34
Infections in the Immunocompromised Host
M. Cristina Vazquez-Guillamet, Joshua J. Mooney, and Joe L. Hsu
BACKGROUND
Recent decades have challenged the emergency physician to manage an evolving spectrum of infections in immune-compromised patients. This comes as a result of an increased use of immune suppression and infectious disease prophylaxis, as well as the improved survival of patients with human immunodeficiency virus (HIV), cancer, and following solid organ transplant (SOT) and hematopoietic stem cell transplantation (HSCT). Early diagnosis and treatment of these individuals with appropriate antimicrobial agents are essential for successful clinical outcomes.
DIAGNOSTIC EVALUATION
The frequent absence of early signs and symptoms traditionally used to define infection presents a major challenge in the care of immune-compromised patients. Cultures—the gold standard for guiding choice of antimicrobial agents—are positive in <50% of patients with invasive fungal diseases (IFDs) or febrile neutropenia and take several days for detection.1,2 This diagnostic uncertainty is particularly concerning since delay in starting appropriate antimicrobial therapy is well established as a cause of increased mortality.3
When performing a targeted evaluation of an immune-compromised patient, the emergency physician should begin with an assessment of the “net state of immune suppression,” including an evaluation of the type of immune suppression, current immune suppressive therapy, level of immune suppression (myeloablative vs. nonmyeloablative regimen prior to HSCT, ongoing immune suppressive therapy), duration (transplant date, last chemotherapy, time from diagnosis of underlying malignancy), and anti-infective prophylaxis. Immune-suppressed patients will typically have unique pathogen susceptibilities based on their underlying immune defect (e.g., defects in T and/or B cells, neutropenia). A thorough patient history should document recent infections, including multidrug-resistant infections (MDRIs), latent and opportunistic infections, and recent surgical procedures including indwelling central venous catheters (CVC). In the patients with HIV, knowledge of a recent CD4 cell count and the use or lack of use of antiretroviral therapy (ART) and anti-infective prophylaxis is essential. The immune-compromised patient may have multiple potential infectious sources including donor acquired, nosocomial, reactivation, and environmental, and community acquired, all of which should be investigated.
In addition to standard infectious laboratory testing, non–culture-based methods including polymerase chain reaction (PCR) assays, antigen and antibody capture assays (Aspergillus galactomannan [GM], (1–3)-β-d-glucan, direct fluorescent antibody [DFA] stains), and microscopy with special stains, if available, should be ordered in consultation with infectious disease. Early use of computed tomography (CT) to identify an infectious source is also indicated since plain radiographs are known to lack sensitivity in the detection of infections in immune-suppressed patients.4–8
INFECTIONS IN PATIENTS WITH HUMAN IMMUNODEFICIENCY VIRUS
The mortality of critically ill HIV-infected patients has decreased to the point that survival rates now approach those seen in non-HIV patients.9,10 Although survival in critically ill patients is independent of ART at the time of admission, the use of ART is associated with higher CD4 counts, increased virologic suppression, and lower rates of opportunistic infections (such as Pneumocystis jirovecii pneumonia [PJP] and others) that independently correlate with survival.11 These changes have shifted the epidemiology of critically ill HIV-infected patients beyond opportunistic infections to include nosocomial and community-acquired infections, comorbid chronic diseases, and medication-related toxicities, including the immune reconstitution inflammatory syndrome (IRIS).12 In general, ART should be continued in critically ill HIV-infected patients with known prior virologic suppression; at the same time, these patients should be monitored for drug side effects (although rare, nucleoside analog reverse transcriptase inhibitors can cause lactic acidosis), proper dosing, and pharmacologic interactions.13
Sepsis in HIV-Positive Patients
The incidence of sepsis in ICU admissions of HIV-infected patients increased from 12% in 1995 to 20% in 2000, but mortality continues to improve.11,14 More than half of these septic episodes were due to respiratory infections, followed by bacteremia, catheter-related blood stream infections (CRBSIs), and urinary tract infections (UTIs). Nosocomial pathogens, including Staphylococcus aureus and Pseudomonas aeruginosa, were the organisms implicated in up to 60% of all infections, followed by opportunistic and community-acquired pathogens.15 Sepsis-associated mortality among HIV-infected patients is correlated to illness severity, rather than degree of immune deficiency; however, an increased risk of nosocomial and opportunistic pathogen infection is observed with CD4 cell counts <200 cells/mm3 and with the absence of ART.15
Treatment of Infection in the HIV-Positive Patient
Treatment of infection in any immune-compromised patient includes goal-directed therapy and broad-spectrum antimicrobials, including empiric coverage for nosocomial organisms in patients at risk for severe sepsis. In HIV-infected patients with suspected sepsis and recent initiation of ART, the possibility of IRIS should be considered. IRIS presents as either a localized or systemic inflammatory reaction that develops after initiation of ART (typically 90 d later) and is due to recovery of the patient's immune response. The syndrome results from either the inflammatory reaction to a recognized preexisting infection (e.g., Pneumocystis jirovecii) or the unmasking of an unrecognized preexisting infection (e.g., Mycobacterium avium complex [MAC], Mycobacterium tuberculosis [MTB], endemic fungal infections) with clinical features related to the prior infection. The diagnosis is one of exclusion. Active infection and drug reaction (e.g., abacavir hypersensitivity) must first be excluded. Management of IRIS is supportive, with continued treatment of the underlying infection and ART. In moderate to severe cases of IRIS, the use of prednisone (1 mg/kg/d) has been employed, although no controlled trials definitively support its benefit.
Respiratory Infections in HIV-Positive Patients
Respiratory infections are the most common reason HIV-infected patients are admitted to the ICU. The clinical presentation, radiographic appearance, and CD4 count help narrow the differential diagnosis (Table 34.1). Streptococcus pneumoniae is the most common etiologic agent.35 Risk factors for MDRIs include CD4 cell counts <200 cells/mm3, underlying lung disease, neutropenia, and recent health care exposures.15
TABLE 34.1 Common Infectious Conditions for Patients Infected with Human Immunodeficiency Virus
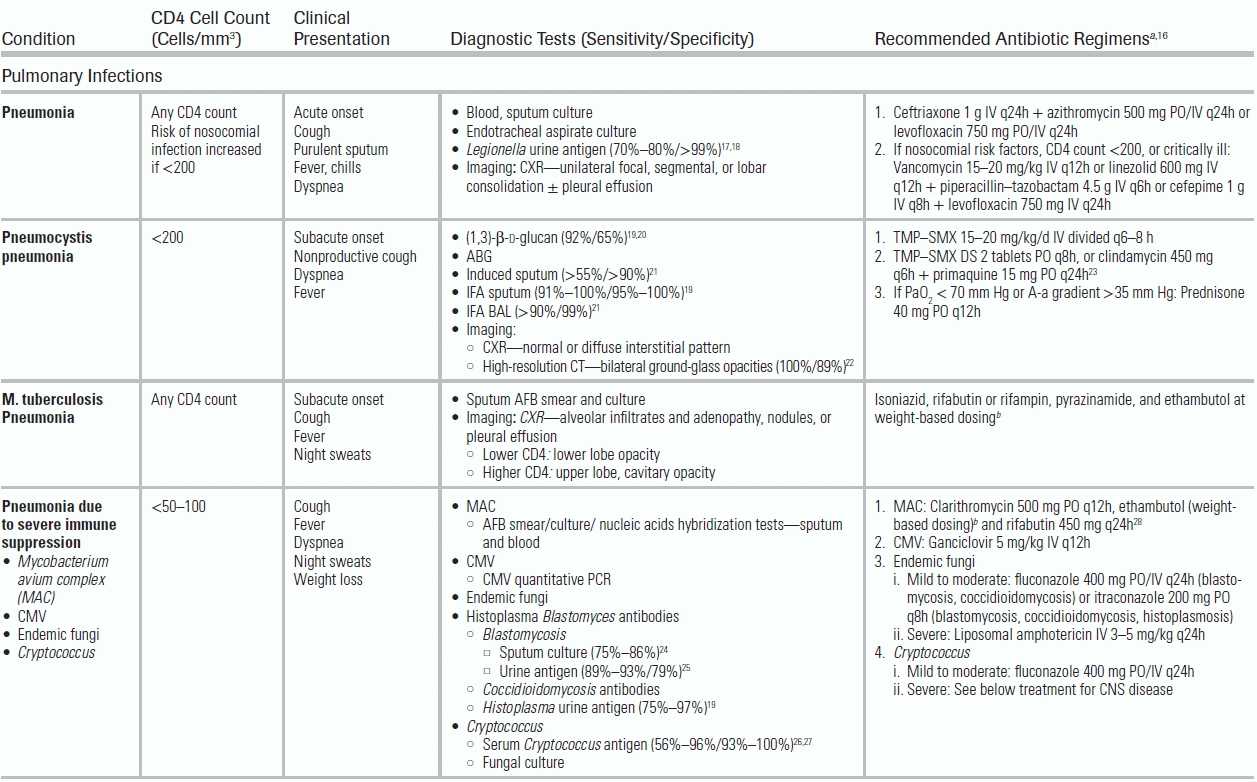
The incidence of PJP has declined with the use of prophylactic antibiotics and ART, but it remains the most common opportunistic respiratory infection and should be considered in those with a CD4 cell count <200 cells/mm3. The sputum immunofluorescent antibody (IFA) test, the presence of ground-glass opacities on high-resolution chest CT scan, and/or elevated serum (1,3)-β-d-glucan36 are reliable noninvasive tests for diagnosing PJP.19,20,22 All patients should undergo arterial blood gas (ABG) sampling on ambient air to determine if adjunct corticosteroids (typically prednisone 40 mg q12h) and low tidal volume ventilation (employed in mechanically ventilated patients to minimize the risk for pneumothorax) are indicated. A PaO2 < 70 mm Hg on ABG or an alveolar–arterial difference (A-a gradient) of >35 mm Hg is the standard cutoff used for corticosteroid initiation.37
HIV-infected patients are also at an increased risk of primary and reactivation of MTB infection. Those with a clinical syndrome suggestive of MTB should be placed in respiratory isolation, and three serial acid-fast bacillus (AFB) sputum samples should be collected. Common radiographic patterns of MTB include upper lung cavitation at higher CD4 cell counts and middle-to-lower lobe infiltrates at lower CD4 cell counts, but any radiographic pattern may be encountered. Severely immune-compromised HIV-infected patients (CD4 cell count <50 to 100 cells/mm3) are also at risk for pulmonary infections from endemic (e.g., Histoplasma capsulatum, Coccidioides immitis) and geographic (e.g., Cryptococcus neoformans) fungi and M. avium complex.
Altered Mental Status in HIV-Positive Patients
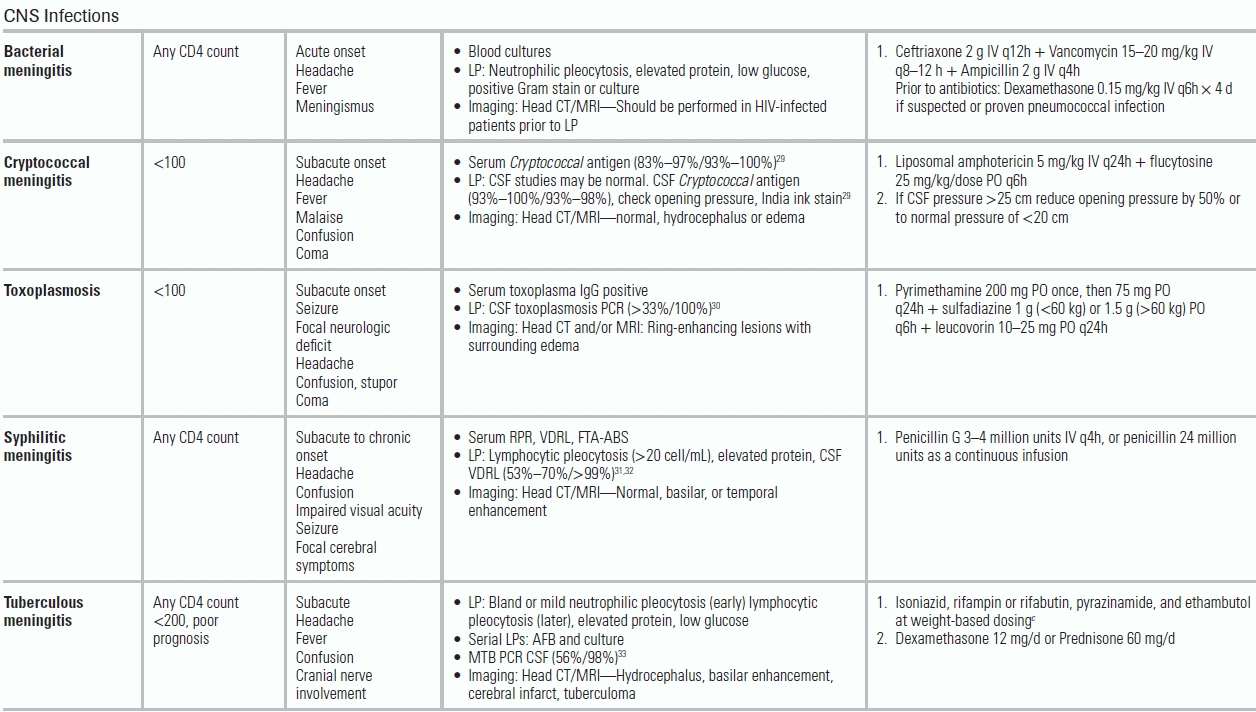
Altered mental status is a common presenting symptom in critically ill HIV-infected patients and results from both infectious and noninfectious etiologies. The following factors can help narrow the differential diagnosis: level of CD4 cell count, status of virologic suppression, toxoplasma serology, and ART regimen and timeline (Table 34.1).
While any infection may result in an altered sensorium, dangerous infections of the central nervous system (CNS) include meningitis (bacterial, cryptococcal, tuberculous, syphilitic), viral encephalitis (Cytomegalovirus [CMV], herpes simplex virus [HSV]), or parenchymal lesions including parasitic infection (toxoplasmosis) and brain abscess (bacterial, fungal). All patients with CD4 cell counts <200 cells/mm3 and focal findings should undergo a CT head with intravenous contrast to evaluate for presence of cerebral toxoplasmosis (characterized by ring-enhancing lesions on CT imaging), CNS lymphoma (usually single lesion), and progressive multifocal leukoencephalopathy (plaques in the white matter). Following head imaging, lumbar puncture should be performed to evaluate for bacterial and fungal meningitis or more indolent CNS processes such as syphilitic and tuberculous meningitis.
Noninfectious etiologies in HIV-infected patients with mental status changes include primary CNS lymphoma, HIV-associated dementia, toxins or medication-side effects (e.g., efavirenz), IRIS, and metabolic encephalopathy.
INFECTIONS IN PATIENTS WITH HEMATOLOGIC MALIGNANCIES AND FOLLOWING HEMATOPOIETIC STEM CELL TRANSPLANTATION
For patients with hematologic malignancies, the primary malignancy dictates the immune defect and pathogen susceptibilities. For example, patients with acute lymphoblastic leukemia have more profound cellular defects than those with acute myeloid leukemia. Multiple myeloma is associated with a reduced humoral immunity. Chronic lymphocytic leukemia is characterized by a prolonged course of T-cell and antibody deficiency. Chemotherapy for hematologic malignancies is uniformly myelosuppressive, resulting in profound neutropenia, which increases susceptibility to bacterial and fungal pathogens. Among chemotherapeutic agents, daunorubicin and cytarabine can produce severe, prolonged neutropenia, while fludarabine and alemtuzumab decrease the T- (especially CD4 cells) and B-cell function.38
For HSCT patients, the preparative regimen for transplantation determines the level of immune suppression. Myeloablative regimens completely suppress the host bone marrow, resulting in profound immune compromise; nonmyeloablative regimens are less suppressive. Posttransplant hematopoietic immune recovery follows a predictable sequence: pre-engraftment (0–1 month), early postengraftment (1 to 3 months) with gradual return of the host's humoral response, and late postengraftment (>3 months) during which cellular immunity returns. In some patients, cellular immune defects may persist 1 to 2 years posttransplant. If graft versus host disease (GVHD) ensues, prolonged and aggressive immune suppression will be required, increasing the risk of infections.
Febrile Neutropenia
Neutropenia is defined as an absolute neutrophil count (ANC) < 1,500 cells/mm3; severe neutropenia as an ANC < 500 cells/mm3 (ANC = [% Neutrophils + % Bands] × [WBC]/100). Febrile neutropenia is defined as one episode of fever >38.3°C or fever >38°C for ≥1 hour with an absolute neutrophil count (ANC) <500 cells/mm3 or with an expected ANC drop to <500 cells/mm3 within 48 hours. The ANC nadir, duration of neutropenia, and rapidity of decline determine infection risk. Table 34.2 provides criteria for risk-stratifying patients with febrile neutropenia.39,40
TABLE 34.2 Classification Scheme for Risk in Patients with Neutropenic Fever

Duthie R, Denning DW. Aspergillus fungemia: report of two cases and review. Clin Infect Dis. 1995;20:598–605. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–431; The Sanford guide to antimicrobial therapy. Sperryville, VA: Antimicrobial Therapy; 2012. Ref.34.
Patients with febrile neutropenia are susceptible to CRBSI, as well as infections that originate in the gastrointestinal tract (most commonly due to bacterial translocation in the setting of mucositis or enterocolitis), lung, kidneys, or soft tissues. The epidemiology of these infections has shifted from gram-negative rods (GNRs) to gram-positive cocci (GPCs, 60% to 75% of positive blood cultures) due to widespread use of antimicrobial prophylaxis that targets GNRs.41 Common pathogens include coagulase-negative Staphylococcus, S. aureus, Streptococcus spp, Enterococcus, Corynebacterium jeikeium, P. aeruginosa, and Enterobacteriaceae. For patients with enterocolitis, potential pathogens include anaerobes (Clostridium septicum, Clostridium tertium, and Bacillus cereus). Neutropenic patients are also at risk for breakthrough infections, resulting from a gap in the pathogen coverage of prophylactic antibiotics (typically fluoroquinolones) leading to infections with Streptococcus spp and anaerobic spp. Breakthrough infection, for example, from Streptococcus viridans, can rapidly lead to severe sepsis and acute respiratory distress syndrome (ARDS).
Diagnostic Evaluation for Febrile Neutropenia
Laboratory Studies
Although only 30% to 40% of neutropenic fever episodes will prove to have a documented infection,42 the clinician must assume an infectious etiology, even when the patient presents with no signs of inflammation (e.g., no meningeal signs, nonproductive cough, no leukocytosis). Table 34.3 highlights diagnostic tests to consider based on the clinical presentation. In a neutropenic host with sepsis, a procalcitonin level may be useful in considering a bacterial etiology (serum cutoff level >0.5 μg/L); it should not be used in localized bacterial or fungal infections, or those due to viral pathogens.55,56 The serum concentration of procalcitonin peaks at 24 hours after development of fever.
TABLE 34.3 Common Infectious Conditions for Patients with Hematologic Malignancies and after Hematopoietic Stem Cell Transplantation
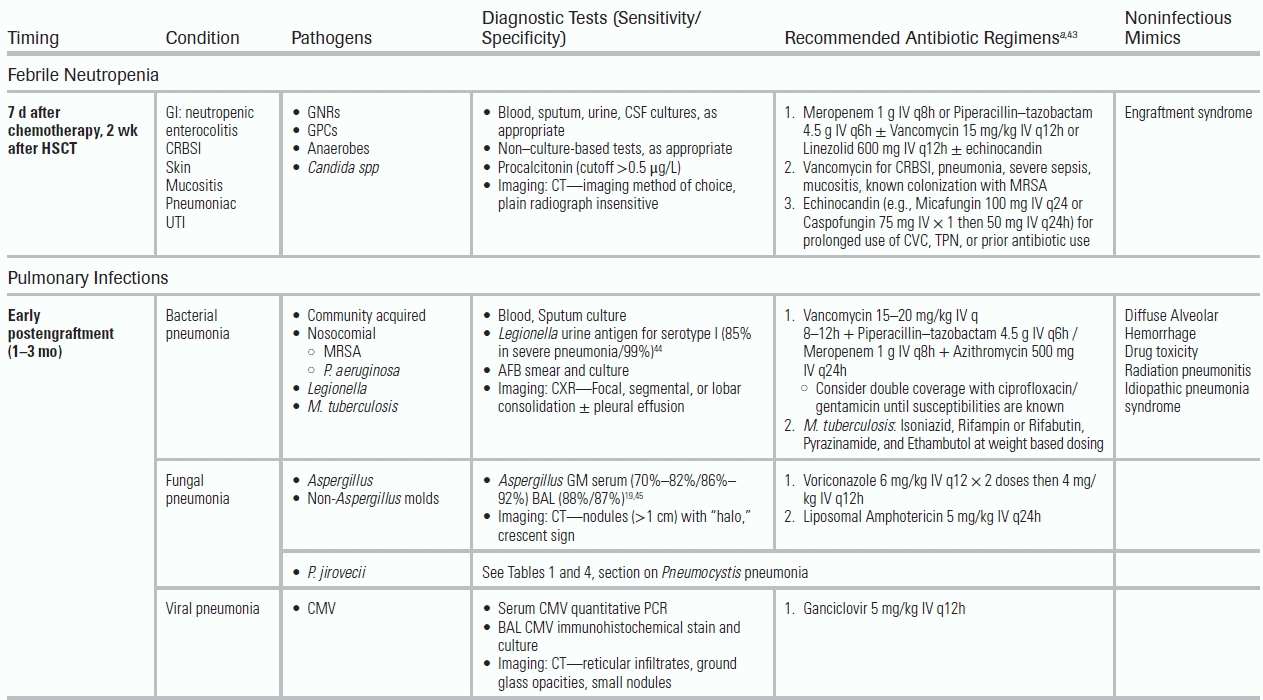
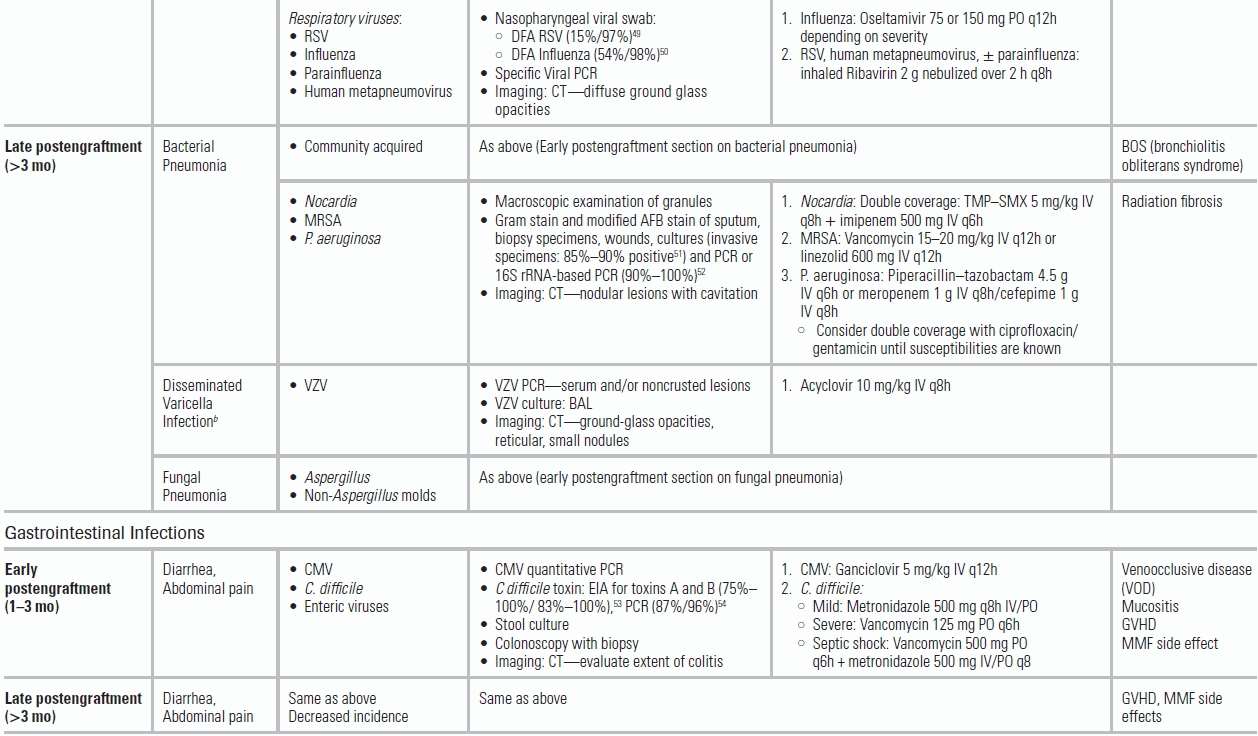
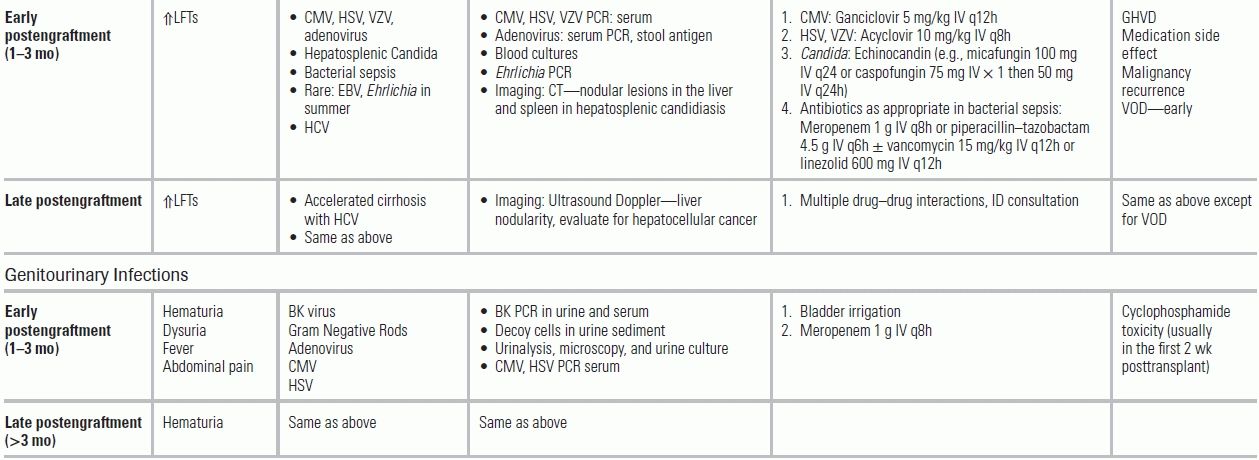
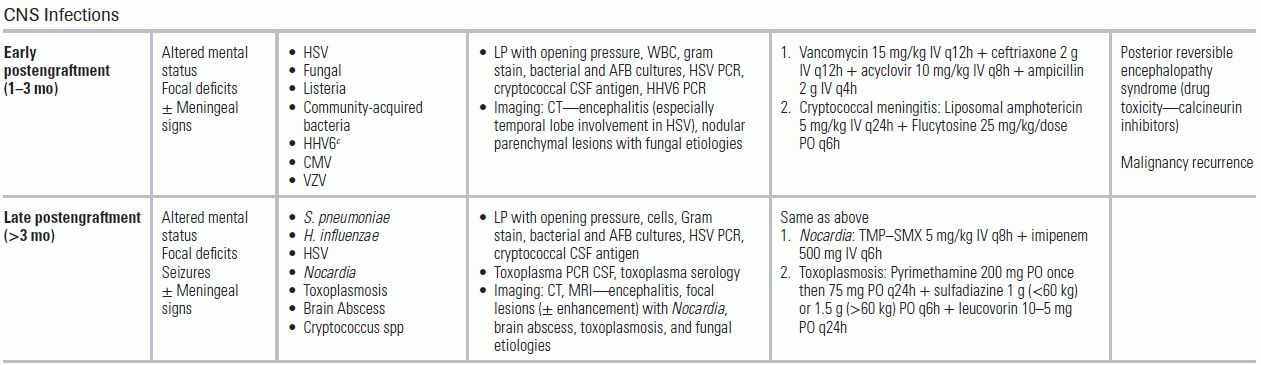
BAL, bronchoalveolar lavage; CMV, cytomegalovirus virus; CRBSI, catheter-related blood stream infection; CSF, cerebrospinal fluid; DFA, direct fluorescent antibody; EBV, Epstein-Barr virus; EIA, enzyme immunoassay; GNRs, gram-negative rods; GPCs, gram-positive cocci; GVHD, graft versus host disease; HCV, hepatitis C virus; HHV6, human herpes virus 6; HSV, herpes simplex virus; MRSA, methicillin-resistant S. aureus; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; TMP–SMX: trimethoprim–sulfamethoxazole; VOD, venoocclusive disease; VZV, varicella-zoster virus.
From The Sanford guide to antimicrobial therapy. Sperryville, VA: Antimicrobial Therapy; 2012. Ref.34.
Imaging
CT imaging should be obtained for patients with focal CNS findings and respiratory or abdominal complaints. For patients with lower quadrant abdominal symptoms, typhlitis, also known as neutropenic enterocolitis, should be suspected. Typhlitis is a chemotherapy-related colonic inflammation that leads to bacterial translocation, resulting in diffuse wall edema and perforation. Abdominal CT imaging determines the extent of typhlitis and the presence of local complications.
Treatment of Infection in Patients with HSCT
Antibacterial Therapy
Initial workup and antibiotic administration should occur as soon as possible after presentation to the emergency department. The Infectious Diseases Society of America (IDSA) guidelines recommend an antipseudomonal β-lactam as the initial regimen.39 Cefepime should be avoided, as it is associated with (a) higher all-cause mortality compared with other β-lactams57; (b) inferior efficacy compared with piperacillin–tazobactam or carbapenems58; and (c) a failure to cover relevant anaerobic pathogens (Bacteroides spp).59 If there is a low index of suspicion for multidrug-resistant GNRs (e.g., extended-spectrum beta-lactamase (ESBL) producing organisms), piperacillin–tazobactam is favored over carbapenems, as carbapenems are associated with higher rates of antibiotic- and C. difficile-associated diarrhea.58 Among the carbapenems, ertapenem should be avoided as it fails to cover P. aeruginosa. The addition of an aminoglycoside, typically gentamicin, to any of the initial β-lactam regimens may be considered for the neutropenic patient in septic shock until culture results are available that will allow more selective antibiotic choice. Vancomycin should be initiated in HSCT critically ill patients or in those with risk factors for resistant GPC infections (oral mucositis, an indwelling catheter, or colonization with methicillin-resistant S. aureus [MRSA]).
Antifungal Therapy
Antifungals are added for HSCT patients at high risk for candidemia; risk factors include prolonged neutropenia, frequent hospitalizations, a protracted antibiotic course, and the presence of CVC, particularly for the administration of total parenteral nutrition. Antifungals are also indicated if fever persists after 5 days of adequate antimicrobial treatment and negative cultures. To date, there are no compelling studies favoring a specific antifungal agent. The initial use of an echinocandin, however, is now recommended given the rising incidence of azole-resistant Candida spp (Candida glabrata, Candida krusei) and the safer side effect profile of echinocandins compared to liposomal amphotericin.60 A suitable alternative would be voriconazole. Both drugs also cover Aspergillus.61
Generally, CVCs should be replaced upon presentation in severe sepsis or septic shock, given the paucity of symptoms in CRBSI. In the presence of barriers to immediate line exchange, emergency physicians may consider sending blood cultures before antibiotic administration to assess “time-to-positivity” cultures (see CRBSIs in Patients with Solid Tumors).47
Adjunctive Therapies
If treatment with colony-stimulating factors has already been initiated, it should be continued. However, a recent meta-analysis did not demonstrate a benefit of colony-stimulating factors in established febrile neutropenia.62 Current guidelines suggest their use in high-risk patients: age >65 years, prolonged and severe neutropenia, and septic shock.63 Surgical consultation should be obtained promptly in enterocolitis, biliary sepsis, necrotizing fasciitis, and gynecologic sepsis.
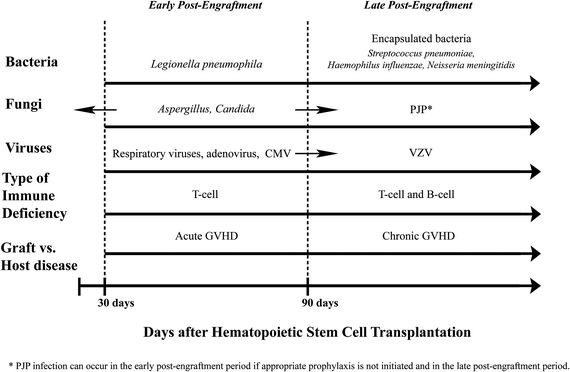
Timing of Infections Following HSCT
Knowledge of the time since transplantation, use of ongoing immune suppression, and previous antimicrobial prophylaxis can help the emergency physician differentiate between noninfectious and infectious etiologies, as well as identify likely pathogens (Fig. 34.1).
Early Postengraftment Period After HSCT
Since the preengraftment period occurs while admitted to the hospital, we will only review the following stages in HSCT that are more relevant to the emergency physician. In the immediate postengraftment period, if no GVHD is encountered, immune suppression is gradually tapered. During this period, respiratory infections predominate. In addition to community-acquired pathogens, HSCT patients are at increased risk from respiratory viruses, including respiratory syncytial virus (RSV), influenza, parainfluenza, and human metapneumovirus.64 Prompt diagnosis is essential in initiating respiratory isolation and appropriate antiviral therapy (e.g., ribavirin or oseltamivir). For RSV and influenza, rapid viral antigen tests are recommended, in conjunction with PCR-based detection (Table 34.3). Prophylaxis for CMV with ganciclovir delays the presentation of CMV syndromes. In addition to fever, interstitial pneumonia, and enteritis, CMV reactivation may manifest indirectly (e.g., concurrent infection such as PJP).65 If CMV is suspected, PCR testing for quantitative CMV viral load is recommended. Adenovirus infection is also encountered in the early postengraftment period and may result in fulminant hepatitis, pneumonitis, and encephalitis.
Fungal Infections in the Early Postengraftment Period
Hepatosplenic candidiasis (chronic disseminated candidiasis) can develop in patients not receiving antifungal prophylaxis and should be considered in a patient recovering from neutropenia who presents with abdominal pain, fever, and increased alkaline phosphatase. Aspergillosis should be suspected in a clinically stable patient with persistent fevers despite prolonged courses of antibiotics and fluconazole. Typical symptoms include a nonproductive cough and pleuritic chest pain. While fungal blood cultures typically do not improve the diagnostic yield over standard blood cultures, they may be helpful in isolating endemic fungi like Histoplasma or molds such as Fusarium.19 Aspergillus GM testing in serum and/or bronchoalveolar lavage (BAL) can also help make the diagnosis.66 Chest CT imaging in patients with pulmonary Aspergillus typically demonstrates multiple, poorly defined macronodules (>1 cm) with or without “halo,” cavitation, or “air-crescent” sign.67 First-line treatment for invasive pulmonary aspergillosis is voriconazole. Other invasive molds (Fusarium, Scedosporium, and agents of mucormycosis) can cause pulmonary, CNS, rhino-orbital, skin, and disseminated disease, and require treatment with liposomal amphotericin.
Late Postengraftment Period After HSCT
Approximately 50% of HSCT patients will develop chronic GVHD and will require prolonged immune suppression. The incidence of bacterial infection decreases substantially in the late posttransplant period, with the exception of infection by encapsulated organisms (e.g., S. pneumoniae) (Fig. 34.1, Table 34.3). Varicella zoster virus (VZV) and PJP also commonly occur in patients receiving corticosteroids for GVHD. Without trimethoprim–sulfamethoxazole (TMP–SMX) prophylaxis, patients are at risk for Nocardia and Toxoplasma infection. Failure of TMP–SMX prophylaxis also can occur (Table 34.3).
INFECTIONS IN PATIENTS WITH SOLID TUMORS
Immune Defect in Patients with Solid Tumors
While at a lower overall risk for infection than those with hematologic malignancies, patients with solid tumors are at risk from infections due to tumor-related immune dysfunction (e.g., mucocutaneous barrier disruption, cellular or humoral deficiencies) or treatment-related immune deficiency resulting from chemotherapy, radiation, or other immune-modulating therapy. Advanced age, malnutrition, comorbid conditions (e.g., chronic obstructive pulmonary disease [COPD]), and frequent health care exposures also increase infection risk.
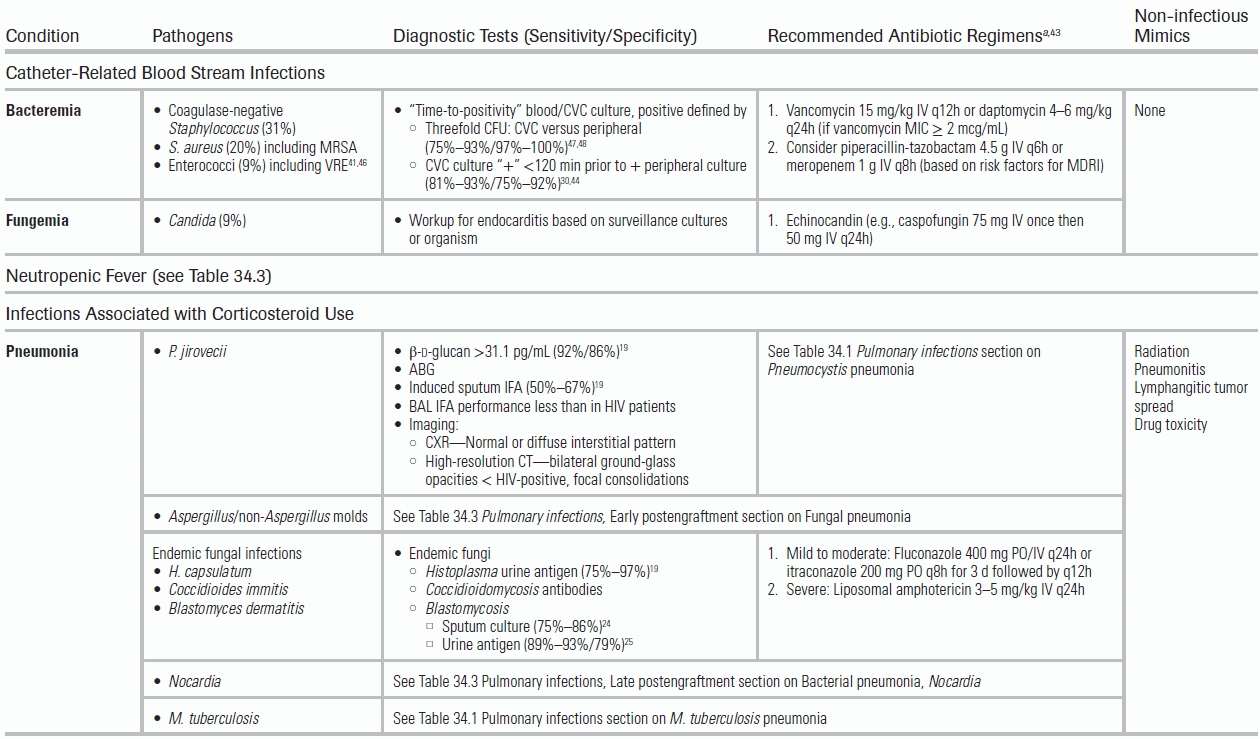
Catheter-Related Blood Stream Infections in Patients with Solid Tumors
CVCs are essential for the administration of chemotherapeutic agents, but may result in CRBSIs. Local inflammatory changes (e.g., warmth, erythema, or purulent exudate) are unreliable (sensitivity <3%); confirmation with a “time-to-positivity” microbiologic culture, simultaneously drawn from a peripheral site and the CVC, is recommended.68 The “time-to-positivity” diagnosis is established either quantitatively by a greater than threefold bacterial colony-forming unit (CFU) yield from the catheter versus the peripheral culture or temporally by catheter culture positivity ≤120 minutes before peripheral culture.47,48 Typical infecting organisms include coagulase-negative Staphylococcus (31%), S. aureus (20%) with increasing rates of methicillin resistance, Candida (9%), and enterococci (9%) with increasing rates of vancomycin resistance.46,69
Treatment of CRBSIs
Antibiotic Therapy
Antibiotic therapy should include vancomycin or daptomycin, and, in the presence of neutropenic fever and/or severe illness, an antipseudomonal β-lactam, such as a carbapenem (recommended if the patient has a history of ESBL infection).70 Based on risk factors for candidemia, empiric treatment may also include an echinocandin.
Catheter Management
Per the IDSA guidelines, long-term CVCs should be removed in the setting of infection with S. aureus, P. aeruginosa, or Candida. However, in the presence of alternative organisms (coagulase-negative S. aureus, Enterococcus) and in the absence of severe sepsis or complicating tunnel or exit-site infection, a trial of antibiotic lock therapy (ALT) and systemic antibiotics may allow for catheter salvage.70,71
Neutropenic Fever in Patients with Solid Tumors
In patients with solid tumors, repeated chemotherapy cycles lead to milder and briefer periods of neutropenia, compared to those with hematologic malignancies. Patients with solid tumors have a lower incidence of fungal infections, including Candida and Aspergillus, compared to patients with hematologic malignancies.
Corticosteroid Use and Risk of Opportunistic Infections in Patients with Solid Tumors
Prolonged corticosteroid use is common among solid tumor patients, particularly those with CNS lesions, increasing the risk for opportunistic infections including oropharyngeal candidiasis, Nocardia, Legionella, MTB, Aspergillus, endemic fungi, and P. jirovecii. Pneumocystis pneumonia occurs in <2% of non-HIV, solid tumor patients and is associated with daily prednisone doses (or corticosteroid equivalent) >15 mg for 4 weeks' duration.72,73
Diagnosis of PJP in HIV-Negative Patients with Solid Tumors
Due to a decreased P. jiroveci burden in HIV-Negative patients, the diagnostic yield of induced sputum, even with indirect fluorescent antibody (IFA) staining, is low. Recommended initial screening tests include (1–3)-β-d-glucan, PCR-based assays, and high-resolution chest CT imaging evaluating for ground-glass opacities.74–76 BAL is the gold standard for obtaining diagnostic samples and should be performed in patients with a high clinical suspicion for PJP.77
PJP Treatment in HIV-Negative Patients with Solid Tumors
TMP–SMX is the treatment of choice for non–HIV-infected PJP patients. Despite limited evidence in HIV-negative patients, adjunctive corticosteroids (prednisone 40 mg q12h) are recommended in patients with moderate to severe PJP characterized by PaO2 ≤ 70 mm Hg on ambient air.
Sepsis in Patients with Solid Tumors
Sepsis is a common cause for ICU admission in patients with solid tumors, particularly in the presence of neutropenia caused by respiratory, blood stream, abdominal, and urinary infections. Although these patients do benefit from ICU admission, it is important to note that common prognostic models, including APACHE II or III and the Simplified Acute Physiology Score (SAPS) II, generally underestimate hospital mortality in cancer patients.78 In patients with advanced cancer, preferences regarding the extent of therapeutic intervention(s) should be elicited early in their ICU course.
GENERAL CONSIDERATIONS BY TYPE OF SOLID TUMOR
Infections in nonneutropenic cancer patients include community-acquired and nosocomial infections with specific predilections by the type of malignancy (Table 34.4).
TABLE 34.4 Common Infectious Conditions for Patients with Solid Tumors
ESBL, extended-spectrum beta-lactamase; MRSA, methicillin-resistant S. aureus; UTI, urinary tract infection. From The Sanford guide to antimicrobial therapy. Sperryville, VA: Antimicrobial Therapy; 2012. Ref.34
Lung Cancer
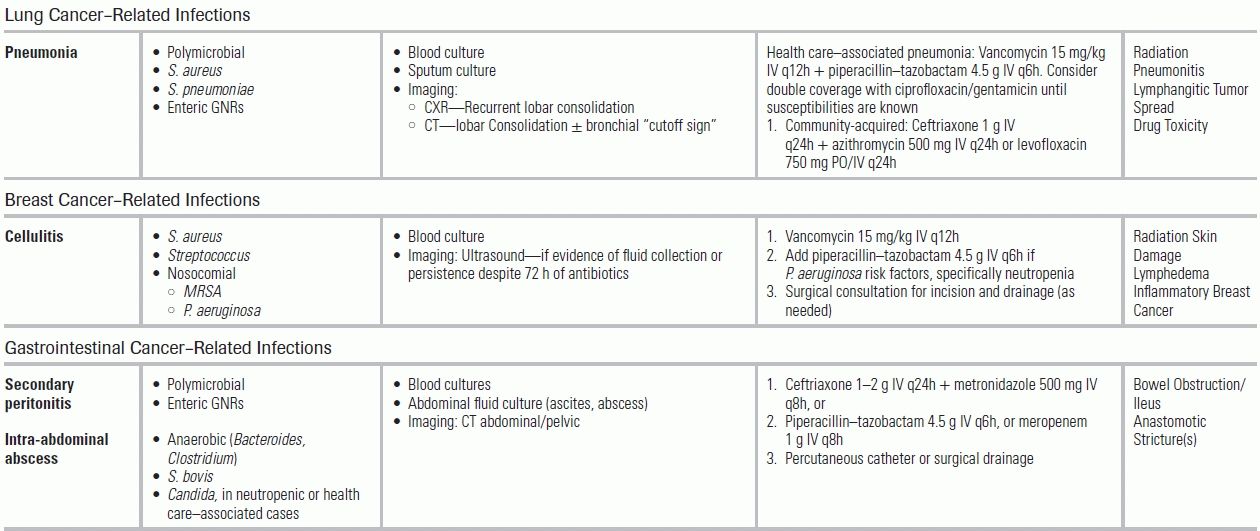
Pneumonia occurs in up to 24% of patients with lung cancer; 27% of these are postobstructive.79 Postobstructive pneumonias are generally polymicrobial; however, there is an increasing incidence of S. aureus and enteric GNR infections due to nosocomial exposures. Antimicrobial treatment should include staphylococcal, anaerobic, and GNR coverage (e.g., vancomycin + piperacillin–tazobactam) to minimize progression to lung abscess or empyema.80
Breast Cancer
Patients with breast cancer are at risk for postoperative skin and soft tissue infections (incidence of 4% to 12%). Lymphedema can also cause delayed episodes of streptococcal cellulitis.81,82 Antimicrobial treatment should include vancomycin, with consideration of antipseudomonal coverage for those with recent chemotherapy or neutropenia. Ultrasound evaluation for an underlying fluid collection should be considered if there is no clinical improvement within 72 hours of antibiotic treatment.
Gastrointestinal Cancer
Persons with gastrointestinal cancers are at risk for bowel obstruction and postsurgical complications, including anastomotic leaks, intra-abdominal abscesses, and peritonitis. Anaerobes (Bacteroides, Clostridium) are common copathogens. Antimicrobial treatment should include broad-spectrum GNR and anaerobic coverage (e.g., piperacillin–tazobactam or a carbapenem). Empiric coverage of Candida with an echinocandin is also recommended. When infectious collections are recognized, percutaneous or surgical drainage (source control) should be initiated within 12 hours.
Genitourinary Cancer
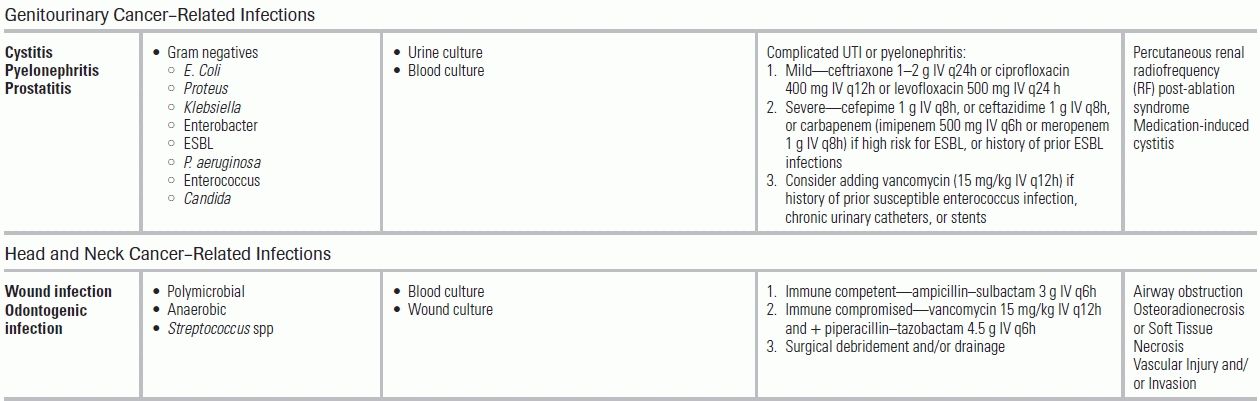
Genitourinary cancer patients have lower rates of infection (<5%); infections in this group are often related to urinary obstruction and/or diversion. Antimicrobial treatment should include coverage of GNRs, including ESBL organisms and vancomycin-resistant enterococci in those with risk factors (urinary procedures, uncontrolled diabetes, colonization with VRE). Urine cultures obtained from ileal conduit are rarely useful. These patients also may experience postsurgical wound infections, ranging from localized cellulitis to extensive infections resulting in pyometra and tuboovarian, intra-abdominal, or pelvic abscesses.
Head and Neck Cancer
For patients with head and neck cancers, wound infections secondary to loss of the protective oral mucosa barrier and subsequent oral anaerobic flora contamination commonly occur (6% to 20% incidence).83 Management includes antimicrobials against oral flora and, where indicated, surgical drainage, which provides diagnostic culture and source control. Pneumonia, from oropharyngeal aspiration, remains a leading cause of death and should be tested for with routine chest radiography.84
Infections in Patients after Solid Organ Transplantation
Immune Defect in Patients After SOT
For SOT recipients, intensified immune suppression has improved survival by reducing rejection, but it also increased the risk for infectious complications. Immune suppression may be divided into discrete phases: induction (high-dose corticosteroids ± antilymphocyte antibodies/IL-2 receptor antibodies), maintenance (corticosteroids, antimetabolites, calcineurin inhibitors), and treatment for episodes of rejection (high-dose corticosteroids, plasmapheresis, antilymphocyte antibodies). During the maintenance phase, immune suppression is gradually tapered; however, continued high levels may be required for transplanted organs with increased environmental exposure, such as the small bowel and lung. Despite continued high level of immune suppression, small bowel and lung recipients still experience the highest rates of acute rejection during the first year (70% and 30%, respectively).85,86 Treatment for acute rejection greatly increases the risk for infection. Plasmapheresis, for example, removes antibodies, increasing the risk for encapsulated bacterial infections. Lymphocyte-depleting therapies result in prolonged B- and T-cell defects, increasing the risk for viral and fungal infections. In general, SOT patients are rarely neutropenic, unless they are receiving chemotherapy for posttransplant lymphoproliferative disease.
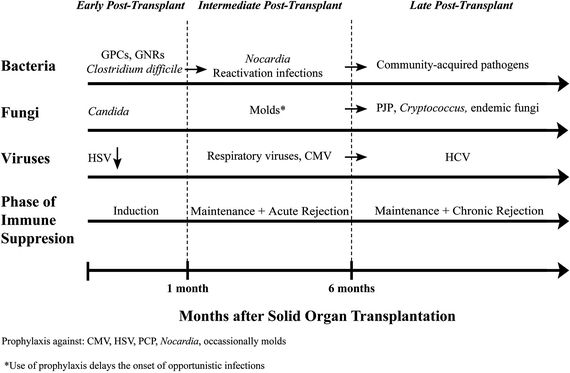
Timeline of Infections in SOT
As is the case with patients post-HSCT, knowledge of the timing since transplantation, intensity of immune suppression, and prophylaxis will allow clinicians to differentiate among likely infectious pathogens for solid organ recipients (Fig. 34.2).87 Typical prophylaxis includes TMP–SMX against P. jirovecii, Nocardia, Listeria, and Toxoplasma and valganciclovir against CMV and HSV. Lung transplant patients often receive antimold prophylaxis with itraconazole or voriconazole.
Early to Intermediate Posttransplantation Period
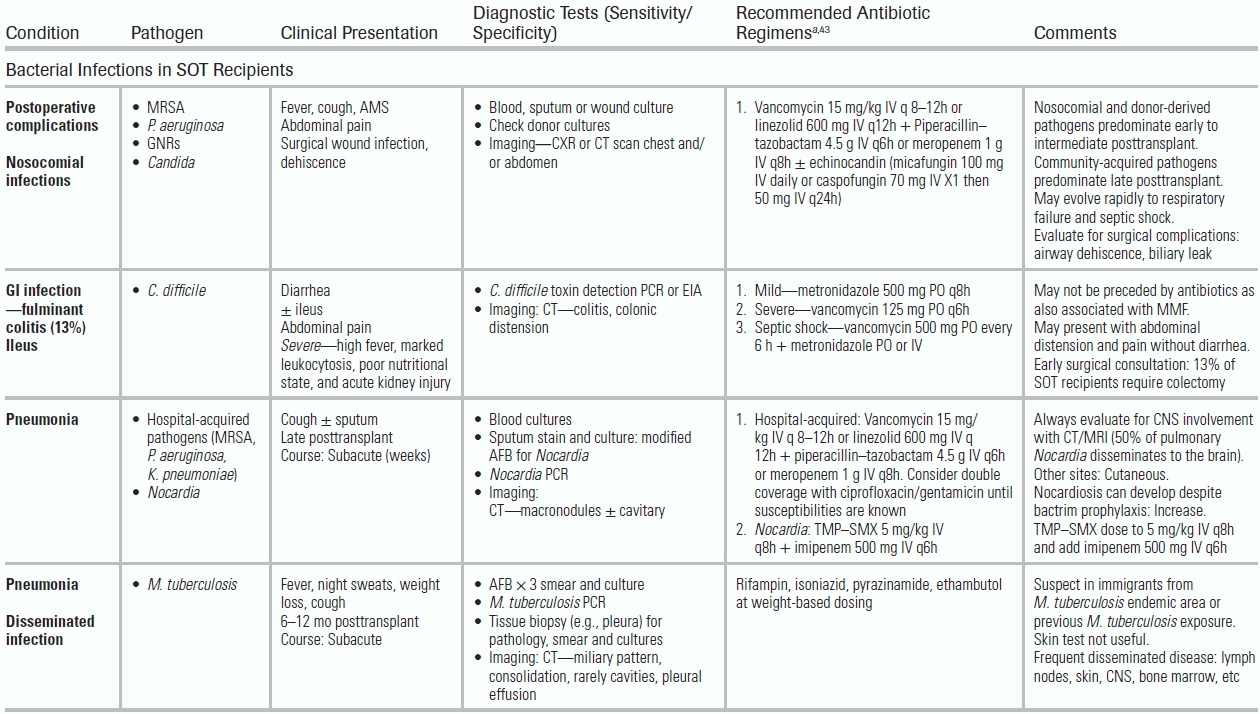
The early posttransplant period (1 month) is dominated by donor-derived infections, nosocomial pathogens (MRSA, P. aeruginosa, Candida, C. difficile), and surgical complications. Postoperative infectious complications may persist into the intermediate posttransplantation period, requiring prolonged antimicrobial treatment. During the intermediate posttransplant period (1 to 6 months), patients are the most vulnerable to opportunistic pathogens, as they are recovering from major surgery and are increasingly immune suppressed as induction therapy is taking effect.
Fungal Infections in the Early to Intermediate Posttransplant Period
For SOT recipients, the incidence of IFD varies by the organ transplanted. Incidence is highest in lung, small bowel, and liver transplant patients and is lowest in renal transplant patients.88 In liver transplants, the incidence of Candida infections ranges from 62% to 91% with risk factors including multiple surgical interventions, use of broad-spectrum antibiotics, and the use of TPN.89 In small bowel transplants, the incidence of Candida infections is 85%.90 In lung transplants, Aspergillus is the most common fungal infection, affecting up to 44% of patients and portending a high mortality (65% to 80%).89–91 Among heart transplant patients, the rate of IFD is 3%.90 During the intermediate posttransplant period, the clinician also must consider geographic and endemic mycoses. For example, disseminated cryptococcus with brain and lung manifestations; disseminated histoplasmosis to the lung, bone marrow, liver, and spleen; and disseminated coccidioidomycosis to the skin, skeletal system, and brain. The incidence of P. jiroveci has decreased with TMP–SMX prophylaxis but can present atypically with negative BAL, requiring biopsy for diagnosis in patients taking non–TMP-SMX–based prophylaxis.92 Infections caused by Aspergillus spp tend to be localized to the lung compared to non-Aspergillus molds that will disseminate in 50% of the cases.93
Diagnosis of Invasive Fungal Diseases
The diagnosis of IFD is particularly difficult in lung transplant patients, who are frequently colonized with Aspergillus. Antifungal treatment is initiated based on clinical presentation, radiographic findings, and results of culture and non–culture-based assays (Table 34.5). Aspergillus (GM) from serum, with an index cutoff of 0.5 in SOT recipients, is not a reliable test to rule out infection.45 Aspergillus GM from BAL has a higher diagnostic yield. For pulmonary aspergillosis, noncontrast CT scans of the thorax often demonstrates multiple pulmonary nodules with or without a “halo sign,” cavitation, or consolidation. For endemic mycoses, such as Cryptococcus and PJP, non–culture-based techniques are available and generally have good test performance (Table 34.5).19
TABLE 34.5 Common Infectious Conditions for Patients After Solid Organ Transplantation

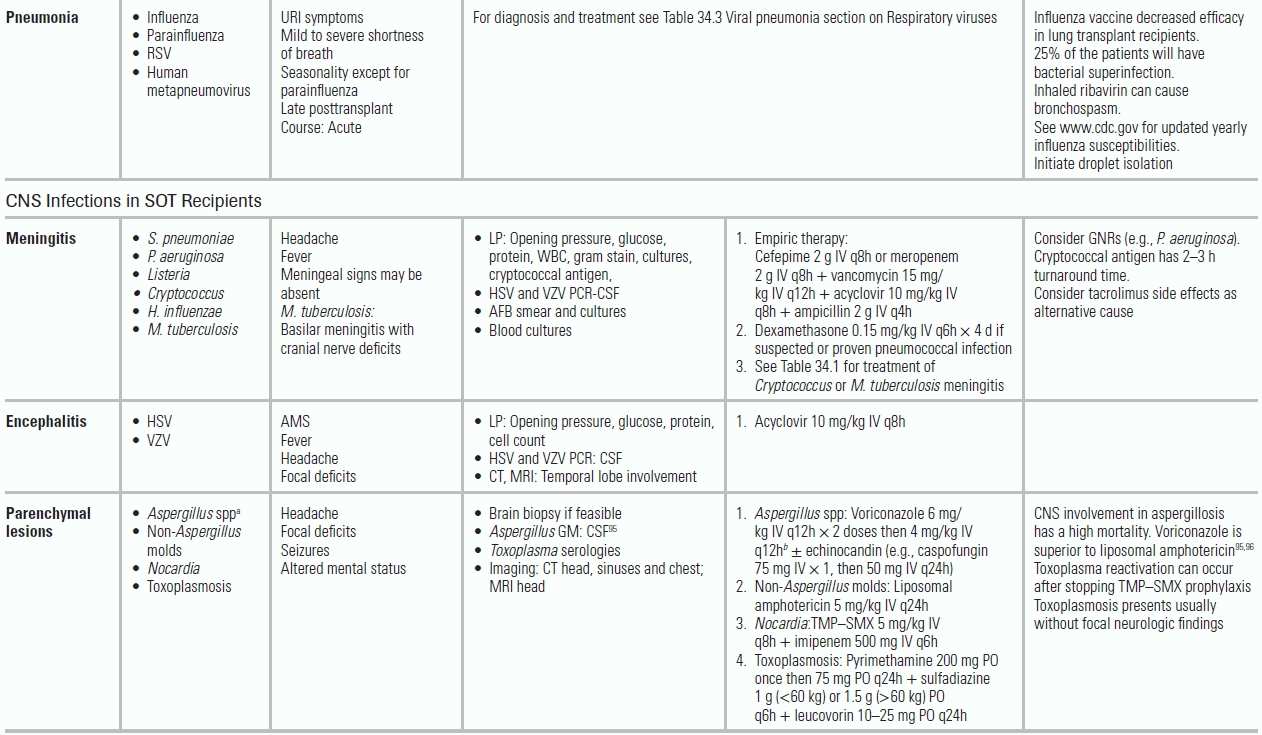

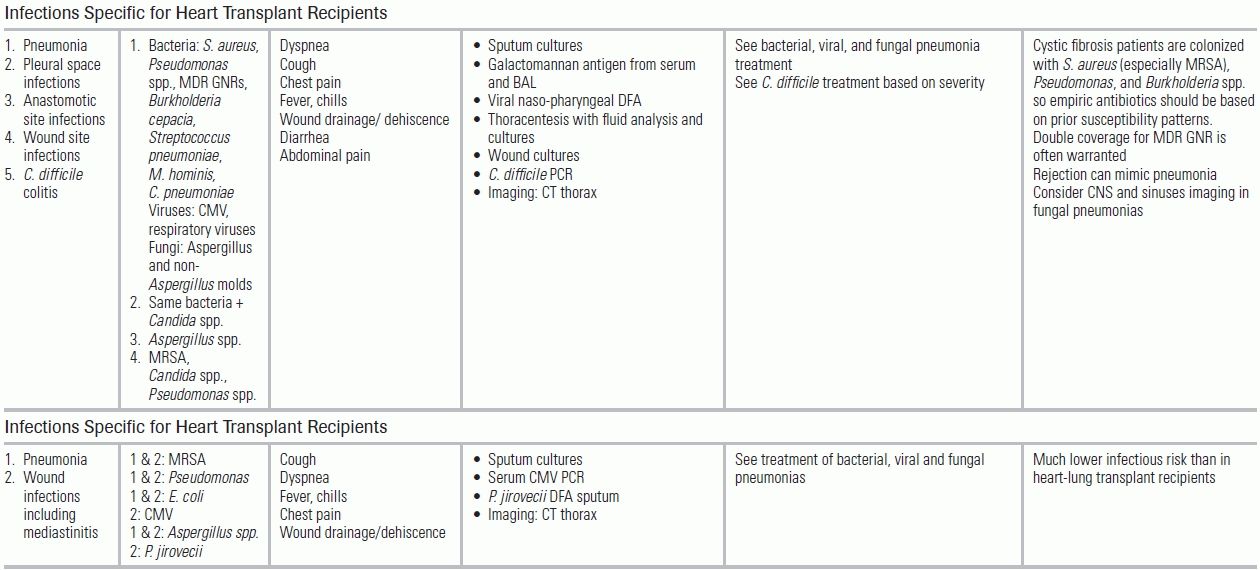
AFB, acid-fast bacilli; AMS, altered mental status; BAL, bronchoalveolar lavage; CMV, cytomegalovirus; CNS, central nervous system; CSF, cerebrospinal fluid; ELISA, enzyme-linked immunoassay; GI, gastrointestinal; GM, galactomannan; GNRs, gram-negative rods (e.g., K. pneumonia, E. Coli); GPCs, gram-positive cocci; HCV, hepatitis C virus; HD, hemodialysis; HHV6, human herpes virus 6; HSV, herpes simplex virus; IS, immune suppression; LFT, liver function tests; LP, lumbar puncture; MMF, mycophenolate mofetil; MRI, magnetic resonance imaging; MRSA, methicillin-resistant S. aureus; Non-Aspergillus molds, mucormycosis, Scedosporium, Fusarium; PCR, polymerase chain reaction; RSV, respiratory syncytial virus; SOT, solid organ transplant; TMP–SMX, trimethoprim–sulfamethoxazole; UA, urinalysis; URI, upper respiratory infection; UTI, urinary tract infection; VRE, vancomycin-resistant enterococcus; VZV, varicella zoster virus. From The Sanford guide to antimicrobial therapy. Sperryville, VA: Antimicrobial Therapy; 2012. Ref.34.
Treatment of Invasive Fungal Diseases
Candida prophylaxis is reserved for high-risk patients, including patients with a history of multiple abdominal surgeries and recipients of small bowel, pancreas, and liver transplants. Lung transplant centers differ in their use of antimold prophylaxis with triazoles, which are thought to promote infections with resistant molds (mucormycosis, Scedosporium).93 Non-Aspergillus molds account for 27% of the mycelial infections in SOTs.93 Rapid microbiologic diagnosis of invasive mold infections is key, given the high mortality rates, medication interactions, and variable susceptibility patterns. Voriconazole is the drug of choice in treating Aspergillus spp. Liposomal amphotericin should be used for almost all other non-Aspergillus molds. Transplant pharmacy consultation should be considered before initiating therapy with antifungal agents, macrolides, metronidazole, and norfloxacin, given significant drug interactions with the immunosuppressive medications.
Clostridium difficile Infections in the Early to Intermediate Posttransplant Period
C. difficile can persist into the intermediate posttransplant period, producing diarrhea and fulminant colitis in 13% of patients.97 The incidence of C. difficile infections varies by type of SOT ranging from 6% in kidney transplants to as high as 15% in heart and lung recipients.43,98,99 Since mycophenolate mofetil (MMF)—an immunosuppressant used extensively in transplant patients—has antibacterial properties and has been associated with C. difficile infections, prior antibiotic exposure is not mandatory. The clinical symptoms (diarrhea, abdominal pain ± fever, ± ileus) can occur while receiving antimicrobials or even weeks after stopping them. Diagnosis is based upon C. difficile toxin detection in the stool using an enzyme immunoassay (EIA) or PCR assay coupled with EIA for C. difficile glutamate dehydrogenase. In the atypical presentation of C. difficile with induced ileus, abdominal CT imaging can demonstrate colitis. In mild cases, metronidazole PO can be used while monitoring for signs of colonic distension. In severe disease characterized by high fever, marked leukocytosis, poor nutritional state, and acute kidney injury, vancomycin PO should be started. For patients with septic shock, treatment requires both high-dose vancomycin PO and metronidazole either PO or IV.100 Surgical consultation should be obtained, as 13% of SOT recipients with C. difficile colitis will require colectomy.101
Viral Infections in the Early to Intermediate Posttransplant Period
Respiratory Viruses
Weeks after transplant, most patients have returned to the community and are exposed to respiratory viruses. Potential pathogens include parainfluenza (no seasonality), influenza, RSV, and human metapneumovirus, but virtually any respiratory virus can cause pneumonia. Incidence of community-acquired respiratory virus disease ranges from 2% to 16%.102 Symptoms vary from a mild viral prodrome to respiratory failure. The protective antibody response to influenza vaccination is not as robust as in immune-competent hosts, and transplant patients can develop respiratory disease even after vaccination. For RSV, influenza, and parainfluenza, rapid viral antigen tests are recommended, in conjunction with PCR-based detection (Table 34.5). The yield is higher with samples obtained from lower in the respiratory tract, and a negative DFA from a nasopharyngeal swab does not rule out viral pneumonia (DFA nasal swab sensitivity is 15%). Respiratory viruses also may contribute to bacterial superinfection and rejection.103 Appropriate antiviral therapy (oseltamivir for influenza and inhaled ribavirin for RSV and human metapneumovirus) should be started and respiratory isolation initiated. Ribavirin therapy in parainfluenza infections remains controversial; however, its use in lung and heart–lung transplant patients in conjunction with methylprednisolone and IVIG has been associated with improved maintenance of lung function.104
Cytomegalovirus Infections
Posttransplantation, most patients receive prophylaxis with valganciclovir to prevent CMV disease. For SOT patients, a donor with a history of CMV and a recipient without previous exposure to CMV (donor positive/recipient negative (D+/R−)) is the combination that carries the highest risk of reactivating CMV. In contrast among HSCT patients, D−/R+ has the highest risk because the recipient losses his or her previous immunity against CMV after bone marrow transplantation. CMV “infection” is viral replication detected in the serum. CMV “disease” is defined as end-organ dysfunction attributed to the virus. This may involve invasive disease (colitis, pneumonitis, hepatitis, retinitis) or CMV viral syndrome (fever, malaise, leucopenia, or thrombocytopenia).105 Patients treated with both universal and preemptive prophylactic therapy can develop late-onset CMV disease. After stopping the prophylactic regimen (usually 3 to 6 months posttransplantation), 25% of the D+/R− SOT patients will develop active CMV infection. Because CMV may affect the allograft, it can cause nephropathy in kidney recipients and pneumonitis in lung recipients. Because of its immune modulatory effects, CMV increases the risk of bacterial and fungal infection, as well as graft rejection and loss.106
Diagnosis of CMV
CMV infection (viremia without end-organ damage) is characterized by nonspecific symptoms of fatigue and low-grade fever. CMV pneumonitis often manifests with dry cough and shortness of breath. CMV colitis presents with abdominal pain and diarrhea. Quantitative PCR-CMV viral load should be obtained in patients at risk for infection (D+/R−, D+/R+, D−/R+). Some patients may require invasive testing and biopsy in cases of normal serum PCR (commonly seen in CMV colitis) or to identify invasive disease.
Treatment and Follow-up of CMV Disease
Based on severity, patients may receive outpatient treatment with oral valganciclovir107 or inpatient therapy with IV ganciclovir. In a patient with stable or worsening viremia after >2 weeks of adequate CMV treatment, infection with a ganciclovir-resistant virus should be suspected and CMV genotype sent.
Reactivation Infections in the Intermediate Posttransplantation Period
Given a patient's intense state of immune suppression during this period, latent infections may reactivate, including MTB, toxoplasmosis (if not on prophylaxis), and leishmaniasis. The risk of MTB varies widely depending on the country of origin, with an incidence (1.2% to 15%) approximately 50 times higher than in the immune-competent population.108 Tuberculosis presents with dry cough, fevers, night sweats, and weight loss, with frequent dissemination to the skin, soft tissues, and lymph nodes. Given that the mortality rate of MTB is 30%, these symptoms demand high suspicion in certain epidemiologic scenarios. The efficacy of pretransplant skin testing and interferon (IFN)-γ release assays is under investigation in transplant patients. Diagnosis of MTB is made by AFB smear and culture. Four-drug therapy is recommended along with immediate respiratory isolation (Table 34.5).
Leishmaniasis is a very uncommon disease in SOT population (62 cases reported)109 and usually arises from reactivation (but can also be transmitted by blood transfusion or organs).
Toxoplasmosis may be acquired via transplanted organs (especially the heart), reactivation, or from exposure to feline excrements. It develops in patients not on TMP–SMX prophylaxis. Infection can occur in the CNS (4% to 29% of the CNS lesions in transplant patients can be attributed to toxoplasmosis),110 heart (myocarditis), lung (pneumonitis), and retina (choroiditis). Fever is present along with headache and altered mental status. Diagnosis relies on positive serologies, presence of the parasite in the histologic specimen, and/or PCR technology.
Late Posttransplantation Period
The late posttransplantation period begins >6 months postoperatively. During this time, most infections are community acquired, with typical pathogens including S. pneumoniae and respiratory viruses. Community-acquired pneumonia presents similarly in both immune-compromised and immune-competent hosts, but in transplant patients, it can evolve rapidly into respiratory failure. The initial antimicrobial regimen should cover nosocomial pathogens (MRSA, P. aeruginosa, Klebsiella pneumoniae) until finalized sputum cultures and blood cultures allow de-escalation or until the patient is clinically improving. This period is also characterized by late-onset infections with CMV, Nocardia, and/or PJP after the discontinuation of prophylaxis, but while the patient is still receiving active immune suppression. Cryptococcosis, with a prevalence of 0.2% to 5% and a mortality of 40%, is an important IFD in the transplant population.89 It usually occurs at approximately 1.5 years posttransplantation.90 Patients can present with localized pulmonary disease (cough and shortness of breath) or with disseminated disease affecting the CNS. Because of the lack of inflammation, meningeal signs may be absent; fever, headache, and altered mentation may be the only findings. Lumbar puncture studies should include a record of the opening pressure and cerebrospinal fluid (CSF) cryptococcal antigen. Cryptococcal meningitis is treated as disseminated disease with liposomal amphotericin and 5-flucytosine for the first 2 weeks. In endemic regions of the United States, emergency physicians should also evaluate for endemic mycoses: histoplasmosis in the Ohio and Mississippi river valleys, coccidioidomycosis in the Southwest, and blastomycosis in the Midwest, Southeast, and South Central states. Serologic studies and antigens along with histopathologic examination provide the diagnosis.
Conclusion
Immune-compromised persons are a highly specialized patient subset whose management is increasingly being returned to community-based physicians. This chapter serves as a guide to their initial management, but the emergency physician is encouraged to consult with infectious disease specialists, oncologists, and transplant physicians familiar with ongoing disease management. In addition, the early involvement of pulmonologists, gastroenterologists, and surgeons may facilitate early diagnosis and improve targeted therapy. Optimization of patient outcomes requires an understanding of pathogen susceptibilities, appropriate empiric anti-infective regimens, and a multidisciplinary approach to patient care.
LITERATURE TABLE
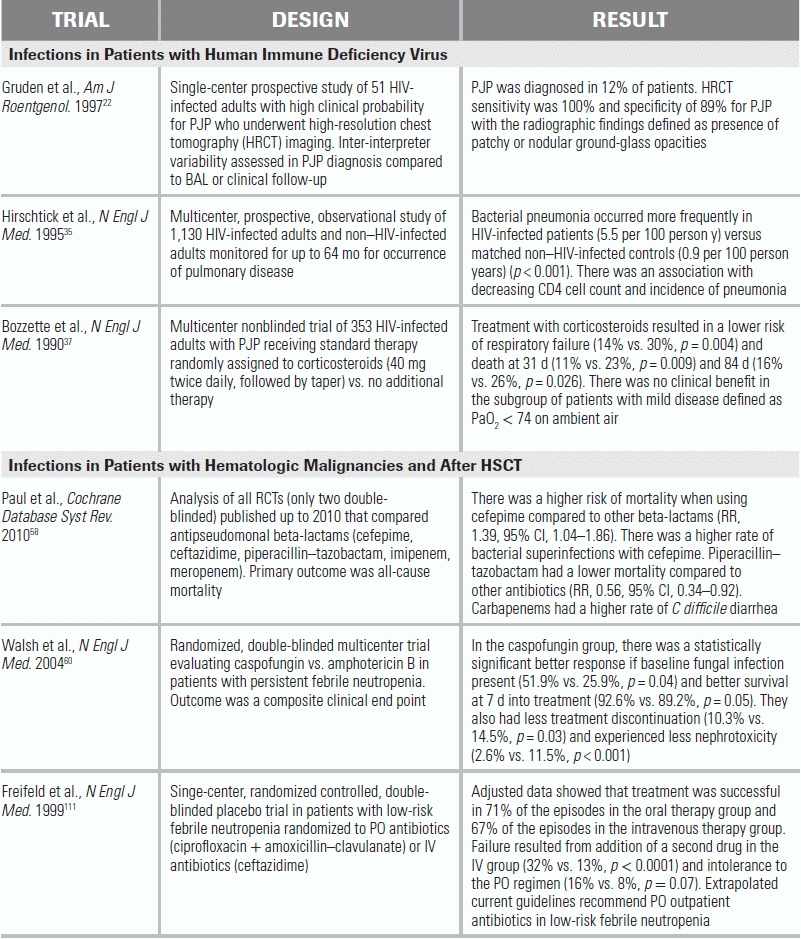
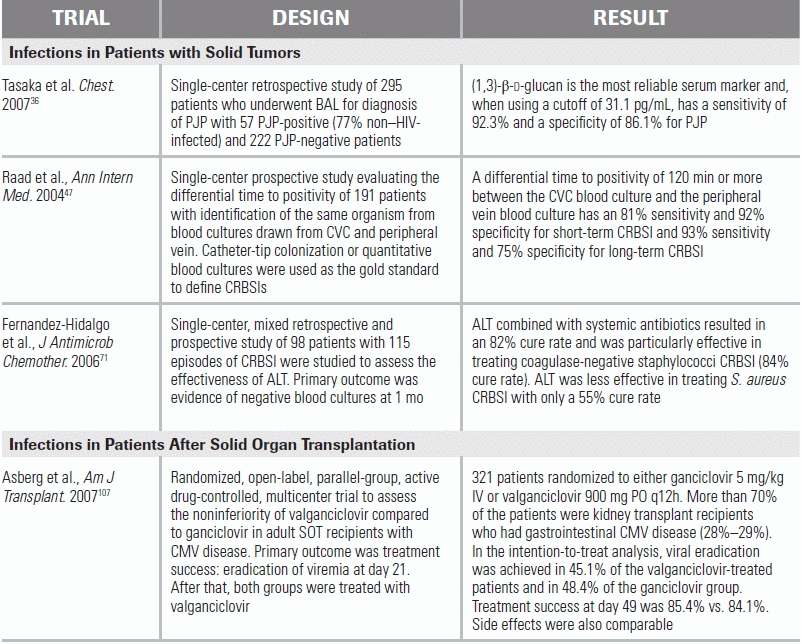
CI, confidence interval; RR, relative risk.
REFERENCES
1.Bodey GP. The emergence of fungi as major hospital pathogens. J Hosp Infect. 1988;11(suppl A):411–426.
2.Duthie R, Denning DW. Aspergillus fungemia: report of two cases and review. Clin Infect Dis. 1995;20:598–605.
3.Morrell M, Fraser VJ, Kollef MH. Delaying the empiric treatment of candida bloodstream infection until positive blood culture results are obtained: a potential risk factor for hospital mortality. Antimicrob Agents Chemother. 2005;49:3640–3645.
4.Blum U, Windfuhr M, Buitrago-Tellez C, et al. Invasive pulmonary aspergillosis. MRI, CT, and plain radiographic findings and their contribution for early diagnosis. Chest. 1994;106:1156–1161.
5.Caillot D, Casasnovas O, Bernard A, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–147.
6.Kuhlman JE, Fishman EK, Siegelman SS. Invasive pulmonary aspergillosis in acute leukemia: characteristic findings on CT, the CT halo sign, and the role of CT in early diagnosis. Radiology. 1985;157:611–614.
7.Mori M, Galvin JR, Barloon TJ, et al. Fungal pulmonary infections after bone marrow transplantation: evaluation with radiography and CT. Radiology. 1991;178:721–726.
8.Staples CA, Kang EY, Wright JL, et al. Invasive pulmonary aspergillosis in AIDS: radiographic, CT, and pathologic findings. Radiology. 1995;196:409–414.
9.Adlakha A, Pavlou M, Walker DA, et al. Survival of HIV-infected patients admitted to the intensive care unit in the era of highly active antiretroviral therapy. Int J STD AIDS. 2011;22:498–504.
10.Dickson SJ, Batson S, Copas AJ, et al. Survival of HIV-infected patients in the intensive care unit in the era of highly active antiretroviral therapy. Thorax. 2007;62:964–968.
11.Powell K, Davis JL, Morris AM, et al. Survival for patients With HIV admitted to the ICU continues to improve in the current era of combination antiretroviral therapy. Chest. 2009;135:11–17.
12.Akgun KM, Pisani M, Crothers K. The changing epidemiology of HIV-infected patients in the intensive care unit. J Intensive Care Med. 2011;26:151–164.
13.Huang L, Quartin A, Jones D, et al. Intensive care of patients with HIV infection. N Engl J Med. 2006;355:173–181.
14.Nickas G, Wachter RM. Outcomes of intensive care for patients with human immunodeficiency virus infection. Arch Intern Med. 2000;160:541–547.
15.Greenberg JA, Lennox JL, Martin GS. Outcomes for critically ill patients with HIV and severe sepsis in the era of highly active antiretroviral therapy. J Crit Care. 2012;27:51–57.
16.Kaplan JE, Benson C, Holmes KH, et al. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207; quiz CE201-204.
17.Den Boer JW, Yzerman EP. Diagnosis of Legionella infection in Legionnaires' disease. Eur J Clin Microbiol Infect Dis. 2004;23:871–878.
18.Helbig JH, Uldum SA, Bernander S, et al. Clinical utility of urinary antigen detection for diagnosis of community-acquired, travel-associated, and nosocomial legionnaires' disease. J Clin Microbiol. 2003;41:838–840.
19.Hsu JL, Ruoss SJ, Bower ND, et al. Diagnosing invasive fungal disease in critically ill patients. Crit Rev Microbiol. 2011;37:277–312.
20.Sax PE, Komarow L, Finkelman MA, et al. Blood (1- > 3)-beta-D-glucan as a diagnostic test for HIV-related Pneumocystis jirovecii pneumonia. Clin Infect Dis. 2011;53:197–202.
21.Cruciani M, Marcati P, Malena M, et al. Meta-analysis of diagnostic procedures for Pneumocystis carinii pneumonia in HIV-1-infected patients. Eur Respir J. 2002;20:982–989.
22.Gruden JF, Huang L, Turner J, et al. High-resolution CT in the evaluation of clinically suspected Pneumocystis carinii pneumonia in AIDS patients with normal, equivocal, or nonspecific radiographic findings. AJR Am J Roentgenol. 1997;169:967–975.
23.Smego RA, Jr., Nagar S, Maloba B, et al. A meta-analysis of salvage therapy for Pneumocystis carinii pneumonia. Arch Intern Med. 2001;161:1529–1533.
24.Martynowicz MA, Prakash UB. Pulmonary blastomycosis: an appraisal of diagnostic techniques. Chest. 2002;121:768–773.
25.Durkin M, Witt J, Lemonte A, et al. Antigen assay with the potential to aid in diagnosis of blastomycosis. J Clin Microbiol. 2004;42:4873–4875.
26.Meyohas MC, Roux P, Bollens D, et al. Pulmonary cryptococcosis: localized and disseminated infections in 27 patients with AIDS. Clin Infect Dis. 1995;21:628–633.
27.Pappas PG, Perfect JR, Cloud GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001;33:690–699.
28.Benson CA, Williams PL, Currier JS, et al. A prospective, randomized trial examining the efficacy and safety of clarithromycin in combination with ethambutol, rifabutin, or both for the treatment of disseminated Mycobacterium avium complex disease in persons with acquired immunodeficiency syndrome. Clin Infect Dis. 2003;37:1234–1243.
29.Tanner DC, Weinstein MP, Fedorciw B, et al. Comparison of commercial kits for detection of cryptococcal antigen. J Clin Microbiol. 1994;32:1680–1684.
30.Cingolani A, De Luca A, Ammassari A, et al. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J Med Microbiol. 1996;45:472–476.
31.Castro R, Prieto ES, da Luz Martins Pereira F. Nontreponemal tests in the diagnosis of neurosyphilis: an evaluation of the Venereal Disease Research Laboratory (VDRL) and the Rapid Plasma Reagin (RPR) tests. J Clin Lab Anal. 2008;22:257–261.
32.Hart G. Syphilis tests in diagnostic and therapeutic decision making. Ann Intern Med. 1986;104:368–376.
33.Pai M, Flores LL, Pai N, et al. Diagnostic accuracy of nucleic acid amplification tests for tuberculous meningitis: a systematic review and meta-analysis. Lancet Infect Dis. 2003;3:633–643.
34.The Sanford guide to antimicrobial therapy. Sperryville, VA: Antimicrobial Therapy; 2012.
35.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333(13):845–851.
36.Tasaka S, Hasegawa N, Kobayashi S, et al. Serum indicators for the diagnosis of pneumocystis pneumonia. Chest. 2007;131(4):1173–1180.
37.Bozzette SA, Sattler FR, Chiu J, et al. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. N Engl J Med. 1990;323(21):1451–1457.
38.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623.
39.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52:427–431.
40.Klastersky J. Management of fever in neutropenic patients with different risks of complications. Clin Infect Dis. 2004;39(suppl 1):S32–S37.
41.Wisplinghoff H, Seifert H, Wenzel RP, et al. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003;36:1103–1110.
42.Pizzo PA. Evaluation of fever in the patient with cancer. Eur J Cancer Clin Oncol. 1989;25(2):S9–S16
43.Munoz P, Giannella M, Alcala L, et al. Clostridium difficile-associated diarrhea in heart transplant recipients: is hypogammaglobulinemia the answer? J Heart Lung Transplant. 2007;26:907–914.
44.Blazquez RM, Espinosa FJ, Martinez-Toldos CM, et al. Sensitivity of urinary antigen test in relation to clinical severity in a large outbreak of Legionella pneumonia in Spain. Eur J Clin Microbiol Infect Dis. 2005;24:488–491.
45.Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis. 2006;42:1417–1427.
46.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805.
47.Raad I, Hanna HA, Alakech B, et al. Differential time to positivity: a useful method for diagnosing catheter-related bloodstream infections. Ann Intern Med. 2004;140:18–25.
48.Flynn PM, Shenep JL, Barrett FF. Differential quantitation with a commercial blood culture tube for diagnosis of catheter-related infection. J Clin Microbiol. 1988;26:1045–1046.
49.Englund JA, Piedra PA, Jewell A, et al. Rapid diagnosis of respiratory syncytial virus infections in immunocompromised adults. J Clin Microbiol. 1996;34:1649–1653.
50.Chartrand C, Leeflang MM, Minion J, et al. Accuracy of rapid influenza diagnostic tests: a meta-analysis. Ann Intern Med. 2012;156:500–511.
51.Couble A, Rodriguez-Nava V, de Montclos MP, et al. Direct detection of Nocardia spp. in clinical samples by a rapid molecular method. J Clin Microbiol. 2005;43:1921–1924.
52.Palmer DL, Harvey RL, Wheeler JK. Diagnostic and therapeutic considerations in Nocardia asteroides infection. Medicine (Baltimore). 1974;53:391–401.
53.Planche T, Aghaizu A, Holliman R, et al. Diagnosis of Clostridium difficile infection by toxin detection kits: a systematic review. Lancet Infect Dis. 2008;8:777–784.
54.van den Berg RJ, Bruijnesteijn van Coppenraet LS, Gerritsen HJ, et al. Prospective multicenter evaluation of a new immunoassay and real-time PCR for rapid diagnosis of Clostridium difficile-associated diarrhea in hospitalized patients. J Clin Microbiol. 2005;43:5338–5340.
55.Kim DY, Lee YS, Ahn S, et al. The usefulness of procalcitonin and C-reactive protein as early diagnostic markers of bacteremia in cancer patients with febrile neutropenia. Cancer Res Treat. 2011;43:176–180.
56.Schuttrumpf S, Binder L, Hagemann T, et al. Utility of procalcitonin concentration in the evaluation of patients with malignant diseases and elevated C-reactive protein plasma concentrations. Clin Infect Dis. 2006;43:468–473.
57.Sanz MA, Lopez J, Lahuerta JJ, et al. Cefepime plus amikacin versus piperacillin-tazobactam plus amikacin for initial antibiotic therapy in haematology patients with febrile neutropenia: results of an open, randomized, multicentre trial. J Antimicrobial Chemotherapy. 2002;50:79–88.
58.Paul M, Yahav D, Bivas A, et al. Anti-pseudomonal beta-lactams for the initial, empirical, treatment of febrile neutropenia: comparison of beta-lactams. Cochrane Database Syst Rev. 2010:CD005197.
59.King A, Boothman C, Phillips I. Comparative in vitro activity of cefpirome and cefepime, two new cephalosporins. Eur J Clin Microbiol Infect Dis. 1990;9:677–685.
60.Walsh TJ, Teppler H, Donowitz GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004;351:1391–1402.
61.Walsh TJ, Pappas P, Winston DJ, et al. Voriconazole compared with liposomal amphotericin B for empirical antifungal therapy in patients with neutropenia and persistent fever. N Engl J Med. 2002;346:225–234.
62.Berghmans T, Paesmans M, Lafitte JJ, et al. Therapeutic use of granulocyte and granulocyte-macrophage colony-stimulating factors in febrile neutropenic cancer patients. A systematic review of the literature with meta-analysis. Support Care Cancer. 2002;10:181–188.
63.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205.
64.Chemaly RF, Ghosh S, Bodey GP, et al. Respiratory viral infections in adults with hematologic malignancies and human stem cell transplantation recipients: a retrospective study at a major cancer center. Medicine. 2006;85:278–287.
65.Freeman RB, Jr. The 'indirect' effects of cytomegalovirus infection. Am J Transplant. 2009;9:2453–2458.
66.Walsh TJ, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–360.
67.Greene RE, Schlamm HT, Oestmann JW, et al. Imaging findings in acute invasive pulmonary aspergillosis: clinical significance of the halo sign. Clin Infect Dis. 2007;44:373–379.
68.Safdar N, Maki DG. Inflammation at the insertion site is not predictive of catheter-related bloodstream infection with short-term, noncuffed central venous catheters. Crit Care Med. 2002;30:2632–2635.
69.Wisplinghoff H, Bischoff T, Tallent SM, et al. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317.
70.Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45.
71.Fernandez-Hidalgo N, Almirante B, Calleja R, et al. Antibiotic-lock therapy for long-term intravascular catheter-related bacteraemia: results of an open, non-comparative study. J Antimicrob Chemother. 2006;57(6):1172–1180.
72.Sepkowitz KA. Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin Infect Dis. 2002;34:1098–1107.
73.Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illness and prior corticosteroid therapy. Mayo Clin Proc. 1996;71:5–13.
74.Azoulay E, Bergeron A, Chevret S, et al. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009;135:655–661.
75.Hardak E, Brook O, Yigla M. Radiological features of Pneumocystis jirovecii Pneumonia in immunocompromised patients with and without AIDS. Lung. 2010;188:159–163.
76.Tasaka S, Tokuda H, Sakai F, et al. Comparison of clinical and radiological features of pneumocystis pneumonia between malignancy cases and acquired immunodeficiency syndrome cases: a multicenter study. Intern Med. 2010;49:273–281.
77.LaRocque RC, Katz JT, Perruzzi P, et al. The utility of sputum induction for diagnosis of Pneumocystis pneumonia in immunocompromised patients without human immunodeficiency virus. Clin Infect Dis. 2003;37:1380–1383.
78.den Boer S, de Keizer NF, de Jonge E. Performance of prognostic models in critically ill cancer patients—a review. Crit Care. 2005;9:R458–R463.
79.Kohno S, Koga H, Oka M, et al. The pattern of respiratory infection in patients with lung cancer. Tohoku J Exp Med. 1994;173:405–411.
80.Perlman LV, Lerner E, D'Esopo N. Clinical classification and analysis of 97 cases of lung abscess. Am Rev Respir Dis. 1969;99:390–398.
81.Keidan RD, Hoffman JP, Weese JL, et al. Delayed breast abscesses after lumpectomy and radiation therapy. Am Surg. 1990;56:440–444.
82.Olsen MA, Chu-Ongsakul S, Brandt KE, et al. Hospital-associated costs due to surgical site infection after breast surgery. Arch Surg. 2008;143:53–60; discussion 61.
83.Papac RJ. Medical aspects of head and neck cancer. Cancer Invest. 1985;3:435–444.
84.Weber RS, Hankins P, Rosenbaum B, et al. Nonwound infections following head and neck oncologic surgery. Laryngoscope. 1993;103:22–27.
85.Abu-Elmagd K, Reyes J, Bond G, et al. Clinical intestinal transplantation: a decade of experience at a single center. Ann Surg. 2001;234:404–416; discussion 416–407.
86.Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086.
87.Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601–2614.
88.Singh N. Fungal infections in the recipients of solid organ transplantation. Infect Dis Clin North Am. 2003;17:113–134, viii
89.Silveira FP, Husain S. Fungal infections in solid organ transplantation. Med Mycol. 2007;45:305–320.
90.Pappas PG, Alexander BD, Andes DR, et al. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis. 2010;50:1101–1111.
91.Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44–69.
92.Fishman JA. Prevention of infection caused by Pneumocystis carinii in transplant recipients. Clin Infect Dis. 2001;33:1397–1405.
93.Husain S, Alexander BD, Munoz P, et al. Opportunistic mycelial fungal infections in organ transplant recipients: emerging importance of non-Aspergillus mycelial fungi. Clin Infect Dis. 2003;37:221–229.
94.Singh N, Forrest G. Cryptococcosis in solid organ transplant recipients. Am J Transplant. 2009;9(suppl 4):S192–S198.
95.Herbrecht R, Denning DW, Patterson TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002;347:408–415.
96.Schwartz S, Ruhnke M, Ribaud P, et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005;106:2641–2645.
97.Riddle DJ, Dubberke ER. Clostridium difficile infection in solid organ transplant recipients. Curr Opin Organ Transplant. 2008;13:592–600.
98.Gunderson CC, Gupta MR, Lopez F, et al. Clostridium difficile colitis in lung transplantation. Transplant Infect Dis. 2008;10:245–251.
99.Keven K, Basu A, Re L, et al. Clostridium difficile colitis in patients after kidney and pancreas-kidney transplantation. Transplant Infect Dis. 2004;6:10–14.
100.Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431–455.
101.Dallal RM, Harbrecht BG, Boujoukas AJ, et al. Fulminant Clostridium difficile: an underappreciated and increasing cause of death and complications. Ann Surg. 2002;235:363–372.
102.Kim YJ, Boeckh M, Englund JA. Community respiratory virus infections in immunocompromised patients: hematopoietic stem cell and solid organ transplant recipients, and individuals with human immunodeficiency virus infection. Semin Respir Crit Care Med. 2007;28:222–242.
103.Ison MG. Respiratory viral infections in transplant recipients. Antivir Ther. 2007;12:627–638.
104.Liu V, Dhillon GS, Weill D. A multi-drug regimen for respiratory syncytial virus and parainfluenza virus infections in adult lung and heart-lung transplant recipients. Transplant Infect Dis. 2010;12:38–44.
105.Humar A, Michaels M. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant. 2006;6:262–274.
106.Rubin RH. The pathogenesis and clinical management of cytomegalovirus infection in the organ transplant recipient: the end of the 'silo hypothesis'. Curr Opin Infect Dis. 2007;20:399–407.
107.Asberg A, Humar A, Rollag H, et al. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7(9):2106–2113.
108.Munoz P, Rodriguez C, Bouza E. Mycobacterium tuberculosis infection in recipients of solid organ transplants. Clin Infect Dis. 2005;40:581–587.
109.Basset D, et al. Visceral leishmaniasis in organ transplant recipients: 11 new cases and a review of the literature. Microbes Infect. 2005;7(13):1370–1375
110.Singh N, et al. Infections of the central nervous system in transplant recipients. Transpl Infect Dis. 2000;2(3):101–111
111.Freifeld A, Marchigiani D, Walsh T, et al. A double-blind comparison of empirical oral and intravenous antibiotic therapy for low-risk febrile patients with neutropenia during cancer chemotherapy. N Engl J Med. 1999;341(5):305–311.