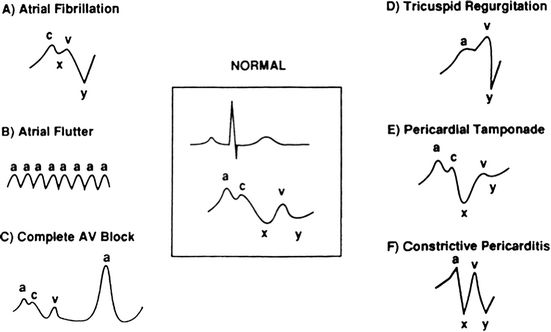
FIGURE 5.1 Center: Normal EKG to CVP waveform relationship. A–F: CVP waveform changes seen with specified pathology.
BACKGROUND
Since their inception, both central venous and pulmonary artery catheters (PACs) have been influential in the management of critically ill patients. Bedside use of a central venous catheter (CVC) was first clearly described by Wilson1 in 1962, who noted the clinical importance of bedside volume assessment and described CVC indications and techniques. Wilson explained the association between extremes of central venous pressures (CVPs) and volume status in the settings of normal or inadequate circulation. He further noted that, although the CVP indicated the circulating blood volume in relation to the pumping capacity of the heart at a given point in time, to maintain a CVP at a “predetermined level” would be clinically misguided.
Bedside use of a flow-directed, balloon-tipped catheter to measure right heart pressures was described in 1970 by Swan and Ganz.2 Early studies in critically ill patients showed improved survival with PAC use in targeting supranormal cardiac output (CO) and oxygen delivery indices; later, more carefully designed studies demonstrated no benefit with this strategy.3–5 Recent studies in geriatric, high-risk surgery, and lung injury patients have also shown no benefit of fluid management based on PAC measurements.6,7
The lack of survival benefit with PAC use may be partially attributable to misinterpretation of waveforms, misguided correlations with preload, and resuscitation to predetermined numeric end points rather than measures of adequate circulation.8–10 The declining use of these invasive monitors has led to decreased familiarity with waveform analysis and a lack of appreciation of the full set of information that can be obtained from these devices. This chapter aims to provide a basic understanding of the information that can be obtained through accurate interpretation of the waveforms and numerical data generated by central venous and PACs and to identify each catheter's appropriate clinical application.
CENTRAL VENOUS CATHETERS
CVCs have traditionally been inserted to assess circulatory volume, to provide intravenous access for vasopressors, and to facilitate simultaneous delivery of multiple medications. CVCs also allow assessment of central venous oxyhemoglobin saturation (ScvO2), a value used to assess the adequacy of oxygen delivery relative to consumption (DO2/VO2). When accurately interpreted, CVP waveforms yield a wealth of hemodynamic data, as well as information about a patient's cardiac health, and can help identify a number of disease states commonly seen in the critically ill. Patient benefits from CVCs are likely maximized in scenarios in which clinicians are versed in the full range of quantitative and qualitative information available from the catheter.
Qualitative analysis of CVP waveforms yields information analogous to the examination of jugular venous pulsation (JVP), but with greater ease and visibility. Insight into both structural and electrical functions of the heart is obtained by analysis of characteristic waveforms present during each cardiac cycle.
Each CVP tracing contains a, c, and v waves and x and y descents. These waves and descents represent the spikes and troughs in pressure that occur in the right atria during each cardiac cycle. The a wave reflects atrial contraction and occurs after the ECG P wave at end diastole. Following atrial contraction, atrial pressures begin to fall due to atrial relaxation and downward right ventricular movement during systole. This fall in pressure—represented by the x descent—is briefly interrupted in early systole by isovolumetric contraction of the right ventricle (RV) against a closed tricuspid valve—represented by the c wave. The time between a and c waves peaks is identical to the PR interval, albeit 80 to 100 ms later than the corresponding ECG.
The v wave follows the x descent and reflects passive atrial filling, which begins at the end of systole and peaks in early diastole. The y descent indicates atrial diastolic emptying and passive ventricular filling.12 While this discussion centers on the CVP, the PAC in the “wedged” position produces identical-appearing waves, but reflects left atrial activity (discussed below). Some examples of the variability of CVP waveforms and their ability to indicate pathology are highlighted below (Fig. 5.1).

FIGURE 5.1 Center: Normal EKG to CVP waveform relationship. A–F: CVP waveform changes seen with specified pathology.
Pathologies that can be detected by analysis of CVP waveforms include the following:
A.Atrial fibrillation: lack of coordinated atrial activity leads to absence of a waves.
B.Atrial flutter: atrial activity leads to high-frequency a waves.
C.AV dissociation: intermittent atrial contraction against a closed tricuspid valve leads to large “cannon” a waves.
D.Tricuspid valve dysfunction: tricuspid valve regurgitation leads to prominent v waves or fusion of the c and v waves without an x descent. In tricuspid stenosis, the mean CVP will be high with large a waves and small slurred y descents due to a continuously elevated atrial diastolic pressure.
E.Pericardial tamponade: CVP values reflect pericardial pressures and change as the size of the heart changes during the cardiac cycle. During diastole, as the heart enlarges, pericardial pressures increase and limit passive ventricular filling; thus, the y descent is impaired. As blood is ejected during systole, the heart becomes smaller, pericardial pressures exert less influence, and the CVP falls, resulting in an isolated x descent.
F.Restrictive and constrictive diseases: pathologies, including constrictive pericarditis, myocarditis, infiltrative diseases, hypertrophy, and ischemia, are typified by high mean filling pressures (tall a and v waves) and short, steep x and y descents, which create an “M” or “W” pattern. The “square root sign” describes an abrupt y descent followed by a plateau when the diastolic pressure limit is met.
Since pressure-based estimates of preload attempt to estimate ventricular volume, CVP is measured when there is a continuous column of fluid between the catheter tip and the ventricle. During the cardiac cycle, this continuity exists when the atrioventricular valves are open and corresponds to the pressure at the end of the a wave just prior to the c wave. If the c wave is not seen, CVP can be inferred from the mean of the a wave's highest and lowest points at end-expiration. If neither the a wave nor the c wave is present, the z point may be used. The z point is a line dropped perpendicularly from the end of the QRS to intersect a simultaneously co-recorded CVP tracing. Respiration will alter CVP measurements, so all quantitative analyses of CVP waveforms and pressures are measured at end-expiration—a time when no net forces are exerted upon the central circulation by the chest wall or lung parenchyma.
CVP is displayed in mm Hg, as opposed to cm H2O, which is generally used in JVP estimation. Using the proper conversion, a JVP of 13.6 cm H2O corresponds to a CVP of 10 mm Hg. A normal CVP is 0 to 4 mm Hg. An elevated CVP results from anything that increases the pressure surrounding the catheter tip, including decreased cardiac function, elevated pericardial pressure, elevated intrathoracic pressures (including elevated extrinsic or intrinsic positive end-expiratory pressure [PEEP]), active exhalation or increased intra-abdominal pressure, vasoconstriction, increased pulmonary artery pressures, and increased venous return (hypervolemia). Decreased CVP results from hypovolemia, vasodilation, or decreased thoracic pressure due to active inspiration (which can generate a CVP of less than 0 mm Hg). Given its predisposition to a number of artifacts, CVP waveforms should be analyzed over several respiratory cycles—ideally using a printout from the monitor. All principles discussed here for CVP, as well as Figure 5.1, are equally applicable to measurements of ‘wedge’ or pulmonary artery occlusion pressure (PAOP) using a PAC.
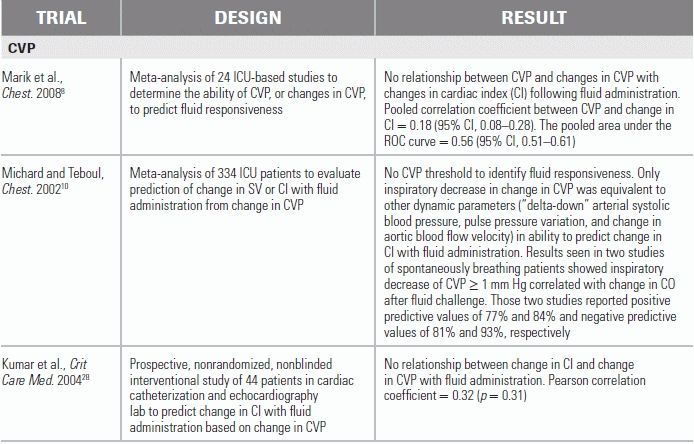
Many intensivists have used CVP to guide fluid therapy in septic patients.11,13,14 Traditionally, the CVP was thought to be a reliable indicator of fluid responsiveness of the left ventricle.1,15 The belief was that if CVP reflected right ventricular preload and therefore stroke volume, CVP should ultimately predict left ventricular preload and stroke volume. The fact that CVP does not accurately reflect the Starling curve of the left ventricle is due to the complex nature of venous return as it relates to CO. Venous return to the right heart is determined by the difference between mean circulatory filling pressure and right atrial pressure; the pressure gradient for left heart filling is affected by transpulmonary pressure, pulmonary venous pressure, and interventricular septal function. Since addition of fluid sufficient to raise CVP does not always augment CO, its role as the measure of preload has been questioned. CVP changes have not been shown to correlate reliably with corresponding changes in blood volume, left heart preload, or fluid responsiveness.8,10,16 In a meta-analysis of 24 ICU-based studies, a poor relationship between CVP (or changes in CVP) and changes in cardiac index following fluid administration were observed (pooled correlation coefficient between CVP and change in cardiac index, 0.18).8 Furthermore, a “normal” CVP does not necessarily reflect euvolemia, as splanchnic circulation allows the venous system to accommodate approximately a 10% intravascular volume gain or loss without a change in CVP. Research has also shown that in critically ill patients, a CVP > 12 mm Hg does not substantially increase CO, suggesting that this value corresponds to the upper, non–fluid-responsive portion of the Starling curve.17,18 Despite good rationale to abandon CVP as a marker of fluid responsiveness, CVP-guided resuscitation persists. The 2012 Surviving Sepsis Campaign (SSC) guidelines continue to endorse CVP as a tool to assure adequate intravascular volume during fluid administration. While acknowledging that “there are limitations to CVP as a marker of intravascular volume status and response to fluid,” the guidelines conclude, “a low CVP generally can be relied upon as supporting a positive response to fluid loading.”14
In managing hemodynamically unstable patients, current clinical evidence calls for avoiding static measures of intravascular pressure such as CVP and PAOP in favor of more accurate indicators of volume responsiveness (see Chapter 3). Monitoring techniques based on dynamic cardiopulmonary interactions—such as pulse pressure, stroke volume variation, and indices derived from Doppler measurements—are better predictors of volume responsiveness and are used increasingly in critical care.10
CVCs allow measurement of the oxyhemoglobin saturation of superior vena caval blood (ScvO2), thus providing an ability to assess the relationship between VO2 and DO2. While the gold standard for this VO2/DO2 assessment is mixed venous oxygen saturation (SvO2) measured in the pulmonary artery, ScvO2 has been demonstrated to provide a reliable surrogate in septic patients.18 Changes in ScvO2 can be used to estimate adequacy of CO and thus to gauge efforts to reverse deficits in tissue perfusion. A decrease in ScvO2 should prompt examination of the components of oxygen delivery (see Chapter 2); if fluid status and hematocrit are determined to be adequate, cardiac contractility should be evaluated and inotropic support initiated when indicated to help normalize DO2/VO2.14 ScvO2 may also be a marker for cardiopulmonary reserve. For example, a decrease in ScvO2 of >4.5% during a spontaneous breathing trial was reported as a sensitive and specific predictor of reintubation in difficult-to-wean patients.19
Significant complications attributable to CVC insertion include infection; arterial or venous injury or fistula; venous thrombosis; DVT/pulmonary embolism; hematoma; hemothorax; pneumothorax; chylothorax; nerve injury; knotting or dislocation of other implanted catheters or equipment; air embolus; and dysrhythmias.20,21 Catheter placement should be justified by a sound physiologic rationale for use, and removal should occur as soon as these indications cease to exist.22
Relative contraindications to CVC placement include coagulopathy, infection at the insertion site, right heart ventricular assist devices, and recent pacemaker placement. Some of these obstacles can be managed by placement of the CVC at an alternative anatomic site (e.g., internal jugular vs. subclavian in the case of coagulopathy). Absolute contraindications are vascular occlusion and patient refusal.
Ultrasound guidance should be used wherever available when placing a CVC. Use of continuous ultrasound guidance for insertion of CVC improves first-pass success, decreases procedure duration, and minimizes number of needle passes.20,21 Placement of a CVC should occur concurrently with therapeutic maneuvers and not delay empiric treatment of extremes in intravascular volume or blood pressure.
PULMONARY ARTERY CATHETERS
PACs are not typically employed as resuscitative or analytic tools in the ED; their use is generally seen in the ICU, operating room, and cardiac catheterization lab. The PAC is capable of providing simultaneous assessment of CO, SvO2, left-sided filling pressures, and continuous right-sided pressures. The PAC is available in thermodilution, pacing, or continuous CO/SvO2 models. The ability to measure SvO2 allows evaluation of adequacy of DO2 in the context of CO measurements. PAC insertion should not delay either resuscitation or ICU admission.
As with CVP, no absolute PAOP has been shown to predict fluid responsiveness, as euvolemic pressures are dependent upon each individual's left ventricular function and compliance.10,23 Thus, the usefulness of the PAC may be limited to specific situations not fully addressed in randomized trials where PAC use failed to show any benefit. In 53 Japanese hospitals, the ATTEND registry showed an in-hospital mortality benefit for patients with acute nonischemic heart failure managed with a PAC (PAC 1.4% vs. non-PAC 4.4%).24 Rationale for PAC placement in registry patients was cardiogenic shock, shock and pulmonary edema, and diagnosis of type of shock; patients were managed individually without a generalized treatment protocol. In a retrospective analysis of the National Trauma Data Bank, a mortality benefit with PAC use was seen in severely injured patients in shock (base deficit ≤ –11) except in patients aged 41 to 60.25 Interestingly, patients older than 60 with severe injury had decreased mortality even with base deficit of −6 to −10, possibly signifying that PAC placement at admission in severely injured patients was associated with earlier resuscitation.
A unique characteristic of the PAC is its ability to provide continuous measurement of pulmonary artery pressures. The ability to titrate pulmonary vasodilators and monitor CO changes remains an attractive advantage of the PAC, although it has not been addressed experimentally. In patients with cardiogenic shock, PACs may help following reperfusion therapy to gauge response to supportive interventions.26 A recent review on left ventricular assist devices advocated use of the PAC to differentiate between right and left heart failure when investigating causes of hypotension in the setting of adequate filling pressures.13 Many of the recent studies on PACs excluded patients that received a PAC based on physician preference—including patients with severe heart failure or pulmonary vascular disease—and thus may have some selection bias against potential beneficiaries of the device.24,27,28,29,31
There are no clear indications regarding PAC use in the emergency department or ICU; in lieu of clinical evidence, existing personal or institutional practices and preferences dictate use. It is not uncommon for institutions to employ PACs in postoperative cardiac surgery patients to provide a broad set of physiologic parameters, allowing practitioners to distinguish between hypovolemia, vasoplegia, and inadequate cardiac contractility.
A PAC may be inserted through any large vein, but the right internal jugular and left subclavian veins are optimal for maintaining the catheter's curvature and are likely the best locations for easy flotation of the tip. Waveform recognition (Fig. 5.2) is typically sufficient for guiding the catheter to its resting position in a central branch of the pulmonary artery. However, fluoroscopy and echocardiography are occasionally used if this approach is unsuccessful. A plain radiograph should be obtained to confirm final position and to rule out right ventricular coiling, aberrant placement, overinsertion, or mechanical complications such as pneumothorax and hemothorax.
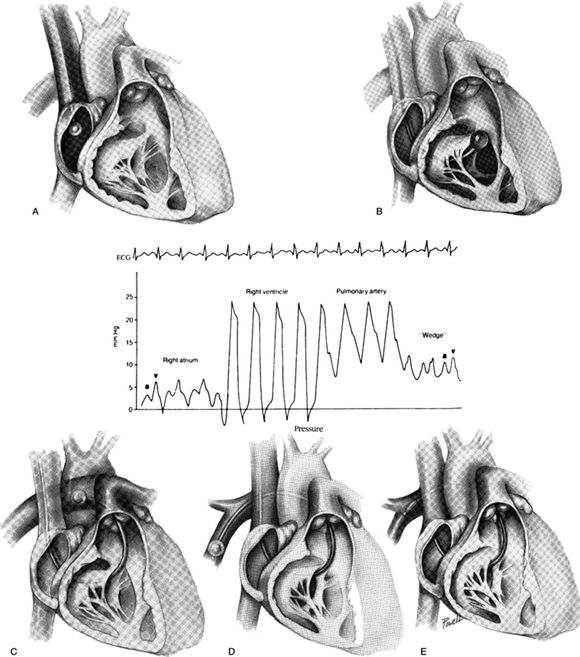
FIGURE 5.2 A: With the PAC tip in the right atrium, the balloon is inflated. B: The catheter is advanced into the right ventricle with the balloon inflated, and right ventricle pressure tracings are obtained. C: The catheter is advanced through the pulmonary valve into the pulmonary artery. A rise in diastolic pressure should be noted. D: The catheter is advanced to the “wedge” or PAOP position. A typical PAOP tracing should be noted with A and V waves. E: The balloon is deflated. Phasic pulmonary artery pressure should reappear on the monitor. Center: Waveform tracings generated as the balloon-tipped catheter is advanced through the right heart chambers into the pulmonary artery. Adapted from Wiedmann HP, Matthay MA, Matthey RA. Cardiovascular pulmonary monitoring in the intensive care unit (Part 1). Chest. 1984;85:537.
Device-specific problems during PAC insertion include failure to zero the transducer, misconnections of pressure tubing and transducer wires to pulmonary artery (PA) and CVP ports causing erroneous display of waveforms, failure to inflate the balloon, advancing too slowly or quickly, and misinterpretation of waveforms. The larger introducer catheter also increases risks of bleeding and carotid injury.
The normal pulmonary arterial tracing has an arterial-like waveform with systolic pulmonary pressures in the 15 to 25 mm Hg range. The PAOP or “wedge” waveform reflects left atrial filling and is recognized by disappearance of the PA waveform and appearance of a CVP-like waveform during catheter advancement. When compared to the CVP tracing, the PAOP tracing normally has two prominent peaks (a and v waves) instead of three. The PAOP should be measured at end-expiration as the average of the a wave's peak and nadir pressures.
In a patient with normal pulmonary vascular resistance (PVR), the PA diastolic pressure (PAD) will be 8 to 15 mm Hg and only slightly higher (1 to 4 mm Hg) than the PAOP. This normal PAD–PAOP gradient often allows the practitioner to follow trends in PAD as estimates of LV filling pressures and eliminates the need for balloon inflation and repeated catheter “wedging.” PAD pressures that are higher than normal (>20 mm Hg) raise concern for either elevated left heart pressures or elevated PVR. For example, with a PAD of 22 mm Hg, a similar PAOP (say 18 mm Hg) would point to elevated left-sided filling pressures as the cause of pulmonary venous hypertension. A PAD of 22 with a PAOP of 10 indicates that the left heart and related structures are not responsible for the elevated pulmonary artery pressure and that the pressure likely comes from high PVR. Elevations in PAOP may also be due to atrial myxomas, mitral valvulopathy, and high PEEP.
As with the CVC, qualitative information regarding left ventricular pump and electrical function can be obtained through the study of waveforms and their intervals. The discussion in the CVP section above is equally applicable here, except that the a and c waves of the PAOP tend to be fused, and an abnormally large v wave would reflect mitral valve regurgitation or poor LV compliance.
PACs had a historical role in defining the hemodynamic profiles associated with prototypic shock states (Table 5.1). Hypovolemic shock is readily recognized by decreased right- and left-sided filling pressures with decreased CO and a high SVR. Cardiogenic shock typically refers to LV failure, which is identified by increased right- and left-sided pressures, decreased CO, and increased SVR. Pure right heart failure is observed with elevated CVP, decreased PAOP and CO, and increased SVR. While a common cause of RV failure is LV failure, other possible etiologies include RV ischemia, pulmonary embolism, and pulmonary hypertension. Distributive shock from sepsis has been clinically described as progressive from an “early” or “warm” to a “late” or “cold” state. A change in measured CO via the PAC can differentiate the prototypically high CO in warm shock versus the low CO of cold distributive shock. This is relevant, as the latter condition may require inotropes while the former may not.
TABLE 5.1 Shock State Identification by PAC Hemodynamic Parameters (Normal Values for Each Parameter Noted in Parentheses)

Complications from pulmonary artery catheterization include those of CVC placement, as well as more PAC-specific problems such as misinterpretation of data, higher likelihood of arrhythmia, pulmonary artery injury, pulmonary infarction, valvular injury, and catheter knotting or tangling with other devices. Of note, misinterpretation of respiratory cycle and corresponding wedge pressures can lead to large over- or underestimates of filling pressures. As with any central catheter, there is always a risk of infection, which is dependent on the length of time a PAC/introducer is maintained and the sterility of the techniques used to place it.
PAC placement is often difficult in patients with pulmonary artery hypertension, where tricuspid regurgitation and a large RV size impede flotation through the right heart. The catheter may coil in the RV, leading to arrhythmias and prolonged insertion times.
Pulmonary artery injury can result from spontaneous wedging (also called “over wedging”) (Fig. 5.3). Spontaneous wedging is evident when the PA waveform transitions to a “wedged” waveform without deliberate catheter advancement or balloon inflation. Over wedging occurs because of inadvertent migration of the catheter tip or occlusion of the PAC tip against a vessel wall.
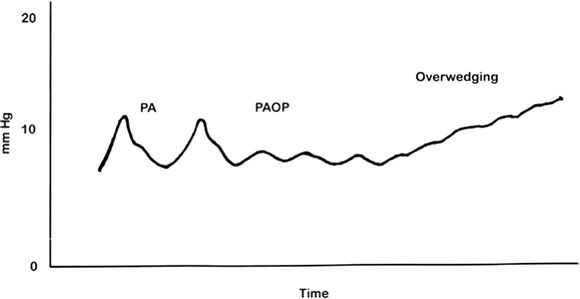
FIGURE 5.3 Overwedging. This condition should be suspected with a rise in PA pressures above known occlusion pressure values. A balloon inflated in this position could lead to pulmonary artery rupture. After assuring balloon deflation, catheters should be withdrawn to the main pulmonary artery and readvanced if necessary. Rarely, a PAC tip thrombus can produce an “overwedging” waveform.
Adapted from Civetta GA. Taylor & Kirby's Critical Care. W. W. Philadelphia, PA: Norton & Company; 2009:175.
Contraindications to PAC placement include left bundle branch block (LBBB) (given the ~5% risk of inducing right bundle branch block [RBBB]), right-sided cardiac mass, or right-sided infectious endocarditis. Contraindications listed for CVCs are also applicable. Attention should be paid to each particular PAC inserted, as several models contain heparin and/or latex and therefore would be contraindicated in patients with heparin-induced thrombocytopenia or a latex allergy. Risks of complications from insertion, manipulation, and interpretation are further increased with operator inexperience.
CONCLUSION
Invasive pressure monitoring is most useful when a change in hemodynamic status demands further clarification and assessment of adequacy of perfusion. CVCs enable the safe administration of vasoconstrictors and measurement of ScvO2 and, with proper interpretation of their waveforms, provide a wealth of hemodynamic data. The PAC's ability to continuously monitor pulmonary artery pressures, CO, and SvO2 remains useful in the management of complex patients, yet awaits a clearly defined population or protocol that demonstrates a clinical benefit. These “upstream” indicators of perfusion, however, have idiosyncrasies and inherent limitations. Careful examination of these capabilities over the last 30 years demonstrates that end points of resuscitation should also include evaluation of “downstream” variables, such as organ function and oxidative metabolism (e.g., lactate). Given the still widespread use of invasive pressure monitoring, understanding the full array of data available from CVC and PAC numerical and waveform data is important in assuring that the patient will derive the greatest benefit from his or her exposure to the risks of catheterization. The clinician at the bedside, not the devices, determines patient outcome.
LITERATURE TABLE
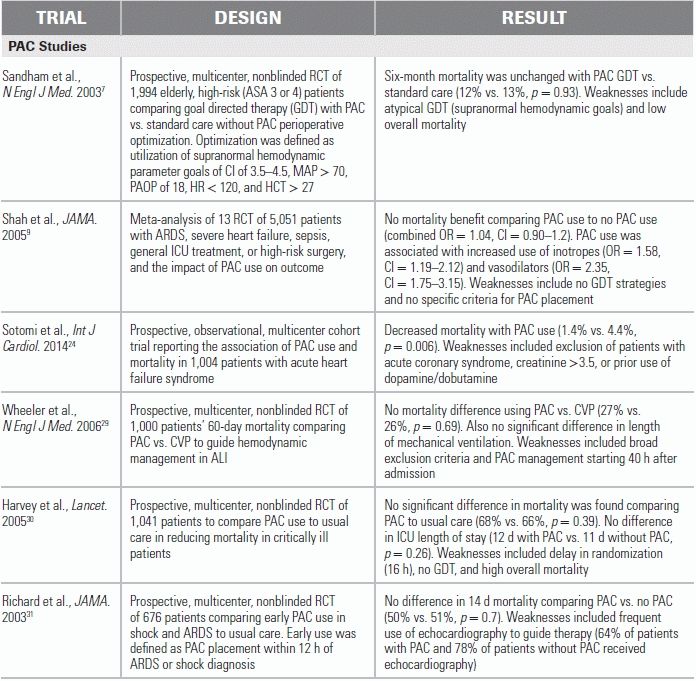
CI, confidence interval; HR, hazard ratio; OR, odds ratio.
1.Wilson JN, et al. Central venous pressure in optimal blood volume maintenance. Arch Surg. 1962;85:563–578.
2.Swan HJ, et al. Catheterization of the heart in man with use of a flow-directed balloon-tipped catheter. N Engl J Med. 1970;283(9):447–451.
3.Hayes MA, et al. Elevation of systemic oxygen delivery in the treatment of critically ill patients. N Engl J Med. 1994;330(24):1717–1722.
4.Gattinoni L, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333(16):1025–1032.
5.Boyd O, Hayes M. The oxygen trail: the goal. Br Med Bull. 1999;55(1):125–139.
6.Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575.
7.Sandham JD, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003;348(1):5–14.
8.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–178.
9.Shah MR, et al. Impact of the pulmonary artery catheter in critically ill patients: meta-analysis of randomized clinical trials. JAMA. 2005;294(13):1664–1670.
10.Michard F, Teboul JL. Predicting fluid responsiveness in ICU patients: a critical analysis of the evidence. Chest. 2002;121(6):2000–2008.
11.Rivers E, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377.
12.Pittman JA, Ping JS, Mark JB. Arterial and central venous pressure monitoring. Int Anesthesiol Clin. 2004;42(1):13–30.
13.McIntyre LA, et al. A survey of Canadian intensivists' resuscitation practices in early septic shock. Crit Care. 2007;11(4):R74.
14.Dellinger RP, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41(2):580–637.
15.Hughes RE, Magovern GJ. The relationship between right atrial pressure and blood volume. AMA Arch Surg. 1959;79(2):238–243.
16.Magder S. Bench-to-bedside review: an approach to hemodynamic monitoring—Guyton at the bedside. Crit Care. 2012;16(5):236.
17.Magder S. More respect for the CVP. Intensive Care Med. 1998;24(7):651–653.
18.Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med. 2011;184(5):514–520.
19.Teixeira C, et al. Central venous saturation is a predictor of reintubation in difficult-to-wean patients. Crit Care Med. 2010;38(2):491–496.
20.Troianos CA, et al. Guidelines for performing ultrasound guided vascular cannulation: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists. J Am Soc Echocardiogr. 2011;24(12):1291–1318.
21.Wigmore TJ, et al. Effect of the implementation of NICE guidelines for ultrasound guidance on the complication rates associated with central venous catheter placement in patients presenting for routine surgery in a tertiary referral centre. Br J Anaesth. 2007;99(5):662–665.
22.Pronovost P, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355(26):2725–2732.
23.Tousignant CP, Walsh F, Mazer CD. The use of transesophageal echocardiography for preload assessment in critically ill patients. Anesth Analg. 2000;90(2):351–355.
24.Sotomi Y, et al. Impact of pulmonary artery catheter on outcome in patients with acute heart failure syndromes with hypotension or receiving inotropes: from the ATTEND Registry. Int J Cardiol. 2014;171(2):165–172.
25.Friese RS, Shafi S, Gentilello LM. Pulmonary artery catheter use is associated with reduced mortality in severely injured patients: a National Trauma Data Bank analysis of 53,312 patients. Crit Care Med. 2006;34(6):1597–1601.
26.Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119(1):147–152.
27.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112(6):1392–1402.
28.Kumar A, et al. Pulmonary artery occlusion pressure and central venous pressure fail to predict ventricular filling volume, cardiac performance, or the response to volume infusion in normal subjects. Crit Care Med. 2004;32(3):691–699.
29.Wheeler AP, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354(21):2213–2224.
30.Harvey S, et al. Assessment of the clinical effectiveness of pulmonary artery catheters in management of patients in intensive care (PAC-Man): a randomised controlled trial. Lancet. 2005;366(9484):472–477.
31.Richard C, et al. Early use of the pulmonary artery catheter and outcomes in patients with shock and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2003;290(20):2713–2720.