved and maintained. Within the diet, dietary cholesterol should be less than 200 mg per day and the saturated fat intake should be reduced substantially (see Table 33-6). Soy protein products (e.g. tofu, miso) can be used in place of animal protein to help lower serum LDL cholesterol and triglycerides.24,25 In addition, dietary sodium should be no more than 2 g per day (see Tables 34-4 to 34-6).
Complementary and alternative therapies
Ginkgo biloba is effective in increasing walking distance for patients with intermittent claudication.25 Side effects of ginkgo biloba include headache, nausea, gastric symptoms, diarrhoea and allergic skin reactions. Patients taking antiplatelet agents (e.g. aspirin), non-steroidal anti-inflammatory agents (e.g. ibuprofen) and anticoagulants (e.g. warfarin) should consult with their healthcare provider before taking ginkgo biloba due to bleeding risks (see the Complementary & alternative therapies box overleaf). Other nutritional supplements show promising results for the treatment of PAD but additional randomised controlled trials are needed. For example, folate, vitamin B6 and vitamin B12 appear to lower homocysteine levels.24,25
Care of the leg with critical limb ischaemia
Critical limb ischaemia is a chronic condition characterised by ischaemic rest pain, arterial leg ulcers and/or gangrene of the leg due to advanced PAD. Conservative management goals for the patient with critical limb ischaemia due to PAD include protecting the extremity from trauma, decreasing vasospasm, preventing and controlling infection and maximising arterial perfusion. Careful inspection, cleansing and lubrication of both feet are advised to prevent cracking of the skin and infection. Although cleansing is important, soaking of the affected foot should be avoided to prevent skin maceration (or breakdown). If ulceration is present, the affected foot should be kept clean and dry. Covering the ulcer with a dry, sterile dressing helps maintain cleanliness and protects the limb. Ulcers with any significant depth may be treated with a variety of wound care products, but without restoration of blood flow healing is unlikely. Footwear should be soft, roomy and protective. Chemicals, heat and cold should be avoided. The patient’s heels should be kept free of pressure. This can be accomplished by placing a pillow under the calves so that the heels do not touch the bed. There are also many commercially available devices that provide heel elevation. In patients with critical limb ischaemia not amenable to interventional or surgical procedures, the rate of primary amputation is between 10% and 40%.26
Herbal and dietary supplements that may affect clotting
COMPLEMENTARY & ALTERNATIVE THERAPIES
Scientific evidence
There is evidence that the following herbs and dietary supplements may affect blood clotting: bilberry, black cohosh, chamomile, chondroitin sulfate, dehydroepiandrosterone, feverfew, garlic, ginger, ginkgo biloba, ginseng, goldenseal, omega-3 fatty acids, melatonin, niacin, psyllium, red yeast rice extract, saw palmetto, soy, turmeric.
Nursing implications
Caution is advised for patients with bleeding disorders or taking drugs, herbs or dietary supplements that increase the risk of bleeding.
Source: Based on a systematic review of scientific literature. Available at www.naturalstandard.com
Several new strategies for the treatment of critical limb ischaemia are under investigation. Prostaglandins have been used in Europe for the treatment of critical leg ischaemia in patients who are not candidates for surgical revascularisation. A recent meta-analysis indicated that patients with severe PAD treated with prostaglandin E1 (PGE1) had better pain relief and healing of arterial leg ulcers compared to those treated with a placebo.26 Furthermore, patients treated with PGE1 were more likely to be alive 6 months post-treatment. Other strategies include immune modulation therapy to reduce the inflammatory response present in PAD and angiogenic gene therapy to enhance the development of collateral circulation.
Interventional radiological procedures
Interventional radiological procedures are indicated when: (1) intermittent claudication symptoms become incapacitating; (2) the patient experiences pain at rest; or (3) ulceration or gangrene is severe enough to threaten the viability of the limb.
Similar to the angiography diagnostic procedure, percutaneous transluminal balloon angioplasty involves the insertion of a catheter through the femoral artery. However, the catheter is special and contains a cylindrical balloon. The end of the catheter is advanced to the narrowed area of the artery and then the balloon is inflated, dilating the vessel by cracking the confining atherosclerotic intimal shell while also stretching the underlying media. This procedure is used selectively in certain patients who have localised, accessible lesions (<10 cm in length).27 Iliac and femoral artery lesions have responded most successfully to balloon angioplasty. It should be noted, however, that balloon angioplasty is not effective on arteries with diffuse or long segment lesions or in smaller vessels below the knee (tibial arteries).27 Furthermore, there is a relatively high rate of restenosis after balloon angioplasty (up to 50% at 1 year). Placement of intravascular stents during angioplasty helps relieve the problems of restenosis and arterial dissection. Stents are expandable metallic devices that are positioned within the artery immediately after the balloon angioplasty is performed. Stents assist in maintaining vessel patency after the procedure. Newer drug-eluting stents using paclitaxel are being studied and may help to minimise the problem of restenosis after angioplasty by reducing the amount of new tissue growth in the stent.28 Antiplatelet agents still are necessary after stenting procedures to reduce the risk of platelet aggregation and subsequent restenosis.
Surgical therapy
Various surgical approaches can be used to improve arterial blood flow beyond a stenotic or occluded artery. The most common is a peripheral arterial bypass operation with autogenous vein or synthetic graft material to bypass or carry blood around the lesion (see Fig 37-6). The Evidence-based practice box explores whether or not stenting can improve symptoms.
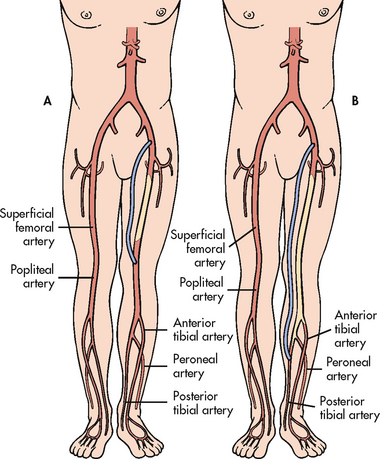
Figure 37-6 A, Femoral–popliteal bypass graft around an occluded superficial femoral artery. B, Femoral–posterior tibial bypass graft around occluded superficial femoral, popliteal and proximal tibial arteries.
Other surgical options include endarterectomy (opening the artery and removing the obstructing plaque) and patch graft angioplasty (opening the artery, removing plaque and sewing a patch to the opening to widen the lumen).28 Antiplatelet agents are often used to prevent thrombosis after arterial bypass surgery. In addition, anticoagulation with heparin in the immediate postoperative period followed by long-term warfarin therapy is sometimes used in patients who have a tendency to occlude grafts secondary to a coagulopathy (clotting abnormality).
Amputation is the least desirable and end-stage surgical option but it may be required if gangrene is extensive, infection is present in the bone (osteomyelitis) or all major arteries in the limb are occluded, precluding the possibility of successful peripheral arterial bypass surgery. Every effort is made to preserve as much of the limb as possible so that the potential for rehabilitation is optimised.
Does femoral artery stenting improve intermittent claudication?
EVIDENCE-BASED PRACTICE
Clinical question
For patients with intermittent claudication due to lesions of the superficial femoral artery (P), does percutaneous transluminal angioplasty (PTA) with stenting (I) versus PTA alone (C) improve treadmill walking distance (O) 2 years later (T)?
Critical appraisal and synthesis of evidence
• 8 RCTS (n = 968) of men and women with similar antiplatelet therapy protocols
• Short-term (6–12 months) improved walking distance for patients with stents was not maintained at 2 years
• Improved treadmill walking distance in patients with PTA plus stent insertion observed at 6 and 12 months, but not at 2 years
• No significant differences at 2 years in treadmill walking distance for patients with femoral artery percutaneous transluminal angioplasty with stenting versus PTA alone
• One RCT showed no significant difference in quality of life between patients treated with PTA alone and patients treated with PTA with stent insertion
Implications for nursing practice
• Advise patient receiving a stent that small short-term gains may diminish with time.
• Future research is needed to determine the best candidates for stenting.
P, patient population of interest; I, intervention or area of interest; C, comparison of interest or comparison group; O, outcome(s) of interest; T, timing.
 NURSING MANAGEMENT: LOWER EXTREMITY PERIPHERAL ARTERIAL DISEASE
NURSING MANAGEMENT: LOWER EXTREMITY PERIPHERAL ARTERIAL DISEASE
 Nursing assessment
Nursing assessment
Subjective and objective data that should be obtained from a patient with PAD are presented in Table 37-2.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses for the patient with PAD of the lower extremities (who has not undergone surgery) may include, but are not limited to, those presented in NCP 37-2.
 Planning
Planning
The overall goals are that the patient with PAD of the lower extremities will have: (1) adequate tissue perfusion; (2) relief of pain; (3) increased exercise tolerance; and (4) intact, healthy skin on extremities.
 Nursing implementation
Nursing implementation
 Health promotion
Health promotion
The patient should be assessed for risk factors and taught how to control them (see Table 33-4). The nursing role in the inpatient care facility includes identifying at-risk patients. Nurses should also be involved at the community level, such as in screening clinics for PAD, hyperlipidaemia, hypertension and diabetes. Young people and adults should be educated about the hazards of cigarette smoking. Nurses should also assist in teaching diet modification to reduce the intake of cholesterol, saturated fat and refined sugars; proper care of the feet; and the avoidance of injury to the extremities. Patients with positive family histories of cardiac, diabetic or vascular disease should be encouraged to obtain regular follow-up care.
 Acute intervention
Acute intervention
After surgical or radiological intervention the patient is placed in a recovery area for close observation. The operative extremity should be checked every 15 minutes initially and then hourly for skin colour and temperature, capillary refill, the presence of peripheral pulses distal to the operative site, and sensation and movement of the extremity. Loss of palpable pulses necessitates immediate notification of the surgeon and intervention. ABI measurements may be ordered and the indices should increase from the patient’s preoperative baseline. They should remain constant if the bypass (or stent) remains patent. All of these findings should be compared with the patient’s preoperative baseline and with findings in the opposite limb.28
After the patient leaves the recovery area, nursing care should focus on continued circulatory assessment and monitoring for the development of potential complications such as bleeding, haematoma, thrombosis, embolisation and compartment syndrome. A dramatic increase in the level of pain, loss of a palpable pulse or pulses distal to the operative site, extremity pallor or cyanosis, numbness or tingling or a cold extremity temperature may indicate occlusion of the bypass graft and should be reported to the surgeon immediately.
Knee-flexed positions should be avoided except for exercise. The patient should be turned and positioned frequently with pillows to cushion the incision. By the first or second postoperative day, the patient should be out of bed several times daily. Sitting for long periods of time should be discouraged because leg dependency may cause significant oedema, resulting in discomfort and stress to suture lines, and increases the risk of deep vein thrombosis. If significant swelling develops, a reclining position is preferred, with the oedematous leg elevated above heart level. Occasionally, elastic bandages or elastic support stockings are used to help control oedema of the limb. Walking even short distances is desirable. The use of a walker may be helpful initially, especially for older patients. If no complications are present, discharge from the hospital can be anticipated 3–5 days postoperatively.
 Ambulatory and home care
Ambulatory and home care
Atherosclerosis is a systemic disease process and not just localised to the lower extremities. Therefore, the overall approach to the control of atherosclerotic occlusive disease involves management of risk factors (see Table 33-4). Tobacco in any form is totally contraindicated, not only because of the vasoconstrictive effects of nicotine but also because tobacco smoke impairs transport and cellular utilisation of oxygen and increases blood viscosity. Continuance of cigarette smoking dramatically decreases the long-term patency rates of the bypass graft, as well as increasing the risk of an MI and stroke.
All patients should be taught the importance of meticulous foot care to prevent injury. Patients should learn to inspect their legs and feet daily for skin colour changes, mottling, alterations in the texture of the skin and subcutaneous fat, and reduction or absence of hair growth. Any ulceration or inflammation must be reported to the healthcare provider. Skin temperature should be noted and capillary refill of the fingers and toes should be tested. In addition, selected patients may be taught to palpate pulses and report any changes to the healthcare provider. Thick or overgrown toenails and calluses are potentially serious lesions that require regular attention by a skilled healthcare provider (e.g. podiatrist). Emphasis on foot care is especially important in diabetic patients with PAD because diabetic neuropathy (i.e. diminished peripheral sensation) increases susceptibility to traumatic injury and results in delay in seeking treatment (see Box 48-10).
Patients should wear clean, light-coloured all-cotton or all-wool socks and comfortable shoes with rounded (not pointed) toes and soft insoles. Shoes should not be laced tightly and new shoes should be broken in gradually. Frequent inspection of the feet should be of paramount importance to this patient population so that prompt attention to problems can be facilitated. Patients with poor eyesight, back problems, obesity or arthritis may need assistance with foot care (see Box 37-3).
BOX 37-3 Peripheral artery bypass surgery
PATIENT & FAMILY TEACHING GUIDE
Include the following in a teaching plan:
1. Reduce your risk factors by stopping smoking and use of tobacco products, controlling your blood pressure and blood sugar levels (if diabetic), and lowering your cholesterol and triglyceride levels.
2. Know the reasons for, and basic mechanism of action of, medications such as antiplatelets, antihypertensives, anticholesterol therapy and pain medication, and how long anticipated therapy will last.
3. Eat a healthy diet—it is essential to your recovery. You need to drink plenty of fluids, eat a well-balanced diet (including high-fibre foods and fresh fruits and vegetables) and eat less fried and high-fat foods.
4. Go for a daily walk and/or participate in an exercise program. In the beginning, take several short walks a day and rest between activities. Gradually increase your walking to 30–40 minutes a day.
5. Care for your feet and legs. Inspect your feet and wash them daily. Wear clean cotton socks and well-fitting shoes. File toenails straight across. Avoid sitting with your legs crossed, extreme hot and cold temperatures, and prolonged standing.
6. Follow routine postoperative wound care that includes keeping the incision site clean and dry, not disturbing Steri-Strips and eating a well-balanced diet that includes foods high in protein, vitamins C and A and zinc.
7. Monitor for signs and symptoms of impaired healing and/or infection of the leg incision, and notify your healthcare provider if any of the following occur:
8. Keep all follow-up appointments with your doctor.
9. Notify your doctor immediately if you experience increased leg or foot pain or a change in the colour or temperature of your foot and leg.
 Evaluation
Evaluation
Expected outcomes for the patient with PAD of the lower extremities are addressed in NCP 37-2.
Acute arterial ischaemic disorders
AETIOLOGY AND PATHOPHYSIOLOGY
Acute arterial ischaemia is a sudden interruption in the arterial blood supply to tissue, an organ or an extremity that if left untreated can result in tissue death. It can be caused by embolism, thrombosis of a pre-existing atherosclerotic artery or trauma. Venous outflow obstruction and low flow states can also result in acute arterial ischaemia.
Embolisation of a thrombus from the heart or an atherosclerotic aneurysm is the most frequent cause of acute arterial occlusion. Heart conditions in which thrombi are prone to develop include infective endocarditis, MI, mitral valve disease, chronic atrial fibrillation, cardiomyopathies and prosthetic heart valves. The thrombi become dislodged and may travel to the lungs (causing a pulmonary embolus) if they originate in the right side of the heart or to anywhere in the systemic circulation if they originate in the left side of the heart. Arterial emboli tend to lodge at sites of arterial branching or in areas of atherosclerotic narrowing. An acute arterial occlusion causes the blood supply distal to the embolus to decrease rapidly. The degree and extent of symptoms depend on the size and location of the obstruction, the occurrence of clot fragmentation with embolism to smaller vessels and the degree of PAD already present.
Sudden local thrombosis may occur at the location of an atherosclerotic plaque. Traumatic injury to the extremity itself may produce partial or total occlusion of a vessel from compression, shearing or laceration. Acute arterial occlusion may also develop as a result of arterial dissection in the carotid artery or aorta or as a result of iatrogenic arterial injury (e.g. after angiography).
CLINICAL MANIFESTATIONS
Signs and symptoms of an acute arterial ischaemia usually have an abrupt onset. The exception is when a sudden occlusion is superimposed on pre-existing PAD. In this case the symptoms may be insidious because collateral circulation is well developed.
Clinical manifestations of acute arterial ischaemia include the ‘six Ps’: pain, pallor, pulselessness, paraesthesia, paralysis and poikilothermia (adaptation of the ischaemic limb to its environmental temperature, most often cool). Without immediate intervention, ischaemia may progress to tissue necrosis and gangrene within hours. It should be noted that paralysis is a very late sign of acute arterial ischaemia and signals the death of nerves supplying the extremity. Because nerve tissue is extremely sensitive to hypoxia, limb paralysis or ischaemic neuropathy may persist after revascularisation and may be permanent.
MULTIDISCIPLINARY CARE
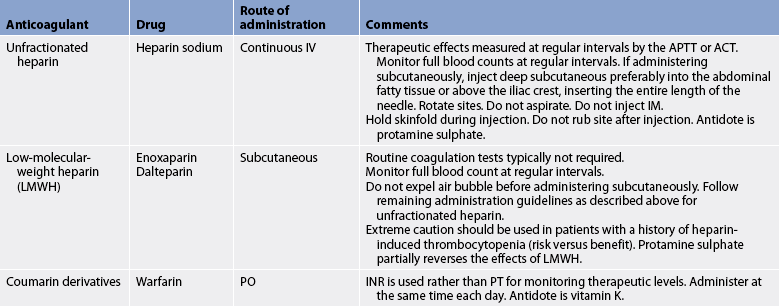
With acute arterial ischaemia due to occlusion, in the absence of adequate collateral circulation, early treatment is essential to keep the affected limb viable. Anticoagulant therapy is initiated immediately to prevent further enlargement of the thrombus and inhibit embolisation. Continuous unfractionated heparin IV is the agent of choice. The thrombus should be removed as soon as possible by embolectomy or thrombectomy. Balloon catheters can be used and are passed distal and proximal to the site to remove the clot. Direct arteriotomy may be necessary to remove the clot.
If the ischaemic limb is stable using heparin, recently formed emboli may be effectively treated with an intraarterial infusion of a thrombolytic agent (e.g. recombinant tissue plasminogen activator, streptokinase or urokinase). A percutaneous catheter is inserted into the femoral artery and threaded to the site of the clot and the drug is infused. Unlike anticoagulants, thrombolytic agents work by directly dissolving the clot over a period of 24–48 hours. (Thrombolytic therapy is discussed in Ch 33.)
After the infusion of the thrombolytic agent, bed rest is maintained and periodic angiography is performed to monitor the dissolution of the clot. The most serious potential problem associated with this procedure is life-threatening bleeding complications (e.g. cerebral haemorrhage). Therefore, patients are carefully selected and monitored by experienced critical care providers.
If the patient remains at risk of further embolisation from a persistent source, such as chronic atrial fibrillation, treatment includes long-term oral anticoagulation to prevent further acute arterial ischaemic episodes (see Table 37-6).
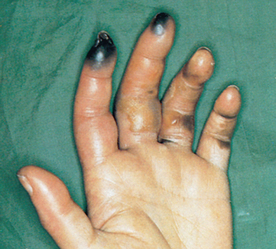
Thromboangiitis obliterans
Thromboangiitis obliterans (Buerger’s disease) is a somewhat rare, non-atherosclerotic, segmental inflammatory disorder of the medium-sized arteries, veins and nerves of the upper and lower extremities. The disorder occurs predominantly in younger men (25–40 years of age) and is more prevalent in the Middle East and Eastern Asia than in Europe and Australasia.29 A familial tendency has also been observed.
The underlying cause of Buerger’s disease remains unknown. Unlike atherosclerosis, lipid accumulation does not occur in the vessel wall. Instead, a highly cellular and inflammatory thrombus forms inside the vessel, causing tissue ischaemia. Buerger’s disease typically begins with ischaemia of the small, distal arteries and veins, progressing to more proximal arteries. Large arteries are rarely involved. The endothelial-dependent vasodilation of blood vessels is impaired in individuals with Buerger’s disease.29 There is a very strong relationship between Buerger’s disease and tobacco use. It is thought that the disease occurs only in smokers and, when smoking is stopped, the disease improves.
The symptom complex of Buerger’s disease is often confused with that of PAD and a variety of other inflammatory or autoimmune diseases.30 Patients may have intermittent claudication of the feet, hands or arms. As the disease progresses, rest pain and ischaemic ulcerations develop. Other signs and symptoms may include colour and temperature changes in the affected limb or limbs, paraesthesia, superficial thrombophlebitis and cold sensitivity. Up to 40% of patients with Buerger’s disease also have Raynaud’s phenomenon.29 There are no laboratory or diagnostic tests specific to Buerger’s disease. Diagnosis is made based on age of onset, history of tobacco usage, clinical symptoms, involvement of distal vessels, the presence of ischaemic ulcerations and the exclusion of diabetes mellitus, autoimmune disease, hypercoagulable states and proximal source of emboli.29
Treatment includes complete cessation of tobacco usage in any form (including second-hand smoke). Nicotine replacement products should not be used and trauma to the extremity must be avoided. Patients are told that they have a choice between their cigarettes and their affected limbs; they cannot have both. Other than smoking cessation, no other therapies have proven successful. Surgical revascularisation is typically not an option because of the diffuse nature of the disease. Painful ulceration may necessitate finger or toe amputations. Amputation below the knee may be necessary in advanced cases. The amputation rate in patients who continue tobacco use is 84.3% compared with only 30.6% in patients who discontinue tobacco use.30 The risk of amputation is eliminated 8 years after discontinuing tobacco use. Patients with Buerger’s disease have a significantly lower survival rate than age- and gender-matched controls. Although the amputation rate is lower in patients who stop using tobacco, risk of death is similar in those who continue to use tobacco and those who quit.30
Other therapies can be considered but have had limited success. The most commonly used medications are antiplatelet agents, followed by calcium channel blockers, α-adrenergic blockers and anticoagulants.30 Surgical options include revascularisation and sympathectomy, with the most common being sympathectomy (transection of a nerve, ganglion and/or plexus of the sympathetic nervous system). While sympathectomy is useful in improving distal blood flow, healing ulcerations and reducing pain, it does not alter the inflammatory process associated with Buerger’s disease. Surgical bypass procedure typically is not an option because of the distal vessel involvement of the disease. However, it may be used in select patients with severe ischaemia.30
Raynaud’s phenomenon
Raynaud’s phenomenon is an episodic vasospastic disorder of small cutaneous arteries, most frequently involving the fingers and toes (see Fig 37-7). The exact aetiology of Raynaud’s phenomenon remains unknown. One popular theory holds that the vasospasm occurs secondary to an exaggerated response to sympathetic nervous system stimulation. An alternative theory suggests that there are abnormalities in the endothelium and endothelium-derived vasoactive substances.31 Other contributing factors include occupationally related trauma and pressure to the fingertips, as noted in typists, pianists and those who use hand-held vibrating equipment. Exposure to heavy metals may also be a contributing aetiological factor. Raynaud’s phenomenon occurs primarily in young women (typically between 15 and 40 years of age). It is also frequently seen in association with collagen diseases such as rheumatoid arthritis, scleroderma and systemic lupus erythematosus; in these cases it is called secondary Raynaud’s phenomenon.
Raynaud’s phenomenon is characterised by vasospasm-induced colour changes (white, blue and red) of the fingers, toes, ears and nose (see Fig 37-7). Decreased perfusion due to arteriole vasospasm results in pallor (white). The digits then appear cyanotic (bluish-purple). These changes are subsequently followed by rubor (redness), caused by the hyperaemic response that occurs when perfusion is restored. The patient usually describes coldness and numbness in the vasoconstrictive phase followed by throbbing, aching pain, tingling and swelling in the hyperaemic phase. This type of episode usually lasts only minutes but in severe cases may persist for several hours. The symptoms are usually precipitated by exposure to cold, emotional upsets, caffeine and tobacco use. After frequent, prolonged attacks the skin may become thickened and the nails brittle. Occasionally, in severe forms of the phenomenon, complications include punctuate (small hole) lesions of the fingertips and superficial gangrenous ulcers in advanced stages.
As with Buerger’s disease, there is no simple diagnostic test for Raynaud’s phenomenon. If the symptoms persist for at least 2 years in the absence of an associated underlying disorder, the diagnosis of primary Raynaud’s disease is made. In contrast, if the symptoms exist in conjunction with a connective tissue or autoimmune disease, the diagnosis of secondary Raynaud’s phenomenon is made.29
Patient teaching should be directed towards reassurance that no serious underlying disorder is present and that prevention of recurrent episodes is possible. Loose, warm clothing should be worn as protection from the cold, including gloves when using the refrigerator/freezer or when handling cold objects. Temperature extremes should be avoided. The patient should stop using all tobacco products and avoid caffeine and other drugs with vasoconstrictive effects (amphetamines, cocaine, ergotamine, pseudoephedrine). If symptoms are exacerbated by stress, the patient needs to develop coping strategies for anxiety-producing situations. Biofeedback, relaxation training and stress management are effective for some patients. Immersing hands in warm water often decreases the vasospasm.
When a patient’s episodes are severe and other therapies are ineffective, drug therapy should be considered. Currently, calcium channel blockers are the first-line drug therapy.31 Calcium channel blockers, such as nifedipine and diltiazem, relax smooth muscles of the arterioles by blocking the influx of calcium into the cells, thus reducing the number of vasospastic attacks. Calcium channel blockers are effective for relieving symptoms in acute episodes. In addition, they can be used on a regular basis in treating patients with chronic Raynaud’s phenomenon. Nifedipine is preferred over diltiazem because it has a stronger vasodilating effect and less effect on the calcium channels in the conduction system of the heart.32 Adverse effects of calcium channel blockers include tachycardia, headache, flushing, dizziness, hypotension, bradycardia and peripheral oedema. Sympathectomy is considered only in advanced cases. Patients with Raynaud’s phenomenon should also receive routine follow-up to monitor possible connective tissue or autoimmune disease.
Venous thrombosis
The most common disorder of the veins is venous thrombosis, the formation of a thrombus (clot) in association with inflammation of the vein. Venous thrombosis is classified as either superficial thrombophlebitis or deep vein thrombosis (DVT) (see Table 37-3). Superficial thrombophlebitis (inflammation of a vein), which occurs in about 65% of all patients receiving IV therapy, is often of minor significance. DVT is a disorder involving a thrombus in a deep vein, most commonly the iliac and femoral veins. It occurs in at least 5% of all surgical patients. It is more serious than superficial thrombophlebitis because it can result in embolisation of thrombi to the lungs. Pulmonary embolism (PE) is a life-threatening condition and, in the best case scenario, results in prolonged hospitalisation.
TABLE 37-3 Comparison of superficial thrombophlebitis and deep vein thrombosis
| Superficial thrombophlebitis | Deep vein thrombosis | |
|---|---|---|
| Usual location | Superficial veins of arms and legs | Deep veins of arms (axillary, subclavian), legs (femoral) and pelvis (iliac, inferior/superior vena cava) |
| Clinical findings | Tenderness, redness, warmth, pain, inflammation and induration along the course of the superficial vein; vein appears as a palpable cord; oedema rarely occurs | Tenderness over involved vein to pressure, induration of overlying muscle, venous distension; oedema usually occurs; may have mild-to-moderate pain; deep reddish colour to area due to venous congestion |
| Sequelae | Embolisation rarely occurs | Embolisation may occur; chronic venous insufficiency may develop |
AETIOLOGY
Three important factors (called Virchow’s triad) in the aetiology of venous thrombosis are: (1) venous stasis; (2) damage to the endothelium (inner lining of the vein); and (3) hypercoagulability of the blood. The patient at risk of developing venous thrombosis usually has predisposing conditions to these three disorders (see Table 37-4).
1. Venous stasis. Normal blood flow in the venous system depends on the action of muscles in the extremities and the functional adequacy of venous valves, which allow unidirectional flow. Venous stasis occurs when the valves are dysfunctional or the muscles of the extremities are inactive. Venous stasis occurs more frequently in people who are obese, have congestive heart failure, have been on long trips without regular exercise, have a prolonged surgical procedure or are immobile for long periods (e.g. with spinal cord injuries or fractured hips). Pregnant women and women in the postpartum period are also at risk.33 Patients with atrial fibrillation are also at high risk because of stagnation of blood in the atria and the eddying in blood flow caused by irregular ventricular contractions in response to the atrial fibrillation. Some medications (e.g. corticosteroids, quinine) predispose a patient to DVT formation.
2. Endothelial damage. Damage to the endothelial surface of the vein may be caused by trauma or external pressure and occurs any time a venipuncture is performed. Damaged endothelium has decreased fibrinolytic properties, predisposing to thrombus development. Increased endothelial damage is sustained when patients on IV therapy are receiving caustic substances such as high-dose antibiotics, potassium, chemotherapeutic agents or hypertonic solutions such as parenteral nutrition or contrast media. Other factors predisposing to endothelial inflammation and damage include prolonged presence (>48 hours) of an IV catheter in the same site, the use of contaminated IV equipment, a fracture that causes damage to the blood vessels, diabetes mellitus, blood pooling, burns and any unusual physical exertion that results in muscle strain.
3. Hypercoagulability of blood. Hypercoagulability of blood occurs in many haematological disorders, particularly polycythaemia, severe anaemias, various malignancies (especially cancers of the breast, brain, pancreas and gastrointestinal tract), antithrombin III deficiency, protein C deficiency, protein S deficiency, antiphospholipid antibodies, elevated homocysteine levels and factor V Leiden mutation.31 A patient with sepsis is hypercoagulable in response to endotoxins that are released. Women of childbearing age who take oestrogen-based oral contraceptives and postmenopausal women who use hormone replacement therapy (HRT) are at increased risk of venous thromboembolism disease.31 Women who use oral contraceptives and smoke double their risk because of the constricting effect of nicotine on the blood vessel walls. Smoking may also cause hypercoagulability. Women who smoke, use oral contraceptives, are over age 35 and have a family history of venous thrombosis are at an extremely high risk of developing a thrombotic event. Women with a known thrombophilia, such as factor V Leiden mutation, should not use HRT, as the risk of thrombotic events may be increased as much as 15-fold.31
PATHOPHYSIOLOGY
Red blood cells (RBCs), WBCs, platelets and fibrin adhere to form a thrombus. A frequent site of thrombus formation is the valve cusps of veins, where venous stasis allows accumulation of blood products. As the thrombus enlarges, increased numbers of blood cells and fibrin collect behind it, producing a larger clot with a ‘tail’ that eventually occludes the lumen of the vein.
If a thrombus only partially occludes the vein, the thrombus will become covered by endothelial cells and the thrombotic process will stop. If the thrombus does not become detached, it undergoes lysis or becomes firmly organised and adherent within 5–7 days. The organised thrombi may detach and result in emboli. Turbulence of blood flow is a major factor contributing to detachment of the thrombus from the vein wall. The thrombus can become an embolus that generally flows through the venous circulation to the heart and lodges in the pulmonary circulation. Thus, it becomes a pulmonary embolus.
Superficial thrombophlebitis
CLINICAL MANIFESTATIONS
The patient with superficial thrombophlebitis may have a palpable, firm, subcutaneous cord-like vein (see Table 37-3). The area surrounding the vein may be tender to the touch, reddened and warm. A mild systemic temperature elevation and leucocytosis may be present. Oedema of the extremity may or may not occur. The most common cause of superficial thrombophlebitis in the upper extremities is trauma to the vein caused by IV therapy. Superficial thrombophlebitis in the lower extremities is usually related to trauma to the varicose veins. This type of superficial thrombophlebitis is more common in older patients with longstanding venous insufficiency and in women during pregnancy.34 Superficial thrombophlebitis is typically diagnosed on the basis of physical examination alone. In the legs, a venous duplex ultrasound may be used.
In rare cases, infectious or suppurative thrombophlebitis occurs at the site of an IV catheter. Unfortunately, this type of superficial thrombophlebitis often has no local signs or symptoms. A high-grade fever or septic pulmonary emboli may be the first indication of infectious thrombophlebitis.34 An elevated WBC count and/or positive blood cultures may also be found.
MULTIDISCIPLINARY CARE
The treatment of superficial thrombophlebitis includes elevation of the affected extremity to promote venous return and decrease the oedema, and the application of warm, moist heat. Heat is used to relieve the pain and treat the inflammation. If the superficial thrombophlebitis is associated with an IV catheter or solution, the IV catheter should be removed immediately. If the superficial thrombophlebitis has occurred in the lower extremity, elastic compression stockings are recommended once the acute thrombophlebitis has resolved.
Mild oral analgesics, such as aspirin or paracetamol, with codeine may be used to relieve pain. Non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen have been used to treat the inflammatory process and accompanying pain. Anticoagulant therapy is usually not indicated unless the proximal greater saphenous vein or the saphenofemoral junction is involved. Antibiotics and/or corticosteroids are occasionally used in complicated situations where an infection or inflammation of the vein exists.34
Deep vein thrombosis
CLINICAL MANIFESTATIONS
The patient with DVT may have no symptoms or have unilateral leg oedema, extremity pain, warm skin, erythema (see Fig 37-8) and a systemic temperature greater than 38°C. If the calf is involved, tenderness may be present on palpation. A positive Homans’ sign (pain on forced dorsiflexion of the foot when the leg is raised) is a classic but very unreliable sign (see Table 37-3). In fact, a positive Homans’ sign appears in only 10% of DVT patients and false positives are frequent.34 If the inferior vena cava is involved, the lower extremities may be oedematous and cyanotic. If the superior vena cava is involved, there may be symptoms on the upper extremities, neck, back and face.
COMPLICATIONS
The most serious complications of DVT are PE, chronic venous insufficiency (CVI) and phlegmasia cerulea dolens. PE is a life-threatening complication of DVT. CVI results from valvular destruction, allowing retrograde flow of venous blood. Persistent oedema, increased pigmentation, secondary varicosities, ulceration and cyanosis of the limb when it is placed in a dependent position may develop in a person with CVI. Signs and symptoms of CVI often do not develop until several years following DVT. Phlegmasia cerulea dolens (swollen, blue, painful leg), a very rare complication, may develop in a patient with severe lower extremity DVT(s). It causes sudden, massive swelling and intense cyanosis of the extremity. Gangrene occurs due to arterial occlusion secondary to venous obstruction, and amputation is required.
DIAGNOSTIC STUDIES
Various diagnostic studies are used to determine the site or location and extent of a deep vein thrombus or an embolism, and these are outlined in Table 37-5.
MULTIDISCIPLINARY CARE
Prevention and prophylaxis
In patients at risk of DVT, a variety of non-pharmacological and pharmacological interventions are used. Early mobilisation is the easiest and most cost-effective method to decrease the risk of DVT. Patients on bed rest need to be instructed to change position, dorsiflex their feet and rotate their ankles every 2–4 hours. Patients who are able to get out of bed need to be in the chair for all meals and ambulate at least three times per day.
Elastic compression stockings (e.g. TED stockings) have long been part of DVT prevention. Studies show that these stockings (which exert about 18 mmHg pressure) decrease distal calf vein thrombosis by decreasing venous stasis and augmenting venous return.35 However, it is unclear whether they are effective in reducing the incidence of proximal DVT and PE. Venous return is impeded by elastic stockings if the top elastic band is too tight.35,36 Thus, it is essential to measure the patient and obtain the appropriate size so that the stockings fit properly.
Intermittent compression devices (ICDs) are used for hospitalised patients at moderate, high or very high risk of DVT and PE. These devices apply intermittent external pressure to the lower extremities, which, in effect, addresses all three aspects of Virchow’s triad.36 First, the compression pushes blood from the superficial veins into the deep veins, thus decreasing venous stasis. Second, the compression decreases venous distension, thus lowering the risk of damage to the endothelium. Third, the increased blood flow velocity enhances fibrinolysis (the body’s natural anticoagulation factors). ICDs will not provide effective DVT prophylaxis if the device is not applied correctly or if the patient does not wear the device continuously except during bathing, skin assessment and ambulation.35,36 ICDs are not to be worn when a patient has an active DVT.
Preventive anticoagulation is used for patients at high or very high risk of DVT and PE and is addressed in the following drug therapy section. Anticoagulation may be used in combination with ICDs.
Non-pharmacological therapy
The usual treatment of DVT in hospitalised patients involves bed rest, elevation of the extremity and drug therapy. Typically, bed rest with elevation of the affected extremity above the level of the heart is indicated until the thrombus is stable and has adhered to the intraluminal wall, therapeutic levels of anticoagulation are achieved and the oedema is resolving. Warm compresses may also be applied to the affected area.
When the patient is allowed to resume ambulation, elastic compression stockings or 12 cm elastic bandages are recommended. Ideally, once the oedema has resolved, the patient should be measured for specialised, custom-fitted, graduated elastic compression stockings (e.g. Jobst stockings), which exert 30–40 mmHg pressure at the ankle.34 The use of elastic compression stockings (or sleeves in the case of an upper extremity) is recommended for several months (at least 3–6 months) to support the vein walls and valves and decrease swelling and pain on ambulation.
Drug therapy
Anticoagulants are routinely used for DVT. The goals of anticoagulation therapy are to prevent propagation of the clot, development of any new thrombus and embolisation. Anticoagulant therapy does not dissolve the clot. Lysis of the clot begins spontaneously through the body’s intrinsic fibrinolytic system (see Ch 29).
The most commonly used anticoagulants are unfractionated heparin, low-molecular-weight heparins (LMWHs) and warfarin compounds (see Table 37-6). Unfractionated heparin (heparin sodium, commonly known as heparin) acts directly on the intrinsic and common pathways of blood coagulation. Heparin inhibits thrombin-mediated conversion of fibrinogen to fibrin. It also potentiates the actions of antithrombin III, inhibits the activation of factor IX and neutralises activated factor X by activating factor X inhibitor.
TABLE 37-6 Anticoagulant therapy
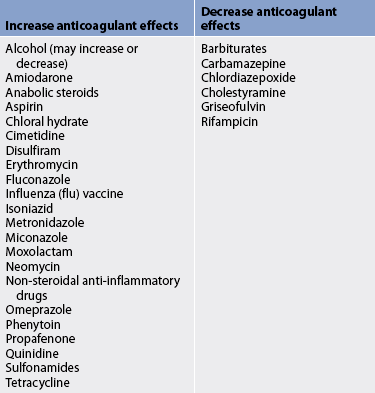
ACT, activated clotting time; APTT, activated partial thromboplastin time; IM, intramuscular; INR, international normalised ratio; IV, intravenous; PO, oral (per os); PT, prothrombin time.
LMWH is effective for the prevention and treatment of DVT. It is derived from heparin but the molecular size is approximately one-third that of heparin. Enoxaparin and dalteparin are examples of LMWH. LMWH has a greater bioavailability, more predictable dose response and longer half-life than heparin with less risk of bleeding complications. LMWH has the practical advantage that it does not require anticoagulant monitoring and dose adjustment.37 It is administered subcutaneously in fixed doses, once or twice daily. Duration of administration depends on the reason for use. Danaparoid, known as a heparinoid, does not contain heparin or heparin fragments. However, like heparin, it has antithrombotic action.
Warfarin, a coumarin compound, exerts its action indirectly on the coagulation pathway. Warfarin inhibits the hepatic synthesis of the vitamin K-dependent coagulation factors II, VII, IX and X by competitively interfering with vitamin K. Vitamin K is normally required for the synthesis of these factors.
Oral anticoagulants are often administered concurrently with heparin. Warfarin requires 48–72 hours to influence the prothrombin time (PT) and it may take several days before maximum effect is achieved. Therefore, an overlap of heparin and warfarin is required for 3–5 days. The clotting status should be monitored by the activated partial thromboplastin time (APTT) for heparin therapy and the international normalised ratio (INR) for warfarin. The INR is a standardised system of reporting PT based on a referenced calibration model and calculated by comparing the patient’s PT with a control value. Other tests to monitor anticoagulation may be used (see Table 37-7).
For DVT prophylaxis, low-dose unfractionated heparin, LMWH or warfarin can be prescribed depending on the patient’s level of risk and weight.38 Unfractionated heparin is typically administered subcutaneously (SC) and prescribed at 5000 units every 12 hours SC for low- and moderate-risk patients or 3500–5000 units every 8 hours SC for high-risk patients. LMWH is usually dosed at 30 mg every 12 hours SC or 40 mg four times daily SC. LMWH is rapidly replacing heparin as the anticoagulant of choice to prevent DVT in high-risk patients. In fact, LMWH is considered the most effective form of prophylaxis in hip and knee surgery and following major trauma.39 Low-dose warfarin is usually reserved for patients with the highest DVT risk.
Patients with a very large DVT are usually hospitalised and receive an initial IV bolus of heparin followed by continuous IV heparin infusion for 5–7 days. Before discontinuing the IV heparin, oral anticoagulant therapy is started. The IV heparin is discontinued once the INR is at the desired level. Oral anticoagulants are prescribed for 3–6 months.
Patients with a small, uncomplicated DVT (in the popliteal vein or a more proximal vein) can be managed on an outpatient basis with LMWH. Current guidelines recommend placing the patient on LMWH at a weight-based dose of 1 mg/kg administered every 12 hours SC.34 Warfarin is also prescribed and given concurrently with the LMWH until the warfarin is at a therapeutic level (INR 2.0–3.0). Warfarin is administered for 3–6 months after the DVT. Recurrent episodes of DVT require lifelong anticoagulation therapy unless contraindicated.
A careful history of pregnancy status and medications should be taken before initiating anticoagulation. Because warfarin is contraindicated in pregnancy, pregnant patients requiring anticoagulation often receive SC heparin or LMWH. Antiplatelet agents (e.g. aspirin) are generally contraindicated while on anticoagulation. Other drugs that interact with warfarin include phenytoin, barbiturates and NSAIDs, such as ibuprofen (see Table 37-8).
Changes in diet can also interact with warfarin. A diet high in vitamin K (e.g. green leafy vegetables) can make it difficult to maintain a patient within a therapeutic range. The patient should be instructed to follow a diet that includes foods containing vitamin K in moderate amounts but to avoid any additional supplements with vitamin K. In addition, the patient should be instructed to avoid excessive amounts of vitamin E and alcohol. While the patient is hospitalised, nursing activities should be adjusted to monitor for and reduce the risk of bleeding that may occur with anticoagulant administration (see Box 37-4).
BOX 37-4 Nursing interventions to prevent bleeding complications in patients receiving anticoagulants
Assessment
• Monitor vital signs as indicated.
• Examine appropriate laboratory coagulation tests for achieved therapeutic levels.
• Inspect skin frequently, especially under splinting devices.
• Perform assessments frequently to observe for signs and symptoms of bleeding and/or clotting.
• Evaluate lower extremity for ecchymosis/haematoma development if sequential compression hose used.
• Test urine and stool for occult blood as indicated.
• Notify the healthcare provider of any abnormalities in assessments, vital signs or laboratory values.
Patient care
• Reposition the patient carefully at regular intervals.
• Use support pads, mattresses, bed cradles and therapeutic beds as indicated.
• Avoid removing/disrupting established clots.
• Instruct the patient not to blow the nose forcefully.
• Administer stool softeners to avoid hard stools and constipation.
• Limit tape application—use paper tape as appropriate.
• Avoid restraints if possible—use only soft, padded restraints as needed.
• Perform care in a gentle manner.
• Use soft toothbrushes or foam swabs for oral care.
• Apply moisturising lotion to the skin.
• Do not use lemon–glycerine swabs.
• Use electric razors, not safety razors.
• Lubricate tubes adequately prior to insertion.
• Apply antiembolism stockings (e.g. TED stockings) as ordered.
Surgical therapy
Although most patients are managed conservatively, small percentages require surgical intervention. The primary indication for surgery is to prevent PE. Surgical procedures include venous thrombectomy (rarely performed) and inferior vena cava interruption (see Fig 37-9). Venous thrombectomy involves the removal of a DVT through an incision in the vein. This procedure is done to prevent PE or to decrease the risk of the development of chronic venous insufficiency.
Figure 37-9 Inferior vena caval interruption technique using Greenfield stainless steel filter to prevent pulmonary embolism.
Vena cava interruption devices, such as the Greenfield or Simon-Nitinol filters, can be inserted percutaneously through superficial femoral or internal jugular veins. The filter device is opened and the spokes penetrate the vessel walls (see Fig 37-9). These devices result in ‘sieve-type’ obstruction, permitting filtration of clots without interruption of blood flow.
Complications after the insertion of the intravascular filter device are rare. They include air embolism, improper placement and migration of the filter more distally into the venous system. Venous congestion is common and results from the accumulation of trapped clots at the filter site. Over time these clots may clog the filter and completely occlude the vena cava. Because this process is gradual, collateral vessels usually develop to maintain venous flow. However, these collateral venous pathways may also provide an alternative route for PE.
 NURSING MANAGEMENT: VENOUS THROMBOSIS
NURSING MANAGEMENT: VENOUS THROMBOSIS
 Nursing assessment
Nursing assessment
Subjective and objective data that should be obtained from a patient with venous thrombosis are presented in Table 37-9.
 Nursing diagnoses
Nursing diagnoses
Nursing diagnoses and collaborative problems for the patient with venous thrombosis include, but are not limited to, the following:
• acute pain related to venous congestion, impaired venous return and inflammation as manifested by complaints of pain and the presence of inflammation
• ineffective health maintenance related to lack of knowledge about the disorder and its treatment
• risk of impaired skin integrity related to altered peripheral tissue perfusion
• potential complication: bleeding related to anticoagulant therapy
• potential complication: pulmonary embolism related to embolisation of thrombus, dehydration and immobility.
 Planning
Planning
The overall goals are that the patient with venous thrombosis will have: (1) relief of pain; (2) decreased oedema; (3) no skin ulceration; (4) no complications from anticoagulant therapy; and (5) no evidence of pulmonary emboli.
 Nursing implementation
Nursing implementation
 Acute intervention
Acute intervention
Nursing care for the patient with venous thrombosis is directed towards the prevention of emboli formation and the reduction of inflammation. While the patient is receiving anticoagulation therapy, the nurse should closely observe for any indication of bleeding, including epistaxis and bleeding gingiva. The patient’s urine should be assessed for gross or microscopic haematuria. A smoky appearance of the urine is sometimes noted if blood is present. A specimen should be checked daily for haematuria. Particular attention should be paid to the protection of skin areas that may be traumatised. Surgical incisions should be closely observed for evidence of bleeding. Stools should be tested to determine the presence of occult blood from the gastrointestinal tract. Mental status changes, especially in the older patient, should be assessed as a possible indication of cerebral bleeding. Intramuscular injections should not be given.
The nurse should review with the patient any medications currently being taken that may interfere with anticoagulant therapy (see Table 37-8) and ask whether the patient is taking any herbal products, as herbs can interfere with anticoagulant therapy (see Complementary and alternative therapies box).39 When a patient is receiving anticoagulant drugs, APTT, INR, haemoglobin, haematocrit and platelet levels should be monitored. Platelet counts are monitored to assess for heparin-induced thrombocytopenia. Medication doses are titrated according to the results of clotting studies. The nurse should be cautious about administering either heparin or warfarin without first checking the results of the clotting studies. The antidote for heparin is protamine sulfate and the antidote for warfarin is vitamin K. These drugs must be immediately available if bleeding occurs. Fresh frozen plasma may be administered for bleeding because it contains multiple clotting factors.
 Ambulatory and home care
Ambulatory and home care
During all phases of care the nurse should evaluate the patient’s psychological response. Many patients are apprehensive that clots will move to the heart or lungs and cause sudden death. Patients should be allowed to verbalise their concerns and an attempt should be made to clarify misconceptions. Patients hospitalised for an extended time require diversional activities.
Discharge teaching should focus on elimination of modifiable risk factors for DVT, the importance of compression stockings and monitoring of laboratory values, medication instructions and guidelines for follow-up. Modifiable risk factors for DVT include cigarette smoking, use of oral contraceptives, use of HRT, a sedentary lifestyle and obesity. Patients should be informed that they need to stop both smoking and the use of nicotine products because nicotine increases the viscosity of the blood. Constrictive clothing, such as girdles or garters, should be avoided. Women with a history of DVT should be instructed to stop using oral contraceptives and HRT. Another important preventive measure is to avoid prolonged standing or sitting in a motionless, leg-dependent position. Frequent knee flexion, ankle rotation and active walking should be done during long periods of sitting or standing, especially on long car or aeroplane trips. Dietary considerations for the overweight patient are aimed at limiting kilojoule intake to achieve and then maintain desired weight.
The patient and family should be taught about signs and symptoms of PE, such as sudden onset of dyspnoea, tachypnoea and pleuritic chest pain. They should be instructed to notify the healthcare provider immediately and/or go to the hospital if such signs appear.
If the patient is continuing on anticoagulant therapy, the patient and family need careful explanations of medication dosage, actions and side effects, as well as the importance of routine blood tests and the need to report symptoms to the healthcare provider (see Box 37-5). Home monitoring devices are now available for immediate testing of PT.40 Patients on LMWH will need to learn how to self-administer the drug or have a friend or family member administer it. The most common problems with LMWH are bruising at the injection site. Active or younger patients need to be instructed to avoid contact sports and other high-risk activities (e.g. roller-blading). Older patients should be taught safety precautions to prevent injuries, such as falls. Should a bleeding episode occur, the patient and family should be instructed to apply pressure for 5 minutes. If the bleeding has not slowed significantly or completely resolved, they should call 000 (Australia) or 111 (New Zealand).
BOX 37-5 Anticoagulant therapy
PATIENT & FAMILY TEACHING GUIDE
Include the following in a teaching plan:
1. Reasons for and basic mechanism of action of anticoagulant therapy and how long anticipated therapy will last.
2. Need to take medication at same time each day (preferably in afternoon or evening).
3. Requirement for frequent follow-up with blood tests to assess blood clotting and whether change in drug dosages is required.
4. Side effects and adverse effects of drug therapy requiring medical attention:
5. Avoid any trauma or injury that might cause bleeding (e.g. vigorous brushing of teeth, contact sports, roller-blading).
6. Do not take aspirin-containing drugs or non-steroidal anti-inflammatory drugs.
7. Limit alcohol intake to small-to-moderate amount.
8. Wear a medical alert bracelet or necklace indicating what anticoagulant is being taken.
9. Avoid marked changes in eating habits, such as dramatically increasing foods high in vitamin K (e.g. broccoli, spinach, kale, greens). Do not take supplemental vitamin K.
10. Consult with healthcare provider before beginning or discontinuing any medication.
11. Inform all healthcare providers, including dentist, of anticoagulant therapy.
12. Correct dosing is essential and supervision may be required (e.g. patients experiencing confusion).
13. Do not use herbal products that may alter coagulation (see Complementary and Alternative Therapies box).
14. Notify doctor immediately if chest pain, shortness of breath, a feeling of passing out or palpitation (heart racing) is experienced.
A well-balanced diet is important because calcium and vitamin E play active roles in the clotting mechanism. Patients on warfarin should be instructed to follow a consistent diet of foods containing vitamin K and to avoid any additional supplements that contain vitamin K. In addition, patients should be instructed to avoid excessive amounts of vitamin E and alcohol. Proper hydration is required to prevent additional hypercoagulability of the blood, which may occur in the presence of deficient fluid intake. The overweight patient needs to not only limit energy intake but also increase physical activity levels to achieve and maintain desired weight. A balanced program of rest and exercise also improves arterial filling and venous return. Exercise programs should be developed with an emphasis on walking, swimming and wading. Water exercises are particularly beneficial because of the gentle, even pressure of the water.
Varicose veins
Varicose veins, or varicosities, are dilated, tortuous subcutaneous veins most frequently found in the saphenous system. They may be small and innocuous or large and bulging. Primary varicosities are those in which the superficial veins are dilated and the valves may or may not be rendered incompetent. This condition tends to be familial, is characteristically found bilaterally and is probably caused by congenital weakness of the veins. Secondary varicosities result from previous DVT of the deep femoral veins, with subsequent valvular incompetence. Secondary varicose veins may occur in the oesophagus (oesophageal varices), in the anorectal area (haemorrhoids) and as abnormal arteriovenous (AV) connections (AV fistulas and malformations). Reticular veins are smaller varicose veins that appear flat, less tortuous and blue–green in colour. Telangiectasias (often referred to as spider veins) are very small visible vessels (generally <1 mm in diameter) that appear bluish-black, purple or red.
AETIOLOGY AND PATHOPHYSIOLOGY
The aetiology of varicose veins is unknown. Superficial veins in the lower extremities become dilated and tortuous, with increased venous pressure. This increased venous pressure may result from a congenital weakness of the vein structure, obesity, pregnancy, venous obstruction resulting from thrombosis or extrinsic pressure by tumours or occupations that require prolonged standing.34 As the veins enlarge, the valves are stretched and become incompetent, allowing venous blood flow to be reversed. As back pressure increases and the calf muscle pump (muscle movement that squeezes venous blood back towards the heart) fails, further venous distension results. The increased venous pressure is transmitted to the capillary bed and oedema develops.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
Discomfort from varicose veins varies dramatically among people and tends to be worsened by superficial thrombophlebitis. In addition, many patients voice concern about cosmetic disfigurement (see Fig 37-10). The most common symptom of varicose veins is an ache or pain after prolonged standing, which is relieved by walking or by elevating the limb. Some patients feel pressure or a cramp-like sensation. Swelling may accompany the discomfort. Nocturnal leg cramps in the calf may occur.
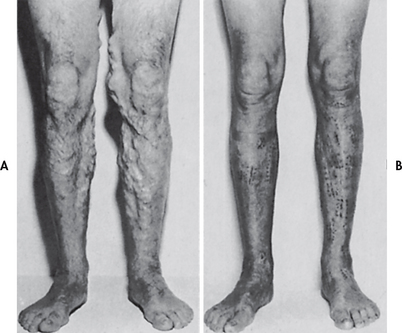
Figure 37-10 Extensive varicosities (incompetency of the greater saphenous systems). A, Appearance preoperatively. B, Appearance 2 weeks postoperatively.
Superficial thrombophlebitis is the most frequent complication of varicose veins and may occur either spontaneously or after trauma, surgical procedures or pregnancy. Rare complications include rupture of the varicose veins from weakening of the vessel wall and ulceration of the skin.
DIAGNOSTIC STUDIES AND MULTIDISCIPLINARY CARE
Superficial varicose veins can be diagnosed by appearance. A duplex ultrasound can detect obstruction and reflux in the venous system with considerable accuracy. It is the most widely used test to diagnose deep varicose veins.
Treatment is usually not indicated if varicose veins are only a cosmetic problem. If incompetency of the venous system develops, multidisciplinary care involves rest with the affected limb elevated, compression stockings and exercise, such as walking.
Sclerotherapy is a technique used in the treatment of unsightly superficial varicosities (see Fig 37-11).41 Direct IV injection of a sclerosing agent such as sodium tetradecyl induces inflammation and results in eventual thrombosis of the vein. This procedure can be performed safely in an office setting and causes minimal discomfort. After injection the leg is wrapped with an elastic bandage for 24–72 hours to maintain pressure over the vein. Local tenderness subsides within 2–3 weeks and eventually the thrombosed vein disappears. After sclerotherapy the patient should be advised to wear compression stockings to help prevent the development of further varicosities.
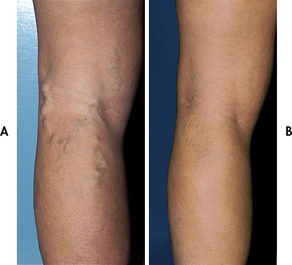
Figure 37-11 Varicose veins and treatment with sclerotherapy. A, Before treatment. B, Clinical appearance 2½ years after treatment.
Newer, more costly, but non-invasive options for the treatment of venous telangiectasias include laser therapy and high-intensity pulsed-light therapy.42 Laser or light therapy is indicated for isolated small telangiectasias or for patients in whom sclerotherapy is contraindicated or has been previously ineffective. Laser treatment typically requires more than one session, scheduled at 6–12 week intervals. Vascular lasers work by heating the haemoglobin in the vessels, which injures the endothelium, resulting in vessel sclerosis. Pulsed light therapy is similar to laser therapy, but uses a spectrum of light rather than a single wavelength. Potential complications associated with both these therapies include pain, blistering, hyperpigmentation and superficial erosions.
Surgical intervention for varicose veins involves ligation of the entire vein (usually the greater saphenous) and dissection and removal of its incompetent tributaries (see Fig 37-11). Surgical intervention is indicated when chronic venous insufficiency cannot be controlled with conservative therapy. Recurrent thrombophlebitis in varicose veins is another indication for surgery. In selected patients, the use of lasers may be an option in the treatment of superficial varicosities instead of sclerotherapy or surgery.42 Surgical treatment for varicose veins is typically done as an outpatient procedure.
 NURSING MANAGEMENT: VARICOSE VEINS
NURSING MANAGEMENT: VARICOSE VEINS
Prevention is a key factor related to varicose veins. The nurse should instruct the patient to avoid sitting or standing for long periods of time, maintain ideal body weight, take precautions against injury to the extremities, avoid wearing constrictive clothing and participate in a daily walking program.
After vein ligation surgery, the nurse should encourage the patient in deep breathing, which helps promote venous return to the right side of the heart. The extremities should be checked regularly for colour, movement, sensation, temperature, the presence of oedema and pedal pulses. Bruising and discolouration are considered normal. Postoperatively, the legs are elevated at a 15° angle to prevent oedema. Compression stockings are applied and removed every 8 hours for short periods and then reapplied.
Long-term management of varicose veins is directed towards improving circulation, relieving discomfort, improving cosmetic appearance and avoiding complications, such as superficial thrombophlebitis and ulceration. Varicose veins can recur in other veins after vein ligation. The patient should be taught proper care of the lower extremities, including cleanliness and the use of individually fitted compression stockings. The patient should be taught to put on the stockings while still lying down just before rising in the morning. The importance of periodic positioning of the legs above the heart should be stressed. The overweight patient may need assistance with weight reduction. Patients whose occupation requires prolonged periods of standing or sitting need to change position frequently.
Chronic venous insufficiency and venous leg ulcers
CVI is a common medical problem in the elderly. It often occurs as a result of previous episodes of DVT and can lead to venous leg ulcers (formerly called venous stasis ulcers or varicose ulcers). Venous ulcer prevalence on admission to nursing homes is 2.5% with an annual incidence in nursing home patients of 2.2%.43 Although CVI and venous ulceration are not life-threatening diseases, they are painful, debilitating and costly chronic conditions that can adversely affect the quality of patients’ lives.
AETIOLOGY AND PATHOPHYSIOLOGY
The causes of CVI include vein incompetence, deep vein obstruction, congenital venous malformation, arteriovenous fistula and calf muscle failure.44 The basic dysfunction is incompetent valves of the deep veins. As a result, hydrostatic pressure in the veins increases and serous fluid and RBCs leak from the capillaries and venules into the tissue, resulting in oedema. Enzymes in the tissue eventually break down RBCs, causing the release of haemosiderin, which results in a brownish skin discolouration. Over time, the skin and subcutaneous tissue around the ankle are replaced by fibrous tissue, resulting in thick, hardened, contracted skin.
Although the causes of CVI are known, the exact pathophysiology of venous ulcers remains unknown. It is known that decreased fibrinolysis, pericapillary fibrin cuffs and WBC trapping occur in venous ulcers.44
CLINICAL MANIFESTATIONS AND COMPLICATIONS
In individuals with CVI, the skin of the lower leg is leathery, with a characteristic brownish or ‘brawny’ appearance from the haemosiderin deposition. Oedema has usually been persistent for a prolonged period. Eczema or stasis dermatitis is often present and pruritus is a common complaint.
Venous ulcers classically are located above the medial malleolus. However, they can occur near the lateral malleolus (see Fig 37-12). The wound margins are irregularly shaped and the tissue is typically a ruddy colour (see Table 37-1). Venous ulcers are typically partial-thickness wounds that extend through the epidermis and portions of the dermis. Ulcer drainage may be extensive, especially when the leg is oedematous. The ulcer is often quite painful, particularly when oedema or infection is present.45 Pain may be worse when the leg is in a dependent position. Ulcer pain adversely affects the patient’s quality of life.46
If the venous ulcer is left untreated, the lesion becomes more extensive, eroding more widely and deeply and increasing the likelihood of wound infection and cellulitis. Recurrent episodes of cellulitis may lead to destruction of the superficial lymphatics, causing a secondary lymphoedema to develop. On very rare occasions, severe CVI with longstanding non-healing venous ulceration may result in the patient’s need to have an amputation.
MULTIDISCIPLINARY CARE
Compression is essential to the management of CVI, venous ulcer healing and the prevention of ulcer recurrence. A variety of options are available to achieve compression, including elastic wraps, custom-fitted compression stockings, elasticised tubular support bandages, Velcro wraps, sequential pneumatic compression devices, paste bandages (Unna’s boot) with an elastic wrap and multilayer (three or four) bandage regimens.44 There are benefits to each type of compression therapy and the nurse must evaluate each patient individually when choosing an extrinsic compression method. Before instituting compression therapy, assessment of the arterial status is necessary to make sure that coexistent arterial disease does not exist. An ABI of 0.8 suggests that the patient also has PAD and should not have high levels of compression.
Moist environment dressings are the mainstay of wound care. A variety of dressings are available that provide a moist environment and include transparent film dressings, hydrocolloids, hydrogels, foams, calcium alginates, impregnated gauze, gauze moistened with saline and combination dressings. When used in conjunction with extrinsic compression, moist environment dressings have proven to be more effective in hastening the healing of venous leg ulcers than dry dressings. (Hydrocolloid dressings and other dressings are discussed in Ch 12.)
Improving quality of life for patients with chronic leg ulcers
NURSING RESEARCH
Citation
Edwards H, Courtney M, Finlayson K, Shuter P, Lindsay E. A randomised controlled trial of a community nursing intervention: improved quality of life and healing for clients with chronic leg ulcers. J Clin Nurs 2009: 18(11):1541–1519.
Purpose
To test the effectiveness of a new community nursing model of care on quality of life, morale, depression, self-esteem, social support, healing, pain and functional ability of patients with chronic lower limb ulcers.
Method
A randomised controlled trial was conducted in Queensland, Australia for 34 patients in an intervention group using the Lindsay Leg Club model of care, which emphasises social and group support for the delivery of care (e.g. during dressing changes). The control group (n = 33) received individual home visits by a community nurse, and both groups received identical wound care including short-stretch compression stockings. Outcomes were measured at 12 and 24 weeks using Spitzer’s Quality of Life Index, the MOS Social Support Scale, the MOS Pain Measure and area measures of wound size.
Results and conclusions
Wounds in patients in the intervention group had significantly smaller (1.54 cm2) ulcers than in the control group (6.17 cm2). Pain levels in the intervention group were significantly lower (18.28) than in the control group (31.46) at 24 weeks. Furthermore, quality of life, morale and self-esteem were significantly higher in the intervention group, although interestingly patients in the intervention group scored their levels of social support lower.
Implications for nursing practice
This study demonstrates the effectiveness of using group delivery-based leg ulcer care for improving multiple important aspects of a patient’s life, without necessarily increasing costs. While further research is required, the model of care shows promise for this chronic illness group.
Nutritional status and intake should be evaluated in patients with a venous leg ulcer.47 A balanced diet with adequate protein, kilojoules and micronutrients is essential for healing. Nutrients most important for healing include protein, vitamins A and C, and zinc. Foods high in protein (e.g. meat, beans, cheese, tofu), vitamin A (green leafy vegetables), vitamin C (citrus fruits, tomatoes, rockmelon) and zinc (meat, seafood) must be provided.47 For patients with coexisting diabetes mellitus, maintaining normal blood glucose levels facilitates the healing process.41 For overweight individuals with CVI and no active venous ulcer, a weight reduction diet may be prescribed.
Routine prophylactic antibiotic therapy is not typically indicated. Clinical signs of infection in a venous ulcer include changes in the quantity, colour or odour of the drainage; the presence of pus; erythema of the wound edges; changes in sensation around the wound; warmth around the wound; increased local pain, oedema or both; dark-coloured granulation tissue; induration around the wound; delayed healing; and cellulitis.47 If signs of infection are present, a culture should be obtained and appropriate antibiotic therapy instituted. The usual treatment for infection is sharp debridement, wound excision and systemic antibiotics. Moist-to-moist saline dressings are indicated until the infection clears, after which other moist environment dressings may be used.48
If the ulcer fails to respond to conservative therapy, alternative treatments include coverage with a split-thickness skin graft, cultured epithelial autografts and allografts or bioengineered skin. Typically when a split-thickness skin graft is used, the ulcer is debrided, varicosities in the area of the lesion are removed and veins are ligated before the tissue from a donor site is applied.
 NURSING MANAGEMENT: VENOUS LEG ULCERS
NURSING MANAGEMENT: VENOUS LEG ULCERS
Long-term management of venous leg ulcers should focus on teaching patients about self-care measures because the incidence of recurrence is high. This is particularly important because research indicates that patients with chronic venous disease lack understanding of appropriate treatments.49 Patient and family teaching should include the importance of avoiding trauma to the limbs, proper skin care measures, the application of prescribed compression stockings, and appropriate activity and limb positioning.
Proper foot and leg care is essential to avoid additional trauma to the skin. In addition, because of the stasis dermatitis, patients with CVI have dry, flaky, itchy skin. Daily moisturising of the skin decreases itching and prevents cracking. Creams or lotions with fragrance or lanolin should be avoided because of the potential for allergic reactions. The wound should be assessed for signs and symptoms of infection during each dressing change.
Patients with CVI with or without a venous ulcer are instructed to avoid standing or sitting with the feet dependent for long periods. Standing or sitting with the legs in a dependent position has been shown to decrease periulcer skin blood perfusion and oxygen levels.50 Venous ulcer patients are also instructed to elevate their legs above the level of the heart. Once an ulcer is healed, a daily walking program is encouraged. Well-fitting shoes are essential.
The patient with peripheral arterial disease
CASE STUDY
Patient profile
Mr Jackson, a 76-year-old man, was admitted to the hospital with pain while at rest and a non-healing ulcer of the big toe on the right foot.
CRITICAL THINKING QUESTIONS
1. What are this patient’s risk factors for peripheral arterial disease?
2. What additional information would you like to know about this patient?
3. Are this patient’s signs and symptoms evidence of an acute or a chronic peripheral arterial disease? Explain your answer.
4. What is the pathophysiology of pain while at rest?
5. What treatment modalities are possible for this patient?
6. What are the primary nursing responsibilities in caring for this patient?
7. Based on the assessment data presented, write one or more appropriate nursing diagnoses. Are there any collaborative problems?
1. A 62-year-old woman weighs 92 kg and has a history of daily alcohol intake, smoking, high blood pressure, high sodium intake and a sedentary lifestyle. The nurse identifies the risk factors most highly related to peripheral arterial disease in this patient as:
2. Significant risk factors for peripheral arterial disease include:
3. A patient is being prepared for an abdominal aortic aneurysm repair. The nurse suspects rupture of the aneurysm when:
4. Important nursing measures after an abdominal aortic aneurysm repair are to:
5. Specific symptoms of aortic dissection vary depending on:
6. Rest pain is a manifestation of peripheral arterial disease that occurs as a result of:
7. A patient with infective endocarditis develops sudden left leg pain with pallor, paraesthesia and a loss of peripheral pulses. The nurse’s initial action should be to:
8. The usual medical treatment of Raynaud’s phenomenon involves:
9. The patient who is most likely to have the highest risk of deep vein thrombosis is a:
10. The nurse suspects the presence of a deep vein thrombosis based on the findings of:
11. Nursing interventions indicated in the plan of care for the patient with acute lower extremity deep vein thrombosis include:
12. The nurse instructs the patient discharged on anticoagulant therapy to:
13. In planning care and patient teaching for the patient with venous leg ulcers, the nurse recognises that the most important intervention in healing and control of this condition is:
1 Hiatt WR, Goldstone J, Smith SC. Atherosclerotic peripheral vascular disease symposium II: nomenclature for vascular diseases. Circulation. 2008;118:2826.
2 Ostchega Y, Paulose-Ram R, Dillon CF, et al. Prevalence of peripheral arterial disease and risk factors in persons aged 60 and older: data from the National Health and Nutrition Examination Survey, 1999–2004. J Am Geriatr Soc. 2007;55:583.
3 Melamed ML, Muntner P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: results from the NHANES, 2001–2004. Arterioscler Thromb Vasc Biol. 2008;28:1179.
4 Australian Institute of Health & Welfare (AIHW). Heart, stroke and vascular diseases: Australian facts. Canberra: AIHW; 2004. Available at www.aihw.gov.au/publications/index.cfm/title/10005 accessed 12 November 2010.
5 Zacharski LR, Chow B, et al. Reduction of iron stores and cardiovascular outcomes in patients with peripheral arterial disease: a randomized controlled trial. JAMA. 2007;297(6):603–610.
6 Australian Institute of Health & Welfare (AIHW). Australia’s health, 2008. Canberra: AIHW; 2008. Available at www.aihw.gov.au/publications/index.cfm/title/10005 accessed 31 August 2010.
7 Matsumura JS. Surgery of the aorta. In Fahey VA, ed.: Vascular nursing, 4th edn., Philadelphia: WB Saunders, 2004.
8 Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 practice guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic). Circulation. 2006;113:e463.
9 Keck School of Medicine, University of Southern California Center for Vascular Care. Abdominal aortic aneurysm. Available at www.surgery.usc.edu/divisions/vas/abdominalaorticaneurysm.html. accessed 31 August 2010.
10 Tinkman M. The endovascular approach to abdominal aortic aneurysm repair. AORN J. 2009;89(2):289.
11 Boult M, Babidge W, et al. Endoluminal repair of abdominal aortic aneurysm: contemporary Australian experience. Eur J Vasc Endovasc Surg. 2004;28(1):36–40.
12 Tinkman M. The endovascular approach to abdominal aortic aneurysm repair. AORN J. 2009;89(2):289.
13 Lederle FA, Larson JC, Margolis KL, et al. Abdominal aortic aneurysm events in the women’s health initiative: cohort study. BMJ. 2008;337:a1724.
14 McPhee J, Hill J, Eslami M. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysms in the US, 2001–2004. J Vasc Surg. 2007;45:891.
15 Lederle FA, Larson JC, Margolis KL, et al. Abdominal aortic aneurysm events in the women’s health initiative: cohort study. BMJ. 2008;337:a1724.
16 Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society consensus for the management of peripheral arterial disease. J Vasc Surg. 2007;45:S5.
17 White C, Gray W. Endovascular therapies for peripheral arterial disease: an evidence-based review. Circulation. 2007;116:2203.
18 Hiatt McDermott MM, Ades P, Guralnik JM, et al. Treadmill exercise and resistance training in patients with peripheral arterial disease with and without intermittent claudication: a randomized controlled trial. JAMA. 2009;301:165.
19 Patel PD, Arora RR. Pathophysiology, diagnosis, and management of aortic dissection. Ther Adv Cardiovasc Dis. 2008;2:439.
20 American Diabetes Association. Standards of medical care in diabetes—2008, Diabetes Care. 2008;31:S1. Available at http://care.diabetesjournals.org/content/31/Supplement_1/S12.full accessed 12 November 2010.
21 Sobel M, Verhaeghe R. Antithrombotic therapy for peripheral artery occlusive disease. Chest. 2008;133:S815.
22 Hatfield J, Gulati S, Rahman A, et al. Nurse-led risk assessment/management clinics reduce predicted cardiac morbidity and mortality in claudicants. J Vasc Nurs. 2008;26:118.
23 Meru AV, Mittra S, Thyagarajan B, et al. Intermittent claudication: an overview. Atherosclerosis. 2006;187(2):221–237.
24 Nicolai S, Kruidenier L, et al. Ginkgo biloba for intermittent claudication. Cochrane Collaboration. 2010. CD006888.
25 Zhou Q, Yuan Z, Chen H, Wu T. Traditional Chinese herbal products for stable angina. Cochrane Collaboration. 2010. CD004468.
26 Amendt K. PGE1 and other prostaglandins in the treatment of intermittent claudication: a meta-analysis. Angiology. 2005;56(4):409–415.
27 Ronayne R. Lower extremity peripheral arterial disease. In: Lewis P, ed. Core curriculum for vascular nursing. Salem, Mass.: Society for Vascular Nursing, 2007.
28 Fahey VA, McCarthy W. Arterial reconstruction of the lower extremity. In Fahey VA, ed.: Vascular nursing, 4th edn., Philadelphia: WB Saunders, 2004.
29 Paraskevas KI, Liapis CD, Briana DD, et al. Thromboangiitis obliterans (Buerger’s disease): searching for a therapeutic strategy. Angiology. 2007;58:75.
30 Chen Z, Takahashi M, Naruse T, et al. Synergistic contribution of CD14 HLA loci in the susceptibility to Buerger’s disease. Hum Genet. 2007;122:367.
31 Suter LG, Murabito JM, Felson DT, et al. The incidence and natural history of Raynaud’s phenomenon in the community. Arthritis Rheum. 2005;52(4):1259–1263.
32 Bakst R, Merola JF, Franks AG, et al. Raynaud’s phenomenon: pathogenesis and management. J Am Acad Dermatol. 2008;59:633.
33 James AH. Venous thromboembolism in pregnancy. Arterioscler Thromb Vasc Biol. 2009;29:326.
34 Cesarone MR, Belcaro G, Agus G, et al. Management of superficial vein thrombosis and thrombophlebitis: status and expert opinion document. Angiology. 2007;58:S7.
35 Crowther M. Deep vein thrombosis: prevention. In: Ackley B, Ladwig G, Swan B, et al, eds. Evidence-based nursing care guidelines: medical-surgical interventions. St Louis: Mosby, 2007.
36 Trujillo-Santos J, Perea-Milla E, Jimenez-Puente J, et al. Bedrest or ambulation in the initial treatment of patients with acute deep vein thrombosis or pulmonary embolism: findings from the RIETE registry. Chest. 2005;127(5):1631–1636.
37 Kearon C, Kahn S, Agnelli G, et al. Antithrombotic therapy for venous thromboembolic disease. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2008;133:454S.
38 Nutescu EA, Shapiro NL, Chevalier A, et al. A pharmacologic overview of current and emerging anticoagulants. Cleve Clin J Med. 2005;72(suppl 1):S2–S6.
39 Vogelzang RL. Percutaneous vascular intervention and imaging techniques. In Fahey VA, ed.: Vascular nursing, 4th edn., Philadelphia: WB Saunders, 2004.
40 Colman R, Marder V, Clowes R, et al. Hemostasis and thrombosis. Philadelphia: Lippincott Williams & Wilkins, 2006.
41 Cesarone MR, Belcaro G, Agus G, et al. Management of superficial vein thrombosis and thrombophlebitis: status and expert opinion document. Angiology. 2007;58:S7.
42 Cosmetic Surgery Australia. Laser spider vein treatment. Available at www.cosmeticsurgeryaustralia.com.au/laserspiderveinremoval.htm. accessed 31 August 2010.
43 Gohel MS, Taylor M, Earnshaw JJ, et al. Risk factors for delayed healing and recurrence of chronic venous leg ulcers—an analysis of 1324 legs. Eur J Vasc Endovasc Surg. 2005;29(1):74–77.
44 Raju S, Neglen P. Chronic venous insufficiency and varicose veins. N Engl J Med. 2009;360:2319.
45 Koupidis SA, Paraskevas KI, Staphopoulos V, et al. The impact of lower extremity venous ulcers due to chronic venous insufficiency on quality of life. Open Cardiovasc Med J. 2008;2:105.
46 Heinen MM, van Achterberg T, op Reimer WS, et al. Venous leg ulcer patients: a review of the literature on lifestyle and pain-related interventions. J Clin Nurs. 2004;13(3):355–366.
47 Hoblyn JC, Brooks JO, 3rd. Herbal supplements in older adults. Consider interactions and adverse events that may result from supplement use. Geriatrics. 2005;60(2):18–23.
48 Kelechi TJ, Bonham PA. Lower extremity venous disorders: implications for nursing practice. J Cardiovasc Nurs. 2008;23:132.
49 Merli GJ. Pathophysiology of venous thrombosis and the diagnosis of deep vein thrombosis—pulmonary embolism in the elderly. Cardiol Clin. 2008;26:203.
50 Lindsay E, White R, eds. Leg ulcers and problems of the lower limb: a holistic approach. Swindon, UK: HealthComm, 2008.
Australian Institute of Health & Welfare. www.aihw.gov.au
Australian Vascular Biology Society. www.avbs.org
Diabetes Australia. www.diabetesaustralia.com.au
Diabetes New Zealand. www.diabetes.org.nz
Everybody.com—Raynaud’s syndrome fact sheet. www.everybody.co.nz/page-5aa1120f-9211-4a22-9b35-dc537159fddb.aspx
Juvenile Diabetes Research Foundation. www.jdrf.org.au
New Zealand Guidelines Group. www.nzgg.org.nz
Peripheral vascular disease fact sheet. www.diabetes.usyd.edu.au/foot/Pvdx1.html
Peripheral Vascular Disease New Zealand. www.vascular.co.nz