59
Neural tube defects and hydrocephalus
Neural tube defects
In the embryo, the flat neural plate folds to become the brain and spinal cord. Neural tube defects (NTDs) arise from a deficiency in this process:
- anencephaly – from failure of cranial development of most of the cranium and brain
- spina bifida – from failure of caudal development of the vertebral bodies
- midline defects – from failure of fusion, e.g. of the skull as an encephalocele.
Most are now diagnosed antenatally, by ultrasound or α-fetoprotein measurement in maternal serum.
Prevalence
NTDs result from a combination of environmental and genetic factors. The risk of having a second affected child is 3–5% and of a third 5–10%. The risk is increased 10–20-fold in mothers taking valproate. In the US, 7 per 10 000 pregnancies are affected, with a birth prevalence of 4 per 10 000 live births, and in the UK, 12 per 10 000 pregnancies, with a birth prevalence of 2 per 10 000 live births.
Maternal folic acid supplementation pre- and periconceptually and during early pregnancy has been shown to reduce prevalence. In the US, but not in the UK or most countries in Western Europe, cereal grain products are fortified with folic acid.
The prevalence of affected infants has decreased markedly over the last 30 years, with better maternal nutrition, folic acid supplements, food fortification in the US, prenatal screening with maternal ultrasound and raised maternal serum a-fetoprotein together with the option of termination of pregnancy.
Trial of open fetal surgery for myelomeningocele is described in Chapter 4.
Anencephaly
The condition is lethal; most are stillborn. There has been considerable debate about the ethics of the use of their organs for donor transplantation. However, the situation rarely arises because few anencephalic infants are now born as most are diagnosed antenatally and parents opt for termination of pregnancy.
Encephalocele
Herniation of sac containing CSF and may contain brain, through a midline skull defect. Most are occipital (Fig. 59.1). Developmental impairment is likely if brain tissue is in the sac or there are other cerebral malformations.
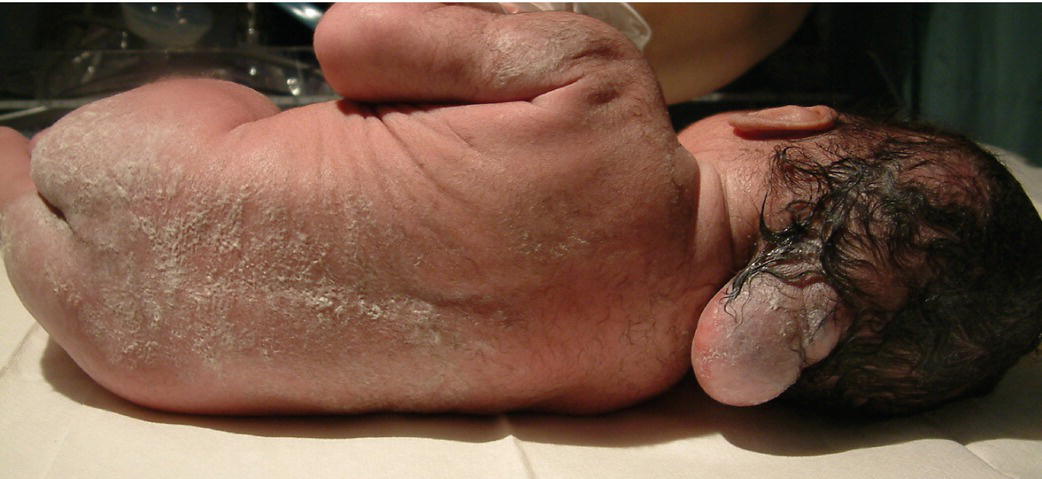
Fig. 59.1 Occipital encephalocele.
Spina bifida
There is a spectrum, of increasing severity:
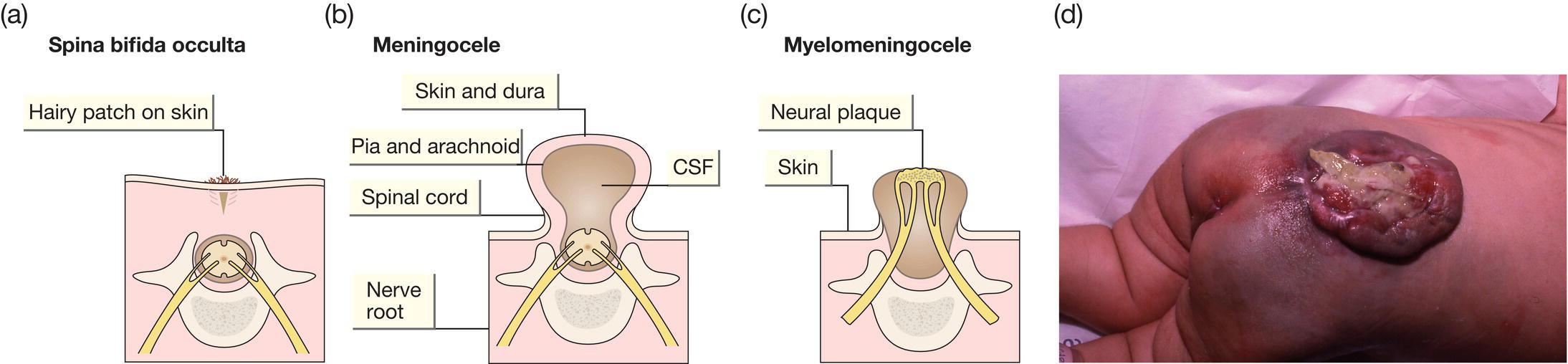
Fig. 59.2 (a) Spina bifida occulta. Defect in the vertebral arch with intact spinal cord. May be an incidental finding on X-rays – asymptomatic. More extensive lesions indicated by overlying patch of hair or nevus or other skin abnormality. (b) Meningocele. Bony defect with herniation of meninges but not the spinal cord. The lesion is covered with skin. (c) Myelomeningocele. Defect in the lumbar or thoracic spine with herniation of the meningeal sac and spinal cord tissue with leakage of CSF. (d) Photograph of myelomeningocele (myelo = cord; meninges = covering, cele = sac) showing exposed neural tissue and patulous, neuropathic anus.
Spina bifida occulta
Spina bifida occulta is often suggested by a skin lesion over the lower spine and is confirmed with further diagnostic imagining such as spinal ultrasound and/or MRI. Usually, there is no neurologic deficit at birth but tethering of the spinal cord may occur during childhood. Neurosurgical opinion should be obtained. May be an incidental finding on X-rays.
Meningocele
Prognosis following surgery is usually good.
Myelomeningocele
Wide range of complications (Fig. 59.3). Most lesions are detected antenatally and a management plan made before the baby is born.

Fig. 59.3 Complications associated with severe myelomeningocele. These depend on the extent and level of the lesion.
Management requires an extensive multidisciplinary team (pediatrics, orthopedics, neurosurgery, urology, child development) working with the family.
The back lesion is usually closed immediately after birth to minimize the risk of infection and surveillance performed for hydrocephalus.
Hydrocephalus
This is from an excessive volume of cerebrospinal fluid (CSF). It is usually from blockage of CSF flow or a defect in CSF reabsorption.
Causes
Congenital
- Aqueduct stenosis.
- Chiari malformation.
- Atresia of outflow foramina of fourth ventricle (Dandy–Walker syndrome).
- Congenital infection.
Acquired
- Post-intraventricular hemorrhage in preterm infants.
- Post-intracranial infection.
- Post-subdural/subarachnoid hemorrhage.
Clinical features
- Ventricular dilatation on imaging precedes symptoms or signs (Fig. 59.4).
- Increasing head circumference.
- Separation of sutures.
- Vomiting.
- Apnea, abnormal muscle tone, seizures, depressed consciousness.
- Dilatation of head veins.
- Sun-downing sign (setting-sun sign, eyes deviate downwards).
- Full then bulging fontanel.
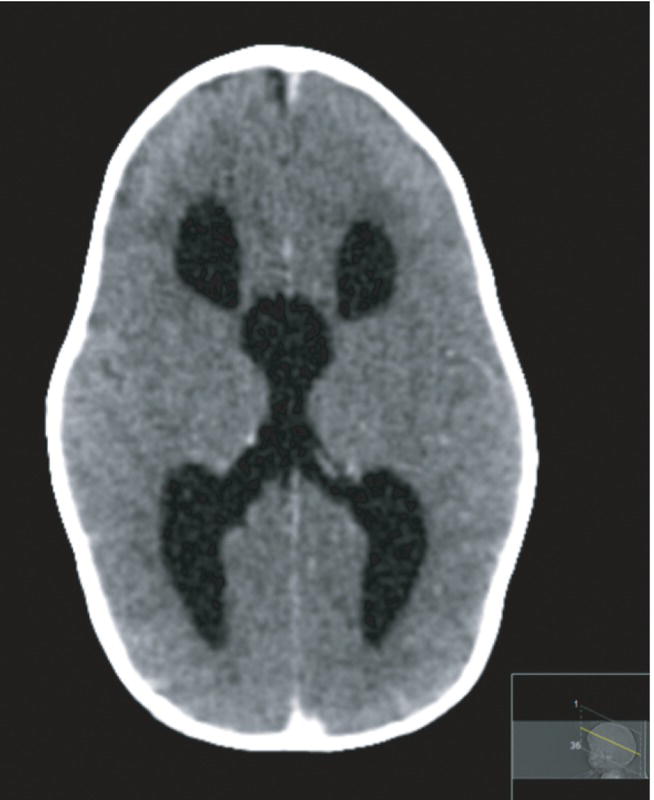
Fig. 59.4 CT scan axial view showing ventricular dilatation in a term infant with aqueductal stenoisis.
(Courtesy of Dr Sheila Berlin.)
Monitoring and treatment
In neonates, hydrocephalus is monitored by serial cranial ultrasound measurements of ventricular size and head circumference (See Chapter 79, Cranial ultrasound).
If severe and progressive or the infant becomes symptomatic, a ventriculoperitoneal shunt is inserted surgically. It carries a significant complication rate.
Hydrocephalus in preterm infants
Usually secondary to intraventricular hemorrhage causing fibrosis and impaired CSF reabsorption. Ventricular dilatation may need treatment if progressing rapidly or causing symptoms. Ventriculoperitoneal shunt insertion in small infants may be delayed because of the risk of skin breakdown or shunt blockage if the CSF protein is high. Instead, CSF may be removed by lumbar or ventricular puncture or from a neurosurgically inserted reservoir. No difference in long-term outcome between repeated lumbar/ventricular taps compared with removal of CSF only when symptomatic. Intraventricular fibrinolytics and acetazolamide, which reduce CSF production, have not been shown to be of benefit.