Family trees
organising materials and processes
Abstract
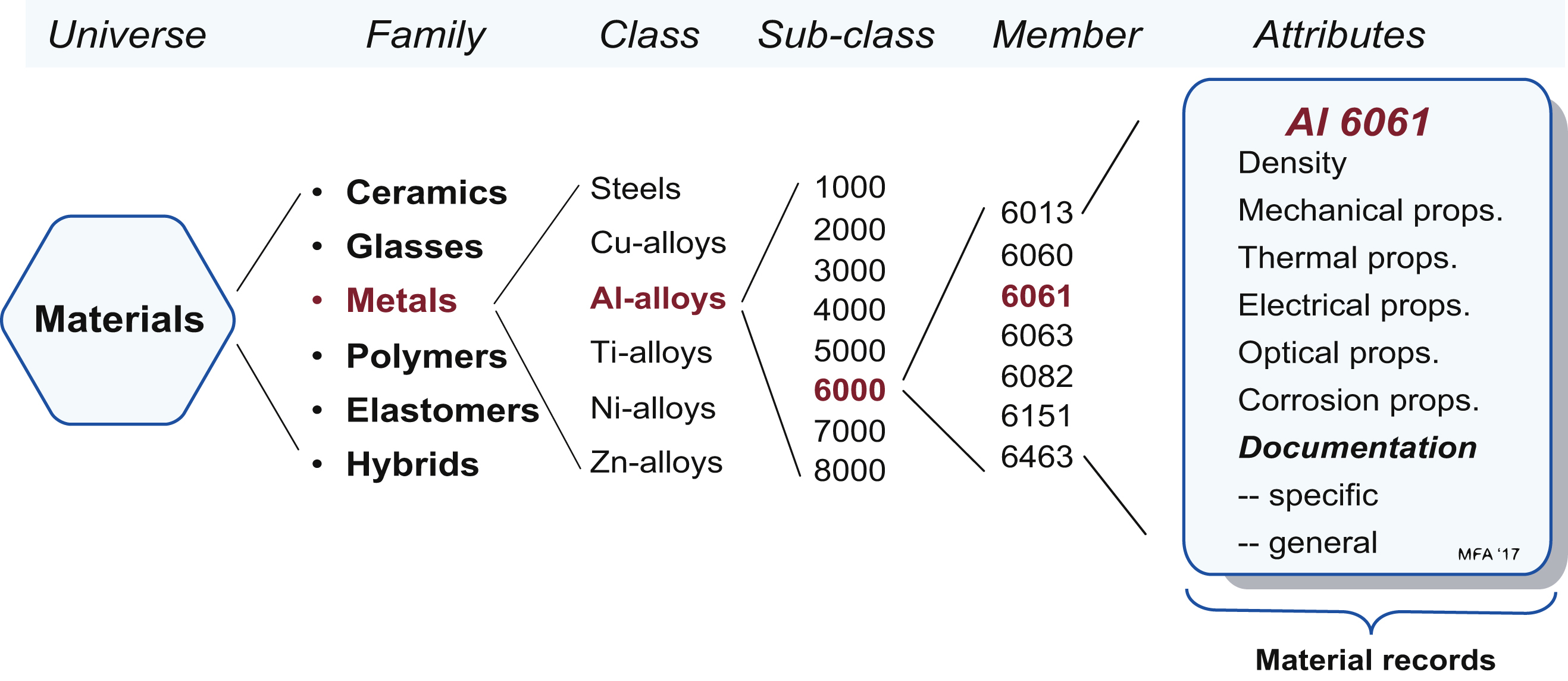
Classification is the first step in creating an information management system for materials and processes. In it, the records for the members of each Universe (materials or processes) are indexed by their position in tree-like hierarchies, making retrieval easy. There are six broad families of materials for mechanical design: metals, ceramics, glasses, polymers, elastomers, and hybrids that combine the properties of two or more of the others. Processes, too, can also be grouped into families: those that create shape, those that join, and those that modify the surface to enhance its properties or to protect or decorate it. The members of the families can be organised into a hierarchical tree-like catalogue, allowing them to be looked up in much the same way that you would look up a member of a company in a management sheet. This structure forms the basis of computer-based material selection systems. It enables a unique way of presenting data for materials and processes as property charts, two of which appear in this chapter.
Keywords
2.1. Introduction and synopsis
2.2. Organising materials: the materials tree
2.3. Organising processes: the process tree
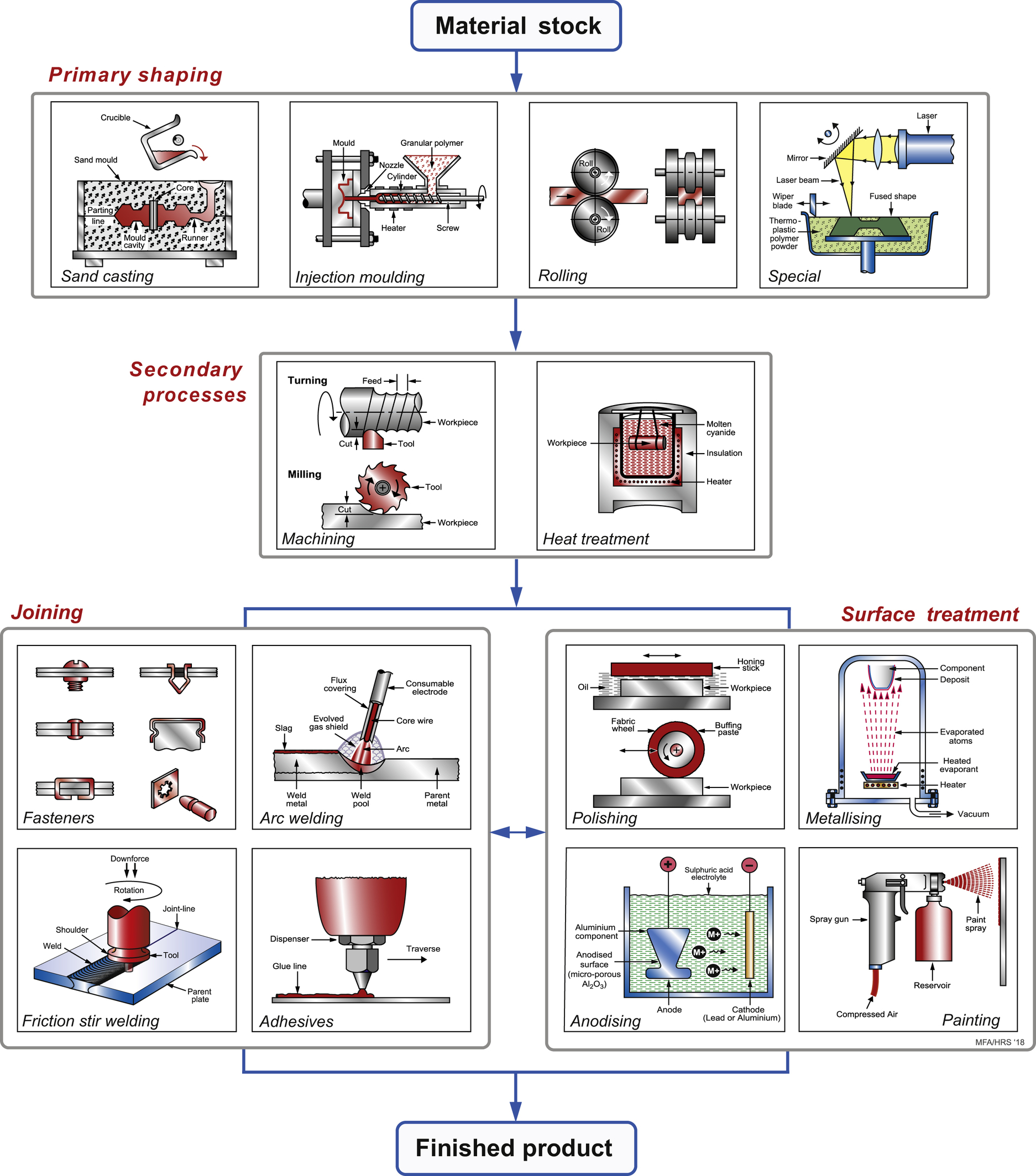
Classifying processes
2.4. Process–property interaction
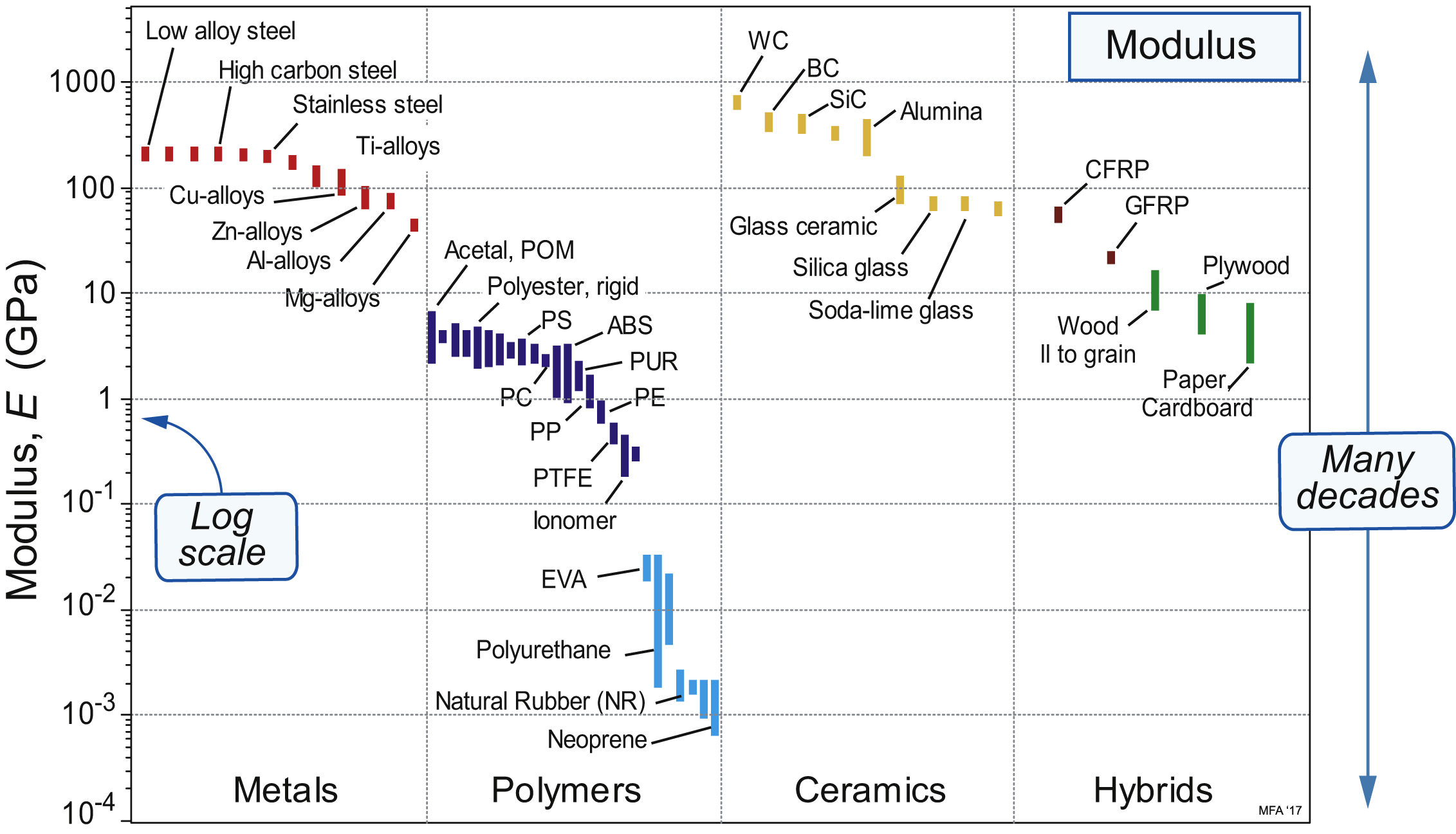
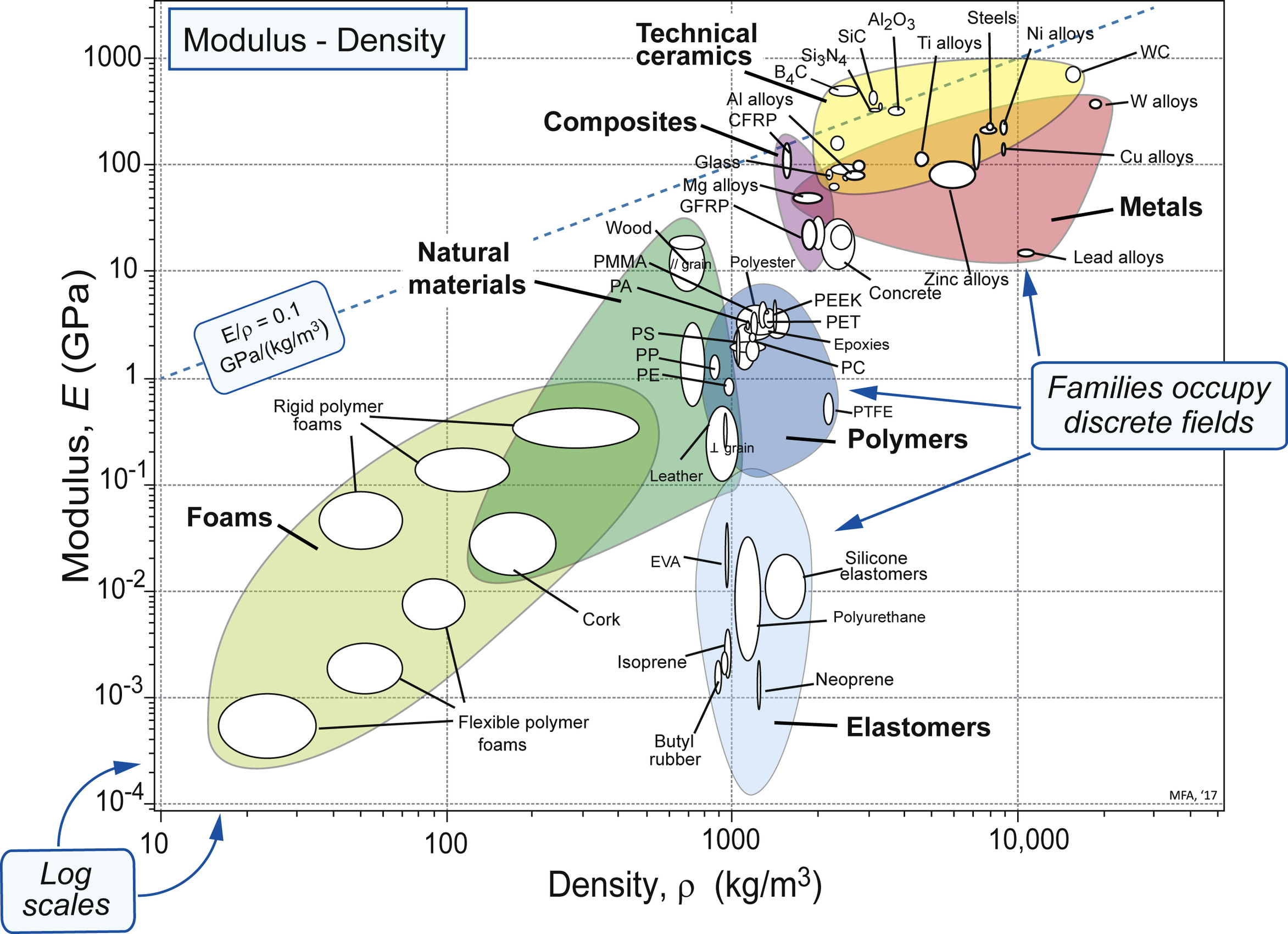
2.5. Material property charts
- • They give an overview of the physical, mechanical and functional properties of materials, presenting the information about them in a compact way.
- • They reveal aspects of the physical origins of properties, a help in understanding the underlying science.
- • They become a tool for optimised selection of materials to meet given design requirements, and they help us understand the use of materials in existing products.
2.6. Computer-aided information sources for materials and processes
Table 2.1
| ASM International (http://www.asminternational.org/online-databases-journals) ASM offers an extensive library of micrographs, phase diagrams, crystallographic structures, and failure case studies. |
| CES EduPack (http://www.grantadesign.com/education/). A comprehensive suite of databases for materials and processes with editions for general engineering, aerospace, polymers engineering, materials science, environmental design and industrial design. It includes powerful search, selection and eco-auditing tools. (The property charts in this book were made with this software). |
| DoITPoMS (https://www.doitpoms.ac.uk/) A free teaching resource created by the Materials Science Department of Cambridge University. |
| F∗A∗C∗T (http://www.crct.polymtl.ca/fact/) Thermodynamic data for engineering alloys and compounds. |
| Matbase (www.matbase.com). A database of the technical properties of materials, originally from the Technical University of Denmark. |
| Matdata (www.matdata.com). A well-documented database of the properties of metals. |
| Materia (www.materia.nl). A database aimed at industrial design, with images of some 2000 products. |
| Material Connexion (www.materialconnexion.com). A materials library emphasising industrial design with records for some 7000 materials, each with an image, a description and a supplier. |
| MatWeb (www.matweb.com). A large database of the engineering properties of materials, drawn from suppliers' data sheets. |
2.7. Summary and conclusions
2.9. Exercises
| Exercise E2.1 | Material properties from experience. List the six main classes of engineering materials. Use your own experience to rank them approximately: (a) by stiffness (modulus, E). A sheet of a material that has a high modulus is hard to bend when in the form of sheet. A sheet of material with a low modulus is floppy. (b) by thermal conductivity (λ). Materials with high conductivity feel cold when you pick them up on a cold day. Materials with low conductivity may not feel warm, but they don't freeze your hands as quickly as those with high conductivity. |
|---|---|
| Exercise E2.2 | Classification (1). A good classification looks simple – think, for instance, of the periodic table of the elements. Creating it in the first place, however, is not so simple. This chapter introduced two hierarchical classifications that work, meaning that every member of the classification has a unique place in it, and any new member can be inserted into its proper position without disrupting the whole. This exercise and the three that follow ask you to create hierarchical classifications following the Universe – Family – Class – Member pattern. Make sure that each level of the hierarchy properly contains all those below it. There may be more than one way to do this, but one is usually better than the others. Test it by thinking how you would use it to find the information you want. Classify the different ways in which sheets of paper can be attached to each other, temporarily or permanently. The Universe is ‘Paper attachment’. Create the Family level by listing the distinctly different mechanisms for attachment. Then add the Class level – the distinct subsets of each family. |
| Table Continued | |

| Exercise E2.3 | Classification (2). In how many ways can wood be treated to change its surface appearance and feel? The Universe is ‘Wood surface treatment’. Develop the classification further using distinct finishing techniques at the Family level and subsets of these at the Class level based on your own observation of wood products. |
|---|---|
| Exercise E2.4 | Classification (3). You run a bike shop that stocks bikes of many types (children's bikes, street bikes, mountain bikes, racing bikes, folding bikes, etc.), prices and sizes. You need a classification system to allow customers to look up your bikes on the internet. The Universe is ‘Bicycles’. Develop the Family and Class levels of the classification and note the further levels of hierarchy that would be needed to uniquely classify a given bike. |
| Exercise E2.5 | Classification (4). You are asked to organise the inventory of fasteners in your company. Fasteners come in various sizes and materials, and there is more than one type. What would be a logical hierarchical classification for the Universe of Fasteners? |
| Exercise E2.6 | Shaping. What is meant by a shaping process? The four products below were shaped using the following processes: A: Additive manufacturing B: Casting C: Forging and milling D: Turning  |
| Exercise E2.7 | Joining. The four products below illustrate the four joining methods: A: Adhesives B: Arc welding C: Brazing D: Threaded fasteners  |
| Exercise E2.8 | Surface treatment. The four products below illustrate the four surface treatment methods: A: Anodising B: Chrome plating C: Enamelling D: Powder coating  |
| Exercise E2.9 | Use of property charts (1). Examine the Modulus chart of Figure 2.7. By what factor, on average, are polymers less stiff than metals? By what factor is neoprene less stiff than polypropylene (PP)? |
| Exercise E2.10 | Use of property charts (2). Wood is a natural polymer, largely made up of cellulose and lignin, and they, like engineering polymers, are almost entirely hydrogen, carbon, oxygen and a little nitrogen. Has nature devised a cocktail of these elements that has a higher modulus than any bulk synthetic polymer? Engineering polymers and woods appear on the charts of Figures 2.7 and 2.8. Use either one to answer the question. |
| Exercise E2.11 | Use of property charts (3). Windows can be made of glass (house windows), polycarbonate (PC) (conservatory roofs) or poly-methyl-methacrylate, PMMA (aircraft cockpit canopies). Would a glass window be more flexible than a replacement of the same thickness made of polycarbonate? Would it be heavier? Use the bubble chart of Figure 2.8 to find out. |
| Table Continued | |

| Exercise E2.12 | Use of property charts (4). Do zinc alloys have a higher specific stiffness E/ρ than polypropylene (PP)? Use the bubble chart of Figure 2.8 to find out (E is the modulus; ρ is the density). Contours of E/ρ are diagonal contours on the axes of this figure – one is shown at E/ρ = 0.1 GPa/(kg/m3). Draw a line parallel to this one, passing through zinc alloys. If PP lies above this line, it has higher E/ρ; if below, it has a lower value. |
|---|