9
THE SAD DEMISE OF A PROMISING CANDIDATE
Nowadays, we picture DNA as a massively long molecule that spells out all the billions of characters of the human genome. Knowing the origins of that understanding, we might also envisage the double helix as the intertwining of strands of research representing ‘DNA chemistry’, ‘DNA structure’ and ‘genes’. At the start of the 1920s, however, there was no hint of any such unity. Each of the research strands appeared to be running in isolation through a conceptual vacuum that excluded all other lines of enquiry. And any attempt to weave them all together into a coherent whole would have been dismissed as an act of folly rather than an inspired leap of lateral thinking.
After the Great War, the thread marked ‘genes’ steadily gained substance. Even William Bateson, who had initially seen ‘formidable difficulties’ in accepting that chromosomes were the receptacles of heredity, was shamelessly seduced by the idea of genes on a string. In December 1921, Bateson went to Toronto to do penance before the American Association for the Advancement of Science. In his keynote address, he praised ‘the marvels of cytology, which until recently I had seen only through a glass darkly’, and added, ‘I come at this Christmas season to lay my respectful homage before the stars that have arisen in the West.’ The brightest of those stars was Thomas Hunt Morgan, the ‘thickhead’ whom Bateson had so despised a decade earlier and who had become a world leader in ‘genetics’, the now well-established science which owed its name to Bateson.
A few months later, Morgan was invited to London by the Royal Society to give the 1922 Croonian Lecture. The Fly Room production line had continued to churn out new mutations, and Morgan was able to report the sequences of over 2,000 ‘factors’ along the four chromosomes of Drosophila (Figure 9.1). His presentation was so brilliant, and the evidence so solid, that nobody now would have thought of attacking his claim that ‘genes are material particles actually lying in and forming part of the chromosome’.
Meanwhile, research into the chemistry and structure of the nucleic acids had not progressed so smoothly. These strands of enquiry had been going nowhere for some time and were about to be cut short – by the two men who knew more about these compounds than anybody else.

Figure 9.1 Mutations affecting the eyes and wings of the fruit fly, Drosophila, described by Thomas Hunt Morgan and his team in 1922.
Zum Gedächtnis
After the war ended for everyone else, hostilities grumbled on for Phoebus Levene. Exchanges of fire continued with the irascible Walter Jones at Johns Hopkins and, across the Rockefeller campus, with Simon Flexner. Jones still insisted that the unidentified sugar in thymus nuclei acid was a hexose, and managed to irritate Levene at every opportunity; naturally, his name remained taboo in Levene’s lab.
Flexner had failed to rein in Levene’s overspending. His irate ‘You are embarrassing the Executive Committee . . . The Bursar will not pay any bills in excess of the budget’ fell on deaf ears; so did the plaintive ‘a single item threatens to spoil our otherwise good relationship: budget’. The pair also crossed swords over a high-profile mugging at Grand Central Station. In July 1923, Ivan Pavlov toured America en route to the Pasteur centenary celebration in Paris and the International Physiological Congress in Edinburgh. Flushed with his conquest of New York, Pavlov had just settled himself on the train to Yale when two ruffians grabbed the seventy-four-year-old and relieved him of his wallet and passport. The incident caused embarrassment for Pavlov’s American hosts and the dramatic headline in the New York Times, ‘RUSSIAN SCIENTIST BARRED FROM BRITAIN’. A friendly Russian émigré saved the day by spiriting up money and a visa, just in time for Pavlov to sail to England. The great man’s saviour was his former student, Phoebus Levene, and the cash came from the coffers of the Rockefeller Institute. True to form, Flexner reprimanded Levene, this time for breaking the Institute’s rule which banned employees from involvement in any ‘political’ activity.
It was thanks to Pavlov that, in 1929 and after twenty years of trying, Levene finally identified the elusive sugar in thymus nucleic acid. A recent report that the dog’s digestive juices broke down nucleic acids prompted Levene to perform a surgical operation which he had watched during a visit to Pavlov’s laboratory in St Petersburg. The procedure allowed him to inject thymus nucleic acid into the small intestine of a living dog and suck out the contents at intervals. The dog was untroubled throughout;
Levene had brought up a loop of its intestine to the skin, and opened a tiny window in the wall.* Conditions in canem were gentler than those in the test tube, and the delicate sugar duly appeared in the intestinal juice.
The mystery ingredient was a previously unknown pentose sugar, and a variation on an existing theme. It was like D-ribose, the pentose in yeast nucleic acid, but with an H (hydrogen atom) instead of an OH (hydroxyl group). As it was effectively ribose minus an oxygen atom, Levene called it ‘desoxyribose’, later shortened to ‘deoxyribose’ (Figure 9.2).
Levene had taken a big step forward by finding the last component of desoxyribonucleic acid, but he had also taken at least two steps back – and had dragged everyone who mattered with him. His ‘tetranucleotide hypothesis’, which postulated that the nucleic acid molecule only contained one of each of the four nucleotides, had somehow become fact. Various structures had been proposed, from Levene’s linear chain (Figure 7.2) to a closed ‘cyclic’ shape in which the nucleotides were linked like four people holding hands. These were no more than doodles on thin air, but they looked believable and carried the stamp of the world’s greatest authority on nucleic acids.
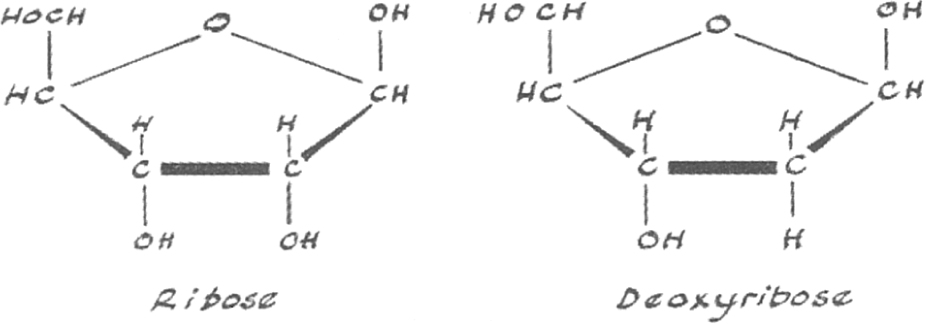
Figure 9.2 Ribose and deoxyribose, the pentose sugars in RNA and DNA. Spot the difference.
Any evidence that challenged Levene’s tetranucleotide was swiftly trampled underfoot. When someone proved that the molecular weight of the nucleic acids was far too high, Levene first tried to discredit the research and then conceded that a nucleic acid molecule could contain more than four nucleotides – as long as it was made up of identical tetranucleotide units, joined together to form a chain. The tetranucleotide hypothesis drove the entire field of nucleic acid chemistry into a cul-de-sac. It spawned the belief that the nucleic acids were much smaller than typical proteins, and had a boring and inflexible structure which ruled out any important biological function.
In 1929, Levene rounded off twenty-five years of research by writing a book. Nucleic Acids was comprehensive and bang up to date, but the preface might have left the reader wondering why he had bothered:
The chemistry of nucleic acids can be summed up very briefly. Indeed, a few graphic formulas which need not even fill a single printed page might suffice to express the entire store of our present-day knowledge on the subject.
In Heidelberg, Albrecht Kossel had already written the definitive book about his own greatest obsession. This had kept him busy during the three years after he retired in the summer of 1924. He finished the manuscript just in time to be knocked flat by a nasty attack of angina, but not quickly enough to hold his labour of love in his hands. The book was published in early autumn 1927, a few weeks after Gertrude and Walter Kossel had placed a brief notice in the newspapers. Headed ‘Zum Gedächtnis’ (In Memoriam), it announced the death of their beloved father on 5 July.
The late editor of Hoppe-Seylers Zeitschrift für Physiologische Chemie was remembered with respect and affection in the obituary which the journal carried in March 1928. The fruits of an outstandingly rich life in science were laid out: Kossel’s discoveries, papers, Nobel and other prizes – and the book by which he wished to be remembered. This was devoted to the molecules which had excited him the most during his forty years in biochemistry and was entitled The Protamines and Histones. Nucleic acids were mentioned only in passing as minor players that happened to share the stage with those fascinating proteins, which were ‘biologically the most important of all the substances in the nucleus’.
‘Zum Gedächtnis’ would also have made a good title for the single page on which Phoebus Levene could have summarised ‘the entire store of our present-day knowledge’ about the nucleic acids. They had been written off as unworthy of further interest by the two greatest experts in the field – just in time to celebrate the sixtieth birthday of nuclein. And inevitably, this negative verdict played directly into the emerging debate about the chemical identity of the ‘hereditable substance’ which transmitted the characteristics of a living organism to its descendants.
Sole contender
Genes had come far since their christening in 1909, but two great mysteries remained completely unsolved as the Roaring Twenties got under way. How did genes work? And as a first step towards answering that question, what were genes made of?
Thinking had moved on from idioplasm, gemmules and other will-o’-the-wisp notions of twenty years earlier. Niels Bohr, the great Danish quantum physicist, had strayed into the alien territory of biology after winning his Nobel Prize in 1922. Bohr argued that life was not infused into an organism by some mysterious ‘vital’ force but, like everything else in the universe, must be grounded in atoms and molecules that behaved according to the laws of physics and chemistry. The same diktat must apply to genetics, even though it was difficult to see how the hard facts of quantum physics could be translated into the inheritance of features as unfathomable as eye colour, height or intelligence.
This meant that genes had to consist of a chemical substance or substances of some sort – hence Herman Muller’s prediction in 1922 that ‘we may be able to grind up genes in a mortar and cook them in a beaker’. There were just two candidates for the ‘genetic material’, namely the only substances so far found in the nucleus: proteins (Kossel’s beloved protamines and histones) and nucleic acids. And there was only one serious contender, because proteins looked the part and nucleic acids did not.
Only proteins appeared to have enough structural diversity to carry the information of heredity. Their Bausteine – by now, nearly twenty different amino acids – could be joined end to end ‘like carriages on a train’ in any order and to any length, thus creating billions of possible structures. Just as the twenty-six letters of the alphabet could be assembled to describe ‘an infinitely large number of thoughts’, so different sequences of amino acids in specific proteins could spell out all the instructions for life. Proteins came in ‘an inexhaustible variety’ of shapes and sizes, and a quick glance around the human body strengthened the impression that proteins could do anything. Collagen held skin and bones together; myosin made muscles contract; haemoglobin carried oxygen to every cell; digestive enzymes such as pepsin and amylase made light work of breakfast; and insulin could quell the metabolic anarchy of diabetes and rescue diabetic children from death row. If proteins did all those things, then surely genes must be made of long-lived ‘hereditary proteins’ which were handed from one generation to the next.
By contrast, nucleic acids lacked that crucial versatility. Levene’s tetranucleotide hypothesis had struck the fatal blow to their candidacy. Even if tetranucleotides were strung into a long chain, the units were all identical and therefore could not carry much useful information. Moreover, nucleic acids appeared ‘remarkably uniform’ in composition, even between vastly different species of animals and plants. This seemed to rule out any controlling role for the nucleic acids. How could the same molecule tell a plant to make chlorophyll, and a mammal to fill its red blood cells with haemoglobin?
The verdict was obvious. Proteins were ‘of the first importance’ in transmitting what Kossel eloquently called ‘the peculiarity of species’ to the next generation, while nucleic acids simply ‘cannot be the hereditable substance’. To shut off any further pointless discussion, Phoebus Levene was characteristically blunt: ‘Nucleic acids carry no individuality, no specificity . . . It may be just to accept the conclusion of the biologist that they do not determine species specificity, nor are they carriers of the Mendelian characters.’
Disconnect
The year of Albrecht Kossel’s death, 1928, also signalled the demise of the Fly Room. Thomas Hunt Morgan had been approached with an unrefusable offer, to set up a new Institute of Biology at the California Institute of Technology (Caltech) in Pasadena. He was sixty-two years old and just two years off retirement at Columbia. Caltech promised generous funding, an environment that had already nurtured two Nobel prizewinners, and a new lease of life. After seventeen years hemmed in by all those milk-bottles, Morgan decided that it was time to go. He took with him his two longest-serving Drosophila veterans, Alfred Sturtevant and Calvin Bridges. Sturtevant, who dreamed up the original chromosomal map, had continued mining that highly profitable seam. Bridges had shown that the mutations in Drosophila were clustered in four groups of different sizes, which corresponded to the fly’s four chromosomes. This was powerful supporting evidence that the chromosomes carried all the information of heredity, and that the genes were arranged along each chromosome in a fixed order.
Morgan and his group prospered at Caltech. He recruited geneticists at the peak of their powers and welcomed in a stream of distinguished visitors, including the novelist H.G. Wells, a visionary in a different dimension, who had written about the dangers of science running free. Unsurprisingly, it was only a matter of time before Morgan’s work came to the attention of the Nobel Prize Committee in Stockholm. By then (1933), he and his team had pinpointed, down to the level of a tiny band on a particular chromosome, the physical locations of almost 3,000 genes.
However, another great mystery in genetics was no closer to being solved. Back at the turn of the century, the gene had been an impenetrable black box; now, almost three decades later, it still was. The nature of the gene was still completely unknown. Towards the end of 1928, the eminent biologist Edmund Wilson described the gene as ‘a complex of specific, autocatalytic, colloidal particles in the germ-cell’ that, ‘in accordance with the recognised principles of physics, can engineer the construction of a vertebrate organism’ – which was a verbose way of saying that he had no idea what it really was.
One thing, however, was blindingly obvious. Faced with the damning verdicts of Phoebus Levene and Albrecht Kossel, the two greatest names from the heroic age of nucleic acid chemistry, only a brave man or a fool would have dared to suggest that the nucleic acids had anything to do with genes or the transmission of inherited characteristics.