17
Controversies in Arrhythmia Management
Sam Senturia
BACKGROUND
Emergency physicians are tasked with managing a wide range of arrhythmias. Rather than reviewing the management of all common arrhythmias, this chapter addresses three controversies of arrhythmia management encountered by emergency and critical care physicians: (1) rate control versus rhythm control in atrial fibrillation (AF), (2) the use of adenosine for the diagnosis and treatment of undifferentiated wide complex tachycardia (WCT), and (3) the use of procainamide versus amiodarone for the treatment of ventricular tachycardia (VT). A review of the evidence relevant to these topics will help physicians make informed evidence-based decisions when these dilemmas arise.
There are no evidence-based guidelines to help the emergency physician decide when to involve critical care services in the management of arrhythmias. It is reasonable to do so when there is concern that an arrhythmia may cause hemodynamic deterioration. Whether an arrhythmia will cause hemodynamic deterioration will depend on both the electrical properties of the arrhythmia and the physiologic reserve of the patient. Many patients, for example, tolerate supraventricular tachyarrhythmias with minimal symptoms, whereas in others with compromised cardiac function, the addition of a supraventricular arrhythmia may lead to life-threatening deterioration. Ventricular arrhythmias always are considered capable of producing hemodynamic deterioration because of the risk of degenerating into pulseless VT or ventricular fibrillation. For example, AF with a wide QRS complex and a ventricular rate exceeding 200 beats per minute may represent atrioventricular (AV) conduction over an accessory pathway, which can precipitate ventricular fibrillation. A commonsense approach to arrhythmia management should involve critical care services in the following situations:
1.Patients with any arrhythmia for which there is concern about the possibility of associated hemodynamic deterioration
2.Patients with ventricular arrhythmias
3.Patients with AF, a wide QRS complex, and a ventricular rate exceeding 200 beats per minute
4.Patients with high-grade AV block, complete heart block, or bradyarrhythmias that require transvenous pacing
5.Patients successfully resuscitated from cardiac arrest
RATE CONTROL VERSUS RHYTHM CONTROL FOR RECENT-ONSET ATRIAL FIBRILLATION
Controversy exists regarding whether rate control or rhythm control is the best management strategy for recent-onset atrial fibrillation (ROAF), a condition commonly defined as AF of <48 hours' duration. Rate control is defined as ventricular rate control without an attempt to convert the patient to sinus rhythm. Rhythm control requires either pharmacologic or electrical cardioversion to sinus rhythm. In a recent survey of members of national emergency medicine associations, 94% (234/249) of American emergency physician respondents indicated that they use rate control, and 26% (65/249) indicated that they use rhythm control.1 In Canada, 71% of respondents indicated that they use rate control, and 66% indicated that they use rhythm control. In the United Kingdom and Australasia, 50% of respondents indicated that they use rhythm control. The American College of Cardiology (ACC)/American Heart Association (AHA)/European Society of Cardiology (ESC) Guidelines for the Management of Patients with AF do not provide recommendations for management in the emergency department (ED).2,3
Large Multicenter Randomized Trials
Several multicenter randomized controlled trials (AFFIRM,4 RACE,5 PIAF,6 STAF,7 HOT CAFÉ8) have compared rate and rhythm control in the general population of patients with AF and have demonstrated equivalent outcomes, including rates of death and thromboembolism. The largest of these was the AFFIRM trial,4 which compared outcomes in 4,060 patients aged 65 or older with AF and risk factors for stroke who were randomized to rate control (RaC) or rhythm control (RhC). After a mean follow-up of 3.5 years, the rhythm control group had an almost significant trend toward increase in the primary endpoint of death (25.9% RaC vs. 26.7% RhC). There was no significant difference between the groups in a composite secondary endpoint composed of death, disabling stroke, disabling anoxic encephalopathy, major bleeding, and cardiac arrest (32.7% RaC vs. 32.0% RhC). There also was no difference in overall frequency of central nervous system events (7.4% RaC vs. 8.9% RhC) or ischemic strokes (5.5% RaC vs. 7.1% RhC). In both groups, the majority of strokes occurred in patients who had discontinued warfarin or had a subtherapeutic international normalized ratio (INR). The rhythm control group had higher hospitalization rates during follow-up (73.0% RaC vs. 80.1% RhC). A subsequent analysis suggested that adverse effects of antiarrhythmic drugs could explain the trend toward increased mortality in the rhythm control group.9 In this analysis, the use of antiarrhythmic drugs was associated with increased mortality (hazard ratio 1.49), and the presence of sinus rhythm was associated with reduced mortality (hazard ratio 0.53).
The AFFIRM trial and the other large trials comparing rate control and rhythm control included very few patients with ROAF. As a result, these studies may have limited applicability to the management of ROAF in the ED. In the AFFIRM trial, the qualifying episode of AF had a duration >48 hours in more than 69% of patients and was the first episode of AF in only 36% of patients.10 The RACE trial included only patients who had persistent AF after a previous cardioversion.5 The median duration of the qualifying episode of AF was >30 days, and the median duration of AF was >300 days. An Annals of Emergency Medicine systematic review abstract reported on a Cochrane review of the RACE, STAF, and HOT CAFÉ trials and found that the mean age in these studies was more than 60 years and the mean duration of AF was more than 200 days. The report concluded that the Cochrane review provided little evidence on which to base decisions in the ED.11
ED Studies Comparing Rate Control with Rhythm Control
Although the large RCTs have provided only limited information relevant to ED management, several studies of ED patients with ROAF support the efficacy and safety of rhythm control. A 2004 multicenter retrospective cohort study reported on 388 stable patients with ROAF who were electrically cardioverted in the ED.12 Eighty-six percent (332/388) were successfully converted to sinus rhythm; of these, 91% (301/332) were discharged from the ED. All patients received IV procedural sedation before cardioversion. Chemical cardioversion was attempted before electrical cardioversion in 29%. Twenty-five cardioversion attempts (6%) were associated with 28 complications: 22 complications from procedural sedation (oxygen desaturation below 90% in 12 patients, use of bag-valve-mask device in 6 patients, and emesis, hypotension, bradycardia, and agitation each in 1 patient) and 6 complications from the cardioversion itself (three minor burns, two episodes of VT, and one episode of bradycardia). Ten percent (39/388) of patients returned to the ED within 7 days, including 6% (25/388) for relapse of AF.
A 2008 prospective controlled study from the Mayo Clinic reported on 153 patients with ROAF who were randomly assigned to either protocolized treatment in an ED observation unit or hospital admission with usual care.13 The ED observation unit protocol consisted of administration of a calcium channel blocker or beta blocker for rate control followed by procedural sedation and electrical cardioversion if AF persisted at 6 hours, followed by observation for an additional 2 hours. Among the ED observation unit cohort, 32% (24/75) reverted to sinus rhythm after rate control, and 51% (38/75) required electrical cardioversion, creating an 85% (64/75) conversion rate. Nine patients (12%) of the ED cohort were admitted. The median length of stay was 10 hours for the ED observation unit group versus 25 hours for the inpatient group. During 6 months of follow-up, there were no differences between the two groups in rates of recurrent AF (10%) or MI, congestive heart failure, stroke, or death (zero patients with each diagnosis except for one MI in the inpatient group).
A recent review considered five ED studies that specifically examined the outcome of patients discharged after cardioversion in the ED.14 No patient in any of the studies suffered a thromboembolic event. Among all five studies, there were only three cardioversion-related complications that resulted in a disposition change, and each of these was an arrhythmia that resolved in the ED.
In Ontario, Canada, emergency physicians have long followed a protocolized management of ROAF called the Ottawa Aggressive Protocol.15 The protocol consists of chemical cardioversion followed, in case of failure, by electrical cardioversion and discharge from the ED (see Table 17.1). A 2010 retrospective cohort study at a university hospital in Ontario evaluated the effectiveness and safety of this protocol. Of 660 ED visits in which the protocol was applied, 40% (261/660) received rate control drugs, 100% (660/660) received IV procainamide, and 37% (243/660) subsequently underwent electrical cardioversion. The rate of conversion to sinus rhythm was 58% (385/660) for procainamide and 92% (223/243) for electrical cardioversion. 97% (639/660) were discharged from the ED, and 90% (595/660) were discharged in normal sinus rhythm. The median lengths of stay from ED arrival to discharge were 4.9 hours (all patients), 3.9 hours (cardioversion with procainamide), and 6.5 hours (electrical cardioversion). Adverse ED events occurred in 7.6% (50/660): 6.6% (44/660) experienced transient hypotension, and 1% (7/66) experienced either bradycardia, AV block, or atrial or ventricular tachyarrhythmia. 3.2% (21/660) required admission. During the 7 day follow-up period, 8.6% (57/660) had relapse of AF. There was no stroke or death.
TABLE 19.1 The Ottawa Aggressive Protocol
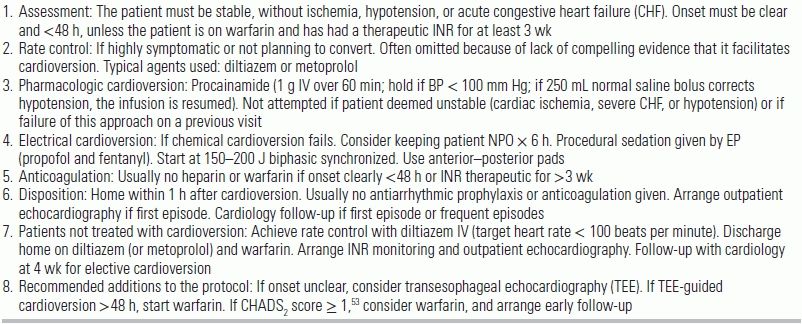
CHF, congestive heart failure; INR, international normalized ratio; IV, intravenously; NPO, nothing by mouth.
Modified from Stiell IG, Clement CM, Perry JJ, et al. Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. CJEM. 2010;12:181–191. Box 1, with permission.
Arguments for Rate Control
Arguments for rate control include the following. The large, randomized controlled trials (AFFIRM, RACE, etc.) have shown that—over the long term—outcomes with rate control are as good as (or better than) outcomes with rhythm control.4–8 In the AFFIRM trial, rhythm control was associated with a higher mortality than rate control among older patients.4 Achieving rate control in the ED may be faster and less operationally complicated than cardioversion.16 Rate control does not expose the patient to the risk of procedural sedation associated with electrical cardioversion. Finally, many patients will spontaneously convert to sinus rhythm. In one study, 32% (24/75) of patients spontaneously converted in the ED within 6 hours of arrival.13 In another study, 29% (59/206) of patients spontaneously converted in the ED, and an additional 11/16 patients discharged in AF returned the next day having converted to sinus rhythm.17
Arguments for Rhythm Control
Arguments for rhythm control include the following. The mean age in the AFFIRM trial was 70 years.4 Younger, more physically active patients would be expected to show benefit from conversion to sinus rhythm. It seems reasonable to offer rhythm control to those patients having a first episode of AF and no known cardiac structural abnormality. There is mounting evidence that AF itself leads to electrical and structural remodeling of the atria that, in turn, make the arrhythmia intractable.18 Current theory suggests that rhythm control should be established as soon as possible to improve the chances of remaining in sinus rhythm.19 Rhythm control strategies allow for high rates of discharge from the ED, usually without warfarin or rate control medication.20 In contrast, patients treated with rate control usually require rate control medications and often warfarin; this frequently requires hospital admission and chronic INR monitoring and carries a risk of serious bleeding.
ADENOSINE IN THE MANAGEMENT OF WIDE-COMPLEX TACHYCARDIA
The 2010 AHA Guidelines for Advanced Cardiovascular Life Support (ACLS) recommend adenosine for the diagnosis and treatment of stable undifferentiated regular WCT.21 The principal effect of adenosine is transient AV nodal blockade. Thus, if a WCT is caused by a supraventricular tachycardia (SVT), administration of adenosine will do one of two things: (1) Terminate the arrhythmia if the AV node is part of a reentry loop, or (2) block AV nodal conduction and reveal the previously hidden atrial activity. If the WCT is VT, administration of adenosine is expected to have no effect on the rhythm (in most cases) and no adverse hemodynamic effect. The AHA executive summary emphasizes that this dual diagnostic/treatment capability is an important change for the 2010 guidelines.22
Early Studies of Adenosine for WCT
Several small studies in the late 1980s and 1990s attempted to clarify the efficacy and safety of adenosine administration for WCT. In a 1994 prospective study of ED patients, adenosine was administered (maximum dose 18 mg) to 12 patients during 29 episodes of WCT.23 Adenosine terminated 59% (17/29) of episodes, all of which were identified as atrioventricular reentry tachycardia (AVRT). Transient AV block occurred in 10% (3/29) of episodes, revealing atrial flutter or AF. No response occurred in 31% (9/29) of episodes, which were identified as AVRT (5) and VT (4). In a second, prospective, study in 2001—also of emergency patients—adenosine was administered (maximum dose 18 mg) to 26 patients with WCT.24 Adenosine terminated 27% (7/26) of episodes. Transient AV block occurred in 42% (11/26) allowing diagnosis of the underlying arrhythmia. No response occurred in 31% (8/26), all of which were VT. No patient suffered serious hemodynamic deterioration in either study.
Two electrophysiology studies, performed in a more controlled lab setting, supported the findings of the ED-based studies. In a 1988 study, adenosine was administered to 26 consecutive patients with WCT.25 Of these, 35% (9/26) were SVT, and 65% (17/26) were VT. Adenosine terminated 67% (6/9) of SVTs and 6% (1/17) of VTs. As a diagnostic test for supraventricular origin of a regular WCT, the investigators calculated that adenosine administration had 89% (8/9) sensitivity and 94% (16/17) specificity. In a 1990 electrophysiology study, adenosine was administered to 34 consecutive patients with WCT.26 Adenosine terminated 7/10 arrhythmias using reentry mechanisms involving the AV node, 1/10 atrial arrhythmias, and 1/14 VTs. As with the ED studies, no patients in the electrophysiology studies experienced adverse hemodynamic effects after adenosine administration.
Studies Demonstrating Adenosine Terminates VT
The studies noted above demonstrate that adenosine can terminate VT. Additional studies have clarified that the episodes of VT terminated by adenosine are predominately exercise-induced VT caused by triggered automaticity in patients with structurally normal hearts and not reentry-related VT in patients with structural (including ischemic) heart disease.27–30 The only known mechanism of adenosine activity on ventricular conduction is antagonism of catecholamine-induced stimulation of intracellular cAMP production. Based on these findings, the authors of one study postulated that the mechanism underlying this form of exercised-induced VT is mediated by cyclic adenosine monophosphate (cAMP).27 Since then, this form of VT has been documented in multiple studies, where it manifests with left bundle branch block (LBBB) pattern in most cases.27–30 In a subsequent retrospective study of patients with WCT who received adenosine, 10/18 patients had VT, and 50% of these (5/10) were terminated with adenosine.30 Four of the five patients whose VT was terminated by adenosine had structurally normal hearts, exercise-induced VT, and LBBB VT pattern consistent with the group of patients described in the initial study.
Concerns About Safety of Adenosine
Concerns have been raised about the safety of adenosine administration because of case reports of persistent bradycardia or asystole, induction of AF, ventricular fibrillation, torsades de pointes, and accelerated ventricular response in patients with AF or atrial flutter with and without preexcitation.31,32 In the 2001 study discussed above, 160 consecutive ED patients were treated with adenosine for narrow complex tachycardia and WCT in order to determine the prevalence of arrhythmogenic effects.24 Of these, 84% (134/160) had narrow complex tachycardia, and 16% (26/160) had WCT. In the narrow complex group, the adenosine-related arrhythmias observed included the following: prolonged AV block (>4 seconds) in 6% (8/134), AF in 1.5% (2/134), and nonsustained VT in 6% (8/134). In the wide complex group, the only adenosine-related arrhythmia observed was prolonged AV block (>4 seconds) in 11% (3/26). All arrhythmias were transient and resolved spontaneously; none required treatment. In a 2001 retrospective study of 187 episodes of tachycardia treated with adenosine in 127 ED patients, VT occurred following successful termination of an arrhythmia of supraventricular origin in 19% (31/160) of episodes.33 All adenosine-related episodes of VT were brief (mean duration 6.0 beats, range 3 to 26 beats) and spontaneously resolved. AF was induced in 5% (8/160) of episodes. There is only one case report of degeneration of VT to ventricular fibrillation (VF) after administration of adenosine for WCT.34
Perhaps the greatest safety concern is that adenosine will accelerate conduction over an accessory pathway in patients with AF or atrial flutter, leading to hemodynamic deterioration or ventricular fibrillation. Neither of the two electrophysiology studies of adenosine use in WCT discussed above reported any adverse hemodynamic effects on patients with AF and preexcitation.25,26 In the first of these two studies, there was also no effect on the mean RR interval.25 In the second of these studies, antegrade accessory pathway conduction was transiently enhanced in all nine patients, and the average RR interval and the shortest RR interval shortened but again without hemodynamic consequence.26 Similarly, in a study of 30 patients with Wolff-Parkinson-White syndrome (WPW), adenosine administration was shown to lead to shortened antegrade refractoriness of the accessory pathway, but again, the effects were brief and no patient suffered clinical deterioration.35
There have been, however, isolated case reports of VF after administration of adenosine to patients with AF and preexcitation.36–38 In one study, four patients who presented to the ED with preexcited AF degenerated to VF after administration of adenosine.38 These four patients were compared to five control patients with preexcitation who underwent induction of AF and administration of adenosine in the electrophysiology lab and did not develop VF. The four patients who developed VF in response to adenosine demonstrated a shorter RR interval during AF and a shorter antegrade effective refractory period of the accessory tract than the five who did not develop VF.
A recently published investigation has attempted to clarify the efficacy and safety of adenosine administered for WCT in ED patients.39 This was a retrospective observational study at nine hospitals in five cities of 197 patients with WCT who received adenosine. Adenosine terminated 15% (29/197) of the WCTs. Overall, 59% (116/197) of the WCTs were diagnosed as SVT, and 41% (81/197) were diagnosed as VT. There was a positive response to adenosine, defined as termination of the WCT or temporary AV block or any other change in rhythm except for retrograde ventriculoatrial block, in 90% (104/116) of patients with SVT and in 2% (2/81) of patients with VT. The investigators calculated that a positive response to adenosine increased the odds of SVT by a factor of 36. A negative response to adenosine, defined as no apparent change in rhythm or transient retrograde ventriculoatrial block, increased the odds of VT by a factor of 9. The rate of primary adverse events, defined as need for emergent electrical or medical intervention in response to adenosine administration, was 0% (0/116) of patients with SVT and 0% (0/81) of patients with VT. In 48% (56/116) of patients diagnosed as SVT, the diagnosis was determined by a positive response to adenosine. As a result, there may have been cases of VT terminated by adenosine that were misdiagnosed as SVT. Therefore, a positive response to adenosine may increase the odds of SVT by less than the factor of 36 calculated by the investigators. Since the calculation that adenosine distinguishes SVT from VT was itself determined by the response to adenosine, this limits the validity of the odds calculation.
Administration of adenosine to patients with stable undifferentiated regular WCT appears to be relatively safe. Except for isolated case reports, no patients in the above studies developed significant arrhythmic or hemodynamic deterioration or required electrical or pharmacologic resuscitation after administration of adenosine. Adenosine is not recommended for irregular WCT, but it is worth noting that none of the 15 patients with WPW and AF in the two electrophysiology studies discussed developed VF or hemodynamic deterioration after administration of adenosine. Some caution needs to be exercised as these observations were based on a very small number of subjects. Because of the possibility of arrhythmic deterioration, defibrillator pads should be attached to patients receiving adenosine for undifferentiated WCT. According to the AHA 2010 guidelines, when faced with a stable WCT, if the mechanism cannot be determined and the rate is regular and the QRS is monomorphic, adenosine is recommended for both diagnosis and treatment.21
The diagnostic use of adenosine to distinguish SVT from VT does have one notable drawback. As a diagnostic test for supraventricular origin of a regular WCT, studies have calculated that adenosine administration has 89% to 90% sensitivity and 93% to 94% specificity.25,40 The less-than-perfect specificity reflects the false-positive VTs that terminated with adenosine. If termination of a WCT by adenosine is accepted as proof of supraventricular origin, some patients with VT will be mislabeled as SVT, with the consequence of not receiving the appropriate workup and treatment for VT (e.g., electrophysiology studies, ablation, implantable cardioverter defibrillator placement). The best approach for the emergency physician probably is to avoid labeling WCTs that terminate with adenosine as SVT and to have these patients receive consultation by a cardiologist. Although SVT is the most likely explanation, a definitive electrophysiologic explanation is warranted.
PROCAINAMIDE VERSUS AMIODARONE FOR TERMINATION OF VENTRICULAR TACHYCARDIA
In the 2010 AHA Guidelines for ACLS, procainamide is the preferred drug for the treatment of stable monomorphic VT.21 Procainamide is rated class IIa (administration is reasonable), whereas amiodarone is now rated class IIb (administration may be considered). Arguments against procainamide have included a long administration time, QT prolongation and hypotension, and a contraindication for use in patients with depressed left ventricular function. Thus, situational variables often dictate pharmacologic choice. Additional agents that have been used in VT include lidocaine and sotalol. The latter agents will be reviewed briefly, followed by a more extensive consideration of evidence surrounding procainamide and amiodarone.
Lidocaine and Sotalol
Lidocaine is mentioned in the 2010 ACLS guidelines as a second-line agent for treatment of VT.21 In multiple studies, lidocaine had poor efficacy in terminating VT, with success ranging between 19% and 29%.41–44 This termination rate is inferior to that seen with sotalol,42 procainamide,44 and amiodarone.45 Sotalol was shown in a single randomized double-blind crossover study of 33 conscious patients with sustained VT to terminate VT in 69% of patients.42 Hypotension required electrical cardioversion in 10% of patients. Since this 1994 study, there appears to be no high-quality evidence addressing the efficacy and safety of IV sotalol for termination of acute hemodynamically stable VT. Until recently, sotalol had little use in the emergency management of VT in the United States because it was not available in intravenous form. The FDA approved an intravenous form of sotalol in July 2009. Sotalol received a class IIb recommendation in the 2010 ACLS guidelines.
Early Studies of Procainamide
Studies of procainamide in the 1990s reported rates of termination of VT as high as 80% to 90%. A 2002 randomized crossover study compared procainamide (10 mg/kg at 100 mg/min) and lidocaine (1.5 mg/kg over 2 minutes) in 29 consecutive patients with hemodynamically stable monomorphic VT.44 The investigators excluded patients with severe heart failure or hypotension during VT (mean LV ejection fraction (LVEF) = 30%; mean systolic BP during VT = 115 mm Hg). When VT did not terminate within 15 minutes of the first drug, the crossover drug was administered. Initial treatment was successful in 80% (12/15) of patients receiving procainamide and in 21% (3/14) of patients receiving lidocaine. After 25 episodes of recurrent VT and 24 episodes that crossed over to the second agent, a total of 79 drug infusions for VT were given; procainamide terminated 79% (38/48), and lidocaine terminated 19% (6/31).
Administration of procainamide was associated with prolongation of the QRS width and QT interval, whereas administration of lidocaine was not. Adverse events requiring termination of the protocol occurred in 13% (2/15) of patients receiving procainamide (hypotension in one patient and acceleration of VT in one patient) and were quickly reversible. The procainamide infusion rate of 100 mg/min in this study is at least double the current 2010 AHA ACLS recommended 20 to 50 mg/min. In this study, a 70-kg patient received the total infusion in 7 minutes, and termination of VT occurred within 15 minutes of finishing the infusion. While this study is limited by the small sample size, the crossover design allowed the testing of the second drug during the same episode when the first was not effective. Procainamide still terminated 70% to 80% of episodes after lidocaine was ineffective. When administered sequentially, a carryover effect of one drug to the other cannot be excluded because only 15 minutes elapsed between the first and second drugs. The authors considered the likelihood of significant crossover effect to be small because lidocaine remained less effective after procainamide. If there had been an interaction between the two drugs, a greater effect would be expected for lidocaine after procainamide than the reverse because of the longer half-life of procainamide.
In a 1992 electrophysiology study, VT was induced by programmed electrical stimulation in 15 patients with prior myocardial infarction (12 with recurrent hemodynamically tolerated VT, 1 with syncope, 2 with history of cardiac arrest and inducible VT).46 Infusion of procainamide at 50 mg/min terminated VT in 93% (14/15) of patients. The total dose of procainamide required to terminate the tachycardia ranged from 100 to 1,080 mg (median 600 mg). The systolic BP during VT was >100 mm Hg in all patients and remained >80 mm Hg in all patients during and after infusion of procainamide. No patients had symptoms related to hypotension.
Early Studies of Amiodarone
The benefit of IV amiodarone in terminating acute hemodynamically stable VT has been extrapolated from studies of prolonged infusions to suppress recurrent unstable ventricular tachyarrhythmias. In a 1996 randomized controlled double-blind dose-range study of amiodarone by continuous infusion in 273 patients with recurrent hypotensive ventricular tachyarrhythmias refractory to lidocaine, procainamide, and bretylium, subjects received 525, 1,050, or 2,100 mg of amiodarone by continuous infusion over 24 hours.47 During VT, all patients had systolic BP < 80 mm Hg with clinical signs or symptoms of shock. All patients had had at least two episodes (mean 5.9) of hypotensive tachyarrhythmias in the 24 hours before admission to the study or were in incessant VT despite attempts at cardioversion. While on continuous amiodarone infusion, 40% (110/273) of patients survived 24 hours without another episode of hypotensive ventricular arrhythmia. There was no clear dose–response relationship with respect to success rate. In a second study that administered prolonged amiodarone infusions to 46 patients with recurrent life-threatening VT or VF that had failed to respond to at least two other antiarrhythmic agents, amiodarone was administered as 5 mg/kg over 30 minutes followed by continuous infusion of 1 g/24 hours for 72 hours, followed by oral amiodarone.48 This protocol led to resolution of recurrent VT or VF in 33% (15/46) of patients within 2 hours and 58.5% (27/46) of patients within 84 hours.
Until recently, few studies examined the use of IV amiodarone to terminate discrete episodes of hemodynamically stable VT. One study in 1989 examined the efficacy of IV amiodarone to terminate sustained VT in 19 patients with depressed LV function (mean EF = 30.1%) who had suffered recurrent sustained VT and VF.49 All patients were hemodynamically stable during VT. Amiodarone was administered as 5 mg/kg over 20 minutes followed by continuous infusion of 1,050 mg over 24 hours. Amiodarone terminated sustained VT in 42% (8/19) of patients within a mean effect time of 31 (±20) minutes.
Recent Studies of Amiodarone and Procainamide
In the past several years, two important studies have provided evidence that neither amiodarone nor procainamide is effective for termination of VT. A retrospective review in 2008 evaluated 41 consecutive patients with hemodynamically tolerated sustained monomorphic VT who were administered bolus dose amiodarone 300 mg IV.50 Amiodarone was administered over <30 minutes in 36 patients and over 30 to 60 minutes in 5 patients. The mean LVEF was 31%, and the mean systolic BP was 112 mm Hg. The median VT duration was 70 min (range 15 to 6,000). VT termination occurred within 20 minutes of the start of the amiodarone infusion in 15% (6/41) and within 1 hour in 29% (12/41). Hemodynamic deterioration requiring emergency cardioversion occurred in 17% (7/41).
A 2010 multicenter historical cohort study evaluated consecutive patients with stable VT treated with IV amiodarone or procainamide.51 Response to the medication was defined as termination of VT within 20 minutes of initiation of the infusion. Rates of termination of VT were 25% (13/53) and 30% (9/30) for amiodarone and procainamide, respectively. The adjusted odds of termination with procainamide compared with amiodarone was 1.2. Eventually, electrical therapy was required to terminate VT in 53% (35/66) of patients receiving amiodarone and 42% (13/31) of patients receiving procainamide. Hypotension requiring cessation of infusion or immediate electrical cardioversion occurred in 6% (4/66) of amiodarone patients and in 19% (6/31) of procainamide patients. The investigators note that the retrospective design of the study, limited data set, and potential for confounders limit the ability to draw firm conclusions about the relative effectiveness of amiodarone and procainamide. The 21-mg/min mean rate of infusion of procainamide is also lower than the 50- to 100-mg/min rate used in other studies and may have limited both the efficacy and the adverse effects of procainamide.
Based on these more recent studies, it appears that neither procainamide nor amiodarone is highly effective or safe in terminating hemodynamically stable VT. The authors of the 2010 study note several limitations that highlight the difficulty of performing their retrospective study, including limited use of procainamide by the physicians at the study hospitals, potential bias in the choice of medicine, and the 20-minute treatment interval allowed for successful termination of VT, which might bias against procainamide since it is often infused over 1 hour. The current 2010 AHA Guidelines for ACLS cite the 2008 study, but not the 2010 study, which was likely unavailable at time of publication.21 Given the unclear effectiveness and risk of significant hypotension associated with procainamide administration, procedural sedation and electrical cardioversion remain the currently recommended approach to hemodynamically stable VT. An early study published in 1973 demonstrated a 98% success rate of electrical cardioversion of 116 episodes of VT in 39 patients, and the incidence of significant complications of electrical cardioversion was low.52
CONCLUSION
Among American emergency physicians, the most common strategy for managing ROAF is rate control. Several studies have, however, demonstrated the efficacy and safety of rhythm control, which offers the advantage of high rates of discharge from the ED, usually without warfarin or rate control medication. No patient in any of the rhythm control studies reviewed suffered a thromboembolic event. The use of adenosine for the diagnosis and treatment of stable undifferentiated regular WCT appears to be safe. Termination of a WCT by adenosine should not be accepted as proof of supraventricular origin because adenosine also can terminate VT. The optimal approach is to consult an electrophysiologist for all episodes of undifferentiated WCT terminated by adenosine. Recent studies have provided evidence that neither amiodarone nor procainamide is highly effective or safe in terminating hemodynamically stable VT. Procedural sedation and electrical cardioversion appears to be the safest and most effective approach.
LITERATURE TABLE


RCT, randomized controlled trial; pts, patients; AF, atrial fibrillation; CHF, congestive heart failure; ED, emergency department; WCT, wide-complex tachycardia; EP, electrophysiology; AVB, atrioventricular block; sens, sensitivity; spec, specificity; VT, ventricular tachycardia; SVT, supraventricular tachycardia; CI, confidence interval; HR, hazard ratio.
REFERENCES
1.Rogenstein C, Kelly AM, Mason S, et al. An international view of how recent-onset atrial fibrillation is treated in the emergency department. Acad Emerg Med. 2012;19:1255–1260.
2.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 Guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for practice guidelines (writing committee to revise the 2001 guidelines for the management of patients with atrial fibrillation): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006;114:e257–e354.
3.Wann LS, Curtis AB, January CT, et al. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (updating the 2006 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:223–242.
4.Wyse DG, Waldo AL, DiMarco JP, et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347(23):1825–1833.
5.Van Gelder IC, Hagens VE, Bosker HA, et al. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347(23):1834–1840.
6.Hohnloser SH, Kuck KH, Lilienthal J. Rhythm or rate control in atrial fibrillation—Pharmacological Intervention in Atrial Fibrillation (PIAF): a randomised trial. Lancet. 2000;356(9244):1789–1794.
7.Carlsson J, Miketic S, Windeler J, et al. Randomized trial of rate-control versus rhythm-control in persistent atrial fibrillation: the Strategies of Treatment of Atrial Fibrillation (STAF) study. J Am Coll Cardiol. 2003;41(10):1690–1696.
8.Opolski G, Torbicki A, Kosior DA, et al. Rate control vs rhythm control in patients with nonvalvular persistent atrial fibrillation: the results of the Polish How to Treat Chronic Atrial Fibrillation (HOT CAFE) Study. Chest. 2004;126(2):476–486.
9.Corley SD, Epstein AE, DiMarco JP, et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation. 2004;109:1509–1513.
10.AFFIRM Investigators. Baseline characteristics of patients with atrial fibrillation: the AFFIRM Study. Am Heart J. 2002;143(6):991–1001.
11.Stead LG, Vaidyanathan L. Rhythm control with electrical cardioversion for atrial fibrillation and flutter. Ann Emerg Med. 2009;54:745–747.
12.Burton JH, Vinson DR, Drummond K, et al. Electrical cardioversion of emergence department patients with atrial fibrillation. Ann Emerg Med. 2004;44:20–30.
13.Decker WW, Smars PA, Vaidyanathan L, et al. A prospective, randomized trial of an emergency department observation unit for acute onset atrial fibrillation. Ann Emerg Med. 2008;52:322–328.
14.von Besser K, Mills AM. Is discharge to home after emergency department cardioversion safe for the treatment of recent-onset atrial fibrillation? Ann Emerg Med. 2011;58:517–520.
15.Stiell IG, Clement CM, Perry JJ, et al. Association of the Ottawa Aggressive Protocol with rapid discharge of emergency department patients with recent-onset atrial fibrillation or flutter. CJEM. 2010;12:181–191.
16.Decker WW, Stead LG. Selecting rate control for recent-onset atrial fibrillation. Ann Emerg Med. 2011;57:32–33.
17.Vinson DR, Hoehn T, et al. Managing emergency department patients with recent-onset atrial fibrillation. J Emerg Med. 2012;42:139–148.
18.Van Gelder IC, Haegeli LM, Brandes A, et al. Rationale and current perspective for early rhythm control therapy in atrial fibrillation. Europace. 2011;13:1517–1525.
19.Cosio FG, Aliot E, Botto GL, et al. Delayed rhythm control of atrial fibrillation may be a cause of failure to prevent recurrences: reasons for change to active antiarrhythmic treatment at the time of the first detected episode. Europace. 2008;10:21–27.
20.Stiell IG, Birnie D. Managing recent-onset atrial fibrillation in the emergency department. Ann Emerg Med. 2011;57:31–32.
21.Neumar RW, et al. Part 8: Adult Advanced Cardiovascular Life Support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S729–S767.
22.Field JM, Hazinski MF, Sayre MR, et al. Part 1: Executive Summary : 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122:S640–S656.
23.Domanovits H, Laske H, Stark G, et al. Adenosine for the management of patients with tachycardias—A new protocol. Eur Heart J. 1994;15:589–593.
24.Camaiti A, Pieralli F, Olivotto I, et al. Prospective evaluation of adenosine-induced proarrhythmia in the emergency room. Eur J Emerg Med. 2001;8:99–105.
25.Griffith MJ, Linker NJ, Ward DE, et al. Adenosine in the diagnosis of broad complex tachycardia. Lancet. 1988;1:672–675.
26.Sharma AD, Klein GJ, Yee R. Intravenous adenosine triphosphate during wide QRS complex tachycardia: safety, therapeutic efficacy, and diagnostic utility. Am J Med. 1990;88:337–343.
27.Lerman BB, Belardinelli L, West A, et al. Adenosine-sensitive ventricular tachycardia: evidence suggesting cyclic AMP-mediated triggered activity. Circulation. 1986;74:270–280.
28.Wilber DJ, Baerman J, Olshansky B, et al. Adenosine-sensitive ventricular tachycardia. Clinical characteristics and response to catheter ablation. Circulation. 1993;87:126–134.
29.Ng KS, Wen MS, Yeh SJ, et al. The effects of adenosine on idiopathic ventricular tachycardia. Am J Cardiol. 1994;74:195–197.
30.Hina K, Kusachi S, Takaishi A, et al. Effects of adenosine triphosphate on wide QRS tachycardia. Analysis in 18 patients. Jpn Heart J. 1996;37:463–470.
31.Mallet ML. Proarrhythmic effects of adenosine: a review of the literature. Emerg Med J. 2004;21:408–410.
32.Pelleg A, Pennock RS, Kutalek SP. Proarrhythmic effects of adenosine: one decade of clinical data. Am J Ther. 2002;9:141–147.
33.Tan HL, Spekhorst HHM, Peters RJG, et al. Adenosine induced ventricular arrhythmias in the emergency room. Pacing Clin Electrophysiol. 2001;24:450–455.
34.Parham WA, Mehdirad AA, Biermann KM, et al. Case report: adenosine induced ventricular fibrillation in a patient with stable ventricular tachycardia. J Interv Card Electrophysiol. 2001;5:71–74.
35.Garratt CJ, Griffith MJ, O'Nunain S, et al. Effects of intravenous adenosine on antegrade refractoriness of accessory atrioventricular connections. Circulation. 1991;84:1962–1968.
36.Exner DV, Muzyka T, Gillis AM. Proarrhythmia in patients with the Wolff-Parkinson-White syndrome after standard doses of intravenous adenosine. Ann Intern Med. 1995;122:351–352.
37.Shah CP, Gupta AK, Thakur RK, et al. Adenosine-induced ventricular fibrillation. Indian Heart J. 2001;53:208–210.
38.Gupta AK, Shah CP, Maheshwari A, et al. Adenosine induced ventricular fibrillation in Wolff-Parkinson-White syndrome. Pacing Clin Electrophysiol. 2002;25:477–480.
39.Marill KA, Wolfram S, Desouza IS, et al. Adenosine for wide-complex tachycardia: efficacy and safety. Crit Care Med. 2009;37:2512–2518.
40.Rankin AC, Oldroyd KG, Chong E, et al. Value and limitations of adenosine in the diagnosis and treatment of narrow and broad complex tachycardias. Br Heart J. 1989;62:195–203.
41.Armengol RE, Graff J, Baerman JM, et al. Lack of effectiveness of lidocaine for sustained, wide QRS complex tachycardia. Ann Emerg Med. 1989;18:254–257.
42.Ho DS, Zecchin RP, Richards DA, et al. Double-blind trial of lignocaine versus sotalol for acute termination of spontaneous sustained ventricular tachycardia. Lancet. 1994;344:18–23.
43.Marill KA, Greenberg GM, Kay D, et al. Analysis of the treatment of spontaneous sustained stable ventricular tachycardia. Acad Emerg Med. 1997;4:1122–1128.
44.Gorgels AP, van den Dool A, Hofs A, et al. Comparison of procainamide and lidocaine in terminating sustained monomorphic ventricular tachycardia. Am J Cardiol. 1996;78:43–46.
45.Somberg JC, Bailin SJ, Haffajee CI, et al. Intravenous lidocaine versus intravenous amiodarone (in a new aqueous formulation) for incessant ventricular tachycardia. Am J Cardiol. 2002;90:853–859.
46.Callans DJ, Marchlinski FE. Dissociation of termination and prevention of inducibility of sustained ventricular tachycardia with infusion of procainamide: evidence for distinct mechanisms. J Am Coll Cardiol. 1992;19:111–117.
47.Levine JH, Massumi A, Scheinman MM, et al. Intravenous amiodarone for recurrent sustained hypotensive ventricular tachyarrhythmias. J Am Coll Cardiol. 1996;27:67–75.
48.Helmy I, Herre JM, Gee G, et al. Use of intravenous amiodarone for emergency treatment of life-threatening ventricular arrhythmias. J Am Coll Cardiol. 1988;12:1015–1022.
49.Schutzenberger W, Leisch F, Kerschner K, et al. Clinical efficacy of intravenous amiodarone in the short term treatment of recurrent sustained ventricular tachycardia and ventricular fibrillation. Br Heart J. 1989;62:367–371.
50.Tomlinson DR, Cherian P, Betts TR, et al. Intravenous amiodarone for the pharmacological termination of haemodynamically-tolerated sustained ventricular tachycardia: is bolus dose amiodarone an appropriate first-line treatment? Emerg Med J. 2008;25:15–18.
51.Marill KA, deSouza IS, Nishijima DK, et al. Amiodarone or procainamide for the termination of sustained stable ventricular tachycardia: an historical multicenter comparison. Acad Emerg Med. 2010;17:297–306.
52.Lown B, Temte JV, Arter WJ. Ventricular tachyarrhythmias. Clinical aspects. Circulation. 1973;47:1364–1381.
53.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–2870.


