CHAPTER 3
ATOMIC IDENTITIES
Everything around you is made of atoms of ninety or so chemical elements. All the atoms of a particular element have the same number of protons in the nucleus (and that same number of electrons surrounding the nucleus). In other words, the proton number determines an atom’s identity. The chemical properties of each element—how it interacts with other elements—are determined by the arrangement of the outermost electrons in their orbitals.

Rough-cut diamonds. Pure diamond is a material made of only carbon atoms, held together in a rigid crystal structure. Most mined diamonds contain impurities—other types of atoms in the crystal structure—that result in slight discoloration.
IDENTIFYING ELEMENTS
Around ninety elements—ninety types of atom—are found naturally on Earth. Uranium (element 92) is the heaviest element for which there is a stable isotope, and there are two unstable elements lighter than uranium that are not found naturally. However, the exact number is vague, because tiny amounts of elements heavier than uranium are found in extreme circumstances. Whatever the number, it is certainly large. How can you tell one from another?
There is a great deal of variety in the properties of pure elements. For example, at room temperature some are invisible gases, while others are shiny, metallic solids or brightly colored liquids. Some elements are highly reactive, others inert; some have extremely high boiling points, others extremely low ones. The exact combination of physical and chemical properties—a result of the configuration of electrons around the nucleus, and the number of protons and neutrons in the nucleus—can identify a pure element. So, for example, if you have a sample of a chemically reactive, colorless gas whose boiling point is −297°F (−183°C), then you have oxygen.

Native elements are elements that sometimes naturally occur in a more-or-less pure state. Each of the small samples shown here is made from many trillions of atoms, nearly all of which are the same. Each one will also contain millions or billions of atoms of other elements.
Most elements are rarely found pure. Instead, they exist in compounds, in which their atoms are bound tightly to atoms of other elements. Of the thirty or so elements that do sometimes exist naturally in their pure state, gold, copper, carbon, sulfur, and silver are relatively easy to identify by sight. To identify the majority of elements, which only exist in nature combined with other elements, you must first separate them into their pure state. Most metals, for example, exist as ores, their atoms typically bound to oxygen atoms. Smelting normally involves heating a metal with carbon, so that the carbon atoms can steal the oxygen atoms away (forming carbon dioxide molecules) and the pure metal is left behind.

Copper metal appears when the bonds between copper atoms and oxygen atoms in copper ore are broken in the presence of heat and carbon atoms in charcoal.
SPECTROSCOPY
In many cases, heating a compound can cause it to dissociate into its elements, the atoms breaking away to form a vapor. One way of identifying the metallic elements present in a compound, the flame test, relies on this fact. The mystery compound is heated in a flame, releasing the metal atoms, which form a vapor. Electrons in the hot atoms are boosted up to higher-energy levels, and then fall down, emitting light of a characteristic frequency (and therefore color). The exact frequency of the light emitted depends upon the difference in energy between the two levels (see here)—and that is unique to each element.
To be sure that a particular element is present, scientists normally study the colored light in a spectroscope, which separates out the individual frequencies present (each one corresponding to a particular pair of energy levels). The same characteristic frequencies are behind many everyday phenomena, including the colors of fireworks and the orange color of sodium lamps used for some types of streetlights. Many of the elements discovered since the 1860s have been identified as new elements—or have had their status as “newly-discovered” verified—by variations of this technique, which is known as spectroscopy.

Mock-up of the colored light produced by (most of) the elements, based on spectroscopic observations. Note how each “emission spectrum” is unique, and contains individual colored lines, instead of a continuum of color.
SORTING BY MASS

Inside a mass spectrometer, atoms from a vaporized gas are ionized by an electron beam and then accelerated past a magnetic field, which bends their path. The lighter the ion, the greater the deflection.
MASS SPECTROMETER
Another way of identifying elements normally combined in compounds is mass spectrometry. Inside an evacuated chamber—in other words, one from which the air has been removed—a sample for testing is first vaporized to break it into individual atoms. A high-powered electron beam knocks electrons off the atoms, turning them into positive ions. These ions are then accelerated by a strong electric field, and they pass along the chamber at speeds of several kilometers per second. Strong electric and magnetic fields inside the chamber force the ions to follow curved paths. Crucially, the heavier the ion, the less it will be deflected—just as blowing a passing tennis ball will change its course much less than doing the same to a passing ping-pong ball. A device at the far end of the curved chamber detects the ions as they arrive, and it is then possible to work out the mass of the ions passing along the chamber, and therefore which elements are present in the sample. Because the ions are separated according to their mass, this technique can even separate different isotopes (that is, different versions of the same element with the same number of protons but different numbers of neutrons). Mass spectrometry has many applications, including in forensics and in purifying a sample of uranium into its two main isotopes, only one of which is useful in nuclear power stations.
Identifying elements is a challenge that scientists have mastered using techniques such as those described above. A far greater challenge was to work out why there are elements at all—which scientists have now done. To answer this we must first find out where the elements came from.
THE ORIGINS OF THE ELEMENTS
All the matter around you is made of atomic nuclei plus electrons, often bound together as atoms (or ions or molecules). The number of protons in a nucleus determines the element to which its atom belongs. Some of the nuclei—and therefore some of the elements—date back to the first seconds and minutes after the beginning of time. Others were formed inside stars, and yet others in extremely energetic supernovas. The rest are the result of radioactive decay.
NUCLIDES FROM THE DAWN OF TIME
Atomic nuclei are composed of protons and neutrons, each unique combination referred to as a nuclide. Before they have electrons bound to them, nuclei are simply clumps of protons and neutrons—tiny objects that could be called “would-be nuclei.” The first would-be nuclei were produced in the early stages of the universe. As far as we know—as far as the evidence and theories of modern cosmology tell us—the universe came from nothing, suddenly, 13.8 billion years ago. Quarks immediately “condensed” out of energy in extremely large numbers. After about a millionth of a second, most of those quarks had formed composite particles, each consisting of a triplet of quarks. These particles were protons and neutrons. An individual proton is the would-be nucleus of a hydrogen-1 atom—so, by default, hydrogen-1 was the first nuclide to come into existence.
Initially, there were equal numbers of protons and neutrons. However, free (unbound) neutrons decay to form a proton plus an electron, so there were soon many more protons than neutrons. In fact, within a few seconds, protons outnumbered neutrons by about seven to one. Over the next few minutes—within minutes of the beginning of time—many of the neutrons became bound to protons, forming nuclides heavier than hydrogen-1.
NEUTRON DECAY

A free (unbound) neutron is unstable. The decay of free neutrons resulted in a dramatic imbalance of protons and neutrons in the early universe. Note how there is no overall electric charge after the decay. For information on antineutrinos, see chapter seven.
PRIMORDIAL ELEMENTS
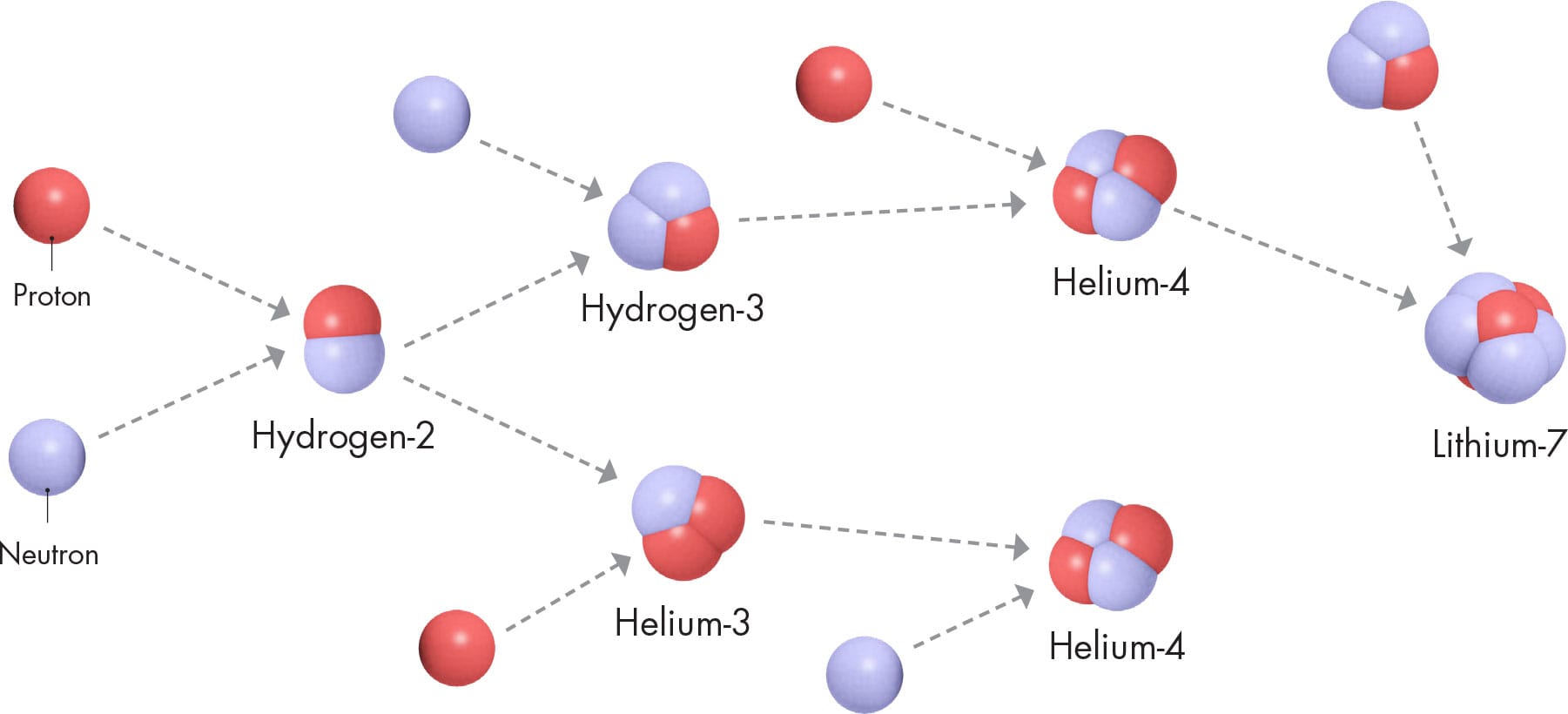
In the first few minutes after the beginning of our universe, protons and neutrons became bound together, forming several nuclides—mostly isotopes of hydrogen and helium.
One proton bound to one neutron makes hydrogen-2 (1p, 1n), which is also called deuterium. Add another neutron, and you get hydrogen-3 (1p, 2n), also called tritium. Add another proton instead, and you have helium-3 (2p, 1n). These were simply intermediaries for a much more stable nuclide, helium-4 (2p, 2n).
If the universe had expanded more slowly, all the neutrons would have ended up in would-be helium-4 nuclei, and the remaining protons would have been left behind as hydrogen-1—these would then have been the only two nuclides present. But the universe expanded extremely rapidly, so small amounts of deuterium and helium-3 also remained (the tritium was unstable and quickly decayed to form helium-3). In addition, a tiny proportion of the first would-be nuclei were lithium-7 (3p, 4n). Overall, though, hydrogen-1 and helium-4 accounted for around 99.9 percent of the would-be nuclei present after those first few minutes. And to this day, these two nuclides are by far the most abundant in the universe.
Electrons were also created in large numbers within the first millionth of a second. But in the intense frenzy of the early universe, both the high temperature and the radiation coursing around space were too great for the electrons to settle neatly into orbitals around the would-be nuclei, so no atoms could exist. Instead, the matter in the universe existed as “plasma,” a mixture of negatively-charged electrons and positively-charged ions. A positively-charged ion is an atom with fewer electrons than protons. In this case, the atoms had no electrons at all; the plasma was a mixture of electrons and completely “naked” would-be nuclei. It was only after 380,000 years that conditions calmed down enough for the first atoms to appear. The would-be nuclei became atomic nuclei at last. With electrons bound to atoms, the universe became transparent—previously, any radiation was absorbed and then reradiated by free electrons, making space opaque and foggy. As well as being transparent, space was also dark, because the initially hot universe had cooled down, and there was nothing to produce light. That all changed about 200 million years later.

STAR BIRTH
The first generation of stars would have been much larger on average than later generations, but new stars being born in the universe right now form in the same way. Gravity pulls the densest regions of vast gas clouds into clumps (1). As the gravitational collapse proceeds, the gas heats up and the pressure at the center becomes so great that nuclear fusion begins. The heat causes the gas to expand, supporting the young “protostar” against further collapse. The energy released by the nuclear fusion heats the gas to a high temperature. The young star emits intense electromagnetic light and other radiation, and throws out a wind of charged particles into the space around it (2). These emanations clear the remaining gas around the star (3).
FIRST LIGHT
In the long dark ages of the universe, some regions of space were filled with huge clouds made almost entirely of hydrogen and helium gas. In other places, the universe was empty. Most of the hydrogen existed as molecules, each consisting of two atoms joined together (see chapter four), while the helium was made of individual atoms. The gases were extremely rarefied. There were a few thousand atoms or molecules per cubic centimeter, which makes it about as dense as the best vacuums scientists can produce here on Earth. Certain parts of the gas clouds were very slightly more dense than the average and, over millions of years, the mutual gravity between the atoms and molecules in these denser regions began to pull the hydrogen and helium mixture together.
This gravitational collapse resulted in spherical blobs of gas that grew ever more dense, and the energy released by the collapse heated up the gas at the centers of the blobs. The rising temperatures in the blobs of gas caused the electrons to leave their atoms, so the gas became plasma once again. Eventually, the temperature and the pressure inside these blobs of gas became high enough to force some of the tiny primordial nuclei to fuse together, creating new would-be nuclei. This “nuclear fusion” released enormous amounts of energy, heating the gas still further. This process had two effects. First, the hot gas expanded, buoying it up against further gravitational collapse. Second, the high temperature caused the enormous blobs to glow with incandescence, becoming fully-fledged stars—bright beacons in an otherwise dark universe.
This first generation of stars began with mostly hydrogen-1 (naked protons) and a little helium-4. For most of their lives, these stars simply made more helium-4 from the hydrogen-1 in a reaction called the proton-proton chain. This “hydrogen burning” is also the predominant reaction in most stars today, including our Sun. So the energy that sustains all life on Earth was released deep inside the Sun as the result of hydrogen nuclei being forced together to form helium.
HYDROGEN BURNING
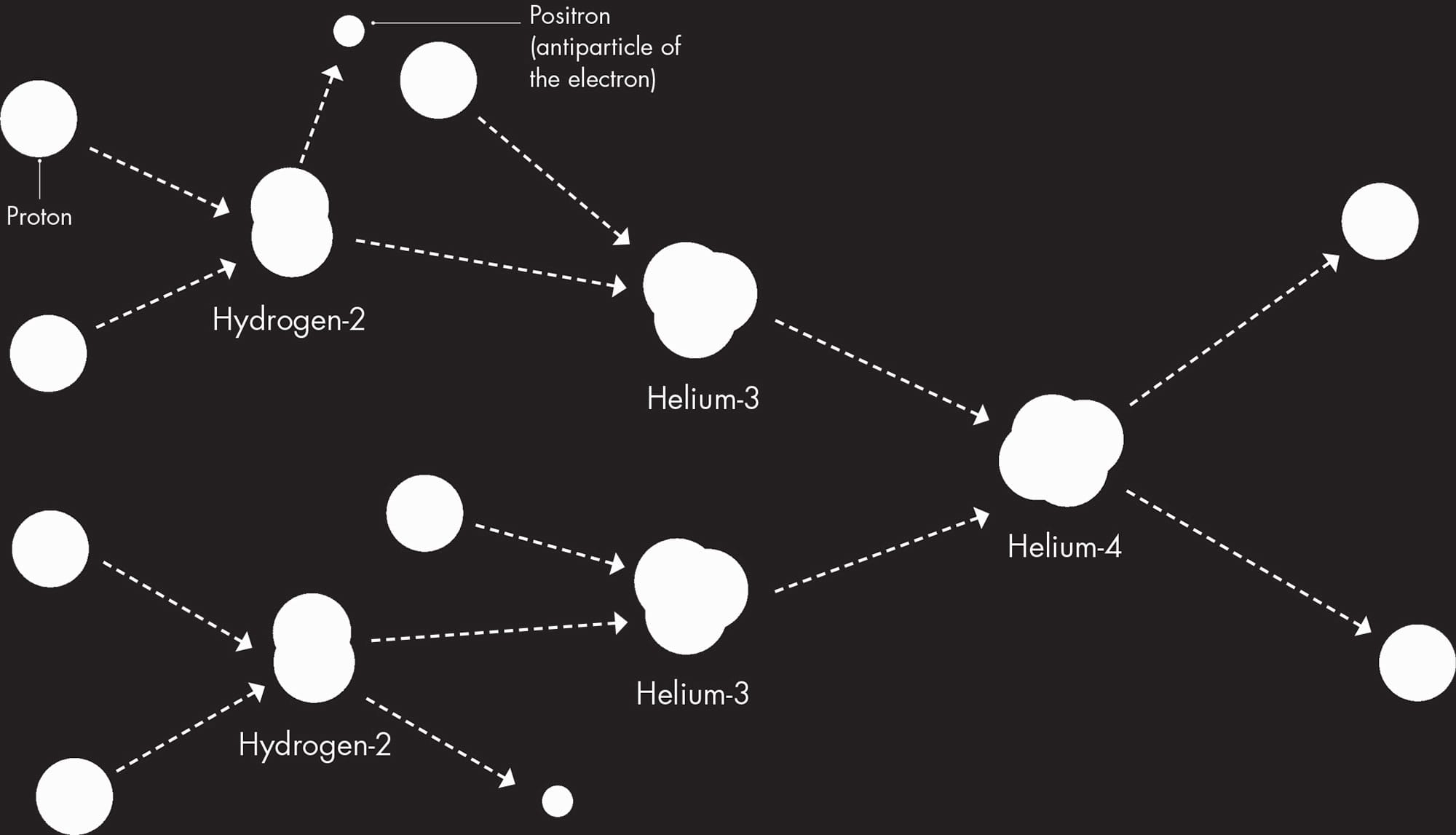
The main fusion reaction going on in most stars is misleadingly called hydrogen burning; strictly speaking, burning is a chemical reaction, involving atoms, not would-be nuclei. The reaction inside stars is a nuclear reaction and is more properly called the proton-proton chain. The result of the reaction is the creation of a would-be nucleus of helium-4.
Once most of the hydrogen in a star’s core is used up—which will happen inside the Sun in a few billion years time—a new reaction can begin. This new reaction is known as the triple alpha process, in which three helium-4 nuclei combine to form carbon-12 (6p, 6n). The process gets its name from the fact that a naked helium-4 nucleus (2p, 2n) is identical to an alpha particle (“α,” see here), and it takes three of those to make carbon-12. Other combinations of alpha particles beyond carbon-12 form different, heavier nuclides. All of them have atomic numbers that are multiples of two and nuclide numbers that are multiples of four; for example, oxygen-16 (8p, 8n; or 4α), neon-20 (10p, 10n; or 5α) and magnesium-24 (12p, 12n; or 6α). These only form in massive stars, where the temperature and pressure is enough to cause these reactions at the core.
TRIPLE ALPHA
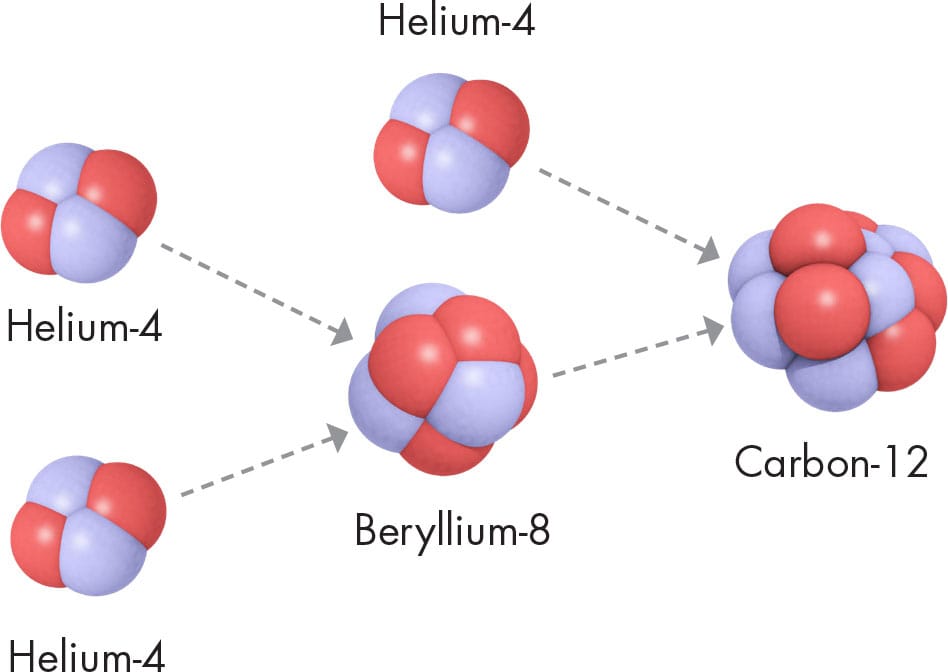
Two alpha particles—helium-4 nuclides produced by hydrogen burning combine to make beryllium-8; add another alpha particle and the result is one would-be nucleus of carbon-12.
Successive rounds of this alpha process take place, building heavier and heavier nuclides. In the meantime, another process creates yet more new nuclides. It is the result of free neutrons dashing around inside the star. When a free neutron hits a nucleus, it has a good chance of sticking to it, creating a nuclide of the same element (there has been no change to the number of protons) but with a mass number increased by one. So, for example, oxygen-16 (8p, 8n) would become oxygen-17 (8p, 9n). In some cases, the new nuclide would be unstable. It might undergo beta decay (see here), in which a neutron inside the nucleus decays to form a proton and releases an electron. If that happens, the nucleus’s proton number increases, because there is one more proton. So in our example, oxygen-17 (8p, 9n) would become fluorine-17 (9p, 8n). There are not many free neutrons in a star, so this process is slow. It is called the s-process, and the “s” really does stand for “slow.” The s-process occurs in the last few tens of thousands of years of a star’s lifetime.
THE S-PROCESS
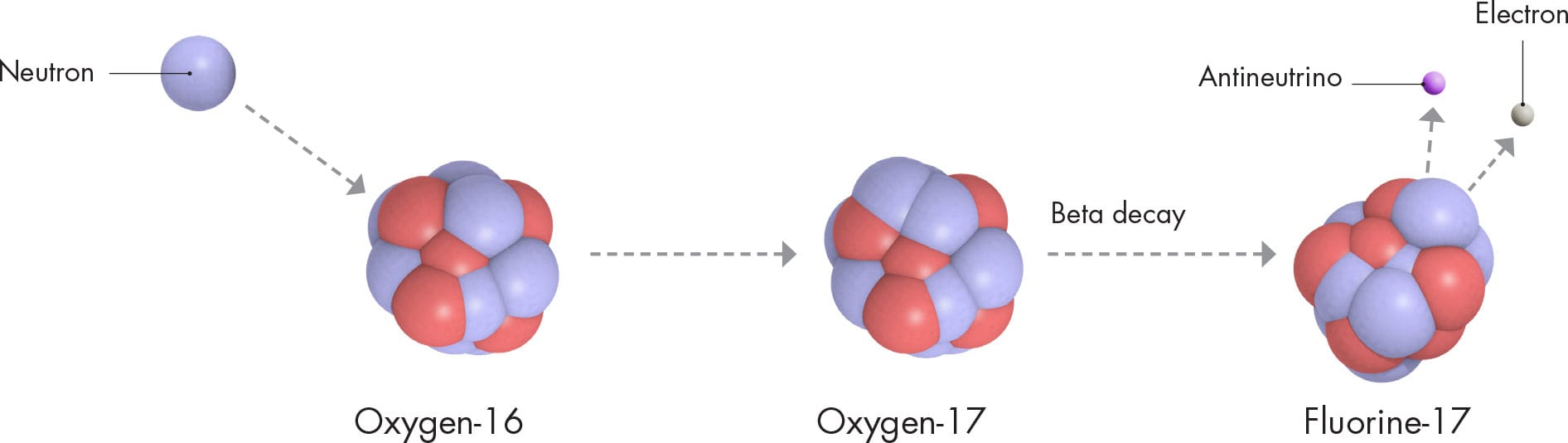
In this example of the slow s-process, the nuclide oxygen-16 transmutes into fluorine-17, after absorbing a neutron and undergoing beta decay.
THE ALPHA PROCESS
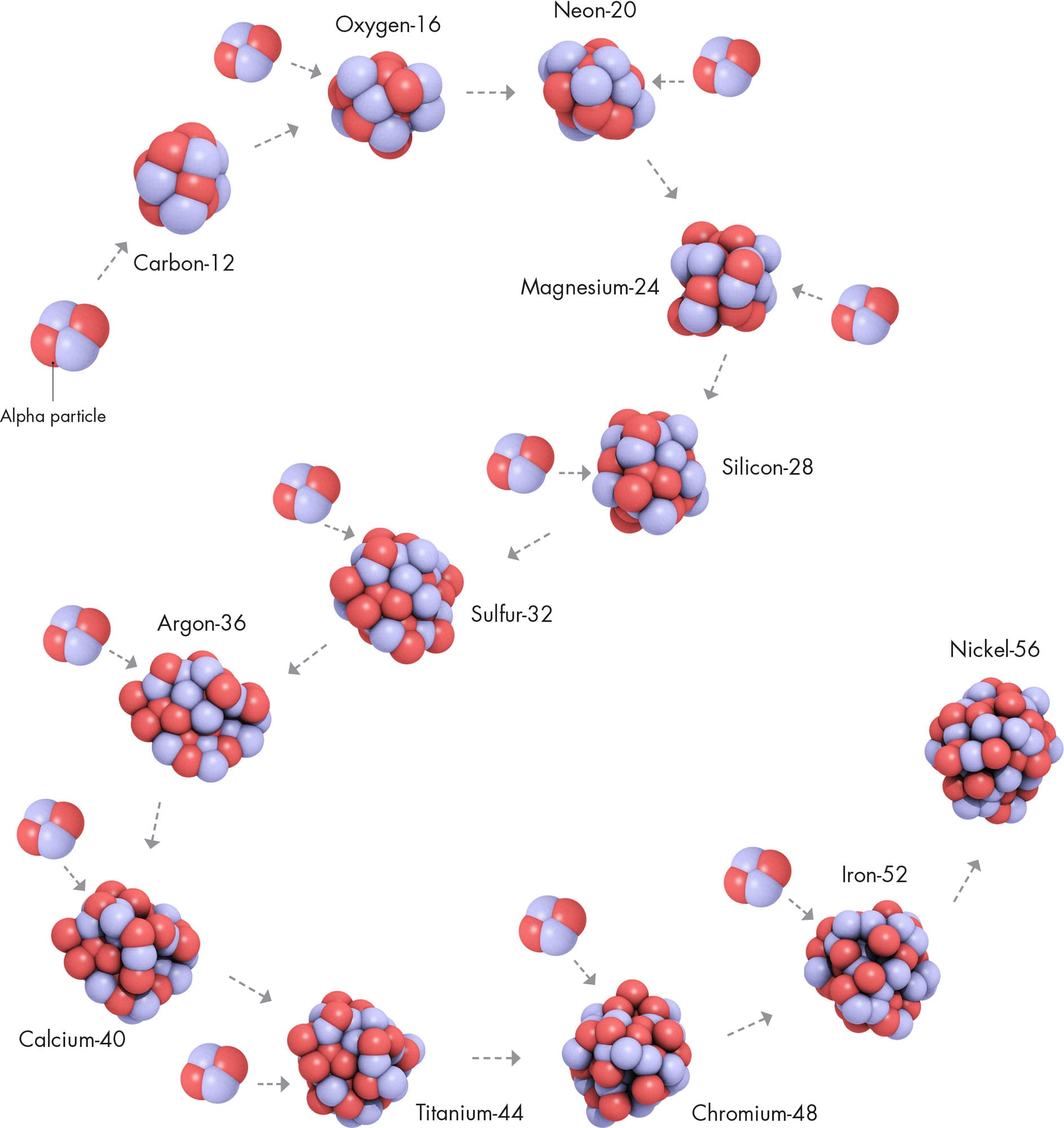
Inside a star, alpha particles are extremely plentiful, and small nuclides can easily absorb them, because the resulting nuclide has less energy than the initial one. This process leads to the creation of larger and larger nuclei, each with multiples of two protons and two neutrons, up to nickel-56. Adding an alpha particle to that nuclide requires a large input of energy instead of a release of energy.
GOING SUPERNOVA
Alongside the s-process, the alpha process continues up to the creation of the nuclide nickel-56. This nuclide requires more energy to form than it releases, unlike the lighter elements that preceded it. At this stage, then, the star has run out of fuel, and it collapses and then blows apart in a dramatic explosion called a supernova. During the supernova, more elements are created by a much more rapid version of the s-process—so rapid it is called the r-process. There are many more free neutrons available in a supernova explosion, and several can be captured by a single nucleus at once. This leads to neutron-heavy nuclei that will decay either immediately or later in successive rounds of beta decay to form any of a large number of brand new nuclides.
Two elements, beryllium and boron, are not produced by any of the processes described above, and yet are not particularly rare. Beryllium-8 (4p,4n) seems like a probable candidate for being made by the alpha process, because its nuclei are the equivalent of two alpha particles stuck together. However, it is very unstable, with a half-life of a fraction of a second. Beryllium-9, on the other hand, is stable. Along with the stable nuclides boron-11 and boron-10, it is produced by the magnificently-named process of “cosmic ray spallation.” Cosmic rays are very fast-moving particles—mostly protons, alpha particles, and electrons. When these particles hit heavier nuclei during or after a supernova explosion, or even during the lifetime of a star, they can cause the nuclei to become unstable, and split, or fission (see here) to form these smaller, lighter nuclei. Some lithium-7 is also made this way.
Supernova explosions have another key role in the story of the origin of the elements. They scatter the whole array of nuclides, old and new, out into deep space. From there, a new generation of stars can form. The new stars may be accompanied by a spinning disk of dust and gas made of the elements created in the dead star’s lifetime and at its fiery end. In many cases, the disk will coalesce into clumps that will become planets. This planetary disk is cool enough for atoms to form once again; would-be nuclei, old and new, become atomic nuclei, as electrons settle down into orbitals. The electrons fill the orbitals from the bottom up—and the best way to understand and represent this pattern of orbital filling is to arrange the elements in a table: the periodic table. Doing so also explains why certain groups of elements have similar chemical and physical properties.
THE R-PROCESS
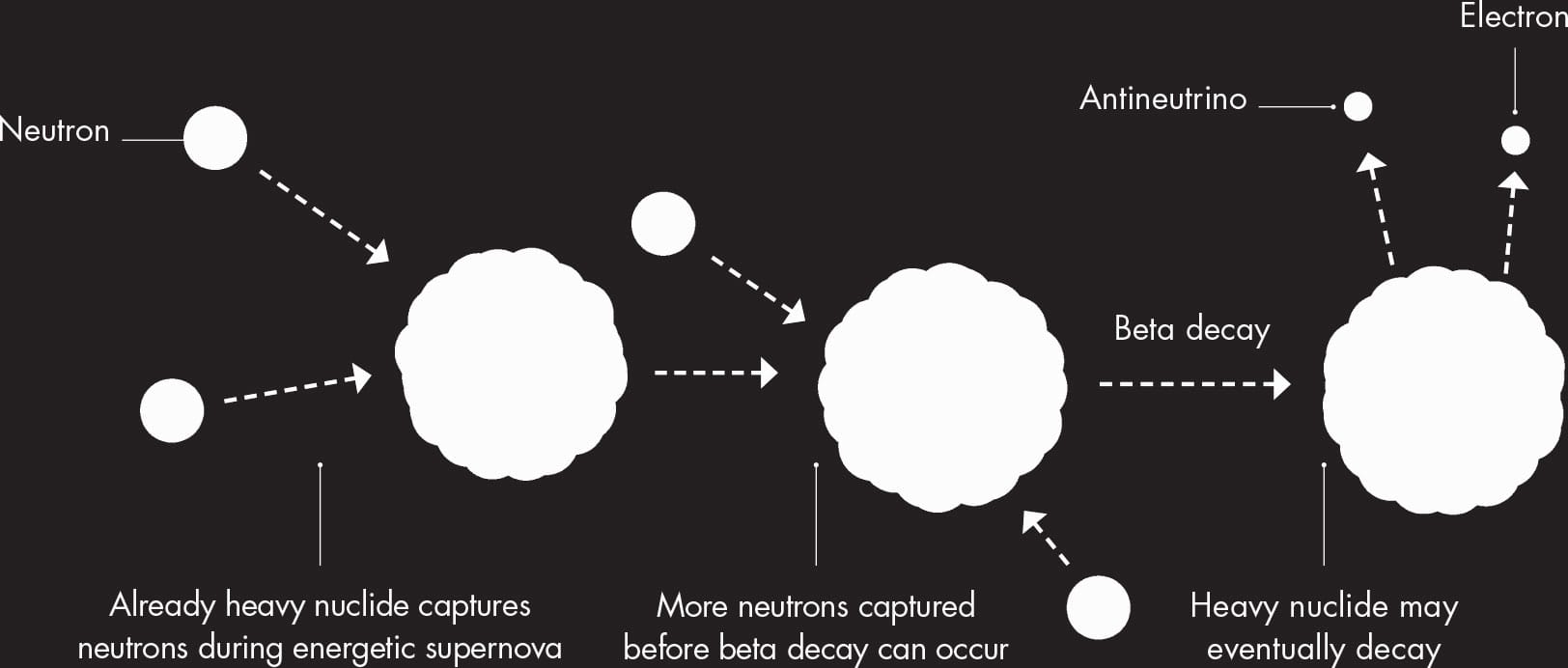
In the rapid r-process, already large nuclides absorb several neutrons before they can undergo beta decay, producing elements with higher atomic numbers.

An artist’s impression of a “kilonova”—an explosion produced by the collision of two neutron stars. Astrophysicists have worked out that many of the heaviest elements, including gold and platinum, are created in such collisions. A neutron star is what is left behind after a massive star has gone supernova and blown its newly-created elements out into space.
THE PERIODIC TABLE
It adorns every chemistry classroom, and is as iconic and instantly recognizable as the map of the United States or the logos that represent global brands. And yet, only a small proportion of people understand the periodic table or realize its explanatory power and its inherent beauty. The periodic table neatly expresses the links between the quantum physics of electrons in their orbitals and the chemical properties of elements in everyday life.
ADDING ELECTRONS
Each row, or period, of the periodic table represents the filling of a particular outer electron shell in the atoms of the elements that inhabit it. A shell is the collection of all the orbitals at a particular energy level (the s-, p-, d-, and f-orbitals, see here). So the first period, at the top of the table, is home to the elements with electrons in only the first shell—in other words, those elements that have electrons at only the first energy level. That first energy level, with the lowest amount of energy, has just one available orbital: the 1s-orbital. And because each orbital can hold up to two electrons, the first period contains just two elements: hydrogen and helium. Hydrogen has one electron, while helium has two. How does this relate to the chemical properties of these elements?

Each cell in the periodic table typically gives the name of the element in question, its symbol, its atomic number (the number of protons in the nucleus of each of its atoms), and its relative atomic mass. Also called atomic weight, relative atomic mass is the mass of a single atom of the element, in atomic mass units (daltons, see here). Different isotopes of the element have different numbers of neutrons, and so different masses, so this figure is an average for all the atoms of that element.
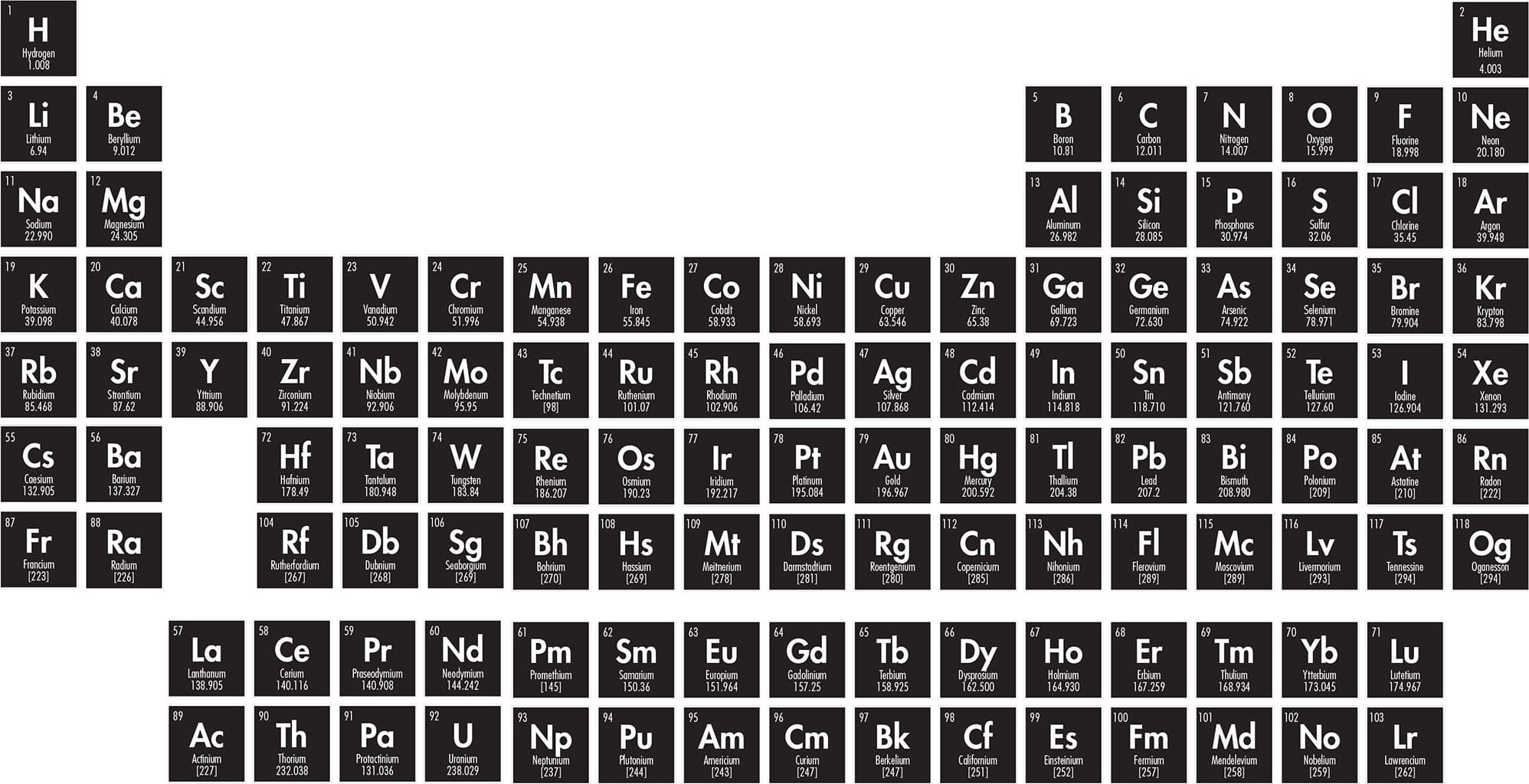
The standard form of the periodic table. Rows are called periods, and columns are called groups. Elements from any particular group have similar properties, because they have the same number of electrons in their outermost shell.

Potassium is in Group 1 of the periodic table, so it has a single outer electron. This makes it extremely reactive—so much so that it reacts explosively with a single drop of water.
Chemical reactions have nothing to do with nuclei and everything to do with electrons. They involve the swapping and sharing of electrons between atoms—something that is explored in more detail in chapter four. Most chemical reactions result in atoms attaining a state in which their outer shell is filled with electrons. An atom with a full outer shell is in a stable, low-energy state. Elements whose atoms naturally have filled shells are therefore unreactive, while the atoms of other elements achieve a full shell by losing or gaining electrons in chemical reactions. Elements that easily lose electrons to attain a full outer shell (and form positive ions in the process) are metals, while those that easily gain electrons (and form negative ions) are nonmetals.
The atoms of elements down the right-hand side of the table, in the last column, all have full outer shells. As a result, those elements are chemically stable, because it would take a huge amount of energy to add or take away electrons from a full shell. Those elements simply do not take part in chemical reactions. The columns of the table are called groups, and those unreactive elements at the right-hand side make up Group 18. This group is also called the “noble gases”—meaning noble as in incorruptible.
At the extreme left-hand side of the table—in Group 1, the alkali metals—are elements whose atoms have just one electron in their outer shell. An atom of a Group 1 element will easily lose its lone outer electron to attain a stable, full-shell state, but it is then no longer an atom: it is a positive ion (having lost one negatively-charged electron). The fact that the electron is so easily lost means that these elements are extremely reactive. Add pure potassium (K) to water, for example, and the potassium atoms will attain a full outer shell by donating their outer electrons to water molecules in a fiery display. Similarly, atoms of the elements in Group 2 are extremely reactive—but not so much as those in Group 1, because they have to lose two electrons before they realize that full outer shell.
Together, Groups 1 and 2 make up what is called the s-block of the periodic table (see here). In the outer shell of their atoms, electrons are only present in an s-orbital. Helium could be in the s-block, because its two electrons are both in an s-orbital. But for the purposes of the periodic table, its status as an atom with a filled shell is more important, so it is placed in with the noble gases in Group 18. Likewise, hydrogen is a little out of place in the periodic table, placed as it is with the alkali metals in Group 1. With just one electron, it is as easy for a hydrogen atom to receive an extra electron to make a full shell of two electrons, as it is for it to lose one. As a result, hydrogen could just as easily be in the penultimate group, next to helium. So the first period of the periodic table is not completely representative of the trends in the rest of the table.
BEYOND THE FIRST ELECTRON SHELL
The fact that the periods become longer after the first period, and the periodic table is wider, is testament to the fact that there are more orbitals available at higher-energy levels. In the second shell (in Period 2), for example, there is the 2s orbital plus three 2p-orbitals—room for a total of eight electrons. That is why there are eight elements in Period 2. At the right-hand end of Period 2 is the element neon (Ne). Neon has electrons in all those orbitals, plus the two electrons in the 1s-orbital below—a total of ten electrons. It is no surprise, then, that neon’s atomic number is 10.
In the next row, Period 3, there are also eight slots available in the outer shell: two in the 3s-orbital and two in each of the three 3p-orbitals. The element at the right-hand end of Period 3 is argon (Ar), and its atomic number is eight more than neon’s: 18 (2 + 8 + 8). The section of the periodic table containing elements whose outermost electrons are in p-orbitals is called the p-block (see here).
In Period 4, a new type of orbital becomes available: the d-orbital. There are five d-orbitals in any shell that has them. So the fourth shell has a total of eighteen slots to fill: two in the 4s-orbital, six in the three 4p-orbitals, and ten in the five 4d-orbitals. That is why the width of the periods suddenly switches from eight to eighteen. The element at the right-hand end of Period 4 is krypton (Kr), with an atomic number eighteen more than that of argon: 36 (1s2 2s2 2p6 3s2 3p6 4s2 4p6 4d10). Period 5 has the same number of elements—a total of eighteen—with the noble gas at the extreme right end being xenon (Xe), with an atomic number of 54, and 54 electrons: (1s2 2s2 2p6 3s2 3p6 4s2 4p6 4d10 5s2 5p6 5d10)—eighteen more electrons than krypton. The section of the periodic table that corresponds to elements whose outer electrons are in d-orbitals is called the d-block (see here).
In Period 6, yet another type of orbital becomes available: the f-orbital. There are seven f-orbitals in each period that has them, so the width of the periodic table should grow yet again, to be 32 elements wide (s2 p6 d10 f14). There are versions of the table that have that full width. In the standard version of the table, however, the f-block is shown separately, under the main table (see here).
The f-block contains elements from two periods: Period 6 and Period 7. The heaviest stable element that occurs naturally, uranium, is found in Period 7. All the remaining “transuranium elements,” all of them in Period 7 (but not all in the f-block), have been made artificially, in particle accelerators, by firing neutrons at other heavy elements, or even by colliding heavy nuclei together. The noble gas at the end of Period 7 (back in the main table) is oganesson (Og), the heaviest element so far discovered or created. It has an atomic number of 118 (2 + 8 + 8 + 18 + 18 + 32 + 32). The only oganesson isotope so far created, oganesson-294, has a half-life of less than one-thousandth of a second.