CHAPTER 7
Chemical Interactions
Structures of Matter
Atomic Particles
All atoms are made up of the same three basic particles: protons, electrons, and neutrons. The atoms of different substances have a different number of particles in each atom.
Protons have a positive charge, and electrons have a negative charge. Neutrons do not have a charge. Another difference between the particles is their mass. Protons and neutrons are each about 2000 times heavier than an electron.
Look at the following model of a helium atom. Notice that the atom is nearly empty and is not a solid object at all. The middle part of the atom is called the nucleus. This is where you always find the protons and neutrons. The nucleus contains most of the mass of an atom and has a positive charge, since all the protons are located here. The electrons orbit around the nucleus.
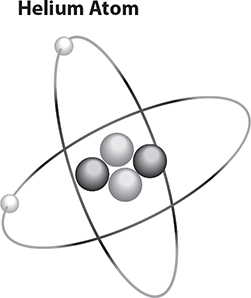
To describe atoms and their nuclei, we use letters and numbers. The letters are a key to the name of the atom. The helium atom is represented by the letters He. The mass number (top number) tells how many protons and neutrons are in the nucleus. The atomic number (bottom number) tells how many protons are in the nucleus. You can see that helium has 2 protons and a mass of 4.

The number of electrons is the same as the number of protons. Because the positively charged protons balance the negatively charged electrons, overall the atom has a neutral charge.
Ions and Isotopes
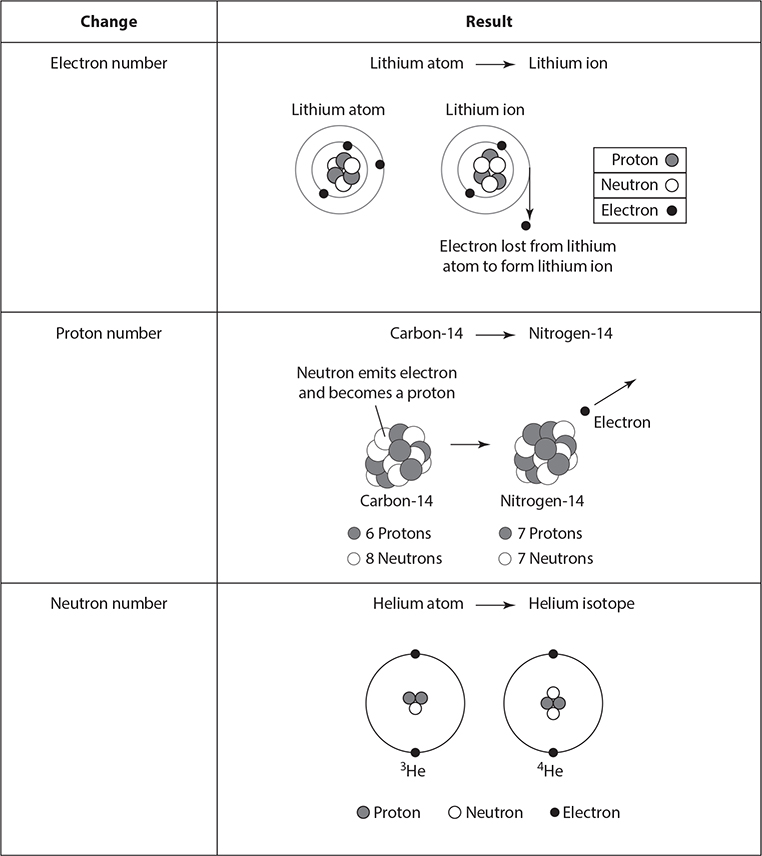
Sometimes the number of particles in an atom can change. The following table illustrates the results of changes to the numbers of electrons, protons, or neutrons in atoms. If the number of electrons in an atom is changed, protons and electrons are no longer in balance, and the atom becomes a charged particle. Charged particles are called ions. If the number of protons in an atom is changed, the atom becomes a completely different element. Atoms with fewer or more neutrons than protons are called isotopes.
Molecules, Elements, and Compounds
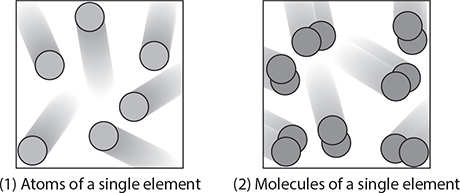
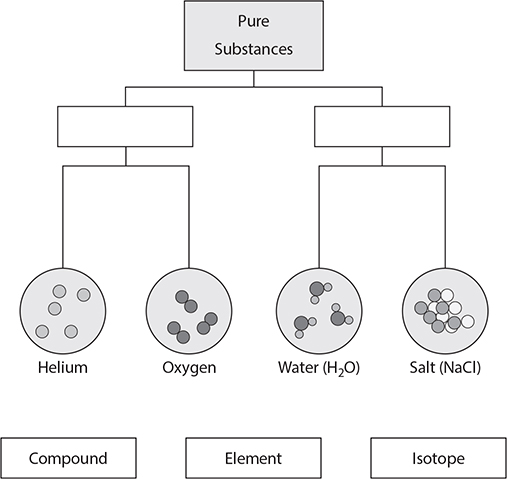
Many times, individual atoms are bonded together with other atoms. A molecule is a particle made of two or more atoms bonded together. If the atoms are the same type, then the molecule is classified as an element.
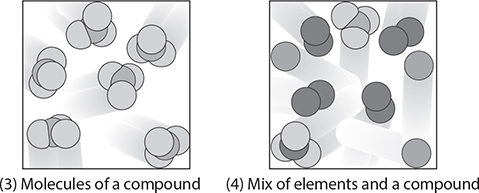
Molecules can also be made of two or more different types of atoms. In this case, the molecule is called a compound. A compound is made of two or more elements that have been chemically bonded.
EXERCISE 1
Structures of Matter
Directions: Choose the best answer for each of the following items.
1. Connect the terms to the correct place on the chart. One term will not be used. (Note: On the real GED® test, you will click on the words you choose and “drag” each one into position in the chart.)
Question 2 is based on the following illustration.
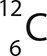
2. How many protons does the carbon atom have in its nucleus?
A. 1
B. 6
C. 12
D. 18
Answers are on page 663.
Physical and Chemical Properties
There are over 100 different elements, each with a unique arrangement of protons, neutrons, and electrons. Scientists use a chart called the periodic table to arrange the elements by their atomic number. Each square of the periodic table supplies four important facts, as shown in the following illustration.
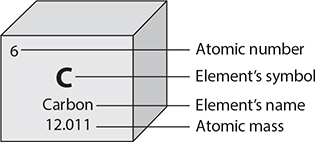
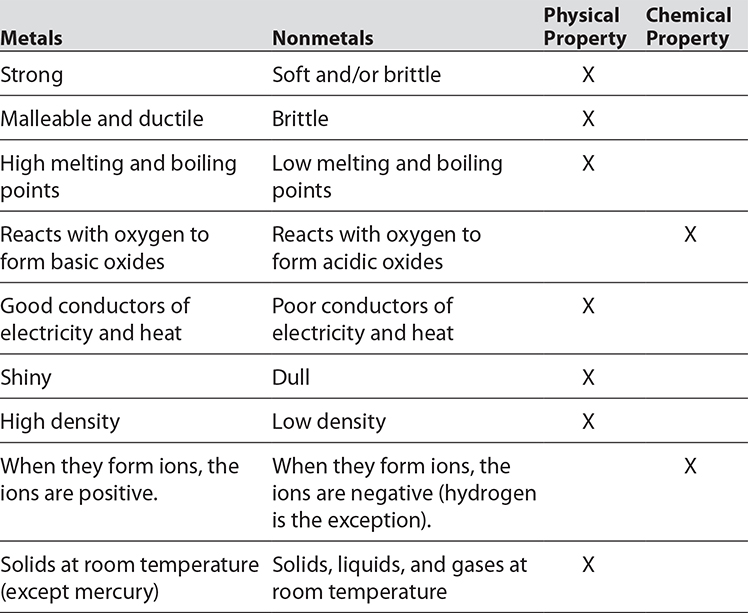
In the periodic table, the elements are organized according to their physical and chemical properties. Metals with similar properties are grouped together on the left side of the table. Nonmetals are grouped on the right side of the table.
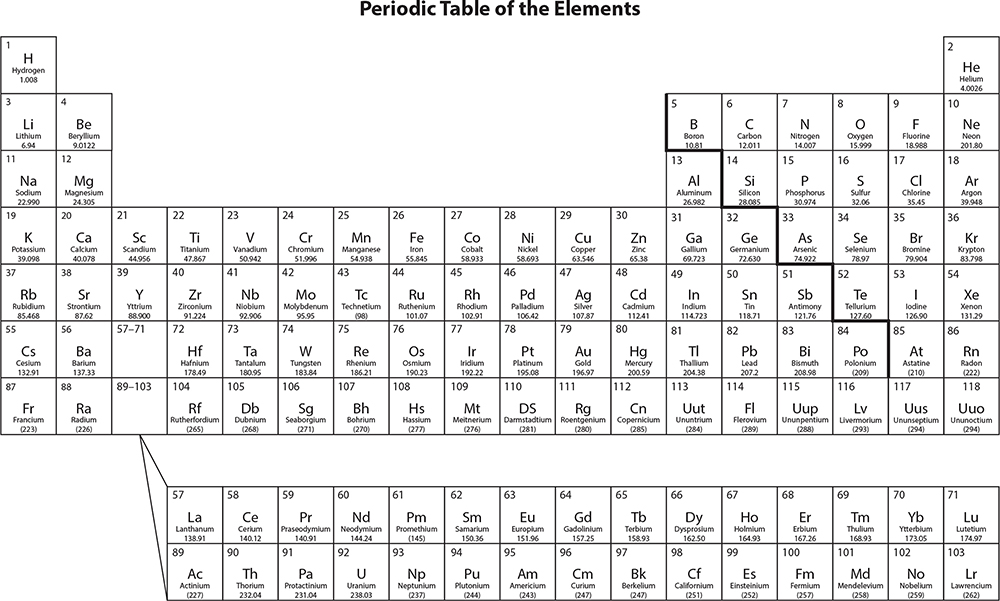
Chemical properties include flammability, combustibility, and reactivity with other chemicals. These properties are observed when one substance interacts with another substance. A physical property can be noted without observing how the substance interacts with others. Color, melting point, and density are a few examples of physical properties.
Density, in particular, is a characteristic physical property of a substance. It is the relationship between the substance’s mass and its volume. Remember that volume is how much space a substance takes up.
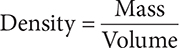
The density of a substance is determined by the mass, size, and arrangement of its atoms. Objects with the same volume but different mass will have different densities.
Look at the following table. It identifies different chemical and physical properties of both metals and nonmetals.
States of Matter
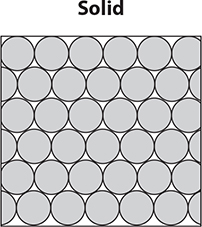
States of matter are considered physical properties. Solids have particles packed in a regular pattern and are dense. There is very little space between the particles. Most metals, such as gold and silver, are in a solid state at room temperature. When a solid is heated, its particles gain energy and expand. When the particles reach the melting point, they break away from their positions and enter a liquid state.
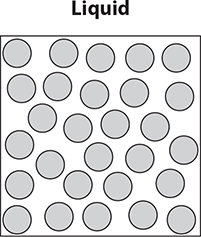
Liquids take the shape of the container in which they are placed, meaning that the particles are not in a fixed position. Liquids are less dense than solids because there is a small amount of space between the particles. Mercury (a metal) and bromine (a nonmetal) are in a liquid state at room temperature. When a liquid is heated to the boiling point, the particles gain energy and expand, changing state into a gas. When the particles contain even more energy, evaporation can take place. When liquids are cooled, they can solidify and enter a solid state.
Gases have very low densities because there are huge amounts of space between the particles. Gases are easily compressed and have no shape, filling up whatever space is available as the particles move around. Many nonmetals, including helium, oxygen, and hydrogen, are in a gas state at room temperature. When a gas is cooled, the particles lose energy, causing them to move more slowly and closer together until the gas becomes a liquid. This change of state is called condensation.
EXERCISE 2
Physical and Chemical Properties
Directions: Choose the best answer for each of the following items.
1. Complete the statement with the appropriate number answer.
The density of a metal with a volume of 30 milliliters and a mass of 120 kilograms is ____________________.
2. Which of the following elements is NOT a solid at room temperature?
A. gold
B. silver
C. mercury
D. nickel
3. Which change of state occurs when a liquid is cooled and the particles lose energy and stop moving?
A. liquefaction
B. solidification
C. evaporation
D. condensation
Answers are on page 663.
Chemical Formulas and Equations
The periodic table of elements uses one- or two-letter symbols to represent each element, but how do you represent a compound? The answer is a chemical formula. A chemical formula is a combination of symbols that represents the elements in a compound. For example, the formula for carbon dioxide is CO2.
The subscript numbers in a formula show the ratio of the atoms of different elements in that compound. In the case of carbon dioxide, for every 1 atom of carbon, there are 2 atoms of oxygen in the compound. If a letter symbol such as the C for carbon does not have a subscript, the number 1 is understood.
Water is a well-known compound with the formula H2O. The subscript 2 shows that there are 2 atoms of hydrogen (H) for every 1 atom of oxygen (O) in the compound.
Using symbols instead of words allows you to write chemical equations. Chemical equations summarize what happens in chemical reactions. They tell you what substances you begin with (the reactants) and what substances you end with (the products). The number of reactants and products can vary, depending on the reaction.
For example, iron reacts with sulfur to produce iron sulfide. This equation would be written as follows:

Conservation of Mass
Even though the arrangement of atoms is different at the end of a reaction from what it was at the start, all the atoms present at the start are still present at the end. In the case of iron and sulfur, there is one atom of iron on the reactant side and one atom of iron on the product side of the equation. The same is true for sulfur.
This principle is known as the conservation of mass. During a chemical reaction, matter is neither created nor destroyed. In other words, during a chemical reaction, the amount of matter involved does not change. The total mass of the reactants will always equal the total mass of the products.
Balancing Chemical Equations
Because mass is conserved during chemical reactions, chemical equations must be balanced. To balance an equation, first look at the formulas. Try writing the equation with words first. Then, use the symbols to write the equation.
Take the reaction resulting in water as an example. Hydrogen gas reacts with oxygen gas to form water when a spark is added to the mixture.
H2 + O2 → H2O
Now, check to make sure that you have the same number of atoms on one side of the reaction as you do on the other side. This is known as balancing the equation.
In this case, there are 2 oxygen atoms on the left but only 1 on the right. To correctly represent the reaction and balance the equation, you use a coefficient. A coefficient is a number placed in front of a chemical formula that tells you how many atoms or molecules of each reactant and product participate in the reaction. If the coefficient is 1, it is understood and not written.
In this case, you need to multiply the H2O on the right by the coefficient 2.
H2 + O2 → 2H2O
This gives you 2 oxygen atoms on both sides, but now you have 2 hydrogen atoms on the left and 4 hydrogen atoms on the right. You also need to multiply the H2 by the coefficient 2 to have a balanced chemical equation.
2H2 + O2 → 2H2O
Limiting Reactants
In chemistry, there is usually too much of one reactant and not enough of another. The reactant that is used up first and prevents more product from being made is referred to as the limiting reactant. The substance that does not get used up as a reactant is called the excess reactant.
Suppose you start with 4 hydrogen gas molecules and 1 oxygen gas molecule. Can you make 4 water molecules?
4H2 + O2 → 4H2O
No, you cannot because you have only 1 oxygen gas molecule. In your reaction, you will run out of O2 before you run out of H2. Oxygen gas is the limiting reactant.
Types of Chemical Reactions
So far, all of the reactions in this section have been examples of synthesis reactions. A synthesis reaction is one in which two or more substances combine to make a more complex substance. Another example is the reaction between magnesium and oxygen to form magnesium oxide.
2Mg + O2 → 2MgO
There are several other types of chemical reactions. A decomposition reaction happens when compounds are broken down into simpler products. An example is the decomposition of hydrogen peroxide into water and oxygen gas.
2H2O2 → 2H2O + O2
A replacement reaction occurs when one element replaces another in a compound, or when two elements in different compounds trade places. An example of a replacement reaction can be seen when rock that contains copper oxide is heated in the presence of charcoal, which is pure carbon, to obtain copper. The carbon found in the charcoal replaces copper in the copper oxide to form carbon dioxide and copper metal.
2CuO + C → 2Cu + CO2
EXERCISE 3
Chemical Formulas and Equations
Directions: Choose the best answer for each of the following items.
Questions 1 through 3 are based on the following information.
Iron oxide reacts with carbon monoxide to produce iron and carbon dioxide.
1. Which of the following word equations is correct?
A. iron + iron oxide → carbon monoxide + carbon dioxide
B. iron oxide → carbon monoxide + iron + carbon dioxide
C. carbon monoxide + iron oxide + iron → carbon dioxide
D. iron oxide + carbon monoxide → iron + carbon dioxide
2. Which of the following equations is correctly balanced?
A. Fe2O3 + CO → 2Fe + 3CO2
B. Fe2O3 + 3CO → 2Fe + 3CO2
C. Fe2O3 + 2CO → 2Fe + CO2
D. Fe2O3 + CO → 2Fe + 2CO2
3. Which type of equation is described?
A. limiting
B. synthesis
C. replacement
D. decomposition
Answers are on page 663.
Solutions and Solubility
When you think about the word solution, what comes to mind? Many people think about something liquid, like a saline solution, a mixture made of salt and water. In chemistry, a solution is a homogenous (same throughout) mixture of two or more substances in which the particles are very small (from 0 to 100 nanometers). The substances can be solids, liquids, or gases. Air is a solution that includes dissolved water vapor in a mixture made up of oxygen, carbon dioxide, nitrogen, and various other gases. Metal alloys such as sterling silver are also solutions.
There are two parts to any solution. The substance that is dissolved is called the solute. The solute is usually present in a smaller amount than the substance in which it is dissolved. The solvent is the substance that does the dissolving and is usually present in a greater amount.
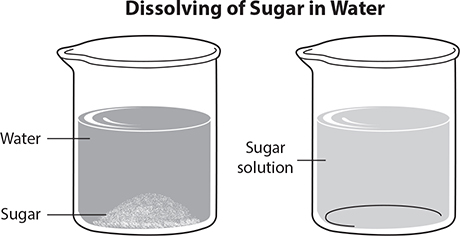
The most common solvent is water. In solutions where water is the solvent, the mixture formed is called an aqueous solution. In the following image, sugar is the solute and water is the solvent. The sugar-water mixture is the solution.
Solubility
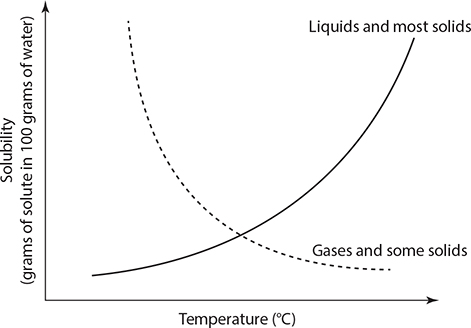
For now, we will focus on aqueous solutions. The concentration of a solution refers to how much of the solute is dissolved in the liquid. Solubility is the maximum amount of solute that will dissolve in a certain amount of water at a given temperature. Take a look at the following solubilities graph. You can see that as temperature increases, most solids increase in solubility. However, gases decrease in solubility as temperature rises.
Saturation
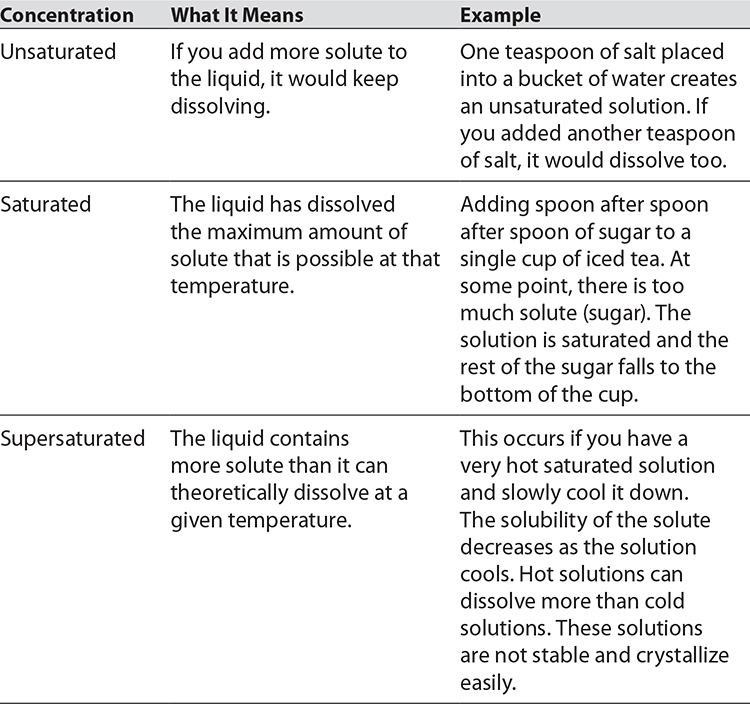
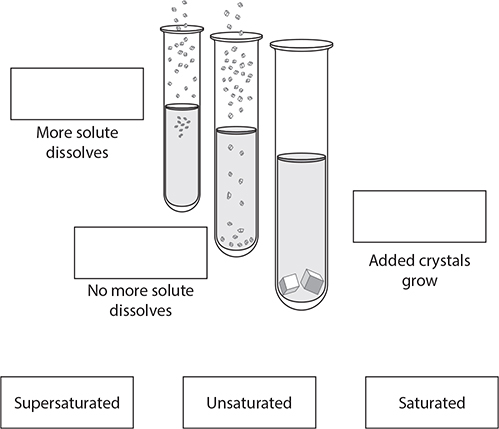
A true solution appears clear. If it has color, you can still see through it. If there are undissolved particles, the solution will be cloudy. What happens when water can no longer dissolve a certain substance? This is referred to as saturation. It is the point at which a solution can dissolve no more of that substance, and any additional amounts of it will appear as undissolved particles. There are three degrees of saturation, as shown in the following table.
Weak and Strong Solutions
Solutions can be described as dilute (weak) or concentrated (strong). Dilute means that a small amount of solute is dissolved in the solvent. Concentrated means that there is a lot of solute dissolved in the solvent.
The acidity, alkalinity, or neutrality of a solution is also described in terms of strength. In a strong acid, nearly all of the acid molecules will form ions, whereas in a weak acid only some of the molecules will form ions. The strength of the solution is shown using a scale of numbers called the pH scale. The pH numbers range from 0 to 14.
• Solutions with pH numbers less than 7 are acidic. Examples include citrus juices. The lower the pH number, the stronger is the acid.
• Solutions with pH numbers greater than 7 are alkaline. An example is milk. A substance with high alkalinity will have a higher pH number.
• A solution with a pH of exactly 7 is considered neutral. Water is neutral.
EXERCISE 4
Solutions and Solubility
Directions: Choose the best answer for each of the following items.
1. Which of the following is NOT an example of a solution?
A. water
B. gasoline
C. salt water
D. 14-karat gold
2. A dilute solution of lemon juice, with a pH of 1.5, would be classified as a
A. neutral.
B. weak acid.
C. strong acid.
D. weak alkali.
3. Connect the terms to the correct image and description. (Note: On the real GED® test, you will click on the words you choose and “drag” each one into position in the diagram.)
Answers are on page 663.