65
Quality improvement
Quality assurance
Clinical governance and quality improvement are frameworks for assuring the quality of clinical services and improving them (Fig. 65.1). They are key issues for all who provide care for newborn infants.
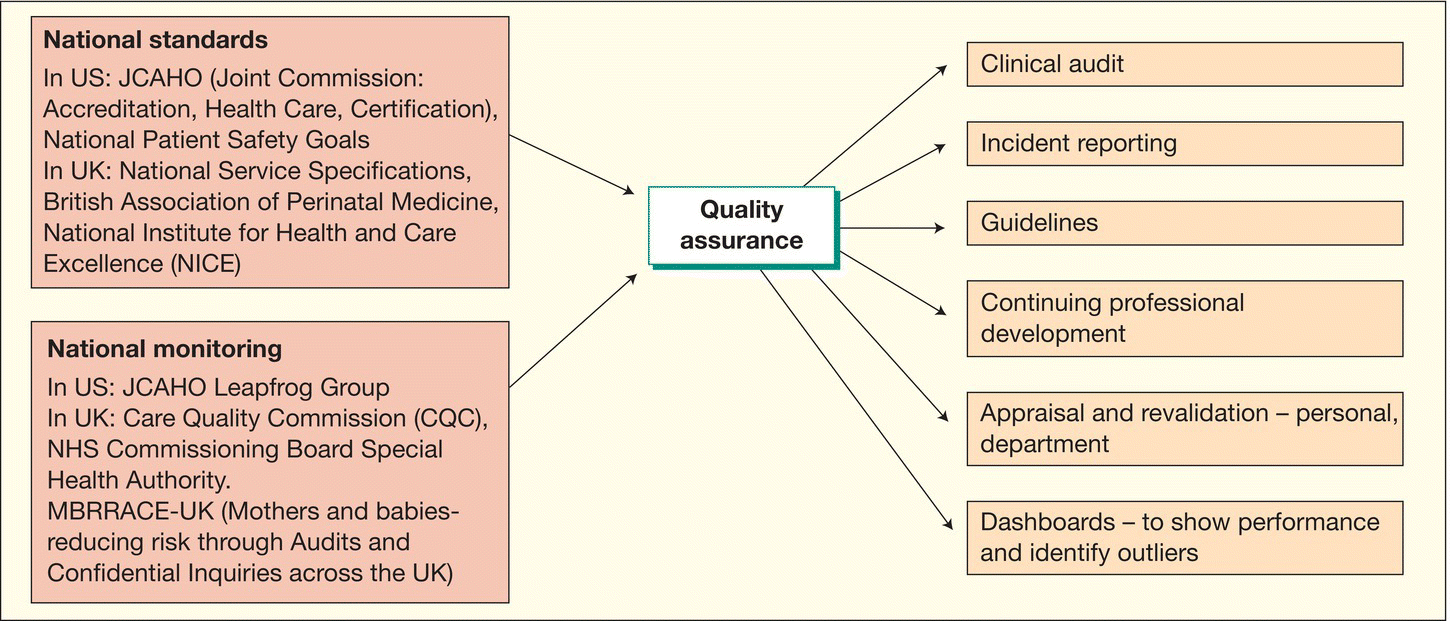
Fig. 65.1 Framework for quality assurance.
Clinical audit
Aims to assess patient care and outcomes through systematic comparison against explicit standards. Change is then implemented and re-audit follows. The audit cycle is shown in Fig. 65.2, and questions about audit are addressed in Table 65.1. More recently neonatal services have been using quality improvement (QI) methodology to implement improvements in care. This uses PDSA (plan-do-study-act) cycles with run-charts to show progress over time. It has the advantage of continuous measurement of outcomes rather than ‘snapshots’ of data at specific time points as measured by audit. PDSA is described in Fig. 65.3. Changes should be evidence based and action plans should be SMART (specific, measurable, achievable, realistic and timely).
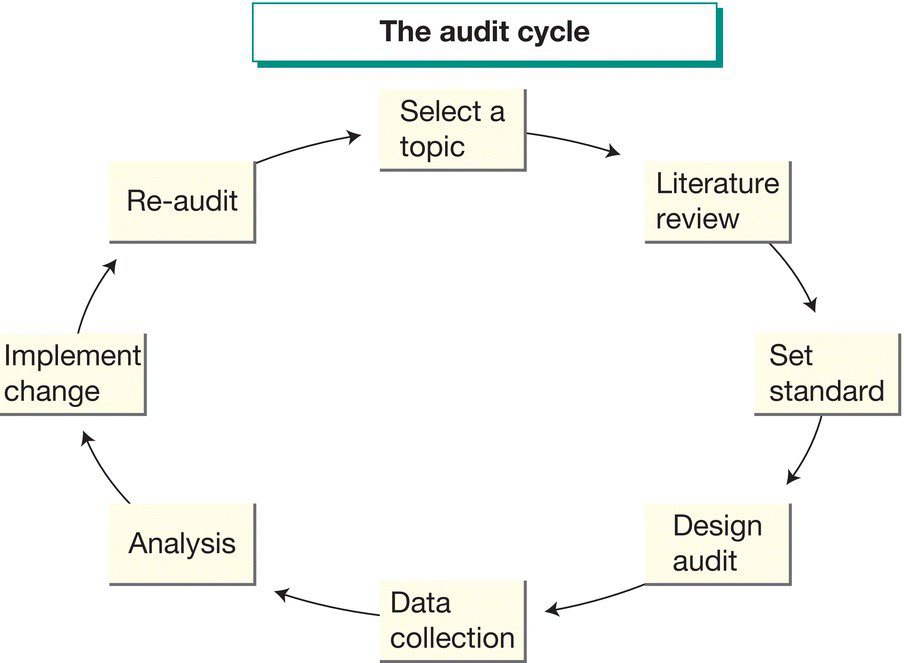
Fig. 65.2 The audit cycle.
Table 65.1 The clinical audit process.
| Who should be involved? | All health professionals (multi-disciplinary) |
| How are topics selected? | Observing current practice |
| Clinical incidents, complaints and claims, etc. | |
| Design? | Set or identify standards |
| Identify data sample | |
| Only collect relevant data | |
| Analysis and recommendations | Were standards met? |
| Feedback results | |
| Identify improvements | |
| Develop an action plan | |
| Re-audit to check improvement |
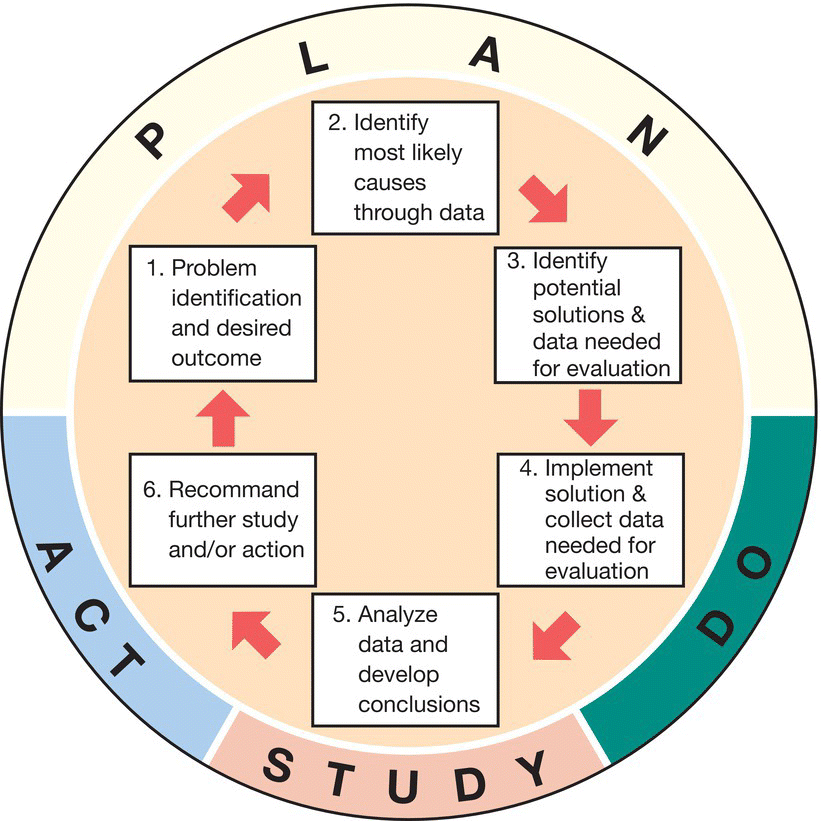
Fig. 65.3 The PDSA cycle to rapidly initiate change in practice and evaluate it.
Simulation
Simulation has become an important component of quality assurance in neonatal care. Clinical scenarios are used to train multidisciplinary teams in a safe and educational environment, sometimes using sophisticated models. Widely used in life support courses, e.g. neonatal resuscitation and emergencies, and increasingly for simulated emergencies in the neonatal unit, delivery room and lying-in (postnatal) wards. It can also be used to develop human factor skills in leadership, teamworking and communication. It also allows training in some practical or uncommon procedures before performing them on patients. Simulation can be used to re-create critical incidents identified through risk reporting systems and can also identify latent risks (accidents waiting to happen) that can be fed back into the risk management system of the unit.
Critical incident reporting (Table 65.2)
It is important that the whole neonatal team develops a culture of quality and safety. Reporting critical incidents, not only those that have caused harm but also those that could have caused harm are a key component of quality assurance. The most common and serious critical incidents in neonatal practice and ways to minimize their occurrence are considered in Chapter 66.
Table 65.2 Questions and answers about critical incidents.
| What are they? | Unexpected events that cause or could cause harm to the patient. Include near-misses |
| Who should report them? | Everyone |
| What should be reported? | The facts |
| Why report? | To identify causes |
| To develop a strategy to prevent recurrence | |
| To act as warning for complaints/litigation | |
| To provide information for external monitoring | |
| Who is to blame? | A no-blame culture should be developed – disciplinary action will not follow except where acts or omissions are malicious, criminal, or constitute professional misconduct |
| What level of investigation is required? | Depends on extent of harm to the patient and assessment of likelihood of recurrence by taking the whole circumstance of the event into account, not just the incident itself |
| If risk of harm or recurrence is high, perform root cause analysis | |
| What is root cause analysis? | A structured method used to analyze serious adverse events with the goal of identifying contributing factors |
| What if major harm has occurred or major damage to the organization? | Because of potential litigation, the hospital risk management group and senior managers should be informed. A more detailed, formal causal analysis (FMEA, failure mode and effect analysis) should be undertaken |