Microbiology and Microbiological Control in the Brewery
Annie E. Hill and Fergus G. Priest
CONTENTS
17.4.2.4 Anaerobic Gram-negative Bacteria
17.5 Microbiological Quality Assurance and Quality Control
17.5.1 Setting Microbiological Standards and Sampling
17.5.2 Traditional Microbiological Procedures
17.5.3.1 Adenosine Triphosphate (ATP) Bioluminescence and Oxidoreductase Tests
17.5.3.2 Direct Epifluorescence Filter Technique (DEFT)
17.5.3.3 Fluorescence In Situ Hybridization (FISH)
17.5.3.4 Polymerase Chain Reaction
17.6 The Microbiological Laboratory Within the Brewery
17.1 INTRODUCTION
Beer is an inherently microbiologically stable product and therefore not subject to the myriad of spoilage microorganisms that can colonize most foods or nonalcoholic beverages. It has been subject to exhaustive yeast growth; and therefore, like other fermented foods, it is largely resistant to further microbial development. The reasons for this resistance are several:
Despite these factors limiting microbial spoilage, there are various yeasts and bacteria that can flourish in beer, particularly if the storage conditions are poor and oxygen is allowed access. To date, no pathogenic organisms have been found to proliferate in beer, although spore-forming Bacillus cereus and coliform bacteria can survive the harsh conditions.1 Consequently, the main issue for the brewing microbiologist is consistency of appearance and organoleptic qualities of the final product (the beer).
In this chapter, we will review the predominant spoilage organisms, outline the available technology for detecting and identifying these organisms, and consider the role of the brewing microbiology laboratory in dealing with these problems and assuring consistent endproduct quality.
17.2 WILD YEASTS
Wild yeasts are generally defined as “yeasts not deliberately used and not under full control.”2 This definition includes brewing strains that are used for a different style of beer and may have been cross-contaminated in the brewery, as well as nonbrewing yeasts that have gained access from the air or raw materials. It is important to emphasize that there are many genera and species of yeast with diverse physiologies—the only unifying feature is that these organisms are predominantly unicellular. However, many types of yeast have a semi-filamentous lifestyle and may form mycelia under various environmental conditions. These hyphae when formed by yeasts contain septa. Most yeasts are members of the ascomycetes, in which spores are produced endogenously in an ascus.
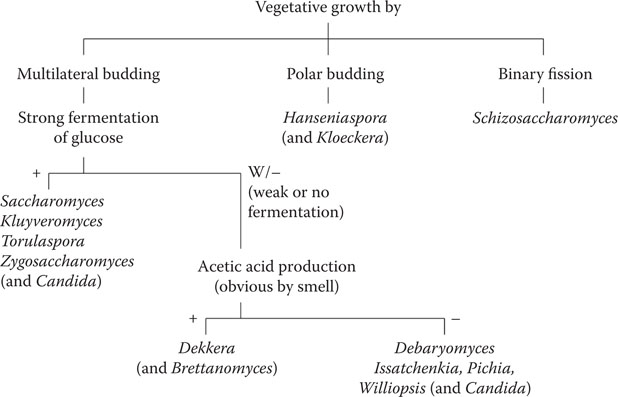
The major genera and types of wild yeast encountered in the brewery are listed in Table 17.13 and a scheme for their identification is presented in Figure 17.1. Some of these yeasts are strictly aerobic and cannot ferment sugars under anaerobic conditions. Pichia membranefaciens is the most common contaminant of beer and wine in this category. The acetic acid-forming Brettanomyces and Dekkera species, although fermentative, do not usually cause a threat to the brewing process because they cannot flourish under anaerobic conditions. However, they form an important component of the yeast flora of fermenting Belgian Lambic beers and can cause problems in ales and lagers if air should gain access. The aerobic yeasts such as Debaromyces, Pichia, and Williopsis produce yeasty or estery flavors that are most unwelcome.
Table 17.1 Physiological Characteristics of Ascomycete Genera of the Brewing Industry
Saccharomycetaceae (vegetative growth by multilateral budding) |
|
Debaryomyces |
Weak or no fermentation |
Dekkera |
Fermentation (but only under aerobic conditions) |
Issatchenkia |
Weak fermentation; forms pseudomycelium and surface film (pellicle) |
Kluyveromyces |
Fermentation, usually vigorous |
Pichia |
Weak or no fermentation; many form true mycelium or pseudomycelium and a surface pellicle |
Saccharomyces |
Vigorous fermentation, no pellicle |
Torulaspora |
Vigorous fermentation |
Williopsis |
Weak or no fermentation |
Zygosaccharomyces |
Vigorous fermentation |
Nadsonioideae (vegetative growth by polar budding) |
|
Hanseniaspora |
Fermentative |
Saccharomycodes |
Fermentative |
Schizosaccharomycetoideae (vegetative growth by fission or mycelium) |
|
Schizosaccharomyces |
Fermentative |
Source: Adapted from Campbell, I., Wild yeasts in brewing and distilling, in Brewing Microbiology, F.G. Priest and I. Campbell, eds., Kluwer Academic, New York, 2003, pp. 247–266.

Figure 17.1 Simplified identification of common brewing yeasts. (From Campbell, I., Wild yeasts in brewing and distilling, in Brewing Microbiology, F.G. Priest and I. Campbell, eds., Kluwer Academic, New York, 2003, pp. 247–266. With permission.)
The fermentative yeasts such as Kluyveromyces, Saccharomyces, Torulaspora, and Zygosaccharomyces, on the other hand, can cause serious problems in the fermentation. They are potentially able to compete with the culture yeast, and although they cannot generally kill it, if they grow just a little faster than the culture yeast, they will displace the brewing yeast over successive generations. Because these wild yeasts neither flocculate well nor interact with finings, they generally pass into conditioning, where they can have deleterious organoleptic effects on post-fermentation beers, as well as causing haze and turbidity.
Most wild yeasts can cause serious flavor effects; for example, many strains are able to decarboxylate substituted cinnamic acids derived from the barley cell wall. P-coumaric and ferulic acids are decarboxylated into their 4-vinyl derivatives, 4-vinylphenol and 4-vinylguaiacol, respectively. Reduction of these molecules produces the 4-ethyl derivatives. These phenolic compounds, which contribute to the characteristic fruity flavor of wheat beers, are most unwelcome in ales and lagers. Other wild yeasts are able to utilize higher malto-oligosaccharides and, as a consequence, cause super-attenuation in the beer.
Generally, wild yeasts are competing with the culture yeast for nutrients, but some yeasts possess the “killer” phenotype and actively kill a sensitive culture yeast. These strains produce zymocins, proteins that are lethal to sensitive cells. Such killer strains can rapidly displace culture yeasts.
17.3 MOLDS
Airborne microorganisms colonize barley in the field soon after the ears emerge from the leaf sheaths. Climatic conditions are important in determining the types of spore and mold that will contaminate the kernels, but Alternaria and Cladosporium species are commonly dominant.4 Fusarium head blight has become a major problem, particularly in the wetter regions of North America and northern Europe.5 Obviously, the growth of head blight fungi occurs at the expense of the grain and, in infected crops, the grain weight is reduced. Fusaria are also responsible for the synthesis of mycotoxins, small molecules with highly toxic characteristics. The trichothecene, deoxynivalenol (DON), is such a mycotoxin originating from Fusarium graminarum and some other species. It has been estimated that the total loss in barley and wheat due to Fusarium head blight since 1990 in the United States amounts to more than US$3 billion.6 In addition to the reduced yields, the farmer receives a lower price for grain containing DON, even at a level of just 1 ppm.
Some of the rarer field fungi, such as the Aspergillus glaucus group (which are anamorphs of Eurotium species) and some penicillia, can grow under various storage conditions to become dominant as storage fungi.4 This can give rise to hot spots in the silo as microbial growth ensues, which results in a rapid decrease in germinative capacity. Malt typically bears Eurotium (Aspergillus) species, including A. fumigatus, Rhizopus species, and penicillia.
Probably the best-known effect of mold-contaminated barley on beer is the reduced gas stability in conditioned beers known as gushing, which results in a rapid loss of beer from a bottle or can on opening. Extensive colonization of barley by F. graminarum and other Fusarium species has long been associated with the gushing phenomenon, which has been attributed to the release of a small peptide-containing substance (hydrophobin). Other field fungi, including Alternaria species, have been associated with gushing. Mold-contaminated grain can also affect the flavor and color of the finished product, but brewers are unlikely to use such poor quality raw materials. Although the occurrence of mycotoxins in finished beer is of major health concern, it has been established that mycotoxins are largely degraded during the brewing process, and the quality of grain used in brewing is such that mycotoxins are avoided. It is therefore important to determine appropriate microbiological quality parameters for malt and adjuncts.7 Initiatives such as the Home Grown Cereals Authority (HGCA) Grain Passport, which includes a requirement to test for mycotoxins, have been extremely valuable in recognizing this.
17.4 BACTERIA
Fortunately, of the many thousands of bacteria that have been described, few are of concern to the brewer. This is largely due to the reasons mentioned in the Introduction, the physicochemical properties of beer are such as to preclude the growth of most bacteria.
Bacteria can be divided into two principal groups dependent on the structure of their cell walls. This was first discovered by the Danish microbiologist Gram and, in deference to his pioneering work, we refer to these as Gram-positive and Gram-negative. Bacteria of the former group possess a thick cell wall composed almost entirely of a polysaccharide-like material called murein or peptidoglycan. This material complexes the crystal violet/iodine dye used in the Gram stain rather like starch complexes iodine, hence their Gram-positive description. Gram-negative cells, on the other hand, contain more lipid in their cell envelopes and do not complex the Gram stain so strongly. This distinction has important implications for brewing microbiology because Gram-positive bacteria are generally sensitive to hop constituents and their growth is inhibited by hop α-acids, while Gram-negative bacteria are not as seriously affected by these compounds.
17.4.1 Gram-positive Bacteria
The lactic acid bacteria are the only group of Gram-positive bacteria likely to cause a significant threat to beer. These bacteria belong to several genera but share common physiological characteristics (reviewed by Priest8 and Suzuki9). They are fermentative bacteria that do not use oxygen to grow; indeed many prefer an anaerobic environment while others will grow in the presence of air. However, they lack respiratory pathways, and none can use oxygen for respiration. Instead, they ferment sugars to predominantly lactic acid as an end product. Some conduct a homofermentative catabolism of sugars in which lactic acid features as the sole end product. The heterofermentative lactic acid bacteria, on the other hand, produce lactate, acetate, and carbon dioxide from sugars. Nevertheless, because both types produce lactate, these bacteria have become adapted to an acid environment and grow at pH 3.5 to about pH 6.0, well fitted to growth in alcoholic beverages. They can generally be recognized by their Gram-positive staining properties and lack of the enzyme catalase. The latter can easily be tested for by adding a drop of dilute hydrogen peroxide to a culture. Catalase-positive bacteria reduce the peroxide to water with the rapid evolution of oxygen bubbles; catalase-negative bacteria have no such action.
The lactic acid bacteria are divided into several genera of which members of Lactobacillus and Pediococcus are the most important to the brewer.
17.4.1.1 Lactobacillus
The heterofermentative bacterium L. brevis is the most common beer spoilage bacterium and is detected at high frequency in beer and breweries.9 Others isolated from spoiled beer include L. buchneri, L. paracasei, and L. plantarum. L. lindneri is a close relative of L. brevis that was isolated from lager beers and is also frequently encountered.10, 11 Other species such as L. amylolyticus, which was isolated from malt and wort, have poor beer spoilage properties, probably because of hop sensitivity.12 It is normally unnecessary to identify lactobacilli to species level; a generic identification is sufficient to be aware that problems may exist. Nevertheless, species identification will give a clearer indication of its spoilage potential and may assist with tracing the origin of the contamination.
Not all lactobacilli can grow in, and consequently spoil, beer. Like most Gram-positive bacteria, lactobacilli are generally sensitive to hop constituents, and their growth is impeded in hopped beers. Trans-isohumulone and the related colupulone are the major antibacterial components derived from hops and cause leakage of the cytoplasmic membrane and inhibition of amino acid uptake in sensitive bacteria.13 However, some lactic acid bacteria are hop resistant, especially strains of L. brevis, due to the presence of the horA gene, which encodes a transporter that expels hop compounds from the cytoplasm.14 This makes them serious contaminants. They are able to grow in conditioning beer, producing a silky turbidity often associated with the buttery flavor of diacetyl. Lactobacilli have also been implicated in the biosynthesis of amines from amino acids in beers.15 Considerable increases in the concentrations of tyramine and to a lesser extent histamine were found in beers inoculated with mixed cultures of brewery lactic acid bacteria and stored until haze formation. Lactobacilli are much more effective amine producers than pediococci.16
Certain thermophilic lactobacilli, especially L. delbrueckii, have been noted as contaminants of sweet wort. Indeed, we have detected these bacteria growing during the mash stage. They are normally killed by the boil, but if the wort is kept sweet (unhopped) for any reason, even stored hot (less than 60°C), then it provides an ideal growth medium for this bacterium that can produce copious amounts of lactic acid.
17.4.1.2 Pediococcus
Spherical lactic acid bacteria, with a homofermentative mode of metabolism and that divide in two planes to form pairs and tetrads, are classified in the genus Pediococcus. These bacteria were noted by Pasteur at the end of the nineteenth century as potent spoilage agents of conditioned beer and one species in particular, P. damnosus, can cause serious spoilage. This bacterium is generally hop-tolerant and can grow in finished beer where it is associated with “sarcina sickness” characterized by the production of diacetyl. Pediococcus inopinatus is also recovered from beer but is less troublesome as a spoilage agent than P. damnosus. P. clausennii has been isolated from spoiled beer.17 In general, pediococci are serious beer spoilage agents and can be responsible for the return of beer from the trade. A simple scheme for the identification of Gram-positive bacteria associated with beer and breweries is shown in Figure 17.2.
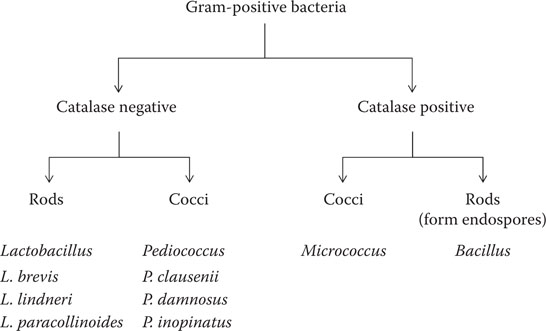
Figure 17.2 Simple scheme for the identification of Gram-positive bacteria from beer and breweries.
17.4.2 Gram-negative Bacteria
17.4.2.1 Acetic Acid Bacteria
These Gram-negative, rod-shaped bacteria obtain their energy for growth by oxidizing ethanol into acetic acid. This is a highly aerobic reaction, which is used commercially for the production of vinegar, but needless to say is very detrimental to the brewer. Because beer should be stored with limited access to air, spoilage by these bacteria should not occur. However, bacteria of the genus Acetobacter are ubiquitous and can cause problems in bars dispensing cask-conditioned beer in which the ale is displaced by air. These bacteria are resistant to hop compounds and are ethanol tolerant and acidophilic; so, given air, they will grow in beer and will produce acetic acid, other off-flavors, and turbidity.
17.4.2.2 Enterobacteriaceae
The family Enterobacteriaceae comprises numerous genera of free-living and sometimes pathogenic bacteria. Fortunately, none of the pathogenic types, such as Salmonella or Shigella species have been found in beer. The enterobacteria are facultative anaerobes able to grow in the presence or absence of air, but they are inhibited by ethanol and low pH so are only responsible for beer spoilage in low alcohol products (less than 2% by volume) with a relatively high pH (greater than 4.2).
One of the characteristic features of all members of the Enterobacteriaceae is the ability to respire under anaerobic conditions with nitrate as an electron acceptor rather than oxygen. In so doing, the nitrate is reduced to nitrite. Unacceptable concentrations of nitrite can be formed in beer if the brewing liquor contains high concentrations of nitrate, as it may do in agriculturally intensive areas. The nitrite reacts with secondary amines of the wort, either chemically or by enzymic catalysis, to form N-nitrosamines18 (Figure 17.3). These molecules, with their carcinogenic properties, are obviously to be avoided in beer, if necessary by removing nitrate from the water (see Chapter 4) and by controlling the populations of enterobacteria.19
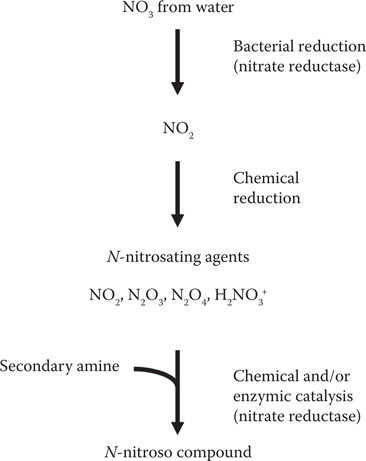
Figure 17.3 Pathway for the formation of nitrosamines in fermenting wort. (From van Vuuren, H.J.J. and Priest, F.G., Gram-negative brewery bacteria, in Brewing Microbiology, F.G. Priest and I. Campbell, eds., Kluwer Academic, New York, 2003, pp. 219–245. With permission.)
Members of the Enterobacteriaceae were first noted for their propensity to grow in wort rather than beer. These bacteria gain entry to the wort from water and can grow during the early stages of fermentation. They will also grow in cooled wort if it left unpitched for any length of time. Typical wort bacteria include species of Citrobacter, Enterobacter, Klebsiella, and Rahnella (reviewed by van Vuuren and Priest20). Their main effect, if allowed to grow in wort, is the production of dimethyl sulfide (DMS), a sulfur molecule with a very low flavor threshold (around 0.06 ppm) and low volatility (35°C) that contributes a parsnip-like, wild garlic, sulfury flavor to beer. They may also introduce low concentrations of diethyl sulfide (DES), which has an even lower flavor threshold (0.003 ppm). A second common, but not invariable, feature of the enterobacteria is their ability to decarboxylate substituted cinnamic acids to produce the phenolic flavor compounds described in Section 17.2.
Obesumbacterium proteus is a unique member of the Enterobacteriaceae that is found exclusively in breweries (although it presumably has habitats outside the brewery but these have never been described). This bacterium was first noted in ale pitching yeasts by J. L. Shimwell as “the short fat rod of pitching yeasts,” but it has also been described in lager yeast cultures. Generally considered harmless, the bacterium is responsible for increased levels of DMS and increased concentrations of some fusel oils in the finished product. There were indications that there are in fact two genetically different groups within O. proteus, one responsible for relatively high levels of DMS and relatively rare, the second more innocuous and more common.21 Beers brewed with yeast containing the former type of O. proteus, at about 1% by number, will typically contain about 14 to 18 µg/L DMS while the less-productive strains produce around 4 µg/L DMS, both below the threshold of about 30 µg/L. A recent study of some strains originating from the 1970s, as well as current isolates, revealed that the two genetic groups represented completely different Gram-negative bacteria, both of which are members of the Enterobacteriaceae. The high DMS producers retained the name Obesumbacterium proteus, while the other group was placed in a new genus, Shimwellia as Shimwellia pseudoproteus.22
The practice of repitching yeasts, often for many generations (cycles), in traditional ale breweries led to yeasts with stable populations of these bacteria, generally containing several different strains but typically of S. pseudoproteus rather than O. proteus. These bacteria would rise to the top of the fermentation in association with the yeast and be repitched into the next fermentation thus continuing their succession. They contributed to the flavor of the beer, but often in a characteristic way, which was not considered detrimental. In the early 1970s, S. pseudoproteus could be isolated from virtually every pitching yeast in use for ale fermentation in the United Kingdom. Today, the situation is different; limited repitching, greater cleanliness, and improved yeast handling have made S. psuedoproteus relatively rare. Much of this has been driven by the need to reduce N-nitrosamine concentrations.
Enterobacter agglommerans strains found in breweries are now generally classified as Rahnella aquatalis.23 These bacteria grow in either hopped or unhopped wort in the presence or absence of yeast. Like S. pseudoproteus, they associate with yeast and can be recycled in pitching yeasts collected from lager fermentations.24 Fermentations contaminated with R. aquatalis generally produce beers with a fruity/sulfury aroma and flavor, largely through the increased levels of acetaldehyde, diacetyl, ethyl acetate, and DMS in the beer.
Other enterobacterial contaminants include strains of Citrobacter, Enterobacter, Hafnia, Klebsiella, and Serratia, generally derived from water supplies. They occur most commonly in wort and the early stages of the fermentation and cannot grow in beer. They are associated with sulfury/phenolic off-flavors.
17.4.2.3 Zymomonas
Bacteria of the genus Zymomonas have a unique mode of catabolism among the bacteria in that they conduct an ethanolic fermentation. This is so efficient that it has been seriously considered for the production of fuel ethanol, but it is not used for potable alcohol except in Africa and Central America. Nevertheless, the bacterium is tolerant of ethanol (up to about 10% by volume) and has been associated with spoilage of primed conditioning ales (reviewed by van Vuuren and Priest20). The organism is very rare in ale breweries and has not been reported in lager breweries where the low conditioning temperatures probably restrict its growth. Beer contaminated with Zymomonas has an estery/sulfury flavor due to the production of acetaldehyde and hydrogen sulfide.
17.4.2.4 Anaerobic Gram-negative Bacteria
With improved technology resulting in very low oxygen levels in packaged beers, a new range of bacterial contaminants has been given the opportunity to proliferate—the strictly anaerobic bacteria that would normally be inhibited by trace amounts of oxygen. First reported by Lee and his colleagues in beers brewed in the United States,25 similar bacteria were subsequently isolated from spoiled beer in Germany, Scandinavia, and Japan. In a comprehensive taxonomic study, the rod-shaped bacteria were assigned to three genera, Pectinatus, Selenomonas, and Zymophilus.26 Gram-negative anaerobic cocci isolated by Weiss in Germany were classified as Megasphaera cerevisiae.27 None of these bacteria will grow under normal laboratory conditions, even given enhanced carbon dioxide (CO2); instead, they must be cultured in a strictly anaerobic environment either by appropriate manipulation of the medium or by the use of anaerobic jars or cabinets.
Pectinatus cerevisiiphilus occurs as slightly curved rods that produce longer helical filaments in older cells. They do not grow in a CO2-enriched atmosphere but must be cultured under strict anaerobic conditions in a modified de Man, Rogosa, and Sharpe (MRS) agar or a similar medium. However, these conditions also enable lactobacilli to grow. As a result, Lee devised a selective medium that inhibits the growth of lactic acid bacteria with crystal violet and sodium fusidate, thus allowing for the recovery of these anaerobes.25
Pectinatus frisingensis is morphologically similar to P. cerevisiiphilus but can be distinguished by molecular and physiological characters. There is evidence that P. frisingensis is more tolerant of ethanol than P. cerevisiiphilus but that the latter grows more quickly in beer.28 Bacteria of both species can contaminate packaged beer, producing considerable amounts of acetic and propionic acids as well as acetoin and hydrogen sulfide (H2S). The beer becomes turbid with an odor of rotten eggs.
Four strains of Selenomonas lactiflex were originally isolated from pitching yeast, but these bacteria have not since been reported as spoilage bacteria. They ferment glucose to acetic, lactic, and propionic acids.26
Zymophilus raffinosivorans and Zymophilus paucivorans were isolated from pitching yeasts. These curved rods have limited ability to grow in beer and cause spoilage, and they have seldom been reported as important spoilage agents.26 Finally, Megasphaera cerevisiae are Gram-negative, slightly elongated, anaerobic cocci that may cause turbidity and off-flavors in bottled beers.
A simplified scheme for the identification of Gram-negative bacteria associated with beer and breweries is given in Figure 17.4.

Figure 17.4 Simple scheme for the identification of Gram-negative bacteria from beer and breweries. (From van Vuuren, H.J.J. and Priest, F.G., Gram-negative brewery bacteria, in Brewing Microbiology, F.G. Priest and I. Campbell, eds., Kluwer Academic, New York, 2003, pp. 219–245. With permission.)
17.5 MICROBIOLOGICAL QUALITY ASSURANCE AND QUALITY CONTROL
Quality control (QC) and quality assurance (QA) in the context of brewing distinguish the act of determining the current or very recent microbiological status of the plant and products (QC) from the actions that are put into place to ensure a quality standard (QA). The results of QC are used to provide QA (e.g., dirty surfaces in fermenters must be cleaned, poor viability yeast discarded). It is important that the process is not entirely reactive, however. A proactive attitude should be adopted to prevent faults occurring, and such an approach is becoming standard practice.
There are essentially two approaches to microbiological testing—the traditional methods that rely on cultivation of yeasts and bacteria in appropriate media followed by identification of the offending organism, if necessary, and newer rapid approaches that have a minimal reliance on prior cultivation. The move toward rapid methods for microbial detection and identification has been driven by technological developments and also by changes in the industry.29 Some key motivating factors are:
Minimizing the time needed to detect a spoilage agent can lead to significant savings through reduced product recalls, extension of shelf life, and consistency of product quality and flavor.
The decision to adopt traditional or rapid methods is to an extent dependent on the critical control point (CCP) under review. For example, processes in the brewhouse, fermenting hall, and storage cellar generally require at least five days and so traditional methods are appropriate to provide the green light for the next step. However, other stages such as filtration, bright beer cellar tanks, clean-in-place (CIP), and water services are more constrained by time, and rapid tests can provide the necessary information for optimization of the process.
17.5.1 Setting Microbiological Standards and Sampling
The most successful brewing companies are those that manage to harmonize productivity with quality, and a range of quality management programs have been employed by brewing companies both large and small, including:
Of these, HACCP has become an essential feature of brewery QC.30 This involves the systematic assessment of all the steps involved in the brewing process and identification of those steps essential to the hygienic quality of the product. In a large, complicated plant, it is advisable to reduce the operations to a series of connected subroutines.
Many of the CCPs are not microbiological (see Chapter 21); the principal microbiological CCPs are shown in Figure 17.5. At these stages, it is essential to monitor for microbiological hazards using either traditional or rapid methods. First, it is necessary to set microbiological standards for each point. What is the maximum allowable level of contamination and by what organisms? Some suggested levels of sensitivity for detection are given in Table 17.2, but these are for guidance only. It is important to establish your own definitive criteria. For example, it may be permissible to use a pitching yeast with limited bacterial contamination by S. pseudoproteus, but a yeast contaminated with Pediococcus should be discarded and the source of contamination determined. Some organizations adopt a “green, amber, red” approach in which green flags indicate adherence to microbiological standards (no action needed), amber indicates minor microbiological concern—the brewing process can continue but some microbiological contamination has occurred and should be investigated, and finally red indicates failure to meet a microbiological standard and production staff must be informed so that corrective action can be taken. It is valuable to prepare trend graphs showing the microbiological status over time for the various stages and products. A trend showing a gradually worsening microbiological situation can give early warning of a problem.
Table 17.2 Suggested Sensitivity Required for Detection of Specific Spoilage Organisms in Brewery Samples
Samples |
Sensitivity |
|---|---|
Cold aerated wort |
1 organism/25 mL |
Pitching yeast |
1 bacterium/mL and 1 wild yeast/106 culture yeast |
Fermenting wort |
1 organism/mL |
Tank bottoms |
1 organism/mL |
Beer in storage |
1 organism/mL |
Filtered beer |
1 beer spoilage organism/100 mL or 10–102 non-beer spoilage organisms per/100 mL |
Packaged beer (non-pasteurized or flash pasteurized) |
10–102 non-beer spoilage organisms/100 mL |
Rinse water (end of cleaning in place) |
1 organism/100 mL |
Source: From Jespersen, L. and Jakobsen, M., Int. J. Food Microbiol., 33, 139–155, 1996. With permission.
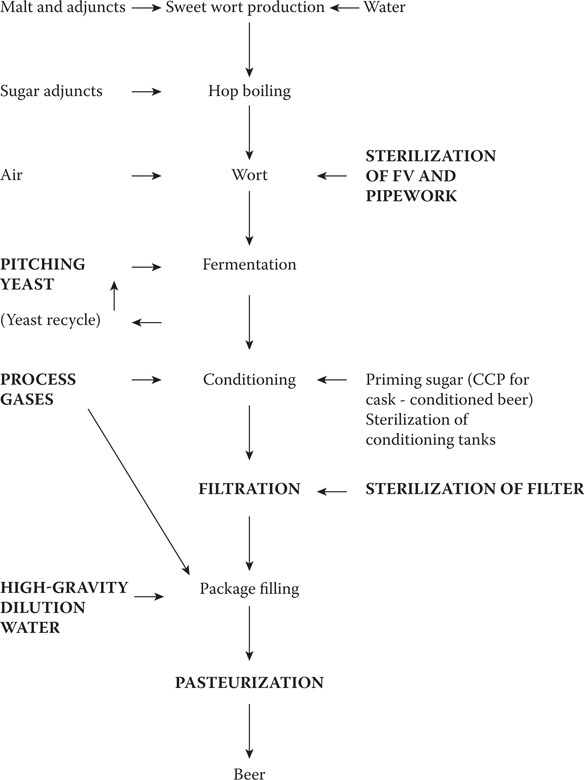
Figure 17.5 Flow diagram of beer production showing critical control points (CCPs) for microbiological testing in bold capital letters.
Sampling at the CCPs requires careful consideration so that the results of the microbiological tests are statistically sufficient. Typical samples are liquids—for example, samples of final water rinses from CIP operations or water rinses of containers. Such samples should be of sufficient scope to provide assurance that the microbiological standard has been achieved. For example, when examining beer, a sufficient quantity should be filtered or forced to allow for the detection of contaminants at the required level, usually 100 to 1,000 mL for testing of packaged beer depending on the size of the container and the sensitivity required (Table 17.2).
17.5.2 Traditional Microbiological Procedures
Culturing of brewery microbes is achieved using a variety of specialist media. These have been discussed in detail elsewhere.31, 32 Here, we will describe the principal types. General-purpose media for the cultivation of yeasts and bacteria can be prepared from wort or beer, but commercial media offer consistency and ease of use. Wallerstein Laboratory Nutrient medium (WLN agar or broth) is commercially available and contains sufficient nutrients for the growth of most brewery microorganisms. The inclusion of a bromocresol green indicator imparts a variety of colors from yellow/green/blue to bacterial and yeast colonies, enabling distinction of different types depending on the pH around the colony.
For distinguishing culture and wild yeasts, it is necessary to suppress the growth of the brewing yeast to enable the detection of the relatively low numbers of wild yeasts. Incorporation of the antibiotic cycloheximide (also known as actidione) at low concentration (10 µg/mL) into WLN agar generally prevents the growth of S. cerevisiae while enabling wild, non- Saccharomyces yeasts to grow. Inclusion of copper salts is preferred by some for this purpose. S. cerevisiae is sensitive to low concentrations of around 6 µg/mL of copper sulfate, but this can be modified according to the sensitivity of the brewing yeast to be examined. Addition of copper sulfate at the determined level to WLN agar will suppress the brewing yeast, allowing other yeasts to grow and form colonies. Lysine agar is a synthetic medium available commercially that prevents the growth of the brewing yeast—not by inhibition (as in cycloheximide and copper sulfate media), but by lack of nutrients. Lysine is the sole nitrogen source in this medium. S. cerevisiae strains are unable to metabolize lysine as a sole source of nitrogen, preventing their growth and allowing various wild yeasts to grow.
Although the aforementioned media generally distinguish non- Saccharomyces wild yeasts from the culture yeast, devising selective media that will prevent the growth of culture yeast while enabling the growth of wild Saccharomyces yeasts is far more challenging. Numerous ingenious media have been formulated for this purpose, but none have been adopted by mainstream brewing laboratories because they fail to work effectively. Instead, the trend has been toward molecular methods for distinguishing brewing strains from wild strains of S. cerevisiae and close relatives (see Chapter 8).
Media for the culture of bacteria generally incorporate cycloheximide at about 100 µg/mL to prevent yeast growth. WLN provides a good general-purpose medium for the cultivation of most bacteria including Enterobacteriaceae. MacConkey agar is a selective medium for coliforms, which allows the differentiation of the lactose-negative S. pseudoproteus and O. proteus from various Lac-positive types such as R. aquatalis. S. pseudoproteus grows slowly on MacConkey agar at 30°C, requiring incubation for 36 to 48 hours.
Lactic acid bacteria require more nutritious media for optimal growth—Raka Ray No. 3 is a highly nutritious medium that is commercially available, and NBB® medium has its proponents.33 The standard medium for lactic acid bacteria is MRS broth. Addition of maltose at about 10 g/L to MRS agar or broth improves this medium for the growth of lactic acid bacteria from beer and breweries. Advanced beer-spoiler detection (ABD) medium has been developed for detection of hard-to-detect beer-spoilage lactic acid bacteria (LAB), and use of at least two culture media is recommended for comprehensive detection of LAB.34 Because the bacteria on recovery from the sample may be stressed, it is generally advisable to cultivate them in a CO2-enriched atmosphere or anaerobically at 30°C for up to five days to encourage full recovery. Acetic acid bacteria and Zymomonas are not usually tested for and require specific media that have been described elsewhere.32 Finally, the strict anaerobes require culturing in an anaerobic cabinet or similar options. Selective Medium for Megasphaera and Pectinatus (SMMP) medium has been developed for the selective isolation of Megasphaera and Pectinatus.25 It contains reducing agents to encourage the growth of anaerobes.
17.5.3 Rapid Methods
Numerous rapid methods for the detection and identification of spoilage microorganisms have been developed and examined in the context of brewing microbiology over the past 10 to 20 years, and many are now being used routinely.29 For the sensitive detection of trace contaminants, membrane filtration or prior enrichment of samples by growth in a suitable medium are often required for these methods, just as they are for the traditional methods, thus lengthening the time needed to obtain a result.
17.5.3.1 Adenosine Triphosphate (ATP) Bioluminescence and Oxidoreductase Tests
The bioluminescence technique has been available since the 1960s and was applied to food in the early 1970s. It depends on the presence of an elevated concentration of adenosine triphosphate (ATP) in viable organisms, which is depleted as soon as the cell dies. Thus, detection of ATP correlates with detection of viable cells. The quantification of the ATP is achieved by using it as the energy source in the luciferase enzyme reaction, adopted from the firefly, which uses luciferase/luciferin to generate light. The amount of light correlates with the amount of ATP and is detected in a luminometer. Yeast are more easily detected than bacteria because a yeast cell contains about 100-fold more ATP than the average bacterium. A variety of kits are available commercially including Ultrasnap (Hygiena), PocketSwab Plus (Charm Sciences), Hy-Lite (VWR), and Clean-Trace (3M). These kits can detect as few as 100 yeast cells per sample without prior enrichment. This approach is used extensively for surface hygiene monitoring and swabbing of process machinery. It can also be used to test the microbiological status of water, which is valuable for assessing the effectiveness of CIP. This approach allows real-time estimation of cleanliness thus making recleaning possible.
An alternative to the ATP test is the oxidoreductase test for determination of nicotinamide adenine dinucleotides (NAD/NADH) and nicotinamide adenine dinucleotide phosphates (NADP/NADPH), which, like ATP, are present in viable cells. HY-RiSE (Merck) uses a test strip that changes color on detection of NAD(P) and/or NAD(P)H.35
17.5.3.2 Direct Epifluorescence Filter Technique (DEFT)
The direct epifluorescence filter technique (DEFT) combines membrane filtration of samples followed by viability staining using fluorochromes and detection of the cells by epifluorescence microscopy or digital image analysis. Acridine orange has been the favored fluorescent dye, but it has its limitations. After excitation, acridine orange fluoresces green in combination with DNA and red with RNA. Viable cells appear orange due to the relatively large amounts of RNA, and dead cells appear green. However, in practice, it is difficult to distinguish live from dead cells. DAPI (4',6-diamidino-2-phenylindole) is currently the standard stain for DEFT, staining the double-stranded DNA in the cell blue. Other stains include the SYTOX® range, FUN,® the Hoechst range, SYBR® green I, and Besbenzimide H. An alternative combination that is claimed to be more discriminatory is the combination of fluorescein diacetate and propidium iodide, which allows for the detection of both yeast and bacteria. Commercial kits are available from Molecular Probes Inc. (www.probes.com) for fluorescent viability staining of both yeast and bacteria (LIVE/DEAD® Yeast Viability kit).
Because the microscopic examination of filters is tiring and labor intensive, automated versions of DEFT using computer-assisted scanning of the membrane coupled to computer-enhanced image analysis have been developed. Such systems can be fully automated.
17.5.3.2.1 Antibody DEFT
One of the drawbacks of DEFT is that although cells can be distinguished microscopically, they cannot be accurately identified. In the case of lactic acid bacteria, this could be very important because only certain species of Lactobacillus are serious spoilage organisms. It is therefore useful to incorporate identification into the procedure. A fluorescent antibody stain can be used for highly specific targeting of spoilage microorganisms, although with loss of the viability assessment. Monoclonal antibodies have proven particularly useful in this respect and have been popular for the detection and identification of Pectinatus and lactic acid bacteria including pediococci.36 The time-consuming aspect of microscopy was circumvented by Yasui and Yoda,37 who linked a chemiluminescent immunoassay to a camera for detection of the luminescent spots.
17.5.3.2.2 Oligonucleotide-DEFT
A further, more sensitive, and specific detection method is Oligonucleotide-DEFT (Oligo-DEFT), which may be used to distinguish among either species or groups of microorganisms.35 In this method, fluorescent-labeled oligonucleotides complementary to target species 16S rRNA are combined with DEFT. It has been demonstrated that the Oligo-DEFT method can achieve a detection limit of 1 CFU/mL for E. coli.38
17.5.3.3 Fluorescence In Situ Hybridization (FISH)
Fluorescence in situ hybridization (FISH) uses a fluorescent nucleic acid probe to specifically hybridize with a nucleic acid target in the cell. These gene probes enter chemically permeabilized cells and bind specifically to nucleic acid targets. A favorite target is the ribosomal RNA because, as a component of the ribosome, it is present in many hundreds of copies. Moreover, when cells die, ribosomes are degraded; so this gives an element of specificity toward living cells. Nucleic acid probes can be designed with various levels of specificity enabling groups of bacteria (e.g., all lactobacilli) or only certain species to be identified. FISH can be applied to beer samples for the detection of Pectinatus without prior cultivation and detection achieved in five hours rather than several days as required by conventional tests.39
This approach has been commercialized as kits by Vermicon (www.vermicon.com); the “VIT-bier plus L. brevis” kit comprises fluorescently labeled probes and reagents for detection of a range of beer spoilage lactobacilli and P. damnosus. Moreover, while these bacteria fluoresce red, L. brevis, the most common beer spoilage bacterium can be distinguished by green fluorescence. Vermicon also market the “VIT-Bier Megasphaera/Pectinatus” kit for FISH analysis of these two anaerobic beer spoilage bacteria.
A relatively new quantitative and qualitative hybridization method similar to FISH is the HybriScan® system. This is based on the detection of rRNA via hybridization and specific capture and detection probes. The use of two probes in this system makes it highly specific, and kits are available for both the detection of beer spoilage bacteria (HybriScan® D Beer) and for yeast (HybriScan® D Yeast).
17.5.3.4 Polymerase Chain Reaction
The polymerase chain reaction (PCR) is a means of amplifying minute amounts of DNA in a highly specific manner. At the molecular level, the PCR comprises repetitive cycles of DNA denaturation by heat into single strands, highly specific primer annealing, and DNA synthesis using a thermostable polymerase. The process is exponential and provides several hundred micrograms of DNA from nanograms of starting material within about 30 cycles. Classical PCR products must be visualized by agarose gel electrophoresis to assess whether material of the correct size has been produced; on occasion, it is necessary to sequence the DNA product to be sure that the correct amplification has taken place. The level of detection is such that prior enrichment by cultivation of the organisms is generally necessary to achieve high sensitivity.
Primers specific for PCR detection and identification of various yeasts and bacteria, including Lactobacillus, Megasphaera, Obesumbacterium, Pectinatus, Pediococcus, and Shimwellia, have been developed over the past 20 years (reviewed by Seigrist et al.34). In general, these primers target the rRNA gene, allowing for varying degrees of specificity according to the region used. Thus, some primers recognize parts of the gene common to all Gram-positive bacteria, or even all bacteria, while other primers are specific at the species level. A particularly innovative approach was to target the hop resistance gene (horA) of lactobacilli for PCR detection. In this way, only hop-tolerant (potential beer spoilage) bacteria were detected.40
The real-time PCR machine, or LightCycler, allows simultaneous amplification and detection of the product without recourse to agarose gel electrophoresis. Several brewing specific kits are available including the “foodproof” Beer Screening kit (BIOTECION diagnostics GmbH). With this kit, it is possible to test for 30 beer spoilage bacteria in a single test after pre-enrichment to ensure detection of trace contaminants. Beer spoilage lactobacilli (L. brevis and L. lindneri), P. damnosus, Pectinantus, Megasphaera, and Selenomonas are all differentiated in the PCR reaction. A more recent system that has been developed by PrimerDesign includes a bespoke primer design (for defined spoilage organisms within each brewery) and a “plug-and-play” PCR thermocycler, which gives a positive/negative result within two to three hours.
17.6 THE MICROBIOLOGICAL LABORATORY WITHIN THE BREWERY
Microbiological records of the plant and its products should be retained for evaluating any deviations from the norm or indications of adverse hygiene trends. The laboratory is not responsible for the hygienic operation of the brewery. The production departments are charged with assuring the quality of the process and the products, but they depend on the data collected by the laboratory and advice of the microbiologist to manage their processes effectively. The modern brewery laboratory is a service providing the functions and assistance required by the production staff to assure quality operations.41 By collecting the necessary data in a reliable and timely fashion, the laboratory provides an essential service to production departments. To achieve this, the laboratory should choose a combination of both traditional and rapid techniques in a cost-effective manner. Automation and rapid data processing are important to indicate adverse trends and to enable microbiologically informed decisions to be made. Good communication with management is then necessary to achieve efficient and hygienically responsible processing throughout the plant.
The scale of the plant will influence the scope of the microbiological analyses that are undertaken. A microbrewery will have limited resources and probably restrict analyses to determination of yeast quality and perhaps microbiological contamination in the finished product. Large breweries, on the other hand, should monitor progress throughout the process and into the finished product; the problems associated with product recall fully justify a comprehensive microbiological service.
The microbiology laboratory also has a responsibility toward the staff of the brewery in terms of providing hygiene training so that the requirements of the health officials are met and that the staff operate in a safe environment. Routine contact between laboratory and production staff will allay potential problems and encourage good working practices.
REFERENCES
1. Jeon, S. H., Kim, N. H., Shim, M. B., Jeon, Y. W., Ahn, J. H., Lee, S. H., Hwang, I. G., and Rhee, M. S., Microbiological diversity and prevalence of spoilage and pathogenic bacteria in commercial fermented alcoholic beverages (beer, fruit wine, refined rice wine, and yakju), J. Food Protect., 78(4):812–818, 2015.
2. Gilliland, R.B., Wild yeast spoilage, Brew. Guardian, 96:37–45, 1967.
3. Campbell, I., Wild yeasts in brewing and distilling, in Brewing Microbiology, Priest, F.G. and Campbell, I. Eds., Kluwer Academic, New York, pp. 247–266, 2003.
4. Flannigan, B., The microbiota of barley and malt, in Brewing Microbiology, Priest, F.G. and Campbell, I. Eds., Kluwer Academic, New York, pp. 113–180, 2003.
5. Steffenson, B.J., Combating Fusarium head blight: An emerging threat to malting barley quality throughout the world. Proc. 27th Eur. Brew. Conv. Congr. Cannes, IRL Press, Oxford, 1999, pp. 531–539.
6. Schmale, D.G., III. and Bergstrom, G.C., Fusarium head blight in wheat, The Plant Health Instructor, 2003, doi: 10.1094. PHI-I-2003-0612-01.
7. Noots, I., Delcour, J.A., and Michiels, C.W., From field barley to malt: Detection and specification of microbial activity for quality aspects, Crit. Rev. Microbiol., 25:121–153, 1999.
8. Priest, F.G., Gram-positive brewery bacteria, in Brewing Microbiology, Priest, F.G. and Campbell, I. Eds., Kluwer Academic, New York, pp. 181–217, 2003.
9. Suzuki, K., Gram-positive spoilage bacteria in brewing, in Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste, Hill, A.E. (Ed.), Woodhead Publishing, London, UK, pp. 141–173, 2015.
10. Back, W., Bohak, I., Ehrmann, M., Ludwig, W., and Schleiffer, K.-H., Revival of the species Lactobacillus lindneri and the design of a species specific oligonucleotide probe, Syst. Appl. Microbiol., 19:322–325, 1996.
11. Hutzler, M., Koob, J., Grammer, M., Riedl, R., and Jacob, F., Statistische Auswertung der PCR Analysen bierschädlicher Bakterien in den Jahren 2010 und 2011, Brauwelt, 152:546–547, 2012.
12. Bohak, I., Back, W., Richter, L., Ehrmann, M., Ludwig, W., and Schleiffer, K.-H., Lactobacillus amylolyticus sp. nov., isolated from beer malt and beer wort, Syst. Appl. Microbiol., 21:360–364, 1998.
13. Simpson, W.J., Cambridge prize lecture, studies on the sensitivity of lactic acid bacteria to hop bitter acids, J. Inst. Brew., 99:405–411, 1993.
14. Sakamoto, K., Margolles, A., van Veen, H.W., and Konings, W.N., Hop resistance in the beer spoilage bacterium Lactobacillus brevis is mediated by the ATP-binding cassette multidrug transporter HorA, J. Bacteriol., 183:5371–5375, 2001.
15. Gasarasi, G., Kelgtermans, M., Verstrepen, K.J., Van Roy, J., Delvaux, F.R., and Derdelinckx, G., Occurrence of biogenic amines in beer: Causes and proposals of remedies, Monat. Brauwiss., 56:58–63, 2003.
16. Kalac, P. and Krizek, M., A review of biogenic amines and polyamines in beer. J. Inst. Brew., 109:123–128, 2003.
17. Dobson, C.M., Deneer, H., Lee, S., Hemmingsen, S., Glaze, S., and Ziola, B., Phylogenetic analysis of the genus Pediococcus, including Pediococcus claussenii sp nov., a novel lactic acid bacterium isolated from beer, Int. J. Syst. Evol. Microbiol., 52:2003–2010, 2002.
18. Calmels, S., Ohshima, H., and Bartsch, H., Nitrosamine formation by denitrifying and non-denitrifying bacteria: Implication of nitrite reductase and nitrate reductase in nitrosation catalysis, J. Gen. Microbiol., 134:221–226, 1998.
19. Smith, N.A., Nitrate reduction and N-nitrosation in brewing, J. Inst. Brew., 100:347–355, 1994.
20. van Vuuren, H.J.J. and Priest, F.G., Gram-negative brewery bacteria, in Brewing Microbiology, Priest, F.G. and Campbell, I. (Eds.), Kluwer Academic, New York, pp. 219–245, 2003.
21. Prest, A.G., Hammond, J.R.M., and Stewart, G.S.A.B., Biochemical and molecular characterization of Obesumbacterium proteus, a common contaminant of brewing yeasts, Appl. Environ. Microbiol., 60:1635–1640, 1994.
22. Priest, F.G. and Barker, M., Gram-negative bacteria associated with brewery yeasts: Reclassification of Obesumbacterium proteus biogroup 2 as Shimwellia pseudoproteus gen. nov., sp. nov. and transfer of Escherichia blattae to Shimwellia blattae comb. nov., Int. J. Syst. Evol. Microbiol., 60:828–833, 2010.
23. Hamze, M., Mergaert, J., and van Vuuren, H.J.J., Rahnella aquatilis, a potential contaminant in lager beer breweries, Int. J. Food Microbiol., 13:63–68, 1991.
24. Magnus, C.A., Ingledew, W.M., and Casey, C.P., High gravity brewing: Influence on the viability of contaminating brewing bacteria, J. Am. Soc. Brew. Chem., 44:158, 1986.
25. Lee, S.Y., SMMP- a medium for selective isolation of Megasphaera and Pectinatus, J. Am. Soc. Brew. Chem., 52:115–119, 1994.
26. Schleiffer, K.-H., Leuteritz, M., Weiss, N., Ludwig, W., Kirchhof, G., and Seidel-Rufer, H., Taxonomic study of anaerobic, Gram-negative, rod-shaped bacteria from breweries: Emended description of Pectinatus cerevisiiphilus and description of Pectinatus frisingensis sp. nov., Selenomonas lactiflex sp. nov., Zymophilus raffinosivorans gen. nov., sp. nov., and Zymophilus paucivorans sp. nov., Int. J. Syst. Bacteriol., 40:19–27, 1990.
27. Engelmann, U. and Weiss, N., Megasphaera cerevisiae sp. nov.: A new Gram-negative obligately anaerobic coccus isolated from spolied beer, Syst. Appl. Microbiol., 6:287–290, 1985.
28. Tholozan, J.L., Membre, J.M., and Grivet, J.P., Physiology and development of Pectinatus cerevisiiphilus and Pectinatus frisingensis, two strict anaerobic beer spoilage bacteria, Int. J. Food Microbiol., 35:29–39, 1997.
29. Russell, I. and Stewart, G.G., Rapid detection and identification of microbial spoilage, in Brewing Microbiology, Priest, F.G. and Campbell, I., Eds., Kluwer Academic, New York, pp. 267–304, 2003.
30. Vrellas, C.G. and Tsiotras, G., Quality management in the global brewing industry, Int. J. Qualt. Reliab. Management, 32(1):42–52, 2015.
31. Hill, A.E., Traditional methods of detection and identification of brewery spoilage organisms, in Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste, Hill, A.E., Ed., Woodhead Publishing, London, UK, pp. 271–286, 2015.
32. Jespersen, L. and Jakobsen, M., Specific spoilage organisms in breweries and laboratory media for their detection, Int. J. Food Microbiol., 33:139–155, 1996.
33. Kindraka, J.A., Evaluation of NBB anaerobic medium for beer spoilage organisms, Tech. Q. Master Brew. Assoc. Am., 24:146–151, 1987.
34. Siegrist, J., Kohlstock, M, Merx, K., and Vetter, K., Rapid detection and identification of spoilage bacteria in beer, in Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste, Hill, A.E., Ed., Woodhead Publishing, UK, 2015, pp. 287–318.
35. Shimokawa, M., Suzuki, K., and Yamagishi, H., Detection and determination of beer spoilage lactic acid bacteria, Brauwelt Int., 34:98–103, 2016.
36. Ziola, B., Ulmer, M., Bueckert, J. Giesbrecht, D., and Lee, S.Y., Monoclonal antibodies showing surface reactivity with Lactobacillus and Pediococcus beer spoilage bacteria, J. Am. Soc. Brew. Chem., 58:63–68, 2000.
37. Yasui, T. and Yoda, K., Imaging of Lactobacillus brevis single cells and microcolonies without a microscope by an ultrasensitive chemiluminescent enzyme immunoassay with a photon-counting television camera, Appl. Environ. Microbiol., 63:4528–4533, 1997.
38. Tortorello, M.L. and Reineke, K.F., Direct enumeration of Escherichia coli and enteric bacteria in water, beverages and sprouts by 16S rRNA in situ hybridization, Food Microbiol., 17(3):305–313, 2000.
39. Yasuhara, Y., Yuuki, T., and Kagami, N., Novel quantitative method for detection of Pectinatus using rRNA targeted fluorescent probes, J. Am. Soc. Brew. Chem., 59:117–121, 2001.
40. Sami, M., Yamashita, H., Kadokura, H., Kitamoto, K., Yoda, K., and Yamasaki, M., A new and rapid method for determination of beer-spoilage ability of lactobacilli, J. Am. Soc. Brew. Chem., 55:137–140, 1997.
41. Nitzsche, F. and Eggers, G., Hygiene monitoring in breweries-the microbiological laboratory as a service provider, Brauwelt Int., 20:96–98, 2002.