After the deadly outbreak of Spanish influenza, health authorities dreaded what would happen next. Would this monster virus continue to circulate, or would it revert to a milder form of influenza? Public health officials worldwide began to investigate these questions through research that one day would lead to the development of suitable vaccines.
The years that followed the 1918 outbreak saw sporadic, severe outbreaks in different parts of the world. In 1920 there were 11,000 influenza deaths in Chicago and New York City, with the latter reporting more influenza deaths in one day that year than on any single day in 1918. On the other side of the world, Australia had enforced the quarantining of ships and had succeeded in keeping out the severe wave until 1919, when it struck and caused societal disruption. However, overall, there were fewer deaths that year (2.3 deaths per 1000 people) than in New Zealand in 1918 (5.8/1000).10 Severe sporadic outbreaks continued through 1919, 1920 and 1921, but by 1922 influenza had declined in severity and returned to so-called ordinary or seasonal influenza.
Ordinary influenza today is similar to – if probably slightly milder than – the influenza of the first wave in 1918. It begins suddenly with headaches, chills, a non-productive cough, fever with temperatures of 38–40°C (100.4–106°F), muscle aches, general weakness and loss of appetite. The fever is usually gone after three days, but general weakness can last up to two weeks. There is a tendency to trivialise seasonal influenza, but in New Zealand it kills 400 people a year in a population of 4.7 million.11
The average direct medical costs of an annual epidemic and the wider economic burden in New Zealand are currently being measured but are estimated to run into the billions of dollars. In the US, on average, 35,000 people die per year in a population of 320 million (based on a 2007 analysis).12 The average direct medical cost for an annual epidemic is US$10.4 billion and the economic burden $87.1 billion. Thus seasonal influenza is not a trivial disease by any measure. People are well advised to be vaccinated annually with the vaccine formulation recommended by the World Health Organization (WHO). National recommendations vary regarding which age groups should be vaccinated.
In the decades after the 1918 Spanish influenza pandemic, the virus responsible – the H1N1 influenza virus – continued to evolve. It caused annual epidemics in humans in the winter months in temperate latitudes and year-round epidemics in the tropics. In 1957 another major change occurred. A different strain of the influenza virus (H2N2) emerged in Asia, causing the second pandemic of the twentieth century, which killed 1.5 million people. In 1968 came the H3N2 virus, which caused the Hong Kong pandemic. The return of the H1N1 virus caused pandemics in 1977 and 2009. A timeline of the epidemics and pandemics of influenza in the past century is shown in Figure 2.1.
There are three types of influenza virus, labelled A, B and C. A fourth type, D, has also been proposed. Type A is found in humans, lower animals and birds; type B is found mainly in humans but has been detected in seals too; type C is found mainly in human children and pigs; and type D is found in bovines. Type A influenza viruses are the ones that cause epidemics and pandemics in humans, including the Spanish influenza of 1918. There are 16 subtypes of influenza A found in aquatic birds and two subtypes recently discovered in bats.
One of the main features of influenza viruses, amply demonstrated during the 1918 pandemic, is that they are extremely variable. The 1918 virus changed from a mild to a deadly virus and then back to mild. What is the secret of this variability? Why does the virus succeed in causing epidemics and pandemics again and again?
As with all disease agents, there is a constant battle between the attacking virus and the host. As the influenza virus attaches to the cell lining of the nose, throat and lungs, the body responds by releasing an array of protective chemicals (cytokines), followed by antibodies that attach specifically to the invading virus so that scavenging macrophages (white blood cells with a ‘clean-up’ function) can remove it. (The virus molecules to which the antibodies attach are known as antigens.) In addition, the body makes killer cells designed to destroy the virus. During this clash, the body temperature rises, and the person begins experiencing the characteristic aches and pains of influenza, due in part to the rather toxic chemicals produced by the human body to kill the virus. Within a week, the body has usually won the battle, the person recovers, and the body keeps a immunological memory of the invader in its protective arsenal, ready to deploy more antibodies if the virus attacks again.
Despite the body’s ability to augment its protective arsenal in this way, the virus is sometimes able to overcome these defences because it can change its surface configuration so that the body no longer recognises it as familiar. There are two ways in which the virus can change. During their multiplication, influenza viruses continually make errors in assembling their genetic building blocks. There is no quality control in the process, so a mixture of viruses is produced, some like the parent virus and many with genetic changes that make it different from the parent. When this mixture of viruses infects a person with antibody memory, the variant influenza viruses circumvent the host’s immune response because they have antigens that the immune system does not recognise. This change in genetic material is called ‘drift’ or ‘genetic drift’, and it is how seasonal or ordinary influenza changes from year to year. As the human population becomes resistant to the prevailing virus, variants with errors survive and cause the next epidemic.

Figure 2.1 Influenza epidemics and pandemics of the past century. (A pandemic is an epidemic that spreads to humans all over the world.) Pandemics occurred in 1918 (H1N1 Spanish), in 1957 (H2N2 Asian), in 1968 (H3N2 Hong Kong), in 1977 (H1N1 Russian) and in 2009 (H1N1). After the emergence of each new pandemic strain, that virus causes epidemics until a new pandemic emerges.
The second phenomenon that yields viral variety is known as ‘reassortment’ (hybridisation), a process by which two influenza viruses mix their gene segments, a bit like mating. An influenza virus has eight separate RNA segments of genetic information (Figure 2.2). When two different influenza A viruses infect the same cell, it is possible to generate 256 genetically distinct offspring. This is the process that led to the emergence of most of the human influenza pandemics in the twentieth century.
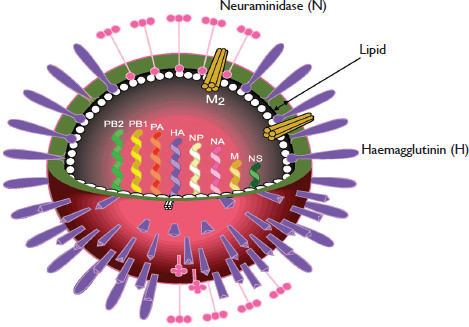
Figure 2.2 (opposite) The surface of an influenza particle is covered in three kinds of projections. The most frequent is the club-shaped haemagglutinin (H) spike (shown in purple), which attaches the virus to docking sites on cells of the human nose, throat and lungs. The second is the neuraminidase (N) spike (pink), an enzyme that serves as molecular scissors to free the virus from the cell surface and facilitate virus spread. The third spike (M2) is a tube-like structure (yellow). Each of these projections is embedded in a lipid (fatty) layer that the virus steals from the human cell as it buds off from that cell. Inside the lipid layer, a membrane (M) protein (white) surrounds the eight RNA segments that contain the virus genetic information.
WHAT IS A VIRUS?
A virus is the ultimate parasite – an obligate intracellular parasite, meaning that it is totally dependent on the living cells of hosts for its existence. It is a tiny piece of genetic information (RNA or DNA) wrapped in a protein overcoat. Viruses infect every class of living organism, including plants, animals and bacteria, often but not always causing disease. There are more viruses in the world than any other biological entity. In the computer age, the term is used to describe commands that replicate themselves and spread extensively, causing disruption (disease), as occurs with biological viruses.
Viruses are the smallest organisms known.
Influenza is caused by an RNA virus that has eight separate pieces of genetic information that permit it to hybridise with influenza viruses of the same type.
Influenza viruses are about 100 nanometres (nm) in diameter (a human hair is 80,000–100,000nm) and vary in shape from spherical to filamentous. The component parts of the virus are made by the infected human cell’s machinery under instructions from the virus’s genetic code. The components are then inserted into the lipid (fatty) membrane that surrounds the cell, turning that membrane into the outer shell of the virus. The end result is a virus particle with a shell sporting a layer of club-like projections or spikes. There are three kinds of spike, the most numerous of which is haemagglutinin (H). This attaches the virus to receptors (docking sites) on human cells. The second type of spike, neuraminidase (N), occurs in clumps. This enzyme acts like molecular scissors and clips the surface of a cell to free the virus, enabling it to spread. The third spike is the matrix 2 protein, a short, tube-like projection. Under the lipid layer, there is a matrix (M) layer that surrounds the eight RNA segments that make up the viral genetic code.
The naming of influenza viruses was standardised by the WHO in 1980. Each name includes the type of virus, the animal host from which the virus was isolated (by convention, humans are not specified), the country, the isolate number and the year. In parentheses are the haemagglutinin and neuraminidase subtypes. For example, A/Madrid/101/1918 (H1N1) would be an influenza A virus isolated from a human in Madrid, isolate number 101 from 1918, the H subtype and N subtype being H1N1. If the influenza virus had been isolated from a pig, the name would be A/swine/Madrid/101/1918 (H1N1).
In 1919–20, public health officials in New Zealand, Britain, the US and other countries undertook major reviews of the medical understanding of influenza. There was general agreement that if a vaccine was to be developed, research on the nature of the causative agent was urgently needed.
When Haemophilus influenzae bacteria were isolated from the throats of infected people during the 1918 pandemic, the consensus among scientists was that these bacteria were the cause of the disease, hence the name. This conclusion was supported by studies showing that vaccines prepared from these bacteria appeared to be effective. The timing of vaccine administration (during the pandemic’s waning phase) was bound to lend support to this notion. In fact, the vaccines probably did provide some protection against the bacterium and the secondary pneumonia it caused – a major cause of death during the pandemic. The bacteria were not, however, the primary cause of influenza.
The first clue that the causative agent was a virus came from an unexpected and completely unappreciated source. In 1901 two Italian scientists, Eugenio Centanni and Ezio Savonuzzi, showed that the highly lethal disease of chickens known as fowl plague was caused by a nonbacterial agent, one of the first pathogens to be classified as a virus.13 Fowl plague is a disease that begins in the nasal tract and lungs of chickens and spreads through the blood to every tissue in the body, including the brain. Up to 100 per cent of infected birds die, with haemorrhages in all organs. But the characteristics of fowl plague were so different from those of human influenza that no connection was suspected. It was not until 1955 that Werner Schäfer from Tubingen, Germany, showed a relationship between the fowl plague and human viruses.14
Meanwhile, John S. Koen, a veterinarian in the US Department of Agriculture at Fort Dodge, Iowa, reported in 1918 that a respiratory disease breaking out in pigs was remarkably similar to the influenza appearing in humans.15 In 1928, transmission studies with unfiltered mucus from infected pigs enabled Charles S. McBryde at the Bureau of Animal Industry to transmit influenza from pig to pig. However, he failed to transmit the disease using mucus passed through a bacteria-proof filter, which was a criterion for identifying a virus at the time. Because viruses are smaller than bacteria, this type of filtration provided the earliest way to distinguish between bacteria and viruses. A few years later Richard E. Shope of the Rockefeller Institute for Medical Research in New York repeated the filtration studies and successfully transmitted influenza between pigs, formally showing that the causative agent was a virus.16
At this time the Medical Research Council (MRC) of Britain was also conducting research on viruses. Since distemper in dogs was affecting the English sport of foxhunting, the magazine The Field gave the MRC generous funding to study the problem. Distemper in dogs begins with a high fever and cough, vomiting and diarrhoea. The dogs develop fits of paralysis and often die. Patrick Laidlaw, who headed the laboratory, had heard that ferrets caught distemper from dogs and in 1921 set up a strict isolation laboratory at Mill Hill Laboratories outside London to study the disease.17
Christopher Andrewes, a young biologist from the MRC, had just spent two years studying rheumatic fever at the Rockefeller Institute, where Shope had studied swine influenza, and had established a friendship with Shope. After Andrewes returned to the MRC, this rather loose professional network came to the fore during a sudden outbreak of seasonal influenza in London in 1933. Andrewes, Laidlaw and their colleague Wilson Smith collected throat samples from influenza patients and, using Shope’s methods, successfully infected ferrets (a good model for investigating influenza because they show the same symptoms of infection as humans). They also showed that the agent was filterable and therefore met the definition of a virus. They also fulfilled Koch’s postulates for identifying the causative agent of a disease, which require isolation of the agent in pure culture, evidence that the isolated agent causes disease when introduced into a healthy animal, and re-isolation of the same agent from the infected animal. Their work proved that a viral agent did cause the disease.
During the ferret transmission studies, a ferret had sneezed on Charles Stuart-Harris, a medical student in Smith’s laboratory, who went on to develop influenza symptoms. The agent was isolated from Stuart-Harris and transmitted back to ferrets, then re-isolated from the animals.18 Back at the Rockefeller Institute, Shope had repeated the ferret studies and noted that it was much easier to infect ferrets if they had first been anaesthetised allowing the virus to be inserted deep into the lungs to contact susceptible cells. This information provided the breakthrough to the team in London, who had moved on from ferrets and had been trying (without success) to infect laboratory mice. Thomas Francis at the Rockefeller Institute also succeeded in infecting mice with influenza under anaesthesia, and thereafter the mouse became the standard laboratory animal for influenza studies, because they are small and easy to breed.
The next big challenge for influenza research was finding a simple way to isolate and grow the viruses. Researchers at the MRC laboratories in London were having limited success in growing the virus in chicken eggs. The method involved getting a 10-day-old developing egg, drilling a hole in the shell and injecting it with a throat wash from an influenza-infected person. Frank MacFarlane Burnet from Australia contributed to this work during his two-year fellowship at the MRC. After his return to Melbourne he discovered that if the samples from the human influenza patients were injected into the liquid-filled amniotic cavity that surrounds the chicken embryo, the influenza virus multiplied quite well.19 The MRC team also found that on subsequent passaging (injecting the fluid from the first egg into a second egg, then into a third egg), the virus would multiply well when injected into the much larger allantoic cavity of the 10–12-day-old chicken embryo.
This readily available source of virus quickly led to the discovery that allantoic fluid containing the virus would agglutinate red blood cells from chickens or humans (cause them to stick together).20 The property of haemagglutination by influenza viruses could be quantified and so provided a simple method for measuring the amount of virus present. Importantly, the reaction was inhibited by serum from people who had recovered from an influenza infection. So, a simple serological assay, the haemagglutination inhibition test, allowed researchers to compare different isolates of influenza virus and determine a vaccine’s efficacy. George Hirst also noticed that haemagglutination of red blood cells by influenza viruses was not permanent and proposed that the virus possessed an enzyme that released it from the red blood cells.21 This astute observation led to the eventual identification of the neuraminidase enzyme on the surface of influenza viruses and to a second serological test for influenza.22
These developments soon led to the isolation of a completely different type of influenza virus, designated type B.23 This new strain was isolated at the Rockefeller Institute by Thomas Francis and sent to the team in London for verification. These two groups could show no relationships between the antigens of earlier strains and those of the new isolate, and they agreed to call the original ‘type A influenza virus’ and the new strain ‘type B influenza virus’.
Willingness to share information and virus strains has always been a feature of influenza research. The first network of influenza scientists in New York, London and Melbourne developed much of the early knowledge about the virus by sharing freely. With the inception of the WHO in 1947, influenza was recognised as a continuing global health problem, one that was complicated by the antigenic variations of the virus from year to year. Andrewes (later Sir Christopher) proposed to the WHO the need for a global network for influenza research and the designation of reference laboratories. Scientists in the existing informal international network agreed, and the WHO collaborating network was established in 1952, when 26 laboratories contributed their influenza isolates.
Andrewes’ own laboratories at Mill Hill were designated the World Influenza Centre. Other collaborating centres were established in Melbourne, Atlanta, Tokyo, Memphis and later in Beijing. National laboratories in any country that wished to participate in the network could send their influenza virus isolates to the designated collaborating laboratory in their region, which would characterise the influenza and send them the results. The collaborating laboratories (known as reference laboratories) prepared sera in ferrets to identify the circulating influenza virusesand provided standardised methods for isolating and identifying them. This information was shared between the centres, facilitating the updating of vaccines.24 Since 1973 the key staff from all collaborating centre laboratories have met to decide on the changes to make to human vaccines to cope with antigenic drift, and WHO has provided a formal recommendation on the strains to include in the vaccine.
The Global Influenza Surveillance Network (GISN) was founded in 1952 and expanded to the Global Influenza Surveillance and Response System (GISRS) in 2011. It currently consists of 152 institutions, including 143 national influenza centres in 113 countries. The influenza WHO network was the prototype for all the other networks that have been subsequently developed by the WHO.
The influenza network faced its first real challenge in 1957, when the second influenza pandemic of the twentieth century emerged in Yunnan Province in southern China. The causative virus was completely different from previous strains yet was still an influenza A virus.25 Fortunately, by 1957 it was known that the causative agent was a virus and that vaccines could be prepared in chicken eggs. The question at that time was whether human influenza pandemics emerged as a result of the great variability of the circulating human influenza virus or whether they were coming from animal sources such as pigs or chickens.

