The kitchen is the ultimate laboratory. Ordinary ingredients are transformed by chemical reactions. These things happen every day in the kitchen, but their magic often goes unnoticed.
Holly’s pick: Exploding Baggies (here) is one of my kids’ favorite experiments. Living in a household with three boys has given me an appreciation for anything that explodes.
Rachel’s pick: I think the Creeping Ink (here) is terrific. Your kids will be mesmerized, as mine were, creating one-of-a kind shirts—with science!
Jamie’s pick: Bursting Soap Cloud (here) is my favorite science experiment of all time. I like washing my hands with it after!


PREP TIME: 1 MINUTE
EXPERIMENT DURATION: 2 MINUTES
 Bar of Ivory soap
Bar of Ivory soap
 Microwave-safe dish
Microwave-safe dish
Charles’s Law (named after a scientist, Jacques Charles) says gases expand when they’re heated. We can watch Charles’s Law in action with a bar of Ivory soap in the microwave.
Unwrap the bar of soap and place it on the microwave-safe dish. Microwave the soap for 1½ to 2 minutes, watching closely to see what happens. (This won’t hurt your microwave, we promise!) Let the soap cool a bit and remove it from the microwave.
When you microwave Ivory soap, it expands as much as six times in size and feels brittle and flaky. Try washing your hands with it.
When you heat up the Ivory soap, you get to see Charles’s Law—the air inside the soap expands when it’s heated. The soap puffs up as the air trapped inside gets bigger.
This experiment won’t work with other brands of soap, because they aren’t as porous as Ivory.
The soap will be very hot when you pull it out of the microwave. Be sure to let it cool.
October 15th is Global Hand-Washing Day. It’s a good idea to wash your hands the other 364 days of the year too, though.

PREP TIME: 10 MINUTES
EXPERIMENT DURATION: 2 MINUTES
 Saucepan
Saucepan
 Water
Water
 Rainbow-colored hard candies
Rainbow-colored hard candies
We can see what happens to water molecules as heat or energy is introduced to the pan.
Fill a saucepan with water and have an adult put it on the stove to boil. When the water warms up a bit, add a few pieces of candy and watch as the water starts to boil. At first, it looks like nothing is happening, but watch closely!
The water starts to boil and your candies bounce all around the saucepan.
As the water heats up, it expands and tiny bubbles form from gas in the water. The super cool color waves around your candy are from the light being bent as it passes through the steam. When the big bubbles show up you’re actually changing the liquid (water) to a gas (steam)!
• Boil two pots of water, one full of cold and the other full of hot. Which one boils first?
Roughly three-quarters of your body is made of water. That’s almost the whole thing!
Boiling water is VERY hot and so is the stove. Make sure you have a parent close by when you’re watching your pot.


PREP TIME: 10 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 Spoon
Spoon
 2 teaspoons (10 g) baking soda
2 teaspoons (10 g) baking soda
 Balloon
Balloon
 ⅓ cup (90 ml) white vinegar
⅓ cup (90 ml) white vinegar
 Old water bottle
Old water bottle
Baking soda is a solid and vinegar is a liquid, but when you mix the two together it creates a whole new substance: gas.
Using the spoon, put the baking soda into the balloon.
Pour the vinegar into the bottle until it’s about one-third full. Keeping the baking soda in the body of the balloon, stretch the mouth over the bottle’s opening. Then dump the baking soda from the balloon into the bottle.
As the vinegar and baking soda mix, a gas—carbon dioxide—is created. It is a by-product of vinegar and baking soda reacting together, and the balloon blows up.
The vinegar is acetic acid, and the baking soda is a base—the opposite pH to an acid. When they mix, it causes a reaction and carbonic acid is formed, separating into bubbles of carbon dioxide (CO2) and water. The CO2 has nowhere to go except up into the balloon, causing it to inflate.
• Change the temperature of your vinegar, making it hotter or colder, and see if that affects the speed at which your balloon grows.
Bounce your balloon. Does it bounce as easily as a regular air-filled balloon? The CO2 gas is heavier than air. Helium is lighter than air, making balloons filled with that gas float.
Vinegar has no expiration date. It never “goes bad.”

PREP TIME: 10 MINUTES
EXPERIMENT DURATION: 20 MINUTES PLUS DRYING TIME
 Spray bottle
Spray bottle
 70% rubbing alcohol
70% rubbing alcohol
 Aluminum foil or cardboard to line the inside of the shirt
Aluminum foil or cardboard to line the inside of the shirt
 White T-shirt
White T-shirt
 Colored permanent markers
Colored permanent markers
A solvent can break the bond of a permanent marker, freeing the ink to spread and burst.
Fill the spray bottle with rubbing alcohol. Cut your foil and put it inside the shirt to keep your marker from bleeding through. Color your shirt with the markers and spray the shirt with alcohol.
Watch as the colors morph into a colorful explosion!
The alcohol acts as a solvent and dissolves the ink as it saturates the shirt, causing the ink to spread.
• If you don’t have an old shirt to spare, this same technique can be used on paper towels or coffee filters.
• Try hydrogen peroxide or water and see how the colors change (or don’t!).
After your shirt is dry, wash it alone so the colors don’t bleed onto the rest of your laundry.
Way back in 12th century BC, the Chinese used ink from squids to write and draw with.

PREP TIME: 2 MINUTES
EXPERIMENT DURATION: 5 TO 20 MINUTES
 A handful of raisins
A handful of raisins
 1 clear glass of water
1 clear glass of water
 1 clear glass of soda
1 clear glass of soda
Even though a glass of water and a glass of clear soda look the same, raisins react very differently due to buoyancy.
Add 5 raisins to each glass and watch what happens.
The raisins in the water will stay at the bottom of the glass, but the raisins in the soda will rise to the top.
Raisins sink in water (and initially in the soda) because they are denser than the liquid. The soda’s carbon dioxide molecules stick to the wrinkles of the raisins, causing them to have increased buoyancy and rise. When the bubbles pop or the raisin gets soggy, it will start to sink again.
• If you’re prepared for a mess, try dropping a whole handful of raisins into a glass of soda.
• You can try heating up your soda or making it very cold, too!
You can make your own raisins by placing grapes on a paper plate on a sunny windowsill or by spreading them onto a cookie sheet in a warm (lowest setting) oven for 24 hours.

PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 7 MINUTES
 Cup
Cup
 Water
Water
 Tablespoon measure
Tablespoon measure
 70% rubbing alcohol
70% rubbing alcohol
 Coin
Coin
 Coffee filter
Coffee filter
 Black marker
Black marker
 Rubber band
Rubber band
Chromatography is a way to separate compounds so they can be seen individually.
Fill the cup with water to about 1 inch (2.5 cm) from the top. Add 1 tablespoon (15 ml) of rubbing alcohol to the cup. Place the coin in the middle of the coffee filter and trace a thick line around it with your marker. Drape the coffee filter over the cup so that the filter just touches the water. Secure it to the cup with the rubber band. Watch as the water creeps up the coffee filter.
As the water moves up the fibers of the coffee filter, it separates the pigments in the marker ink into their components.
The lighter particles of ink travel the fastest and farthest, and the heavier color pigments are slower to move.
Can you tell how many colors are in the black pen?
• Try different brands of black markers. Different companies use different combinations to get black!
Do this experiment faster by increasing the amount of rubbing alcohol in your water.


PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 2 MINUTES
 ⅓ cup (80 ml) white vinegar
⅓ cup (80 ml) white vinegar
 1 zipper-lock bag
1 zipper-lock bag
 10 drops of food coloring (optional)
10 drops of food coloring (optional)
 Clothespin
Clothespin
 2 tablespoons (28 g) baking soda
2 tablespoons (28 g) baking soda
Combine a solid and a liquid to see if the resulting chemical reaction is bigger than the components by themselves.
Pour the vinegar into the bag and add a few drops of food coloring. Twist the plastic above the liquid, and hold the twist secure with a clothespin. Now, above the clothespin, add the baking soda and close the bag’s zipper.
Take off the clothespin and let the vinegar drop into the bag. Shake it and watch the reaction!
The bag blows up like a balloon.
When baking soda and vinegar mix together, it causes a reaction that creates gas. Gas molecules take up more space than the liquid and the solid do, which is why the bag expands.
Do this in an easy-to-clean location. We suggest outside with a hose handy—or maybe in the bathtub!

PREP TIME: 2 MINUTES
EXPERIMENT DURATION: 2–3 HOURS
 Ruler
Ruler
 6 gummy worms
6 gummy worms
 Paper and pencil
Paper and pencil
 2 glass bowls
2 glass bowls
 ⅔ cup (160 ml) warm water
⅔ cup (160 ml) warm water
 ¼ teaspoon salt
¼ teaspoon salt
 Spoon
Spoon
Most of the water on our planet is salt water, yet we don’t drink it. Let’s use gummy worms to show how different types of water are absorbed by our body.
With the ruler, measure the length of the gummy worms. Write this on a piece of paper. In a bowl, combine ⅓ cup (80 ml) of the water and the salt, and mix together with a spoon. Add the remaining ⅓ cup (80 ml) water to the other bowl. Write which bowl is which on a piece of paper and place under the bowl. Put 3 gummy worms in each bowl. Let the worms sit in the bowls for a couple of hours.
Pull out the worms and measure them again. Are your worms bigger?
The gummy worms in both bowls absorbed water. However, the worms in the plain water absorbed more water and grew much larger than the worms in the salt water.
When gummy worms are added to water, the water molecules move through the tiny holes in the surface of the candy. The gelatin keeps the gummy worms from dissolving.
Plain water is easier for our bodies to absorb. That’s why we don’t drink salt water.
• Try different liquids. Will your gummy worms dissolve in soda or orange juice? What about if you heat your water or put it in the refrigerator first?
Gummy bears were invented 60 years before the gummy worm, and July 15 is National Gummy Worm Day.


PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 Tonic water
Tonic water
 2 clear plastic cups
2 clear plastic cups
 Black light
Black light
 Pliers
Pliers
 Neon yellow highlighter
Neon yellow highlighter
A lot of things that glow in the dark do so because they contain chemicals called quinine and pyranine. These chemicals absorb light and create a new wave of light at a lower wavelength, creating a cool glow.
Pour tonic water in one of the cups and turn off the lights. Now shine a black light onto your cup. What do you see?
Fill the other cup with tap water and use pliers to take the bottom off the highlighter. Pull out the ink tube and drop it into the cup. The ink will bleed into the water and make it change color. Now, shine the black light on the water. Whoa!
The black light makes your water glow. The tonic water will be blue, and the marker water will glow whatever color your highlighter is.
Tonic water contains the chemical quinine that glows a blue color and the highlighter ink contains the fluorescein chemical pyranine. You can’t see these chemicals, but when the black light hits them, the light is converted into a new form that you can see! That’s what makes it glow.
• Make Jell-O with tonic water for a glow-in-the-dark dessert!
If you can’t get your highlighter water to glow, cut off the plastic tubing.
Some scorpions glow bright blue under a black light. Scientists aren’t sure why they glow, but they know it happens because of a chemical called betacarabine in the scorpion’s back.


PREP TIME: 5 TO 10 MINUTES
EXPERIMENT DURATION: OVERNIGHT
 2 cups (470 ml) water
2 cups (470 ml) water
 Microwave-safe bowl
Microwave-safe bowl
 3 packages of plain gelatin
3 packages of plain gelatin
 Spoon
Spoon
 Cookie sheet
Cookie sheet
 Straw
Straw
 Food coloring
Food coloring
When molecules move from a place where there are lots of molecules and spread out to a much larger space where there are fewer molecules, that’s called diffusion.
Pour the water into a microwave-safe bowl and have a parent microwave it until the water is boiling, and then carefully remove the bowl with oven mitts. Pour in all 3 packages of gelatin. Stir with a spoon until dissolved. Pour your mixture onto a cookie sheet and let it harden overnight.
Take your straw and poke holes in the gelatin mixture about 3 inches (7.5 cm) apart. Drop 2 or 3 drops of food coloring into each hole and let it sit for 2 to 3 hours.
The food coloring spreads out in a perfect circle around each hole.
This is diffusion, when the food coloring spreads from one place to over a larger area.
• Add glitter to your mix or use cookie cutters to make gelatin window clings!
In the early 1900s immigrants were offered Jell-O when they moved to the United States as a “Welcome to America” gift.

PREP TIME: 10 MINUTES
EXPERIMENT DURATION: 20 TO 30 MINUTES
 1 cup (235 ml) whipping cream
1 cup (235 ml) whipping cream
 ½ teaspoon vanilla extract
½ teaspoon vanilla extract
 2 tablespoons (25 g) sugar
2 tablespoons (25 g) sugar
 Small coffee can with lid
Small coffee can with lid
 Spoon
Spoon
 Packing tape
Packing tape
 Large coffee can with lid
Large coffee can with lid
 4 cups (800 g) ice
4 cups (800 g) ice
 ½ cup (145 g) kosher salt
½ cup (145 g) kosher salt
In this experiment, we will see how salt makes it quicker and easier to freeze things by making the ice super cold. We are making ice cream.
Pour the cream, vanilla and sugar into the small coffee can, stir with the spoon and seal the lid with the tape.
Place the smaller can inside the large coffee can. Fill the space between the two cans with ice and then salt. Tape the lid on the larger can and roll the can. After 30 minutes has passed, remove the lid from the large can, remove and rinse off the small can and then open it.
You get ice cream!
When you mix ice and salt, the ice becomes even colder than normal, making it perfect to freeze even moving liquid, like the cream in the can. Because it freezes so quickly, the ice cream has crystals of ice dispersed between the cream, and doesn’t freeze into a solid block.
• Try to make ice cream in zipper-lock bags instead of cans. Pour the ingredients into a quart-size zipper-lock bag. Double bag it with a larger gallon-size freezer bag. Fill the big bag with ice and salt. Gently shake the bag until your ice cream forms.
Fill one ice cube tray or plastic container with plain water and fill another with a salt water solution. Put them in the freezer. Which do you think will freeze first?
Vanilla is the most popular flavor of ice cream.

PREP TIME: 10 TO 15 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 Cotton swab
Cotton swab
 Dish soap
Dish soap
 Paper towel
Paper towel
In this experiment you can see the effects of a water-hating (or hydrophobic) substance when water that has become a gas (or steam) hits it.
Dip the cotton swab into the soap and blot the swab onto the towel so nothing drips. Use the cotton swab to write a message on the mirror.
Take a hot shower—hot enough to fill the room with steam. Check out the mirror.
Your words and drawings show through the steam.
When you take a hot shower, the mirror in your bathroom gets covered in a thin layer of steam. The droplets of water from the steam attached to the mirror, making it appear “foggy.” But, when you wrote on the mirror with soap, the soap prevented the water from clinging to that part of the mirror.
Water loves to stick to things. This is why your skin feels clammy when it’s raining outside, even if you’re under an umbrella.
To keep a mirror from fogging up, fill a spray bottle half with water and half with vinegar. Add a drop or two of dishwashing soap and spray the mirror. Wipe clean with a paper towel. No more fog!


PREP TIME: 10 MINUTES
EXPERIMENT DURATION: 10 MINUTES
 Clear container or bottle
Clear container or bottle
 Vegetable oil
Vegetable oil
 Water
Water
 10 drops food coloring (red is best)
10 drops food coloring (red is best)
 Small cup
Small cup
 Knife
Knife
 Effervescent or fizzing tablets, such as Alka-Seltzer
Effervescent or fizzing tablets, such as Alka-Seltzer
Have you ever seen a lava lamp? They are groovy! Gobs of wax move through lit water. Here we will use science to create our own wax-less lava lamp!
Fill the bottle three-fourths full with oil. Mix some water and food coloring together in a small cup and add some of the colored water to the oil bottle, leaving 1 to 2 inches (2.5 to 5 cm) of air space at the top of the bottle. Watch for a few minutes while the oil and water separate.
Have a parent use the knife to cut the tablet into three or four pieces. Drop one piece into the mixture and watch what happens. To keep the effect going, add another piece or two.
As you watch, colorful blobs of water will rise and fall through the oil.
Oil is made up of non-polar molecules, but water is pure polar molecules. No matter how hard you might shake oil and water together, they will never mix. Oil also rises to the top of the bottle. This is because water is denser than oil.
The tablet, after being dropped into the bottle, begins to dissolve and creates gas bubbles. As the bubbles rise, they take a bit of the colored water along with them to the surface. When the blob of water reaches the top, the gas escapes. Down goes the water.
• For an even greater lava lamp effect, you can shine a flashlight through the bottom of the bottle.
A British accountant named Edward Craven Walker created the first lava lamp. He called them “Astro Lamps.”
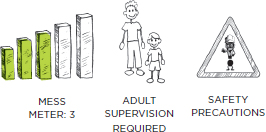

PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 15+ MINUTES
 6 large marshmallows (we used Peeps)
6 large marshmallows (we used Peeps)
 Glass bowl
Glass bowl
 Spoon
Spoon
 1 tablespoon (15 ml) melted butter or coconut oil
1 tablespoon (15 ml) melted butter or coconut oil
 1 cup (120 g) powdered sugar
1 cup (120 g) powdered sugar
Often we can’t see molecules moving. In this experiment we will watch molecules expand and contract in marshmallows.
Observe the size of the marshmallows, then place them in a bowl and heat in the microwave for 15 to 25 seconds, until the marshmallows have doubled in size. See how the marshmallows expanded with the heat?
Now push on the marshmallows with the spoon and watch them deflate with the pressure.
Add the butter or coconut oil to the melted marshmallows and stir it up. Spoon in the powdered sugar using just enough to create a non-sticky dough consistency.
You now have edible play dough that is a treat!
Heat is energy and will cause the molecules in the marshmallows to move faster, expanding the space between them. As the molecules are compressed by stirring and cooling, the space contracts or gets smaller.
• Place a marshmallow in the freezer for a few hours. Can you guess what will happen to it?
The mashmallows will be very hot and sticky when you pull them out of the microwave. Do not touch them!
The first marshmallows were made from the sap of the marsh mallow plant, and people have been eating marshmallows for thousands of years.


PREP TIME: 2 MINUTES
EXPERIMENT DURATION: 2 MINUTES
 2-liter plastic bottle of diet soda (any soda works, but a diet soda won’t leave a sticky mess)
2-liter plastic bottle of diet soda (any soda works, but a diet soda won’t leave a sticky mess)
 Mentos mints
Mentos mints
The three states of matter are liquid, gas and solid. In this experiment we will see what happens when you release the gas from a liquid.
Put your bottle on the ground outdoors, and have a hose handy—this is messy. Open the bottle and drop in 2 Mentos.
A geyser of liquid will shoot out of the bottle.
Soda is made by dissolving carbon dioxide (CO2) into liquid, usually by pressure. But CO2 is nonpolar and water is polar. That means they don’t like to stay mixed. When the mint is dropped into the soda, it falls to the bottom and carbon dioxide bubbles are freed from the soda. Because the bubbles are lighter than soda, they rise to the surface of the soda—FAST, like in a geyser.
• Try changing up one of the ingredients in this experiment. You can either use a different type of candy, a different brand of soda or even change the temperature of the soda How do these changes affect the “geyser”?
The average American drinks 44 gallons (167 liters) of soda in a year.


PREP TIME: 15 MINUTES, PLUS 30 MINUTES COOLING TIME
EXPERIMENT DURATION: 5 MINUTES
 1 cup (235 ml) whole milk
1 cup (235 ml) whole milk
 Mug
Mug
 Cup
Cup
 Food coloring (optional)
Food coloring (optional)
 4 teaspoons (20 ml) white vinegar
4 teaspoons (20 ml) white vinegar
 Spoon
Spoon
 Scrap of fabric
Scrap of fabric
 Rubber band
Rubber band
Creating a sort of plastic from common kitchen ingredients is a great way to watch molecules rearrange themselves right before your eyes.
Pour the milk into the mug and heat in the microwave for a minute or two. You want the milk to be hot, but not boiling.
In a separate cup, combine the food coloring and the vinegar. Stir with a spoon. Pour the vinegar into the hot milk. Watch the milk curdle and clumps form as you stir the ingredients together.
After the milk has curdled strain the curds, pouring the liquid out. We used the fabric and the rubber band to make a strainer. After the curds cool, about 30 minutes, smush this plastic-like substance into a shape, like a ball.
You will get blobs of a plastic-like substance.
You can find a protein called “casein” in milk. When you add an acid (like vinegar) to the casein, it causes the proteins to clump together so the liquid can be poured off. Those are the blobs in the milk, and they are like plastic.
Buttons in the early 19th century were made with this type of plastic. People “wore” their milk!


PREP TIME: 10 MINUTES
EXPERIMENT DURATION: 1 HOUR OR MORE
 Your favorite cookie recipe
Your favorite cookie recipe
 Enough ingredients to double the recipe
Enough ingredients to double the recipe
Baking is chemistry! Check out how just one ingredient can drastically change the way a cookie looks or tastes.
With an adult, assemble most of the ingredients for your doubled recipe, leaving out the eggs, baking powder and butter. Separate into four different bowls.
The goal is to leave out just one ingredient from each portion—one bowl will be without eggs, another without baking powder, the third without butter; the fourth will have all ingredients, making it a normal cookie recipe.
Add the other non-excluded ingredients to each bowl and bake according to the recipe.
When you leave out even just one ingredient, your favorite cookie recipe turns out different. Each ingredient is important! Leaving out eggs, baking powder or butter can change the results dramatically in the way the cookie tastes, feels or looks.
Each ingredient plays a special role in the cookie recipe’s chemistry. The ingredients all work together. Baking powder helps the batter rise so the cookies aren’t flat disks. Butter gives the cookie texture and tenderness. Eggs do a lot of different things in a recipe. They help the batter bind together, rise when baked and give the baked cookie a lovely toasty color.
• If you leave out vanilla, it won’t change the structure of the cookies, but it will change the flavor and smell. If you leave out flour, your cookies will lack structure and be a gooey mess. If you leave out the sugar, you might complain about the taste!
Americans on average eat almost one cookie per day!
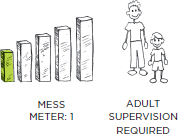

PREP TIME: 1 MINUTE
EXPERIMENT DURATION: 5 MINUTES
 Dried corn still on the cob
Dried corn still on the cob
 1 teaspoon (5 ml) canola oil
1 teaspoon (5 ml) canola oil
 Paper lunch sack
Paper lunch sack
Using a cob of dried corn, you are going to make microwave popcorn!
Place your corncob and oil into the paper bag and fold down the top. Cook in the microwave for 2 to 4 minutes. Listen for the pops to slow down.
You made popcorn! It will pop right off the cob.
A tiny bit of moisture is in every kernel of popcorn. When the kernel is heated, the moisture turns to steam and expands, causing a mini explosion.
If you can’t find dried popcorn, don’t worry—you can use any corn on the cob to make your own. Just boil it in hot water for 5 minutes and then bake in the oven at 200°F (100°C) for 3 to 4 hours. Dried popcorn can store for up to 12 months!
Microwave popcorn was first invented in 1981 and had to be stored in the freezer.
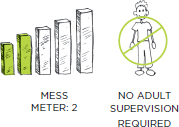

PREP TIME: 3 MINUTES
EXPERIMENT DURATION: 3 TO 30 MINUTES
 White casserole dish
White casserole dish
 Cooking oil (enough to completely cover the bottom of the dish at least ½ inch [1.3 cm] deep)
Cooking oil (enough to completely cover the bottom of the dish at least ½ inch [1.3 cm] deep)
 Liquid food coloring
Liquid food coloring
 Whisk
Whisk
 Broken egg yolk
Broken egg yolk
Everyone knows that oil and water don’t mix, but when you put them in the right company they can be combined.
Fill the bottom of the dish with oil and whisk in a few drops of food coloring. The oil and the coloring (which is mainly water) will not mix. The drops of food coloring may get smaller and smaller, but they are still separate from the oil. Let the liquid sit for a few minutes. The drops of food coloring will grow back together into bigger blobs.
Add 2 teaspoons (10 ml) of egg yolk to the oil and food coloring mixture and whisk it again.
Your oil and water will now mix into a cloudy concoction.
Oil and water don’t mix well together. Oil is hydrophobic, which means its molecules repel water. Water is hydrophilic, meaning it loves itself and will mix with other hydrophilic liquids. The egg yolk contains a fatty substance that acts as an emulsifier. An emulsifier can combine these two liquids that are usually unmixable.
Most eggs have a single yolk. But some eggs have two! The chances of getting a double-yolk egg is 0.1 percent.
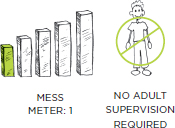

PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 2 oranges
2 oranges
 Scale
Scale
 Clear plastic or glass bowl
Clear plastic or glass bowl
 Water
Water
If you drop an orange into water, will it float? Definitely not! But could you do something to an orange to make it float? In this experiment, you will find out!
Pick two oranges that are roughly the same weight and peel one and leave the other with its peel on. Put both oranges on the scale and weigh them. The one with the peel should be heavier.
Fill the bowl with water. Drop in the peeled orange. Next, drop in the unpeeled orange.
The peeled orange should have sunk like a rock to the bottom of your bowl, while the unpeeled orange floats.
The unpeeled orange floated because the orange rind is actually filled with tiny pockets of air, making it buoyant. Even though the unpeeled orange is heavier than the peeled one, it floats!
• Try this with other fruits. Can you make a lemon float? How about a grapefruit? An apple?
Next time you are in a pool, try to make yourself more buoyant by puffing yourself up with air and floating on your back.
It is easier to carry something heavy in the pool. This is called the Archimedes’ Principle. The object will feel lighter because the displaced water applies pressure against the object, carrying it with you.


PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 1 cup (235 ml) 3% hydrogen peroxide
1 cup (235 ml) 3% hydrogen peroxide
 ¼ cup (60 ml) bubble solution
¼ cup (60 ml) bubble solution
 Tall bottle or vase
Tall bottle or vase
 Food coloring
Food coloring
 Cup
Cup
 ¼ cup (60 ml) warm water
¼ cup (60 ml) warm water
 1 tablespoon (9 g) active dry yeast (or 1 packet)
1 tablespoon (9 g) active dry yeast (or 1 packet)
 Spoon
Spoon
 A place to be messy
A place to be messy
According to the law of thermodynamics, chemical compositions eventually fall apart. With hydrogen peroxide we can watch the molecules break down. All we need is a catalyst—yeast.
Pour the hydrogen peroxide and bubble solution into your tall bottle. Add a LOT of drops of food coloring (at least 15 drops). In a separate cup, mix the warm water and the yeast and stir with a spoon. Once the yeast is dissolved into the water, pour the yeast mixture into the bottle and stand back and watch!
A ton of bubbles emerge from the bottle.
The yeast is a catalyst. It will break down the hydrogen peroxide. The bubble solution thickens the surface tension, capturing the escaping oxygen in lots and lots of frothy bubbles.
• Replace the 3% hydrogen peroxide with an 8% solution for a bigger reaction. You may be able to find these at your local beauty supply store.
• If you want a less-mess version, consider sprinkling some yeast in the bottom of a dish, add a couple of drops of food coloring, and pour a couple of tablespoons of hydrogen peroxide over the yeast. You will experience the experiment on a smaller scale.
Water is also a mixture of the hydrogen (2 atoms) and oxygen (1 atom) and is a relatively stable chemical. Unlike water, hydrogen peroxide has two oxygen atoms, making it less stable than water.
Try having an adult light a match over the potion as it is bubbling out of the bottle. Listen closely: what do you hear?

PREP TIME: 15 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 A large handful of spinach
A large handful of spinach
 Saucepan
Saucepan
 Water
Water
 Tongs
Tongs
 Blender
Blender
 1 tablespoon (15 ml) vegetable oil
1 tablespoon (15 ml) vegetable oil
 Fine-mesh strainer
Fine-mesh strainer
 Bowl
Bowl
 Black light
Black light
Did you know chlorophyll doesn’t always look green? In this experiment, you’ll use spinach to find out what color it can also look.
First we need to extract the chlorophyll from the spinach. Boil the leaves in a saucepan of water for 1 minute. Take the spinach out of the water with tongs and put it in the blender with the vegetable oil. Blend until smooth.
Strain the green liquid through a fine-mesh strainer into the bowl. The leaf goo will stay in the strainer, and the green chlorophyll-infused oil will fall into the bowl. Shine the black light onto the green liquid.
The chlorophyll in plants looks green under normal light, but it glows red under UV light, such as a black light!
Boiling the spinach pulls the chlorophyll, which is used by the plant to capture sunlight, out of the spinach cells. Chlorophyll absorbs all wavelengths of visible light—except green. Plants look green because the leaves don’t absorb the green light, so it is reflected back to us. When you shine a black light at the spinach slush, the chlorophyll absorbs the UV light and you see a bright red color.
In the 1930s Americans bought 33 percent more spinach because of the cartoon Popeye!
Did you notice how much the spinach shrank when cooked it? Guess how much fresh spinach would be needed to end up with ½ cup (90 g) of cooked spinach.

PREP TIME: 5 MINUTES, PLUS OVERNIGHT TO FREEZE (IF DOING VARIATION)
EXPERIMENT DURATION: 30 TO 40 MINUTES
 Blocks and cubes of frozen ice
Blocks and cubes of frozen ice
 Large bowl
Large bowl
 Salt
Salt
 Droppers
Droppers
 Food coloring
Food coloring
Salt affects the temperature at which water freezes. We will see how salt affects the freezing rate in this experiment.
Place the ice in a large bowl. Sprinkle the salt over the ice. Using droppers, squirt food coloring over the melting pits left by the salt.
The food coloring will help you see the effects of the melting more clearly.
When you sprinkle the salt on the ice, the ice starts to melt. The salt makes it harder for the water molecules to form ice crystals. The freezing point of water lowers as more salt is added.
• Fill two identical plastic containers halfway with warm water. Add salt (⅓ cup [75 g]) to one of the containers and stir. Place both containers in the freezer and check them every few hours. Which container froze first, the salt-filled one or the one with just tap water?
This is why you sprinkle salt on sidewalks in winter to melt the ice. Tap water freezes at 32°F (0°C), but salt water freezes at about 15°F (-9°C).

PREP TIME: 15 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 2 clear glasses
2 clear glasses
 1½ cups (360 ml) warm water, divided
1½ cups (360 ml) warm water, divided
 ½ cup (120 g) salt
½ cup (120 g) salt
 Spoon
Spoon
 2 eggs
2 eggs
 Collection of items, such as pens, small balls, toys, rubber bands and paper clips
Collection of items, such as pens, small balls, toys, rubber bands and paper clips
Most objects are denser (heavier) than water, so they sink. But when you add salt to the water, it changes how dense the water is, allowing some objects to float. In this experiment we will change the density of water and float objects.
Into one glass, pour ¾ cup (180 ml) of the warm water and set it aside. This will be your control sample.
Into the other glass, pour the remaining ¾ cup (180 ml) warm water. Slowly pour in the salt. Using the spoon, stir it up until it has dissolved. Place 1 egg into each glass. Compare how easily they float between the two glasses of water. Test again with the other items you collected.
The egg in the glass with salt water floats.
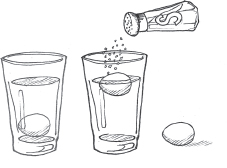
Objects float more easily in salt water. When you added salt to the water, you increased its density, making it easier for some objects to float.
• Make an egg float in the middle of a glass of water! Fill a glass halfway with water. Add ⅓ cup (75 g) salt and a few drops of liquid food coloring. Stir to dissolve the salt. Slowly add water to fill the glass to right below the brim. Try to not disturb the colored water as you pour the fresh water in. Drop the egg into the water. The egg should drop through the tap water but be suspended above the colorful salt solution.

PREP TIME: 5 MINUTES, PLUS DRYING TIME
EXPERIMENT DURATION: 3 TO 4 MINUTES
 ¼ cup (60 ml) lemon juice
¼ cup (60 ml) lemon juice
 Small bowl
Small bowl
 Cotton swab
Cotton swab
 White construction paper
White construction paper
 Hair dryer or incandescent light bulb
Hair dryer or incandescent light bulb
With science we are going to create invisible ink and a super secret way to read the message.
Pour the lemon juice into the bowl. Dip one end of the cotton swab into the lemon juice. Using the swab, write a secret message on the paper. Let the paper dry fully, then hold it a few inches (5 to 10 cm) away from a heat source, such as hair dryer on low or a light bulb.
Your message will appear!
Heat causes the citric acid in lemon juice to decompose, freeing the carbon. When the carbon touches the air, it turns brown; this is called “oxidation.” The magic of oxidation allows someone to read your message!
• Compare “secret ink” solutions. Try the experiment with other fruits, or a different liquid like vinegar, milk or apple juice. Is your message more or less clear than the one with the lemon juice?
Light bulbs can get hot fast. Make sure you don’t touch it.
The same process of oxidization we see here is what causes apples to turn brown or when something left outside gets rusty, but in those cases no heat is needed.

PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 5 MINUTES
 Scissors
Scissors
 Disposable diaper
Disposable diaper
 Disposable cup
Disposable cup
 ¼ to ⅓ cup (60 to 75 ml) water
¼ to ⅓ cup (60 to 75 ml) water
Diapers have a lot of different parts to them that help absorb liquid. We are going to dissect a diaper to see how they work.
Using the scissors, cut open the diaper. Separate the cotton to find sandy particles. These are silica grains. Dump as many grains as you can from the diaper into the cup. Add the water to the cup. Wait a second, and then turn your cup upside down.
The liquid will solidify, so it won’t pour out of the cup.
When you turned the cup upside down, the water did not dump out as you may have expected. The sand particles of silica absorbed the water, making a gel. When you turn the cup upside down, the gel stays in the cup. It’s solid! That’s how the diaper keeps the baby dry!
Avoid touching and playing with the silica water gel. It can irritate skin.
The National Geographic Society says that the average baby uses 3,796 diapers before he or she is potty trained. That is a LOT of diapers!


PREP TIME: 5 MINUTES
EXPERIMENT DURATION: 15 MINUTES
 Bowl
Bowl
 Liquid starch
Liquid starch
 White glue
White glue
 Liquid watercolor paint or food coloring
Liquid watercolor paint or food coloring
In this experiment, you will make an easy slime recipe and explore its colloidal properties.
In the bowl, combine equal parts of starch, glue and paint or food coloring. Use your hands to mix it all up. The key to making good slime is mixing it really, really well.
If after a lot of mixing, the slime hasn’t formed well, add a small amount of glue and mix some more. If it’s still not forming, add a small amount of the liquid starch.
When you use your hands to combine the ingredients, it forms a solid, stretchy blob.
Slime is a colloid, or substance that is evenly dispersed through another substance. Your slime has the characteristics of both a solid and a liquid. It moves more slowly than water, yet it is wet, and it slides quickly!
• What do you think would happen if you added more starch to the mix? How about more glue? How would the slime feel and act differently?
Don’t taste the slime!
Different types of fluids flow at different rates. This is called their viscosity. For instance, ketchup is much more viscous than water. Slime has an interesting property in that its viscosity isn’t always the same. It can change, such as if you add stress. This type of fluid is called a non-Newtonian fluid, and other examples are ketchup, yogurt and mud.

PREP TIME: 1 DAY
EXPERIMENT DURATION: SECONDS TO SEE A REACTION
 Red cabbage, chopped
Red cabbage, chopped
 Slow cooker
Slow cooker
 Water
Water
 Strainer
Strainer
 Sturdy white paper towels
Sturdy white paper towels
 Safety goggles
Safety goggles
 Substances to test (see variations)
Substances to test (see variations)
We create a test from cabbage juice to show whether a chemical is an acid or a base. This experiment is smelly and colorful!
This is a stinky experiment—we suggest you do it outside. Place the cabbage leaves in the slow cooker, add water to cover the cabbage, turn it on low and leave it on overnight. The water will turn dark purple.
Strain out the leaves and dip the paper towels into the purple water. Pull them out and hang them to dry. Once dry, the paper towels should be lavender.
Put on your safety goggles. Drip drops of the different substances onto the towels. Watch colors appear on the towel.
If a solution has a high level of acid, it will turn your paper towel bright pink. If a solution is alkaline, it will leave a blue-green stain.
Red cabbage contains a pigment called flavin, which changes colors when it comes into contact with an acid or a base chemical. Neutral solutions are purple. Acids, such as lemon juice, will turn the flavin red, and bases, such as baking powder, will turn the flavin green or blue. As you drip the solutions, your towel pH test sheet will be covered in a tie-dye of colors.
• Test apple juice, lemon juice, laundry detergent, soda, baking soda, potato slices, window cleaner, toothpaste, even spit. See what else you can come up with!
Be sure to be outdoors or in a place where the ventilation is good, only mix a drop or two at a time and be sure to avoid inhaling the fumes as the chemicals are mixing.


PREP TIME: 10 MINUTES PLUS OVERNIGHT DRYING TIME
EXPERIMENT DURATION: 15 MINUTES
 Fine sand
Fine sand
 Shallow disposable container
Shallow disposable container
 Fabric protector spray
Fabric protector spray
 Large glass bowl of water
Large glass bowl of water
 Spoon or other utensils (optional)
Spoon or other utensils (optional)
 Plastic container with a lid
Plastic container with a lid
Wet sand is heavy and messy! In this experiment, we will coat the sand with a hydrophobic layer to waterproof sand.
In a well-ventilated area, spread the sand out in the disposable container. Spray the sand with a heavy coat of fabric protector spray. Shake the box a few times to make sure it’s thoroughly coated. Allow the sand to dry.
Pour the dried sand into the bowl of water. Play with the sand in the water, using spoons to dunk it in and out of the water. Store the sand in a lidded sealed container.
The sand clumps together in the water.
When you sprayed the sand with the fabric protector, you coated the grains of sand with a hydrophobic layer (or a layer that hates water). This causes the grains of sand to clump to each other in water.
Hydrophobic sand was discovered near ocean oil spills. People would sprinkle the sand on floating oil, and the sand would mix with the oil and make it heavy enough to sink.