CHAPTER 7
A NEW CONTINENT IN SCIENCE: THE DISSIPATIVE STRUCTURE
Christopher Columbus threw himself down on his knees and thanked God, then stood up and announced the discovery of a new route to India. Everyone appreciates the importance of the discovery of the new continent. For those in Europe, Asia, and Africa, this historic discovery significantly expanded the known world and enabled the birth of new nations. Similarly, discerning the structure that corresponds to the function of acupuncture required the discovery of a new realm in science, namely, the dissipative structure.
Dissipative Structure Occurs Everywhere
While the term dissipative structure is academic and unfamiliar to most people, the phenomena it describes exist everywhere. Despite being unaware of the underlying scientific theory, we have all encountered numerous examples of dissipative structures. A waterfall is a typical example: it can only exist when there is permanent and continuous flow of water from an elevated position. In other words, it requires a supply of water with higher potential energy. The waterfall permanently dissipates this energy and can be called a dissipative structure. The flame of a candle is another example. It can exist only when there is a continuous supply of energy—in other words, it perpetually dissipates energy. The natural spring, the artificial fountain, the whirlpool in a river, the terrible hurricane, and the beautiful clouds in the sky are all dissipative structures. Lightning is another example; it dissipates its store of potential energy so quickly that it only survives for a brief moment.
Dissipative structures exist in contrast to the more common “static” category of structures, examples of which include buildings, mountains, and trees as well as moving cars, trains, and rockets. It is worth noting the car, the train, and the rocket are static structures, even though they can be fast-moving and consume energy. The distinction is that these vehicles can be stored while isolated from a source of energy. Conversely, isolation is fatal for any dissipative structure. A waterfall will immediately disappear if isolated from its upriver water supply. In essence, the dissipative structure is vital, while the static structure is dead. This may seem a somewhat simple and self-evident truth, but it took more than one hundred years for scientific theory to come to terms with it.
The Beautiful Dream of Perpetual Motion
The beginning of the industrial revolution brought newfound awareness of the importance of energy. Talented scientists and inventors pursued the dream of building a machine that could run forever, sustained by its own power alone, not requiring any external energy. It became fashionable for aspiring and respected thinkers at the time to consider how to invent such a machine. Even in modern times this is a popular endeavor; for example, the U.S. Patent and Trademark Office received 252 patent applications involving perpetual motion between March and September 2001.1
While most of these inventions are extremely ingenious, none of them have satisfied the requirements of running perpetually without access to external energy. After many failures of perpetual motion, scientists finally deduced the law of conservation of energy, which, to date, nothing is able to violate.
The Compromised Dream of Perpetual Motion of the Second Kind
Once the law of conservation of energy was acknowledged, people tried to invent what became known as a perpetual motion machine of the second kind. They theorized that if a machine could be designed to spontaneously convert an abundant source of low-temperature thermal energy into movement, it could essentially operate perpetually without violating the law of conservation of energy. For example, if a machine could be constructed that harnessed the thermal energy in the ocean, this energy could be converted into mechanical work. To put the abundance of this energy source in context, the energy in half a degree in temperature of thermal energy from the ocean could power all the machines that currently exist in the world for about three thousand years. In a practical sense, this would enable perpetual motion.
Even the compromised dream of perpetual motion of the second kind is unachievable. After many failed attempts, scientists deduced another important law in physics, the second law of thermodynamics. Perhaps the most understandable expression of this law is, “Heat cannot be transferred from a heat source at lower temperature to a heat source at higher temperature without external energy input.” As this transfer of heat energy from a higher temperature area to a lower temperature area is required to convert thermal energy to mechanical work, this expression clearly indicates the failure of perpetual motion of the second kind.
An academic way to express the second law of thermodynamics is, “In an isolated system, entropy irreversibly increases.” The first important concept is an isolated system, which exchanges neither matter nor energy with its surroundings. Isolating the system in question from its surroundings has been a fundamental, conventional, and routine method in scientific investigation for a long time. Isolation allows the conditions and variations within a system to be clarified and prevents external disturbances. The second concept is entropy, which is a measure of the degree of disorder. Quantifying a variable as nebulous as disorder appears counterintuitive, but mathematicians have successfully been able to do so with clarity. The following example of the degree of disorder in a simple system illustrates how entropy can be quantified and why entropy always increases irreversibly in an isolated system. Figure 7.1 shows a plate with 50 small balls on it. The plate is enclosed by a wall, and a partition divides the plate into two sections.
Initially the balls are limited to moving in the left section, even when the plate is continuously shaken (top image in fig. 7.1). If a gap is made in the partition, over time many balls—approximately half—will move from the left section to the right (bottom in fig. 7.1). In terms of the degree of disorder, it can be seen that there is more disorder in the second scenario.
Once the situation of higher disorder has been established, is it possible for random shaking to return all the balls to the left section? Mathematically, it is possible, but with an extremely low probability. The probability is 1 in 250, or about 0.00000000000000089. This means we might have a reasonable chance of having all the balls returning to the left part after shaking the plate every second, day and night, for 35,702,052 years. In a practical sense, this is impossible. If more balls are involved in the system, the possibility of achieving this declines exponentially. This is the mathematical basis for the concept of an irreversible process, or an irreversible increase in entropy in an isolated system.
Ilya Prigogine, the Modern Columbus
One hundred years after the discovery of the disorder-destined second law of thermodynamics, and just as science’s journey of discovery appeared to be approaching its end, a development occurred that moved in the opposite direction—toward order. In the 1970s, the theories of quantum physics and molecular biology were close to perfection, or in other words, close to the end of their spectacular development. Physicists were considering how to unify the four fundamental interactions—gravity, electromagnetism, the strong nuclear force, and the weak nuclear force—in order to establish a final unified theory, or Theory of Everything, that would be able to explain and calculate every phenomenon in the universe, from the most minute quantum aspects to the whole universe itself.
Once achieved, no major questions would remain for theoretical physicists to ponder. Having no major mysteries to explore, theoretical physics research departments could be wound down and, given the existence of a complete and perfect theoretical framework, the exploration of physics theory could be abandoned. Coming generations would be left to study the theory, research its application in niche areas, and search for new technological applications.
Meanwhile, 1970s biology also seemed close to perfection. After the great success of discerning the genetic code in DNA and the revelation of the three-dimensional structure of protein, biologists were considering how to study every molecule in living systems. It would be a massive project but, in principle, would not pose any problems. Once this colossal undertaking was completed, the departments of biology in universities could also be wound down, or perhaps adopt new names, such as the department of genetic engineering or department of biomolecule engineering, as many universities have done in contemporary times. In other words, new ideas in modern science were dying out in the 1970s.

Figure 7.2. Ilya Prigogine (1917–2003), the modern Columbus.
At that point, a modern-day Columbus, Ilya Prigogine (fig. 7.2), made several revolutionary advances in science. He can be regarded as having discovered a new continent of scientific exploration.
From Isolated System to Open System
The first daring step taken by Prigogine was to study open systems instead of isolated systems. In contrast to an isolated system, an open system has an intimate relationship and constant exchange with its surroundings. This includes an exchange of matter, energy, information, and entropy, which can be both positive and negative. In other words, it is possible to import negative entropy into a system in order to reduce the entropy inside the system.
The term negative entropy is academic, but it is essentially a simple concept. In the example in figure 7.1, touching the balls on the plate is prohibited, because it is an isolated system. Under this restriction, we can only witness the irreversible increase of entropy. In other words, we can only witness the “decay” of the system and are powerless to reverse it. In an open system, this restriction is lifted, allowing us to pick up the balls in the right section and put them back in the left. This simple procedure reduces the entropy in the system. Expressed in academic terms, we have imported some negative entropy into the system.
This simple idea represented a decisive step. It is illustrated by a story, almost certainly apocryphal, involving Columbus. Upon his return from his historic voyage, some asserted that it was a simple achievement—all that was required was to head west instead of east; anyone could have done it. Instead of arguing, Columbus asked whether anybody could place an egg vertically on the dinner table. When no one was able to, Columbus took the egg and struck it gently on the table. The shell was broken, but it stood vertically on the table. Finding the new continent was an essentially uncomplicated task, but nobody else had been able to do it. This illustrates why it was so difficult for countless intelligent scientists to venture beyond the old continent of the isolated system to the new one of the open system.
The concept of an open system has been accepted and is taken for granted by the scientific community. However, this new realm contains countless unknown phenomena that remain unexplored, particularly in relation to living systems. It is well established that living systems are open systems. As early as 1944, the iconic scientist Erwin Schrödinger (1887–1961) had already pointed out that what we eat and breathe is actually a kind of negative entropy, which allows a high degree of order to be maintained in our bodies. Unfortunately, research into this aspect of biology is still desperately needed. Most biologists are unfamiliar with the terms entropy, open system, and dissipative structure.
From Equilibrium State to Far from Equilibrium
The second brave step taken by Prigogine was moving from considering an equilibrium state to considering far from equilibrium state. A system categorized as being in an equilibrium state has already reached maximum entropy. This means that it is in a perfectly homogeneous state and exhibits no internal variation.
Figure 7.3 is a cross-sectional view of cooking pots resting on an electric stove. The water in the pot on the left, sitting for some time with the stove off, exhibits homogeneous composition. As such, it is in an equilibrium state.
If the stove is turned to a low temperature, the pot of water will change to the situation shown in the middle pot. The higher temperature at the bottom will gradually and smoothly diffuse to the surface. The heat energy from the stove has shifted the water only slightly from its equilibrium state; this state is referred to as quasi-equilibrium. It still allows for the calculation of entropy, temperature, and other parameters by visualizing the water as many thin slices and regarding each slice as a system in an equilibrium state. This was how experts in thermodynamics dealt with this situation before Prigogine.
However, if the temperature of the stove is increased significantly, the water will boil, as in the pot on the right. Boiling water is in a completely chaotic and turbulent state. Prior to Prigogine, scientists believed that there was no law that could find order in this situation, but Prigogine found that this turbulent state is not as exceedingly chaotic as once believed. Some dynamic structure exists in the chaos, particularly when the energy input is stable.
When viewed from above, boiling water reveals a pattern on the surface of the water that remains stable while the energy supply is stable. These stable patterns illustrate the essential idea behind Prigogine’s discovery. In generalized terms, he deduced that a new order would spontaneously arise from disorder. In other words, new structures will occur in a far from equilibrium state.
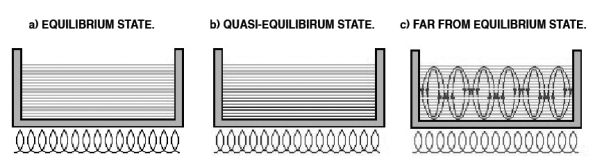
Figure 7.3. From equilibrium to far from equilibrium.
From Static Structure to Dissipative Structure
Prigogine named these new structures dissipative structures. As discussed at the beginning of the chapter, everyday examples include waterfalls, whirlpools, clouds, and lightning. Given their commonplace and ongoing existence, some might question the importance of the theory of dissipative structures. In this context, however, its discovery is akin to the discovery of the Americas.
The Americas were not new for their indigenous inhabitants, of course, who had lived there for tens of thousands of years. The importance of the discovery of the New World was that it was new to Europeans. The discovery enabled modern European civilization to greatly extend its territory, and the new seeds of European culture planted in the New World grew so fast, successfully, and powerfully that they completely merged with and enriched Western culture. Old World culture expanded beyond its historic European, West Asian, and North African roots.
Comparing Prigogine to Columbus demonstrates the importance that the discovery of dissipative structures has to science, medicine, and modern civilization. While dissipative structures existed before Prigogine and modern scientific theory, their discovery by science greatly expanded its territory and that of the disciplines of physics, chemistry, biology, and medicine.
In addition to enabling the mysteries of acupuncture and other traditional forms of medicine, which had been discovered intuitively in ancient times, to be rationally understood in the eyes of modern science, this rediscovery of old “new structures” will also facilitate a paradigm shift in scientific thinking. It will enable venturing beyond the old continent of conventional Western medicine, with its associated reductionist thinking, to the simultaneously ancient and new continent of holistic medicine, with its new worldview and inclusive thinking. Furthermore, this new way of thinking and new outlook will profoundly influence not only the development of medical technology, but also the direction of the development of our civilization.
