
FIGURE 18.1 Pulsatile pump and continuous-flow pump. A: Pulsatile (HeartMate XVE, left) and continuous flow (HeartMate II, right) B: Internal mechanics of HeartMate II. Reprinted with the permission of Thoratec Corporation.
BACKGROUND
Heart failure affects over 6 million people in the United States and accounts for 1 million hospital admissions annually.1,2 Despite this prevalence, the number of hearts transplanted annually in the United States has remained fixed over the past decade at approximately 2,000 per year.3 In response to the disparity between need for transplantation and organ availability, in 1994, the U.S. Food and Drug Administration approved the use of left ventricular assist devices (LVADs) for patients awaiting heart transplantation and more recently for long-term support (i.e., destination therapy) in 2010. Although left ventricular (LV) assist technology to provide mechanical circulatory assistance for the failing heart has existed since 1963, it has only been in the last decade, with the advent of a continuous-flow pump, that these devices have become capable of providing reliable long-term support.4,5 Based on data from the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS), the number of LVADs implanted increased nearly sixfold from 276 in 2006 to an estimated 1,600 patients in 2011.6 Among the 5,407 patients with LVADs registered in INTERMACS, approximately ¾ had devices placed as a bridge to transplant, and ¼ had them placed as a destination therapy.6 As the number of patients with LVADs continues to rise and as their survival improves, the emergency physician will increasingly be tasked with their acute care. This review focuses on a single LVAD system, the HeartMate II (Thoratec, Pleasanton, CA); this is the most commonly-installed assist device worldwide, and knowledge of this system is applicable to the management of other continuous-flow systems. This chapter addresses (1) the evolution of the LVAD, (2) the management of LVAD-associated complications, and (3) the use of radiographic imaging in diagnosing these complications.
EVOLUTION AND GENERAL FUNCTION OF LEFT VENTRICULAR ASSIST DEVICES
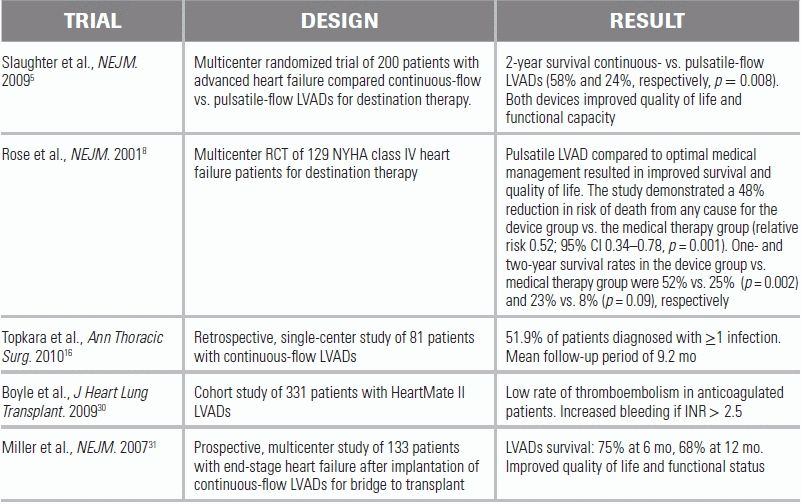
The two main groups of LVADs are distinguished by pump type: pulsatile and continuous flow (Fig. 18.1). Pulsatile pumps are analogous to the heart: a pumping chamber, once filled, activates a pusher plate technology.7 Newer continuous-flow pumps utilize a valveless system, in which centrifugal or axial pumping propels blood forward (Fig. 18.1B). Compared to the pulsatile system, continuous-flow pumps are smaller, lighter (0.75 vs. 2.6 pounds), and quieter. The Randomized Evaluation of Mechanical Assistance for Congestive Heart Failure (REMATCH) trial demonstrated the pulsatile LVAD HeartMate XVE superior to medical therapy.8 Patients with the HeartMate XVE had a 1-year survival rate of 52% and a 2-year survival rate of 23%; patients with medical therapy had a 1-year survival of 25% and a 2-year survival rate of 8%.8 A follow-up trial compared the HeartMate XVE to the continuous-flow HeartMate II for use as destination therapy.5 Patients with the pulsatile-flow pump had a 1-year survival rate of 55% and a 2-year survival rate of 24%; patients with the continuous-flow pump had a 1-year survival rate of 68% and a 2-year survival rate of 58%. Continuous-flow devices outperformed pulsatile pumps in rates of rehospitalization, pump replacement, and LVAD- and non-LVAD–related infections.5 Adverse events associated with continuous-flow devices included hemorrhagic stroke (9%), right heart failure (5%), sepsis (4%), and bleeding (3%); the rate of these complications was, however, not significantly different from that of pulsatile-flow devices.5 This trial highlighted the primary advantages of continuous-flow over pulsatile devices, namely, improved reliability and decreased pump wear, a lighter weight and less cumbersome design, and lower infection risk.9 Newer second-generation LVADs include the HeartWare system (HeartWare, Framingham, MA, approved for bridge to transplant); this smaller “wearless” device is implantable within the pericardium, suspended by a passive magnet and a hydrodynamic thrust-bearing system.10

FIGURE 18.1 Pulsatile pump and continuous-flow pump. A: Pulsatile (HeartMate XVE, left) and continuous flow (HeartMate II, right) B: Internal mechanics of HeartMate II. Reprinted with the permission of Thoratec Corporation.
PUMP PARAMETERS
All LVAD flow parameters are set at the time of implantation; for the emergency physician needing to diagnose and manage acute illness and device-related complications in LVAD users, understanding the parameters displayed and their significance is essential. The HeartMate II control monitor displays the following parameters: pump speed (revolutions per minute [RPM]), pump power (watts [W]), flow estimate (liters per minute [LPM]), and pulse index (dimensionless value). Commonly, clinicians inexperienced with LVAD management will make decisions based on single parameters (e.g., decreased flow), failing to understand the significance of this parameter in the context of an acute change in condition. Instead, when troubleshooting a patient with an LVAD, clinicians should gather data from the whole patient, assessing volume status, presence of arrhythmias, mean arterial pressure (MAP), date of LVAD placement, recent echocardiography results, pump parameters, and history of LVAD alarms (e.g., suction events). Deviation from a functional baseline is more significant than the specific value of each parameter. Acquisition of additional hemodynamic data often requires use of echocardiography and pulmonary artery catheters (PACs).
Pump speed is a fixed value set intraoperatively and often reassessed prior to hospital discharge in a process known as a “ramp study.” This involves empirically adjusting pump speed under echocardiographic guidance to determine the patient's optimal LV cavity size and output at a given speed. Pump speed governs flow through the device and is a measure of assistance provided to the patient. Only an experienced VAD clinician should adjust pump speed, and always under echocardiographic guidance. Excessive pump speeds may be associated with ventricular arrhythmias.
The HeartMate II controller directly measures the amount of power delivered to maintain pump speed. This parameter is analogous to myocardial workload in normal individuals. An increase in speed, preload, or afterload will increase power consumption. In the absence of these conditions, a gradual increase in power use may indicate the formation of clot on the rotor (see Thrombotic Complications). Conversely, a decrease in afterload, preload, or speed as well as a blockage of inflow or outflow cannula will decrease power consumption. There is no generalizable power level, as it can vary from patient to patient; rather, it is the change (>2 W) from a previous level that may indicate a change in device or patient status.
The flow on the HeartMate II is derived from power and speed. Flow is not directly measured but rather is an estimate of the amount of fluid passing through the pump, assuming normal pump function. Using an ultrasonic probe, one study evaluated the differences between the “flow estimate,” as reported on the HeartMate II control monitor, and the “absolute flow” measured by the probe. The study showed that at a flow of 4 to 6 LPM, there was a variable 15% to 20% difference between the estimated and absolute flow values.11 Several factors affect “absolute flow” for continuous pumps; these include preload (LV preload and right ventricular [RV] function), speed, and afterload (the difference between outlet cannula and inlet cannula pressure).9 Thus, hypervolemia, increased LV contractility, increased speed, and decreased pressure difference across the pump will increase flow. As mentioned, situations such as a clot on the rotor will cause power to increase, resulting in an erroneously high flow displayed as “+++.” Flows displayed as “+++” or “−−−” (for high and low flows, respectively) are considered outside the range of the expected physiologic limits based on speed.9 The HeartMate II low-flow alarm will signal when flow is <2.5 LPM. It is important to recognize that “flow estimate” is not analogous to cardiac output or “absolute flow” through the LVAD and thus should be used as a trended, directional value rather than a diagnostic tool to be used alone and without other patient and LVAD values.11
Pulse index refers to the amount of flow that passes through the pump during a cardiac cycle as averaged over 15 seconds. It is calculated as [(flow max – flow minimum)/flow average] × 10.9 It is a dimensionless value that is derived from the LVAD estimated flow. The degree of LVAD support is the primary variable that correlates with pulse index, and the two are inversely related. During LV systole, the flow through the pump increases due to an increased pressure at the pump inlet approximating the pressure at the outlet cannula (aortic pressure). During cardiac diastole, this inlet pressure drops, while the outlet pressure remains high (increased pressure difference) and, consequently, flow decreases. Therefore, pulse index is directly proportional to LV contractility (increases in which are due to preload, inotropic support, and myocardial recovery) and inversely proportional to the assistance provided by the pump.
ADVERSE EVENTS AND COMPLICATIONS
Common LVAD-related complications include hemorrhage, arrhythmias, infections, hemodynamic instability, and thrombosis (Table 18.1).5,13 When any of these are encountered in an LVAD-supported patient, a multidisciplinary approach to management is required; the patient's cardiologist and/or VAD coordinator should be contacted to discuss the plan of care. If hospitalization is indicated, then the patient should be transferred to a VAD center when stable for transport.
TABLE 18.1 Diagnosis and Management of Adverse Events and Complications
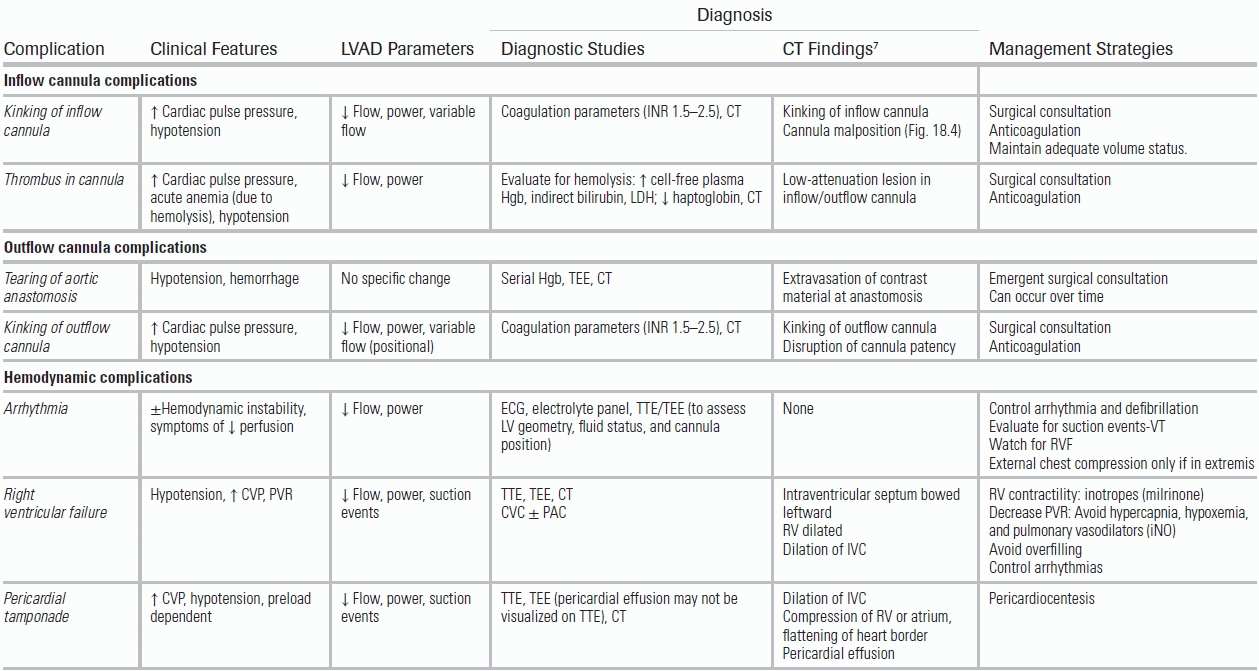
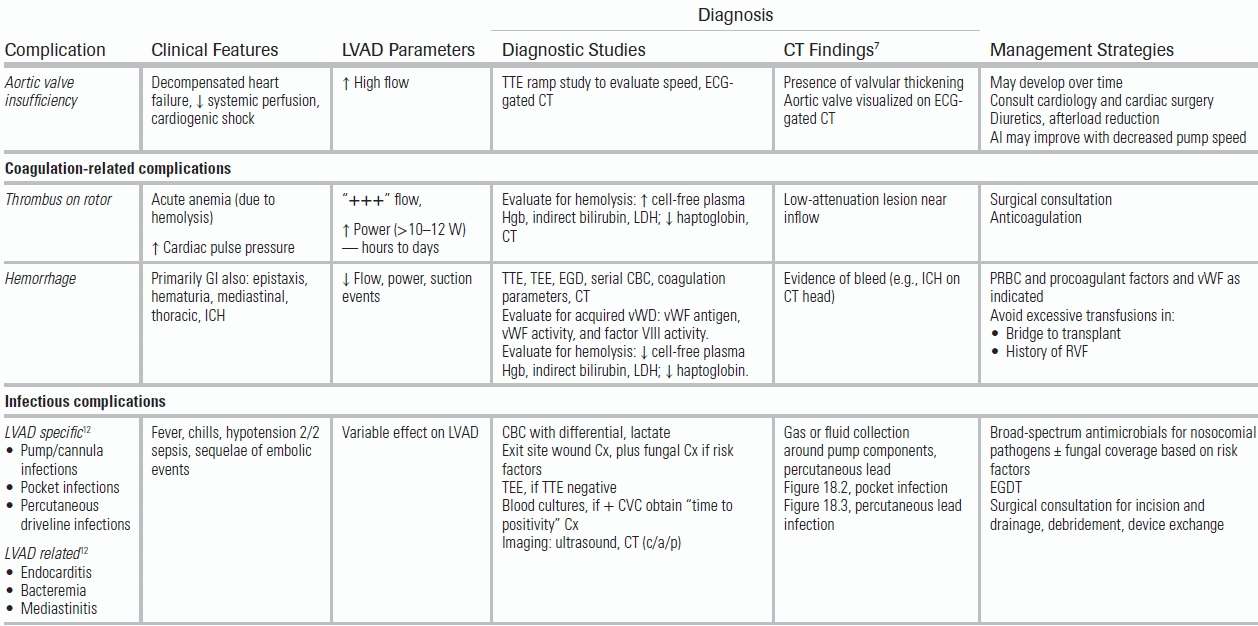
CVC, central venous catheter; CVP, central venous pressure; Cx, culture; EGDT, early goal-directed therapy; GI, gastrointestinal; Hgb, hemoglobin; ICH, intracranial hemorrhage; LDH, lactate dehydrogenase; PAC, pulmonary artery catheter; PVR, pulmonary vascular resistance; RV, right ventricle; RVF, right ventricular failure; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram; vWD, von Willebrand disease; vWF, von Willebrand factor; W, watts.
Infections are common in LVAD patients and are a leading cause of hospital readmission and mortality.5,13–17 Based on an analysis of 2,006 patients registered in the INTERMACS database, nearly 19% of patients will develop a percutaneous site infection within 1 year.14 The percutaneous lead acts as a portal of entry for pathogens, which can progress to the subcutaneous tunnel, pump pocket, device, heart (i.e., endocarditis), and bloodstream (Figs. 18.2 and 18.3). Recommendations for the evaluation of an LVAD patient with suspected infection are outlined in Table 18.1. Patients with suspected LVAD-related infections should be treated with empiric broad-spectrum antimicrobials to cover nosocomial pathogens, including Methicillin-resistant Staphylococcus aureus (MRSA) and Pseudomonas aeruginosa.16,18 Fungemia has also been reported in LVAD-supported patients.19 Surgical consultation should be obtained, as incision and drainage, debridement, and/or percutaneous lead revision may be required.16 The ongoing development of LVADs that do not require a percutaneous lead should reduce the risk of these infections.
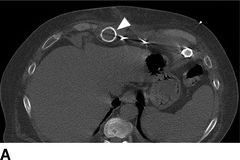

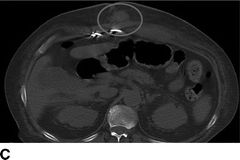

FIGURE 18.2 Computed tomography images of pump pocket infection. A: Gas bubble (arrows) in outflow cannula. B: Gas bubbles in pocket space surrounding LVAD pump. C: Hyperdense area inferior to pump pocket (oval). D: Sagittal view of pump pocket infection; gas bubbles can be seen in outflow cannula and pump pocket.
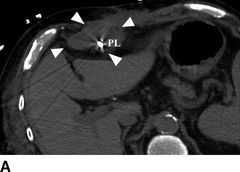
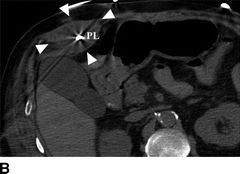

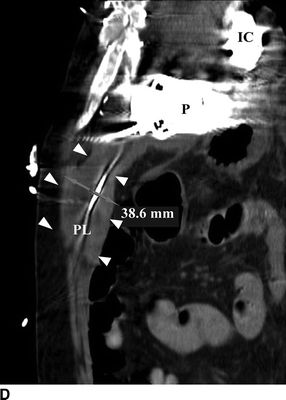
FIGURE 18.3 Computed tomography images of percutaneous lead infection. A, B: Hyperattenuated area surrounding percutaneous lead (PL), borders marked by arrows. C: Local erythema at exit site of PL. D:Sagittal view of hyperattenuated area surrounding PL (diameter: 38.6 mm). IC, inflow cannula; P, pump; PL, percutaneous lead.
Continuous-flow pumps unload the LV throughout the cardiac cycle, resulting in a diminished or absent pulse pressure.20 Thus, noninvasive measurement of blood pressure and pulse oximetry are often unreliable.9 Blood pressure is best measured as a mean arterial pressure (MAP) obtained by Doppler and sphygmomanometer or, alternatively, by placement of an arterial catheter. Goal MAP in most LVAD patients is 70 to 80 mm Hg. In general, MAP should not exceed 90 mm Hg; LVAD patients are sensitive to increases in afterload, and higher blood pressures increase their risk for adverse neurological events.9 For the hypertensive LVAD patient, beta-blockers and angiotensin-converting enzyme inhibitors are generally used for blood pressure control. For the hypotensive patient, the etiology should be identified (hypovolemic, vasodilatory, or cardiogenic shock due to RV or LV failure) and treated accordingly with volume repletion, vasopressor, and/or inotropic medications. Echocardiography and pulmonary artery catheterization may provide valuable data for diagnosis and management. Additionally, problems intrinsic to the device, such as oversuctioning, may contribute to diminished blood flow and should be considered when evaluating the hypotensive LVAD patient.
Right ventricular failure (RVF) is one of the more dreaded etiologies of hypotension after LVAD placement. RVF is estimated to occur in up to 20% of LVAD patients21 and is associated with a 1-year mortality of 83%.22 While this condition is usually recognized in the immediate perioperative period, the emergency physician may have to contend with the management of LVAD patients with RV dysfunction. Because of the complexity of managing an LVAD patient with RVF, early consultation with a VAD specialist or a cardiologist is recommended, as the patient may require more advanced mechanical circulatory assistance. Causes of RVF specific to LVADs include (1) leftward bowing of the intraventricular septum due to LVAD-related LV emptying, reducing its capacity to participate in RV contractility, and (2) increased venous return from the LVAD, outmatching the capacity of a failing right heart.23 Table 18.1 details the management strategy for patients with RVF, including transfer to a critical care unit for placement of a pulmonary artery catheter (PAC) and/or transesophageal echocardiography. Among inotropes, milrinone is particularly beneficial because it decreases pulmonary vascular resistance (PVR) and improves RV contractility and matching of RV and LVAD outputs.24 Additional therapies for RVF include pulmonary vasodilators, such as inhaled nitric oxide (iNO) and/or aerosolized prostacyclin, that lower PVR. Avoidance of conditions that aggravate pulmonary vasoconstriction, such as hypercapnia and hypoxemia, is also essential. In a randomized trial of 11 patients with increased PVR after LVAD placement, iNO significantly reduced PVR and increased LVAD flow in patients with pulmonary hypertension.25 Avoidance of excessive preload is also important in the management of patients with RVF; thus, particular caution should be paid to the LVAD patient with a history of RVF who presents to the emergency department with hemorrhagic shock and who may require large-volume blood and factor transfusions. Patients who do not respond to these medical therapies for RVF (e.g., iNO, inotropes) may require more advanced mechanical circulatory support including the placement of a right ventricular assist device (RVAD). Risk factors that predict the need for an RVAD placement include female gender, low right ventricular stroke work index, history of pulmonary hypertension, and intraoperative high central venous pressure.21,22,26,27
A suction event occurs when there is excessive LV unloading due to a pump speed that is too high relative to LV volume. Suction events may manifest as a decrease in pump flow, arrhythmias, or transient and intermittent decreases in the pump speed to the low-speed limit (a result of LVAD auto-correction). During a suction event, echocardiography will demonstrate a leftward shift of the intraventricular septum with an underfilled LV. Other potential causes of poor LV filling include hypovolemia, RVF, pulmonary hypertension, and malposition of the inflow cannula toward the intraventricular septum or the lateral wall (Fig. 18.4). Suction events can be alleviated by volume repletion or by decreasing the pump speed, tasks best performed by an LVAD specialist using echocardiographic guidance.

FIGURE 18.4 Inflow cannula malposition. CT images depicting malposition of the inflow cannula toward the posterior–lateral cardiac wall (arrows). Cannula orientation depicted by rectangle.
Patients with advanced heart failure have a high prevalence of cardiac arrhythmias both prior to and following LVAD placement.28 When patients develop new arrhythmias postimplantation, an underlying etiology, such as ischemia, suction events, or electrolyte imbalances, should be excluded. Although the LVAD continues to unload the LV during arrhythmic events, the right heart is unsupported and is at risk of acute dysfunction. The management of most arrhythmias in LVAD patients is similar to that of patients with advanced heart failure.
Medications used for rate and rhythm control in patients with advanced heart failure are also appropriate for LVAD-supported patients. In addition to impairing right heart function (resulting in decreased LVAD preload), atrial fibrillation increases the risk of thromboembolism. Due to the risk of embolic events, which may occur peripherally or within the LVAD pump itself, warfarin is typically dosed to achieve a target INR of 2 to 2.5.29
Because the LVAD continues to function during otherwise life-threatening arrhythmias, patients with these arrhythmias may present with hemodynamic stability. However, the unsupported RV is at high risk of failure, placing the patient at risk of cardiogenic shock or sudden cardiac death. All ventricular arrhythmias require immediate treatment. Beta-blockers and other antiarrhythmic medications may be beneficial; however, defibrillation is indicated in patients with persistent ventricular arrhythmias or arrhythmia-induced hypotension. Given the high prevalence of ventricular arrhythmias in end-stage heart failure patients, the majority of LVAD patients will have implanted cardiac defibrillators (ICDs). Patients who do not have ICDs should be considered for this intervention.
External chest compressions may disrupt the aortic anastomosis or LVAD inflow tract and are generally contraindicated, particularly shortly after implant when the sternum has not healed; however, they may be helpful in patients in extremis. Prior to mediastinal wound healing, direct cardiac massage by a qualified surgeon may be effective in patients with recent device implantation. External defibrillation should be performed with the pump running and the system controller connected to the percutaneous lead. The system controller should be disconnected only if open-chest defibrillation is required. All drugs routinely given per advanced cardiac life support (ACLS) protocols may be administered.
The routine use of systemic anticoagulation and antiplatelet therapy has resulted in a low incidence of device thrombosis and thromboembolism.30–33 Current guidelines recommend administering warfarin with a target INR of 1.5 to 2.5 alongside daily aspirin. In a study of 331 HeartMate II patients treated with warfarin and antiplatelet therapy, ischemic strokes occurred in 2.4% of patients and pump thrombosis in 0.9%.30 The risk of thrombosis was highest when the INR was <1.5. Hemorrhagic complications, including hemorrhagic stroke or blood loss requiring transfusions of ≥2 units PRBC or surgical intervention, were more common than thrombotic events (2.1%, 15.4%, and 1.2%, respectively) particularly in patients with an INR >2.5. Pump thrombus is a rare but serious complication, as it may result in obstruction of blood flow. Gradual increases in pump power (often >10 to 12 W) occurring over hours to days may herald a thrombus in contact with the rotor or bearings.9 Thrombus may be initially noted as unexplained hemolysis (see Hemolysis).
Acute anemia in an LVAD patient may be due to hemorrhage caused by anticoagulation and/or acquired coagulopathies and hemolysis. In some cases, hemorrhage may be severe enough to require blood transfusions or surgery.5 The gastrointestinal (GI) tract is a particularly common source of bleeding, and in a retrospective study of 154 LVAD patients, GI bleeds were most often due to peptic ulcer disease and vascular malformations.34 Esophagogastroduodenoscopy (EGD) is a reasonable first diagnostic study in most LVAD patients with a suspected upper GI bleed, as it is well tolerated, diagnostic, and therapeutic. Bleeding may also manifest as epistaxis, hematuria, or mediastinal, thoracic, or intracranial hemorrhage. Depending on the severity and location of the hemorrhage, anticoagulants should be reduced or withheld. Patients should be transfused as clinically indicated with packed red blood cells and procoagulant factors. Unnecessary or excessive transfusions in candidates for heart transplantation should be avoided, as there is an increased risk of graft rejection due to transfusion-related allosensitization. As previously discussed, invasive monitoring (e.g., PAC) may be useful to avoid exacerbating RV dysfunction in patients requiring large-volume transfusions.
In addition to bleeding due to therapeutic anticoagulation, other derangements in hemostasis are associated with LVADs. Acquired von Willebrand disease (vWD) may occur following continuous-flow LVAD placement.35 A postulated mechanism involves excessive cleavage of high molecular weight von Willebrand factor (vWF) multimers by continuous-flow, pump–related shear stress forces,36 a process analogous to the acquired vWD associated with severe aortic stenosis.37 At this time, no studies have clarified the potential benefits of vWF replacement therapy for LVAD patients with active hemorrhage. Generally, empiric use of desmopressin is both appropriate and efficacious in reducing hemorrhage with acquired vWD.
Acute anemia in the LVAD patient may also be the result of hemolysis. Hemolysis may be diagnosed by laboratory findings, including increased cell-free plasma hemoglobin, indirect bilirubin, and lactate dehydrogenase, and decreased haptoglobin. Although the incidence of hemolysis due to mechanical shearing from the pump is low (3%), it may be observed with pump-related thrombus.33,38 Thus, the observation of hemolysis should prompt a workup for a pump or cannula-related thrombus.
Aortic insufficiency (AI) adversely affects pump function by causing rapid LV filling and high pump flow.9 Studies have demonstrated the development of de novo AI, as well as the progression of AI to at least moderate severity, in up to 64% of patients within 18 months after HeartMate II implantation.39,40 The patient with clinically significant AI may present with decompensated heart failure, decreased systemic perfusion, cardiogenic shock, and high pump flow. Mild AI can often be managed with diuretics and afterload reduction. As long as systemic perfusion is not compromised, decreasing pump speed may reduce regurgitant flow across the valve. Moderate or severe symptomatic AI after LVAD implantation requires surgical repair, including bioprosthetic aortic valve replacement, coaptation of the aortic valve leaflets, or complete oversewing of the aortic valve outflow tract.41
RADIOGRAPHIC EVALUATION OF THE HEARTMATE II
For the emergency physician, radiographic imaging is one of the most accessible and useful tools for diagnosing potential problems in an LVAD patient (Figs. 18.5 and 18.6). Figure 18.6 depicts the typical contrast computed tomography (CT) appearance of the LVAD in situ. CT imaging can inform diagnoses both anatomic (e.g., pocket and percutaneous lead infections, Figs. 18.2 and 18.3, respectively) and, with ECG gating, dynamic (e.g., AI). The HeartMate II is implanted anterior to the rectus sheath, preperitoneally.9 The distal end is attached via an end-to-side anastomosis to the ascending aorta (Fig. 18.6A). Both the inflow and outflow cannula can be visualized using CT with intravenous contrast and should be patent without evidence of kinking or obstruction (Fig. 18.6B and C). The inflow cannula attaches to the LV apex (Fig. 18.6C). A bend relief allows the inflow cannula to attach to the pump in the upper abdomen pocket without kinking. Similarly, the outflow cannula attaches to the pump with a bend relief. The pump itself cannot be visualized on CT (Fig. 18.6D). Table 18.1 lists potential complications and their characteristic CT findings.
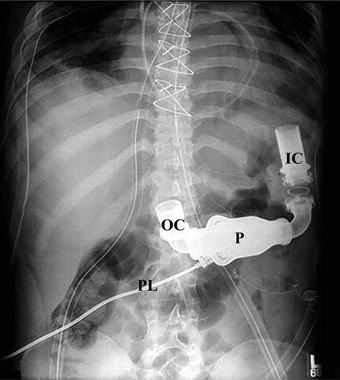
FIGURE 18.5 Plain radiograph of HeartMate II. IC, inflow cannula; OC, outflow cannula; P, pump; PL, percutaneous lead. Reprinted with the permission of Thoratec Corporation.
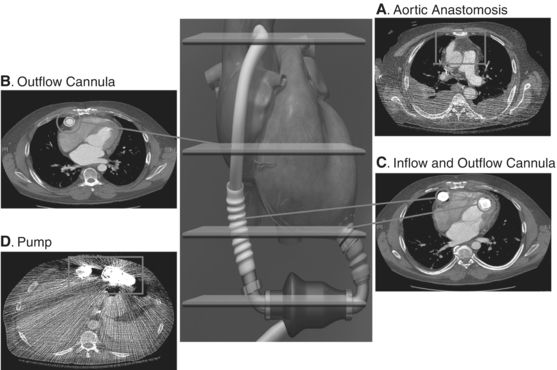
FIGURE 18.6 Computed tomography images of HeartMate II pump. Cross-sectional CT images of HeartMate II pump, in situ. A: Aortic anastomosis: end-to-side anastomosis of outflow cannula to ascending aorta. B: Outflow cannula: Cannula is patent without evidence of obstruction or kinking. C: Outflow and inflow cannula: Note position at ventricular apex oriented toward mitral valve. D: Pump itself cannot be visualized. HeartMate II graphic images reprinted with the permission of Thoratec Corporation.
CONCLUSION
As the prevalence of LVADs increases, more of these patients will present to the emergency department, challenging the emergency physician to recognize and manage the complications that can arise in this complex group of patients. An emergency medical service (EMS) LVAD guide, produced by the Mechanical Circulatory Support Organization, provides instructions and important facts for the emergency personnel (www.mylvad.com). For the HeartMate II, 24-hour clinical and technical support and manuals with information pertaining to routine operating procedures, alarms, and emergencies are available to clinicians via the Thoratec website (www.thoratec.com). A multidisciplinary approach including the patient's cardiologist, VAD coordinator, and, when indicated, cardiothoracic surgeon and/or infectious disease specialist, is necessary for successful clinical outcomes in this patient population.
LITERATURE TABLE
CI, confidence interval.
We would like to thank Dipanjan Banerjee, MD, MS; Greg Rosselini, MD; and Robert Smith, CCP, RN, for their careful review of the manuscript. We would also like to thank Dominik Fleischmann, MD for providing radiographic images used in the figures.
1.Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000–2010. NCHS Data Brief. 2012;108:1–8.
2.Rathi S, Deedwania PC. The epidemiology and pathophysiology of heart failure. Med Clin North Am. 2012;96:881–890.
3.Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. Am J Transplant. 2012;12 (suppl 1):1–156.
4.DeBakey ME. Left ventricular bypass pump for cardiac assistance. Clinical experience. Am J Cardiol. 1971;27:3–11.
5.Slaughter MS, Rogers JG, Milano CA, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251.
6.Kirklin J, Naftel DC, Myers SL, et al. INTERMACS Interagency Registry for Mechanically Assisted Circulatory Support Quarterly Statistical Report Implant dates: June 23, 2006–December 31, 2011. 2012:15.
7.Carr CM, Jacob J, Park SJ, et al. CT of left ventricular assist devices. Radiographics. 2010;30:429–444.
8.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443.
9.Slaughter MS, Pagani FD, Rogers JG, et al. Clinical management of continuous-flow left ventricular assist devices in advanced heart failure. J Heart Lung Transplant. 2010;29:S1–S39.
10.Slaughter MS, Sobieski MA II, Tamez D, et al. HeartWare miniature axial-flow ventricular assist device: design and initial feasibility test. Tex Heart Inst J. 2009;36:12–16.
11.Slaughter MS, Bartoli CR, Sobieski MA, et al. Intraoperative evaluation of the HeartMate II flow estimator. J Heart Lung Transplant. 2009;28:39–43.
12.Hannan MM, Husain S, Mattner F, et al. Working formulation for the standardization of definitions of infections in patients using ventricular assist devices. J Heart Lung Transplant. 2011;30:375–384.
13.Hasin T, Marmor Y, Kremers W, et al. Readmissions after implantation of axial flow left ventricular assist device. J Am Coll Cardiol. 2013;61:153–163.
14.Goldstein DJ, Naftel D, Holman W, et al. Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant. 2012;31:1151–1157.
15.Gordon RJ, Weinberg AD, Pagani FD, et al. Prospective, multicenter study of ventricular assist device infections. Circulation. 2013;127:691–702.
16.Topkara VK, Kondareddy S, Malik F, et al. Infectious complications in patients with left ventricular assist device: etiology and outcomes in the continuous-flow era. Ann Thorac Surg. 2010;90:1270–1277.
17.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10.
18.Maniar S, Kondareddy S, Topkara VK. Left ventricular assist device-related infections: past, present and future. Expert Rev Med Devices. 2011;8:627–634.
19.Bagdasarian NG, Malani AN, Pagani FD, et al. Fungemia associated with left ventricular assist device support. J Card Surg. 2009;24:763–765.
20.Myers TJ, Bolmers M, Gregoric ID, et al. Assessment of arterial blood pressure during support with an axial flow left ventricular assist device. J Heart Lung Transplant. 2009;28:423–427.
21.Dang NC, Topkara VK, Mercando M, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6.
22.Drakos SG, Janicki L, Horne BD, et al. Risk factors predictive of right ventricular failure after left ventricular assist device implantation. Am J Cardiol. 2010;105:1030–1035.
23.Haddad F, Couture P, Tousignant C, et al. The right ventricle in cardiac surgery, a perioperative perspective: II. Pathophysiology, clinical importance, and management. Anesth Analg. 2009;108:422–433.
24.Kihara S, Kawai A, Fukuda T, et al. Effects of milrinone for right ventricular failure after left ventricular assist device implantation. Heart Vessels. 2002;16:69–71.
25.Argenziano M, Choudhri AF, Moazami N, et al. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann Thorac Surg. 1998;65:340–345.
26.Fukamachi K, McCarthy PM, Smedira NG, et al. Preoperative risk factors for right ventricular failure after implantable left ventricular assist device insertion. Ann Thorac Surg. 1999;68:2181–2184.
27.Ochiai Y, McCarthy PM, Smedira NG, et al. Predictors of severe right ventricular failure after implantable left ventricular assist device insertion: analysis of 245 patients. Circulation. 2002;106:I198–I202.
28.Raasch H, Jensen BC, Chang PP, et al. Epidemiology, management, and outcomes of sustained ventricular arrhythmias after continuous-flow left ventricular assist device implantation. Am Heart J. 2012;164:373–378.
29.Boyle A. Arrhythmias in patients with ventricular assist devices. Curr Opin Cardiol. 2012;27:13–18.
30.Boyle AJ, Russell SD, Teuteberg JJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–887.
31.Miller LW, Pagani FD, Russell SD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896.
32.Struber M, Sander K, Lahpor J, et al. HeartMate II left ventricular assist device; early European experience. Eur J Cardiothorac Surg. 2008;34:289–294.
33.John R, Kamdar F, Liao K, et al. Improved survival and decreasing incidence of adverse events with the HeartMate II left ventricular assist device as bridge-to-transplant therapy. Ann Thorac Surg. 2008;86:1227–1234; discussion 1234–1225.
34.Kushnir VM, Sharma S, Ewald GA, et al. Evaluation of GI bleeding after implantation of left ventricular assist device. Gastrointest Endosc. 2012;75:973–979.
35.Crow S, Chen D, Milano C, et al. Acquired von Willebrand syndrome in continuous-flow ventricular assist device recipients. Ann Thorac Surg. 2010;90:1263–1269; discussion 1269.
36.Uriel N, Pak SW, Jorde UP, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213.
37.Vincentelli A, Susen S, Le Tourneau T, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349.
38.Bhamidipati CM, Ailawadi G, Bergin J, et al. Early thrombus in a HeartMate II left ventricular assist device: a potential cause of hemolysis and diagnostic dilemma. J Thorac Cardiovasc Surg. 2010;140:e7–e8.
39.Aggarwal A, Raghuvir R, Eryazici P, et al. The development of aortic insufficiency in continuous-flow left ventricular assist device-supported patients. Ann Thorac Surg. 2013;95:493–498.
40.Cowger J, Pagani FD, Haft JW, et al. The development of aortic insufficiency in left ventricular assist device-supported patients. Circ Heart Fail. 2010;3:668–674.
41.Park SJ, Liao KK, Segurola R, et al. Management of aortic insufficiency in patients with left ventricular assist devices: a simple coaptation stitch method (Park's stitch). J Thorac Cardiovasc Surg. 2004;127:264–266.