TABLE 20.1 Stroke Mimics and Their Features
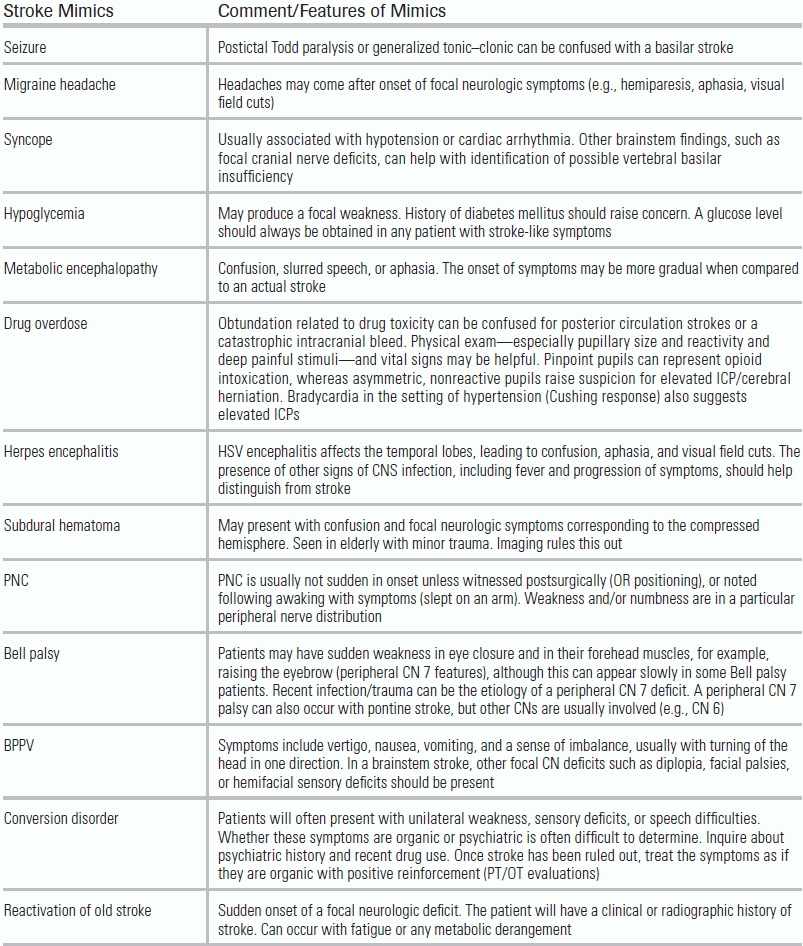
BPPV, benign paroxysmal positional vertigo; PNC, peripheral nerve compression; CN, cranial nerves ; HSV, herpes simplex virus; ICP, intracranial pressure; CPP, cerebral perfusion pressure.
BACKGROUND
Stroke is the fourth leading cause of mortality in the United States and incurs an estimated cost of 38.6 billion dollars anually.1 Although the overall mortality from this disease has declined over the last decade, 50% of individuals who suffer a stroke at an age of >65 will die within 5 years.1 Traditionally a clinical diagnosis, evaluation of stroke is now highly reliant on imaging. While new multimodality radiologic technologies are being developed to determine brain ischemia and penumbral tissue, the most important imaging for acute care remains the noncontrast computed tomography (CT) of the head. With this in mind, this chapter reviews both the pathogenesis and clinical manifestation of stroke as well as a basic approach to its image interpretation in the acute setting.
HISTORY AND PHYSICAL EXAM
Obtaining a quick and accurate history in patients with stroke facilitates optimal clinical care. It is essential to determine the exact time at which brain ischemia started. Because this can be a difficult task unless bystanders are present at the onset of symptoms, the practitioner should rely on the time of “last seen normal,” as opposed to when the patient was first witnessed having symptoms. For example, if a patient were to wake up with symptoms of a stroke, his or her time last seen normal would be the night before when he went to sleep. This information is required to determine a patient's eligibility to receive specific interventions, including intravenous tissue plasminogen activator (IV tPA) or endovascular intra-arterial (IA) thrombolysis/thrombectomy. In addition to the time of onset and duration of symptoms, delineating the progression of neurologic findings is also important. Most vascular events result in immediate deficits; exceptions to this include stuttering transient ischemic attacks and flow related symptoms caused by an intracranial or extracranial stenosis.
The emergency provider should also attempt to identify nonstroke conditions, referred to as “stroke mimics” that can produce focal neurologic deficits (Table 20.1).
TABLE 20.1 Stroke Mimics and Their Features

BPPV, benign paroxysmal positional vertigo; PNC, peripheral nerve compression; CN, cranial nerves ; HSV, herpes simplex virus; ICP, intracranial pressure; CPP, cerebral perfusion pressure.
A thorough history and exam can help distinguish brain infarction from stroke mimics, and, in the event of a true central ischemic process, help pinpoint the specific etiology of the neurologic insult. Stroke symptoms accompanied by severe chest pain radiating to neck are suggestive of a myocardial infarction with associated cardiac emboli. Stroke symptoms accompanied by severe chest and back pain can suggest an aortic dissection with extension into the carotid or the vertebral arteries. Such combinations of findings not only help identify a specific pathologic process but also may dramatically alter patient management by avoiding hemorrhagic complications of thrombolytic therapy in patients with specific contraindications, for example, an intracerebral tumor.
The diagnosis of stroke mimics can often be difficult during the acute phase. Studies show anywhere between 3% and 16% of patients treated with tPA are stroke mimics.2,3 Fortunately, multiple studies have reported stroke mimics treated with tPA to have no increased rate of symptomatic intracranial bleeds.3 The pathogenesis of intracranial hemorrhage after tPA is secondary to reperfusion hemorrhage into a region of infarcted brain tissue. Because stroke mimics do not have actual brain ischemia, they are less likely to have hemorrhage after tPA. The exception to this is patients with intracranial tumors, but these are typically visualized on a CT scan prior to IV tPA administration.
In addition to accompanying symptoms, the patient's past medical and surgical history needs to be quickly established, focusing on exclusion and inclusion criteria for administration of IV tPA. IV tPA is the current standard of care for patients who present within 3 hours of symptoms and is recommended in patients up to 4.5 hours if they meet the criteria (Table 20.2).4
TABLE 20.2 Pertinent History in Stroke Patients
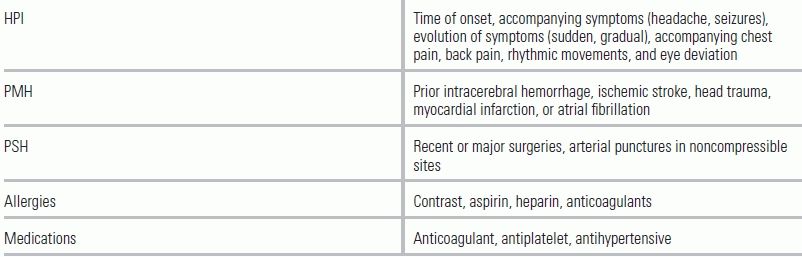
HPI, history of present illness; PMH, past medical history; PSH, past surgical history.
Assessment of the ABCs (airway, breathing, circulation) and vital signs may also provide clues as to the nature and cause of the stroke (whether hemorrhagic or ischemic). Tachycardia with an irregular heartbeat may support a cardioembolic ischemic stroke. Elevated blood pressure in the setting of headache, nausea/vomiting, and obtundation is more likely to be indicative of a hemorrhagic stroke, although posterior circulation strokes (e.g., a basilar artery occlusion) can present similarly. Once the ABCs are stabilized, a rapid neurologic examination using the National Institutes of Health Stroke Scale (NIHSS) should be obtained (Table 20.3).5,6
TABLE 20.3 NIHSS (National Institutes of Health Stroke Scale)
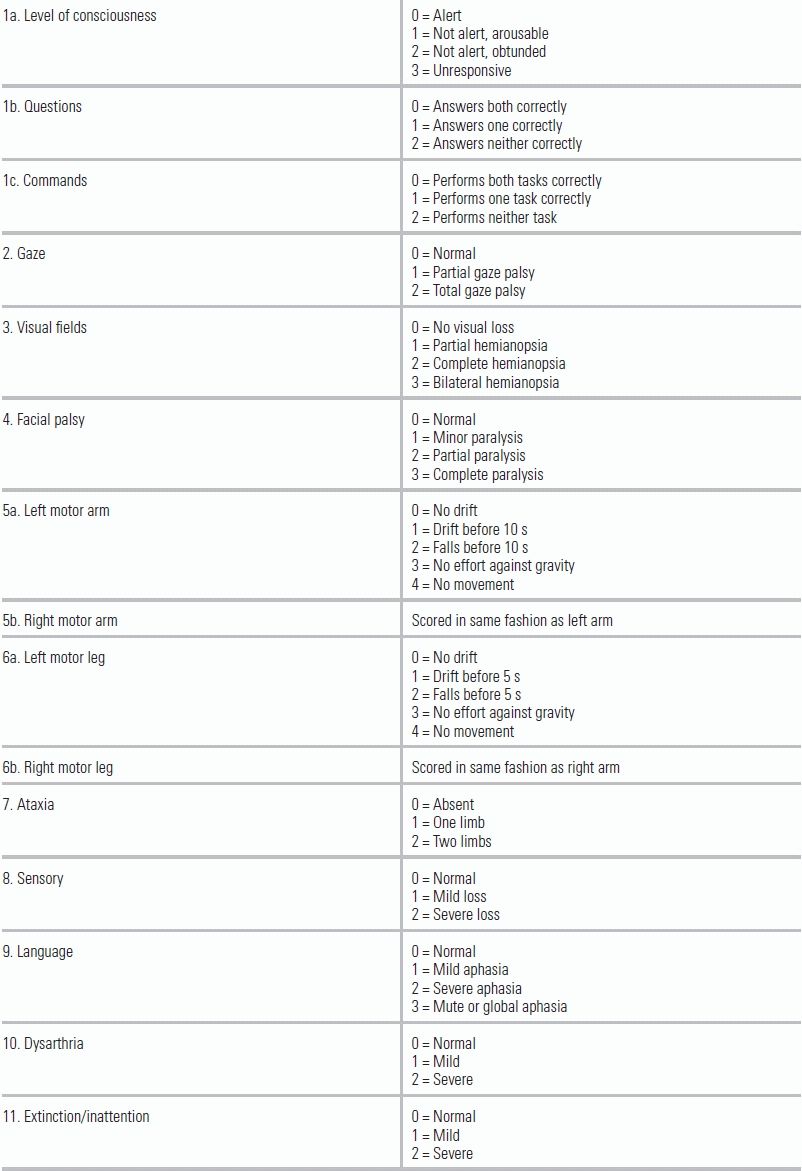
Available at www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf
The NIHSS is easy to perform, helps predict short-term and long-term outcomes, and can help identify large-vessel occlusion (LVO) strokes.7,8 The scale has been demonstrated to be reliable and reproducible, but proper use requires training and certification, which can be obtained through the American Stroke Association's Web site (www.strokeassociation.org).9,10
Common clinical syndromes ascribed to specific subtypes of stroke are listed in Table 20.4.11–13 While not an exhaustive list, familiarity with these signs and symptoms can help providers localize the region of ischemia. Stroke patients often present with dramatic findings, such as hemiparesis; in isolation, however, weakness can be representative of both large- and small-territory strokes. The presence of cortical signs, such as aphasia, visual field deficits, or a gradient in weakness (face and arm greater than leg involvement), suggests a LVO that may eventually require endovascular therapy. The presence of cranial nerve deficits or cerebellar findings, such as ataxia or dysmetria, may help localize a stroke to the brainstem or posterior fossa.14
TABLE 20.4 Common Signs and Symptoms by Type of Stroke
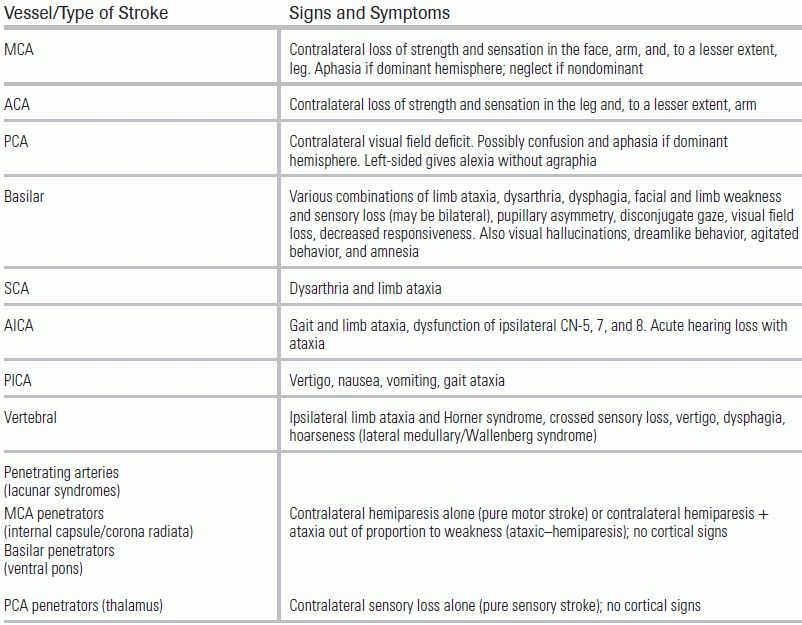
MCA, middle cerebral artery; ACA, anterior cerebral artery; PCA, posterior cerebral artery; SCA, superior cerebellar artery; AICA, anterior inferior cerebellar artery; PICA, posterior inferior cerebellar artery.
From Levine J, Johnston K. Diagnosis of stroke and stroke mimics in the emergency setting. Continuum Lifelong Learning Neurol. 2008; Jones HR, Srinivasan J, Allam GJ, et al. Netter's Neurology. W. B. Saunders Co; 2011; Uchino K, Pary J, Grotta J. Acute Stroke Care. 2011.
PATHOGENESIS
Ischemic stroke is commonly classified according to the following subtypes: small-vessel atherosclerosis, large-artery atherosclerosis, cardioembolic, cryptogenic, or other.15 Other known causes include arterial dissections, infections, trauma, sickle cell disease, and hypercoagulable states. A patient's particular diagnostic course will depend in part on his or her history of illness and clinical presentation. For example, a patient who presents with heart palpitations followed by stroke symptoms will require a cardiac evaluation for arrhythmias; whereas a patient with stroke symptoms who has sustained neck trauma would need vascular imaging of the chest, neck, and head to identify potential arterial dissections.
DIAGNOSTIC EVALUATION
The initial ED diagnostic workup should consist of a focused history, exam, labs, and imaging. Per the American Heart Association/American Stroke Association (AHA/ASA) guidelines, only a noncontrast CT of the head is required, even though advanced imaging, if available, may help delineate the stroke.4 The rationale for this recommendation is that a noncontrast head CT is sufficient to determine a patient's eligibility for IV tPA, provided that clinical criteria are already met. Although other imaging such as CT angiogram or MRI may ultimately be required, the decision to give tPA should be made immediately following noncontrast CT so as to avoid delays that can lessen benefit from IV tPA (Table 20.5).
TABLE 20.5 Diagnostic Approach by Time Line
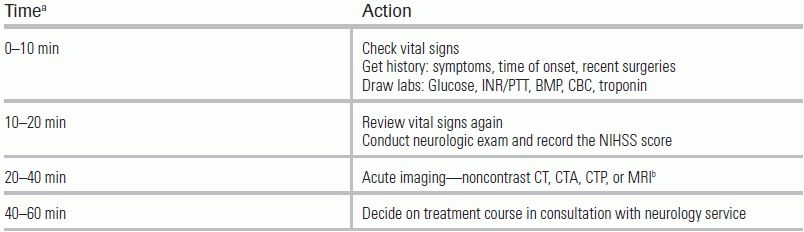
aThis is a relative time scale and defines the maximum allotted time to complete each step.
bImaging will differ depending on the institution. Only CT of the head is required.
Recommended initial orders and labs include (per AHA/ASA guidelines)
The noncontrast CT allows a radiologist to distinguish hemorrhagic from ischemic stroke; the relative acuity of an ischemic stroke; the presence of mass effect or imminent midline shift that would necessitate more aggressive treatment; and the degree of brain parenchyma that is unsalvageable. In patients with stroke onset >3 hours, a hypodensity of greater than or equal to one-third of middle cerebral artery (MCA) territory excludes use of tPA. For ischemic stroke, the emergency physician should be aware of the following radiographic findings indicative of a stroke (Fig. 20.1); these findings may sway the decision to administer IV tPA based on the presumed degree of infarcted brain tissue:
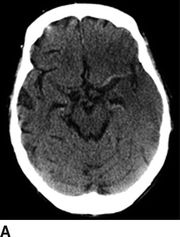
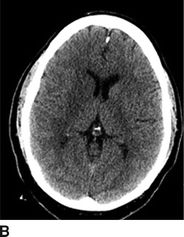
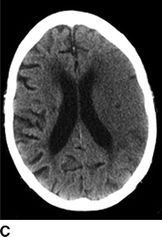
FIGURE 20.1 A: A hyperdense left MCA with blurring of the insular ribbon and sulcal effacement of the left temporal lobe. B: Blurring of the gray–white junction involving the right caudate, internal capsule, and putamen. C: Sulcal effacement and blurring of the gray–white junction over the entire left MCA territory.
In addition to the subtle findings above, one should evaluate for midline shift, masses/mass effect, blood (in the brain or at the base of the skull), cerebral edema, or herniation. A frequently used tool for the evaluation of ischemic stroke on a head CT is the Alberta Stroke Program Early CT Score (ASPECTS).16 The ASPECTS tool evaluates 10 commonly viewed areas of the MCA territory. Every area of hypodensity is subtracted from 10; a lower composite score indicates more areas of infarcted brain. This tool can be used to evaluate functional outcome (a score of 7 or less associated with poor functional outcome) as well as to estimate the size of any MCA stroke (Fig. 20.2).
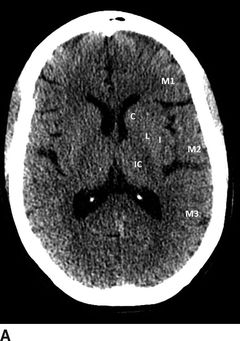
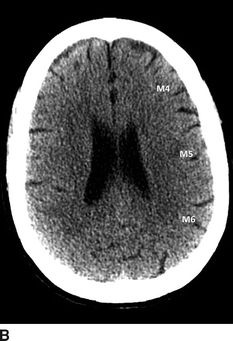
FIGURE 20.2 ASPECTS stroke regions (10 regions assessed). (A) Lower cross-sectional region of interest with deep structures. (B) Higher cross-sectional region of interest for cortex evaluation. C, caudate; L, lentiform nucleus; IC, internal capsule; I, insula; M1–6, corresponding regions of the MCA territory.
MANAGEMENT GUIDELINES
The treatment of acute ischemic stroke centers on urgent revascularization of occluded vessels or augmentation of collateral cerebral blood flow in order to minimize brain infarct size and salvage penumbra. Equally important is the provision of supportive care to minimize stroke complications, including intracerebral hemorrhage, increasing stroke size, and brain herniation, as well as identification of concomitant disease processes such as myocardial infarction, aortic dissection, aspiration pneumonia, or drug intoxication. Management aims to provide appropriate treatment, and to proceed as rapidly as possible to improve neurologic outcome with an acceptably low risk of complications. A decision tree for the management of ischemic stroke is provided in Figure 20.3. Guidelines for standard medical management are detailed in Table 20.6. Potential complications of stroke therapy and their management are reviewed in Table 20.7. Additional standard ischemic stroke protocols, criteria, and order sets are provided in Table 20.8.4,17–19 Indications and contraindications to IV tPA are listed in Table 20.9.
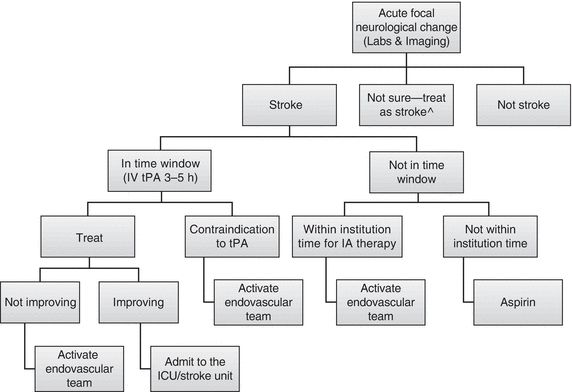
FIGURE 20.3 Acute-Stroke Flow Chart. ^Meta-analysis of stroke mimics treated with IV tPA revealed no adverse events (involve stroke team in decision). From Chang J, Teleb M, Yang JP, et al. A model to prevent fibrinolysis in patients with stroke mimics. J Stroke Cerebrovasc Dis. 2011;21(8):839–843. doi:10.1016/j.jstrokecerebrovasdis.2011.04.018; Tsivgoulis G, Alexandrov AV, Chang J, et al. Safety and outcomes of intravenous thrombolysis in stroke mimics: a 6-year, single-care center study and a pooled analysis of reported series. Stroke. 2011;42(6):1771–1774. doi:10.1161/STROKEAHA.110.609339
TABLE 20.6 Medical Management of Ischemic Stroke
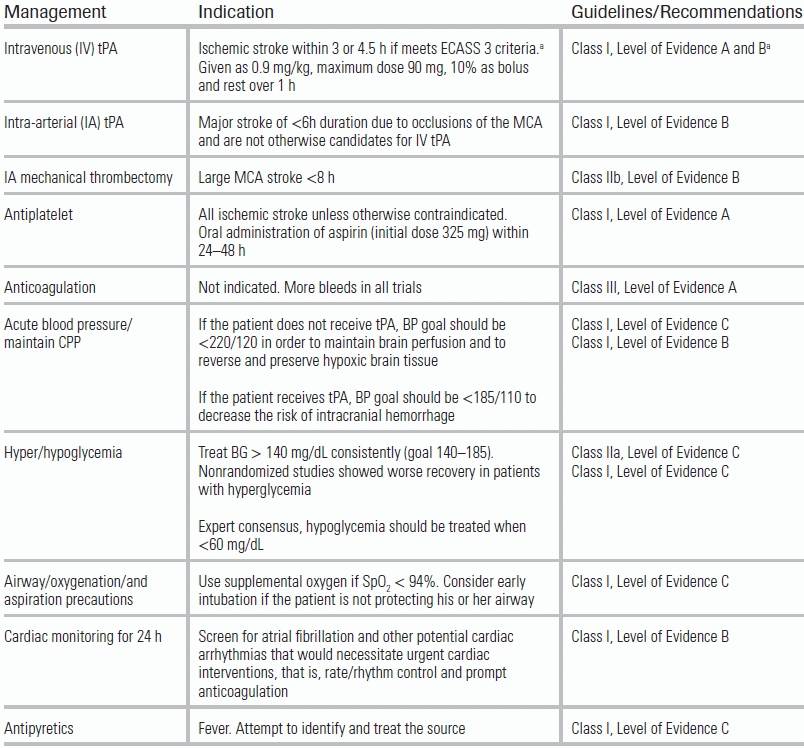
From Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a
aAge >80, combination of previous stroke and diabetes, NIHSS >25, >1/3 MCA infarct on CT are the exclusions for 3–4.5 h as these patients were not in trial. From Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi:10.1056/NEJMoa0804656.
TABLE 20.7 Potential Complications and Treatment of Stroke Therapy
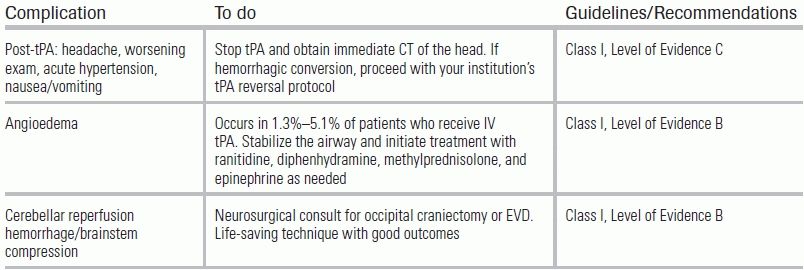
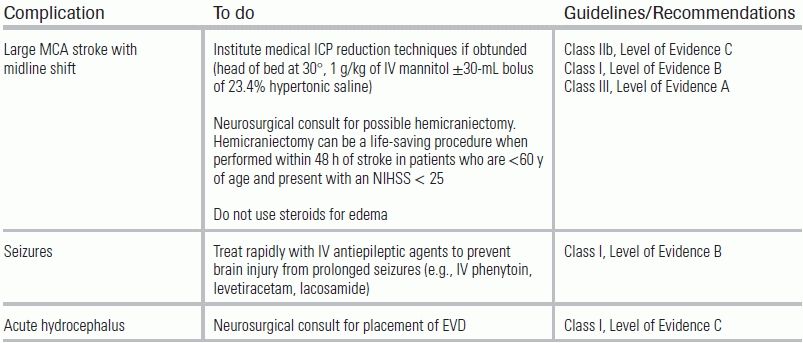
From Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a
EVD, extraventricular drain.
TABLE 20.8 Important Ischemic Stroke Protocols, Criteria, and Order Sets
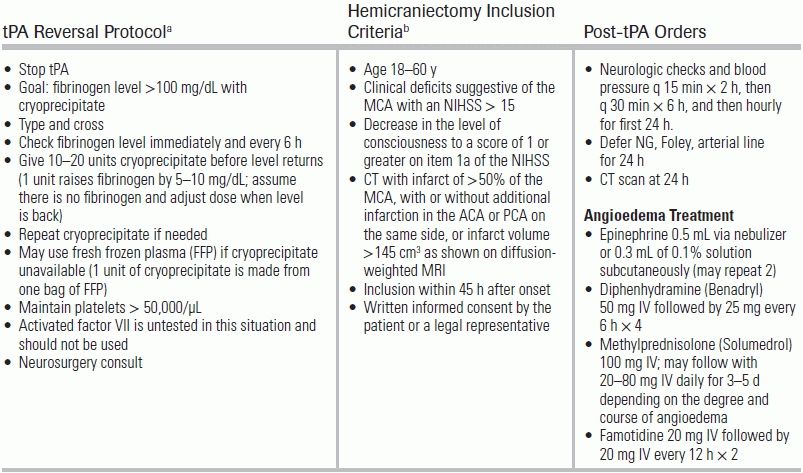
aConsensus recommendations based on the AHA/ASA 2007 Guidelines. From Broderick J, Connolly S, Feldmann E, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage in Adults: 2007 Update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(6):2001–2023. doi:10.1161/STROKEAHA.107.183689; Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. doi:10.1161/STR.0b013e3181ec611b
bEvidence from Pooled Analysis of Hemicraniectomy Trials. From Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215–222. doi:10.1016/S1474-4422(07)70036-4.
MCA, middle cerebral artery, ACA, anterior cerebral artery, PCA, posterior cerebral artery, hours (hours), tPA, tissue plasminogen activator.
TABLE 20.9 Indications and Contraindications for IV tPA
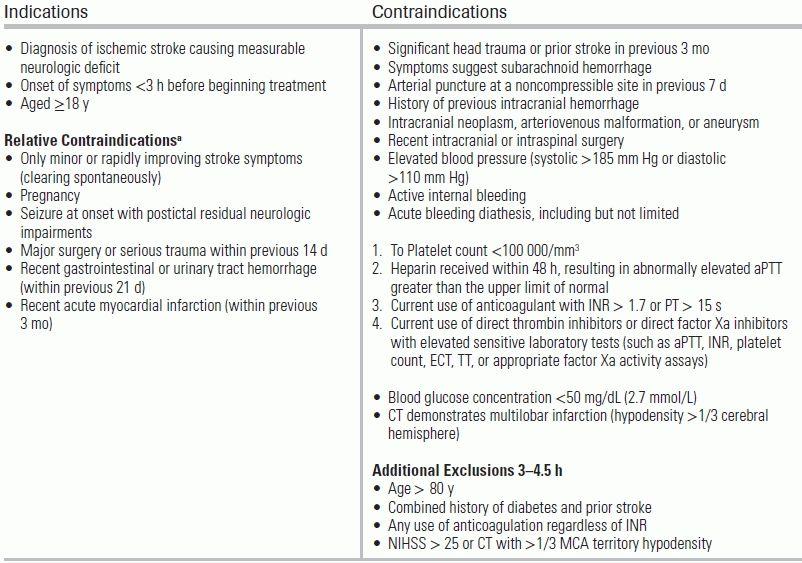
aUnder some circumstances, patients may receive fibrinolytic therapy despite one or more relative contraindications. Consider risk to benefit of IV tPA carefully if any the relative contraindications are present. Based on the 2013 AHA/ASA Guidelines. From Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a
The most commonly asked questions regarding the acute management of ischemic stroke relate to the risks and benefits of IV tPA. Major clinical trials evaluating the efficacy of IV tPA include ECASS 3, NINDS 2, and IST 3.20,21,32,33 In order to better understand the results of these trials, it is important to recognize the outcome parameters being measured. The modified Rankin Scale (mRS) is a 6-point functional outcome parameter that ranges from no disability (0) to death (6). It is typically obtained 90 days after a patient has a stroke. The NIHSS, which is not a functional outcome scale but rather a 42-point measure of neurologic deficit, is more valuable during the initial evaluation. There is some controversy regarding these IV tPA trials, because in none of them were NIHSS scores at 24 hours different following IV tPA. At 3 months, however, the NIHSS and mRS demonstrated a significant benefit in patients treated within the 3-hour time window. These benefits were replicated in the 3- to 4.5-hour time window in the ECASS 3 trial. Importantly, earlier trials that extended IV tPA therapy to 6 hours revealed no difference in outcome and potential harm, and a 2010 meta-analysis of the ECASS, ATLANTIS, NINDS 2, and EPITHET trials demonstrated the same findings (no difference/potential harm) for IV tPA given after 4.5 hours.33 Because of this, it is imperative to determine the time of onset of a patient's symptoms as well as to abide by the contraindications to therapy. The following is a summary of IV tPA outcome data:
Intra-arterial tPA (IA-tPA) and mechanical thrombectomy (IA-thrombectomy) are two endovascular therapeutic options that may be considered in patients with large vessel occlusions and NIHSS ≥ 8. The latest trial of endovascular therapy, in which patients within the IV tPA time window received either IV tPA alone or IV tPA and an endovascular intervention, did not show a significant difference in the outcome.31 This study, however, did not use advanced perfusion imaging to confirm a large vessel clot, and nearly 20% of cases proved to have no large vessel occlusion. In addition, the majority of devices used in this trial are no longer used because of their inferior recanalization rates compared to newer models. Improved recanalization rates with the Trevo and Solitaire devices24,25 have lead to continued research—not available at the time of this publication—regarding the benefits of endovascular therapy in large vessel occlusion (SWIFT PRIME and POSITIVE Trials). At this time, IA-tPA and IA–-thrombectomy cannot be considered standard of care, but may be considered for patients with an NIHSS ≥ 8 who are either not candidates for IV tPA or who show no improvement following IV tPA.
For patients with a large MCA stroke seen on CT (>1/3 of MCA), admission to the ICU and neurosurgical consultation for consideration of hemicraniectomy may be considered, provided family members favor aggressive care. Hemicraniectomy trials have universally shown a significant mortality benefit, but have not demonstrated consistent improvement in functional status.19 In patients with large MCA strokes, if signs or symptoms of herniation are encountered in the ED, ICP management should be implemented (see Table 20.7), including definitive airway management. Other potential complications that can arise in this setting include seizures and acute hydrocephalus.
CONCLUSION
Ischemic stroke continues to increase in prevalence, and is the cause of considerable morbidity in the United States. Fortunately, advances in diagnosis and in the armamentarium of therapeutic options, such as IV tPA and endovascular therapy, make stroke a highly treatable disease in the acute care setting. The protocols reviewed in this chapter can help guide a rapid diagnostic workup and facilitate the time-sensitive interventions necessary to minimize death and disability from to this devastating disease.
LITERATURE TABLE

CI, confidence interval; OR, odds ratio SB, symptomatic bleed NNT, number needed to treat.
1.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad
2.Chang J, Teleb M, Yang JP, et al. A model to prevent fibrinolysis in patients with stroke mimics. J Stroke Cerebrovasc Dis. 2011;21(8):839–843. doi: 10.1016/j.jstrokecerebrovasdis.2011.04.018
3.Tsivgoulis G, Alexandrov AV, Chang J, et al. Safety and outcomes of intravenous thrombolysis in stroke mimics: a 6-year, single-care center study and a pooled analysis of reported series. Stroke. 2011;42(6):1771–1774. doi: 10.1161/STROKEAHA.110.609339
4.Jauch EC, Saver JL, Adams HP, et al. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. doi: 10.1161/STR.0b013e318284056a
5.Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864–870.
6.Lyden PD, Lu M, Levine SR, et al. A modified National Institutes of Health Stroke Scale for use in stroke clinical trials: preliminary reliability and validity. editorial comment: The NIH stroke scale: is simpler better? Stroke. 2001;32(6):1310–1317.
7.Adams H Jr, Davis PH, Leira EC, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke. Neurology. 1999;53(1):126–131.
8.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke. 2005;36(10):2121–2125.
9.Lyden P, Raman R, Liu L, et al. NIHSS training and certification using a new digital video disk is reliable. Stroke. 2005;36(11):2446–2449. doi: 10.1161/01.STR.0000185725.42768.92
10.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46(6):660. doi: 10.1001/archneur.1989.00520420080026
11.Levine J, Johnston K. Diagnosis of stroke and stroke mimics in the emergency setting. Continuum Lifelong Learning Neurol; 2008:13–20.
12.Jones HR, Srinivasan J, Allam GJ, et al. Netter's Neurology. W. B. Saunders Co; 2011:496–517.
13.Uchino K, Pary J, Grotta J. Acute Stroke Care. 2011:3–6.
14.Searls DE, Pazdera L, Korbel E, et al. Symptoms and signs of posterior circulation ischemia in the New England Medical Center Posterior Circulation registry. Arch Neurol. 2012;69(3):346–351. doi: 10.1001/archneurol.2011.2083
15.The Publications Committee for the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) Investigators. Low molecular weight heparinoid, ORG 10172 (danaparoid), and outcome after acute ischemic stroke: a randomized controlled trial. JAMA. 1998;279(16):1265–1272.
16.Pexman JH, Barber PA, Hill MD, et al. Use of the Alberta Stroke Program Early CT Score (ASPECTS) for assessing CT scans in patients with acute stroke. AJNR Am J Neuroradiol. 2001;22(8):1534–1542.
17.Broderick J, Connolly S, Feldmann E, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage in Adults: 2007 Update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group: the American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Stroke. 2007;38(6):2001–2023. doi:10.1161/STROKEAHA.107.183689
18.Morgenstern LB, Hemphill JC, Anderson C, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–2129. doi:10.1161/STR.0b013e3181ec611b
19.Vahedi K, Hofmeijer J, Juettler E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6(3):215–222. doi:10.1016/S1474-4422(07)70036-4
20.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333(24):1581–1588. doi:10.1056/NEJM199512143332401
21.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317–1329. doi:10.1056/NEJMoa0804656
22.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282(21):2003–2011
23.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39(4):1205–1212. doi:10.1161/STROKEAHA.107.497115
24.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380(9849):1241–1249. doi:10.1016/S0140-6736(12)61384-1
25.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380(9849):1231–1240. doi:10.1016/S0140-6736(12)61299-9
26.Bath PM, Lindenstrom E, Boysen G, et al. Tinzaparin in acute ischaemic stroke (TAIST): a randomised aspirin-controlled trial. Lancet. 2001;358(9283):702–710
27.International Stroke Trial Collaborative Group. The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. Lancet. 1997;349(9065):1569–1581. doi:10.1016/S0140-6736(97)04011-7
28.Berge E, Abdelnoor M, Nakstad PH, et al. Low molecular-weight heparin versus aspirin in patients with acute ischaemic stroke and atrial fibrillation: a double-blind randomised study. HAEST Study Group. Heparin in acute embolic stroke trial. Lancet. 2000;355(9211):1205–1210.
29.Vahedi K, Vicaut E, Mateo J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke. 2007;38(9):2506–2517. doi:10.1161/STROKEAHA.107.485235
30.Jüttler E, Unterberg A, Woitzik J, et al. Hemicraniectomy in older patients with extensive middle-cerebral artery stroke. N Engl J Med. 2014;370(12):1091–100.
31.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368(10):893–903. doi:10.1056/NEJMoa1214300.
32.IST-3 Collaborative Group, Sandercock P, Wardlaw JM, Lindley RI, et al. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet. 2012;379(9834):2352–2363. doi:10.1016/S0140-6736(12)60768-5
33.Lees KR, Bluhmki E, Kummer von R, et al. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375(9727):1695–1703. doi:10.1016/S0140-6736(10)60491-6.
34.Saver JL. Number needed to treat estimates incorporating effects over the entire range of clinical outcomes: novel derivation method and application to thrombolytic therapy for acute stroke. Arch Neurol. 2004;61(7):1066–1070. doi:10.1001/archneur.61.7.1066
35.Saver JL, Gornbein J, Grotta J, et al. Number needed to treat to benefit and to harm for intravenous tissue plasminogen activator therapy in the 3- to 4.5-hour window: joint outcome table analysis of the ECASS 3 Trial. Stroke. 2009;40(7):2433–2437. doi:10.1161/STROKEAHA.108.543561