41
Jaundice
Visible jaundice occurs in more than 80% of term and preterm infants during the first week. Bilirubin metabolism is shown in Fig. 41.1. Elevated bilirubin levels are due to:
- High hemoglobin (Hb) concentration at birth, so considerable heme degradation.
- A newborn’s red blood cell lifespan in shorter than an adult’s.
- Liver enzyme conjugation is reduced.
- Enterohepatic circulation is enhanced. This ‘physiologic’ jaundice peaks at 2–5 days and then usually clears by 14 days, but may persist for several weeks in breast-fed infants.
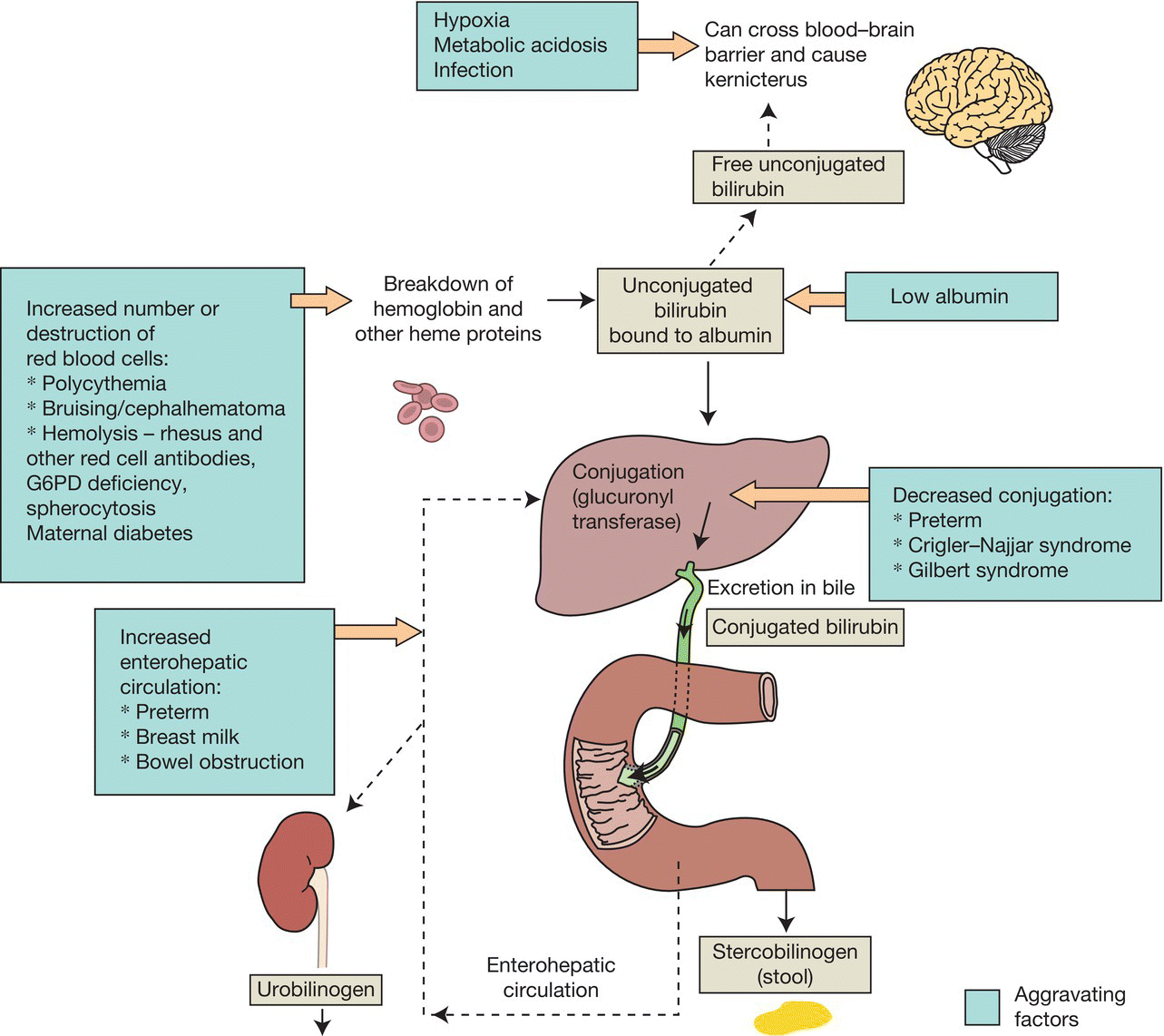
Fig. 41.1 Metabolism of bilirubin. Bilirubin is the product of the metabolism of hemoglobin and other heme proteins. The initial breakdown product is unconjugated bilirubin (indirect bilirubin), which is carried in the blood bound to albumin. When the albumin binding is saturated, free, unconjugated, lipid-soluble bilirubin can cross the blood–brain barrier. Unconjugated bilirubin bound to albumin is conjugated in the liver (direct bilirubin), which is excreted via the biliary tract into the gut. Some bilirubin is reabsorbed from the gut (enterohepatic circulation). Risk factors for jaundice are shown in green.
Significance of severe hyperbilrubinemia
Kernicterus describes bilirubin encephalopathy. In acute bilirubin encephalopathy there may be hyptonia, lethargy, poor feeding, irritability, high-pitched cry, fever, apnea, hypertonia with arching of the neck and trunk-opisthotonus (Fig. 41.2), seizures, coma and death. In chronic bilirubin encephalopathy there is permanent neurologic injury resulting from the deposition of unconjugated bilirubin in the basal ganglia and brainstem nuclei (Fig. 41.3). Long-term consequences include dental dysplasia with yellow staining of the teeth, high-frequency sensorineural hearing loss (auditory neuropathy), paralysis of upward gaze of the eyes, choreoathetoid cerebral palsy and learning difficulties. Kernicterus is rare in developed countries.
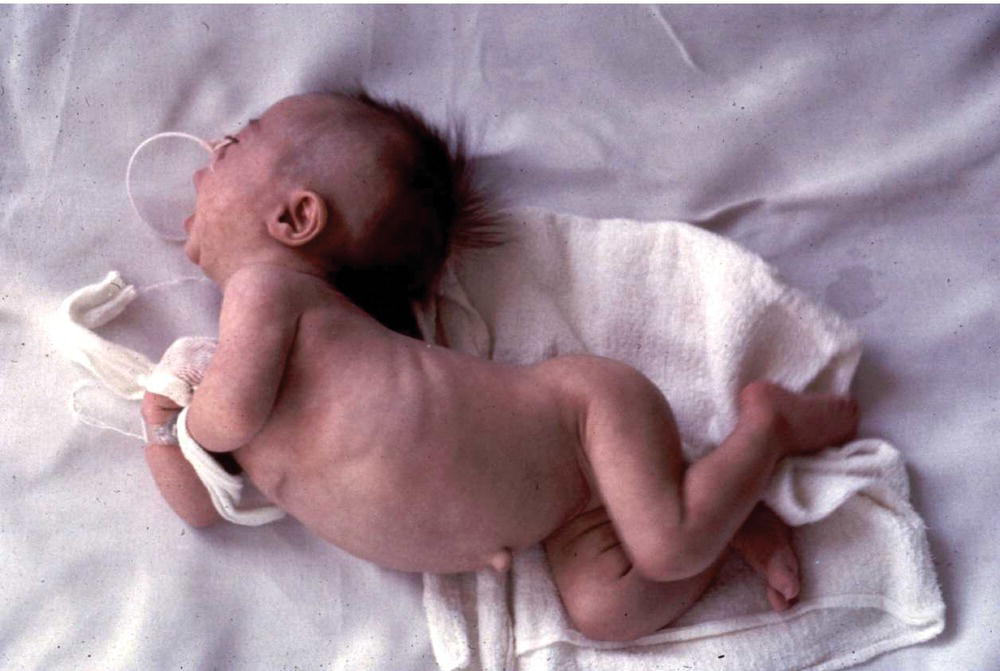
Fig. 41.2 Opisthotonus from kernicterus. This is now rarely seen in developed countries.
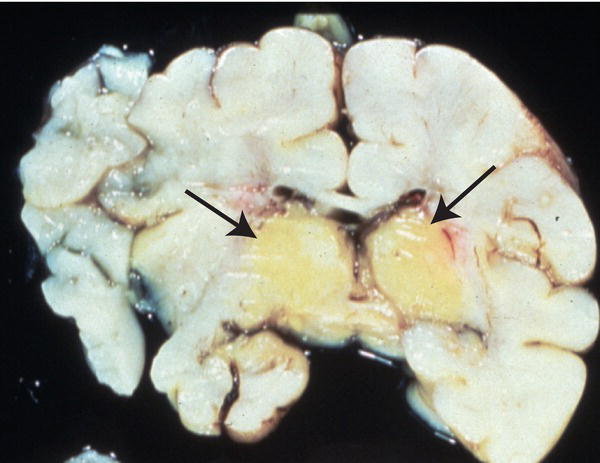
Fig. 41.3 Cross-section of the brain at autopsy showing yellow staining, predominantly in basal ganglia from deposition of unconjugated bilirubin.
Causes of early-onset jaundice (<24 hours) (Table 41.1)
Table 41.2 Indications for phototherapy and exchange transfusion in infants ≥35 weeks’ gestation.
(Adapted from American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004; 114: 297–316.)
| Age (hours) | Phototherapy | Exchange transfusion | ||||
| Higher risk | Medium risk | Lower risk | Higher risk | Medium risk | Lower risk | |
| 24 | >8 mg/dL (137 µmol/L) |
>10 mg/dL (171 µmol/L) |
>12 mg/dL (205 µmol/L) |
>15 mg/dL (257 µmol/L) |
>17 mg/dL (291 µmol/L) |
>19 mg/dL (325 µmol/L) |
| 48 | >11 mg/dL (188 µmol/L) |
>13 mg/dL (222 µmol/L) |
>15 mg/dL (257 µmol/L) |
>17 mg/dL (291 µmol/L) |
>19 mg/dL (325 µmol/L) |
>22 mg/dL (376 µmol/L) |
| 72 | >13 mg/dL (222 µmol/L) |
>15 mg/dL (257 µmol/L) |
>18 mg/dL (308 µmol/L) |
>18 mg/dL (308 µmol/L) |
>21 mg/dL (359 µmol/L) |
>24 mg/dL (410 µmol/L) |
| 96 | >14 mg/dL (239 µmol/L) |
>17 mg/dL (291 µmol/L) |
>20 mg/dL (342 µmol/L) |
>19 mg/dL (325 µmol/L) |
>22 mg/dL (376 µmol/L) |
>25 mg/dL (428 µmol/L) |
Lower risk: ≥38 weeks and well. Medium risk: ≥38 weeks and risk factor listed below or 35–37 weeks and well. Higher risk: 35–37 weeks and risk factor listed below.
Risk factors: isoimmune hemolytic disease, G6PD deficiency, asphyxia, significant lethargy, temperature instability, sepsis, acidosis or albumin <3.0 g/dL (30 g/L) if measured.
Hemolysis
Jaundice within 24 hours of birth is most likely to be hemolytic. Bilirubin levels may rise rapidly.
Rhesus disease (see Chapter 5)
This is the most severe form of hemolytic disease with onset in utero. At birth, infants may have anemia, hydrops (edema), jaundice and hepatosplenomegaly. It is now uncommon because of anti-D prophylaxis (0–2/100 000 live births in developed countries but more common in resource-limited countries).
ABO incompatibility
- Mother’s blood type O, infant’s blood type A or B. Maternal anti-A or anti-B IgG crosses the placenta, causing hemolysis.
- Direct antibody test (DAT or Coombs test) is positive, but positive test is poor predictor of significant jaundice.
- Generally less severe than rhesus, but can still cause significant hemolysis and jaundice. Onset is after birth.
- Hemolysis may progress during the first few weeks of life, and requires monitoring for anemia.
Minor antigen Incompatibility (Kell, Duffy, Kidd, etc.)
- Infant’s direct antibody test (DAT or Coombs test) is positive.
- Usually moderate hemolysis and jaundice.
G6PD (glucose-6-phosphate dehydrogenase) deficiency
This X-linked disorder is the most common enzyme defect in the world, affecting 200–400 million people. It affects males, but females can have a mild form, especially if they also have Gilbert syndrome (liver enzyme defect). It can cause severe jaundice and kernicterus in people originating from central Africa, the Mediterranean or Middle or Far East. It is diagnosed by measuring G6PD activity in red blood cells. However, during hemolytic crises this may be misleadingly elevated owing to the increased reticulocytes, which have a higher enzyme concentration. A repeat assay is required to avoid missing the diagnosis. Affected infants should avoid certain medications, i.e. some antimalarials and antibiotics, contact with mothballs (naphthalene) and eating fava (broad) beans when older.
Hereditary spherocytosis
Red blood cells are spherical with limited deformability, causing splenic sequestration and hemolysis. Autosomal dominant inheritance – family history positive in 75%. Uncommon.
Congenital infection
Increases hemolysis and may impair conjugation, causing elevated conjugated bilirubin. Other stigmata of congenital infection will be present.
Causes of jaundice 24 hours to 2 weeks
Breast-feeding jaundice
Common. Exacerbated if there is difficulty in establishing breast-feeding. Cause uncertain; may be related to low volume of breast milk and increased enterohepatic circulation of bilirubin. Breast-feeding should be continued but support may be needed. Continues beyond 2 weeks of age in up to 15% of breast fed infants.
Infection
Always consider infection, including urinary tract infection. Jaundice occurs because of hemolysis, impaired conjugation, reduced fluid intake and increased enterohepatic circulation.
Other causes
These include:
- hemolysis – may develop after first 24 hours of life
- bruising, cephalhematoma
- polycythemia
- liver enzyme defects, e.g. Crigler–Najjar syndrome, Gilbert syndrome
- gastrointestinal obstruction, e.g. pyloric stenosis
- metabolic disorders, e.g. galactosemia.
Clinical examination and assessment
Jaundice is clinically detectable from skin color on blanching the skin with digital pressure or yellow color of the sclerae when bilirubin exceeds 5 mg/dL (85 μmol/L).
It starts on the head, spreads to the abdomen and then to the limbs. It is harder to detect in preterm and dark-skinned infants. The severity of jaundice cannot be reliably assessed by clinical examination. However, an infant who is not jaundiced clinically is unlikely to have significant hyperbilirubinemia.
If jaundiced, also check for:
- pallor
- evidence of infection
- bruising, cephalhematoma
- hepatosplenomegaly (hemolysis)
- weight loss, dehydration
- family history of neonatal jaundice.
Investigations
Term infants who become jaundiced should have a transcutaneous bilirubin (TcB) measured. However, a serum measurement should be obtained if:
- the infant is <24 hours old
- transcutaneous bilirubinometer measurement >13 mg/dL (230 μmol/L) (>14.5 mg/dL, 250 μmol/L in the UK)
- transcutaneous bilirubinometer (TcB) not available
- infant ≤34 weeks’ gestational age
- on phototherapy treatment.
Further tests, other than total bilirubin, that may be required (but in most infants no pathologic cause is found)
- direct (conjugated) bilirubin
- complete blood count, reticulocyte count and smear for red cell morphology
- blood packed cell volume or hematocrit
- blood group (mother and baby)
- direct antibody test (DAT or Coombs test)
- G6PD testing
- microbiological cultures of blood, urine and/or cerebrospinal fluid for infection.
Management
The need for treatment is determined by plotting the total bilirubin level on a gestation-specific graph of bilirubin against age. This will determine whether:
- no treatment is needed
- bilirubin should be repeated in 6–12 hours
- to start phototherapy
- to perform exchange transfusion.
Treatment will change according to the absolute level of bilirubin reached and the rate of rise on serial measurements (start if bilirubin rising at >0.5 mg/dL/h, 8.5 μmol/L/h). The evidence for treatment thresholds is very limited but national guidelines assist uniformity of practice (see American Academy of Pediatrics in US – Table 41.2; NICE guidelines in the UK). Different cut-off criteria are used for preterm infants, for whom the treatment threshold is lower (NICE guidelines include graphs for different gestational ages – see Appendix).
If an exchange transfusion is being considered, a low serum albumin may be an additional risk factor for kernicterus.
Other treatment to be considered:
- Ensure baby is well hydrated.
- Sepsis – requires investigation and treatment.
Phototherapy
Conventional phototherapy units use a blue–green light (wavelength 425–475 nm) above the baby, which converts unconjugated bilirubin to harmless isomers. If the bilirubin is rising rapidly or does not fall after 6 hours of treatment, then add in multiple units, ideally with one source underneath the infant. Phototherapy is most effective when there is an effective light source (LED lights), high irradiance (usually ≥30 μW/cm2/nm), the light is as close to the infant as possible (if LED lights are used, can be as close as 10 cm from the infant) with maximum skin exposure.
Disadvantages of phototherapy:
- Separates baby and parents.
- Eyes need to be covered.
- Bronze-baby syndrome if phototherapy given with elevated conjugated bilirubin.
- Unstable temperature while in open crib (cot) with majority of skin exposed.
- Increased insensible water loss (less with modern LED light sources).
- Slightly loose, more frequent stools.
Exchange transfusion
Baby’s blood is removed in aliquots (usually twice blood volume, ‘double volume exchange’ = 2 × 90 mL/kg) and replaced with transfused blood (see Chapter 78). Removes bilirubin and hemolytic antibodies and corrects anemia. Complications include thrombosis, embolus, volume overload or depletion, metabolic abnormalities, infection, coagulation abnormalities and death (<1%).
Intravenous immunoglobulin (IVIG)
Can be used in rhesus disease or ABO incompatibility when total bilirubin levels are rising despite continuous multiple phototherapy to try to prevent the need for exchange transfusion.
Discharge and follow-up
In view of the re-emergence of kernicterus in otherwise healthy infants, particularly at 35–37 weeks’ gestation, the American Academy of Pediatrics (2004) recommends predischarge measurement of bilirubin and/or assessment of clinical risk factors for the development of jaundice for all infants. The risk of developing significant hyperbilirubinemia in healthy term and near-term newborns can be determined by plotting the bilirubin level on an hour-specific chart (Fig. 41.4). It also recommends a follow-up assessment for jaundice depending on their length of stay in the nursery:
- discharge at <24 hours, follow-up by 72 hours of life
- discharge at 24–48 hours, follow-up by 96 hours of life
- discharge at 48–72 hours, follow-up by 120 hours of life.
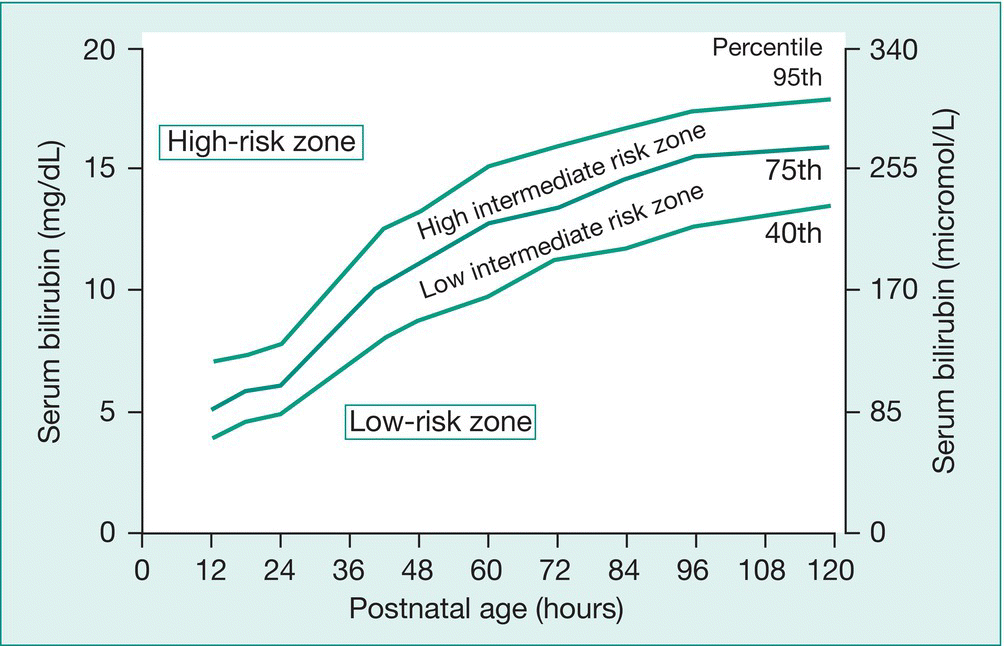
Fig. 41.4 Nomogram for determination of risk of development of severe hyperbilirubinemia for infants ≥35 weeks’ gestation and ≥2.5 kg birthweight.
(From Bhutani V.K. et al. Predictive ability of a predischarge hour-specific serum bilirubin for subsequent significant bilirubinemia in healthy term and near-term newborns. Pediatrics 1999; 103: 6–14.)
Earlier assessment may be needed if risk factors are present.
In the UK, the recommendation is further assessment by 48 hours of age if risk factors are present (gestational age <38 weeks, a previous sibling had neonatal jaundice requiring phototherapy, breast-fed, visible jaundice in the first 24 hours of life), otherwise by 72 hours of age.
Parents should also be given written and verbal information about jaundice.
Prolonged jaundice (>14 days)
Jaundice present at more than 2 weeks of age for term or 3 weeks for preterm infants can be considered prolonged jaundice and requires further assessment. First, it needs to be determined if the jaundice is unconjugated or conjugated.
Unconjugated jaundice Causes are:
- breast milk jaundice – due to increased enteric reabsoption; can last for several months
- hypothyroidism – usually identified on newborn blood spot screening
- gastrointestinal obstruction, e.g. pyloric stenosis
- sepsis
- liver enzyme disorders.
Conjugated jaundice (direct bilirubin >1.5 mg/dL, 25 μmol/L) The infant will pass pale, clay-colored stools (no stercobilinogen) and dark urine (from bilirubin).
Caused by:
- biliary atresia – uncommon, but important to identify as delay in surgery adversely affects outcomeneonatal hepatitis syndrome.
- Detailed investigation of infants with conjugated jaundice is required.