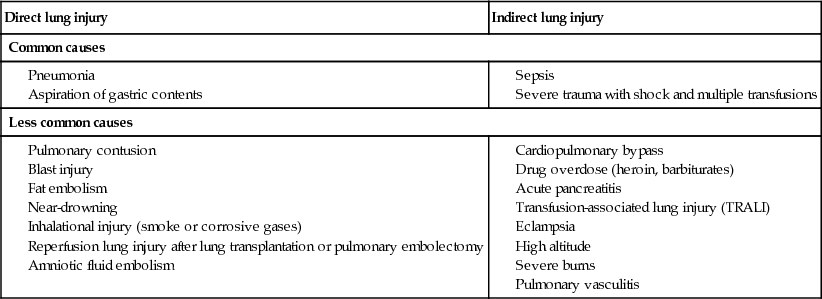Myocardial contractility and inotropic agents
Myocardial contractility can be impaired by many factors, such as hypoxaemia and hypocalcaemia, as well as by some drugs (e.g. beta-blockers, antiarrhythmics and sedatives).
Severe metabolic acidosis conventionally is said to depress myocardial contractility and limit the response to vasopressor agents. Attempted correction of acidosis with intravenous sodium bicarbonate, however, generates additional carbon dioxide, which diffuses across cell membranes, producing or exacerbating intracellular acidosis. Other disadvantages of bicarbonate therapy include sodium overload and a left shift of the oxyhaemoglobin dissociation curve. Ionized calcium levels may be reduced and, combined with the fall in intracellular pH, this may impair myocardial performance. Treatment of lactic acidosis should therefore concentrate on correcting the cause. Bicarbonate should only be administered to correct extreme persistent metabolic acidosis (see pp. 179–180).
If the signs of shock persist despite adequate volume replacement, and perfusion of vital organs is jeopardized, inotropic/vasopressor agents should be administered to improve cardiac output and blood pressure. Vasopressor therapy may also be required to maintain perfusion in those with life-threatening hypotension, even when volume replacement is incomplete. All inotropes increase myocardial oxygen consumption, particularly if a tachycardia develops, and this can lead to an imbalance between myocardial oxygen supply and demand, with the development or extension of ischaemic areas. Inotropes should therefore be used with especial caution, particularly in cardiogenic shock following myocardial infarction and in known ischaemic heart disease.
Many of the most seriously ill patients become increasingly resistant to the effects of pressor agents, an observation attributed to ‘downregulation’ of adrenergic receptors and nitric oxide-induced ‘vasoplegia’ (see p. 1159).
All inotropic agents should be administered via a large central vein and their effects continually monitored (Box 25.12).
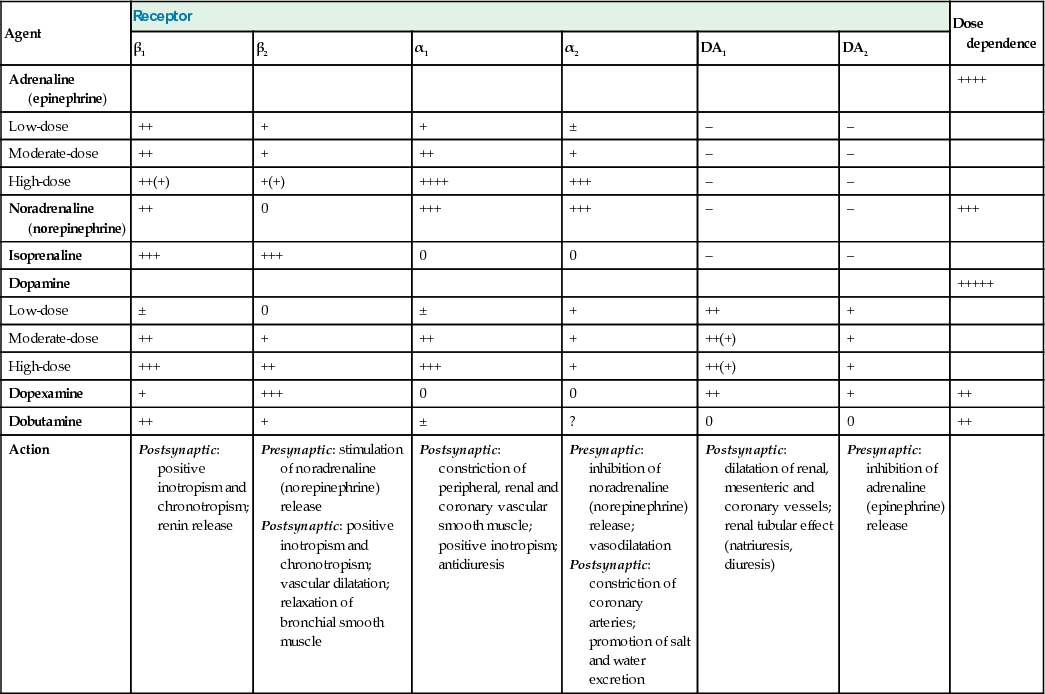
Adrenaline (epinephrine)
Adrenaline stimulates both α- and β-adrenergic receptors but β effects predominate at low doses. Heart rate and cardiac index increase, while peripheral resistance is reduced. If there is an associated increase in perfusion pressure, urine output may improve. Adrenaline at higher doses can cause excessive (α-mediated) vasoconstriction with reductions in splanchnic flow, and cardiac output may fall. Prolonged high-dose administration can cause peripheral gangrene and lactic acidosis. The minimum effective dose of adrenaline should therefore be used for as short a time as possible.
Noradrenaline (norepinephrine)
This is predominantly an α-adrenergic agonist. It is particularly useful in patients with hypotension and a low systemic vascular resistance, such as is seen in septic shock. There is a risk of producing excessive vasoconstriction with impaired organ perfusion and increased afterload, particularly if noradrenaline is administered when fluid resuscitation is inadequate and the circulating volume is reduced.
Dopamine
The haemodynamic effects of dopamine are dose-dependent. At low doses, dopamine is a positive inotrope with vasodilator actions on the renal and splanchnic circulation. Higher doses may be complicated by tachycardia, arrhythmias and vasoconstriction. Dopamine may be used as an alternative to noradrenaline in patients with a low risk of tachyarrhythmia and absolute or relative bradycardia.
Dopexamine
Dopexamine is an analogue of dopamine that activates β2 receptors, as well as DA1 and DA2 receptors. Dopexamine is a weak positive inotrope but a powerful splanchnic vasodilator, reducing afterload and improving blood flow to vital organs, including the kidneys. It has been used as an adjunct to the perioperative management of high-risk surgical patients (see below).
Dobutamine
Dobutamine is closely related to dopamine and has predominantly β1 activity. Dobutamine has no specific effect on the renal vasculature but urine output often increases as cardiac output and blood pressure improve. It reduces systemic vascular resistance, as well as improving cardiac performance, thereby decreasing afterload and ventricular filling pressures. Dobutamine is therefore useful in patients with cardiogenic shock and cardiac failure. In septic shock, it can be used to increase cardiac output and oxygen delivery.
Phosphodiesterase inhibitors (e.g. milrinone, enoximone)
These agents have both inotropic and vasodilator properties. Because the phosphodiesterase type III inhibitors bypass the β-adrenergic receptor they do not cause tachycardia and are less arrhythmogenic than β agonists. They are useful in patients with receptor ‘downregulation’, those receiving beta-blockers, those being weaned from cardiopulmonary bypass and those with cardiac failure.
Vasopressin
Patients with septic shock have inappropriately low circulating levels of vasopressin. Low-dose vasopressin can increase blood pressure and systemic vascular resistance in patients with vasodilatory septic shock and a high cardiac output unresponsive to other vasopressors (‘vasoplegia’). Low-dose vasopressin is sometimes added to conventional vasopressors in patients with septic shock.
Levosimendan
Levosimendan is a myofilament calcium sensitizer. Unlike other inotropes, levosimendan does not exert its action through increases in intracellular Ca2+ and, as a result, does not impair diastolic relaxation of the heart. Levosimendan binds to troponin C with high affinity but only during systole when the intracellular calcium concentration is high. Levosimendan has phosphodiesterase inhibitor actions but these are not thought to be clinically significant. The dose is usually infused over 24 hours but, significantly, a long-acting metabolite of levosimendan has similar calcium-sensitizing actions, maintaining the inotropic effect of levosimendan once an infusion is stopped. Adverse cardiovascular effects of levosimendan include tachycardia and hypotension; as a consequence, the addition of a vasopressor may be required.
Summary for use of inotropic and vasopressor agents
A combination of dobutamine and noradrenaline (norepinephrine) is used for the management of patients who are shocked with a low systemic vascular resistance (e.g. septic shock).
• Dobutamine is given to achieve an optimal cardiac output.
• Noradrenaline, sometimes supplemented by vasopressin, is used to restore an adequate blood pressure by reducing vasodilatation.
In vasodilated septic patients with an adequate cardiac output, noradrenaline can be used alone. There is evidence to suggest that adrenaline (epinephrine) may be equally safe and effective as a dobutamine/noradrenaline combination. Because of its potency, adrenaline is particularly useful in patients with refractory hypotension. Phosphodiesterase inhibitors can be used in the management of cardiac failure, especially when associated with pulmonary hypertension, and perioperatively in those undergoing cardiac surgery. Dobutamine is an alternative that is also used in septic patients with fluid overload or myocardial failure. Dopamine is used much less frequently than in the past. The role of levosimendan in the management of shock has yet to be established but it may be useful in resistant cardiogenic shock.
Targeting haemodynamics and oxygen transport
Although resuscitation has conventionally aimed at achieving normal haemodynamics, many of the critically ill patients that survive have raised values for cardiac output, DO2 and VO2. However, elevation of DO2 and VO2 to these ‘supranormal’ levels following admission to intensive care produces no benefit and may be harmful. Early goal-directed therapy to resuscitate patients in the emergency room, aimed at maintaining a central venous oxygen saturation of more than 70%, does not appear to improve outcome in patients with severe sepsis or septic shock.
High-risk surgical patients
These patients benefit from intensive perioperative monitoring and circulatory support (Box 25.13). In particular, maintenance of an adequate circulating volume and postoperative admission to a critical care area are advocated. Many believe that volume replacement and administration of inotropes or vasopressors should be guided by monitoring of stroke volume/cardiac output, usually using an oesophageal Doppler or pulse contour analysis. The value of the routine use of inodilators such as dopexamine remains unclear.
Vasodilator therapy
In selected cases, afterload reduction is used to increase stroke volume and decrease myocardial oxygen requirements by reducing the systolic ventricular wall tension. Vasodilatation (see p. 987) also decreases heart size and the diastolic ventricular wall tension, so that coronary blood flow is improved. The relative magnitude of the falls in preload and afterload depends on the pre-existing haemodynamic disturbance, concurrent volume replacement and the agent selected (see below). Vasodilators also improve microcirculatory flow.
Vasodilator therapy can be particularly helpful in patients with cardiac failure in whom the ventricular function curve is flat (see Fig. 25.7), so that falls in preload have only a limited effect on stroke volume. This form of treatment, combined in selected cases with inotropic support, is therefore useful in cardiogenic shock and in the management of patients with cardiogenic pulmonary oedema or mitral regurgitation.
• Sodium nitroprusside (SNP) dilates arterioles and venous capacitance vessels, as well as the pulmonary vasculature, by donating nitric oxide. SNP therefore reduces the afterload and preload of both ventricles and can improve cardiac output and the myocardial oxygen supply/demand ratio. The effects of SNP are rapid in onset and spontaneously reversible within a few minutes of discontinuing the infusion. Cyanide poisoning is a risk with high-dose, prolonged infusions.
• Nitroglycerine (NTG), at low doses, is predominantly a venodilator, but as the dose is increased, it also causes arterial dilatation, thereby decreasing both preload and afterload. Nitrates are particularly useful in the treatment of cardiac failure with pulmonary oedema and are usually used in combination with intravenous furosemide. NTG reduces pulmonary vascular resistance, an effect that can be exploited in patients with a low cardiac output secondary to pulmonary hypertension.
Mechanical support of the myocardium
Intra-aortic balloon counterpulsation (IABCP) is the technique used most widely for mechanical support of the failing myocardium. It is discussed on pages 961–962. In specialized centres, ventricular assist devices and veno-arterial extra-corporeal membrane oxygenation (ECMO; see below) may be used in the treatment of cardiac failure.
Adjunctive treatment
Initial attempts to combat the high mortality associated with sepsis concentrated on cardiovascular and respiratory support in the hope that surgery, antibiotics and the patient's own defences would eradicate the infection. Despite some success, mortality rates remained unacceptably high. So far, attempts to improve outcome by modulating the immune response (including high-dose steroids) or neutralizing endotoxin (Box 25.14) have proved disappointing and, in some cases, may even have been harmful.
The administration of relatively low ‘stress’ doses of hydrocortisone to selected patients with refractory vasopressor-resistant septic shock may assist shock reversal and perhaps improve outcome.
The aim of current sepsis guidelines (see ‘Further reading’) is to combine evidence-based interventions with early effective resuscitation (aimed especially at achieving an adequate circulating volume, combined with the rational use of inotropes and/or vasoactive agents to maintain blood pressure, cardiac output and oxygen transport), in order to create ‘bundles of care’ delivered within specific time limits.
Respiratory Failure
(See also Chapter 24.)
 Classification and aetiology
Classification and aetiology
The respiratory system consists of a gas-exchanging organ (the lungs) and a ventilatory pump (respiratory muscles/thorax), either or both of which can fail and precipitate respiratory failure. Respiratory failure occurs when pulmonary gas exchange is sufficiently impaired to cause hypoxaemia with or without hypercarbia. In practical terms, respiratory failure is present when the PaO2 is <8 kPa (60 mmHg) or the PaCO2 is >7 kPa (55 mmHg). It can be divided into:
• type I respiratory failure, in which the PaO2 is low and the PaCO2 is normal or low
• type II respiratory failure, in which the PaO2 is low and the PaCO2 is high.
Type I or ‘acute hypoxaemic’ respiratory failure occurs with diseases that damage lung tissue. Hypoxaemia is due to right-to-left shunts or 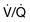 mismatch. Common causes include pneumonia, acute lung injury, cardiogenic pulmonary oedema and lung fibrosis.
mismatch. Common causes include pneumonia, acute lung injury, cardiogenic pulmonary oedema and lung fibrosis.
Type II or ‘ventilatory failure’ occurs when alveolar ventilation is insufficient to remove the volume of carbon dioxide being produced by tissue metabolism. Inadequate alveolar ventilation may be due to reduced ventilatory effort, inability to overcome an increased resistance to ventilation, failure to compensate for an increase in dead space and/or carbon dioxide production, or a combination of these factors. The most common cause is COPD. Other causes include chest-wall deformities, respiratory muscle weakness (e.g. Guillain–Barré syndrome) and depression of the respiratory centre (e.g. drug overdose).
Deterioration in the mechanical properties of the lungs and/or chest wall increases the work of breathing and the oxygen consumption/carbon dioxide production of the respiratory muscles. Respiratory muscle fatigue is a factor in the pathogenesis of respiratory failure.
 Clinical assessment
Clinical assessment
The general assessment and recognition of an acutely ill ward patient have been discussed above on pages 1139–1140. A clinical assessment of respiratory distress should be made on the following criteria:
• the use of accessory muscles of respiration
• pulsus paradoxus (rarely present)
• inability to speak, unwillingness to lie flat
• agitation, restlessness, diminished conscious level
• asynchronous respiration (a discrepancy in the timing of movement of the abdominal and thoracic compartments)
• paradoxical respiration (abdominal and thoracic compartments move in opposite directions)
• respiratory alternans (breath-to-breath alteration in the relative contribution of intercostal/accessory muscles and the diaphragm).
Blood gas analysis should be performed to guide oxygen therapy and to provide an objective assessment of the severity of the respiratory failure. The most sensitive clinical indicator of increasing respiratory difficulty is a rising respiratory rate. Measurement of tidal volume is a less sensitive indicator. Vital capacity is often a better guide to deterioration and is particularly useful in patients with respiratory inadequacy that is due to neuromuscular problems such as the Guillain–Barré syndrome or myasthenia gravis, in which the vital capacity decreases as weakness worsens. Measurement of forced expiratory volume in 1 second (FEV1) is useful in the assessment of patients suffering acute asthma attacks.
 Monitoring
Monitoring
Pulse oximetry
Lightweight oximeters can be applied to an earlobe or finger. They measure the changing amount of light transmitted through the pulsating arterial blood and provide a continuous, non-invasive assessment of arterial oxygen saturation (SpO2). These devices are reliable and easy to use, and do not require calibration.
Pulse oximetry is not a sensitive guide to changes in oxygenation. An SpO2 within normal limits in a patient receiving supplemental oxygen does not exclude the possibility of hypoventilation with carbon dioxide retention. Readings can be inaccurate in those with poor peripheral perfusion.
Blood gas analysis
Normal values of blood gas analysis are shown in Box 25.8. See online for examples of partially compensated respiratory alkalosis.
Errors can result from malfunctioning of the analyser or incorrect sampling of arterial blood. Disposable pre-heparinized syringes are available for blood gas analysis.
• The sample should be analysed immediately. Alternatively, the syringe should be immersed in iced water (the end having first been sealed with a cap) to prevent the continuing metabolism of white cells causing a reduction in PO2 and a rise in PCO2.
• Air almost inevitably enters the sample. The gas tensions within these air bubbles will equilibrate with those in the blood, thereby lowering the PCO2 and usually raising the PO2 of the sample. However, provided the bubbles are ejected immediately by inverting the syringe and expelling the air that rises to the top of the sample, their effect is insignificant.
Interpretation of the results of blood gas analysis can be considered in two separate parts:
• disturbances of acid–base balance (see pp. 176 and 1149–1150)
Correct interpretation requires a knowledge of the patient's clinical history and age, the inspired oxygen concentration and any other relevant treatment (e.g. the ventilator settings for those on mechanical ventilation or the administration of sodium bicarbonate). The oxygen content of the arterial blood is determined by the percentage saturation of haemoglobin with oxygen. The relationship between the latter and the PaO2 is determined by the oxyhaemoglobin dissociation curve (see Fig. 25.2).
PaO2/FIO2 ratio
This calculated variable is a simple approximation to the PA–aO2 and can be used to assess the severity of respiratory failure (see p. 1143). It is calculated by taking the arterial PO2 in mmHg and dividing by the FIO2 expressed as a fraction of 1. The ‘P/F’ ratio has been used to define respiratory impairment in patients with ARDS (see pp. 1167–1168).
Capnography
Continuous breath-by-breath analysis of the expired carbon dioxide concentration can be used to:
• Confirm tracheal intubation.
• Continuously monitor end-tidal PCO2, which approximates to PaCO2 in normal subjects. Continuous capnography is recommended for all mechanically ventilated patients to detect acute airway problems, e.g. tracheal tube/tracheostomy blocked or dislodged (essential when transporting critically ill patients).
• Detect apparatus malfunction.
• Detect acute alterations in cardiorespiratory function (e.g. sudden fall in cardiac output).
 Management
Management
Standard management of patients with respiratory failure includes:
• administration of supplemental oxygen through a patent airway
• treatment for distal airways obstruction
The load on the respiratory muscles should be reduced by improving lung mechanics. Correction of abnormalities that may lead to respiratory muscle weakness, such as hypophosphataemia and malnutrition, is also necessary.
Oxygen therapy
Methods of oxygen administration
Oxygen is initially given via a face mask. In the majority of patients (except those with COPD with chronically elevated PaCO2), the precise concentration of oxygen given is not vital and oxygen can therefore be given by a ‘variable performance’ device such as a simple face mask or nasal cannulae (Fig. 25.24).
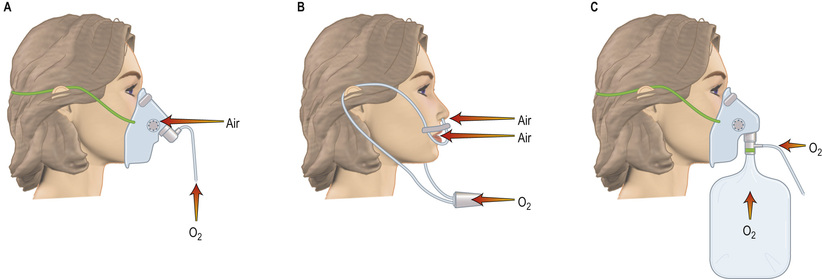
With these devices, the inspired oxygen concentration varies from about 35% to 55%, with oxygen flow rates of between 6 and 10 L/min. Nasal cannulae are often preferred because they are less claustrophobic and do not interfere with feeding or speaking, but they can cause ulceration of the nasal or pharyngeal mucosa and the inspired oxygen concentration is diluted by mouth breathing. Higher concentrations of oxygen can be administered by using a mask with a reservoir bag attached (Fig. 25.24C). Figure 25.24 should be compared with the fixed-performance mask shown in Figure 24.25; the oxygen concentration can be controlled with the latter and it is used in patients with COPD and chronic type II failure. The hazards of reducing hypoxic drive can be overemphasized and are less dangerous when the patient is in a critical care unit; remember, severe hypoxaemia is more dangerous than hypercapnia.
Oxygen toxicity
Experimentally, mammalian lungs have been shown to be damaged by continuous exposure to high concentrations of oxygen, but oxygen toxicity in humans is less well proven. Nevertheless, it is reasonable to assume that high concentrations of oxygen might damage the lungs, and so the lowest inspired oxygen concentration compatible with adequate arterial oxygenation should be used. Although dangerous hypoxia should never be tolerated through a fear of pulmonary oxygen toxicity, oxygen saturations between 90 and 92% are probably adequate for most patients and there is some suggestion that targeting higher oxygen saturations using greater concentrations of inspired oxygen may be detrimental. There has also been concern that, in some circumstances (e.g. following myocardial infarction), hyperoxia associated with routine administration of supplemental oxygen may be harmful, perhaps because of associated vasoconstriction, and it is now not routinely given.
Respiratory support
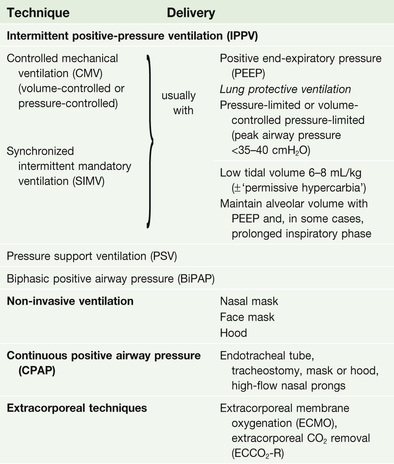
If, despite the above measures, the patient continues to deteriorate or fails to improve, the institution of some form of respiratory support is necessary (Box 25.15). Non-invasive ventilation via a mask or hood (see p. 1166) can be used, particularly in respiratory failure due to COPD, but in many critically ill patients, invasive ventilation through an endotracheal tube or tracheostomy is required.
Intermittent positive pressure ventilation (IPPV) is achieved by intermittently inflating the lungs with a positive pressure delivered by a mechanical ventilator. There have been a number of refinements and modifications to the manner in which positive pressure is applied to the airway, and to the interplay between the patient's respiratory efforts and mechanical assistance (see pp. 1165–1166).
Controlled mechanical ventilation (CMV), with the abolition of spontaneous breathing, rapidly leads to atrophy of respiratory muscles, so that assisted modes that are triggered by the patient's inspiratory efforts (see below) are preferred.
The rational use of mechanical ventilation depends on a clear understanding of its potential beneficial effects, as well as the dangers.
Beneficial effects of mechanical ventilation
• Relief from exhaustion. Mechanical ventilation reduces the work of breathing, ‘rests’ the respiratory muscles and relieves the extreme exhaustion present in patients with respiratory failure; this exhaustion may culminate in respiratory arrest.
• Effects on oxygenation. Application of positive pressure can prevent or reverse atelectasis. In those with severe pulmonary parenchymal disease, the lungs may be very stiff and the work of breathing is therefore greatly increased. Under these circumstances, the institution of respiratory support significantly reduces total body oxygen consumption; consequently, 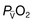 and thus PaO2 may improve. Because ventilated patients are connected to a leak-free circuit, it is possible to administer high concentrations of oxygen (up to 100%) accurately and to apply a positive end-expiratory pressure (PEEP). In selected cases, the latter may reduce shunting and increase PaO2 (see below).
and thus PaO2 may improve. Because ventilated patients are connected to a leak-free circuit, it is possible to administer high concentrations of oxygen (up to 100%) accurately and to apply a positive end-expiratory pressure (PEEP). In selected cases, the latter may reduce shunting and increase PaO2 (see below).
• Improved carbon dioxide elimination. By adjusting the minute volume, the PaCO2 can usually be controlled.
Indications for mechanical ventilation
• Acute respiratory failure, with signs of severe respiratory distress (e.g. respiratory rate >40 breaths/min, inability to speak, patient exhaustion) persisting despite maximal therapy. Confusion, restlessness, agitation, a decreased conscious level, a rising PaCO2 (>8 kPa, >60 mmHg) and extreme hypoxaemia (<8 kPa, <60 mmHg), despite oxygen therapy, are further indications.
• Acute ventilatory failure due, for example, to myasthenia gravis or Guillain–Barré syndrome. Mechanical ventilation should usually be instituted when the vital capacity has fallen to 10 mL/kg or less. This will avoid complications such as atelectasis and infection, as well as preventing respiratory arrest. The tidal volume and respiratory rate are relatively insensitive indicators of respiratory failure in the above conditions and change late in the course of the disease. A high PaCO2 (particularly if rising) is an indication for urgent mechanical ventilation.
Not all patients with respiratory failure and/or a reduced vital capacity require ventilation; clinical assessment of each individual case is essential. The patient's general condition, degree of exhaustion, level of consciousness and ability to protect the airway are often more useful than blood gas values.
Other indications include:
• postoperative ventilation in high-risk patients
• head injury: to avoid hypoxia and hypercarbia, which increase cerebral blood flow and intracranial pressure (see below)
• trauma: chest injury and lung contusion
• severe left ventricular failure with pulmonary oedema
• coma with breathing difficulties, e.g. following drug overdose.
Institution of invasive respiratory support
This requires tracheal intubation. If the patient is conscious, the procedure must be fully explained and written consent obtained before anaesthesia is induced. The complications of tracheal intubation are given in Box 25.16.
Intubating patients in severe respiratory failure is a hazardous undertaking and should be performed only by experienced staff. In extreme emergencies, it may be preferable to ventilate the patient by hand using an oropharyngeal airway and a face mask with added oxygen until experienced help arrives. An alternative is insertion of a laryngeal mask airway.
Intubating a critically ill patient is very different to intubating a patient in the operating theatre prior to elective surgery. The patient is usually hypoxic and hypercarbic, with increased sympathetic activity; the stimulus of laryngoscopy and intubation can precipitate dangerous arrhythmias, bradycardia and even cardiac arrest. Except in an extreme emergency, therefore, the ECG and oxygen saturation should be monitored, and the patient pre-oxygenated with 100% oxygen before intubation. Resuscitation drugs should be immediately available. If time allows, the circulating volume should be optimized and, if necessary, inotropes commenced before attempting intubation. In some cases, it is appropriate to establish intra-arterial and CVP monitoring before instituting mechanical ventilation, although many patients will not tolerate the supine or head-down position. In some deeply comatose patients, no sedation is required, but in the majority, a short-acting intravenous anaesthetic agent, usually with an opiate followed by muscle relaxation, will be necessary. Capnography must be used to confirm tracheal intubation.
Sedation, analgesia and muscle relaxation
Most critically ill patients will require analgesia and many will receive sedatives. The combination of an opiate with a benzodiazepine or propofol is often used to facilitate mechanical ventilation and to obtund the physiological response to stress. Heavy sedation is indicated in those with severe respiratory failure, especially since ‘lung-protective’ ventilatory strategies (see p. 1166) are inherently uncomfortable. A few may require neuromuscular blockade. It is recognized, however, that minimizing sedation levels using ‘sedation scores’ and ‘daily wakening’, or even the avoidance of sedatives altogether, often in combination with spontaneous breathing modes of respiratory support (see pp. 1165–1166), is associated with reductions in the duration of mechanical ventilation and more rapid discharge from the ICU and hospital.
Tracheostomy
Tracheostomy may be required for the long-term control of excessive bronchial secretions, and in those with a chronically reduced conscious level in order to maintain an airway and protect the lungs if pharyngeal and laryngeal reflexes are impaired. Tracheostomy is also performed when intubation is likely to be prolonged, for patient comfort, reduction of sedation requirements and facilitation of weaning from mechanical ventilation.
Tracheostomy was once performed only in the operating room by a surgeon but it is now usual for this procedure to be carried out by intensive care specialists in the ICU using a percutaneous dilatational approach. This approach is quicker, less resource-intensive, safe and associated with a greatly reduced wound infection rate.
A life-threatening obstruction of the upper respiratory tract that cannot be bypassed with an endotracheal tube can be relieved by a cricothyroidotomy, which is safer, quicker and easier to perform than a formal tracheostomy.
Tracheostomy has a small but significant mortality rate. Complications associated with the technique are shown in Box 25.17.
Complications associated with mechanical ventilation
See Boxes 25.16 and 25.17.
Disconnection, failure of gas or power supply, mechanical faults
These are unusual but dangerous. A method of manual ventilation, a face mask and oxygen must always be available by the bedside.
Cardiovascular complications
The application of positive pressure to the lungs and thoracic wall impedes venous return and distends alveoli, thereby ‘stretching’ the pulmonary capillaries and causing a rise in pulmonary vascular resistance. Both of these mechanisms can produce a fall in cardiac output.
Respiratory complications
Mechanical ventilation can be complicated by a deterioration in gas exchange because of 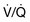 mismatch, fluid retention and collapse of peripheral alveoli. Traditionally, the latter was prevented by using high tidal volumes (10–12 mL/kg) but high inflation pressures, with over-distension of compliant alveoli, perhaps exacerbated by the repeated opening and closure of distal airways, can disrupt the alveolar–capillary membrane. There is an increase in microvascular permeability and release of inflammatory mediators, leading to ‘ventilator-associated lung injury’. Extreme over-distension of the lungs during mechanical ventilation with high tidal volumes and high airway pressures can rupture alveoli and cause air to dissect centrally along the perivascular sheaths. This ‘barotrauma’ and ‘volutrauma’ may be complicated by pneumomediastinum, subcutaneous emphysema, pneumoperitoneum, pneumothorax and intra-abdominal air. The risk of pneumothorax is increased in those with destructive lung disease (e.g. necrotizing pneumonia, emphysema), asthma or fractured ribs.
mismatch, fluid retention and collapse of peripheral alveoli. Traditionally, the latter was prevented by using high tidal volumes (10–12 mL/kg) but high inflation pressures, with over-distension of compliant alveoli, perhaps exacerbated by the repeated opening and closure of distal airways, can disrupt the alveolar–capillary membrane. There is an increase in microvascular permeability and release of inflammatory mediators, leading to ‘ventilator-associated lung injury’. Extreme over-distension of the lungs during mechanical ventilation with high tidal volumes and high airway pressures can rupture alveoli and cause air to dissect centrally along the perivascular sheaths. This ‘barotrauma’ and ‘volutrauma’ may be complicated by pneumomediastinum, subcutaneous emphysema, pneumoperitoneum, pneumothorax and intra-abdominal air. The risk of pneumothorax is increased in those with destructive lung disease (e.g. necrotizing pneumonia, emphysema), asthma or fractured ribs.
A tension pneumothorax can be rapidly fatal in ventilated patients. Suggestive signs include the development or worsening of hypoxia, hypercarbia, respiratory distress and an unexplained increase in airway pressure, as well as hypotension and tachycardia, sometimes accompanied by a rising CVP. Examination may reveal unequal chest expansion, mediastinal shift away from the side of the pneumothorax (deviated trachea, displaced apex beat) and a hyper-resonant hemithorax. Although breath sounds are often diminished over the pneumothorax, this sign can be misleading in ventilated patients. If there is time, the diagnosis can be confirmed by chest X-ray prior to definitive treatment with chest tube drainage.
Ventilator-associated pneumonia
Hospital-acquired pneumonia occurs in as many as one-third of patients receiving mechanical ventilation and is associated with a significant increase in length of stay and mortality. The diagnosis is difficult and controversial because many ventilated patients develop a pyrexia, and infiltrates on the chest X-ray that may not be infective are common. The measurement of serum procalcitonin, a marker of severe bacterial infections, may be helpful. Various organisms may be isolated, such as aerobic Gram-negative bacilli (e.g. Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Acinetobacter spp.) and Staphylococcus aureus, including meticillin-resistant Staph. aureus (MRSA). Deciding whether an organism that has been isolated is causing ventilator-associated pneumonia or is simply colonizing the respiratory tract can also be difficult. Leakage of infected oropharyngeal secretions past the tracheal tube cuff is thought to be largely responsible for ventilator-associated pneumonia. Bacterial colonization of the oropharynx may be promoted by regurgitation of colonized gastric fluid and so the risk of ventilator-associated pneumonia can be reduced by nursing patients in the semi-recumbent, rather than the supine, position and by oropharyngeal decontamination. Treatment is with an appropriate broad-spectrum antibiotic, which can be modified if a causative organism is isolated.
Techniques for respiratory support
(See Box 25.15.)
Controlled mechanical ventilation
Controlled mechanical ventilation (CMV) is used when respiratory efforts are absent or have been abolished.
Ventilation involves one of two types:
• Volume-controlled ventilation. The tidal volume and respiratory rate are preset on the ventilator. The airway pressure varies according to both the ventilator setting and the patient's lung mechanics (airways resistance and compliance).
• Pressure-controlled ventilation. Both the inspiratory pressure and the respiratory rate are preset but the tidal volume varies according to the patient's lung mechanics.
Positive end-expiratory pressure
A positive airway pressure can be maintained at a chosen level throughout expiration using a threshold resistor valve in the expiratory limb of the circuit. Positive end-expiratory pressure (PEEP) re-expands under-ventilated lung units, and redistributes lung water from the alveoli to the perivascular interstitial space, thereby reducing shunt and increasing PaO2. The inevitable rise in mean intrathoracic pressure associated with the application of PEEP may, however, further impede venous return, increase pulmonary vascular resistance and reduce cardiac output. The fall in cardiac output can be ameliorated by expanding the circulating volume, although, in some cases, inotropic or vasopressor support is required. Thus, although arterial oxygenation is often improved by the application of PEEP, a simultaneous fall in cardiac output can lead to a reduction in total oxygen delivery.
Traditionally, the application of PEEP was considered only if it proved difficult to achieve adequate oxygenation of arterial blood despite raising the inspired oxygen concentration above 50%. Low levels of PEEP (5–7 cmH2O) are now used in the majority of mechanically ventilated patients in order to maintain lung volume, as well as in those with basal atelectasis and in selected cases with airways obstruction.
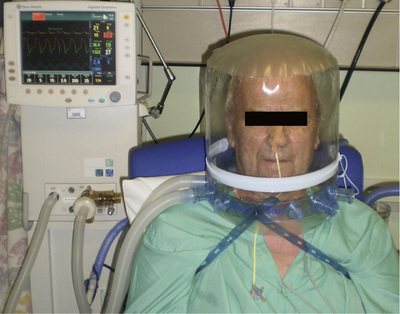
Continuous positive airway pressure
The application of continuous positive airway pressure (CPAP) achieves for the spontaneously breathing patient what PEEP does for the ventilated patient. Oxygen and air are delivered under pressure via an endotracheal tube, a tracheostomy, a tightly fitting face mask or a hood (Fig. 25.25). Not only can CPAP improve oxygenation, but also the lungs become more compliant and the work of breathing is reduced.
An alternative is to use a very high flow of oxygen and air delivered via large-diameter nasal prongs to create a modest amount of CPAP. This system is often better tolerated, communication is easier and the patient can eat. Commencing therapy with high-flow nasal prongs in patients with type I respiratory failure may be equivalent to, or more effective than, non-invasive ventilation in preventing patient deterioration and avoiding subsequent invasive ventilation. However the application of positive airway pressure is compromised if the patient mouth-breathes.
Pressure support ventilation
In pressure support ventilation (PSV), spontaneous breaths are augmented by a preset level of positive pressure (usually between 5 and 20 cmH2O), triggered by the patient's spontaneous respiratory effort and applied for a given fraction of inspiratory time or until inspiratory flow falls below a certain level. Tidal volume is determined by the set pressure, the patient's effort and pulmonary mechanics. The level of pressure support can be reduced progressively as the patient improves.
Intermittent mandatory ventilation
The intermittent mandatory ventilation (IMV) technique allows the patient to breathe spontaneously between the ‘mandatory’ tidal volumes delivered by the ventilator. These mandatory breaths are timed to coincide with the patient's own inspiratory effort (synchronized intermittent mandatory ventilation, or SIMV). SIMV can be used with or without CPAP, and spontaneous breaths may be assisted with pressure support ventilation.
‘Lung-protective’ ventilation
This is designed to avoid exacerbating or perpetuating lung injury by avoiding over-distension of alveoli, minimizing airway pressures, and preventing the repeated opening and closure of distal airways. Alveolar volume is maintained with PEEP, and sometimes by prolonging the inspiratory phase, while tidal volumes are limited to 6–8 mL/kg ideal bodyweight. Peak airway pressures should not exceed 35–40 cmH2O. An alternative is to deliver a constant preset inspiratory pressure for a prescribed time in order to generate a low tidal volume at reduced airway pressures (‘pressure-limited’ mechanical ventilation). Respiratory rate can be increased to improve CO2 removal and avoid severe acidosis (H+ >63 nmol/L; pH <7.2), but hypercarbia is frequent and should usually be accepted (‘permissive hypercarbia’). Both techniques can be used with SIMV. Ventilation with low tidal volumes has been shown to improve outcome in patients with ARDS (see pp. 1168–1169). Lung-protective ventilation should be used in almost all patients undergoing mechanical ventilation.
High-frequency oscillation
High-frequency oscillation (HFO) is administered using a purpose-designed ventilator. With HFO, there is no bulk flow of gas; rather, gas oscillates to and fro at rates of 60–3000 cycles/min with a VT of 1–3 mL/kg. Both inspiration and expiration are actively controlled with a sine wave pump. The mechanism of gas exchange is not fully understood but lung volume is well maintained and oxygenation may be improved. Whilst this is a well-established and proven treatment in the neonatal ICU, there is some doubt as to its usefulness in adults with acute respiratory failure.
Extracorporeal gas exchange
In patients with severe refractory respiratory failure, pumped, high-flow, veno-venous bypass through a membrane lung is used in specialized centres to achieve adequate oxygenation and increase CO2 removal (extracorporeal membrane oxygenation, ECMO). Extracorporeal carbon dioxide removal (ECCO2-R) is more easily administered, and uses lower flow rates to provide effective CO2 removal but less efficient oxygenation. Both techniques have been used to reduce ventilation requirements, thereby minimizing further ventilation-induced lung damage and encouraging resolution of the lung injury.
Non-invasive ventilation
Non-invasive ventilation (NIV) is suitable for patients who are conscious, cooperative and able to protect their airway; they must also be able to expectorate effectively. Positive pressure is applied to the airways using a tight-fitting full-face/nasal mask or a hood. The most popular ventilators for this purpose are those that deliver bi-level positive airway pressure (BiPAP), which are simple to use, cheap and flexible. With the latter technique, inspiratory and expiratory pressure levels and times are set independently and unrestricted spontaneous respiration is possible throughout the respiratory cycle. BiPAP can also be patient-triggered. There is a reduced risk of ventilator-associated pneumonia and improved patient comfort, with preservation of airway defence mechanisms, speech and swallowing (which allows better nutrition). Spontaneous coughing and expectoration are not hampered, permitting effective physiotherapy, and sedation is usually unnecessary. Institution of non-invasive respiratory support can rest the respiratory muscles, reduce respiratory acidosis and breathlessness, improve clearance of secretions and re-expand collapsed lung segments. The intubation rate, length of ICU and hospital stay, and, in some categories of patient, mortality may all be reduced. NIV is particularly useful in acute hypercapnic respiratory failure associated with COPD, provided the patient is not profoundly hypoxic or obtunded. NIV may also be valuable as a means of avoiding tracheal intubation in immunocompromised patients with acute respiratory failure. Evidence suggests that early NIV after extubation of hypercapnic patients with respiratory disorders can reduce the risk of subsequent respiratory failure and mortality. Box 25.18 shows some indications for the use of NIV when standard medical treatment has failed.
Weaning
Weakness and wasting of respiratory muscles are inevitable consequences of the catabolic response to critical illness and are often exacerbated by the reduction in respiratory work during mechanical ventilation (‘disuse atrophy’). Often, abnormalities of gas exchange and lung mechanics persist. Not surprisingly, therefore, many patients experience difficulty in resuming unsupported spontaneous ventilation. In a significant proportion of patients who have undergone a prolonged period of respiratory support, the situation is further complicated by the development of a neuropathy, a myopathy or both.
Neuromuscular weakness complicating critical illness
Polyneuromyopathies have most often been described in association with persistent sepsis and multiple organ failure.
Critical illness polyneuropathy is characterized by a primary axonal neuropathy involving both motor and, to a lesser extent, sensory nerves. Clinically, the initial manifestation is often difficulty in weaning the patient from respiratory support. There is muscle wasting, the limbs are weak and flaccid, and deep tendon reflexes are reduced or absent. Cranial nerves are relatively spared. Nerve conduction studies confirm axonal damage. The CSF protein concentration is normal or minimally elevated. These findings differentiate critical illness neuropathy from Guillain–Barré syndrome, in which nerve conduction studies nearly always show evidence of demyelination and CSF protein is usually high.
The cause of critical illness polyneuropathy is not known and there is no specific treatment. Weaning from respiratory support and rehabilitation are likely to be prolonged. With resolution of the underlying critical illness, recovery can be expected after 1–6 months but weakness and fatigue frequently persist.
Critical illness myopathies can also occur, often in association with a neuropathy. A severe quadriplegic myopathy has been associated particularly with the administration of steroids and muscle relaxants to mechanically ventilated patients who have acute, severe asthma.
Criteria for weaning patients from mechanical ventilation
Clinical assessment is the best way of deciding whether a patient can be weaned from the ventilator. The patient's conscious level, mood and cardiovascular performance, as well as the effects of drugs, must all be taken into account. A subjective evaluation by an experienced clinician of the patient's response to a short period of spontaneous breathing (spontaneous breathing trial) is the most reliable predictor of weaning success or failure. Objective criteria are based on an assessment of pulmonary gas exchange (blood gas analysis), lung mechanics and muscular strength.
Techniques for weaning
Patients who have received mechanical ventilation for <24–48 hours, e.g. after elective major surgery, can usually resume spontaneous respiration immediately and no weaning process is required. This procedure can also be adopted for those who have been ventilated for longer periods but who tolerate a spontaneous breathing trial and clearly fulfil objective criteria for weaning. Techniques of weaning include the following:
• The traditional method is to allow the patient to breathe entirely spontaneously for a short time, following which respiratory support is resumed. The periods of spontaneous breathing are gradually increased and the periods of respiratory support are progressively reduced. Initially, it is usually advisable to ventilate the patient throughout the night. This method can be stressful and tiring for both patients and staff, although it is sometimes successful when other methods have failed.
• SIMV (see p. 1166) involves a progressive reduction in the frequency of mandatory breaths. Spontaneous breaths are usually pressure-supported.
• Gradual reduction of the level of pressure support is thought to be the preferred technique.
• CPAP (see p. 1165–1166) can prevent the alveolar collapse, hypoxaemia and fall in compliance that might otherwise occur when patients start to breathe spontaneously. It is therefore used during weaning with SIMV or pressure support, during spontaneous breathing trials and in spontaneously breathing patients prior to extubation.
• Tracheostomy is often used to facilitate weaning from mechanical ventilation.
• Non-invasive respiratory support (BiPAP, CPAP) can be used following extubation to prevent respiratory failure and re-intubation.
Extubation and tracheostomy decannulation
These should not be performed until patients can cough, swallow and protect their own airway, and are sufficiently alert to be cooperative. Patients who fulfil these criteria can be extubated, provided their respiratory function has improved sufficiently to sustain spontaneous ventilation indefinitely. Similar considerations guide the elective removal of tracheostomy tubes.
The ratio of respiratory rate (in breaths/minute) to tidal volume (in litres) can be used to predict which patients will tolerate extubation, provided all other preconditions have been met. This ‘rapid shallow breathing index’ quantifies the clinical assessment of a patient who breathes slowly and comfortably with adequate tidal volumes. A score of <100 is a relatively good predictor of extubation success.
Acute Respiratory Distress Syndrome
 Definition and aetiology
Definition and aetiology
The acute respiratory distress syndrome (ARDS; Box 25.19) can be defined as follows:
• Stiff lungs (reduced pulmonary compliance resulting in high inflation pressures).
• Chest X-ray: new bilateral, diffuse, patchy or homogeneous pulmonary infiltrates.
• Cardiac factors: no apparent cardiogenic cause of pulmonary oedema (pulmonary artery occlusion pressure <18 mmHg if measured or no clinical evidence of left atrial hypertension).
– mild: PaO2/FIO2 ratio 300–200 mmHg
– moderate: PaO2/FIO2 200–100 mmHg
– severe: PaO2/FIO2 ratio <100 mmHg
all with a PEEP ≥5 cmH2O (Berlin definition) (in all cases, despite normal arterial carbon dioxide tension).
ARDS can occur as a non-specific reaction of the lungs to a wide variety of direct pulmonary and indirect non-pulmonary insults. By far the most common predisposing factor is sepsis, and 20–40% of patients with severe sepsis will develop ARDS (Box 25.19).
 Pathogenesis and pathophysiology
Pathogenesis and pathophysiology
ARDS can be viewed as an early manifestation of a generalized inflammatory response with endothelial dysfunction and is therefore frequently associated with the development of multiple organ dysfunction syndrome (MODS; see p. 1155).
Non-cardiogenic pulmonary oedema
This is the cardinal feature of ARDS and is the first and clinically most evident sign of a generalized increase in vascular permeability caused by the microcirculatory changes and release of immune mediators described previously (see pp. 1151–1153), with activated neutrophils playing a key role. The pulmonary epithelium is also damaged in the early stages, reducing surfactant production, predisposing to alveolar collapse and lowering the threshold for alveolar flooding.
Pulmonary hypertension
Pulmonary hypertension, sometimes complicated by right ventricular failure (see p. 1030), is a common feature of ARDS. Initially, mechanical obstruction of the pulmonary circulation may occur as a result of vascular compression by interstitial oedema, while local activation of the coagulation cascade leads to thrombosis and obstruction in the pulmonary microvasculature. Later, pulmonary vasoconstriction may develop in response to increased autonomic nervous activity and circulating substances such as catecholamines and thromboxane. Those vessels supplying alveoli with low oxygen tensions constrict (the ‘pulmonary hypoxic vasoconstrictor response’), diverting pulmonary blood flow to better-oxygenated areas of lung, thus limiting the degree of shunt.
Haemorrhagic intra-alveolar exudate
This exudate is rich in platelets, fibrin, fibrinogen and clotting factors, and may inactivate surfactant and stimulate inflammation, as well as promoting hyaline membrane formation and the migration of fibroblasts into the air spaces.
Resolution, fibrosis and repair
Within days of the onset of lung injury, formation of a new epithelial lining is under way and activated fibroblasts accumulate in the interstitial spaces. In some cases, there is progressive interstitial fibrosis. In those who recover, the lungs are substantially remodelled.
Physiological changes
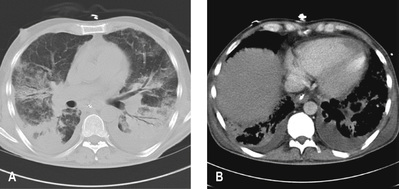
Shunt and dead space increase, compliance falls and there is evidence of airflow limitation. Although the lungs in ARDS are diffusely injured, the pulmonary lesions, when identified as densities on a CT scan, are predominantly located in dependent regions (Fig. 25.26). This is partly explained by the effects of gravity on the distribution of extravascular lung water and areas of lung collapse. Pleural effusions are common.
 Clinical features
Clinical features
The first sign of the development of ARDS is often an unexplained tachypnoea, followed by increasing hypoxaemia with central cyanosis and breathlessness. Fine crackles are heard throughout both lung fields. Later, the chest X-ray shows bilateral diffuse shadowing, interstitial at first, but subsequently with an alveolar pattern and air bronchograms (Fig. 25.27). The differential diagnosis includes cardiac failure and lung fibrosis.
 Management
Management
This is based on treatment of the underlying condition (e.g. eradication of sepsis), supportive measures, and avoidance of complications such as ventilator-induced lung injury and ventilator-associated pneumonia.
Mechanical ventilation
This is the cornerstone of management; strategies designed to minimize ventilator-induced lung injury and encourage lung healing should be used (see p. 1166).
Pulmonary oedema limitation
Pulmonary oedema formation should be limited by fluid restriction, the use of diuretics and, if these measures fail, prevention of fluid overload by haemofiltration. The aim should be to achieve a consistently negative fluid balance.
Prone position
When the patient is changed from the supine to the prone position, lung densities in the dependent region are redistributed and shunt fraction is reduced. More uniform alveolar ventilation, caudal movement of the diaphragm, redistribution of perfusion and recruitment of collapsed alveoli all contribute to the improvement in gas exchange. Repeated position changes between prone and supine allow reductions in airway pressures and the inspired oxygen fraction. Recent findings suggest that proning early in the course of the disease process and spending longer periods prone than supine are associated with substantial mortality benefits.
Inhaled nitric oxide
This vasodilator, when inhaled, may improve matching by increasing perfusion of ventilated lung units, as well as reducing pulmonary hypertension. It has been shown to improve oxygenation in so-called ‘responders’ with ARDS but has not been shown to increase survival.
Aerosolized prostacyclin
This appears to have similar effects to inhaled nitric oxide and is easier to deliver. As with inhaled nitric oxide, the response to aerosolized prostacyclin is, however, variable; although it improves oxygenation, its effect on outcome is unclear.
Aerosolized surfactant
Surfactant replacement therapy reduces morbidity and mortality in neonatal respiratory distress syndrome and is beneficial in animal models of ARDS. In adults with ARDS, however, the value of surfactant administration remains uncertain.
Steroids
Administration of steroids to patients with persistent ARDS does not appear to improve outcome.
 Prognosis
Prognosis
Mortality from ARDS has fallen over the last decade, from around 60% to between 20% and 40%, perhaps as a consequence of improved general care, lung-protective ventilation strategies, the increasing use of management protocols, and attention to infection control and nutrition. Prognosis is, however, still very dependent on aetiology. When ARDS occurs in association with intra-abdominal sepsis, mortality rates remain very high, whereas much lower mortality rates are to be expected in those with ‘primary’ ARDS (pneumonia, aspiration, lung contusion). Mortality rises with increasing age and failure of other organs. Most of those dying with ARDS do so as a result of MODS and haemodynamic instability rather than impaired gas exchange.
Acute Kidney Injury
Acute kidney injury (AKI) is a common and serious complication of critical illness, and is strongly associated with increased morbidity and mortality. The presence and severity of AKI are now formally classified by relative increases in serum creatinine and/or a persistent reduction in urine output (see Box 20.27).
The importance of preventing renal injury by rapid and effective resuscitation, the avoidance of nephrotoxic drugs (especially non-steroidal anti-inflammatory drugs, NSAIDs), and control of infection cannot be overemphasized. While shock and sepsis are the most common causes of AKI in the critically ill, causes requiring specific treatment should be excluded, especially urinary tract obstruction and acute intrinsic renal disease such as rapidly progressive glomerulonephritis (see p. 772).
Acutely, oliguria often accompanies shock and renal hypoperfusion and should prompt attempts to optimize cardiovascular function by expanding the circulating volume and restoring blood pressure, with restoration of urine output a good indicator of successful resuscitation. However, when AKI is established, urine output is often normal or high due to impaired concentrating capacity, so that development of oliguria then implies almost complete loss of renal function; in this case, continued fluid resuscitation will be ineffective and potentially harmful.
There is no evidence to support specific pharmacological interventions to prevent or treat AKI; in particular, low-dose dopamine is ineffective for preventing or reversing renal impairment in sepsis (see p. 772). Thus, treatment of those developing or at risk of AKI should focus on prompt resuscitation with fluid and/or vasopressor therapy, as well treatment of the underlying causes of shock and sepsis. If these measures fail to reverse oliguria, loop diuretics such as furosemide (see p. 156) may be used to treat or prevent fluid overload. However, there is no evidence that diuretics alter the clinical course of AKI, and renal replacement therapy (RRT) should not be delayed if indicated.
In AKI, RRT is indicated for refractory fluid overload, major electrolyte disturbances (especially hyperkalaemia), severe acidosis and, less often, uraemia. There is no evidence to support commencement of RRT for less severe AKI in the absence of these indications. Continuous renal replacement therapy (CRRT; see p. 773) is associated with greater haemodynamic stability and better control of fluid balance than intermittent haemodialysis, while use of peritoneal dialysis is unsatisfactory in critically ill patients (although this may be an option in resource-limited environments). Thus, CRRT is now the preferred method of renal support in the critically ill.
In critical illness complicated by AKI, if the underlying problems resolve, renal function usually recovers over a period of a few days to several weeks. However, there is good evidence to suggest that, after recovery, subtle defects in renal function persist, so that patients requiring RRT in the ICU have a significantly increased risk of developing chronic kidney disease in the months and years after critical illness.
Neurocritical Care
 Physiology
Physiology
Neurones are particularly susceptible to acute ischaemic/hypoxic insults, as they have very limited capacity for anaerobic metabolism and are irreversibly lost when blood flow is restricted for as little as 3–8 minutes.
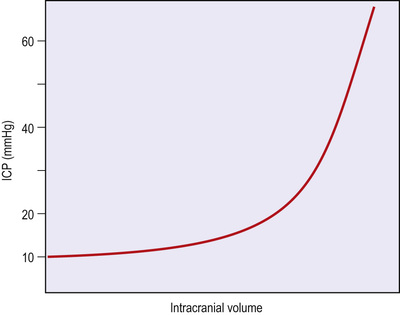
Within the rigid vault of the skull are housed the intracranial contents, which consist of brain parenchyma, blood and CSF. The intracranial pressure (ICP, pressure within the skull) is approximately 7–15 mmHg in a resting, supine adult. Changes in intra-abdominal and intrathoracic pressures, such as occur during coughing or a Valsalva manœuvre, may result in transient increases in ICP that are of no consequence. The Monro–Kellie doctrine states that, because the cranial compartment is rigid, any increase in the volume of one of the intracranial constituents (blood, CSF, brain tissue) must be compensated by a decrease in the volume of the others. As space-occupying lesions expand or brain tissue swells, the rise in ICP is limited by displacement of CSF and venous blood from the intracranial compartment. Once these compensatory mechanisms are exhausted, further increases in intracranial volume cause exponentially greater increases in ICP (Fig. 25.28).
In health, autoregulation maintains constant cerebral blood flow over a range of blood pressures. However, these mechanisms are lost following significant intracranial injury, and cerebral blood flow then becomes directly dependent on cerebral perfusion pressure (CPP). CPP is calculated as the MAP minus the ICP. Occasionally, a very high CVP, greater than the ICP, will also reduce CPP (Fig. 25.29).
 Clinical assessment
Clinical assessment
The causes of coma are listed in Box 21.27. Conscious level should be documented and any changes noted. The Glasgow Coma Scale (GCS) score (Box 21.26) is widely used as a semi-quantitative, yet crude, means of gauging the level of consciousness. Assessment of the airway is of particular importance. Patients with airway obstruction due to lax oropharyngeal musculature should be managed as discussed on page 1156. Similarly, patients without a cough or gag reflex may need to be intubated in order to protect the airway. Pupillary signs are crucial. Documentation of pupillary size and reactivity is crucial. A unilaterally dilated pupil that reacts sluggishly or not at all is frequently a sign of increasing ICP. This is caused by compression of the IIIrd cranial nerve by herniating brain; if untreated, it can be rapidly fatal. Immediate treatments to decrease ICP (Box 25.20) are required whilst a CT scan of the brain is obtained. In some situations, patients may be taken directly to the operating theatre in order to alleviate the raised ICP and to remove expanding space-occupying lesions such as a haematoma. Small or pinpoint pupils may be a sign of mid-brain injury or opioid overdose. New focal neurological signs should be sought, such as cranial nerve palsies, limb weakness or hemiparesis. Seizures should be recognized and treated appropriately (see p. 826).
 Monitoring
Monitoring
Regular clinical assessments by the bedside nurse are essential, including Glasgow Coma Scale score and pupillary size and reaction to light at least every hour. Neurological assessments are inevitably limited in patients requiring sedation and mechanical ventilation. In these patients, invasive monitoring of ICP may be necessary.
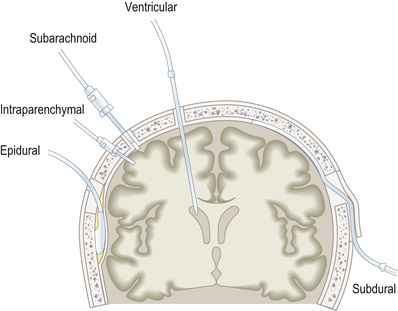
Invasive ICP monitoring devices may be extradural, subdural, subarachnoid, intraparenchymal or intraventricular (Fig. 25.30). The external ventricular drain (EVD) is the most difficult to insert, particularly when increased ICP causes the ventricles to collapse. EVDs are normally placed through the non-dominant (usually right) hemisphere into the lateral ventricle in the operating theatre. Their main advantage over the other monitoring devices is that they can also be used to treat raised ICP by draining CSF from the ventricular system. They are more prone to infection, however.
Multi-electrode electroencephalography (EEG) monitors give both a waveform and an analogue display that can help in the diagnosis of non-convulsive seizures and are also useful in monitoring the depth of sedation.
Oximeter-tipped catheters can be placed via the internal jugular vein and advanced in a cranial direction to lie in the jugular bulb just external to the skull. These ‘jugular bulb oximetry’ catheters monitor venous saturation in blood leaving the brain and give an indication of the balance between cerebral oxygen supply and demand. Measurements can be interpreted similarly to the mixed venous oxygen content (see pp. 1143–1144).
Cerebral oximetry uses near-infrared spectroscopy (NIRS) technology to estimate the oxygenation of a small portion of the cerebral cortex. Adhesive pads that both emit and capture near-infrared light waves are placed over the frontal cortex. Cerebral oximetry can provide an early warning of decreased cerebral oxygen delivery.
 Management
Management
Because little can be done to reverse the immediate primary brain injury and irreversible loss of neurones that occur as a direct consequence of intracerebral haemorrhage, infarct or traumatic brain injury, ICU care is focused on minimizing secondary brain injury (Box 25.20). This commonly occurs as a result of increases in ICP and consequent decreases in CPP. Cerebral oedema, expanding mass lesions (e.g. haemorrhage), prolonged seizure activity and hypercarbia (which causes cerebral vasodilatation) are all examples of mechanisms leading to secondary injury. Neurocritical care has therefore focused on monitoring ICP and maintaining CPP. The Brain Trauma Foundation (BTF) guidelines state that an ICP of <20 mmHg and a CPP of between 50 and 70 mmHg should be the aim, with vasopressors and fluid given as required in conjunction with supportive care; the latter should include airway protection, maintenance of normocarbia, reduction of the risk of nosocomial infections and prevention of thromboembolic complications.
Outcomes
Withholding and withdrawing treatment
(See also Ch. 3.)
For many critically ill patients, intensive care is undoubtedly life-saving and resumption of a normal lifestyle is to be expected. It is also widely accepted that the elective admission of high-risk patients into an ICU or HDU, particularly in the immediate postoperative period, can minimize morbidity and mortality and lower costs, as well as reducing the demands on medical and nursing personnel on general wards. In the most seriously ill patients, however, mortality rates are high and a significant number die soon after discharge from the ICU. Mortality rates are particularly high in those who require re-admission to intensive care. Moreover, patients surviving a prolonged ICU admission often do not regain their premorbid functional status, and longer-term mortality rates (for at least 5 years post discharge) remain higher than in a general population matched for age and illness severity. Many centres have established specialist follow-up clinics to address long-term sequelae of critical illness.
Inappropriate use of intensive care facilities has other implications. The patient may experience unnecessary suffering and loss of dignity, while relatives may also have to endure considerable emotional pressures. In some cases, treatment may simply prolong the process of dying or sustain life of dubious quality, and in others the risks of interventions outweigh the potential benefits. Lastly, intensive care is expensive, particularly for those with the worst prognosis, and resources are limited.
To ensure both a humane approach to the management of critically ill patients and the appropriate use of limited resources, it is necessary to:
• avoid admitting patients who cannot benefit from intensive care
• limit further aggressive therapy when the prognosis is clearly hopeless.
Such decisions are extremely difficult; every case must be assessed individually, taking into account previous health and quality of life, primary diagnosis, medium- and long-term prognosis of the underlying condition, and survivability of the acute illness. Age alone should not be the sole consideration. When in doubt, active measures should continue but with regular review in the light of response to treatment and any other changes.
Decisions to limit therapy, not to resuscitate or to withdraw treatment should be made jointly by the ICU medical staff, the primary physician or surgeon, the nurses and, if possible, the patient, normally in consultation with the patient's family. Limitation of active treatment is not the cessation of medical or nursing care; rather, a caring approach must be adopted to ensure a dignified death, free of pain and distress, with support for family and friends (see p. 41).
Scoring systems
A variety of scoring systems have been developed that can be used to evaluate the severity of a patient's illness. Some have included an assessment of the patient's previous state of health and the severity of the acute disturbance of physiological function (acute physiology, age, chronic health evaluation, or APACHE; and the simplified acute physiology score, or SAPS). Other systems have been designed for particular categories of patient (e.g. the injury severity score for trauma victims).
The APACHE and SAPS scores are widely applicable and have been extensively validated. They can accurately quantify the severity of illness and predict the overall mortality for large groups of critically ill patients, and are therefore useful for defining the ‘case mix’ of patients when auditing a unit's clinical activity, for comparing results nationally or internationally, and for characterizing groups of patients in clinical studies. Although the APACHE and SAPS methodologies can also be used to estimate risks of mortality, no scoring system has yet been devised that can predict with certainty the outcome in an individual patient. They must not, therefore, be used in isolation as a basis for limiting or discontinuing treatment.
Brain death and organ donation
Brain death means ‘the irreversible loss of the capacity for consciousness combined with the irreversible loss of the capacity to breathe’. Both of these are essentially functions of the brainstem. Death, if thought of in this way, can arise either from causes outside the brain (i.e. respiratory and cardiac arrest) or from causes within the cranial cavity. With the advent of mechanical ventilation, it became possible to support such a dead patient temporarily, although, in all cases, cardiovascular failure eventually supervenes and progresses to asystole.
Before deciding on a diagnosis of brainstem death, it is essential for certain preconditions and exclusions to be fulfilled.
Exclusions
• The possibility that unresponsive apnoea is the result of poisoning, sedative drugs or neuromuscular blocking agents must be excluded.
• Hypothermia must be excluded as a cause of coma. The central body temperature should be >35°C.
• There must be no significant metabolic or endocrine disturbance that could produce or contribute to coma or cause it to persist.
• There should be no profound abnormality of the plasma electrolytes, acid–base balance or blood glucose levels.
Diagnostic tests for the confirmation of brainstem death
All brainstem reflexes are absent in brainstem death.
The following tests should not be performed in the presence of seizures or abnormal postures.
• Oculocephalic reflexes should be absent. In a comatose patient whose brainstem is intact, the eyes will rotate relative to the orbit (i.e. doll's eye movements will be present). In a brainstem-dead patient, when the head is rotated from side to side, the eyes move with the head and therefore remain stationary relative to the orbit.
• The pupils are fixed and unresponsive to bright light. Both direct and consensual light reflexes are absent. The size of the pupils is irrelevant, although most often they will be dilated.
• Corneal reflexes are absent.
• There are no vestibulo-ocular reflexes on caloric testing (see p. 810).
• There is no motor response within the cranial nerve territory to painful stimuli applied centrally or peripherally. Spinal reflex movements may be present.
• There is no gag or cough reflex in response to pharyngeal, laryngeal or tracheal stimulation.
• Spontaneous respiration is absent. The patient should be ventilated with 100% O2 (or 5% CO2 in 95% O2) for 10 min and then temporarily disconnected from the ventilator for up to 10 min. Oxygenation is maintained by insufflation with 100% oxygen via a catheter placed in the endotracheal tube. The patient is observed for any signs of spontaneous respiratory efforts. A blood gas sample should be obtained during this period to ensure that the PaCO2 is sufficiently high to stimulate spontaneous respiration (>6.7 kPa, 50 mmHg).
The examination should be performed and repeated by two senior doctors.
In the UK, it is not considered necessary to perform confirmatory tests, such as EEG or carotid and vertebral angiography.
The primary purpose of establishing a diagnosis of brainstem death is to demonstrate beyond doubt that it is futile to continue mechanical ventilation and other life-supporting measures.
In suitable cases, and provided the assent of relatives has been obtained (easier if the patient was carrying an organ donor card or is on the organ donor register), the organs of those in whom brainstem death has been established may then be retrieved whilst the heart is still beating and be used for transplantation.
Organ donation may be possible in situations where ongoing life-sustaining treatment in the ICU is deemed futile and a decision is reached in conjunction with the family (and, in rare circumstances, the patient) to withdraw treatments such as invasive ventilation and inotropes that are simply delaying an inevitable death. If the patient and family wish to consider organ donation, then withdrawal of life-sustaining therapies usually occurs in a planned fashion and often in the operating department suite. If the patient does indeed die in a relatively brief period, then organ retrieval may occur immediately following a cardiac death.
Both of these situations require rapport and trust to be established with the family, and many hospitals have a separate team of nurse specialists to help the families through these very difficult situations. In the UK, each region has a transplant coordinator who can help with the process, as well as providing information, training and advice about organ donation. These transplant coordinators should be informed of all patients undergoing brainstem testing and all patients in whom withdrawal of life-sustaining treatment is being considered, so that all families and patients can be offered the opportunity to donate organs if they so wish. The medical teams involved in the ICU care of the patient and those involved in the organ retrieval process must remain completely independent.
Significant websites
http://www.esicm.org European Society of Intensive Care Medicine.
http://www.ficm.ac.uk Faculty of Intensive Care Medicine.
http://www.icnarc.org Intensive Care National Audit & Research Centre.
http://www.ics.ac.uk UK Intensive Care Society.
http://www.sccm.org/Research Society of Critical Care Medicine guidelines.