In February 2013 a second bird flu emerged in people and poultry in Shanghai, caused by an influenza virus of the H7 subtype. This was an H7N9 influenza virus, and it caused very mild or unnoticeable disease in chickens but killed about 30 per cent of infected humans. The symptoms in humans were almost identical with those of the H5N1 bird flu: high fever and sore throat, progressing rapidly to pneumonia.75 While most people infected with H5N1 were otherwise healthy middle-aged women, the people who first contracted H7N9 influenza were older men who had other compromising diseases, such as heart disease, or health issues such as asthma.
The absence of disease or symptoms in poultry in the LBMs at the time meant that humans were again serving as the canaries in the coal mine. The response of Chinese health officials this time was dramatic. Outbreaks of disease in humans and poultry were immediately reported to the WHO, and all available information about the H7N9 influenza virus, including its full genetic code, was published. Chinese health authorities are to be complimented and thanked by the rest of the world for such openness and sharing. The outcome demonstrated the tremendous importance of the ‘one world, one health’ initiative and was light years ahead of the official response to the 2006 outbreak of severe acute respiratory syndrome (SARS) in China.
It is worth a little detour here from the outbreak of H7N9 to look at SARS, because the infrastructure developed at the University of Hong Kong for responding to H5N1 bird flu served perfectly for the detection of SARS in 2003. SARS is another respiratory disease, caused by a group of viruses related to the coronaviruses, which cause the common cold. SARS is characterized by chills, muscle aches, headaches and loss of appetite, and was initially thought to be caused by the H5N1 bird flu virus. The SARS coronavirus was spread from human to human by respiratory droplets, by faecal contamination and through urine. Mortality was age related, being less than 1 per cent in people under 25 years old and greater than 50 per cent in those aged 65 and older. Originally an animal virus, the virus spread to humans in Guangzhou Province and then to visitors from Hong Kong, who spread the virus to humans in Singapore and Canada. It spread rapidly through hotels and hospitals and showed all the signs of becoming a new pandemic.
Malik Peiris isolated the virus responsible for SARS and identified it as a coronavirus, raising the possibility of making vaccines in the future and developing hygiene strategies for its control.76 Meanwhile, Yi Guan established that the civet cat (Paradoxurus hermaphroditus) in southern China’s live animal markets was the intermediate host transmitting the virus to humans.77 Civet cats were sold in the markets as exotic wildlife for human consumption. They were removed from the markets, and the farms where the animals were bred were closed down. Later it was shown by Kwok Yung Yuen, also from Hong Kong University, that the ultimate source of the SARS virus was the tiny horseshoe bat (Rhinolophus species) that is native to Hong Kong. The bat colony was left undisturbed, and people now know the risks of visiting the bat’s lodging places.
The rapidity with which the SARS virus had acquired the ability to spread from human to human surprised researchers and was a lesson that influenza virologists must continue to heed. Fortunately an epidemiological study quickly established that hand washing, wearing face masks and practising good hygiene could prevent the spread of the virus. In total, some 8096 people were infected with SARS, and 724 died – 648 in China, 43 in Canada and 33 in Singapore. Once again, containing the outbreak had been hindered by the initial reluctance of health officials in China to share information on the new disease.
***
Now let’s turn back to the emergence of H7N9, the second bird flu virus, in Shanghai in 2013. Our knowledge of the role of LBMs in the 1997 H5N1 bird flu led us to suspect that LBMs were the source of the H7N9 virus. Local health officials recommended that LBMs in Shanghai be closed. When that action was taken, the effect was the same as it had been in Hong Kong 16 years earlier: the number of new human influenza cases rapidly fell to zero. It is unclear what happened to all the poultry from the Shanghai LBMs. There are rumours that at least a portion of the H7N9-infected chickens that showed no visible disease were trucked to markets in cities to the south. If this it true, it would explain why the virus spread quickly in cities where the LBMs were not closed.
Laboratory examinations of the virus showed a remarkable similarity to H5N1. Six of the eight components of H7N9 had come from the H9N2 influenza virus. The haemagglutinin surface spike came from a wild duck influenza virus and the neuraminidase from a different wild duck influenza virus (Figure 13.1).

Figure 13.1 The second bird flu virus, H7N9, is a triple reassortant (hybrid) virus. The haemagglutinin (H7) gene segment came from an influenza virus in Asian wild ducks, which spread to domestic ducks; the neuraminidase (N9) gene segments came from a different influenza virus in Asian wild ducks, which also spread to domestic ducks; and the other six segments came from H9N2 in domestic chickens. This new virus, H7N9, began to spread to humans, proving lethal in over 30 per cent of cases.
The H9N2 influenza virus was not new, but here it was the enabler, providing H7N9 the ability to spread from poultry to people and to cause severe and even lethal disease. A study of H9N2 viruses in China from 2010 to 2013 showed that they were widespread across most of China and were causing reduced egg production on chicken farms.78 Back in the early 1990s, poultry vaccines against H9N2 had been developed and administered. Those vaccines were effective at reducing the drop in egg production but also caused the virus to change. As the H9N2 virus changed, new vaccines were made. However, the H9N2 virus eventually reassorted with an H7N9 influenza virus to produce the bird flu virus that was transmitted to humans in 2013.79
In the first wave of H7N9 infection in the Shanghai region, 135 people were infected, and 45 died. Studies of the H7N9 influenza virus in the laboratory showed that the virus was picking up features that might allow it to spread from human to human. To determine which of the bird flu viruses (H5N1 or H7N9) had the greatest potential to spread human to human, we did risk-assessment studies in ferrets (the best model we have for testing human transmission risk). Two ferrets were infected (via the nose) with either H5N1 or H7N9, and each was put into a cage containing four healthy ferrets to test for transmission through direct contact. In an adjacent cage, separated by 20 centimetres, were four additional healthy ferrets to test whether the virus was spread by aerosol.
The H5N1 virus spread to all of the ferrets in direct contact with the two infected animals, but not to the ferrets in the adjacent cage. In contrast, the H7N9 virus spread to both the direct-contact ferrets and two of the four ferrets in the adjacent cage, indicating aerosol spread. So the H7N9 influenza virus has greater potential to spread through the air.
After the number of human cases of H7N9 fell to zero, LBMs in Shanghai were reopened, and there were occasional reports of additional human infection. Meanwhile the virus spread to the south of China, and the first human infections were detected in Guangzhou in January 2014. The spread was inevitable, given that affected poultry showed no signs of disease. There was no evidence that migratory birds were involved in the virus’s spread, but small domesticated birds like canaries and budgerigars, and small birds such as sparrows were infected experimentally in the high containment laboratory, and they may have spread the virus locally. Since elderly men were the age group initially affected, we wondered if the Chinese tradition of walking and talking with pet birds might have contributed.80

Figure 13.2 A timeline showing the numbers of human cases of H7N9 avian influenza in China since 2013. The numbers peak each year in the winter months. The majority of H7N9 human infections occur through direct contact between humans and poultry, usually in live bird markets. When the LBMs are closed the number of human cases declines dramatically. Over this time there have been a total of 1623 human cases, with 620 deaths. To date the virus has not acquired the ability to spread human to human, but the large increase in the number of cases in 2016–17 is of great concern. Courtesy of World Health Organization
To date, the H7N9 virus has not spread to countries adjacent to China, but humans infected in China have taken the virus to Taiwan. Still, this virus has not acquired the ability to spread human to human – yet! Each winter since 2014, the H7N9 influenza virus has spread from LBMs and caused severe disease and deaths in humans in China. The pattern of this disease has changed, and it infects a wider age range and also otherwise healthy individuals (see Figure 13.2).
***
In early February 2017, when I was in Hong Kong writing about the H7N9 outbreak, there was a larger than usual peak of disease caused by H7N9 influenza virus in humans across China, and the virus underwent a dramatic change. From February 2013 until late 2016, the H7N9 virus had caused imperceptible disease in poultry. Then it became a killer of chickens, like the lethal H5N1 virus. Scientists in China and elsewhere had been expecting this type of change – from benign to killer strain in chickens – because it is one of the known characteristics of the H5 and H7 subtypes of influenza viruses.
We know the exact changes in the H7 spike necessary for it to become a chicken killer and had been watching for this to happen. At the time of writing in 2017, the H7N9 virus was confined to chickens and had not spread to other hosts. Stamping it out, while a huge task, would be merited.
While the H7N9 bird flu virus has flared up every winter in China, its counterpart H5 has also been on the move. A group of highly lethal H5N1 viruses now seems to be permanently entrenched in poultry in many countries and is endemic in China, Vietnam, Indonesia, the Indian subcontinent and Egypt. Back in 2015 a major outbreak in Egypt caused 136 human infections and 39 deaths.
And still this group of viruses continues to change, and many hybrids have emerged. One of these has the ability to spread easily in wild ducks, and in Korea in 2014 it picked up a new neuraminidase spike, becoming an H5N8 influenza virus. This virus literally took wing, and in January–March of 2014 it was detected in wild aquatic birds in Japan. By April–May it had spread to wild aquatic birds in Siberia and Alaska, and by September–October the virus was evident in both wild and domestic poultry in Europe and North America.
This was the first time the dreaded Asian H5 virus had spread to the Americas: the earlier H5N1 had not succeeded in doing so. H5N8 was first detected in a captive-reared gyrfalcon in Washington State. The virus was identical with the duck H5N8 influenza virus from South Korea and had probably been spread by migratory waterfowl. Immediately after arriving in the Americas, the virus reassorted with influenza viruses already present in wild waterfowl to produce two offspring: an H5N2 and an H5N1 influenza virus. Suddenly there were three deadly H5 influenza viruses in the wild birds in the Washington State area – H5N8, H5N1 and H5N2. The autumn (October–November) southern migration of wild ducks from Alaska and Canada then carried these viruses south, down the Pacific Flyway, and spread all three to commercial poultry flocks in Washington, Idaho, Oregon, and California, resulting in up to 100 per cent mortality in chickens and turkeys (Figure 13.3).
The most devastating and highly contagious virus was the H5N2 strain. In April–May 2015, ducks migrating from Central America to Canada carried the deadly H5N2 virus to the upper Mississippi Valley, an area bristling with poultry farms. Despite warnings about the need for high levels of biosecurity, H5N2 and H5N8 successfully infected over 220 poultry farms. The good news, if there was any, was that no human infections were detected. This may be due to the absence of LBMs in the Midwestern United States. The other possible reason is that H5N8 may have lost some of its ability to spread to humans (obtained from H9N2 viruses) when its genes reassorted with those of the many influenza viruses present in the Americas.
The strategy adopted by agricultural authorities in the US was culling, quarantine, and compensation for the farmers. Over 42 million chickens were culled – about 10 per cent of the total number of chickens in the country – and 7.5 million turkeys – about 3 per cent of the national flock. While this was going on, the H5 influenza viruses were detected in 85 wild birds.
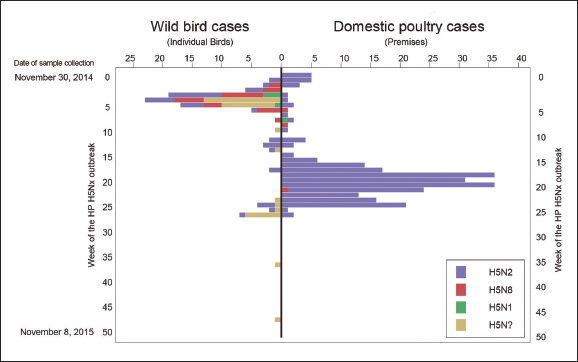
Figure 13.3 Outbreaks of H5Nx influenza in wild and domestic birds in North America. The deadly H5N8 virus on arrival in North America from Korea reassorted with influenza virus in wildfowl and developed H5N1 and H5N2 hybrid viruses, which spread among migratory waterfowl and to domestic poultry farms. The most deadly was H5N2 – more than 42 million chickens and 7.5 million turkeys were culled to contain its spread and stamp it out.
By the summer (July–August) of 2015 the numbers of new outbreaks of H5 influenza viruses in poultry farms had fallen to zero, and agricultural authorities were holding their breath while waiting for the migratory ducks from Canada. Would they bring the H5 influenza viruses with them again? Poultry farmers had been begging the Department of Agriculture (USDA) to let them protect their flocks with the poultry vaccine. The USDA had indeed prepared millions of doses of H5 vaccine, but they did not permit the poultry farms to use them (for fear of the viruses becoming endemic – see Chapter 11). The wild ducks arrived on schedule on their way south, but they did not bring the H5 influenza viruses with them. There have been no outbreaks of H5 influenza in poultry farms in North America since June 2016. Footprints of the H5 viruses (gene sequences like that for the H spike on the H5N2 virus) have been found twice in wild birds in North America, but no live virus has been found.
The apparent disappearance of the lethal H5 influenza viruses from wild aquatic birds is a mystery, and proposed explanations have caused controversy. My research group has been surveying influenza in wild ducks in the US and Canada for over 40 years, and in Alberta, Canada, no lethal H5 or H7 influenza viruses have ever been detected.81 We believe that wild ducks have an as-yet unknown mechanism to keep the killer H5 and H7 influenza viruses out of the wild duck breeding areas. Yet benign versions of H5 and H7, as well as almost all influenza viruses found anywhere in the world, are regularly found in apparently healthy young ducks.
One possible explanation is that exposure to the vast array of influenza viruses, including non-lethal H5 and H7 viruses, has somehow provided the ducks with herd immunity. Other mechanisms may also be involved, such as the gene in ducks that provides them with inbuilt immunity to influenza. Unfortunately, chickens lost this gene during their evolution from the jungle fowl. However, some scientists disagree with these suggested explanations and believe the lethal H5 virus is still lurking in wild waterfowl. Time will tell. Clearly further scientific research is needed to explain why lethal H5 and H7 influenza viruses are not pathogenic in wild aquatic birds.
Meanwhile, the lethal viruses of the H5 type that persist in Asia have been actively reassorting with influenza viruses in the region to produce an H5N6 influenza virus that caused outbreaks of lethal disease in poultry in China, Myanmar, Taiwan and Vietnam in 2017. These endless changes in the H5 and H7 influenza viruses and their tendency to hybridise and acquire components from other viruses have given rise to grave concerns that eventually they will acquire the ability to spread from human to human. Until very recently, some researchers have believed that since bird flu has been present in the world for 20 years without spreading from human to human, it simply cannot and will not do so. This complacency was shattered when two groups of scientists made an H5N1 bird flu virus that could spread between cages of ferrets and cause severe disease (see Chapter 16).
With all of the active evolution, the numerous subtypes of H5 and H7 and the persistence of these ‘hot’ influenza viruses in at least four regions of Eurasia, the influenza research community know that it is essential to be prepared and that we urgently need better strategies to deal with the inevitable pandemic in humans.


