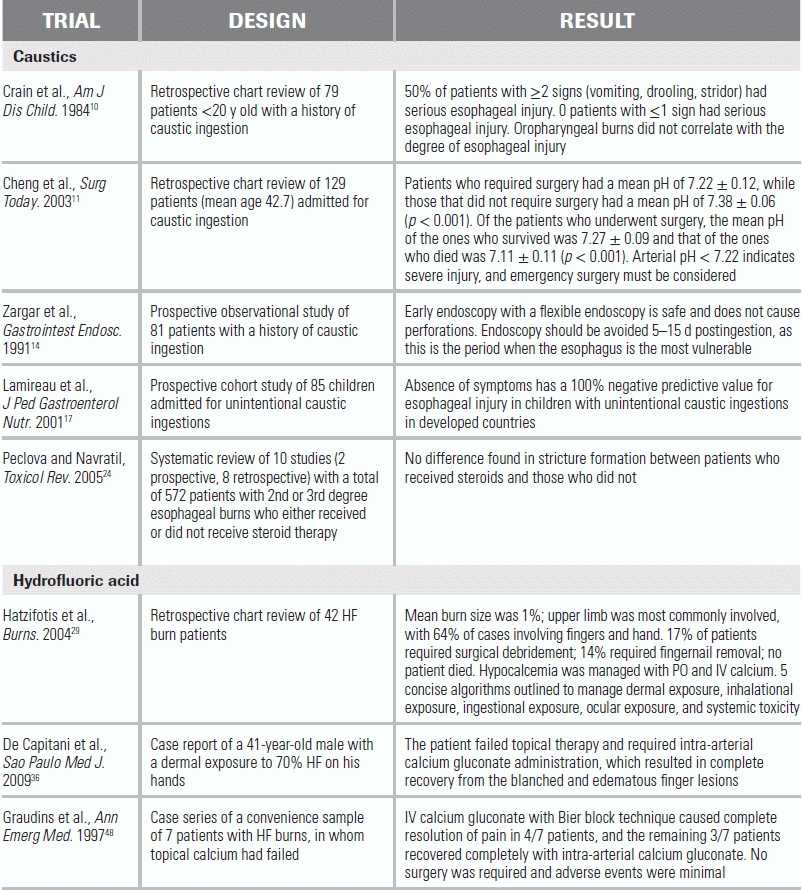
LITERATURE TABLE
BACKGROUND
In 2010, the American Association of Poison Control Centers documented 201,750 reported exposures to caustic household cleaning substances. Most acid, alkali, and other caustic exposures occur via ingestion: 85% are unintentional, and more than 90% occur in children.1 Intentional exposures, although less common, are more likely to result in severe damage.2,3 Immediate risks of caustic exposure include esophageal perforation and death. Delayed risks include stricture formation and esophageal carcinoma.2,4
PATHOPHYSIOLOGY
The extent of tissue injury caused by contact with caustics is determined by four factors: the amount of caustic ingested, the specific caustic ingested, the caustic's pH, and its titratable acid/alkaline reserve (TAR).5,6 Significant damage can be caused by strong acids (pH < 3) and strong bases (pH > 11), as well by certain substances with near-neutral pH, such as phenol, because of high TAR.6 TAR is defined as the amount of HCl or NaOH needed to titrate a given caustic to a pH of 8, which is close to normal esophageal pH.5 In evaluating the potential of a substance to cause esophageal injury, TAR may be more accurate than pH; however, TAR values can vary greatly among similar substances and even between solid and liquid forms of the same substance. TAR values may be unavailable to the emergency physician during the initial evaluation of a patient; however, TARs of common household substances are published and can be accessed as needed.5 While the practical use of TARs in the ED is limited, it is important for the emergency physician to be mindful that a substance can cause significant caustic injury despite having a near-neutral pH.
Strong alkalis (or bases) produce liquefactive tissue necrosis when hydroxide (OH−) ions penetrate deeply into tissue surfaces. This necrotic tissue is then hydrolyzed by enzymes and forms a soft, purulent fluid mass.7 Alkalis commonly encountered in the home that can cause significant caustic injury in small amounts include lye-containing liquids typically used in oven cleaners and drain openers (historically composed of potassium hydroxide (KOH), but now more commonly sodium hydroxide (NaOH)). Solid caustics that contain strong alkalis, such as laundry powders and dishwasher detergents, can also cause severe injury in small volumes because of prolonged adherence of the solid to the mucosa.8 Household strength ammonium hydroxide (NH4OH, found in multiple household cleaning products) and sodium hypochlorite (NaOCl, found in household bleach) are generally dilute enough not to cause significant esophageal damage except when ingested in large volumes (e.g., in an intentional ingestion).
Liquid detergent capsules, only relatively recently available in North America, deserve special mention for their unique ability to cause serious injury. Introduced in Europe in 2001, these detergent capsules became available in North America in 2011. They contain a concentrated liquid detergent, composed of anionic and ionic detergents, propylene glycol, and ethanol, in a water-soluble polyvinyl alcohol sachet.9 Although the pH of the detergents is close to physiologic (pH 7 to 9), they have caused severe esophageal, skin, and eye irritation and damage. A recent retrospective review attempted to catalogue the presenting symptoms and outcomes of patients exposed to these capsules.9 The majority of exposures were unintentional ingestions in children, and vomiting was the most common presenting complaint. Several children presented with severe respiratory distress and central nervous system (CNS) depression, typically shortly after exposure. Consistent follow-up was not possible in a majority of the cases, and thus the long-term effect of these liquid detergent ingestions is unclear; as such, these exposures should be managed cautiously.
Finally, the ingestion of button batteries historically posed a serious threat because of their tendency to leak sodium and potassium hydroxide upon contact with the esophageal mucosa. Newer batteries, although still of clinical concern, are significantly more resistant to leakage.6
Acids dissociate into hydrogen (H+) ions and cause coagulation necrosis, which results in desiccation of tissues and a firm eschar formation. Eschar formation may, in fact, protect against further injury by limiting deeper penetration of the acid. Common strong household acids include hydrochloric acid (found in toilet bowl cleaners and other cleaning products) and hydrofluoric acid (HF) (present in rust removers and wall and tile cleaners).1 HF has unique clinical manifestations and management guidelines, which will be discussed at the end of the chapter.
HISTORY AND PHYSICAL EXAM
Alkalis cause liquefactive necrosis, and acids cause coagulation necrosis; however, exposure to both caustics presents similarly. When managing a case of caustic exposure, the EP should obtain a detailed patient history, including intent of ingestion; type, formulation (solid or liquid) and concentration of compound ingested; amount of compound ingested; and time of ingestion. The physical exam should observe for the following:
These findings may be indicative of esophageal, gastric, or airway injury.10 Details of the specific injury patterns associated with each clinical presentation are outlined in the endoscopy and management sections that follow.
DIAGNOSTIC EVALUATION
In addition to the history and physical exam, certain blood tests should be performed. A 2003 study found an arterial pH < 7.22 and a base deficit of 12 to be reliable predictors of severe esophageal injury requiring surgical intervention, such as transhiatal esophagectomy and total gastrectomy.11 The same study reported a pH < 7.11 and a base deficit of 16.1 to be predictors of death despite intervention, suggesting that severe acidosis correlates well with the degree of tissue necrosis and subsequent lactic acid production.
Chest and abdominal x-rays are recommended following any caustic ingestion to exclude obvious esophageal perforation; however, the sensitivity of these tests is limited.6 Computed tomography (CT) is more sensitive and should be considered in patients with negative x-rays despite a high clinical suspicion for perforation. Contrast esophagography can also be used to detect perforations, which can be visualized on a radiograph as extravasation of the contrast medium. The choice of contrast medium remains controversial; some experts advocate a water-soluble contrast like gastrografin to minimize mediastinal and peritoneal irritation upon extravasation, while others advocate barium, which is less likely to cause aspiration pneumonitis.6 The appropriate contrast medium should be chosen in conjunction with radiology, toxicology, and gastroenterology consult.
For grading of esophageal injury, recent studies suggest benefits of using CT instead of endoscopy; currently, however, endoscopy remains the current standard.12,13
For the emergency physician, the greatest challenge in treating caustic ingestion is determining which patients require immediate endoscopy. Although it carries a potential risk of further esophageal damage, endoscopic evaluation can differentiate low-grade injuries in patients who may be safely discharged from patients with high-grade injuries requiring more extensive management, including potential surgery or other intervention to reduce the risk of stricture formation.
In the past, endoscopy with rigid endoscopes carried a high risk of perforation. Newer flexible endoscopes have lowered this risk and made endoscopy easier to perform14; nevertheless, the indication and timing of the procedure remain controversial. Several studies have evaluated history and physical exam criteria as indicators for emergent endoscopy in patients with caustic ingestions. Their conclusions vary widely. A retrospective review of 378 children found no statistically significant relationship between the presence of symptoms (such as vomiting, excessive drooling, abdominal pain, oropharyngeal burns, dysphagia, nausea and refusal to drink) and the severity of esophageal lesions and thus advocated endoscopy in all ingestions.15 A second retrospective study of 156 children arrived at similar conclusions, excepting a correlation of vomiting with second- and third-degree esophageal lesions.16
Two other studies reported opposite findings in a similar population. In a review of 79 patients younger than 20 years of age, the absence of vomiting, drooling, and stridor was found to have a 100% negative predictive value in identifying esophageal injury, while the presence of two or more of the above three symptoms had a 50% positive predictive value for esophageal injury.10 Based on these findings, the authors recommended limiting the use of endoscopy to patients with clinical symptoms (vomiting, drooling, stridor) rather than performing endoscopy on all patients. A second prospective study of 85 children likewise reported the absence of symptoms to have a 100% negative predictive value for esophageal injury, and the presence of respiratory symptoms and hematemesis to have a significant positive predictive value for injury.17 This study also advocated withholding endoscopy in the asymptomatic patient with an unintentional ingestion. These studies did not consider suicidal ingestions, but consensus is that endoscopy is necessary in these cases regardless of symptoms, given the high morbidity associated with intentional ingestions.1
Consensus recommendation is that the unintentionally exposed, asymptomatic patient—with no respiratory complaints, drooling, stridor, hoarseness, dysphagia, or vomiting—can be safely discharged after appropriate observation in the ED and a trial of oral intake.10 Symptomatic patients, whether exposed intentionally or unintentionally, should have formal gastroenterology evaluation for endoscopy. The suicidal patient, regardless of symptoms, deserves consultation for endoscopy.
A limited number of studies have considered the time period within which endoscopy should be performed or avoided. Mucosal sloughing typically occurs 4 to 7 days after injury, and collagen deposition does not begin until after 14 days; therefore, the esophagus is considered most vulnerable to endoscopic-induced perforation during the 5- to 15-day period following caustic ingestion.3,14,18 A 1991 prospective cohort study of 81 patients with caustic ingestions who underwent endoscopy reported that of the 381 total (initial and follow-up) endoscopies performed, no patient experienced perforation in close proximity to endoscopy.14 Perforation did occur in three patients—on the 9th, 11th, and 15th day following endoscopy, respectively—although these perforations were likely due to the use of rigid endoscopes. The study concluded that endoscopy could safely be performed between 6 and 96 hours following caustic ingestion. Of note, none of the study's patients underwent endoscopy during the 5- to 15-day post-ingestion period due to the assumption of esophageal friability. There are no studies that specifically evaluate endoscopy during the 5- to 15-day interval, and it is recommended to avoid the procedure whenever possible during this time.3,14,18
One benefit of early endoscopy is the placement of a nasogastric tube (NGT) (always done under direct visualization), which allows for early enteral nutrition. Early nutrition aids rapid healing of the caustic injury, which in turn reduces hospital length of stay.19 Enteral nutrition has advantages over parenteral nutrition, including preservation of intestinal mucosa, reduced risk of infection, reduced hepatic and biliary complications, more effective monitoring of electrolytes and nutrients, and more cost-efficient delivery.19
Grade I injuries involve superficial tissue damage, such as edema and erythema. These injuries do not progress to stricture formation or carcinoma, and these patients can be discharged safely if able to tolerate a regular diet. No other therapy is required.
Grade IIa injuries involve transmucosal damage with superficial ulceration, sloughing, and mucosal hemorrhage of the esophagus. These patients may be able to tolerate a soft diet, or may need the placement of a NGT for enteral feeding. Grade IIb injuries are similar to IIa injuries, but are circumferential, affecting all sides of the esophagus.
Grade III injuries involve deep ulcerations, tissue necrosis, severe hemorrhage, and perforation. Patients with IIb and III injuries are at a risk of perforation, infection, and stricture formation, and are at a 1,000 times increased risk of developing carcinoma over the following 40-year period.
MANAGEMENT GUIDELINES
Management begins with the airway. Caustics can produce significant airway edema, which can lead to rapid airway compromise. Hoarseness, stridor, and drooling are all signs of upper airway injury and require fiberoptic inspection of the vocal cords by an otolaryngologist in the ED. Although not investigated, the consensus recommendation is to use dexamethasone to treat airway edema at a one-time dose of 10 mg IV.6 If the edema progresses to airway compromise and respiratory distress, orotracheal intubation must be performed, preferably with a fiberoptic laryngoscope.
Although decontamination is generally contraindicated in caustic ingestions, it is essential in treating caustic exposure to the eye and skin. Dry, powdered caustics should be brushed off the skin before washing, as dissolution of the caustic in water may cause further injury. Ophthalmic exposures should be managed with copious irrigation of the eye using a Morgan lens and normal saline (NS), or lactated Ringer's (LR), until the pH of the eye is close to physiologic pH (7.40). Visual acuity should be assessed after irrigation, and a slit-lamp examination should be performed to look for corneal abrasions and ulcerations. These injuries require ophthalmic antibiotics and timely follow-up with an ophthalmologist.
Activated charcoal, a commonly used gastrointestinal decontaminant, is contraindicated in caustic ingestions. Its use impedes endoscopic visualization of the esophageal mucosa, and it can cause pneumonitis if perforations are present. Ingested caustics should never be neutralized, as this reaction is exothermic and can cause further tissue injury.
Surgical management is necessary in patients with caustic ingestion who present with perforation, persistent hypotension, and metabolic acidosis.11,20 Early surgical management (within 24 hours of ingestion) is associated with a lower morbidity and mortality than delayed surgery.20 Surgery may be also required in grade II and III esophageal injuries.21
Steroids have been considered for the prevention of caustic ingestion associated esophageal stricture formation, but their use is controversial. A meta-analysis of 361 patients demonstrated a 19% rate of stricture formation in the steroid-treated group and a 41% rate in the untreated group; as a result, the study advocated the use of steroids in high-degree esophageal injuries.22 They study did not, however, differentiate between grade II and III injuries. Other studies have failed to show a benefit from the use of steroids. A prospective study of 60 children with a range of grade I, II, and III injuries found no statistically significant difference in stricture formation between steroid-treated and untreated groups, even after considering each injury grade separately.23 A recent review study also reported no difference in stricture formation between steroid-treated and untreated patients.24 It is important to note that steroid therapy not only lacks proven efficacy but also is potentially harmful, as steroids may suppress immunity in patients with injuries already prone to infection. Current guidelines thus recommend against steroid therapy for prevention of esophageal strictures.24
There are limited data on the use of antibiotics for esophageal injuries. If there is a known source of infection, antibiotics should be administered.6 Giving antibiotics to patients receiving steroids is reasonable, although in general prophylactic antibiotic therapy is not recommended.
Placement of a NGT may be necessary to provide enteral nutrition in patients unable to tolerate an oral diet because of esophageal injury. In patients with esophageal injuries, an NGT should be placed only under endoscopic visualization.
Placement of intraluminal stents, usually made of silicone, may prevent stricture formation and ensure patency of the esophageal lumen.3,6,25,26 Stents can cause increased trauma at the insertion site and can cause increased gastrointestinal reflux, which may impede healing.27 Use of stents is decided on a case-by-case basis.
No significant scientific evidence exists to suggest a benefit of using sucralfate in caustic ingestions.28
The use of proton pump inhibitors and H2 antagonists reduces the amount of acid that comes into contact with the esophageal mucosa, aids in healing, and is recommended in all cases.6
HYDROFLUORIC ACID
Hydrofluoric acid (HF) is present in multiple products, including oven cleaners, rust removers, aluminum brighteners, heavy-duty cleaners, and laundry detergents. It is also used in plastic dye and electronics manufacturing, as well as in the synthesis of Teflon and Freon.1,6,29,30 Although technically a weak acid, HF produces a unique systemic toxicity, unrelated to its causticity, which merits special discussion.
Aqueous hydrofluoric acid is a weak acid, with a pKa of 3.5.6 HF toxicity is typically the result of dermal, ocular, or inhalational exposure, although ingestions do also occur. HF penetrates deeply into tissues and dissociates into hydrogen (H+) and fluoride (F−) ions. Localized hypocalcemia and hypomagnesemia occur when F− ions bind to Ca and Mg6,31–34 and form insoluble salts, such as calcium fluoride (CaF2), that deposit in the tissues.31 The pain of HF exposure is due to the corrosive burns of the H+ ions, as well as the calcium dysregulation, which can result in neuroexcitation and vasospasm with associated pain and ischemia.6,34
HF's deep penetration produces systemic toxicity regardless of the route of exposure. In addition to hypocalcemia and hypomagnesemia, hyperkalemia can occur. This is postulated to be due to F−-induced increased intracellular Ca2+, which induces Ca2+-dependent K+ channels to produce a K+ efflux.35 Hypocalcemia, hypomagnesemia, and hyperkalemia can, in turn, cause potentially fatal cardiac dysrhythmias.
Dermal exposure to HF can cause a delayed onset of pain and visible tissue damage. Hyperemia may occur, followed by a white discoloration due to calcium precipitation. Pain may precede tissue changes; therefore, a high level of clinical suspicion is required for the patient who presents with severe hand pain but without obvious skin damage.6,36–38
Inhalational exposure to HF can produce symptoms ranging from mild upper respiratory tract irritation to dyspnea, hypoxemia, and hypocalcemia.39 A retrospective chart review of 939 patients with inhalational exposure to HF released from a petrochemical plant revealed subjective toxicity including eye and throat irritation, headache, and shortness of breath, as well as objective toxicity, including decreased pulmonary function testing, hypoxemia, and hypocalcemia.39
Ingestion of HF results in gastritis and systemic toxicity, including possible cardiac dysrhythmia due to hypocalcemia and hyperkalemia.40,41 Local tissue damage may result in airway compromise. Intentional ingestion of HF often results in death.42,43
HF is highly caustic to the eye; it penetrates deeply and causes corneal stromal edema, conjunctival chemosis, hemorrhage, ischemia, inflammation, and stromal opacification.44,45 Long-term effects can include corneal revascularization and dry eyes.
Serum calcium, magnesium, and potassium should be monitored. Low serum pH is a sign of worsening systemic toxicity and can be monitored via blood gas analysis.31 Serum fluoride concentrations are not clinically relevant because of the time it takes to obtain results.6
An electrocardiogram should be obtained in all cases of HF exposure to evaluate the effects of hypocalcemia (prolonged QTc) and hyperkalemia (peaked T waves).
All patients with digital exposures require 4 to 6 hours of ED observation to monitor for recurrence of pain and need for repeat calcium administration.6
Cardiac dysrhythmias due to hypocalcemia and hypomagnesemia should be managed with intravenous calcium and magnesium. Hyperkalemia should be aggressively treated using standard therapies. Urinary alkalinization with intravenous sodium bicarbonate can enhance fluoride elimination.55 Patients who cannot tolerate a large volume load, who are severely ill, or who have renal dysfunction may require hemodialysis for definitive fluoride elimination.56,57
CONCLUSION
A majority of lethal caustic exposures are intentional and occur in adults. A majority of accidental caustic exposures occur in children. It is challenging to risk stratify the extent of tissue injury after caustic exposures, and special attention should be directed towards the type of product involved, the intent of exposure, and signs and symptoms such as vomiting, stridor, and drooling. Gastroenterology and surgical consults should be involved early in the care of any significant or symptomatic ingestion. Although decontamination is usually contraindicated in caustic ingestions, it is important in ocular and dermal exposures. Hydrofluoric acid exposures may also require decontamination to prevent systemic toxicity, followed by appropriate calcium therapy.
LITERATURE TABLE

1.Bronstein AC, Spyker DA, Cantilena LR, et al. 2010 Annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 28th Annual Report. Clin Tox. 2011;49:910–941.
2.Gumaste VV, Dave PB. Ingestion of corrosive substances by adults. Am J Gastroenterol. 1992;87(1):1–5.
3.Ramasamy K, Gumaste VV. Corrosive Ingestion in Adults. J Clin Gastroenterol. 2003;37(2):119–124.
4.Moore WR. Caustic ingestions. Pathophysiology, diagnosis, and treatment. Clin Pediatr (Phila). 1986;25(4):192–196.
5.Hoffman RS, Howland MA, Kamerow HN, et al. Comparison of titratable acid/alkaline reserve and pH in potentially caustic household products. J Toxicol Clin Toxicol. 1989;27(4–5):241–246.
6.Nelson LS, Lewin NA, Howland MA, et al. Goldfrank's Toxicologic Emergencies. 9th ed. New York, NY: McGraw-Hill; 2011.
7.Kumar V, Abbas AK, Fausto N, et al. Robbins and Cotran Pathological Basis of Disease. 8th ed. Pennsylvania: Saunders Elseveir; 2010.
8.Kirsh MM, Ritter F. Caustic ingestion and subsequent damage to the oropharyngeal and digestive passages. Ann Thoracic Surg. 1976;21:74–82.
9.Williams H, Bateman DN, Thomas SHL, et al. Exposure to liquid detergent capsules: a study undertaken by the UK National Poisons Information Service. Clin Tox. 2012;50:776–780.
10.Crain EF, Gershel JC, Mezey AP. Caustic ingestions. Symptoms as predictors of esophageal injury. Am J Dis Child. 1984;138(9):863–865.
11.Cheng YJ, Kao EL. Arterial blood gas analysis in acute caustic ingestion injuries. Surg Today. 2003;33:483–485.
12.Isbister GK, Page CB. Early endoscopy or CT in caustic injuries: a re-evaluation of clinical practice. Clin Tox. 2011;49:641–642.
13.Ryu HH, Jeung KW, Lee BK, et al. Caustic injury: can CT grading system enable prediction of esophageal stricture? Clin Tox. 2010;48:137–142.
14.Zargar SA, Kochhar R, Mehta S, et al. The role of fiberoptic endoscopy in the management of corrosive ingestion and modified endoscopic classification of burns. Gastrointest Endosc. 1991;37(2):165–169.
15.Gaudreault P, Parent M, McGuigan MA, et al. Predictability of esophageal injury from signs and symptoms: a study of caustic ingestion in 378 children. Pediatrics. 1983;71:667–770.
16.Previtera C, Giusti F, Guglielmi M. Predictive value of visible leasions (cheeks, lips, oropharnyx) in suspected caustic ingestion: may endoscopy reasonably be omitted in completely negative pediatric patients? Pediatr Emerg Care. 1990;6(3): 176–178.
17.Lamireau T, Rebousissoux L, Denis D, et al. Accidental caustic ingestions in children: is endoscopy always mandatory? J Pediatr Gastroenterol Nutr. 2001;33(1):81–4.
18.Cheng HT, Cheng CL, Lin CH, et al. Caustic ingestion in adults: the role of endoscopic classification in predicting outcome. BMC Gastroenterol. 2008;8(31).
19.Chibishev A, Simonovska-Veljanovska N, Pereska Z. Artificial nutrition in therapeutic approach of acute caustic poisonings. Maced J Med Sci. 2010;3(2).
20.Javed A, Pal S, Krishnan EK, et al. Surgical management and outcomes of severe gastrointestinal injuries due to corrosive ingestion. World J Gastrointest Surg. 2012;4(5):121–125.
21.Estrera A, Taylor W, Mills LJ, et al. Corrosive burns of the esophagus and stomach: a recommendation for an aggressive surgical approach. Ann Thor Surg. 1986;41:276–283.
22.Howell JM, Dalsey WC, Hartsell FW, et al. Steroids for the treatment of corrosive esophageal injury: a statistical analysis of past studies. Am J Emerg Med. 1992;10:421–425.
23.Anderson KD, Rouse TM, Randolph JG. A controlled trial of corticosteroids in children with corrosive injury of the esophagus. N Engl J Med. 1990;323(10):637–640.
24.Peclova D and Navratil T. Do corticosteroids prevent oesophageal stricture after corrosive ingestion? Toxicol Rev. 2005;24(2):125–129.
25.Berkovits RN, Bose CE, Wijburg FA, et al. Caustic injury of the esophagus. J Laryngol Otol. 1996; 110:1041–1045.
26.De Peppo F, Zaccara A, DallOglio L, et al. Stenting for caustic strictures. J Pediatr Surg. 1998;22:54–57.
27.Sinar DR, Fletcher JR, Cordova CC, et al. Acute acid-induced esophagitis impairs esophageal persitalsis in baboons. Gastroenterology. 1981;80:1286.
28.Reddy AN, Budhraja M. Sucralfate therapy for lye-induced esophagitis. Am J Gastroenterol. 1988;83:71–73.
29.Hatzifotis M, Williams A, Muller M, et al. Hydrofluoric acid burns. Burns. 2004;30:156–159.
30.Huisman LC, Teijink JAW, Overbosch EH, et al. An atypical chemical burn. Lancet. 2001;358:1510.
31.Boink AB, Wemer J, Meulenbelt J, et al. The mechanism of fluoride-induced hypocalcemia. Hum Exp Toxicol. 1994;13(3):149–155.
32.Greco RJ, Hartford CE, Haith LR, et al. Hyrdofluoric acid-induced hypocalcemia. J Trauma 1988;28:1593–1596.
33.Sanz-Gallen P, Nogue S, Munne P, et al. Hypocalcaemia and hypomagnesaemia due to hydrofluoric acid. Occup Med. 2001;51(4):294–295.
34.Thomas D, Jaeger U, Sagoschen I, et al. Intra-arterial calcium gluconate treatment after hydrofluoric acid burn of the hand. Cardiovasc Intervent Radiol. 2009;32:155–158.
35.Cummings CC, McIvor ME. Fluoride-induced hyperkalemia: the role of Ca2+-dependent K+ channels. Am J Emerg Med. 1988;6(1):1–3.
36.De Capitani EM, Hirano ES, Zuim Ide SC, et al. Fingers burns caused by concentrated hydrofluoric acid, treated with intra-arterial calcium gluconate infusion: case report. Sao Paolo Med J. 2009;127(6):379–381.
37.Anderson WJ, Anderson JR. Hydrofluoric acid burns of the hand: mechanism of injury and treatment. J Hand Surg Am. 1988;13(1):52–57.
38.Sheridan RL, Ryan CM, Quinby WC Jr, et al. Emergency management of major hydrofluoric acid exposures. Burns. 1995;21:62–64.
39.Wing JS, Brender JD, Sanderson LM, et al. Acute health effects in a community after a release of hydrofluoric acid. Arch Environ Health. 1991;46(3):155–160.
40.Stremski ES, Grande GA, Ling LJ. Survival following hydrofluoric acid ingestion. Ann Emerg Med. 1992;21(11):1396–1399.
41.Yu-Jang S, Li-Hua L, Wai-Mau C, et al. Survival after a massive hydrofluoric acid ingestion with ECG changes. Am J Emeg Med. 2001;19(5):458–460.
42.Whiteley P, Aks SE. Case files of the toxicon consortium in Chicago: survival after intentional ingestion of hydrofluoric acid. J Med Toxicol. 2010;6:349–354.
43.Kao WF, Dart RC, Kuffner E, et al. Ingestion of low-concentration hydrofluoric acid: an insidious and potentially fatal poisoning. Ann Emerg Med. 1999;34(1).
44.McCulley JP, Whiting DW, Petitt MG, et al. Hydrofluoric acid burns of the eye. J Occup Med. 1983;25(6):447–450.
45.McCulley JP. Ocular hydrofluoric acid burns: animal model, mechanism of injury and therapy. Trans Am Ophthalmol Soc. 1990;88:649–684.
46.Baltazar RF, Mower MM, Reider R, et al. Acute fluoride poisoning leading to fatal hyperkalemia. Chest. 1980;78:660–663.
47.Manoguerra AS, Neuman TS. Fatal poisoning from acute hydrofluoric acid ingestion. Am J Emerg Med. 1986;4:362–363.
48.Graudins A, Burns MJ, Aaron CK. Regional intravenous infusion of calcium gluconate for hydrofluoric acid burns of the upper extremity. Ann Emerg Med. 1997;30(5):604–607.
49.Ryan JM, McCarthy GM, Plunkett PK. Regional intravenous calcium—an effective method of treating hydrofluoric acid burns to limb peripheries. J Accid Emerg Med. 1997;14:401–404.
50.Vance MV, Curry SC, Kunkel DB, et al. Digital hydrofluoric acid burns: treatment with intraarterial calcium infusion. Ann Emerg Med. 1986;15(8):890–896.
51.Schiettecatte D, Mullie G, Depoorter M. Treatment of hydrofluoric acid burns. Acta Chir Belg. 2003;103:375–378.
52.Kono K, Watanabe T, Dote T, et al. Successful treatments of lung injury and skin burn due to hydrofluoric acid exposure. Int Arch Occup Environ Health. 2000;73:S93–S97.
53.Heard K, Delgado K. Oral decontamination with calcium or magnesium salts does not improve survival following hydrofluoric acid ingestion. J Toxicol Clin Toxicol. 2003;41:789–792.
54.Heard K, Hill RE, Cairns CB, et al. Calcium neutralizes fluoride bioavailability in a lethal model of fluoride poisoning. J Toxicol Clin Toxicol. 2001;39(4):349–353.
55.Proudfoot AT, Krenzelok EP, Vale JA. Position paper on urine alkalinization. J Toxicol Clin Toxicol. 2004;42:1–26.
56.Berman L, Taves D, Mitra S, et al. Inorganic fluoride poisoning treatment by hemodialysis. N Engl J Med. 1973;289:922.
57.Juncos LI, Donadio JV. Renal failure and fluorosis. JAMA. 1972;222:783–785.