
Where Is Reproductive Medicine Heading?
WE’VE SPENT NEARLY an entire book discussing what’s happening today. But what can you expect in the future? Imagine the possibilities. Just about anything is conceivable. Are you ready for a world where women don’t need men (or their sperm) to have a baby? Or a world where two men can biologically father the same child minus a genetic mother? Or perhaps a world where you can carry and deliver a baby using the same uterus you were born from? Or a world where clones walk among us? Or what about a world where animals are part human (or humans are part animal)? Although most of these examples seem like science fiction, they’re all closer to reality than you think.

![]() The Importance of Asking, “What If?”
The Importance of Asking, “What If?”
Reproductive medicine needs free thinkers. If scientists didn’t ask “what if?” there would be no freezing of embryos for future use, no prebirth detection of chromosomal abnormalities, and probably no babies born through IVF.
There is no question that recent advances in genetics, experimental embryology, and reproductive medicine have surpassed even our wildest imaginations. But because these technological breakthroughs test ethical, moral, and spiritual concerns, we felt that the best way to present each is to categorize them according to how they might affect you: probable (in common use in the next decade), possible (in common use in the next few decades) and plausible (could become a mainstay in your grandchildren’s lifetime if public opinion shifts).
Please note that all techniques described in the following sections are currently experimental, and the researchers behind many of them have not yet proven them safe even for laboratory animals. We don’t recommend participating in any of these procedures until more research is available and the FDA has authorized controlled clinical trials to study their effectiveness.
Stem Cell Therapy
Have you ever watched a loved one die of cancer, seen a youngster cry at the sight of an insulin needle, heard an arthritic relative groan, or felt the agony of not being able to bear your own child? These are just some who could benefit from stem cell therapy. Never before has medicine had the potential to develop cures for such a wide range of illnesses as it has since scientists first isolated stem cells in the 1990s. Ever since, biomedical science has emerged as a hotbed of research potential and public controversy.
What Are Stem Cells?
They are cells from which all other cells develop.
Scientists have been growing stem cells in the lab for nearly 15 years now. In that time span they have learned to transform stem cells into just about anything they wanted to, such as heart cells, liver cells, and brain cells. The use of stem cells remains an important area of research. In fall of 2012 newspapers around the globe reported how Japanese researchers demonstrated in mice that eggs and sperm can be grown from stem cells and combined to produce healthy offspring. This is amazing news for the field of reproductive medicine, as it suggests infertile women can have children simply by growing eggs in laboratory dishes. It also suggests that many older women can turn back the clock and have children even after menopause. It’s worth noting we have yet to find a way to make old eggs young, but we have found a way to potentially replace old eggs with laboratory-grown eggs. This latest discovery will likely create new, innovative fertility treatments.
The aim of stem cell research is to determine how to create specific tissues like blood, cell, organ, skin, and so on. Researchers currently obtain stem cells from unused embryos or aborted fetuses but are looking for other ways to get them. If scientists can learn to control and direct stem cell development, then they could cure many common and debilitating diseases and conditions. Stem cells have the potential to repair damaged spinal cords, generate healthy insulin-producing cells in diabetics, and many other wonderful things.
Public fear and misunderstanding over stem cells has greatly slowed progress in this area. There is increasing government intervention to prevent stem cell research because aborted fetuses were the source of early stem cells. The fact is that surplus embryos created during fertility treatment could provide all the stem cells needed without requiring the government to give tacit endorsement of abortion. If the government allowed stem cell therapy to flourish under their regulation, medical practice would change. Instead of treating your symptoms, your doctor could focus on correcting the underlying causes of your disease. Medicine would become proactive instead of reactive.

![]() What Critics Say about Stem Cell Therapy
What Critics Say about Stem Cell Therapy
A number of people believe stem cell therapy is immoral, unethical, and an irreprehensible waste of human life. Even though the number of frozen embryos is reaching one million and most of those embryos will never produce a child, critics of stem cell therapy believe that each embryo is a human life and no one has the right to end it.
But take a minute to think about what this therapy means for people waiting for organ donations. Most can enjoy additional years and many may live long enough for doctors to cure what ails them (coincidentally, Jennifer’s brother-in-law, Bob, was denied a liver transplant as we completed the first edition of this book). Sadly, it seems that there are not enough organ donations to go around. Without deep pockets, most realize that their life will end before a donation becomes available.
Doctors worldwide use donated organs and tissues to replace diseased or destroyed tissue, but they haven’t been able to keep up with growing demand. Science has an opportunity (and supporters would say an obligation) to address that need and others by directing stem cells to grow into specific tissues and organs to treat diseases, including Parkinson’s, Alzheimer’s, paralysis due to spinal cord injury, stroke, heart disease, burns, diabetes, osteoporosis, osteoarthritis, and rheumatoid arthritis.
WHO MIGHT BENEFIT FROM STEM CELL RESEARCH?
• tens of thousands of infertile couples
• 30,000 with Lou Gehrig’s disease
• quarter of a million paralyzed with spinal cord injuries
• 1 million children with juvenile diabetes
• 2.4 million burn victims per year
• 4 million with Alzheimer’s disease
• 8.2 million people with cancer
• 10 million with osteoporosis
• 43 million with arthritis
• 58 million with heart disease
Stem cells are early cells that could each grow into a complete human being with diverse organs and functions. As embryonic cells begin to differentiate, specific functions and properties arise as various genes turn on or off. These stem cells are totipotent because they retain the ability to produce all cells in the body (total potential). In an early embryo, cells remain totipotent until they reach blastocyst stage (around the fifth day of development). Once a blastocyst, they begin to specialize into pluripotent cells. These are versatile cells that can create many tissues but are limited to a specific line of cells (e.g., internal organs; blood, muscle, and bones; skin and nerves). Pluripotent cells undergo further specialization before they turn into multipotent cells, which produce cells with a specific function. For instance, multipotent blood stem cells give rise to red cells, white cells, and platelets in your blood but cannot develop into bone cells.
After a number of cell divisions, multipotent cells transition into terminally differentiated cells. These make up the mainstays of your body like your nerves, heart, and blood.
Scientists can grow cultures of stem cells. Current research focuses on how stem cells “know” what to form. If scientists can control this factor, then they can create new organs for people, grow specialized cells (like eggs and sperm) and even help regrow tissues like the spinal cord.
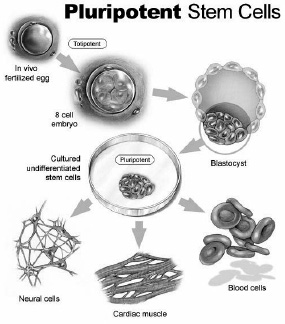
Figure 16.1. Pluripotent stem cells
Source: Stem Cell Research Foundation
Artist: Robert F. Morreale, CMI, Visual Explanations, LLC
The collision between science and ethics occurred when President George W. Bush announced that he would make federal money available only to approved stem cell lines. Bush restricted these lines to those in which scientists had destroyed original embryos prior to August 9, 2001, the announcement date. Each cell line had to have donor consent. Although some considered this move politically astute, critics in both camps claimed foul play.
Those against stem cell research leveled that the ruling allowed taking human lives for the sake of research and gave the private sector free rein to fund additional stem cell lines as they saw fit.
Supporters claimed that what was being called a human life was no more than a ball of cells or a dividing egg (embryologists assert that gene expression or unique traits of embryos do not occur until after day three, well past embryo stage) and that now millions of people afflicted with diseases ranging from heart disease to infertility will not benefit from probable cures.

![]() What Supporters Say about Stem Cell Therapy Research
What Supporters Say about Stem Cell Therapy Research
A majority of scientists believe stem cells hold promise for treating and curing an array of currently incurable diseases. In fact, most experts believe that stem cell research has the potential to help up to half of all Americans who suffer from an incurable disease, injury, or birth defect. Experts in reproductive medicine believe stem cells will give couples lacking sperm or eggs the opportunity to have a biological child.
Other countries are still grappling with how to define stem cell research and whether to allow it within their borders. Whereas the European Union voted to allow its countries to conduct human embryonic stem cell research, some countries forbid this practice. Others are unclear on whether they support it.
Research in the United States was at a standstill until voters passed a controversial bond in California in November 2004. Now $3 billion is available to fund human embryonic stem cell research experiments. This is the largest state-supported scientific research program and may very well open floodgates for other states to support similar research.
Many debilitating diseases garnered attention over the last few years when actor Michael J. Fox made Parkinson’s a household name and the late Christopher Reeve put a face on paralysis. Both spent years drumming up community support and urging government and the medical community to take action and find cures. Although both actors helped pass the bond issue in the Golden State, it’s unfortunate that Reeve did not survive to see the fruits of his labor.
Table 16.1. Difference between reproductive and therapeutic cloning
Somatic Cell Nuclear Transfer
You’ve probably heard of “therapeutic cloning,” but you may not know how this application differs from traditional cloning. Therapeutic cloning is the lay term for somatic cell nuclear transfer (SCNT). This technique can potentially improve and save lives not to mention create them for infertile couples who can’t have a child of their own.
SCNT is fundamentally different from reproductive cloning. In SCNT a scientist removes the nucleus from an emptied donor egg and replaces it with a nucleus from your somatic cell (body cell). The nucleus is the brain of your egg. It provides genetic information, and the emptied donor egg provides nutrients and other energy-producing materials to help an egg mature into an embryo. At this point the egg begins to divide until a blastocyst forms. If an RE implanted this blastocyst in you or a surrogate and it survived to full term, you would have a clone of yourself. But with SCNT, we never get that far. Scientists harvest stem cells from the blastocyst and can use them for any number of stem cell therapies. So unlike embryonic stem cell therapy, SCNT produces stem cells with your own DNA.
Patients’ immune systems sometimes reject donor organs, so scientists could get around this by growing organs through SCNT. Scientists do not use sperm in this procedure, so there is no chance of harming what some people consider human beings. Scientists never transplant these cells into a woman’s womb but instead place them in a Petri dish so that they’re available to treat life-threatening medical conditions.
Figure 16.2. Somatic cell nuclear transfer
Illustrator: Adam Hanin

![]() What Critics Say about SCNT
What Critics Say about SCNT
There are many psychological side effects associated with growing up with a diminished sense of individuality and autonomy. Human cloning could degrade the quality of parenting and family life. They believe that because scientists can replicate life so easily, human cloning could create the potential for people to view clones as objects instead of people.
Besides helping treat incurable diseases, SCNT is also useful to agricultural science because it can improve the quality of livestock and prevent the extinction of many endangered animals. Unfortunately for those who support SCNT, it’s legally banned by statute for reproductive purposes.

![]() What Supporters Say about SCNT
What Supporters Say about SCNT
Scientists and many others believe stem cells hold promise for treating an array of diseases, from diabetes to Parkinson’s. Most scientists in reproductive medicine believe that government should allow somatic cell nuclear transfer (SCNT) to continue under government regulation. They contend that the technology saves and improves lives and that SCNT produces stem cells, not babies.
Hearing you have cancer can seem like a death sentence. What follows may be even more of shock when you learn that the treatment that may save your life will leave you sterile. But now researchers have developed a way to not only preserve your fertility so you can get the lifesaving treatment you need but also restore it so you can have a baby.
In ovarian tissue transplant your doctor surgically extracts and freezes a portion of your ovarian tissue. When you’re healthy and ready to have a child, your doctor thaws and reimplants the frozen tissue. As long as the tissue transplant takes, the implanted tissue will begin producing eggs just as it did before removal. A donor can also provide tissue for transplant as long as there is a tissue match.

![]() What Critics Say about Ovarian Tissue Transplant
What Critics Say about Ovarian Tissue Transplant
Critics of ovarian tissue transplant are concerned that this technique has significant limitations. For example, if you’re a cancer patient, you’ll require up to three surgeries—two alone by your RE to remove and reinsert your ovarian tissue once your oncologist declares you cancer free. But between those two surgeries your oncologist may need to remove your cancer surgically, and the last thing you want after you’ve won a battle with cancer is to reseed your disease. The consequences of learning you have cancer a second time and possibly when you’re carrying a child would be catastrophic.

Table 16.2. Preserving your fertility If you have cancer, these steps will help preserve and restore your fertility
Oncologists today realize that young women facing cancer-induced infertility need options that allow them to have a biological child. Most oncologists would agree that ovarian tissue cryopreservation is one of many fertility options that should be recommended to patients who desire children. Fertility specialists are also working toward preserving patient’s fertility with innovative treatments. One such clinician’s research in fertility preservation has earned him an international reputation. Kutluk Oktay, MD, has spent more than the last decade developing techniques to intervene before a patient’s fertility is jeopardized.
Most fertility specialists would agree that a woman’s fertility is preserved prior to cancer treatment only by freezing her eggs, embryos, or ovarian tissues and then returning those to her body when chemotherapy and/or radiation are over and she decides to get pregnant. Candidates receiving fertility preservation are not only of childbearing age with cancer but also young girls with leukemia, lymphoma, or tumors whose parents want to safeguard their future ability to have children. Currently the best option for men or young boys is testicular tissue freezing.

![]() What Supporters Say about Ovarian Tissue Transfer
What Supporters Say about Ovarian Tissue Transfer
Many claim that this treatment was designed to help young female cancer patients become mothers later in life, but the technology could also be used for women past childbearing years who wish to have children by freezing their tissue when they’re young and transplanting it later. Most are thrilled that men and boys who develop cancer can benefit from this treatment too.
Another reason you may wish to consider ovarian tissue transplant is if you have unexplained infertility or if you experience early menopause. In either of these cases you’ll need a donor who is a tissue match with you before moving forward.
It’s worth mentioning that the first successful ovarian tissue transfer occurred in September 2004. Doctors were quick to praise a 32-year-old Belgium woman, Quarda Touirat, for delivering an eight-pound, three-ounce baby girl. Although doctors deemed the birth at the Universitaires Saint Luc hospital a breakthrough, experts say the science driving this procedure has a long way to go before it’s applicable to patients across the board.
Since then dozens of kids have been born using ovarian tissue transplants or ovary transplants. This procedure also has significant benefits for older women who are well beyond their childbearing years. Menopausal women will be able to turn back their biological clock and give birth either through an ovarian tissue transplant or an ovary transplant well after their 50s.
Like ovarian tissue transplants, ovarian tissue grafts restore ovarian function, but with a different process. If you have undergone chemotherapy or are menopausal and wish to reestablish ovarian function, your RE would remove sections of another woman’s ovaries and implant them in your forearm or abdomen. If the procedure worked, the grafted tissue would begin producing eggs, which your RE could then harvest for IVF.
WHO BENEFITS FROM TISSUE GRAFTS?
• women who want to resume their fertility after chemotherapy
• postmenopausal women wanting to reverse menopause
Embryo Cloning
Like identical twinning (essentially natural cloning), an embryologist takes an IVF embryo at the four-cell stage and separates the cells. Each of these cells is then allowed to grow into separate embryos that your RE can also split. It’s unlikely that you’ll find artificial twinning at a fertility center near you anytime soon because it’s so controversial, but technology exists to do this today. Researchers have successfully performed embryo cloning in many species, including primates. This technique could greatly reduce the expense of fertility treatment by allowing a couple to create as many embryos as needed to achieve pregnancy from a single stimulation. Additionally, PGD on one embryo would identify the genetic health of the others.
Manufactured Eggs
Just when you thought reproductive medicine couldn’t get more creative, someone proves you wrong. Scientists in the United States and Australia have developed a technique that offers hope to millions of infertile women who want to become mothers long after they can produce viable eggs. The technique is still in preliminary stages, but the goal is to give you a child who has genes from you and your partner rather than one whose genes come from your partner and a donor.
This technique borrows the nuclear transfer process from SCNT. Once an approved fertility treatment, an embryologist would select a healthy donor egg and suction out the nucleus. She would then carefully inject the nucleus from your healthy somatic cell into the emptied donor egg.
Figure 16.3. Manufacturing eggs
Illustrator: Adam Hanin
Every cell in your body except your egg (and, for men, sperm) has two copies of each chromosome that house your genes, one from your mom and one from your dad. Because a mature egg has one set of chromosomes and your somatic cell has two, she’ll have to remove one set of chromosomes from the new egg to make it viable for fertilization; without this step, you would have a clone of yourself. She would then apply an electrical shock to your egg just as she injects your partner’s sperm to force out the extra set of chromosomes.

![]() What Critics Say about Manufacturing Eggs
What Critics Say about Manufacturing Eggs
Some scientists disagree that manufactured eggs will give couples a healthy baby anytime soon. They assert that this technique is likely to produce babies with severe genetic abnormalities. They contend that age is a factor because older women face a higher probability of having children with major birth defects.
The main benefit to you is the ability to create an unlimited supply of eggs long after menopause. This is essential if you want children later in life or if you’ve experienced infertility due to cancer or other diseases. This technique offers hope if you don’t have any eggs or you need eggs because your egg quality is poor. It’s also ideal if your doctor removed your ovaries due to disease or if you were born without ovaries or a functional uterus.

![]() What Supporters Say about Manufacturing Eggs
What Supporters Say about Manufacturing Eggs
Some scientists say this is one of the biggest accomplishments in reproductive medicine since the development of ICSI. This technique addresses the biggest concern scientists have dealt with in recent years: reversing a woman’s declining egg supply.
Like cytoplasmic transfer, the end result is an embryo with DNA from three people—you, your partner, and the donor. Keep in mind that mitochondrial DNA from the donor is miniscule but does show up, and some government officials equate DNA from a third party with cloning. So the jury is still out on when a technique like this will be available for popular use or whether the government will ban it like cytoplasmic transfer.
Eggs from Stem Cells
For years scientists thought the egg cell was a cell that they could never produce. Previous attempts failed miserably. But all that changed in 2003 when they created a mammalian egg outside the body for the first time. Researchers created the egg using cells taken from mouse embryos. What does a faux mouse egg have to do with human reproduction? Just about everything. Scientists predict that if they can create synthetic eggs using embryonic stem cells from mice, they can do the same thing with human embryonic stem cells.

![]() What Critics Say about Eggs from Stem Cells
What Critics Say about Eggs from Stem Cells
The controversy over using embryonic stem cells to grow eggs is the same as using embryonic stem cells to grow anything—an embryo dies in the process, making it, for some, an act of murder.
This technology could have a huge impact on infertile couples. Researchers used a method that allows stem cells to grow at a high speed. They coax stem cells to create ovarian tissue. Once the cell mass begins to divide, smaller cells nurture a larger cell that grows into an egg. Scientists then add a gonadotropin to the mass, which simulates “ovulation” and forces the egg cell into the dish.
This discovery led scientists to uncover some amazing facts about creating eggs. They found that eggs can form from either male or female embryonic stem cells. This occurs because mammalian germ cells grow into eggs unless they receive a signal from the testes instructing them to turn into sperm. So it’s conceivable that in the future two men could biologically father a child together without a genetic mother; it could also work for two women. Scientists in Tokyo have already created an embryo by using two eggs and tricking genes in one egg to act as though they had come from sperm (see page 281).

![]() What Supporters Say about Eggs from Stem Cells
What Supporters Say about Eggs from Stem Cells
Researchers believe this technique will allow you to have babies long after you stop producing viable eggs. No longer would you equate menopause with the inability to bear your own children. Once perfected, this technique would make donor eggs a thing of the past.
What does all this mean for you? The most obvious benefit of growing stem cells into synthetic eggs is to give you an alternative way to produce healthy eggs. If you have poor egg quality and repeated attempts at traditional IVF fail, this technology could be your best bet. The second benefit is that creating synthetic eggs could have profound implications for understanding and treating disease. Stem cells can grow into any type of tissue, and scientists plan to direct blank cells into specific cell types needed for transplants.
Transplanted or Artificial Wombs
Women who are born without a uterus or had it removed because of disease often find themselves at a loss when they want to have a baby. Until recently the only options women in this situation had were adoption or surrogacy. But some researchers believe they have an answer: uterine transplants.

![]() What Critics Say about Transplanted or Artificial Wombs
What Critics Say about Transplanted or Artificial Wombs
Many experts say they just don’t see uterine transplants becoming a common practice anytime soon. So far there’s no proof (besides animal studies) that this technique actually works. The operation is risky because the uterus is a dynamic and complex organ filled with many blood vessels.
As far as researchers can determine, uterine age has little bearing on a woman’s ability to get pregnant. As we discussed in chapter 9, using a healthy donor egg makes the age of the intended mother irrelevant. This means that you could theoretically receive your mother’s uterus in a transplant and carry and deliver a child from the same uterus you spent your first nine months in. Quite a thought!
But as with any other type of organ transplant, women must take antirejection drugs to prevent their immune system from attacking the new organ. The bottom line is that presently the risks outweigh benefits for this surgery, and it seems unlikely that it will ever be a safe, acceptable fertility treatment. The few documented attempts in humans have failed miserably. If a transplant saved a life, then it could be an acceptable risk. But having a uterine transplant is not life saving. Uterine transplant is plausible but very unlikely to ever become available.

![]() What Supporters Say about Transplanted or Artificial Wombs
What Supporters Say about Transplanted or Artificial Wombs
Some doctors are optimistic that uterine transplants could help women who have had a hysterectomy or who were born either without a uterus or with a malformed one with normally functioning ovaries. They believe further clinical experience and advancements in surgical techniques could make uterine transplantation useful in treatment of infertility, especially in communities that forbid surrogates due to a religious or ethical standpoint.
This has many wondering whether science will ever get to a point at which a woman could have an artificial womb. Artificial wombs are not likely anytime soon, but we have slowly closed the gap during which growth inside the mother is necessary. The length of time that embryos are able to grow and remain viable in the laboratory is slowly increasing as our knowledge of embryo physiology improves. On the other end, women have delivered babies as early as 22 weeks who have survived. At this point there is no technology available to bridge this 20-week gap, but, knowing science, this gap will continue to narrow as our knowledge develops. The artificial womb is certainly a possibility in the next 100 years or so, but it’s by no means inevitable.
Sperm from Stem Cells
Researchers working with cells from mice have succeeded in overcoming yet another fertility obstacle by creating sperm cells outside the body. They transplanted stem cells from a donor mouse into infertile mice that were then able to produce sperm and mate, resulting in mice genetically related to the donor. What this means is that researchers could extend the reproductive life of animals indefinitely and that doctors could eventually use this technology to treat infertile men.

![]() What Critics Say about Sperm from Stem Cells
What Critics Say about Sperm from Stem Cells
Critics say success rates are problematic. Only one in five embryos develops properly. They cite vanishing success rates as impractical, unsafe, and poor science. They don’t believe anyone should spend research dollars on dismal results. But their biggest concern rests on killing embryos.
Besides finding new ways to treat male infertility, researchers are confident this technology would allow them to introduce new genetic traits. For instance, researchers could implant a new gene into a sperm cell, reproduce large numbers of sperm cells in the lab, and then implant them into specific animals. These animals would then pass the new trait on to their offspring. Doing this would greatly assist breeders improve the quality of livestock and laboratory animals.

![]() What Supporters Say about Sperm from Stem Cells
What Supporters Say about Sperm from Stem Cells
Supporters feel that this technology has a range of possibilities, from treating male infertility to enhancing the survival of endangered species.
Making Babies without Sperm
Dads were once chastised for not helping with dirty diapers, warming bottles, or manning 3 a.m. feedings, but moms have always counted on their genetic contribution to make a baby. Now a new fertility technique could make dad’s genetic contribution a thing of the past.
Scientists in Japan created the first fatherless mice, including a female mouse that reached adulthood and reproduced. They accomplished this by combining genetic contents of two eggs after altering the genetic contents of one.

![]() What Critics Say about Making Babies without Sperm
What Critics Say about Making Babies without Sperm
Many religious groups claim this technology mirrors cloning. The Apologetics, a Christian organization, view this technology the same way they view cloning and cytoplasmic transfer. They contend that the effect of receiving DNA from two mothers is unknown. They have also suggested that any form of human cloning is morally and ethically reprehensible.
A number of amphibians, fish, insects, and reptiles are able to reproduce asexually from eggs alone using a process called parthenogenesis. This process does not occur in mammals like humans and mice, and scientists had little luck in recreating it in the lab. The researchers’ goal from the start was to uncover why sperm and eggs were required for reproduction in mammals. They believed they could use two eggs to produce a viable mouse embryo. Somehow, though, they had to fool imprinted genes in one of the eggs to act like they had come from sperm.

![]() What Supporters Say about Sperm from Stem Cells
What Supporters Say about Sperm from Stem Cells
Supporters (mainly women and many in the field of reproductive medicine) like the idea of women having babies without sperm, as this means that women without partners don’t have to worry about finding one or locating a sperm donor.
Generally mammals can’t produce offspring without a genetic contribution from their biological parents. For years scientists believed that we couldn’t reproduce unless we had two copies of each gene, one set from mom and one from dad. But in the subset of genes that regulate development, only one copy is activated. This is genomic imprinting, and it guarantees genetic input from both parents.
The team came close to success in an earlier experiment when they combined a mature egg from an adult with an immature egg from a newborn. The embryo survived longer than any former mouse embryo without sperm, more than midway through gestation. What caused this embryo to die early? Scientists concluded that imprinted genes killed the mouse embryos. The team had to determine how to make genes in the egg behave as though they were genes from sperm.
They turned their attention to two genes: Igf2, which controls growth and fetal development and is only found in sperm, and H19, which controls Igf2 in eggs. The team knew that if they modified H19, they could turn on Igf2 in the immature egg. To do this they genetically altered an immature egg. They had to forgo using eggs from adult mice, as these eggs have already been imprinted.

![]() What Critics Say about Genomic Imprinting
What Critics Say about Genomic Imprinting
Critics of this technology see no difference between genetic imprinting and cloning. They make many religious and moral cases against it and do not want it to ever come to fruition. They chalk it up to “playing God.”

![]() What Supporters Say about Genomic Imprinting
What Supporters Say about Genomic Imprinting
Many claim this technology could help infertile women have babies. Most researchers are confident that one day even sterile women and men will be able to have biological children. This technology also suggests that someday two women might be able to produce a biological child together.
Although the results are promising, we won’t see human babies born from two eggs for many years. The team started with 457 manufactured fertilized eggs. These grew into blastocysts, of which only 357 survived. After transfer into female mice, ten embryos were born, but only two survived after birth—leaving a success rate of less than 1 percent.
With such a poor success rate, scientists say it would be irresponsible to offer it to infertile couples anytime soon. Nonetheless, until scientists know more about imprinted genes, many of which cause diseases, chances are that Dad’s genetic contribution is not going away anytime soon.
Cloning
Scientists use the term cloning to describe different processes that involve making replicas of animal, plant, or human genetic material. Researchers have been cloning mice in experiments since the late 1970s and animal breeding since the late 1980s. Most debate over cloning has occurred since the 1996 birth of the first cloned sheep, Dolly.

![]() What Happened to Dolly?
What Happened to Dolly?
Researchers euthanized Dolly in February 2003 after she developed a serious lung infection. She was only 6 and half years old. Dolly suffered from arthritis and seemed to have aged prematurely. Most sheep live to 11 or 12. Some experts believe she was actually 12 at the time of her death after adding her age to the age of the 6-year-old ewe that her DNA came from.
Scientists used SCNT to create Dolly. Most debate over cloning centers on whether our government should ban the practice altogether or allow it only for therapeutic purposes (making reproductive cloning illegal).

![]() What Critics Say about Cloning
What Critics Say about Cloning
Critics of cloning cite ethical, moral, and safety concerns as reasons to ban it from public use. Many religious groups believe any type of cloning is wrong, especially cloning used to kill innocent lives for “spare parts.” They cite that scientists who do this are “playing God.” Most critics believe cloning is morally irresponsible and that the government should ban both reproductive and therapeutic uses, especially uses involving embryonic stem cells for research.
To date, fifteen states have passed laws regarding human cloning. California was first, when it banned reproductive cloning in 1997 but allowed cloning research. Since then Arkansas, Connecticut, Indiana, Iowa, Maryland, Massachusetts, Michigan, Rhode Island, New Jersey, North Dakota, South Dakota, and Virginia have passed measures to prohibit reproductive cloning. Arizona and Missouri passed measures to ban the use of public funds for cloning, and Maryland prohibits the use of state stem cell research funds for reproductive cloning and potentially therapeutic cloning (depending on how the definition of human cloning is interpreted). Louisiana also enacted legislation that prohibits reproductive cloning.
States Allowing Therapeutic Cloning
Arkansas, Indiana, Iowa, Michigan, North Dakota and South Dakota have laws that prohibit therapeutic cloning for research purposes. Virginia’s law likely bans human cloning for any purpose, but it remains unclear in its current definition. Rhode Island does not prohibit cloning for research purposes. Of these 15 states, only California and New Jersey specifically allow cloning for research purposes. (For each state’s human cloning laws visit www.ncsl.org/programs/health/genetics/rt-shcl.htm.)
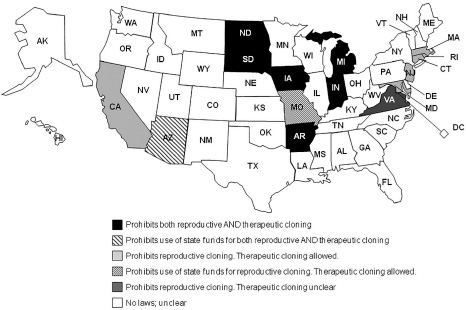
Figure 16.4. Cloning map
It may come as a surprise to learn that clones have walked the Earth for centuries. Most of them live, work, love, and play just like the authors or readers of this book. Society accepts them because their parents created them naturally, not in a lab. These clones are none other than identical twins.

![]() Cloning Around the World
Cloning Around the World
European nations enacted similar laws as the United States after signing a ban on human cloning on January 12, 1998. These countries include Denmark, Estonia, Finland, France, Greece, Iceland, Italy, Latvia, Luxembourg, Moldova, Norway, Portugal, Romania, San Marino, Slovenia, Spain, Sweden, Macedonia, and Turkey. Germany did not because their officials believed their laws following Nazi genetic engineering experiments are stricter than the ban. Britain and Belgium allow therapeutic cloning for research, like the United States. Since then the following countries have allowed therapeutic cloning: Canada, Mexico, Colombia, Finland, Hungary, Poland, Spain, United Kingdom, China, Japan, Singapore, South Korea, Sweden, Thailand, New Zealand, Israel, and Turkey.
With the swarm of ethical, moral, and safety issues surrounding cloning, it’s unlikely that the United States will reverse laws on reproductive cloning in the near future. So far success rates for cloning mammals have been anything but positive. Nearly 90 percent of clones fail to develop into live animals. A large percentage of those animals die at birth, and those that do survive have age-related genetic defects and develop other fatal diseases. Besides that, many people believe that cloning could cause a variety of problems, including psychological damage springing from a diminished sense of individuality and personal autonomy. They believe it could severely interrupt and degrade family life. Others are concerned with abuse because people may treat cloned individuals as objects.
Embryo cloning (see page 276) is a more probable occurrence. It does not involve replication of an adult animal; instead, it involves making copies of newly created embryos. This technique would help decrease the cost of IVF by creating an ongoing source of embryos for couples undergoing IVF after a single stimulated cycle. Accelerated aging would be unlikely because the cells are embryonic and no older than other cells of embryos at the same stage.

![]() What Supporters Say about Cloning
What Supporters Say about Cloning
A majority of supporters believe there should be limits to cloning. They cite that the government has an obligation to ban reproductive cloning due to obvious genetic defects and psychological repercussions but that therapeutic cloning should be legal. A smaller majority of supporters believe that government regulation of any kind limits personal choice, freedom of scientific investigation, and the potential for new biomedical breakthroughs. Scientists believe stem cells hold promise for treating an array of diseases, including cancer, diabetes, ALS, Parkinson’s, and paralysis due to spinal cord injuries. Most reproductive medicine researchers believe that SCNT should continue under government regulation.
Therapeutic cloning is a completely different story and will likely become a common practice over the next decade, especially if California succeeds in swaying public opinion as it has with everything from computer chips to sushi. This means medicine would get an overdue facelift: cells would have the potential to cure any illness, ranging from aging to paralysis. In essence, stem cell research not only could mean the end of disease but could also one day become the modern-day fountain of youth.
Insurance companies surprise couples each year by agreeing to pay for all or part of their office visits, ultrasounds, fertility medication, and treatments. But in the United States how much or how little your insurance company pays largely falls on your employer. Some employers exclude part or all infertility diagnosis from their benefits package. Infertility medications will be covered only if your employer purchased a drug rider that includes these costly medications.
So what can we do to change how insurers and employers view fertility coverage? First, we need to understand why they’re reluctant to cover infertility. Employers want to keep cost down, so they’ll usually opt for less comprehensive coverage whenever they can. Some small to midsize employers would go bankrupt if they offered comprehensive health packages that include infertility coverage. Keep in mind that infertility is only one of the diseases medical plans cover and most people don’t see it as a disease in the same sense as cancer or heart disease. Most employers, insurers, and pretty much the rest of the world look at infertility treatment as voluntary. They understand that for cancer or heart disease, you have to get treatment or you’ll die. But they don’t consider a broken heart from being childless a fatal condition. Sadly, most insurers and employers treat it with the same irreverence as a psychiatric condition or cosmetic surgery.
Insurance companies fear that if they offer widespread coverage, couples will pour into fertility centers and bankrupt them. They know treatment can become quite costly, and they have no way of knowing how effective treatment really is. This is because while the FDA has set guidelines for US fertility centers, not all fertility centers report their yearly success rates (birth rates per treatment cycle) to the Society for Reproductive Medicine (SART) or to the Centers for Disease Control (CDC). Without knowing how much couples are paying out of pocket for treatment, there is no way insurance companies can estimate what the cost will be if they pick up the tab and what it would be if more couples had access to coverage. If this wasn’t enough to scare them off, the thought of paying for more multiple births might. Insurers know that neonatal care for even one premature baby can be quite costly; imagine paying for three or more per couple? It’s no wonder that insurers contemplating fertility coverage would be a little gunshy.
Employers are gunshy for similar reasons. They’re watching costs and concerned about productivity. If you work for a company and you become pregnant with twins, you might be on bed rest at 29 weeks, and if you’re pregnant with triplets, it might be at 16 weeks. This means you’ll be out of the office a lot longer than any of your coworkers taking maternity leave at 39 or 40 weeks. And your situation affects your partner’s job too, because who else is going to take time off to take care of you? Either way, employers lose all the way around.
So how can we persuade more employers and insurance companies to cover fertility treatments? Contacting your state legislator is a good place to start. Without national health insurance, what insurance covers and what it doesn’t is up to individual state legislators. Unfortunately, there is no quick fix to this problem, but laws and policies can continue to become more fertility-friendly with your help. Some progressive companies are including insurance coverage for fertility treatments, but it’s still not the norm. If you want infertility to have the same coverage as other diseases, this is the time to take action. We’ve seen recent changes in mental health coverage for similar reasons.
CAN WE POLICE A FUTURE SOCIETY?
Until recently your local cosmetologist had more licensing than your fertility specialist. This is not to say that your specialist doesn’t have all the degrees, accreditations, and training he needs to help you have a baby; what it means is the government doesn’t regulate fertility centers in the United States. It set forth guidelines for fertility centers to follow but did not impose any recourse if centers failed to meet them. But the FDA began pulling in the reins in 2005. It started imposing rules and regulations on fertility centers nationwide that allow the government to shut down practices that don’t meet its criteria.
There is little doubt that researchers will refine techniques for IVF, IVM, and cryopreservation. Scientists and REs backed by large pharmaceutical companies conduct ongoing research to test the limits of how drugs and therapies can improve birth rates.

![]() Understanding the Goal of Science
Understanding the Goal of Science
Scientists must think the unthinkable. Otherwise we would never have IVF, ICSI, IVM, or any techniques that help infertile couples have children.
Although reproductive scientists strive to help couples have their own genetic children, they know that there is no technology available to replace natural procreation. Instead, their goal has been steadfast for over a quarter of century: to make reproduction more efficient. This means it will get not only more accurate and reliable but also safer and more accessible for couples who need it but can’t afford it. Techniques like IVM are already making this a possibility. But regardless of pro-life and religious groups who want to stop scientists in their tracks, research must continue under the watchful eye of the government; anything less would create an unsafe underground movement aimed at helping couples conceive no matter what the risks are, much like the days of back-alley abortions.
But what about routine tests like the sperm penetration assay (see page 50)? We’ve known for years that human sperm can fertilize hamster eggs. Crossing humans with animals is a notion researchers at biotech companies have tinkered with for years. Scientists at Advanced Cell Technology took cells from a fellow scientist in 1998 and combined it with cells from a cow. They allowed the embryo to divide up to 32 cells before destroying it. If this cloned human had lived, its genes would have been 1 percent cow. This brings up the question: what’s next on the experimental embryology horizon? Are we ready for a new race of folks whom we will have no understanding for?
WHAT QUESTIONS SHOULD YOU ASK?
Reproductive medicine radically changed the way many of us make babies. What was once a private affair reserved for the bedroom now takes place in a sterile lab. But one thing remains the same: anyone you ask has an opinion on what reproductive technologies our government should allow and which ones they should ban.
Keep in mind that most people lobbying for or against these technologies will never personally use them. But as concerned citizens, legislatures, and religious leaders, we take up causes even when the end result never personally touches us.
But suppose the government would have listened to IVF critics 27 years ago? There were plenty of skeptics at that time who thought creating a human life in a Petri dish (test tubes were never used) was unconscionable. If this occurred, millions of couples would still be childless and the authors of this book would not delight in the fact that every day they share “firsts” with their children.
If you answer yes to any of the following questions, consider contacting your state representative to see how you can help.
• Do you think consumers need universal health coverage that includes fertility treatments?
• Do you think insurance companies should cover most or all fertility treatments?
• Do you believe more states should have insurance mandates regarding infertility?
• Do you think government should oversee regulation of US fertility clinics?
• Reproductive medicine needs freethinkers. If scientists didn’t ask “what if?” there would be no frozen eggs for future use, no prebirth detection of chromosomal abnormalities, and probably no babies born through IVF.
• There is no doubt technological breakthroughs in reproductive medicine have put ethical, moral, and spiritual concerns to the test.
• Stem cell therapy, somatic cell nuclear transfer, and ovarian and testicular tissue transplants are technologies that you’ll see more of in the next decade.
• Ovarian tissue grafts, embryo cloning, manufactured eggs, and eggs from stem cells are technologies that you may see in use in the next few decades.
• Transplanted or artificial wombs, sperm from stem cells, genetic engineering, and cloning could become a mainstay in your grandchildren’s lifetime if public opinion shifts in that direction.
• Insurance companies fear that widespread infertility coverage will bankrupt them.
• Employers are reluctant to offer insurance packages that cover fertility treatments. They’re watching costs and concerned about productivity. Yet more are offering coverage.
• Until recently your local cosmetologist had more licensing than did your fertility specialist. But the FDA pulled in the reins in 2005 by imposing rules and regulations on fertility centers.
• Reproductive scientists know that there is no technology available to replace natural procreation; instead, their goal has been to make reproduction more efficient.